Abstract
Human cytomegalovirus (HCMV) is the leading cause of congenital disease in the developed world. Transmission of HCMV to the fetus can occur through the villous placenta. Previously, we have shown that although syncytiotrophoblast (ST) can be productively infected, it is more likely that HCMV reaches the fetus through breaks in the ST than through basal release of progeny virus from infected ST. Progeny virus released on the maternal side could interact back with the ST and accumulate. In pregnancy, the organ distribution of disease burden is dramatically shifted, with the placenta reported as a reservoir for some pathogens. Thus, we propose that the ST layer functions as a viral reservoir, where HCMV is harbored and ultimately protected from degradation. Using primary cytotrophoblasts differentiated into an ST culture in vitro and challenged with HCMV, we have defined reversible binding between the virus and trophoblasts that protects the virus from degradation. This is blocked by treatment with low pH and neutralizing intravenous immunoglobulin. This reversible binding likely is to heparan sulfate proteoglycans, because heparin treatment blocks it. Importantly, we show that bound and released virus maintained in culture for at least 48 h results from inoculum and not progeny virus. Thus, the placenta has the potential to accumulate a relatively high steady-state level of virus within the intervillous space resulting from localized binding and release at the ST. A better understanding of the molecular interactions between HCMV and ST will provide insights regarding interventions to prevent or minimize congenital transmission.
Keywords: fetal, human cytomegalovirus, infection, maternal, microvilliation, placenta, pregnancy, syncytiotrophoblast, viral reservoir
The syncytiotrophoblast layer of the placenta functions as a viral reservoir where HCMV is harbored and ultimately protected from degradation.
INTRODUCTION
Human cytomegalovirus (HCMV), from the Herpesviridae family, is transmitted in utero to approximately 40% of fetuses from mothers who develop a primary infection during pregnancy [1]. Whereas the most severe adverse neonatal outcomes are reported in this group, congenital infections are also found in approximately 1% of newborns from women with prepregnancy immunity [2, 3]. Neonatal sequelae range from no symptoms to significant neurodevelopmental problems, including sensorineural hearing loss, and in some cases can also lead to death. Importantly, asymptomatic congenital infections can also lead to late-onset, long-term complications [4, 5]. The importance of preventing HCMV congenital infections is exemplified in the considerable societal economic burden of caring for and supporting these children; in the 1990s, the estimated annual cost in the United States was $1.86 billion [6]. Seale et al. [7] reported that the Advisory Committee on Immunization Practices in the year 2000 estimated that implementation of an effective and fully utilized HCMV vaccine program could equate to a savings of $4 billion in U.S. health care costs.
Primary infections, reactivation, or reinfection with another strain during pregnancy are risk factors for intrauterine HCMV transmission to the fetus [8]. These types of infections can in part be differentiated by the presence of immunoglobulin M (primary, reactivation, and reinfection) or detection of multiple viral strains (reinfection). All lead to an active infection; however, viral load in blood [9, 10] or urine [11] does not correlate with intrauterine transmission, which occurs even when the virus level in these compartments is low or undetectable.
In pregnancy, the organ distribution of disease burden for some infections is dramatically shifted [12]. For example, the intracellular bacteria Listeria monocytogenes continues to replicate in the placenta of resistant female mice even though it is effectively cleared from the livers of these same mice [13]. In the guinea pig, a continuous placental infection with L. monocytogenes leads to repeated reinfection of the maternal organs until removal of the placenta [14]. Similarly, the intracellular parasite Plasmodium falciparum, which causes malaria, parasitizes red blood cells, which are predominantly found in the villous placenta of pregnant women. They bind to chondroitin sulfate A, which is highly expressed in the placental syncytiotrophoblast (ST) layer, where they cause placental damage associated with low-birth-weight babies [15, 16]. Furthermore, lymphocytic choriomeningitis virus, a single-stranded RNA virus, in the mouse is rapidly cleared from all tissues except for the placenta [17]. The above are striking examples of possible adverse consequences of the 12-m2 surface area of placental trophoblast present in term pregnancies that has been characterized as an immune-privileged site [18, 19]. To our knowledge, whether the placenta sequesters HCMV even when cleared from maternal blood and urine, as happens with L. monocytogenes, and thereby facilitates fetal infection or prolongs maternal disease has not been explored. It has been suggested that the guinea pig placenta acts as a viral reservoir for guinea pig CMV [20, 21]. Here, the virus is protected from circulating maternal antibodies, thereby maintaining the infection throughout gestation [22].
In the placenta, maternal blood is separated from fetal blood vessels and stromal tissue by trophoblasts. The villous trophoblast consists of a mature, multinucleated ST layer that faces maternal blood and an underlying layer of replicative mononuclear cells, called cytotrophoblasts (CT), that replenish the ST by fusion to its basal surface. Using sensitive PCR, viral DNA has been detected in all cell types of the placenta at much higher rates than initially believed: 63% of first-trimester placentas [23], 74% of second-trimester placentas [24], and 9.8%–62% of term placentas [23–26]. However, evidence for a widespread, productive infection as detected by viral gene expression, particularly in the ST layer, is limited [27, 28], even though mature virus is often found associated with ST. Collectively, these animal models and human studies suggest that the placenta acts as a repository to collect and protect mature and infectious virus, which increases the risk of fetal infection [29]. The ST layer is highly microvilliated and functions as a barrier between maternal and fetal circulations, in part through several mechanisms that contribute to immune privilege. These characteristics suggest that HCMV is being sequestered and thereby protected from inactivation in and around the ST layer of the placenta. The initial attachment of HCMV to cells involves tethering by viral envelope glycoproteins to cell surface heparan sulfate proteoglycans (HSPGs) that act as low-affinity receptors [30, 31]. Using a model of highly purified villous CT differentiated with epidermal growth factor (EGF) into ST-like cultures [28, 32], we tested the hypothesis that HCMV binds to HSPGs on ST and is protected from degradation.
MATERIALS AND METHODS
Cell Preparations and Culture
Isolation and culture of term villous CT.
