Abstract
Relaxin (RLN) is a systemic hormone from the corpus luteum, and its levels remain low during normal human gestation. Indeed, elevation of circulating RLN has long been associated with preterm birth, for which there has been no physiological explanation. Recent studies have shown that RLN suppresses endotoxin-induced cytokine secretion from THP-1 monocytic cells by acting on the glucocorticoid receptor (GR), but its effects on primary macrophages are unknown. Therefore, in the present study, we examined the effects of RLN on cytokine secretion from primary decidual macrophages (DMs) obtained at term before labor. Unlike THP-1 cells, RLN had no effects on the cytokine responses induced by either lipopolysaccharide (LPS) or interleukin (IL) 1B, mimicking infection-induced or sterile inflammation, respectively. However, RLN alone for 4 h significantly decreased (P < 0.05) colony-stimulating factor 2 (CSF2; also known as granulocyte-macrophage colony-stimulating factor) and IL8 but for 24 h significantly increased IL6 (P < 0.01). We show that DMs express both the RLN receptor (RXFP1) and the GR. RLN suppression of CSF2 and IL8 was sensitive to the GR-antagonist mifepristone (RU-486). However, RLN activation of RXFP1 induced a dose-dependent cAMP response, which when mimicked by forskolin also caused significantly increased (P < 0.05) secretion of IL6. Thus, RLN may be anti-inflammatory in DMs via activation of the GR but proinflammatory via activation of RXFP1 and cAMP. In summary, we have shown that RLN targeting DMs may modulate proinflammatory cytokine secretion at the maternal-fetal interface and contribute to the localized inflammatory response associated with parturition in women.
Keywords: cytokines, decidua, immunology, parturition, relaxin
In decidual macrophages isolated from human fetal membranes at term, treatment with relaxin is anti-inflammatory after 4 h but is proinflammatory after 24 h.
INTRODUCTION
Elevated systemic human relaxin (RLN) levels have long been associated with preterm birth [1]. Although work in animals has clearly shown that RLN causes uterine quiescence and cervical dilatation [2], it has recently become apparent that neither of these actions of RLN is important for either maintenance of human pregnancy or timely parturition [3, 4]. It is generally agreed that serum RLN originates from the corpus luteum in most animals and humans, hence the reported association between elevated RLN levels and patients with twin gestations [5], in vitro fertilization pregnancies [6], and in singleton gestations after superovulation [7]. More recent studies have shown that in healthy women with singleton pregnancies but with premature uterine contractions or preterm premature rupture of the membranes before 34 wk of gestation, serum RLN levels were raised [8]. Subsequent studies by the same group have shown that in those patients with preterm delivery, RLN levels had a different course of secretion throughout gestation compared with the course in those patients delivering at term [9]. To date, however, there has been no physiological explanation for any of these observations or associations.
We have spent many years showing that RLN is an autocrine/paracrine hormone in the human fetal membranes and that this may be significant to the outcome of pregnancy in the human [10]. Our focus has been primarily upon the action of RLN to remodel or degrade the extracellular matrix of the fetal amnion and chorion. Because chorioamnionitis is a major cause of preterm premature rupture of the fetal membranes and preterm birth [11], we have shown that the action of RLN is distinct from the infection-induced pathway to preterm birth. This is based upon in vivo evidence that RLN expression was not increased in the fetal membranes of patients with severe chorioamnionitis [12] or in rhesus monkeys when lipopolysaccharide (LPS) was infused into the amniotic fluid [13]. However, accumulating evidence supports the idea that some intrauterine inflammation is associated with even normal human parturition, unrelated to the presence of microorganisms [14]. We have recently shown that RLN can stimulate the production of interleukin (IL) 6 and IL8 from the extraplacental chorionic cytotrophoblast and have suggested that it is one of a number of sterile stimuli capable of causing an increase in proinflammatory cytokines [10, 13]. At the same time, it has recently been shown that RLN has the reverse effect and suppresses the endotoxin-induced inflammatory cytokine production in the human monocytic cell line THP-1 [15, 16]. This anti-inflammatory effect of RLN was shown to be caused by its entering the cell [15], then binding and activating the glucocorticoid receptor (GR) with subsequent transrepression of the proinflammatory transcription factor AP-1 [17]. Such an effect of a peptide hormone is novel and represents a new mechanism of RLN action, distinct from its action on its cognate cell-membrane receptor (RXFP1).
In the present work, we have therefore studied the effects of RLN on human decidual macrophages (DMs), because these comprise up to 18% of the decidual cells throughout pregnancy [18] and are highly specialized immune cells ideally positioned at the maternal-fetal interface to facilitate communication and homeostasis between the maternal decidua and fetal chorion. Such an action could be important in further understanding the role of RLN in pregnancy maintenance and/or parturition.
MATERIALS AND METHODS
Reagents
RPMI 1640, phenol-free Modified RPMI 1640, charcoal-stripped fetal calf serum (FCS), fetal bovine serum (FBS), Hanks balanced salt solution (HBSS), and antibiotics mix (100×) were obtained from Gibco Invitrogen Corporation. Modified RPMI 1640 for THP-1 cells was from American Type Culture Collection (ATCC). RBC Lysis Buffer and Accumax were from eBioscience. Recombinant human IL1β (IL1B) was purchased from R&D Systems. Bradford reagent, forskolin, mifepristone (RU-486), phorbol 12-myristate 13-acetate (PMA), and LPS from Salmonella abortus equi were obtained from Sigma-Aldrich. Rabbit anti-human RXFP1 antibody (LS-A566) was from MBL International, rabbit anti-human GR antibody (sc-1003) was purchased from Santa Cruz Biotechnology, nonimmune rabbit immunoglobulin (Ig) G (X0936) was from DAKO, and mouse monoclonal phycoerythrin/Texas Red (PE/TR)-conjugated anti-human CD14 (MHCD1714) and goat anti-rabbit Alexa Fluor 488 (A11070) antibodies were from Molecular Probes (Invitrogen). Normal horse serum, ImmPRESS reagent, diaminobenzidine (DAB) peroxidase substrate solution, and Gill hematoxylin were purchased from Vector Laboratories, and Pro-Texx mounting medium was from Thermo Fisher Scientific. Recombinant human relaxin H2 (RLN) was a generous gift from Corthera, Inc. All other chemicals were purchased from Sigma-Aldrich unless otherwise specified.
Tissue Collection and Primary Cell Isolation
Human fetal membranes were collected at Kapiolani Medical Center for Women and Children (Honolulu, HI). Informed consent was determined by the Institutional Review Board to be unnecessary, because the tissues were collected anonymously. Each tissue was collected within 30 min of elective cesarean section at term (>37 wk of gestation) in the absence of labor and pathological complications.
Decidual macrophages were isolated using the digestion method for decidua as described previously [19]. Briefly, the tissue was thoroughly rinsed in PBS and the amnion peeled off. The adherent decidua was scraped using a glass slide, minced, and digested in RPMI 1640 containing 0.2% collagenase A and 0.2% DNase I (Roche Diagnostics) for 1 h at 37°C with gentle shaking. The digest was depleted of erythrocytes with RBC Lysis Buffer, then enriched for macrophages using immunomagnetic selection for CD14 (STEMCELL Technologies) according to the manufacturer's instructions. The positively enriched fraction was further labeled for fluorescence-activated cell sorting (FACS) by incubating with PE/TR-conjugated mouse anti-human CD14 (1:50; 1 × 106 cells) for 20 min after blocking with 3% bovine serum albumin (BSA). Labeled cells were then washed with BD Pharmingen Stain Buffer (BSA; BD Biosciences), resuspended in equal parts of Accumax and RPMI containing 10% FBS and antibiotics (100 U/ml of penicillin and 0.1 mg/ml of streptomycin), and filtered through a 30-μm CellTrics filter (Partec). CD14-positive cells were analyzed and sorted using a FACSAria cell sorter with FACSDiva software (BD Biosciences). Analysis of sorted cells showed a purity of 94.6% ± 0.8% (mean ± SEM) for CD14-positive cells, with postsort viability of 67.9% ± 3.3% viability established by trypan blue assay (data not shown). Sorted DMs were washed in HBSS to remove sheath fluid and either used for RNA isolation, whole-cell lysate preparation for Western blot analysis, or seeding at a density of 0.1 × 106 cells/well in 24-well culture plates and cultured in RPMI supplemented with 10% FBS and antibiotics mix at 37°C under 5% CO2/95% O2 for 48 h to allow the cells to return to a more baseline state. Serum-free RPMI was then used to replace the culture medium. After starvation for 12–16 h, the cells were used as described. To control for any possible affects on cell viability as a result of treatment, the appropriate vehicle-treated cells (controls) were used for normalization of the results in each experiment. After incubation, the medium was harvested, and cells were used for protein measurements. All samples were stored at −80°C until further assay.
