Abstract
Traditionally, oxytocin (OT) is well known to play a crucial role in the regulation of cyclic changes in the uterus, implantation of the embryo, and parturition. Recently, an additional role for OT has been identified in several types of cancer cells in which OT acts as a growth regulator. In endometrial cancer cells, OT is known to efficiently inhibit cellular proliferation. In the present study, we show that OT increases invasiveness of human endometrial carcinoma (HEC) cells, which are otherwise resistant to the growth-inhibiting effects of OT. Using pharmacological inhibitors, invasion assay, RNA interference, and immunofluorescence, we found that OT enhances the invasive properties of HEC cells through up-regulation of X-linked inhibitor of apoptosis protein (XIAP), matrix-metalloproteinase 2 (MMP2), and matrix-metalloproteinase 14 (MMP14). In addition, we show that OT-mediated invasion is both cyclooxygenase 1 (PTGS1) and cyclooxygenase-2 (PTGS2) dependent via the phosphatidylinositol 3-kinase/AKT (PIK3/AKT) pathway. PTGS2 knockdown by shRNA resulted in XIAP down-regulation. We also show that OT receptor is overexpressed in grade I to III endometrial cancer. Taken together, our results describe for the first time a novel role for OT in endometrial cancer cell invasion.
Keywords: cancer, cell culture, endometrium, invasion, MMP14, MMP2, oxytocin, PTGS1, PTGS2, XIAP
Oxytocin regulates endometrial cancer cell invasion through phosphatidylinositol 3-kinase/AKT-dependent up-regulation of cyclooxygenase-1, -2, and X-linked inhibitor of apoptosis protein.
INTRODUCTION
Endometrial carcinoma is the fourth commonly diagnosed cancer among women in the Western world [1]. The capacity for invasion and dissemination of tumor cells is a significant turning point in the survival of endometrial carcinoma. Various growth factors, extracellular matrix components (ECM), and tumor-secreted factors are known to stimulate the motility of tumor cells [2].
Oxytocin (OT) is a nonapeptide hormone playing a crucial role in many reproductive and behavioral processes [3]. In recent years, OT has been found to inhibit cell proliferation as well as to promote cell proliferation and invasion, depending on cancer cell type [4, 5]. OT operates through the activation of a specific membrane G-coupled receptor, the oxytocin receptor (OTR) [6]. The OTR could activate different signal transduction pathways: a) the traditional signaling pathway that results in the hydrolysis of phosphatidylinositol and cytosolic Ca2+ increase [6], leading to transcriptional activity by phosphorylation and activation of mitogen-activated protein kinases (MAPKs) [7] and extracellular signal-regulated kinases (ERKs), or b) the unconventional pathway using the formation of cAMP, resulting in the induction of cyclin kinase inhibitor p21WAF1/CIP1 [4, 8].
It has been reported that OT could stimulate prostaglandin E (2) (PGE2) synthesis in endometrial epithelial cells under physiologic conditions [9, 10] as well as in cancer cells [11]. PGE2, a cyclooxygenase 2 (PTGS2)-derived eicosanoid, has been shown to influence the hallmark of cancer cells by inducing proliferation, survival, angiogenesis, immunosuppression processes, migration, and invasion through activating multiple cellular pathways [12–14]. In the current study, we evaluated if OT could modulate the invasive properties of human endometrial carcinoma (HEC) cell lines (Hec-1-A and Ishikawa) and investigated the involvement of the PTGS/PGE2 and PIK3/AKT survival pathway in this process.
MATERIALS AND METHODS
Cell Line and Reagents
Hec-1-A cell line was purchased from ATCC (www.atcc.org). Hec-1-A cells were derived from a poorly differentiated endometrial carcinoma (grade 3). Cells were maintained in McCoy 5A media supplemented with 2.438 g/L NaHCO3, 10% bovine growth serum (BGS), and 50 μg/ml gentamycin. Ishikawa cells were generously provided by Dr. Sylvie Mader (Université de Montréal, QC). Ishikawa cells were maintained in Dulbecco modified Eagle medium-Ham F12 medium supplemented with 2% BGS and 50 μg/ml gentamycin. All of the antibodies were from Cell Signaling Technology (Beverly, MA) except for COX-1 (PTGS1) and COX-2 (PTGS2), which were purchased from Cayman Chemical (Cedarlane, Burlington, ON). Horseradish peroxidase (HRP)-conjugated anti-rabbit and anti-mouse secondary antibodies were obtained from Bio-Rad Laboratories (Mississauga, ON). Antibody for OTR was from Sigma Aldrich (St. Louis, MO), and MMP14 was from Abcam (Cambridge, MA). OT, Indomethacin, SB203580, MTT (3-[4,5-dimethyl-thiazolyl-2]-2,5-diphenyltetrazolium bromide), and Hoechst 33258 were obtained from Sigma Aldrich. Prostaglandin E2, SC-19220, and NS-398 were purchased from Cayman Chemical. LY294002 and PD98059 were obtained from Cell Signaling Technology. PTGS2 shRNA was purchased from SA Biosciences (Frederick, MD).
Western Blot Analysis
Cells were trypsinized, lysed in cold radio-immunoprecipitation assay lysis buffer (PBS 1× [pH 7.4], 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and Protease Inhibitor Cocktail Tablets [Roche, Indianapolis, IN]), frozen and thawed three times, and centrifuged (16 100 × g, 20 min at 4°C) to remove insoluble material. Supernatant was recovered and stored at −20°C till further analysis. Protein content was determined with the Bio-Rad DC Protein Assay (Bio Rad). Protein extracts (35–50 μg) were denatured (3 min, 95°C) and resolved by 8%, 10%, or 14% w/v SDS-PAGE, followed by semidry electrotransfer to nitrocellulose membranes (30 min, 15 V) using the Bio-Rad apparatus. Membranes were then blocked (1 h, room temperature [RT]) with PBS (1×)-Tween 20 (0.06%) containing 5% w/v nonfat milk powder, then incubated with primary antibody overnight at 4°C and subsequently with HRP-conjugated anti-rabbit secondary antibody (45 min) or HRP-conjugated anti-mouse secondary antibody (45 min, RT). Peroxidase activity was visualized with the SuperSignal West Femto substrate (Pierce, Arlington Heights, IL) according to the manufacturer's instructions.
MTT Proliferation Assay
Cells were plated at a density of 1.5 × 104 cells per well in 96-wells plates and incubated overnight at 37°C until they reached 80% confluence. Cells were cultured for 24, 48, and 72 h in the presence of increasing concentrations of OT (0, 0.01, 0.1, 1, and 10 μM in culture media) at 37°C. MTT reagent (Sigma Aldrich) was added to the wells (10 μl of 5 mg/ml thiazolyl blue tetrazolium bromide in PBS) 3.5 h before the end of the incubation period. The conversion of yellow tetrazolium salt to blue thiazol crystals by metabolically active cells was stopped by adding 100 μl of solubilization solution (10% w/v SDS in 0.01 M HCl) to each well. The microplate was incubated overnight (37°C, 5% CO2/air) to allow the complete solubilization of thiazol crystals. The optical density of blue emission (600 nm) was read with the FluoStar Optima reader (BMG Laboratories, Durham, NC). Percentage of proliferating cells was calculated as the ratio of optical densities of treated to control-treated cells. The experiments were repeated three times in duplicate.