Placentas were obtained with patient consent as approved by the Human Ethics Research Board at the University of Alberta and Alberta Health Services after normal term delivery or elective cesarean section from uncomplicated pregnancies. Isolation of villous CT (purity, >99.99%) by trypsin/DNase digestion of minced chorionic tissue and immunoabsorption onto immunoglobulin-coated glass bead columns has been previously described [33]. Immunoelimination was carried out with anti-CD9 (Clone 50H.19), anti-major histocompatibility complex (MHC) class I (W6/32; Harlan Sera-Lab), and anti-MHC class II (Clone 7H3) antibodies. Trophoblasts were isolated from five different placentas and cryopreserved for use in the present study. Isolated CT were cultured in Iscove modified Dulbecco medium (IMDM; Gibco) supplemented with 10% fetal bovine serum (FBS) and 50 μg/ml of gentamicin in the absence of recombinant human EGF (rhEGF; Pepro-Tech). An ST culture is defined as CT that are syncytialized by treatment with 10 ng/ml of rhEGF for 5 days [28, 32]. Syncytialization was confirmed by immunohistochemically staining fixed cells with anti-desmoplakin monoclonal antibody (Sigma Immunochemicals) to visualize desmosome-containing tight junctions as previously described [34]. All trophoblast preparations contained fewer than five vimentin-positive cells (nontrophoblasts) per well in a 96-well culture plate (seeded with 1 × 105 cells/well) as assessed by immunohistochemistry with vimentin antibody (Clone V9; DAKO Corporation).
Human embryonic lung fibroblasts.
Human embryonic lung (HEL) fibroblasts (a gift from Dr. J. Preiksaitis, Dept. of Medicine, University of Alberta) are highly sensitive to HCMV infection and were used in two ways for the present study. First, they were used as a comparison for binding and release studies in trophoblast cultures. Second, they were used as an indicator cell line to quantify viral titer of stock virus and experimental virus released into supernatants or remaining cell adherent [28]. HEL fibroblasts (4 × 104 cells) were plated in 100 μl of Eagle minimum essential medium supplemented with 10% FBS and 50 μg/ml of gentamicin in 96-well tissue culture wells and cultured to confluency. All cultures were used within 48 h of plating.
CaCo2 cells.
Human epithelial colorectal adenocarcinoma cells (CaCo2 cells; a gift from Dr. T. Hobman, Dept. of Cell Biology, University of Alberta) represent another tight junctioned, microvilliated, epithelial cell type [35]. They were used as a comparison for binding and release studies in trophoblast cultures. CaCo2 cells (2 × 105 cells) were plated in 100 μl of Dulbecco modified Eagle medium supplemented with 10% FBS and 50 μg/ml of gentamicin in 96-well tissue culture wells and cultured to confluency. All cultures were used within 48 h of plating.
Stock Virus Preparation and Quantification of Viral Titer
Virus stock preparations.
An HCMV laboratory strain, AD169, and a low-passage (maximum, seven passages) HCMV clinical isolate, Kp7 (a gift from Dr. J. Preiksaitis, Dept. of Medicine, University of Alberta), were propagated in HEL fibroblasts as previously described [28]. Briefly, infected cells and supernatant were removed from the flask with a cell scraper (Becton Dickinson) and sonicated on ice with a Vibracell sonicator (Sonics and Materials, Inc.). The virus preparation was purified by passage through a filter (pore size, 0.45 μm; MILLEX-HV; Millipore Products Division) and stored in liquid nitrogen until use.
Quantification of viral titer.
Stock virus titer was assessed by serial dilution into HEL fibroblast microcultures, which were then centrifuged to increase the sensitivity of detection [36] and cultured for a further 18 h as previously described. Infectious virions were quantified by counting nuclei positive for HCMV immediate early (IE) antigen [28, 37]. The titer was determined within a linear dose-response concentration range as infectious virions per milliliter.
Experimental Protocols
Addition of inoculum virus.
Inoculum virus was added at various multiplicity of infection (MOI), as described in each figure legend, in 2% FBS/IMDM at 37°C in 5% CO2 in the presence (10 ng/ml; ST cultures) or absence (CT, CaCo2, and HEL fibroblast cultures) of rhEGF. The ST, CT, and CaCo2 were challenged with 1, 10, or 20 MOI, and HEL fibroblasts, which are more readily infected in culture, were challenged with 0.2 MOI. The plates were immediately centrifuged at 2500 rpm for 30 min [36], followed by incubation for 4 or 24 h before treatment as outlined below and in the individual figure legends. MOI was calculated as the number of infectious virions per nuclei per well. The number of nuclei per well was calculated in parallel wells stained with hematoxylin (Sigma) by multiplying the mean number of nuclei counted in five 0.25-mm2 fields by the total number of fields per well (123 fields at 200× magnification).
Culture treatments.
After initial incubation with inoculum virus, before any treatment listed below, all cultures were thoroughly washed five times with serum-free IMDM to remove any loose and nonadherent virus.
After washing, cultures with adherent inoculum virus were refed with 2% FBS/IMDM and further incubated as noted in individual figure legends. Removal of adherent inoculum virus was performed as follows: For low-pH treatment, cultures after washing were incubated with a PBS solution (pH 3.0) for 2 min to remove adherent virus [38], washed three times with IMDM, refed with fresh 2% FBS/IMDM, and further incubated as noted in the individual figure legends. For intravenous immunoglobulin (IVIG) treatment, cultures after washing were incubated with IVIG (a gift from Talecris Biotherapeutics), which contains neutralizing antibodies to HCMV [39], at a concentration of 10 mg/ml for 48 h (see Figs. 1 and 2) or for varying periods of time (0–360 min), followed by a thorough wash and a further 6-h incubation (see Fig. 6). The lowest concentration of this batch of IVIG that gives more than 95% inhibition of infection on HEL fibroblasts after a 10-min preincubation is 10 mg/ml (results not shown). These experiments were repeated at 4°C to prevent viral entry [30]. Thirty minutes before incubation with or without IVIG, cell culture plates were placed at 4°C, and the remainder of the experiment was carried out at this temperature.
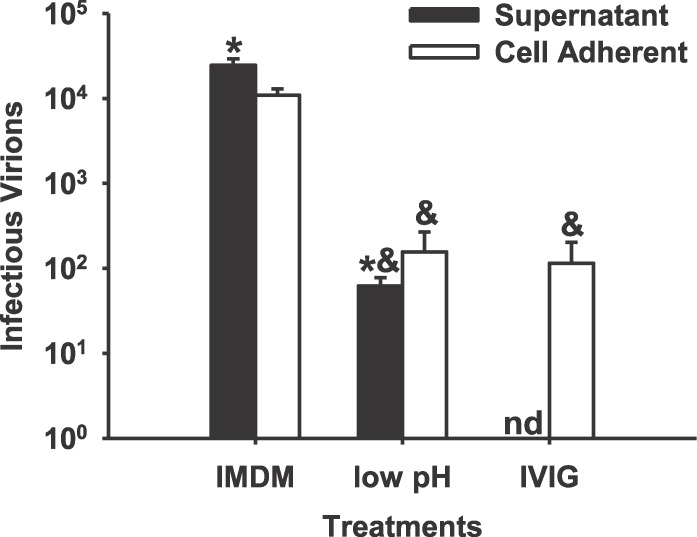
FIG. 1.