THP-1 Monocytic Cell Line
The human monocytic cell line THP-1 (ATCC) is widely used as a model system for macrophages, because these cells are similar in morphology and secretory phenotype once differentiated [20]. THP-1 cells between passages 5 and 10 were cultured in Modified RPMI 1640 supplemented with 10% FBS, 0.05 mM 2-mercaptoethanol (Gibco Invitrogen), and antibiotics mix. Cells were subcultured when they reached 0.8–1 × 106 cells/ml and reseeded at 2–4 × 104 cells/ml. For differentiation into macrophages (differentiated THP-1 cells, hereafter referred to as diff THP-1), cells were treated with 0.02 μg/ml of PMA for 72 h, washed and incubated in phenol-free Modified RPMI 1640 supplemented with 10% charcoal-stripped FCS and antibiotics mix, and used for experiments after 24 h of rest.
Multiplex Cytokine Assay and IL6/IL8 ELISA
Cells were treated with increasing doses of RLN (1, 10, or 100 nM) either alone or in combination with 1 ng/ml of IL1B or 1 ng/ml of LPS for 4 or 24 h at 37°C. For the GR blocking experiments, cells were incubated with 10 nM RLN in the absence or presence of 0.5 μM RU-486 or with vehicle control (0.001% ethanol) for 4 h at 37°C. Secretion of proinflammatory cytokines (IL1B, colony-stimulating factor 2 [CSF2], IL6, IL8, and tumor necrosis factor [TNF]) was measured with the Human Inflammatory 5-Plex Cytokine Panel (Invitrogen) according to the manufacturer's instructions and read on a Luminex 200 instrument. Results were analyzed with xPONENT (Luminex) or Beadview (Upstate) software, normalized to total protein as determined by Bradford assay, and expressed relative to the untreated controls as the mean ± SEM.
In experiments when only IL6 or IL8 secretion needed to be determined, this was measured by specific ELISA (R&D) according to the manufacturer's instructions. To show whether increasing intracellular cAMP could affect IL6 secretion, DMs were stimulated with 100 μM forskolin for 24 h, and secreted IL6 was measured by ELISA. Diff THP-1 samples were rerun using IL8 ELISA, because values were outside the IL8 standard range using the Luminex multiplex assay. Results were normalized to total protein as measured by Bradford assay and were expressed relative to untreated controls as the mean ± SEM.
RNA Purification and Real-Time PCR
Total RNA was isolated with the RNeasy Mini or Micro Kit (Qiagen) according to the manufacturer's instructions. Complementary DNA was prepared by reverse transcription with the High Capacity RNA-to-cDNA Master Mix (Applied Biosystems). The reaction mixture was incubated at 25°C for 5 min, 42°C for 30 min, and 85°C for 5 min. For quantitative real-time PCR (qPCR), primers for RXFP1 and the housekeeping gene 18S were purchased from Applied Biosystems (TaqMan Assays on Demand). These were designed to span an exon junction and to be of the highest specificity and sensitivity possible. The ABI protocol for qPCR was used with the following incubation: one cycle at 95°C for 10 min and 40 cycles at 95°C for 15 sec and 60°C for 1 min using an Opticon Continuous Fluorescence Detector (MJ Research). Each reaction was performed in triplicate, and the data were analyzed as described in ABI User's Bulletin 2 (http://www3.appliedbiosystems.com/cms/groups/mcb_support/documents/generaldocuments/cms_040980.pdf). Results were normalized to the expression of 18S and presented as the mean ± SEM of relative gene expression.
Immunolocalization
The specificity of the primary antibody to RXFP1 was assessed on sections of fetal membranes with nonimmune IgG used as a control, because the decidual cells and cytotrophoblasts have been shown to express RXFP1 [21]. Briefly, membranes were fixed in Bouin fixative, paraffin embedded, and sectioned at 6 μm onto slides. These tissue sections were deparaffinized and hydrated through xylene and a graded series of alcohols, rinsed in distilled water, and treated for 20 min with 0.5% hydrogen peroxide to reduce endogenous peroxidase activity. Antigen retrieval was performed by heating slides in sodium citrate buffer (10 mM, pH 6.0) for 30 min. Slides were blocked with 2.5% normal horse serum for 20 min and incubated with rabbit anti-human RXFP1 antibody (10 μg/ml) for 1 h or nonimmune rabbit IgG for negative controls. After washing, slides were incubated with ImmPRESS reagent for 30 min and DAB peroxidase substrate solution for 5 min. Slides were rinsed, counterstained with Gill hematoxylin, cleared, and mounted in Pro-Texx mounting media. Sections were viewed under bright-field microscopy.
Digested decidua containing a mixture of decidual cells before immunomagnetic enrichment for macrophages were used, because the purified DMs were fragile and easily washed off of the chamber slides during immunolabeling. Thus, decidual cells containing macrophages were cytospun onto slides to permit immunovisualization. These were suspended in PBS and centrifuged onto a Shandon Cytoslide for 6 min at 1500 × g in a Shandon CytoSpin cytocentrifuge (Thermo Fisher Scientific) and air-dried. Slides were washed with PBS and blocked with 3% BSA-PBS for 1 h before incubation with PE/TR-conjugated mouse anti-human CD14 (1:200) for 20 min. After washing with PBS, cells were fixed for cell surface staining or fixed and permeabilized for total staining using the Fix & Perm Cell Permeabilization Kit (Invitrogen). Cells were then washed with PBS and incubated with rabbit anti-human RXFP1 (20 μg/ml) for 1 h. Following additional PBS washes, they were incubated with anti-rabbit Alexa Fluor 488 secondary antibody (1:1000) for 1 h. Slides were washed with PBS and mounted with ProLong Gold antifade reagent containing 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes, Invitrogen).
Images were acquired using a multispectral imaging system comprised of an Olympus BX51 fluorescence microscope and a Nuance spectral analyzer (Cambridge Research and Instrumentation, Inc.). The Nuance analyzer contains a liquid-crystal tunable filter (LCTF) and a charge-coupled device camera. The LCTF allows discrete wavelengths of light to pass to the camera, which acquires images. Exposure settings were determined for each sample using the autoexpose feature of the Nuance software (Version 2.10.0) for optimal signal intensity. For each slide, a series of images was collected at wavelength intervals of 10 nm for each channel as follows: 450–720 nm (DAPI), 500–720 nm (fluorescein isothiocyanate [FITC]), and 580–720 nm (tetramethylrhodamine isothiocyanate [TRITC]). Each image file contains the spectral information at each wavelength interval per pixel and forms a concatenated three-dimensional stack or spectral “cube,” with dimensions x, y, and wavelength. Analysis of the acquired spectral data was performed with the Real Component Analysis feature within the Nuance software to allow algorithmic spectral unmixing and digital removal of autofluorescence. Following spectral deconvolution, the separate spectral contributions to the data cube were output as composite pseudocolorized images to visualize staining intensity and distribution for each fluorochrome, revealing signal distribution otherwise obscured by autofluorescence.