Invasion Assays
Transwell Permeable support (Costar 3432; Corning, Corning, NY) with an 8.0-μm pore size polycarbonate membrane was coated with 2 mg/ml of BD Matrigel Low (BD Biosciences, Franklin Lakes, NJ) diluted 1:5 in fresh media without serum. The cells were pretreated for 1 h with 20 μM of LY294002, 100 μM of Indomethacin, 10 μM of NS-398, or 10 μM of SC-19220 or transfected with XIAP siRNA followed by a 24-h treatment with 100 nM of PGE2 or 1 μM of OT. After the completion of treatment period, cells were collected, washed, and resuspended in basal medium without serum. The lower chambers were filled with 600 μl of respective fresh culture medium containing 5% of BGS with or without 1 μM of OT, and 1 × 105 cells were added to the upper chamber inserts. The plates were incubated for 24, 48, or 72 h at 37°C. After the incubation period, invasive cells had reached the porous insert, where they adhered. The nonmigrated cells on the upper surface of the filter were removed by scraping. Invasive cells were fixed in methanol, and nuclear staining was performed with Hoechst 33258 followed by densitometric analysis. The assays were run in duplicate.
PGE2 Enzyme Immunoassay
The procedure for the PGE2 enzyme immunoassay kit (Cayman Chemical) described by the manufacturer was followed. Briefly, a 50-μl aliquot from culture medium was used for PGE2 determination in a 96-wells plate coated with goat anti-rabbit secondary antibody. A volume of 50 μl of PGE2 tracer and 50 μl of the PGE2 antibody were added to each sample, and the plate was incubated overnight at 4°C. Wells were washed with 10 mM phosphate buffer (pH 7.4) containing Tween 20 (0.05%) at pH 7.4; 200 μl of Ellman reagent (69 mM acetylthiocholine and 54 mM 5,5′-dithio-bis [2-nitrobenzoic acid] in 10 mM phosphate buffer, pH 7.4) was added to each well, and samples were incubated in the dark at RT. This allows the bound enzyme tracer to react with Ellman reagent to yield a yellow solution that can be measured photometrically with a microplate reader at 410 nm. A standard curve was developed simultaneously, with standards ranging from 50 to 1000 pg/ml of PGE2. The presence of PGE2 was undetectable in the culture media in the absence of cells.
XIAP siRNA
Cells (2 × 106) were seeded in a petri dish to adhere and in order to reach ∼80% confluence in 24 h. At the time of experiment, XIAP (5′-ccaagugguaguccuguuucagcau-3′ and 5′-augcugaaacaggacuaccacuugg-3′), or control (5′-acucuaucugcacgcugacuu-3′ and 5′-agucagcgugcagauagagu-3′) siRNA were mixed with Trans-it TKO transfection reagent (Mirus, Madison, WI) according to the manufacturer's instructions and added to the cells for a working concentration of 100 nM. Following 8 h of transfection, fresh medium containing 1 μM of OT was added, and cells were collected after an additional incubation period of 24 h.
PTGS2 shRNA
One day before transfection, cells (0.3 × 106) were seeded in six-well culture plates. After 24 h, cells were transiently transfected with 1 μg PTGS2 shRNA per well using Fugene 6 (Roche). Following 48 h of transfection, medium was replaced with fresh medium containing 1 μM OT. Cells were further incubated for 24 h. After 24 h of OT treatment, cells were harvested for Western blot analysis.
Hoechst Nuclear Staining
Treated cells were collected, washed, and resuspended in 1 μg/ml Hoechst 33258 (Sigma Aldrich) in 10% formalin and incubated for 24 h at 4°C before blind cell counts were performed using an Olympus BX60 fluorescence microscope (Olympus, Center Valley, PA). At least 300 cells were counted for each sample, and the percentage of apoptotic cells was calculated as the ratio of apoptotic cells (with characteristic apoptotic morphology such as nuclear shrinkage and condensation, as revealed by fluorescence microscopy) to total cell count.
RNA Isolation and RT-PCR
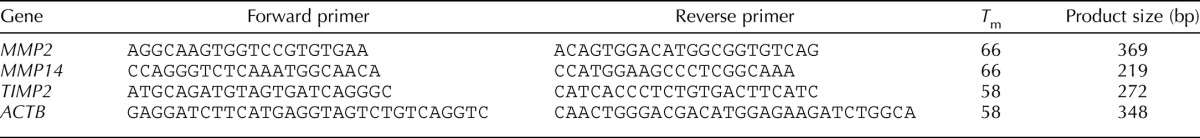
Total RNA was isolated from cells using Trizol Reagent (Invitrogen, Carlsbad, CA) as described by the manufacturer. First-strand cDNA synthesis was done using 1 μg of RNA. Briefly, the RNA samples were incubated (10 min, 65°C) with 2:1 oligo dT (deoxythymidine) primers in a final volume of 10:1. Samples were then incubated (1 h, 37°C) in 20:1 of a reaction buffer 10× containing dithiothreitol (100 mM), deoxynucleotide triphosphate (dNTPs; 5 mM) and Muloney Murine Leukemia Virus Reverse Transcriptase (200 U; Invitrogen). The reaction volumes were brought up to 60:1 with autoclaved water. Each PCR reaction mixture (final volume 50:1) contained 1× Buffer, RT template or negative control (5:1), MgCl2 (50 mM), dNTPs (5 mM), primers (25 pmoles, 1:1 each), and Taq polymerase (5 U/μL). PCRs were performed in an MJ Research Thermal cycler (model PTC-100; Bio-Rad) using the following parameters: 30 sec at 94°C, 30 sec at melting temperature (Tm), and 30 sec to 1 min at 72°C for 36–40 cycles, except for β-actin (25 cycles). Tm and primer sequences are given in Table 1. Reaction products were analysed on 1% w/v agarose gels. Bands were visualized using SYBR-Safe (Invitrogen) staining upon ultraviolet transillumination.
TABLE 1.
Primers for PCR amplification.
Gelatin Zymography
Serum-free conditioned medium from cells was assessed by zymography for the detection of gelatinolytic enzymes. The samples were applied without heating to a 10% polyacrylamide gel with 0.1% gelatine (Sigma Aldrich). After electrophoresis, the gels were washed twice for 30 min with 2.5% and 1% Triton X-100 to remove SDS and incubated for 24 h in 50 mM Tris, 5 mM CaCl2, and 1% Triton-X at 37°C. The gels were stained with Coomassie Brilliant Blue for 30 min and destained in 30% v/v methanol and 5% acetic acid. The gelatinase activity was identified as a clear band on a blue background.
Immunofluorescence-Based Detection of OTR in Clinical Samples
HEC tissue section slides (Cybrdi, Frederick, MD) containing 16 grade I tumor specimens, 34 grade II tumor specimens, five grade III tumor specimens, two smooth muscles, and three normal endometrial specimens were used. The tissues, obtained from biopsies, were already formalin-fixed and paraffin-embedded. The slides were deparaffinized by heating at 55°C for 30 min, followed by two washes in NeoClear solvent (VWR Canlab, Mississauga, ON), and they were progressively hydrated with successive washes at RT in 100%, 95%, and 70% ethanol and two washes in water. After antigen retrieval for 20 min in boiling citrate solution (10 mM sodium citrate [pH 6] and 0.05% Tween in water), the tissue slides were cooled down for 20 min at RT and then washed twice in PBS. Nonspecific binding sites were blocked by 1-h incubation with 5% normal donkey serum and 0.3% Triton X-100 in PBS at room temperature in a humidified chamber, and the tissues were probed with rabbit anti-human OTR primary antibody (Sigma Aldrich) or rabbit IgG isotype control (Vector Laboratories) overnight at 4°C. The slides were washed three times in PBS, and the tissues were probed with Alexa Fluor 488-conjugated anti-rabbit secondary antibody (Molecular Probes) for 1 h at room temperature in a humidified chamber protected from light. The slides were washed once in PBS and counterstained with Hoechst nuclear dye followed by two rinses in water. The tissues were mounted with p-phenylenediamine in glycerol and observed under a fluorescence microscope.