Strongly adherent inoculum virus that is released into ST culture supernatants and remains infectious over 48 h is dramatically reduced by low-pH or neutralizing antibody treatment. An HCMV laboratory strain, AD169, was added to ST cultures at an MOI of 10 for 24 h, then washed five times with IMDM and further incubated for 48 h. Some cultures were then incubated for 2 min in PBS (pH 3.0), washed well, and further incubated for 48 h (low pH). Other cultures were incubated in 10 mg/ml of IVIG for 48 h following the initial IMDM wash (IVIG). Cell adherent and supernatant infectious virus was assessed as described in Materials and Methods. Results are presented as the mean ± SEM from three independent experiments. &, significantly different from IMDM (P < 0.05); *, significantly different from cell adherent virus (P < 0.05); nd, not detected.
FIG. 2.
Release of strongly adherent infectious inoculum virus in CT, HEL, and CaCo2 culture supernatants. AD169 was added to CT (A) and HEL (B) cultures, and Kp7 was added to CaCo2 cultures (C), at an MOI of 10 for 24 h, then extensively washed five times with IMDM. Some cultures were then incubated for 2 min in PBS (pH 3.0) and again washed well (low pH). After a further 48 h (A and C) or 24 h (B) of incubation in 2% FBS/IMDM, cell adherent and supernatant infectious virus was measured as described in Materials and Methods. Other cultures were incubated in 10 mg/ml of IVIG for 48 h (A and C) or 24 h (B) following the initial IMDM wash. Results are presented as the mean ± SEM from three independent experiments. &, significantly different from IMDM (P < 0.05); *, significantly different from cell adherent virus (P < 0.05); nd, not detected.
FIG. 6.
Neutralizing antibody treatment time dependently reduces the amounts of released and cell adherent virus. After addition of Kp7 for 4 h at an MOI of 10 followed by five IMDM washes, IVIG was added to ST cultures for varying periods of time (x-axis) followed by a thorough wash and a further 6-h incubation in 2% FBS/IMDM. Supernatants (A) and cell lysates (B) were assessed for infectious virus as described in Materials and Methods. Cell-adherent virus after IVIG treatment was graphed as the natural log over time (C). The slope of this line was used to calculate the rate of release as a percentage of the bound virus at time zero. Results are presented as the mean ± SEM from three independent experiments. *, significantly different from zero time point (P < 0.05).
Following addition of inoculum virus and centrifugation at 2500 rpm for 30 min, some cultures were treated with 40% polyethylene glycol for 30 sec and then extensively washed to increase adherence of virus to cells [40]. Some cultures were treated with cycloheximide (1 μg/ml) to prevent viral replication 30 min before addition of inoculum virus and throughout the culture period.
Virus was treated for 30 min at 37°C with 20 μg/ml of heparin sodium salt (H9399; Sigma-Aldrich) before addition to cell cultures.
Some cultures were fixed in 4% paraformaldehyde before addition of inoculum virus to provide evidence that protection of HCMV by reversible binding is an active cellular process.
Quantification of inoculum virus remaining cell adherent or released into supernatants.
Culture supernatants were removed at various times after the above-mentioned treatment protocols and frozen at −80°C until assessed for viral titer. Cell cultures were washed three times with PBS, lysed in 100 μl of 2% FBS/IMDM by freezing and thawing three times, and stored at −80°C until assessment of viral titer (cell adherent virus). Viral titers were quantified on confluent HEL fibroblast cultures as described above.
Assessment of HCMV IE antigen production in ST, CT, HEL, or CaCo2 cultures after treatment.
Evidence for infection of all cell cultures after the above-mentioned treatments was determined by the presence of HCMV IE-positive nuclei using immunohistochemistry. The average percentage of IE-positive nuclei from five 0.25-mm2 fields in each microwell was assessed and reported as the mean ± SD of three microwells. All primary trophoblast preparations were assessed for a preexisting HCMV infection by staining control cultures unchallenged by virus for IE antigen expression as described below. Additionally, all trophoblast preparations were negative for HCMV glycoprotein B as assessed by real-time PCR. None of the trophoblast preparations used in the present study stained positive for IE antigen before addition of inoculum virus. Productive infection (production of progeny virus) in ST or CT cultures has previously been shown in our lab to be slower than that found in fibroblast cultures (96 h compared to 48 h) [28].
Immunohistochemistry
Infected and uninfected cultures were washed twice with PBS, fixed in ice-cold methanol for 10 min at −20°C, and washed three times with PBS. Endogenous peroxidase activity was neutralized by a 30-min incubation at room temperature with 3% H2O2 and followed by a 1-h blocking incubation at room temperature in 10% nonimmune goat serum (Zymed/Intermedico). Primary antibodies detecting HCMV IE (p72; Specialty Diagnostics, Dupont) or its isotype control, IgG2a (Zymed/Intermedico), were added, after which the plates were sealed with parafilm and incubated overnight at 4°C. After thorough washing with PBS, secondary antibody (biotinylated goat anti-mouse IgG) and streptavidin-peroxidase conjugate (Streptavidin Biotin System, Histostain-SP Kit; Zymed) were added according to the manufacturer's instructions. Following a PBS wash, amino-ethyl carbazole substrate was added for 2–5 min, yielding a red precipitate. The wells were then washed with double-distilled water and the number of IE-positive nuclei assessed per microwell.
Statistics
Cryopreserved trophoblasts from five different placentas were used in these experiments. Whereas the ability of HCMV to infect a given trophoblast preparation is consistent in experiments repeated on different days, the fraction of infected cells in different preparations can be considerably variable even if examined at the same time. However, the trends remain consistent between different trophoblast preparations: Regardless of what the infected fraction of a cell preparation is on Day 2, it is 20%–50% higher on Day 8. However, the large variability in absolute number between placental preparations prevents pooling results from multiple experiments done with cells isolated from different placentas. Thus, the results presented here are from triplicate experiments performed with a single representative trophoblast preparation. When statistics are carried out, Student t-test is used to compare the error between two groups, and one-way ANOVA with Fisher post-hoc test is used for multiple comparisons (P < 0.05).