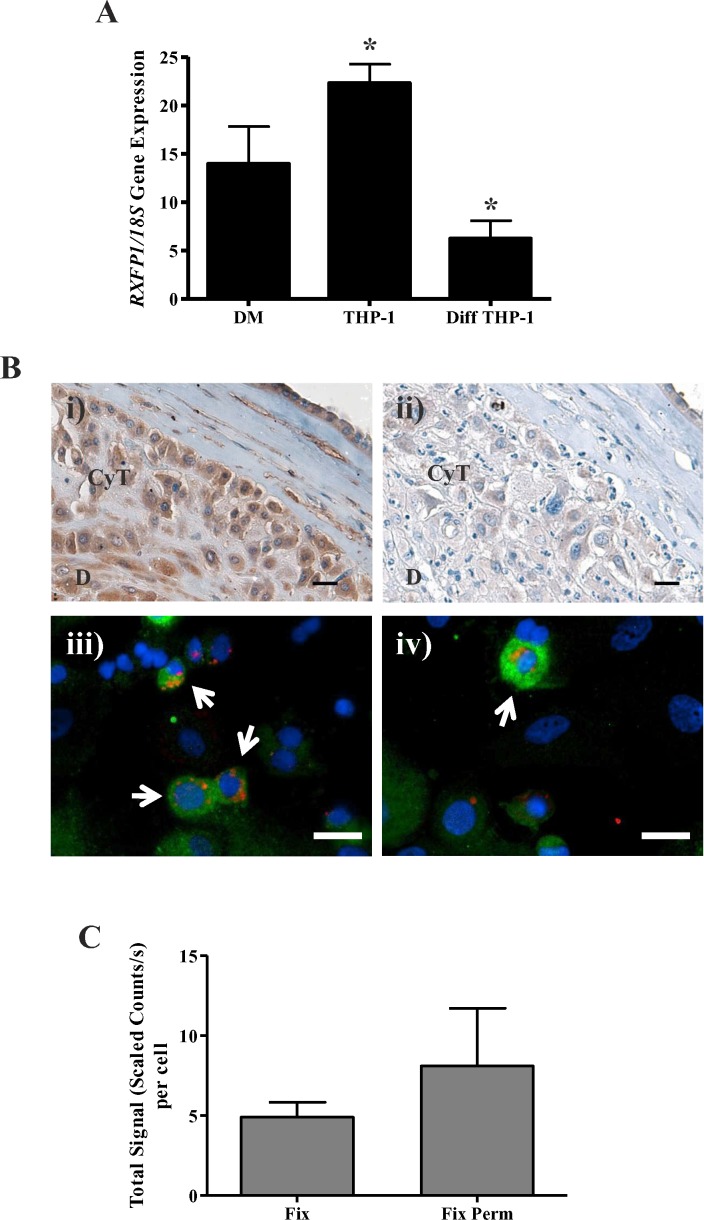
Quantitative image analysis was performed with the Nuance software. First, DMs were identified by setting an automated, default threshold for TRITC signal, distinguishing positive CD14 immunolocalization. Next, thresholded FITC signal was analyzed within these regions to yield a quantitative measure of RXFP1 expression within DMs. Total signal intensity per cell was normalized to exposure time to allow comparison between different exposure settings. Numeric data from a minimum of four fields from cells from each of four different patients were averaged, with results expressed as the mean ± SEM of signal (scaled counts/sec) per cell.
Intracellular cAMP Determination
Cells were incubated with increasing doses of RLN (0.01, 0.1, 1, or 10 nM) or forskolin (0.1, 1, 10, or 100 μM) for 30 min at 37°C in medium containing 0.25 mM 3-isobutyl-1-methylxanthine to prevent phosphodiesterase degradation of cAMP. Cells were lysed, and intracellular cAMP was measured with the Amersham cAMP Biotrak EIA System (GE Healthcare) according to the manufacturer's instructions. Results were normalized to protein content as determined by DC Protein Assay (Bio-Rad) and expressed relative to untreated controls. Each experiment was performed in duplicate and expressed as the mean ± SEM for a minimum of three observations.
Western Blot Analysis
Whole-cell lysates were prepared in modified RIPA buffer (50 mM Tris [pH 7.4], 1% NP40, 0.2% deoxycholate, 150 mM NaCl, and 1 mM ethyleneglycoltetra-acetic acid, with addition of 1× protease inhibitor [Roche], 1 mM Na3VO4, and 1 mM NaF immediately before lysis). Total protein (20 μg) was suspended in SDS-PAGE loading buffer (50 mM Tris [pH 6.8], 2% SDS, 15% glycerol, 2% β-mercaptoethanol, 1 mM ethylenediaminetetra-acetic acid, and 0.02% bromophenol blue) and denatured by heating at 95°C for 5 min. The proteins were resolved in SDS-PAGE (7.5% acrylamide gel) and transferred electrophoretically to nitrocellulose membranes (Amersham Biosciences). The membrane was blocked in Odyssey Blocking Buffer (LI-COR) for 1 h and then incubated with polyclonal rabbit antibody to GR (1:500 in blocking buffer with 0.2% Tween) at 4°C overnight. The membrane was then incubated with IRDye 800CW goat anti-rabbit secondary antibody (1:10 000; LI-COR). Immunoreactive proteins were visualized using the Odyssey Infrared Imaging System (LI-COR). Densitometric analysis was performed with the Odyssey software and expressed as integrated intensity counts, which correct for background signal. Membranes were stripped and reprobed for the loading control, β-actin (1:5000; Abcam). GR signal intensity was normalized to that of β-actin and expressed as the =-3pt?>mean ± SEM (n = 3) for each cell type.
Statistical Analysis
Data were analyzed for statistical significance by Kruskal-Wallis (nonparametric ANOVA), Friedman (nonparametric repeated measures ANOVA), or Mann-Whitney tests using GraphPad InStat software (GraphPad Software, Inc.). Results are expressed as the mean ± SEM for the number of different experiments. Statistical significance was set at P < 0.05.
RESULTS
Effects of RLN on LPS- and IL1B-Induced IL6 Secretion
We first confirmed the observation that in diff THP-1 treated with LPS, RLN causes a significant reduction in the amount of IL6 secreted [15]. As shown in Figure 1A, LPS treatment for both 4 and 24 h caused significant increases in secreted IL6 (P < 0.01 and P < 0.05, respectively). This effect was then significantly reduced when RLN at 1 or 10 nM was added to the cells for 4 h. No significant effect was found at 24 h, in contrast to previous results with RLN treatment for 8, 24, or 48 h [15]. This was then repeated using DMs (Fig. 1B), and although LPS treatment of these cells significantly increased IL6 secretion at both 4 and 24 h (P < 0.05), the addition of RLN up to 100 nM had no effect on the secreted IL6 induced by LPS in these cells. Treatment of DMs with LPS did not induce a significant increase in either CSF2, IL8, or IL1B (data not shown) at either 4 or 24 h. Furthermore, addition of RLN did not affect secretion of these cytokines by DMs (data not shown). When DMs were stimulated with IL1B, which is considered to be more like a nonbacterially induced inflammatory response, only a significant increase in IL6 secretion after 24 h of treatment was observed (P < 0.001) (Fig. 1C). The addition of RLN at any dose tested had no significant effect on the amount of IL1B-induced IL6 secreted (Fig. 1C).
FIG. 1.
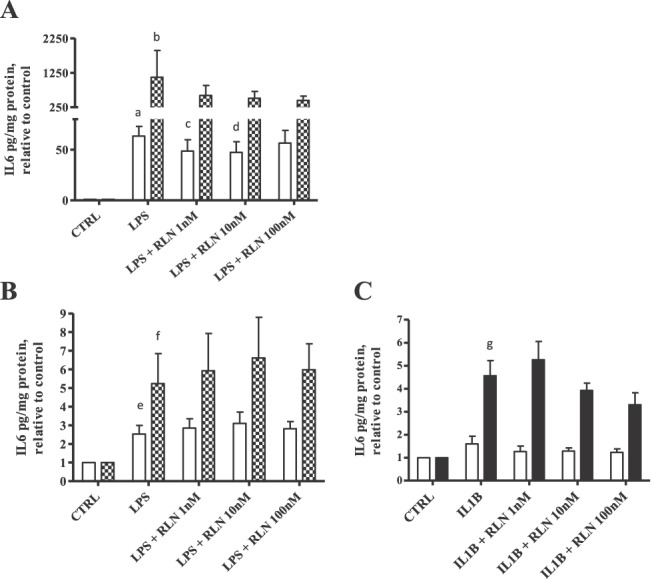
Effect of RLN on LPS or IL1B-induced cytokine secretion in diff THP-1 and DMs. Cells were stimulated with either LPS (Salmonella abortus equii, 1 ng/ml) or IL1B (1 ng/ml) in the absence or presence of RLN (1, 10, or 100 nM) for 4 h (open bars) or 24 h (filled bars). Secreted IL6 was measured by Luminex multiplex assay, with results normalized to protein concentration and expressed relative to untreated controls (CTRL; mean ± SEM). A) PMA-differentiated THP-1 cells stimulated with LPS (n = 6). a, P < 0.01; b, P < 0.05 compared to respective CTRL; c and d, P < 0.05 compared to LPS alone. B) DM isolated from a minimum of five different patients, stimulated with LPS. e and f, P < 0.05 compared to respective CTRL. C) DM isolated from a minimum of eight patients, stimulated with IL1B. g, P < 0.001 compared to respective CTRL.