Statistical Analysis
All experiments were repeated at least three times. Data were subjected to one-way ANOVA (PRISM software version 3.03; GraphPad, San Diego, CA). Differences between experimental groups were determined by the Tukey test. Statistical significance was accepted when P < 0.05.
RESULTS
HEC Cells Are Resistant to the Growth-Inhibitory Effect of OT
Expression of the OTR and the antiproliferative effect of OT in endometrial carcinoma have been reported [15]. We examined whether Hec-1-A and Ishikawa cells expressed the OTR, and we have evaluated the growth inhibitory effect of OT using MTT proliferation assays. We found that OTR was abundantly expressed (Fig. 1A) in both Hec-1-A and Ishikawa cell lines. Hec-1-A cells did not respond to the growth inhibitory effect of OT, even when the concentration of OT was increased up to 10 μM or after a prolonged exposure of 72 h (Fig. 1B). These results indicate that OT does not affect the proliferation of endometrial carcinoma cells.
FIG. 1.
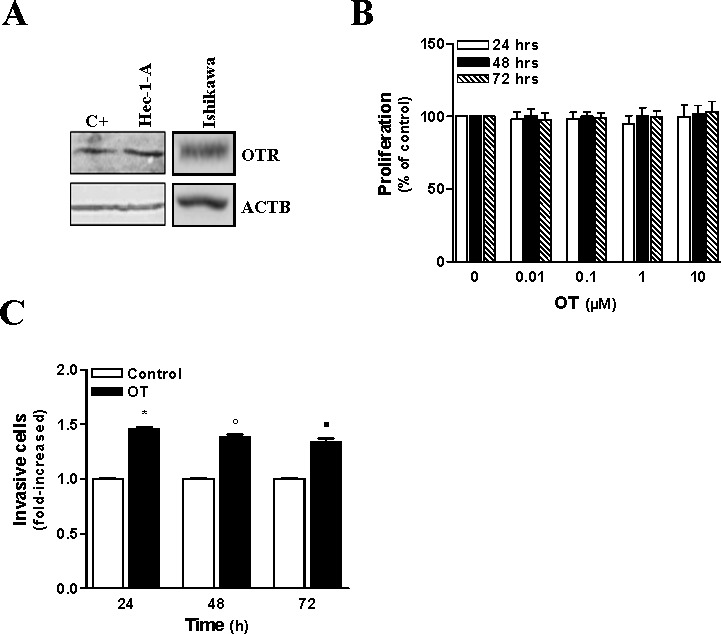
OT increases invasive properties of HEC cells. A) OTR expression was detected by Western blot in Hec-1-A and Ishikawa cells. HeLa cells were used as a positive control, and ACTB was used as loading control. Representative result is shown. B) Hec-1-A cellular proliferation after treatment with different doses of OT for 24, 48, and 72 h was assessed using MTT proliferation assay. The results are the mean ± SEM of four independent experiments, each performed in duplicate. Statistical significance was not achieved. C) The effect of OT (1 μM) on the invasive properties of Hec-1-A cells was determined by using Matrigel invasion assay for the indicated times. The results are the mean ± SEM of three independent experiments performed in duplicate. *P < 0.05 when compared to untreated cells (control) after 24 h of treatment. °P < 0.05 when compared to untreated cells (control) after 48 h of treatment. ▪P < 0.05 when compared to untreated cells (control) after 72 h of treatment.
OT Increases Invasion in HEC Cells
The ability of OT to stimulate motility and invasion had been reported in different cells [16, 17]. We have evaluated the effect of OT on the invasiveness of Hec-1-A cells using the Matrigel invasion assay. We found that OT increased Hec-1-A cell invasion (Fig. 1C) by 45%, which was poorly invasive in basal conditions. The maximal induction in cell motility was observed with 1 μM of OT; therefore, this concentration was chosen for all subsequent experiments.
OT Mediates Invasion by Up-Regulating PTGS Isoforms and PGE2 Production
Because PTGS enzymes represent the rate-limiting step in prostaglandin biosynthesis and it is predominantly PGE2 production that has a strong association with carcinogenesis as well as tumor growth, invasion, and metastasis [18, 19], we investigated whether OT could stimulate PTGS1, PTGS2, and PGE2 synthesis in HEC cells. The results showed that OT treatment significantly increased PTGS1 expression (Fig. 2, A and B). Interestingly, PTGS2, which is not detectable or poorly expressed in HEC cells, was dramatically up-regulated in both Hec-1-A and Ishikawa cells (Fig. 2, A–D). The lower band in the PTGS2 blot (Fig. 2A) represents a nonspecific band and has been previously reported [20]. A similar increase was observed in PGE2 production by Hec-1-A cells following exposure to OT (Fig. 2E). To confirm that OT increases invasiveness of Hec-1-A cells via PGE2 production, we performed an invasion assay by using SC-19220 (a selective antagonist of PGE2), which blocks the activity of EP1 receptor. We had previously demonstrated the presence of this receptor in the Hec-1-A endometrial carcinoma cell line [21]. We found that SC-19220 blocks OT- and PGE2-induced Hec-1-A cell invasion (Fig. 2F). These results indicate that OT increases invasion of HEC cells through the upregulation of PTGS isoforms and subsequent PGE2 production.
FIG. 2.
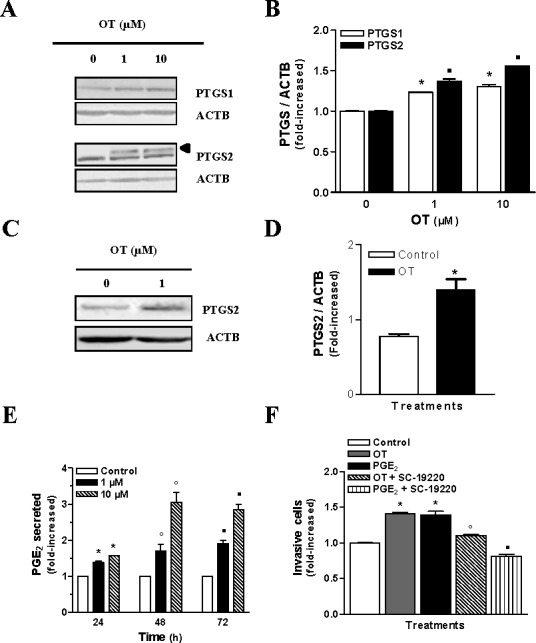
OT-induced up-regulation of PTGS isoforms and PGE2 production increases the invasiveness of HEC cells. A) PTGS1 and PTGS2 (arrow indicates upper band) expression were analyzed by Western blots in Hec-1-A cells after 24 h of treatment with indicated concentrations of OT. ACTB was used as a loading control; representative results are shown. B) Densitometric analysis of results obtained in A. The results are the mean ± SEM of three independent experiments. *P < 0.05 when compared with untreated (control) cells for PTGS1. ▪P < 0.05 when compared with untreated (control) cells for PTGS2. C) PTGS2 expression was analyzed by Western blot analysis in Ishikawa cells after 24 h of treatment with 1 μM of OT. D) Densitometric analysis of results obtained in C. The results are the mean ± SEM of three independent experiments. *P < 0.05 when compared with untreated (control) cells. E) The effect of OT on PGE2 production by Hec-1-A cells was determined using enzyme immunoassay following 24, 48, or 72 h of treatment with 1 or 10 μM of OT. Data represent the mean ± SEM of three independent experiments. *P < 0.05 when compared to untreated cells (control) after 24 h of treatment. °P < 0.05 when compared to untreated cells (control) after 48 h of treatment. ▪P < 0.05 when compared to untreated cells (control) after 72 h of treatment. F) Involvement of the PGE2 and EP1 receptor in the invasion induced by OT in the Hec-1-A cell line were determined by Matrigel invasion assay without pretreatment or following 1 h of pretreatment with 10 μM of SC-19220 before adding 100 nM of PGE2 for 24 h or 1 μM of OT in the lower chamber when the assays were performed. *P < 0.05 when compared to untreated cells (control). °P < 0.05 when compared to OT-treated cells. ▪P < 0.05 when compared to PGE2-treated cells.