RESULTS
Inoculum Virus Strongly Adheres to ST, Is Released Back into Culture Supernatants, and Remains Infectious
AD169, a laboratory strain of HCMV, was added to ST cultures at an MOI of 10 for 24 h. The cultures were extensively washed with IMDM to remove loosely adherent virus and further incubated with fresh 2% FBS/IMDM for an additional 48 h. Equivalent amounts of infectious inoculum virus were detected in both culture supernatants (released virus) and washed cell lysates (cell adherent virus) (Fig. 1). Treatment of cultures with PBS (pH 3.0) for 2 min before the extensive washing step to remove surface-bound virus dramatically reduced cell adherent and released virus after 48 h (Fig. 1). Treatment with neutralizing antibodies to HCMV (10 mg/ml of IVIG) after the extensive washing step to capture any released virus also reduced cell adherent and released virus after 48 h of additional incubation. Neither a 2-min PBS treatment at pH 3.0 nor a 2-day IVIG treatment affected the total number of nuclei in any culture (results not shown).
To determine if inoculum virus could remain infectious over a similar time period in the presence of other types of cells, we repeated these experiments in less differentiated trophoblasts (CT), fibroblasts (HEL), and CaCo2 (Fig. 2). Both CT and CaCo2 showed results similar to those found with ST. In contrast, the remaining inoculum virus levels in HEL cultures were approximately 100-fold less (Fig. 2). HEL cultures were incubated for 24 h rather than 48 h to ensure no progeny virus would be produced.
Infectious Virus Detected in Supernatants and Cell Lysates Is Not Due to Progeny Virus Produced by Infected Cells
It is important to note that infectious virus recovered at this time point in ST cultures likely is inoculum virus (that added at the beginning of the experiment) and not progeny virus (that produced by infected cells), because we have previously shown in these cells that production of progeny virus requires at least 4 days [28]. Two further experiments were done to confirm this. First, a shorter time for initial viral contact with the cells (4 h) before extensive washing was followed by further incubation in a time course from 0.5 to 24 h to ensure the total length of time was less than that required for production of progeny virus in these cultures. In these and other experiments, we used a low-passage clinical isolate, Kp7, rather than the laboratory strain, AD169, to confirm that this effect was not caused by the many gene deletions found in AD169 [41]. Both cell adherent and released virus were detected at all time points (Fig. 3) and were significantly reduced by low-pH treatment (results not shown), as seen in Figures 1 and 2. However, the total amount of Kp7 detected was significantly less than that of AD169, likely because of the reduced viral challenge time in the Kp7 experiments. As well, only 25.2% of total Kp7 recovered 24 h after extensive washing was found in the supernatants (Fig. 3), compared to 69.2% of total AD169 (Fig. 1). The second experiment to show that virus detected in these experiments was not due to progeny virus was to treat the ST cultures with cycloheximide to inhibit viral protein synthesis. This treatment had no effect on released or cell adherent virus (Fig. 4). Taken together, the results with AD169 and Kp7 demonstrate that strongly adherent virus (i.e., virus that remains after the extensive washing step) is released into the supernatant quickly (<30 min), with equilibration occurring between released and adherent infectious inoculum virus for at least 48 h after initial exposure of the cultures to virus.
FIG. 3.
Time course of cell adherent and released virus in ST cultures. An HCMV clinical isolate, Kp7, was added to ST cultures at an MOI of 10 for 4 h, then washed five times with IMDM. The cultures were then further incubated in 2% FBS/IMDM for various periods of time (0.5–24 h). Cell adherent and supernatant infectious virus was assessed as described in Materials and Methods. Results are presented as the mean ± SEM from three independent experiments. *, significantly different from cell adherent virus (P < 0.05).
FIG. 4.
Strongly adherent inoculum virus and its release in ST cultures is not affected by cycloheximide treatment. An HCMV clinical isolate, Kp7, was added to ST cultures at an MOI of 10 for 4 h, followed by further incubation for 24 h in the presence and absence of 1 μg/ml of cycloheximide. Results are presented as the mean ± SEM from three independent experiments. *, significantly different from cell adherent virus (P < 0.05).
Low Infection Levels in ST Are Not Affected by Low-pH or IVIG Treatments
Further support for detection of inoculum rather than progeny virus in these experiments is the finding that the ST is poorly infected, as previously seen [28]. The total amount of recovered virus from extensively washed but otherwise untreated cultures was 3.55 × 104 plaque-forming units. The total number of nuclei in these cultures is approximately 4 × 104. Thus, the maximum expected infection efficiency would be approximately 90%. The finding of a low level of infection in these cultures 48 h after the extensive washing step (2.6% ± 0.5%) that is not affected by low-pH treatment (2.4% ± 0.3%) or treatment with IVIG (1.9% ± 0.2%) suggests that the majority of strongly adherent virus in ST cultures does not enter and infect the cells. However, increasing the contact time of virus to cells or centrifugation of virus onto cell cultures significantly increases infection efficiency in all cell types tested (Fig. 5). Likewise, increasing adherence and fusion of the virus to the cells using polyethylene glycol treatment [40] also increased infection efficiency (data not shown). Overall, ST cultures showed the poorest infection efficiency, followed by CT cultures, with the highest infection efficiency in HEL cultures (Fig. 5A). The infection efficiency in CaCo2 cultures was similar to that of CT cultures (Fig. 5).
FIG. 5.
HCMV infection efficiency in cell cultures as a function of initial virus exposure time. AD169 was added to ST and CT cultures (A), and Kp7 was added to ST and CaCo2 cultures (B), at an MOI of 10. AD169 was added to HEL fibroblast cultures (A) at an MOI of 0.2. After a 2- or 24-h challenge time, each culture was washed five times in IMDM, incubated for a further 48 h (ST, CT, or CaCo2) or 18 h (HEL), and immunohistochemically stained for HCMV IE antigen. Percentages were calculated by dividing the total number of IE-positive nuclei by the total number of nuclei per well and these are reported as the mean ± SEM of triplicate wells from three independent experiments. *, significantly different from 2-h time point (P < 0.05).