RLN Modulation of Proinflammatory Cytokine Secretion from Unstimulated DMs and Diff THP-1
We then investigated the effects of RLN alone directly on cytokine secretion by DMs and compared this with its effects on diff THP-1 in the absence of stimulation with either LPS or IL1B. In DMs, 10 nM RLN caused a significant decrease in the amount of CSF2 secreted into the medium at 4 h, with no significant effect at 24 h of treatment, although this was close to significance (P = 0.06) (Fig. 2A). A higher dose of RLN (100 nM) also significantly (P < 0.05) inhibited the secretion of IL8 at 4 h of treatment (Fig. 2C), with no effects at 24 h. In contrast, no significant reduction of IL6 secreted into the medium as a result of RLN was found at the shorter time (Fig. 2B), although the longer treatment (24 h) with 10 nM RLN significantly increased (P < 0.01) the secretion of IL6 (Fig. 2B). However, RLN at any of the doses tested had no effect on IL1B or TNF secretion from DMs (data not shown). These effects of RLN on CSF2, IL8, and IL6 in DMs in the absence of an inflammatory stimulus were absent in diff THP-1 (Fig. 2, D–F).
FIG. 2.
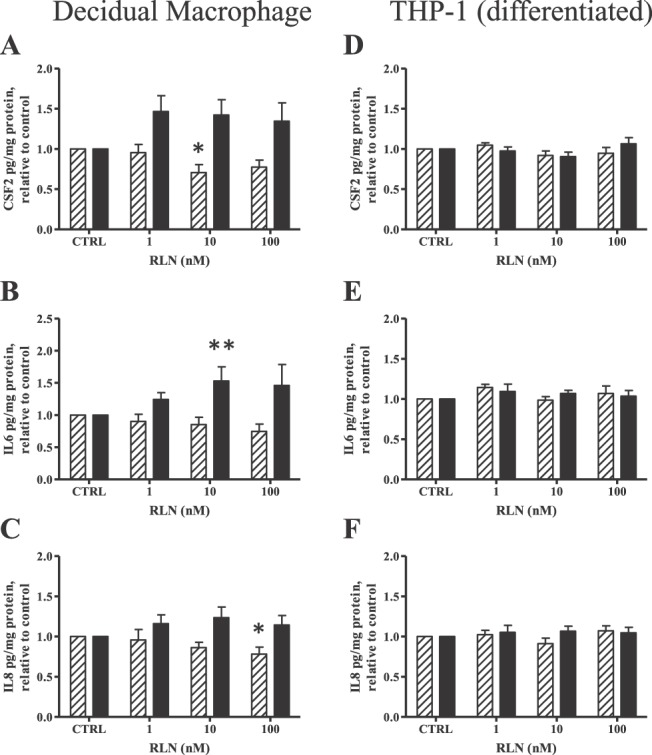
Effect of RLN alone on cytokine secretion in DMs and diff THP-1. Cells were treated with increasing doses of RLN (1, 10, or 100 nM) for 4 h (striped bars) or 24 h (solid bars). Secreted proinflammatory cytokines were measured with Luminex multiplex assay or IL8 ELISA (diff THP-1 only). Results for CSF2, IL6, and IL8 are shown as the mean ± SEM of cytokine concentration normalized to protein content and expressed relative to untreated controls (CTRL). A–C) Secretion of CSF2 (A), IL6 (B), and IL8 (C) in DMs (n = 8 from different patients). *P < 0.05, **P < 0.01 compared to respective CTRL. D–F) Secretion of CSF2 (D), IL6 (E), and IL8 (F) in diff THP-1 (n = 6).
RXFP1 Expression
We next measured expression of RXFP1 in DMs, THP-1 cells, and diff THP-1. As shown in Figure. 3A, THP-1 cells had the highest expression of RXFP1, which was significantly greater (P < 0.05) than for the diff THP-1. Whereas DMs are considered to be closer in phenotype to diff THP-1 than to THP-1 cells [20], DMs had a level of RXFP1 gene expression that was between those of THP-1 cells and diff THP-1, but these differences were not significant.
FIG. 3.
RLN receptor (RXFP1) gene and protein expression in DMs. A) Baseline RXFP1 gene expression in DM (n = 6 from different patients) measured by qPCR normalized to 18S, shown with THP-1 cells (n = 12) and diff THP-1 (n = 7). Data presented as the mean ± SEM of relative mRNA levels. *P < 0.01. B) Representative immunostaining of baseline RXFP1 protein expression in decidual cells. Paraffin-embedded sections of human fetal membranes were labeled with rabbit anti-human RXFP1 to confirm primary antibody specificity (i) or with nonimmune IgG for control (ii), then visualized with DAB and hematoxylin counterstaining and viewed under bright-field microscopy. RXFP1 localization is in brown and nuclear counterstaining in blue. A mixture of primary decidual cells was cytospun onto slides, then fixed or fixed and permeabilized to show cell surface (iii) or cell surface and intracellular (total; iv) protein expression, respectively. Cells were labeled with mouse anti-human CD14 PE-TR, rabbit anti-human RXFP1, and a rabbit FITC secondary antibody and mounted in a medium containing DAPI. Slides were imaged with the Nuance Multispectral Imaging System, spectrally analyzed to reduce autofluorescence, and assigned artificial colors to display CD14 localization in red, RXFP1 in green, and nuclei in blue. Arrows show examples of CD14-positive DMs with RXFP1 immunolocalization. CyT, chorionic cytotrophoblast; D, decidua. Bar = 20 μm. C) RXFP1 quantitation in DMs. CD14-positive cells were identified by default threshold TRITC signal, and those regions were analyzed for FITC signal intensity, with data expressed as the mean ± SEM of the total signal (scaled counts per second)/cell for a minimum of six fields using cells from four different patients. Fix, fixed (cell surface); Fix Perm, fixed and permeabilized (cell surface and intracellular).
Because gene expression is not always indicative of protein expression, we measured RXFP1 protein expression in DMs by immunolocalization (Fig. 3B). A commercial polyclonal antibody to a synthetic peptide for an epitope of the N-terminal extracellular domain of human RXFP1 was used. However, the complete range of potential cross-reactivities of this antibody has not been fully explored. Therefore, we first confirmed its ability to immunostain the cells of the full-thickness fetal membranes. As shown in Figure 3Bi, the decidual cells and chorionic cytotrophoblasts were positively stained as previously described [21]. A serial section shows lack of staining (control) with nonimmune IgG at the same concentration (Fig. 3Bii). In DMs (arrows), RXFP1 expression was evident both at the cell surface (Fig. 3Biii) as well as intracellularly, which is shown as the total expressed, including the cell surface expression (Fig. 3Biv), and was more intense than expression at the cell surface alone. Quantitation of the immunostaining in the DMs for both cell surface (fixed) and cell surface and intracellular (fixed and permeabilized) is shown in Figure 3C. However, the greater expression of total RXFP1 in DMs compared to that at the cell surface was not significant.
Cyclic AMP Response to RLN and Involvement in IL6 Secretion by DMs
The functionality of the RXFP1 was then assessed by measuring the production of intracellular cAMP after RLN treatment. Mirroring the RXFP1 gene expression results (Fig. 3A), THP-1 cells showed a robust RLN dose-dependent increase in cAMP production (Fig. 4A) that was significant at 0.1 and 1 nM RLN (P < 0.05 and P < 0.01, respectively). DMs also showed a RLN dose-dependent cAMP induction significantly elevated compared to controls with 0.1 and 1 nM RLN (P < 0.05) (Fig. 4A). However, the magnitude of this cAMP response was significantly less at these doses when compared to those of the THP-1 cells (P < 0.01). In contrast, diff THP-1 failed to induce any elevation in cAMP (Fig. 4A).
FIG. 4.