OT Up-Regulates PTGS Isoforms by Activating PIK3
We have previously reported that the PIK3/AKT survival pathway is involved in the regulation of PTGS2 and PGE2 synthesis in HEC cells [22, 23]. Next we evaluated the effect of specific PIK3 inhibitor LY294002, PTGS1 inhibitor Indomethacin, and PTGS2 activity inhibitor NS-398 on OT-induced invasion in Hec-1-A cells. Interestingly, we found that these three inhibitors prevented OT-induced invasion of Hec-1-A cells (Fig. 3A). LY294002 totally blocked the effect of OT in terms of PTGS1 and PTGS2 up-regulation (Fig. 3, B–E), suggesting that PIK3 activation takes place upstream of PTGS1 and PTGS2 up-regulation in Hec-1-A cells. Moreover, we observed that under OT stimulation, Indomethacin increased PTGS2 expression (Fig. 3, D and E), whereas NS-398 increased PTGS1 expression (Fig. 3, B and C), suggesting a counterbalance mechanism between these two cyclooxygenases. As previously reported, we observed that NS-398 did not alter PTGS2 protein expression [24, 25]. These results indicate that OT confers invasive properties to Hec-1-A cells via activation of PIK3/AKT pathway, which leads to the up-regulation of PTGS isoforms.
FIG. 3.
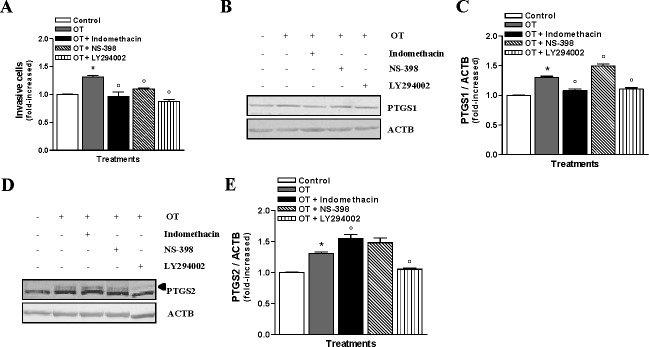
OT-induced invasiveness occurs via the activation of PIK3 in HEC cells. A) The involvement of PTGS1, PTGS2, and PIK3 on the invasive properties of Hec-1-A cells upon exposure to OT (1 μM) was determined using Matrigel invasion assay following 1 h of pretreatment with 20 μM LY294002, 100 nM Indomethacin, or 10 μM NS-398 and 24 h OT treatment; the results are the mean ± SEM of three independent experiments. *P < 0.05 when compared to untreated cells (control). °P < 0.05 when compared to OT-treated cells. B) Western blot analysis was also performed for PTGS1; the cells were pretreated for 1 h with or without 20 μM LY294002, 100 nM Indomethacin, or 10 μM NS-398 before adding 1 μM of OT for 24 h. β-actin was used as a loading control; a representative result is shown. C) Densitometric analysis of results presented in B. The results are the mean ± SEM of three independent experiments. *P < 0.05 when compared to untreated cells (control). °P < 0.05 when compared to OT-treated cells. D) Western blot analysis for PTGS2 (arrow indicates upper band); the cells were pretreated for 1 h with or without 20 μM LY294002, 100 nM Indomethacin, or 10 μM NS-398 before adding 1 μM of OT for 24 h. ACTB was used as a loading control; a representative result is shown. E) Densitometric analysis of results presented in D. The results are the means ± SEM of three independent experiments. *P < 0.05 when compared to untreated cells (control). °P < 0.05 when compared to OT-treated cells.
XIAP Is Involved in OT-Induced Invasion in HEC Cells via PIK3 and PTGS2
We have recently reported that PIK3 activity was involved in the up-regulation of XIAP, which plays an important role in invasiveness of endometrial cancer cells in response to TGFB [26]. XIAP is constitutively expressed in resting HEC cells; however, treatment with OT leads to XIAP up-regulation in these cells (Fig. 4, A–D). As hypothesized, PIK3 inhibitor LY294002 blocked the OT-induced up-regulation of XIAP (Fig. 4, E and F). Because activation of PIK3 by OT leads to up-regulation of PTGS1 and PTGS2 levels, we have examined the ability of these enzymes to regulate XIAP expression. We found that PTGS1 inhibitor Indomethacin did not prevent OT-induced XIAP expression; however, PTGS2 activity inhibitor NS-398 effectively blocked OT-induced XIAP expression (Fig. 4, G and H). Next, we down-regulated PTGS2 levels using PTGS2 shRNA; we observed a greater than 50% reduction in PTGS2 levels. Reduced PTGS2 levels also led to the down-regulation of XIAP levels, suggesting that PTGS2 might regulate XIAP levels in Hec-1-A cells under OT stimulation (Fig. 4, I and J). Further, we investigated if XIAP is a key player involved in the OT-induced invasiveness in Hec-1-A cells. To test this, Hec-1-A cells were transfected with XIAP siRNA. We observed an approximately 50% reduction in the endogenous XIAP protein levels (Fig. 5, A and B). Reduced XIAP levels completely blocked the ability of OT to increase the invasiveness of Hec-1-A cells (Fig. 5C). Moreover, reducing XIAP levels increased the apoptosis of Hec-1-A cells upon treatment with OT (Fig. 5D), suggesting a protective role of XIAP during OT-induced apoptosis. Taken together, these results imply that XIAP is regulated by PTGS2 via PIK3/AKT activation and plays a crucial role in OT-induced invasion and survival of HEC cells.
FIG. 4.