Neutralizing HCMV Antibody Treatment Time Dependently Reduces Cell Adherent and Released Virus in ST Cultures
One mechanism for the equilibration of inoculum virus between cell adherent virus and that found in supernatants over time is through repeated binding and release or reversible binding, as previously shown over a much shorter time frame in mouse fibroblasts [42]. To evaluate this possibility in ST cultures, neutralizing antibodies to HCMV were used to inactivate any virus reaching the supernatant (Fig. 6). The half-life of supernatant virus was 6.91 ± 0.10 min, and the half-life of cell adherent virus was 9.24 ± 1.24 min over a 60-min time frame (no significant difference), showing that equilibrium had been reached (Fig. 6, A and B). Released virus is inactivated by IVIG and, thus, is unable to rebind to the ST, resulting in a continual decrease in the amount of cell adherent virus compared to the amount found with no IVIG treatment over the same time (Fig. 6, A and B). By treating the cultures with IVIG for various time points after the extensive washing step followed by another thorough wash and further incubation, we found approximately 4.98% of the inoculum was released per minute (Fig. 6C). The rate of release was calculated from the slope of the natural log of the bound virus versus time of IVIG treatment (Fig. 6C). This experiment was repeated at 4°C with equivalent results (data not shown).
Pretreatment of Virus with Heparin Significantly Reduces Reversible Binding
The HSPGs are low-affinity receptors used for initial binding by HCMV on a variety of cell types [30, 31]. In vitro, HCMV virions are initially dissociable from the cell surface of fibroblasts by soluble heparin, suggesting that HSPGs could be the receptors involved in reversible binding [30, 31]. To determine if reversible binding is dependent on HSPGs, we pretreated virus with 20 μg/ml of heparin for 30 min at 37°C and then challenged ST cultures with pretreated or untreated virus at an MOI of 10 for 4 h. The cultures were extensively washed with IMDM to remove loosely adherent virus and further incubated with fresh 2% FBS/IMDM for 30 min or 2, 4, or 24 h. Equivalent amounts of infectious inoculum virus were detected in both culture supernatants (released virus) and washed cell lysates (cell adherent virus) from cultures receiving untreated virus (Fig. 7A). However, the amount of virus detected in the supernatants and cell lysates from cultures receiving the heparin-treated virus was dramatically less than that detected in cultures receiving untreated virus (Fig. 7B).
FIG. 7.
Pretreatment of virus with heparin dramatically reduces reversible binding of HCMV to ST. The ST cultures were challenged for 4 h at an MOI of 10 with untreated Kp7 or with Kp7 pretreated with 20 μg/ml of heparin for 30 min. Cultures were then extensively washed and further incubated as described in Materials and Methods, and infectious virus was isolated from supernatants and cell lysates at the respective time points. A) Untreated virus. B) Virus pretreated with heparin. Results are presented as the mean ± SEM from three independent experiments. The lack of detectable error bars is due to very little interexperimental error. *, significantly different from cell adherent virus (P < 0.05).
Maintenance of Infectious Cell Adherent and Supernatant Virus Over Time Is Dependent on the Presence of Live Cells
To determine if the maintenance of cell adherent virus and that found in the supernatants in ST cultures over time was an active process requiring live cells, experiments were repeated either in the absence of cells or in the presence of cells fixed with paraformaldehyde. The rate of loss of infectious virus over a 24-h time period was significantly greater in cell-free (Fig. 8A) or fixed ST cultures (Fig. 8B) compared to live ST cultures (P < 0.05).
FIG. 8.
HCMV is protected from inactivation by live ST cultures. A) Kp7 was added at an MOI of one to ST cultures or to microwells containing medium only (no cells). Supernatants were removed from triplicate wells at each time point and assessed for infectious virus as described in Materials and Methods. Results are presented as the percentage (mean ± SEM) of infectious virus remaining relative to the original inoculum. B) Kp7 was added (at an MOI of 10) to live ST cultures or ST cultures fixed with 4% paraformaldehyde. Infectious virus from combined cell lysates and supernatants were assessed as described in Materials and Methods. Results are presented as the mean ± SEM from three independent experiments. *, significantly different from live ST (P < 0.05); nd, not detected.
DISCUSSION
Human cytomegalovirus is the most common cause of congenital infection in newborns [1]. It was initially thought that almost all symptomatic congenital HCMV infections occurred after a primary maternal infection; however, it is now known that recurrent maternal infections also lead to symptomatic congenital infections [43]. These two scenarios create a danger for every pregnant woman, because developing an effective means for screening and/or prevention for HCMV remains an obstacle [21]. In the present study, we examined the relationship between HCMV and the cell layer that forms a barrier between maternal and fetal circulations, the ST. Using neutralizing antibodies, we showed that HCMV can reversibly bind to ST cultures and be protected from degradation for over 48 h in culture. Importantly, we confirmed that the detected infectious virus was inoculum virus and not progeny virus. Although microvilliation may be important for the reversible-binding process, ST cultures were poorly infected even after longer exposure times. This demonstrated that even when virus was continuously present, the ST still functioned as a barrier to HCMV by preventing infection. However, these data support the concept that virus being sequestered within the ST would have ready access to the fetus through breaks in this layer [44].
After thorough washing of ST cultures in which HCMV had been incubated, we found a pool of infectious HCMV released into culture supernatants and a pool remaining adherent to ST. This distribution of virus was maintained for at least 48 h. Both cell adherent and supernatant virus were significantly reduced by low-pH treatment and by treatment with immunoglobulin containing neutralizing antibodies to HCMV. Because low-pH treatment removes strongly adherent virus from the cell surface, these results indicate that strongly adherent virus does not enter the ST for the most part but remains infectious and is released back into culture supernatants. Infectious virus that remains adherent after extensive washing binds reversibly to the ST and when released is neutralized by IVIG treatment, preventing rebinding. In a clinical study conducted by Nigro et al. [45], pregnant women with primary HCMV infections, as defined by seroconversion and HCMV DNA-positive amniotic fluid, were treated by IVIG at a one-time dose of 200 U/kg maternal weight. It was concluded that IVIG may be effective in the treatment and potential prevention of congenital HCMV infection, because only 3% of babies born to the IVIG-treated group were born with HCMV disease, compared to 50% of babies born to the untreated group [45]. It has been suggested that the function and health of the placenta is improved by IVIG treatment [46]. Our results support these conclusions by providing a mechanism whereby IVIG neutralizes the active virus and prevents it from readhering to the ST in the placenta.