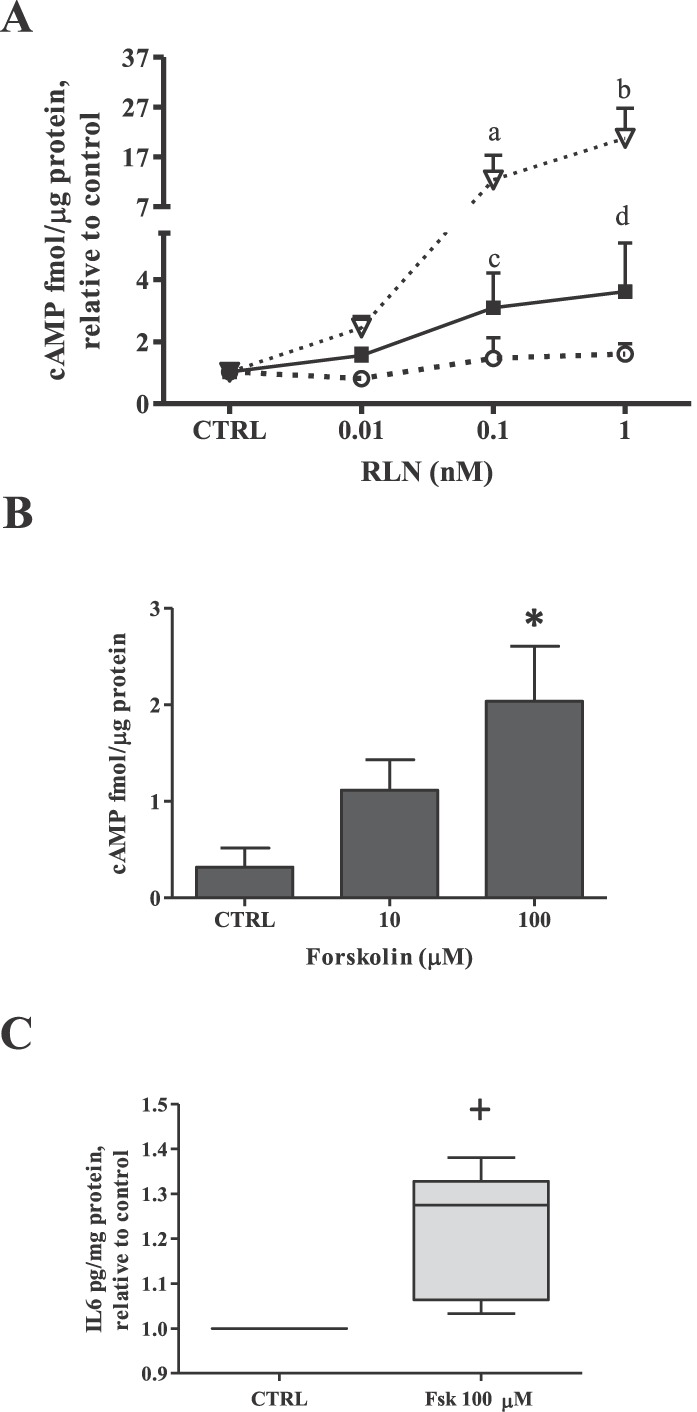
Effect of RLN or forskolin on cAMP production and forskolin-induced IL6 secretion in DMs. A) DMs, THP-1 cells, and diff THP-1 were incubated with RLN (0.01, 0.1, or 1 nM) for 30 min, and intracellular cAMP production measured by ELISA. Data are expressed as the cAMP concentration (mean ± SEM) relative to total protein and normalized to untreated controls (CTRL) for each cell type (n ≥ 4). Inverted open triangles, THP-1 cells; filled squares, DMs; open circles, diff THP-1. a, c, and d, P < 0.05; b, P < 0.01 compared to cTRL. For comparison, a vs. c, 0.1 nM; b vs. d, 1 nM; P < 0.01. B) DMs from a minimum of three different patients were treated with forskolin (10 or 100 μM) for 30 min before measurement of intracellular cAMP by ELISA. *P < 0.05 compared to CTRL. C) DMs from four different patients treated with forskolin (100 μM) for 24 h and IL6 secretion as measured by ELISA, with results expressed as the mean ± SEM normalized to total protein. +P < 0.05 compared to CTRL.
Because the promoter region of the IL6 gene has several regulatory elements that can be influenced by transcription factors activated downstream of cAMP [22], we next examined the possibility that RLN-induced IL6 secretion in DMs might be due to RXFP1-mediated cAMP production. When DMs were incubated for 30 min with increasing doses of forskolin, which is known to elevate intracellular cAMP levels through the activation of adenylate cyclase [23], a dose-dependent increase in cAMP was demonstrated (Fig. 4B) that was significant at 100 μM (P < 0.05) compared to controls. In addition, the magnitude of this cAMP response with 100 μM forskolin was similar to that caused by 1 nM RLN (Fig. 4A) and has been used by others for RLN-related cAMP experiments [24]. The stimulation of cAMP production in DMs by this dose of forskolin for 24 h, the time in which RLN caused significant IL6 secretion (Fig. 2B), resulted in significantly increased IL6 secretion compared to controls (P < 0.05) (Fig. 4C). Taken together, these results suggest that in DMs, RLN-induced IL6 secretion may be due, in part, to RXFP1-mediated cAMP production.
GR Expression and Involvement in RLN-Induced CSF2 and IL8 Suppression by DMs
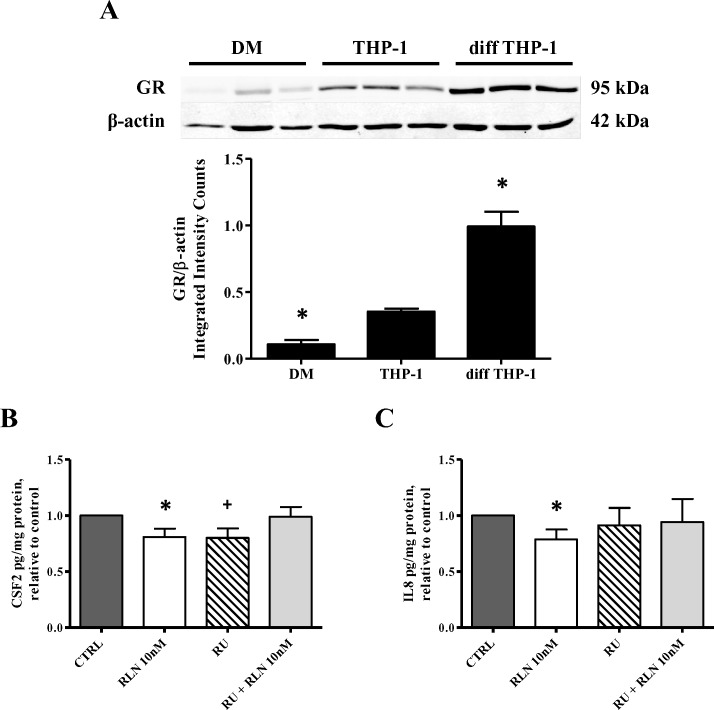
Relaxin is capable of binding and activating the GR in addition to activating its cognate receptor (RXFP1) [15]. This has been shown to be the mechanism by which RLN mediates immunosuppression in diff THP-1 [15, 17]. To determine whether the RLN-induced decrease in CSF2 and/or IL8 secretion by DMs (Fig. 2, A and C) was due to GR activation, we first demonstrated GR expression in these cells in comparison to THP-1 cells and diff THP-1 by Western blot analysis (Fig. 5). Basal levels of total GR were barely visible in immunoblotted samples from DMs compared to those from THP-1 cells and diff THP-1 (Fig. 5A, top); diff THP-1 had the strongest signal. Quantitation confirmed these results (Fig. 5A, bottom), with a significantly higher level of GR expressed in diff THP-1 compared to DMs (P < 0.05).
FIG. 5.
GR protein expression and the effect of RU-486 on RLN-induced cytokine suppression in DMs. A, top) Baseline GR protein expression by Western blot analysis. Whole-cell lysates (20 μg/lane) from DMs (n = 3), THP-1 cells (n = 3), and diff THP-1 (n = 3) were separated by gel electrophoresis and probed with anti-GR or β-actin followed by a near-infrared secondary antibody. Detection was performed with the LI-COR Odyssey Infrared Imaging System and densitometric analysis with the Odyssey software. Results are presented as the mean ± SEM of total GR signal relative to β-actin, expressed as integrated intensity counts. *P < 0.05. A, bottom) Effect of RU-486 on RLN-induced cytokine suppression. DMs from four different patients were treated with RLN (10 nM), RU-486 (0.5 μM), or a combination of both for 4 h. B and C) CSF2 (B) or IL8 (C) secretion were measured by Luminex multiplex assay, normalized to total protein, and expressed relative to untreated control (CTRL). *+P < 0.05 compared to CTRL.