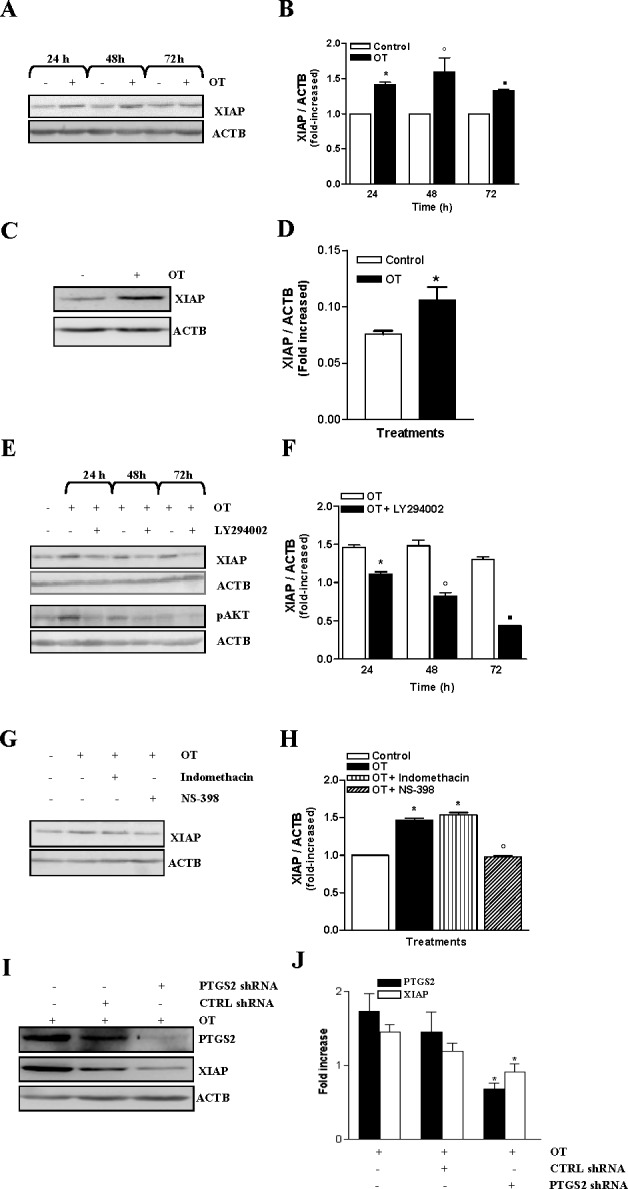
OT up-regulates XIAP via PIK3 and PTGS2 in HEC cells. A) XIAP expression was measured by Western blot analysis in Hec-1-A cells after exposure of 1 μM of OT for the indicated times. ACTB was used as a loading control; a representative result is shown. B) Densitometric analysis of results presented in A. The results are the mean ± SEM of three independent experiments. *P < 0.05 when compared to untreated cells (control) after 24 h of treatment. °P < 0.05 when compared to untreated cells (control) after 48 h of treatment. ▪P < 0.05 when compared to untreated cells (control) after 72 h of treatment. C) XIAP expression was measured by Western blot analysis in Ishikawa cells after exposure of 1 μM of OT for 24 h. ACTB was used as a loading control. D) Densitometric analysis of results presented in C. The results are the mean ± SEM of three independent experiments. *P < 0.05 when compared to untreated cells (control) after 24 h of treatment. E) To determine the involvement of PIK3 in XIAP up-regulation by OT, Western blot analysis was performed following a pretreatment for 1 h in Hec-1-A cells with 20 μM of LY294002, a PIK3-specific inhibitor, before adding 1 μM of OT for the indicated times. ACTB was used as a loading control; a representative result is shown. F) Densitometric analysis of results presented in E. The results are the means ± SEM of three independent experiments. *P < 0.05 when compared to untreated cells (control) after 24 h of treatment. °P < 0.05 when compared to untreated cells (control) after 48 h of treatment. ▪P < 0.05 when compared to untreated cells (control) after 72 h of treatment. G) The ability of PTGS isoforms to up-regulate XIAP was determined by using Western blot analysis. The cells were pretreated with 100 nM of Indomethacin or 10 μM of NS-398 for 1 h before treatment with 1 μM of OT for 24 h. β-actin was used as a loading control; a representative result is shown. H) Densitometric analysis of results presented in G. The results are the means ± SEM of three independent experiments. *P < 0.05 when compared to untreated cells (control). °P < 0.05 when compared to OT-treated cells. I) PTGS2 levels were down-regulated using shRNA against PTGS2. Protein levels of PTGS2, XIAP, and ACTB were determined by Western blot analysis. J) Densitometric analysis of results presented in I. The results are the mean ± SEM of three independent experiments. *P < 0.05 when compared to untransfected cells (control).
FIG. 5.
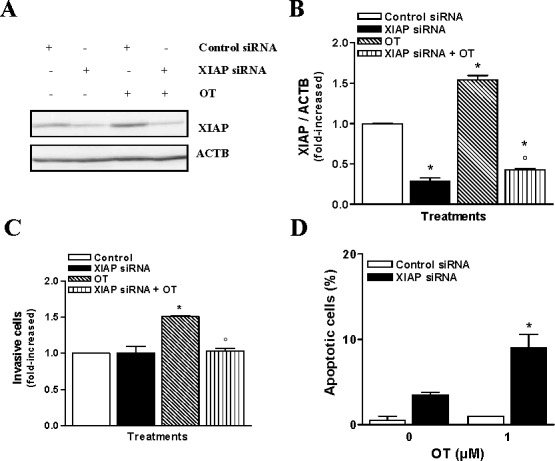
XIAP is involved in the invasiveness of HEC cells by OT. A) Western blot analysis was performed to determine XIAP content following XIAP siRNA targeting in the presence or absence of 1 μM of OT. ACTB was used as a loading control; a representative result is shown. B) Densitometric analysis of results presented in A. The results are the mean ± SEM of three independent experiments. *P < 0.05 when compared to untreated cells (control). °P < 0.05 when compared to OT-treated cells. C) The effect of reduction of XIAP on Hec-1-A invasion was determined by Matrigel invasion assay after treatment with 1 μM of OT added in the lower chamber of the assay for 24 h. *P < 0.05 when compared to untreated cells (control). °P < 0.05 when compared to OT-treated cells. D) Apoptotic index following a XIAP reduction in the presence and absence of 1 μM of OT was determined using Hoechst nuclear staining. The results are the mean ± SEM of three independent experiments. *P < 0.05.
OT-Mediated Up-Regulation of MMP14 and MMP2 Are PTGS1- and PTGS2-Dependent, Respectively
Because MMP14 and MMP2 have been identified as important participants in tumor cell invasion [27], we have examined whether OT-induced invasiveness of Hec-1-A cells also occurs via these two matrix metalloproteinases. We found that resting Hec-1-A cells expressed detectable levels of both MMP14 and MMP2, but exposure to OT induced an up-regulation of their transcripts (Fig. 6A) and proteins (Fig. 6B). Treatment with Indomethacin did not impede the up-regulation of MMP2 expression in Hec-1-A cells by OT (Fig. 6, C and D), but the treatments with NS-398 or LY294002 blocked OT-induced MMP2 up-regulation, indicating a regulatory role of PTGS2 and PIK3 in OT-induced MMP2 expression. In addition, we found that Indomethacin treatment decreased MMP14 expression in Hec-1-A cells (Fig. 6, E and F). Pretreatment with LY294002 for 1 h inhibits OT-induced MMP14 expression, whereas NS-398 could not inhibit OT-induced MMP14 expression. Collectively, these results indicate that up-regulation of MMP14 and MMP2 following OT stimulation is a result of PIK3-dependent up-regulation of PTGS1 and PTGS2 in HEC cells.
FIG. 6.