Importantly, the infectious virus detected in supernatants and adherent to cells was found to be from the original inoculum and not progeny virus produced by infected cells. Indeed, our experiments done in the presence of cycloheximide, to inhibit viral protein synthesis, did not affect reversible binding. This is further supported by our previous study showing that progeny virus production in ST cultures does not occur until 4 days after exposure to HCMV [28]. The inoculum virus remained infectious only in the presence of live cells and not in cultures fixed in formaldehyde or in cell-free medium. Interestingly, when reversible-binding experiments utilizing IVIG treatment were done at 4°C, the results were similar to those at 37°C. Whereas the trophoblast is the most metabolically active tissue in the placenta [47] and HCMV entry into cells is not metabolically silent [48], these results suggest that metabolic activity is not necessary for reversible binding to occur.
Reversible binding of HCMV to the ST in the local environment of the placenta could lead to sequestration of the virus even in the presence of a properly established immune response. In a murine model, lymphocytic choriomeningitis virus can be effectively removed from the maternal system through an active virus-specific, CD8 T-cell response yet still remain present in the placenta [17]. This indicates that the lack of clearance from the murine placenta is not caused by the lack of a maternal immune response [17]. The increased and prolonged presence of HCMV within the placenta increases the probability of entry into the fetal compartment even when viral levels are low or undetectable in maternal blood or urine [20]. Thus, the placenta has the potential to accumulate a relatively high steady-state level of virus within the intervillous space resulting from localized binding and release events at the ST. These data are consistent with those of previous studies that suggest the placenta functions as a viral reservoir for guinea pig CMV, which can cross the guinea pig placenta and cause an infection in utero [12, 49, 50]. It appears as though the physical structure of the ST layer in the human placenta, which provides protection for the fetus, inadvertently also protects HCMV by allowing it to bind in and around its receptors in the ST layer. Thus, a viral reservoir is created where HCMV is maintained, although ST is not readily infectable.
Considerable reversible binding occurs in ST and CaCo2 cultures, which are both highly microvilliated [35, 44]. In contrast, fibroblasts with no microvilliation showed 100-fold less reversible binding but the highest infection levels. CT cultures show reversible binding similar to that of the ST, which likely is attributable to the differentiation that immediately begins as soon as CT cells are plated in culture [32]. Indeed, CT cells at term show some microvilliation when examined under the electron miscroscope, although not to the extent of that seen in the ST [51]. CT cells isolated using the same methodology as we have described and cultured for 24 h spontaneously develops microvillae [32]. Thus, we speculate that microvilliation may be an important cellular requirement for reversible binding of HCMV. Interestingly, ST cultures display the lowest infection levels, with CaCo2 cultures showing a 2.5-fold higher infection efficiency at 2 h and 3-fold higher at 24 h. Thus, ST is functioning as a strong protective barrier to viral infection even in the prolonged presence of HCMV through reversible binding. The difference between infection efficiency of ST and CaCo2 could be attributed to the differences in expression of cell-specific receptors for HCMV tethering, docking, and entry.
The initial tethering of HCMV is to HSPGs, which act as low-affinity receptors, and this is followed by high-affinity binding to cell surface receptors, committing the virus to cell entry [30, 31]. It has previously been shown that HSPGs are a vital first step in HCMV infection; for example, HCMV does not bind to or infect fibroblast cells lacking HSPGs [30]. We postulated that the reversible binding in ST cultures occurs by continuous reversible interactions of the virus with HSPGs. We found that pretreatment of virus with heparin dramatically reduced reversible binding in ST cultures. Heparin binds to viral glycoproteins, blocking HCMV binding to HSPGs and implicating HSPGs as the viral receptor responsible for reversible binding. It is possible, however, that the initial binding of HCMV to HSPGs is required for subsequent binding to another unidentified, low-affinity receptor that is responsible for the reversible binding. This intermediate step is unlikely, because at least in fibroblasts, progression from low-affinity binding to HSPGs progresses directly to high-affinity binding to other cell surface receptors that commits the virus to cell entry [30, 31]. HSPGs can act as either receptors or coreceptors for various ligands, stimulating signal transduction leading to gene regulation [52]. It is therefore likely that in addition to sequestering virus in the placenta, reversible binding of HCMV to HSPGs also stimulates cell signaling. Viral attachment alone is known to stimulate the cell to produce prostaglandins, reactive oxygen species, and activation of nuclear factor kappa B, leading to production of inflammatory cytokines [53, 54]. This has important implications for normal placental function, particularly with respect to immune privilege.
Placental infection precedes fetal infection, as shown by the presence of RNA transcripts for viral proteins in each trimester and in all cell types [25, 55]. We and others have previously shown that term ST can be productively infected in culture [28, 56]. However, progeny virus is mostly retained by these cells, with less than 30% released apically (maternal side) and less than 1% released basally (fetal side) [57]. It is therefore unlikely that an ST infection leads directly to a fetal infection; however, progeny virus released into the local placental environment on the maternal side from either intact or sloughed infected ST could interact back with the ST, accumulate through reversible binding, and be protected from inactivation. Interestingly, one study showed that mouse CMV binds to fibroblasts in a stable but reversible manner before infecting, which leads to an equilibrium of bound and free virus [42]. In this case, however, the reversible binding only occurs over a short time frame of 5 min in vitro [42]. We find a similar interaction with trophoblasts, which suggests that virus generated during maternal viremia or released from infected ST could be sequestered and thereby inadvertently protected by the 12-m2 ST layer and increase the viral load in the placenta. This occurs in the presence of an established immune response to which the placenta is not susceptible, but the ST still effectively prevents a high infection level through some cellular mechanism yet undefined. It has been shown that the interaction of HCMV with innate immune coreceptors CD14 and TLR2, on the surface of cultured ST, increases tumor necrosis factor alpha expression and induces apoptosis in neighboring uninfected cells, ultimately leading to damage of the villous trophoblast [44, 58]. Thus, the continual proximity of infectious virus in or around the ST would increase the potential for breaks and also accessibility of HCMV to fetal tissues through breaks in the ST layer [44] or through Fc receptor uptake of virus-antibody complexes [59] and ultimately increase fetal infection. Indeed, high levels of virus localized to the placenta increased the risk of in utero transmission in the guinea pig [49].
In the present study, we demonstrate that the placenta can serve as a reservoir for HCMV through reversible binding of virus to HSPGs in and around the ST layer. Reversible binding protects HCMV, thereby facilitating its prolonged presence in and around the placenta. This observation and the knowledge that during an active infection, correlation is lacking between viral load in blood [2, 4] or urine [11] and intrauterine transmission have important implications for prevention and prophylactic treatment of viral infections during pregnancy. It is important to focus on the correlation of viral load in the placenta to clinical diagnosis of congenital HCMV infection. Insights regarding the interaction of the placenta and virus will provide data to further understand the progression of congenital HCMV infections.