The possibility that the RLN-induced decreases in CSF2 and IL8 secretion (Fig. 2, A and C) were mediated through the GR was then examined. We repeated our initial experiments in the presence of the GR antagonist RU-486. As expected, treatment of DMs with 10 nM RLN significantly decreased levels of secreted CSF2 (Fig. 5B) and IL8 (Fig. 5C) compared to the respective controls (P < 0.05). Treatment with the GR inhibitor RU-486 alone also significantly reduced CSF2 secretion (P < 0.05) (Fig. 5B) but had no effect on IL8 (Fig. 5C). However, when RU-486 was added together with 10 nM RLN, the suppressive effect of RLN on both cytokines was lost, and the secretion levels were the same as the unstimulated or baseline levels, suggesting that RU-486 inhibited RLN binding of the GR. These results suggest that the RLN-induced suppression of CSF2 and IL8 was probably through its binding and activation of the GR.
DISCUSSION
In the present study, we have confirmed the anti-inflammatory effects of RLN in diff THP-1 stimulated with LPS, as originally shown by Dschietzig et al. [15]. However, when extended to DMs, RLN failed to have any such effect on LPS-stimulated IL6 secretion. On the other hand, DMs responded to RLN treatment alone, whereas the THP-1 cells did not. These responses of DMs were cytokine-specific and time-dependent, with RLN causing suppression of CSF2 and IL8 at 4 h of treatment but stimulation of IL6 after 24 h. These data suggest that RLN, if delivered to these cells at low levels during gestation, could have an important anti-inflammatory effect, whereas more sustained or higher levels of exposure to RLN could result in a sterile inflammatory response [10, 13]. Indeed, it has recently been shown that serum RLN levels are significantly elevated in women during late pregnancy and the early puerperium [25].
We used LPS to mimic bacterially induced inflammation and IL1B to model nonmicrobial or sterile inflammation, both of which have been associated with preterm labor [26]. The lack of any effect of RLN on DMs stimulated by either LPS or IL1B was not due to a lack of stimulatory capacity of the DMs, because LPS treatment significantly increased the secretion of IL6 at both 4 and 24 h, whereas IL1B had a significant effect only at 24 h, on these cells. However, the magnitude of the DM response to both stimuli was modest in comparison to that of THP-1 cells, which were robustly stimulated by both LPS and IL1B. This difference between THP-1 cells and primary DMs is consistent with their immunosuppressive phenotype, as shown by others using gene expression profiling [27]. Thus, although macrophages are similar in morphology and phenotype to diff THP-1 [20], these monocyte-derived macrophages have a unique genotypic profile [28] and have been programmed in vivo during the course of gestation [29] to become highly specialized DMs. Indeed, the DMs used here were probably a heterogeneous population of cells [29], and the results therefore most likely reflect an average of values for a mixture of macrophage phenotypes. Thus, it could be that the anti-inflammatory and proinflammatory effects of RLN were due to the responses of different macrophage populations. It has been shown that in vivo, the macrophages resident in tissue respond to their environment as the needs change [30], and the suggestion has been made that in the decidua in vivo, their phenotype alters during the course of pregnancy [29]. Our use of term decidua before labor and without infection was an attempt to limit, as much as possible, the influence of the environment, but as with any study in which cells are isolated, especially from human tissue, it is impossible to show whether any of the manipulations during the isolation procedure altered their in vitro responses. However, the use of these cells is more likely to represent the in vivo situation than the use of THP-1 cells.
In the absence of any inflammation, we have shown here that RLN was capable of modulating CSF2, IL8, and IL6 in DMs but had no effect on either IL1B or TNF. In a nonhuman primate model, it has recently been shown that the intra-amniotic infusion of IL1B or TNF caused rapid preterm labor [31]. Thus, RLN is responsible for expression of a selective immune profile by these cells, which may be protective and directed toward pregnancy maintenance. The differences in the patterns of cytokine secretion caused by RLN may be the result of separately regulated secretory pathways, as has been demonstrated for IL6 and TNF secretion in macrophages [32]. In the uterus, CSF2 is a local mediator of the trafficking and activation of macrophages, granulocytes, and dendritic cells [33]. In uterine epithelial cells, CSF2 acts in synergy with IL8, one of the most important chemoattractants, to promote neutrophil chemotaxis [34]. Thus, the parallel decrease in both CSF2 and IL8 caused by RLN treatment for 4 h in DM may support a protective effect of RLN, whereby neutrophil infiltration and acute inflammation are suppressed. In contrast, the longer (24 h) exposures of the DMs to RLN caused a significant increase in IL6 secretion. IL6 is a proinflammatory cytokine involved in the induction of acute-phase reactions and in the proliferation and differentiation of lymphocytes [35]. It is elevated in amniotic fluid and gestational tissues in both normal term and preterm labor [11,36]. We suggest that RLN is one of several factors capable of fine-tuning the macrophage activation phenotype. We recognize that the cytokine responses caused by RLN in the present study were relatively modest and the dose-responses sometimes less than ideal. Unfortunately, the numbers of cells isolated from the decidua of each patient limited the number of RLN doses that could be used, and the inherent variability of the cells from different patients made these studies challenging. However, the production of even low levels of cytokines within gestational tissues is very important because of the multiple cytokine loops that feed-forward and are important for the initiation of labor.
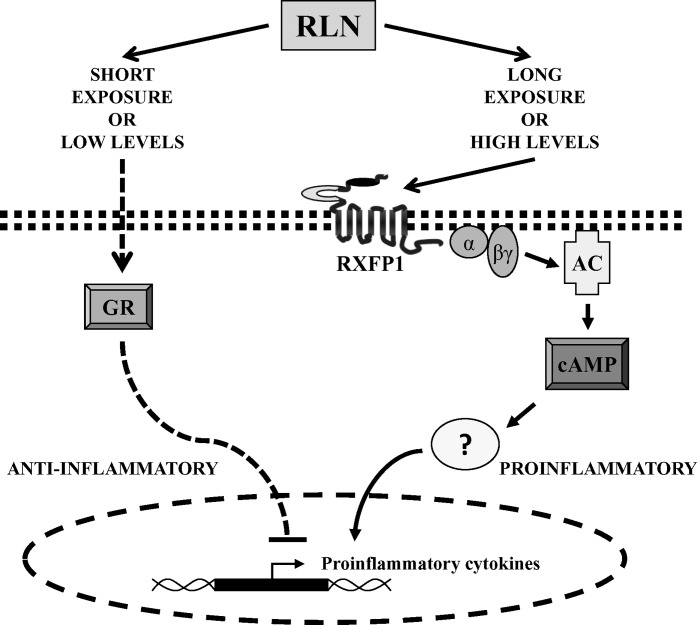
The proinflammatory effect of RLN shown here is consistent with our recent work showing that RLN, both in vivo in rhesus monkeys and in vitro in human tissues and cells, stimulated secretion of both IL6 and IL8 from extravillous chorionic cytotrophoblasts but not decidual cells [13]. In contrast to amnion and chorion, the decidua is vascular and, therefore, receives RLN directly from the systemic circulation in addition to that which is produced locally within the fetal membranes. Thus, we may begin to explain how altered levels of circulating RLN in normal compared to pathological pregnancies may target the gestational tissues with different immune consequences. The very low systemic RLN levels in human serum measured during normal gestation (∼1 ng/ml, or ∼0.2 nM) [37] may contribute to the immunosuppressive milieu in the fetal membranes. However, if the systemic RLN levels are elevated or abnormally high, this could be immunostimulatory, in agreement with literature showing that high systemic RLN levels are associated with preterm delivery [1, 8, 9] and normal labor at term [25]. This is schematically represented in Figure 6. At this time, we do not know how the DMs in vivo respond to a short versus a longer-term exposure to RLN, or to low versus higher RLN doses. Neither do we know whether these cells perceive the systemic and local RLN identically or have the ability to differentiate between them. Certainly, the action of RLN is controlled at the level of its receptor (RXFP1) and by the expression of its splice variants, which have been shown to have a dominant negative effect on the cell surface expression of the wild-type RXFP1 [38]. We chose the short (4-h) RLN treatment, based upon its immunosuppression of THP-1 cells [15], and the longer (24-h) RLN treatment, which we had shown to be immunostimulatory for the chorionic cytotrophoblast [13]. Further work is required to clarify the mechanisms involved in how these cells respond to differing RLN levels over different lengths of time.
FIG. 6.
Schematic representation of the possible signaling pathways involved in RLN-induced cytokine secretion in DMs. RLN likely causes the activation of multiple signaling pathways, which are time- and dose-dependent. In the absence of an inflammatory setting, RLN at low doses or short exposures may enter DMs to bind and activate the GR, which would suppress proinflammatory cytokine production. Longer exposures and potentially higher doses of RLN are proinflammatory and may involve its cognate cell surface receptor, RXFP1, initiating cAMP production and release of proinflammatory cytokines, such as IL6. The cell membrane is shown as a line of double squares and the nuclear membrane as dashed lines. The actions of cAMP may involve several downstream kinases and transcription factors and, therefore, are represented by a question mark. AC, adenylate cyclase; α and βγ, G-protein subunits.