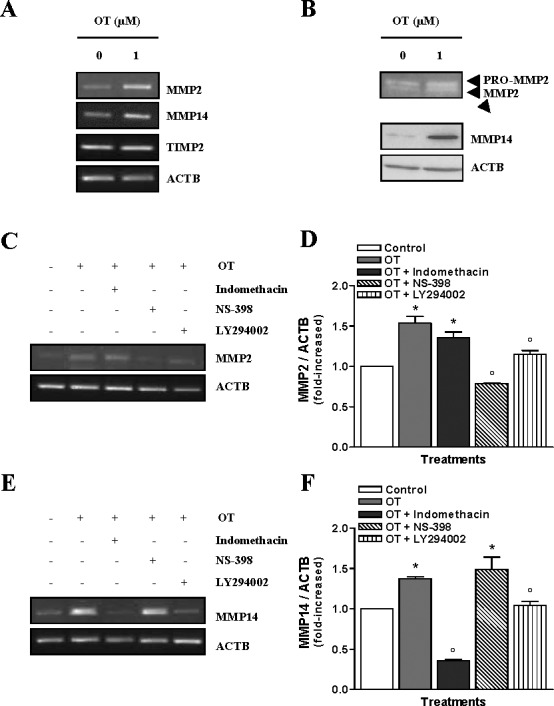
Involvement of PTGS1 and PTGS2 in the OT-mediated up-regulation of MMP14 and MMP2. A) The mRNA levels of MMP2, MMP14, and TIMP2 were determined in Hec-1-A cells by RT-PCR following treatment with 1 μM of OT for 24 h. β-actin was used as a loading control; a representative result is shown. B) Gelatin zymography was performed to analyze MMP2 activity upon treatment with 1 μM of OT for 24 h, whereas MMP14 activity was determined by Western blot analysis. ACTB was used as a loading control; a representative result is shown. C) The involvement of PTGS1, PTGS2, and PIK3 on MMP2 was determined by RT-PCR after a pretreatment of 1 h with 20 μM LY294002 or 100 nM of Indomethacin or 10 μM of NS-398 followed by 24 h incubation with 1 μM of OT. ACTB was used as a loading control; a representative result is shown. D) Densitometric analysis of results presented in C. The results are the mean ± SEM of three independent experiments. *P < 0.05 when compared to untreated cells (control). °P < 0.05 when compared to OT-treated cells. E) The involvement of PTGS1, PTGS2, and PIK3 in the regulation of MMP14 was determined by RT-PCR after a pretreatment of 1 h with 20 μM LY294002 or 100 nM of Indomethacin or 10 μM of NS-398 followed by 24 h incubation with 1 μM of OT. ACTB was used as a loading control; a representative result is shown. F) Densitometric analysis of results presented in C. The results are the mean ± SEM of three independent experiments. *P < 0.05 when compared to untreated cells (control). °P < 0.05 when compared to OT-treated cells.
OTR Is Expressed in Endometrial Carcinoma Tumors In Vivo
To correlate our findings for a role of OTR in the invasiveness of HEC cells with the clinical data, we have performed immunofluorescence analysis on an endometrial carcinoma tissue panel representing various grades of the disease (Fig. 7). OTR expression was observed in epithelial and glandular cells from the grade I (Fig. 7A) and grade II (Fig. 7B) of endometrial carcinoma specimens and even in poorly differentiated grade III (Fig. 7C). Moreover, few cells in the stroma compartment showed OTR signals in the grade I tumors. Additionally, we found that the immunostaining for OTR decreases with advanced grades. In the normal endometrium and in smooth muscles tissue samples, OTR immunoreactivity was undetectable (Fig. 7, D and E). Altogether, these results indicate that OTR is expressed in all the grades of endometrial tumors samples in vivo.
FIG. 7.
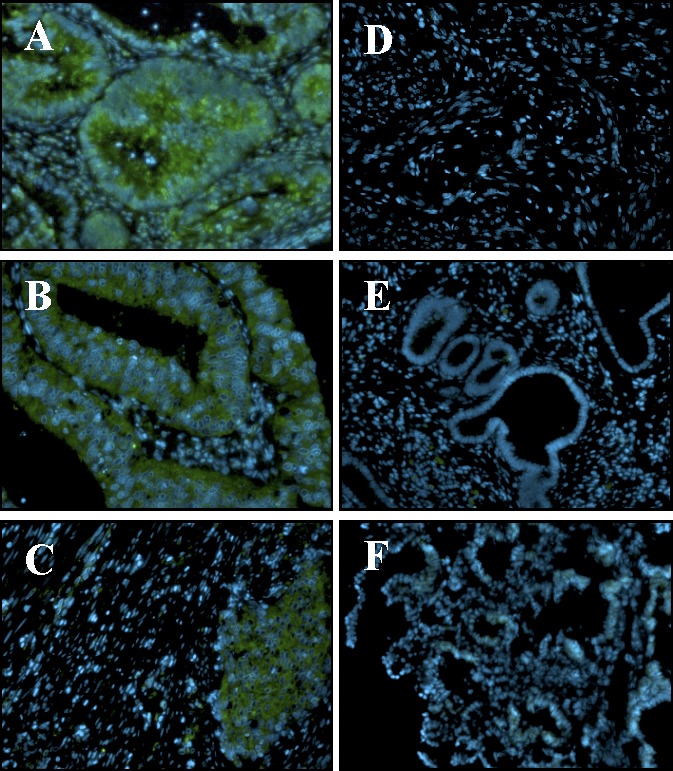
OTR is expressed in endometrial carcinoma tumors in vivo. OTR immunoreactivity (green) in human endometrial carcinoma grade I (A), grade II (B), and grade III (C), smooth muscles (D), and normal endometrial tissue (E) was assessed using Cybrdi human endometrial tissue array slides. For negative control, primary antibody was substituted with rabbit IgG (F). Hoechst dye was used to visualize nuclei (blue). Results shown are representative of 16 grade I, 34 grade II, five grade III tumors, two smooth muscles, and three normal endometrial specimens. Original magnification ×200.
DISCUSSION
Recent findings have greatly expanded the classical roles of OT, which were mainly associated with reproductive functions [28–33]. Moreover, novel sites of OTR expression have been identified in many peripheral organs [28, 34–39], suggesting that OT has physiological functions. OTR has also been described in various carcinoma cells [15, 40–45] where OT may regulate cell proliferation depending on cancer cell type. Cassoni et al. [15] reported that OT significantly inhibited cell proliferation of endometrial carcinoma without inducing apoptosis. Beyond the growth-inhibiting aspect of OT, the purpose of this study was to investigate the possibility that OT may promote the invasiveness of resistant endometrial cancer cells by up-regulating or stimulating selected factors of tumor aggressiveness such as PTGS2/PGE2, PIK3/AKT, and XIAP.
We have worked with Hec-1-A and Ishikawa cell lines commonly used as a model of endometrial carcinoma cells and found to be OTR positive, poorly invasive in basal conditions, and resistant to many molecules such as TGFB and tumor necrosis factor [26, 46]. We show for the first time that OT can induce invasion in endometrial cancer cells, which are also resistant to the growth-inhibitory effects of OT.
It is well accepted that production of prostaglandins by endometrial epithelial cells under basal conditions is regulated through the constitutive expression of PTGS1, whereas under stimulated conditions prostaglandin production is a result of the up-regulation of PTGS2 expression [47]. Our results support this concept because resting HEC cells express high levels of PTGS1 but very low levels of PTGS2 and PGE2 concentration. After OT stimulation, the expression of both the isoforms of cyclooxygenases and PGE2 is up-regulated in HEC cells. Together, these results suggest that OT-induced PGE2 production occurs via the up-regulation of PTGS2 expression rather than PTGS1. Moreover, we showed that OT increased the invasiveness of endometrial carcinoma cells through production of PGE2 and EP1 receptor.
Further, we reported the specific contribution of each cyclooxygenase isoform to the OT-induced invasion using specific pharmacological inhibitors. We found that both PTGS1 and PTGS2 are necessary for the OT-induced invasion of HEC cells, which was inhibited by PIK3 inhibitor LY294002. Furthermore, OT was able to induce AKT phosphorylation/activation, which was also inhibited by LY294002, thus suggesting that PK3/AKT pathway plays a central role in endometrial cancer cell invasion. The ability of OT to increase invasion, however, is not same for all tissue types. Previously, OT was found to inhibit proliferation, migration, and invasion of ovarian cancer cells [45]. The reason for this difference as compared to endometrial cancer cells could be tissue specificity and dose dependence, as the authors have used lower concentration of OT compared to the concentration used in the present study.