ACKNOWLEDGMENTS
We thank the research nurse, Donna Dawson, R.N., at the Tissue Collection Core of the Women and Children's Health Research Institute and the delivery room staff at the Royal Alexandra Hospital in Edmonton for their assistance in timely collection of placentas for the present study.
Footnotes
Supported by Women and Children's Health Research Institute (WCHRI), Natural Sciences and Engineering Research Council of Canada (NSERC), and the Canadian Institutes of Health Research (CIHR).
REFERENCES
- Demmler GJ. Infectious Diseases Society of America and Centers for Disease Control. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev Infect Dis 1991; 13: 315 329. [DOI] [PubMed] [Google Scholar]
- Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med 2001; 344: 1366 1371. [DOI] [PubMed] [Google Scholar]
- Stagno S, Pass RF, Cloud G, Britt WJ, Henderson RE, Walton PD, Veren DA, Page F, Alford CA. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 1986; 256: 1904 1908. [PubMed] [Google Scholar]
- Ahlfors K, Ivarsson SA, Harris S. Report on a long-term study of maternal and congenital cytomegalovirus infection in Sweden. Review of prospective studies available in the literature. Scand J Infect Dis 1999; 31: 443 457. [DOI] [PubMed] [Google Scholar]
- Williamson WD, Demmler GJ, Percy AK, Catlin FI. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics 1992; 90: 862 866. [PubMed] [Google Scholar]
- Porath A, McNutt RA, Smiley LM, Weigle KA. Effectiveness and cost benefit of a proposed live cytomegalovirus vaccine in the prevention of congenital disease. Rev Infect Dis 1990; 12: 31 40. [DOI] [PubMed] [Google Scholar]
- Seale H, Booy R, MacIntyre CR. Trends in hospitalizations for diagnosed congenital cytomegalovirus in infants and children in Australia. BMC Pediatr 2009; 9: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med 1992; 326: 663 667. [DOI] [PubMed] [Google Scholar]
- Lazzarotto T, Gabrielli L, Lanari M, Guerra B, Bellucci T, Sassi M, Landini MP. Congenital cytomegalovirus infection: recent advances in the diagnosis of maternal infection. Hum Immunol 2004; 65: 410 415. [DOI] [PubMed] [Google Scholar]
- Revello MG, Zavattoni M, Sarasini A, Percivalle E, Simoncini L, Gerna G. Human cytomegalovirus in blood of immunocompetent persons during primary infection: prognostic implications for pregnancy. J Infect Dis 1998; 177: 1170 1175. [DOI] [PubMed] [Google Scholar]
- Stagno S. Remington JS, Klein JO. (eds.), Infectious Diseases of the Fetus and Newborn Infant. Philadelphia: W.B. Saunders Co.; 2001: 389 424. [Google Scholar]
- Griffith BP, McCormick SR, Fong CK, Lavallee JT, Lucia HL, Goff E. The placenta as a site of cytomegalovirus infection in guinea pigs. J Virol 1985; 55: 402 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med 2000; 6: 589 593. [DOI] [PubMed] [Google Scholar]
- Bakardjiev AI, Theriot JA, Portnoy DA. Listeria monocytogenes traffics from maternal organs to the placenta and back. PLoS Pathog 2006; 2: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 1996; 272: 1502 1504. [DOI] [PubMed] [Google Scholar]
- Lucchi NW, Koopman R, Peterson DS, Moore JM. Plasmodium falciparum-infected red blood cells selected for binding to cultured syncytiotrophoblast bind to chondroitin sulfate A and induce tyrosine phosphorylation in the syncytiotrophoblast. Placenta 2006; 27: 384 394. [DOI] [PubMed] [Google Scholar]
- Constantin CM, Masopust D, Gourley T, Grayson J, Strickland OL, Ahmed R, Bonney EA. Normal establishment of virus-specific memory CD8 T cell pool following primary infection during pregnancy. J Immunol 2007; 179: 4383 4389. [DOI] [PubMed] [Google Scholar]
- Medawar P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol 1953; 7: 320 338. [Google Scholar]
- Trowsdale J, Betz AG. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol 2006; 7: 241 246. [DOI] [PubMed] [Google Scholar]
- Goff E, Griffith BP, Booss J. Delayed amplification of cytomegalovirus infection in the placenta and maternal tissues during late gestation. Am J Obstet Gynecol 1987; 156: 1265 1270. [DOI] [PubMed] [Google Scholar]
- Revello MG, Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev 2002; 15: 680 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CJ, Myers MG. Relation of maternal CMV viremia and antibody response to the rate of congenital infection and intrauterine growth retardation. J Med Virol 1990; 31: 222 228. [DOI] [PubMed] [Google Scholar]
- Pereira L, Maidji E, McDonagh S, Genbacev O, Fisher S. Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J Virol 2003; 77: 13301 13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazaki K, Ozono K, Yahara T, Wada Y, Suehara N, Takeuchi M, Nakayama M. Detection of cytomegalovirus DNA in human placenta. J Med Virol 2002; 68: 363 369. [DOI] [PubMed] [Google Scholar]
- McDonagh S, Maidji E, Chang HT, Pereira L. Patterns of human cytomegalovirus infection in term placentas: a preliminary analysis. J Clin Virol 2006; 35: 210 215. [DOI] [PubMed] [Google Scholar]
- Muhlemann K, Miller RK, Metlay L, Menegus MA. Cytomegalovirus infection of the human placenta: an immunocytochemical study. Hum Pathol 1992; 23: 1234 1237. [DOI] [PubMed] [Google Scholar]
- Fisher S, Genbacev O, Maidji E, Pereira L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol 2000; 74: 6808 6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings DG, Kilani R, Nykiforuk C, Preiksaitis J, Guilbert LJ. Permissive cytomegalovirus infection of primary villous term and first trimester trophoblasts. J Virol 1998; 72: 4970 4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyaeyan M, Alborzi A, Abbasian A, Kalani M, Moravej A, Nasiri J, Amiri A, Hashemi N, Sefiddashti F. Detection of HCMV DNA in placenta, amniotic fluid and fetuses of seropositive women by nested PCR. Eur J Pediatr 2007; 166: 723 726. [DOI] [PubMed] [Google Scholar]
- Compton T, Nowlin DM, Cooper NR. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 1993; 193: 834 841. [DOI] [PubMed] [Google Scholar]