In the present study, we have demonstrated that DMs express the cognate cell surface receptor for RLN (RXFP1) and have shown considerable intracellular and cell surface localization. DMs are small cells with low levels of RXFP1 expression. It is known that THP-1 cells only express approximately 275 receptors per cell at their cell surface [39], and this is more than that expressed by the DMs. The intracellular localization of RXFP1 has also been shown for other cell types [40] and appears to be common for G protein-coupled receptors (GPCRs) [41]. However, any significance of the intracellular RXFP1 for the action of RLN is not yet known. It is well documented in multiple cell types that RLN signaling through RXFP1 involves G-protein coupling and adenylate cyclase signaling to increase intracellular cAMP levels [42]. In DMs, RLN caused only a modest dose-dependent elevation in cAMP compared to its greater effects in THP-1 cells. These cells also showed a much higher level of RXFP1 expression, but their differentiation to a macrophage-like phenotype caused a significant loss of RXFP1 expression and a reduction in RLN-induced cAMP production. It is possible that different signaling mechanisms account for the differences in cAMP levels induced by RLN in DMs and THP-1. Indeed, the same GPCR can couple to different signaling pathways in different cells, and binding of the same ligand can induce activation of different pathways [43]. In any event, the production of cAMP by RLN is significant in the context of the term decidua, because cAMP is recognized as a universal regulator of cellular function in a multitude of organisms and is involved in the mediation of many biological processes, including immune function [44]. Whereas some evidence exists for the involvement of cAMP as an anti-inflammatory agent [45–47], it is also capable of stimulating IL6 production from different cell lines, including FS-4 fibroblasts [48], Caco-2 cells [49], and 3T3 adipocytes [50]. In the present study using forskolin to increase cAMP, this caused concurrent stimulation of IL6 secretion from DMs. Thus, we infer from this that RLN-induced cAMP production through RXFP1 might be responsible for the increased IL6 secretion by DMs. We elected here to stimulate cAMP endogenously with forskolin, showing that this stimulated IL6 production. On the other hand, we could also have attempted to inhibit cAMP to prevent IL6 production. This approach was considered to be more high risk, because it would have been necessary to first stimulate cAMP to demonstrate an inhibitory effect.
The novel action of RLN to enter the cell through an unknown mechanism and compete with other GR agonists to bind the GR and effect nuclear translocation and suppression of proinflammatory cytokines appears to be independent from its action on RXFP1 [17]. It is not yet understood if there is any preference for RLN to bind and activate its cell surface RXFP1 or to enter the cell and activate the GR and, if so, how that preference is determined. We have shown that the expression of the GR by DMs was modest compared to that of diff THP-1, which may explain why RLN suppressed LPS-induced IL6 secretion in diff THP-1 but not in DMs. However, like diff THP-1 [15], DMs were sensitive to the GR antagonist RU-486, suggesting that the anti-inflammatory effects of RLN alone on CSF2 and IL8 could be due to its action on the GR rather than on RXFP1. Interestingly, RU-486 by itself significantly inhibited CSF2 secretion in DMs to a similar extent as RLN did. However, RU-486 has previously been shown to act as an agonist in other cell types [51, 52], especially in the presence of cAMP-induced protein kinase A (PKA) [53]. Whereas RU-486 is a potent antagonist of both the GR and the progesterone receptor (PR), DMs have been reported to lack PR expression [54]. Thus, our results with RU-486 may be attributed solely to its binding of GR.
In conclusion, we have shown that the DMs located at the maternal-fetal interface of the fetal membranes may be a pivotal target for RLN, either from a systemic source or from local production. The findings presented here show an anti-inflammatory role for RLN in the fetal membranes, where low RLN exposure would add to the general suppression of cytokine secretion and support pregnancy. On the other hand, in the event of either elevated RLN or a different secretory profile acting in concert with its known stimulatory effects on the cells of the chorionic cytotrophoblast, the resulting increased secretion of IL6 would contribute to a localized sterile inflammatory milieu and stimulate matrix metalloproteinase production, promoting extracellular matrix remodeling and membrane weakening associated with parturition.
ACKNOWLEDGMENTS
We thank the nurses and staff of the labor and delivery ward at Kapiolani Medical Center for Women and Children (Honolulu, HI) for their help with tissue collection and Corthera, Inc., for the generous gift of RLN.
Footnotes
Open Access Article: No; PMC Deposit Required: Yes; Release date in months: 12
Supported by National Institutes of Health (NIH) grant HD24314, a grant from the Hawaii Community Foundation, a fellowship to J.S.H. from the U.S. Department of Homeland Security, and grant U54 RR014607-10 “Pacific Research Center for Early Human Development” from the National Center for Research Resources (NCRR), a component of the NIH. Contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
REFERENCES
- Bell RJ, Eddie LW, Lester AR, Wood EC, Johnston PD, Niall HD. Antenatal serum levels of relaxin in patients having preterm labor. Br J Obstet Gynaecol 1988; 95: 1264 1267. [DOI] [PubMed] [Google Scholar]
- Sherwood OD. Relaxin's physiological roles and other diverse actions. Endocr Rev 2004; 25: 205 234. [DOI] [PubMed] [Google Scholar]
- Brennand JE, Calder AA, Leitch CR, Greer IA, Chou MM, MacKenzie IZ. Recombinant human relaxin as a cervical ripening agent. Br J Obstet Gynaecol 1997; 104: 775 780. [DOI] [PubMed] [Google Scholar]
- Weiss G, Teichman S, Stewart D, Nader D, Wood S, Unemori E. A randomized, double-blind, placebo-controlled trial of relaxin for cervical ripening in postdelivery date pregnancies. Ann N Y Acad Sci 2009; 1160: 385 386. [DOI] [PubMed] [Google Scholar]
- Haning RV, Jr, Canick JA, Goldsmith LT, Shahinian KA, Erinakes NJ, Weiss G. The effect of ovulation induction on the concentration of maternal serum relaxin in twin pregnancies. Am J Obstet Gynecol 1996; 174: 227 232. [DOI] [PubMed] [Google Scholar]
- Haning RV, Jr, Goldsmith LT, Seifer DB, Wheeler C, Frishman G, Sarmento J, Weiss G. Relaxin secretion in in vitro fertilization pregnancies. Am J Obstet Gynecol 1996; 174: 233 240. [DOI] [PubMed] [Google Scholar]
- Mushayandebvu TI, Goldsmith LT, Von Hagen S, Santoro N, Thurston D, Weiss G. Elevated maternal serum relaxin concentrations throughout pregnancy in singleton gestations after superovulation. Obstet Gynecol 1998; 92: 17 20. [DOI] [PubMed] [Google Scholar]
- Vogel I, Glavind-Kristensen M, Thorsen P, Armbruster FP, Uldbjerg N. S-relaxin as a predictor of preterm delivery in women with symptoms of preterm labor. BJOG 2002; 109: 977 982. [DOI] [PubMed] [Google Scholar]
- Vogel I, Thorsen P, Hundborg HH, Uldbjerg N. Prediction of preterm delivery using changes in serum relaxin in low risk pregnancies. Eur J Obstet Gynecol Reprod Biol 2006; 128: 113 118. [DOI] [PubMed] [Google Scholar]
- Bryant-Greenwood GD, Kern A, Yamamoto SY, Sadowsky DW, Novy MJ. Relaxin and the human fetal membranes. Reprod Sci 2007; 14: 42 45. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000; 342: 1500 1507. [DOI] [PubMed] [Google Scholar]
- Millar LK, Boesche MH, Yamamoto SY, Killeen J, DeBuque L, Chen R, Bryant-Greenwood GD. A relaxin-mediated pathway to preterm premature rupture of the fetal membranes that is independent of infection. Am J Obstet Gynecol 1998; 179: 126 134. [DOI] [PubMed] [Google Scholar]
- Bryant-Greenwood GD, Yamamoto SY, Sadowsky DW, Gravett MG, Novy MJ. Relaxin stimulates interleukin-6 and interleukin-8 secretion from the extraplacental chorionic cytotrophoblast. Placenta 2009; 30: 599 606. [DOI] [PubMed] [Google Scholar]
- Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol 2006; 195: 394.e1 394.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dschietzig T, Bartsch C, Stangl V, Baumann G, Stangl K. Identification of the pregnancy hormone relaxin as glucocorticoid receptor agonist. FASEB J 2004; 18: 1536 1538. [DOI] [PubMed] [Google Scholar]
- Figueiredo KA, Rossi G, Cox ME. Relaxin promotes clustering, migration, and activation states of mononuclear myelocytic cells. Ann N Y Acad Sci 2009; 1160: 353 360. [DOI] [PubMed] [Google Scholar]
- Dschietzig T, Bartsch C, Baumann G, Stangl K. RXFP1-inactive relaxin activates human glucocorticoid receptor: further investigations into the relaxin-GR pathway. Regul Pept 2009; 154: 77 84. [DOI] [PubMed] [Google Scholar]
- Vince GS, Starkey PM, Jackson MC, Sargent IL, Redman CW. Flow cytometric characterization of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Methods 1990; 132: 181 189. [DOI] [PubMed] [Google Scholar]
- Blanco O, Tirado I, Muñoz-Fernández R, Abadía-Molina AC, García-Pacheco JM, Peña J, Olivares EG. Human decidual stromal cells express HLA-G: effects of cytokines and decidualization. Hum Reprod 2008; 23: 144 152. [DOI] [PubMed] [Google Scholar]
- Daigneault M, Preston JA, Marriott HM, Whyte MKB, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 2010; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowndes K, Amano A, Yamamoto SY, Bryant-Greenwood GD. The human relaxin receptor (LGR7): expression in the fetal membranes and placenta. Placenta 2006; 27: 610 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NF-kappaB Richmond A. chemokine gene transcription and tumor growth. Nat Rev Immunol 2002; 2: 664 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel PA, Ostrom RS. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol 2003; 23: 305 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halls ML, Bathgate RAD, Summers RJ. Relaxin family peptide receptors RXFP1 and RXFP2 modulate cAMP signaling by distinct mechanisms. Mol Pharmacol 2006; 70: 214 226. [DOI] [PubMed] [Google Scholar]
- Lafayette RA, Hladunewich MA, Derby G, Blouch K, Druzin ML, Myers BD. Serum relaxin levels and kidney function in late pregnancy with or without preeclampsia. Clin Nephrol 2011; 75: 226 232. [DOI] [PubMed] [Google Scholar]
- Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, Petraglia F. Inflammation and pregnancy. Reprod Sci 2009; 16: 206 215. [DOI] [PubMed] [Google Scholar]
- Gustafsson C, Mjösberg J, Matussek A, Geffers R, Matthiesen L, Berg G, Sharma S, Buer J, Ernerudh J. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS ONE 2008; 3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohro T, Tanaka T, Murakami T, Wada Y, Aburatani H, Hamakubo T, Kodama T. A comparison of differences in the gene expression profiles of phorbol 12-myristate 13-acetate differentiated THP-1 cells and human monocyte-derived macrophage. J Atheroscler Thromb 2004; 11: 88 97. [DOI] [PubMed] [Google Scholar]
- McIntire RH, Ganacias KG, Hunt JS. Programming of human monocytes by the uteroplacental environment. Reprod Sci 2008; 15: 437 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 2007; 204: 1057 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol 2006; 195: 1578 1589. [DOI] [PubMed] [Google Scholar]
- Manderson AP, Kay JG, Hammond LA, Brown DL, Stow JL. Subcompartments of the macrophage recycling endosome direct the differential secretion of IL-6 and TNFalpha. J Cell Biol 2007; 178: 57 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA. GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev 2007; 18: 287 298. [DOI] [PubMed] [Google Scholar]
- Shen L, Fahey JV, Hussey SB, Asin SN, Wira CR, Fanger MW. Synergy between IL-8 and GM-CSF in reproductive tract epithelial cell secretions promotes enhanced neutrophil chemotaxis. Cell Immunol 2004; 230: 23 32. [DOI] [PubMed] [Google Scholar]
- Saito S, Kasahara T, Kato Y, Ishihara Y, Ichijo M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony-stimulating factor (G-CSF) in term and preterm parturition. Cytokine 1993; 5: 81 88. [DOI] [PubMed] [Google Scholar]
- Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin-6 in preterm labor. Association with infection. J Clin Invest 1990; 85: 1392 1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RJ, Eddie LW, Lester AR, Wood EC, Johnston PD, Niall HD. Relaxin in human pregnancy serum measured with an homologous radioimmunoassay. Obstet Gynecol 1987; 69: 585 589. [PubMed] [Google Scholar]
- Kern A, Hubbard D, Amano A, Bryant-Greenwood GD. Cloning, expression, and functional characterization of relaxin receptor (leucine-rich repeat-containing G protein-coupled receptor 7) splice variants from human fetal membranes. Endocrinology 2007; 149: 1277 1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Samuel CS, Binder C, Hewitson TD, Tregear GW, Wade JD, Bathgate RA. The chemically synthesized human relaxin-2 analog, B-R13/17K H2, is an RXFP1 antagonist. Amino Acids 2010; 39: 409 416. [DOI] [PubMed] [Google Scholar]
- Kern A, Bryant-Greenwood GD. Characterization of relaxin receptor (RXFP1) desensitization and internalization in primary human decidual cells and RXFP1-transfected HEK293 cells. Endocrinology 2008; 150: 2419 2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyaloglu AC, Von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol 2008; 48: 537 568. [DOI] [PubMed] [Google Scholar]
- Halls ML, Hewitson TD, Moore X-L, Du X-J, Bathgate RA, Summers RJ. Relaxin activates multiple cAMP signaling pathway profiles in different target cells. Ann N Y Acad Sci 2009; 1160: 108 111. [DOI] [PubMed] [Google Scholar]
- Baker JG, Hill SJ. Multiple GPCR conformations and signaling pathways: implications for antagonist affinity estimates. Trends Pharmacol Sci 2007; 28: 374 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo JA, Brunton LL. Cyclic nucleotide research—still expanding after half a century. Nat Rev Mol Cell Biol 2002; 3: 710 718. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Lichtenstein LM, Melmon KL, Henney CS, Weinstein Y, Shearer GM. Modulation of inflammation and immunity by cyclic AMP. Science 1974; 184: 19 28. [DOI] [PubMed] [Google Scholar]
- Moore AR, Willoughby DA. The role of cAMP regulation in controlling inflammation. Clin Exp Immunol 1995; 101: 387 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol 2008; 39: 127 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lin JX, Vilcek J. Synthesis of interleukin 6 (interferon-beta 2/B cell stimulatory factor 2) in human fibroblasts is triggered by an increase in intracellular cyclic AMP. J Biol Chem 1988; 263: 6177 6182. [PubMed] [Google Scholar]
- Hershko DD, Robb BW, Luo G, Hasselgren PO. Multiple transcription factors regulating the IL-6 gene are activated by cAMP in cultured Caco-2 cells. Am J Physiol Regul Integr Comp Physiol 2002; 283: R1140 R1148. [DOI] [PubMed] [Google Scholar]
- Tchivileva IE, Tan KS, Gambarian M, Nackley AG, Medvedev AV, Romanov S, Flood PM, Maixner W, Makarov SS, Diatchenko L. Signaling pathways mediating beta3-adrenergic receptor-induced production of interleukin-6 in adipocytes. Mol Immunol 2009; 46: 2256 2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibay-Tupas JL, Okazaki KJ, Tashima LS, Yamamoto S, Bryant-Greenwood GD. Regulation of the human relaxin genes H1 and H2 by steroid hormones. Mol Cell Endocrinol 2004; 219: 115 125. [DOI] [PubMed] [Google Scholar]
- Beck CA, Estes PA, Bona BJ, Muro-Cacho CA, Nordeen SK, Edwards DP. The steroid antagonist RU486 exerts different effects on the glucocorticoid and progesterone receptors. Endocrinology 1993; 133: 728 740. [DOI] [PubMed] [Google Scholar]
- Vassen L, Wegrzyn W, Klein-Hitpass L. Human insulin receptor substrate-2 (IRS-2) is a primary progesterone response gene. Mol Endocrinol 1999; 13: 485 494. [DOI] [PubMed] [Google Scholar]
- King A, Gardner L, Loke YW. Evaluation of estrogen and progesterone receptor expression in uterine mucosal lymphocytes. Hum Reprod 1996; 11: 1079 1082. [DOI] [PubMed] [Google Scholar]