Previously, the involvement of PTGS2/PGE2/MMP pathway in increasing cell migration and invasion has been reported [48]. Here, we demonstrated that both PTGS1 and PTGS2 are required for the increase in MMP2 and MMP14 activities. Indeed, the action of the two cyclooxygenases seems to act synergistically: PTGS1 triggers MMP14, which has been shown to be one of the major activators of MMP2 in invasive tumors [49], while PTGS2 up-regulates MMP2 expression. To our knowledge, this is the first report of such collaborative regulation of MMP2 and MMP14 by COX isoforms. A recent study [50] showed that PTGS1 and PTGS2 play essential roles in gonadotropin-induced migration and invasion in human ovarian cancer cells, supporting our findings that the expression of the two isoforms of cyclooxygenases were critical for OT-induced invasive properties in endometrial carcinoma cells.
We had recently reported the involvement of XIAP in the regulation of invasion of endometrial carcinoma cells that had been exposed to TGFB [26], given the indication that XIAP could be a critical factor in the invasiveness of endometrial carcinoma cells. We have investigated a putative role of XIAP in the OT-induced invasion of HEC cells. OT increased XIAP protein level in Hec-1-A and Ishikawa cells in a PTGS2-dependent manner, which is in accordance with activation of the NFKB pathway, as shown in other cell types [22, 51]. In addition, we also found that XIAP is required to induce invasion in HEC cells exposed to OT. XIAP is a ubiquitous protein known to protect cells against apoptosis by binding and inhibiting caspase pathway [52]. Although no toxic effects or apoptosis were reported with OT [15], it is not surprising that inhibition of XIAP by RNA interference increased the number of apoptotic cells. However, we cannot rule out the possibility that the reduction in invasion followed by XIAP knockdown could be due to increased apoptotic count. Taken together, these results highlight the importance of XIAP in invasion as well as in resistance to apoptosis in endometrial carcinoma cells.
Finally, we have correlated the presence of OTR in endometrial carcinoma tumors with the grade of the disease in vivo. In the normal cycling endometrium, OTR is shown to be under sex steroid regulation and high levels of OTR are present in the luteal phase [53]. It is, therefore, not surprising that the OTR signal detected by immunofluorescence analysis and confirmed by immunohistochemistry was low/undetectable in normal endometrium tissue samples. By contrast, OTR immunoreactivity was strongly expressed in tumor tissue. Upon tumor progression from grade I to grade III, the OTR was localized mostly in the epithelial and glandular compartment, which is consistent with a role for OTR in the invasiveness of HEC cells. These results indicate that overexpression of the OTR is an important factor to induce cancer progression, and further, metastasis in the presence of OT in the system.
In summary, we provide the first evidence that OT could efficiently increase invasion in HEC cells through different factors such as PTGS1, PTGS2, and XIAP, which all seem to be dependent on the activation of PIK3. Moreover, we have identified a key role for XIAP in OT-induced invasion, which is PTGS2-dependent. The present study further suggests that the presence of OTR in endometrial cancer cells may, therefore, be considered as a major factor involved in the progression of endometrial cancer.
Footnotes
Supported by a research grant from the Canadian Institute of Health Research (CIHR). E.A. is Chairholder of the Canada research chair in molecular gyneco-oncology.
REFERENCES
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277 300. [DOI] [PubMed] [Google Scholar]
- Holland JF, Frei E III. Endometrial cancer. Kufe DW, Bast RC, Hait WN, Hong WK, Pollock RE, Weichselbaum RR, Holland JF, Frei E III. (eds.), Cancer Medicine, 7th ed, Hamilton, Ontario: BC Decker; 2006: 1522 1538. [Google Scholar]
- Silverman AJ, Zimmerman EA. Magnocellular neurosecretory system. Annu Rev Neurosci 1983; 6: 357 380. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Marrocco T, Deaglio S, Sapino A, Bussolati G. Biological relevance of oxytocin and oxytocin receptors in cancer cells and primary tumors. Ann Oncol 2001; 12 (suppl 2): S37 S39. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Sapino A, Marrocco T, Chini B, Bussolati G. Oxytocin and oxytocin receptors in cancer cells and proliferation. J Neuroendocrinol 2004; 16: 362 364. [DOI] [PubMed] [Google Scholar]
- Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Structure and expression of a human oxytocin receptor. Nature 1992; 356: 526 529. [DOI] [PubMed] [Google Scholar]
- Zingg HH, Laporte SA. The oxytocin receptor. Trends Endocrinol Metab 2003; 14: 222 227. [DOI] [PubMed] [Google Scholar]
- Bussolati G, Cassoni P. Editorial: the oxytocin/oxytocin receptor system—expect the unexpected. Endocrinology 2001; 142: 1377 1379. [DOI] [PubMed] [Google Scholar]
- Asselin E, Goff AK, Bergeron H, Fortier MA. Influence of sex steroids on the production of prostaglandins F2 alpha and E2 and response to oxytocin in cultured epithelial and stromal cells of the bovine endometrium. Biol Reprod 1996; 54: 371 379. [DOI] [PubMed] [Google Scholar]
- Tithof PK, Roberts MP, Guan W, Elgayyar M, Godkin JD. Distinct phospholipase A2 enzymes regulate prostaglandin E2 and F2alpha production by bovine endometrial epithelial cells. Reprod Biol Endocrinol 2007; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copland JA, Jeng YJ, Strakova Z, Ives KL, Hellmich MR, Soloff MS. Demonstration of functional oxytocin receptors in human breast Hs578T cells and their up-regulation through a protein kinase C-dependent pathway. Endocrinology 1999; 140: 2258 2267. [DOI] [PubMed] [Google Scholar]
- Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009; 30: 377 386. [DOI] [PubMed] [Google Scholar]
- Kennedy TG. Prostaglandins and uterine sensitization for the decidual cell reaction. Ann N Y Acad Sci 1986; 476: 43 48. [DOI] [PubMed] [Google Scholar]
- Wu WK. Yiu Sung JJ, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett 2010; 295: 7 16. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Fulcheri E, Carcangiu ML, Stella A, Deaglio S, Bussolati G. Oxytocin receptors in human adenocarcinomas of the endometrium: presence and biological significance. J Pathol 2000; 190: 470 477. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Marrocco T, Bussolati B, Allia E, Munaron L, Sapino A, Bussolati G. Oxytocin induces proliferation and migration in immortalized human dermal microvascular endothelial cells and human breast tumor-derived endothelial cells. Mol Cancer Res 2006; 4: 351 359. [DOI] [PubMed] [Google Scholar]
- Cattaneo MG, Chini B, Vicentini LM. Oxytocin stimulates migration and invasion in human endothelial cells. Br J Pharmacol 2008; 153: 728 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narko K, Ristimaki A, MacPhee M, Smith E, Haudenschild CC, Hla T. Tumorigenic transformation of immortalized ECV endothelial cells by cyclooxygenase-1 overexpression. J Biol Chem 1997; 272: 21455 21460. [DOI] [PubMed] [Google Scholar]
- Ohno S, Ohno Y, Suzuki N, Inagawa H, Kohchi C, Soma G, Inoue M. Multiple roles of cyclooxygenase-2 in endometrial cancer. Anticancer Res 2005; 25: 3679 3687. [PubMed] [Google Scholar]