- Feire AL, Koss H, Compton T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Pro Natl Acad Sci U S A 2004; 101: 15470 15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrish DW, Dakour J, Li H, Xiao J, Miller R, Sherburne R, Berdan RC, Guilbert LJ. In vitro cultured human term cytotrophoblast: a model for normal primary epithelial cells demonstrating a spontaneous differentiation program that requires EGF for extensive development of syncytium. Placenta 1997; 18: 577 585. [DOI] [PubMed] [Google Scholar]
- Yui J, Garcia-Lloret M, Brown AJ, Berdan RC, Morrish DW, Wegmann TG, Guilbert LJ. Functional, long-term cultures of human term trophoblasts purified by column-elimination of CD9 expressing cells. Placenta 1994; 15: 231 246. [DOI] [PubMed] [Google Scholar]
- Douglas GC, King BF. Differentiation of human trophoblast cells in vitro as revealed by immunocytochemical staining of desmoplakin and nuclei. J Cell Sci 1990; 96 (pt 1): 131 141. [DOI] [PubMed] [Google Scholar]
- Collett A, Walker D, Sims E, He YL, Speers P, Ayrton J, Rowland M, Warhurst G. Influence of morphometric factors on quantitation of paracellular permeability of intestinal epithelia in vitro. Pharm Res 1997; 14: 767 773. [DOI] [PubMed] [Google Scholar]
- Osborn JE, Walker DL. Enhancement of infectivity of murine cytomegalovirus in vitro by centrifugal inoculation. J Virol 1968; 2: 853 858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SW, Scott KM. Rapid quantitation of cytomegalovirus and assay of neutralizing antibody by using monoclonal antibody to the major immediate-early viral protein. J Clin Microbiol 1988; 26: 504 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T, Nepomuceno RR, Nowlin DM. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 1992; 191: 387 395. [DOI] [PubMed] [Google Scholar]
- Zaia JA. Prevention and treatment of cytomegalovirus pneumonia in transplant recipients. Clin Infect Dis 1993; 17 (suppl 2): S392 S399. [DOI] [PubMed] [Google Scholar]
- Ryckman BJ, Jarvis MA, Drummond DD, Nelson JA, Johnson DC. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J Virol 2006; 80: 710 722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha TA, Tom E, Kemble GW, Duke GM, Mocarski ES, Spaete RR. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol 1996; 70: 78 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin PD, Scalzo AA, Swaminathan N, Price P, Shellam GR. Murine cytomegalovirus binds reversibly to mouse embryo fibroblasts: implications for quantitation and explanation of centrifugal enhancement. J Virol Methods 1988; 22: 215 230. [DOI] [PubMed] [Google Scholar]
- Ornoy A, Diav-Citrin O. Fetal effects of primary and secondary cytomegalovirus infection in pregnancy. Reprod Toxicol 2006; 21: 399 409. [DOI] [PubMed] [Google Scholar]
- Chan G, Hemmings DG, Yurochko AD, Guilbert LJ. Human cytomegalovirus-caused damage to placental trophoblasts mediated by immediate early gene-induced tumor necrosis factor-alpha. Am J Pathol 2002; 161: 1371 1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro G, Adler SP, La Torre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 2005; 353: 1350 1362. [DOI] [PubMed] [Google Scholar]
- Schleiss MR. The role of the placenta in the pathogenesis of congenital cytomegalovirus infection: is the benefit of cytomegalovirus immune globulin for the newborn mediated through improved placental health and function? Clin Infect Dis 2006; 43: 1001 1003. [DOI] [PubMed] [Google Scholar]
- Broeder JA, Smith CH, Moe AJ. Glutamate oxidation by trophoblasts in vitro. Am J Physiol 1994; 267: C189 C194. [DOI] [PubMed] [Google Scholar]
- Compton T. Receptors and immune sensors: the complex entry path of human cytomegalovirus. Trends Cell Biol 2004; 14: 5 8. [DOI] [PubMed] [Google Scholar]
- Griffith BP, Chen M, Isom HC. Role of primary and secondary maternal viremia in transplacental guinea pig cytomegalovirus transfer. J Virol 1990; 64: 1991 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiss MR. Animal models of congenital cytomegalovirus infection: an overview of progress in the characterization of guinea pig cytomegalovirus (GPCMV). J Clin Virol 2002; 25 (suppl 2): S37 S49. [DOI] [PubMed] [Google Scholar]
- Boyd JD, Hamilton WJ. Electron miscroscopic observations on the cytotrophoblast contribution to the syncytium in the human placenta. J Anat 1966; 100: 535 548. [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss JL, Regatieri CV, Jarrouge TR, Cavalheiro RP, Sampaio LO, Nader HB. Heparan sulfate proteoglycans: structure, protein interactions and cell signaling. An Acad Bras Cienc 2009; 81: 409 429. [DOI] [PubMed] [Google Scholar]
- Fortunato EA, McElroy AK, Sanchez I, Spector DH. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol 2000; 8: 111 119. [DOI] [PubMed] [Google Scholar]
- Speir E, Shibutani T, Yu ZX, Ferrans V, Epstein SE. Role of reactive oxygen intermediates in cytomegalovirus gene expression and in the response of human smooth muscle cells to viral infection. Circ Res 1996; 79: 1143 1152. [DOI] [PubMed] [Google Scholar]
- Trincado DE, Munro SC, Camaris C, Rawlinson WD. Highly sensitive detection and localization of maternally acquired human cytomegalovirus in placental tissue by in situ polymerase chain reaction. J Infect Dis 2005; 192: 650 657. [DOI] [PubMed] [Google Scholar]
- Maidji E, Percivalle E, Gerna G, Fisher S, Pereira L. Transmission of human cytomegalovirus from infected uterine microvascular endothelial cells to differentiating/invasive placental cytotrophoblasts. Virology 2002; 304: 53 69. [DOI] [PubMed] [Google Scholar]
- Hemmings DG, Guilbert LJ. Polarized release of human cytomegalovirus from placental trophoblasts. J Virol 2002; 76: 6710 6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri S, Lowen B, Chan G, Davey A, Riddell M, Guilbert LJ. Human cytomegalovirus interacts with toll-like receptor 2 and CD14 on syncytiotrophoblasts to stimulate expression of TNFalpha mRNA and apoptosis. Placenta 2009; 30: 994 1001. [DOI] [PubMed] [Google Scholar]
- Maidji E, McDonagh S, Genbacev O, Tabata T, Pereira L. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am J Pathol 2006; 168: 1210 1226. [DOI] [PMC free article] [PubMed] [Google Scholar]