- Moore AE, Greenhough A, Roberts HR, Hicks DJ, Patsos HA, Williams AC, Paraskeva C. HGF/Met signalling promotes PGE(2) biogenesis via regulation of COX-2 and 15-PGDH expression in colorectal cancer cells. Carcinogenesis 2009; 30: 1796 1804. [DOI] [PubMed] [Google Scholar]
- Sexton E, Van Themsche C, Leblanc K, Parent S, Lemoine P, Asselin E. Resveratrol interferes with AKT activity and triggers apoptosis in human uterine cancer cells. Mol Cancer 2006; 5: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Germain ME, Gagnon V, Parent S, Asselin E. Regulation of COX-2 protein expression by Akt in endometrial cancer cells is mediated through NF-kappaB/IkappaB pathway. Mol Cancer 2004; 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Germain ME, Gagnon V, Mathieu I, Parent S, Asselin E. Akt regulates COX-2 mRNA and protein expression in mutated-PTEN human endometrial cancer cells. Int J Oncol 2004; 24: 1311 1324. [PubMed] [Google Scholar]
- Amir M, Agarwal HK. Role of COX-2 selective inhibitors for prevention and treatment of cancer. Pharmazie 2005; 60: 563 570. [PubMed] [Google Scholar]
- Gao J, Mazella J, Tang M, Tseng L. Ligand-activated progesterone receptor isoform hPR-A is a stronger transactivator than hPR-B for the expression of IGFBP-1 (insulin-like growth factor binding protein-1) in human endometrial stromal cells. Mol Endocrinol 2000; 14: 1954 1961. [DOI] [PubMed] [Google Scholar]
- Van Themsche C, Mathieu I, Parent S, Asselin E. Transforming growth factor-beta3 increases the invasiveness of endometrial carcinoma cells through phosphatidylinositol 3-kinase-dependent up-regulation of X-linked inhibitor of apoptosis and protein kinase c-dependent induction of matrix metalloproteinase-9. J Biol Chem 2007; 282: 4794 4802. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Song T, Jin Y, Pan J, Zhang L, Wang L, Li P. Epidermal growth factor receptor regulates MT1-MMP and MMP-2 synthesis in SiHa cells via both PI3-K/AKT and MAPK/ERK pathways. Int J Gynecol Cancer 2009; 19: 998 1003. [DOI] [PubMed] [Google Scholar]
- Blanks AM, Thornton S. The role of oxytocin in parturition. BJOG 2003; 110 (suppl 20): 46 51. [DOI] [PubMed] [Google Scholar]
- Chandrasekher YA, Fortune JE. Effects of oxytocin on steroidogenesis by bovine theca and granulosa cells. Endocrinology 1990; 127: 926 933. [DOI] [PubMed] [Google Scholar]
- Einspanier A, Ivell R, Hodges JK. Oxytocin: a follicular luteinisation factor in the marmoset monkey. Adv Exp Med Biol 1995; 395: 517 522. [PubMed] [Google Scholar]
- Einspanier A, Jurdzinski A, Hodges JK. A local oxytocin system is part of the luteinization process in the preovulatory follicle of the marmoset monkey (Callithrix jacchus). Biol Reprod 1997; 57: 16 26. [DOI] [PubMed] [Google Scholar]
- Flint AP, Lamming GE, Stewart HJ, Abayasekara DR. The role of the endometrial oxytocin receptor in determining the length of the sterile oestrous cycle and ensuring maintenance of luteal function in early pregnancy in ruminants. Philos Trans R Soc Lond B Biol Sci 1994; 344: 291 304. [DOI] [PubMed] [Google Scholar]
- Kiss A, Mikkelsen JD. Oxytocin—anatomy and functional assignments: a minireview. Endocr Regul 2005; 39: 97 105. [PubMed] [Google Scholar]
- Frayne J, Nicholson HD. Localization of oxytocin receptors in the human and macaque monkey male reproductive tracts: evidence for a physiological role of oxytocin in the male. Mol Hum Reprod 1998; 4: 527 532. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev 2001; 81: 629 683. [DOI] [PubMed] [Google Scholar]
- Ivell R, Balvers M, Rust W, Bathgate R, Einspanier A. Oxytocin and male reproductive function. Adv Exp Med Biol 1997; 424: 253 264. [DOI] [PubMed] [Google Scholar]
- Jankowski M, Hajjar F, Kawas SA, Mukaddam-Daher S, Hoffman G, McCann SM, Gutkowska J. Rat heart: a site of oxytocin production and action. Proc Natl Acad Sci U S A 1998; 95: 14558 14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre DL, Giaid A, Bennett H, Lariviere R, Zingg HH. Oxytocin gene expression in rat uterus. Science 1992; 256: 1553 1555. [DOI] [PubMed] [Google Scholar]
- Lefebvre DL, Giaid A, Zingg HH. Expression of the oxytocin gene in rat placenta. Endocrinology 1992; 130: 1185 1192. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Sapino A, Negro F, Bussolati G. Oxytocin inhibits proliferation of human breast cancer cell lines. Virchows Arch 1994; 425: 467 472. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Sapino A, Fortunati N, Munaron L, Chini B, Bussolati G. Oxytocin inhibits the proliferation of MDA-MB231 human breast-cancer cells via cyclic adenosine monophosphate and protein kinase A. Int J Cancer 1997; 72: 340 344. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Sapino A, Stella A, Bussolati G. Antiproliferative effect of oxytocin through specific oxytocin receptors in human neuroblastoma and astrocytoma cell lines. Adv Exp Med Biol 1998; 449: 245 246. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Sapino A, Stella A, Fortunati N, Bussolati G. Presence and significance of oxytocin receptors in human neuroblastomas and glial tumors. Int J Cancer 1998; 77: 695 700. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Sapino A, Munaron L, Deaglio S, Chini B, Graziani A, Ahmed A, Bussolati G. Activation of functional oxytocin receptors stimulates cell proliferation in human trophoblast and choriocarcinoma cell lines. Endocrinology 2001; 142: 1130 1136. [DOI] [PubMed] [Google Scholar]
- Morita T, Shibata K, Kikkawa F, Kajiyama H, Ino K, Mizutani S. Oxytocin inhibits the progression of human ovarian carcinoma cells in vitro and in vivo. Int J Cancer 2004; 109: 525 532. [DOI] [PubMed] [Google Scholar]
- Van Themsche C, Lafontaine L, Asselin E. X-linked inhibitor of apoptosis protein levels and protein kinase C activity regulate the sensitivity of human endometrial carcinoma cells to tumor necrosis factor alpha-induced apoptosis. Endocrinology 2008; 149: 3789 3798. [DOI] [PubMed] [Google Scholar]
- Parent J, Villeneuve C, Fortier MA. Evaluation of the contribution of cyclooxygenase 1 and cyclooxygenase 2 to the production of PGE2 and PGF2 alpha in epithelial cells from bovine endometrium. Reproduction 2003; 126: 539 547. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Choi EM, Kim SR, Park JH, Kim H, Ha KS, Kim YM, Kim SS, Choe M, Kim JI, Han JA. Cyclooxygenase-2 promotes cell proliferation, migration and invasion in U2OS human osteosarcoma cells. Exp Mol Med 2007; 39: 469 476. [DOI] [PubMed] [Google Scholar]
- Mignon C, Okada A, Mattei MG, Basset P. Assignment of the human membrane-type matrix metalloproteinase (MMP14) gene to 14q11-q12 by in situ hybridization. Genomics 1995; 28: 360 361. [DOI] [PubMed] [Google Scholar]
- Lau MT, Wong AS, Leung PC. Gonadotropins induce tumor cell migration and invasion by increasing cyclooxygenases expression and prostaglandin E(2) production in human ovarian cancer cells. Endocrinology 2010; 151: 2985 2993. [DOI] [PubMed] [Google Scholar]
- Xiao CW, Ash K, Tsang BK. Nuclear factor-kappaB-mediated X-linked inhibitor of apoptosis protein expression prevents rat granulosa cells from tumor necrosis factor alpha-induced apoptosis. Endocrinology 2001; 142: 557 563. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature 1997; 388: 300 304. [DOI] [PubMed] [Google Scholar]
- Fuchs AR, Fuchs F, Soloff MS. Oxytocin receptors in nonpregnant human uterus. J Clin Endocrinol Metab 1985; 60: 37 41. [DOI] [PubMed] [Google Scholar]


