Abstract
To explore the relationship between signal-stimulated increases in intracellular calcium ([Ca2+]i) and depletion and refilling of the endoplasmic reticulum (ER) Ca2+ stores ([Ca2+]L) in human myometrial cells, we measured simultaneous changes in [Ca2+]i and [Ca2+]L using Fura-2 and Mag-fluo-4, respectively, in PHM1-41 immortalized and primary cells derived from pregnant myometrium and in primary cells derived from nonpregnant tissue. Signal- and extracellular Ca2+-dependent increases in [Ca2+]i (SRCE) and ER refilling stimulated by oxytocin and cyclopiazonic acid were not inhibited by voltage-operated channel blocker nifedipine or mibefradil, inhibition of Na+/Ca2+ exchange with KB-R7943, or zero extracellular Na+ in PHM1-41 cells. Gadolinium-inhibited oxytocin- and cyclopiazonic acid-induced SRCE and slowed ER store refilling. TRPC1 mRNA knockdown specifically inhibited oxytocin-stimulated SRCE but had no statistically significant effect on ER store refilling and no effect on either parameter following cyclopiazonic acid treatment. Dominant negative STIMΔERM expression attenuated oxytocin- and thapsigargin-stimulated SRCE. Both STIM1 and ORAI1–ORAI3 mRNA knockdowns significantly attenuated oxytocin- and cyclopiazonic acid-stimulated SRCE. The data also suggest that reduction in STIM1 or ORAI1–ORAI3 mRNA can impede the rate of ER store refilling following removal of SERCA inhibition. These data provide evidence for both distinct and overlapping influences of TRPC1, STIM1, and ORAI1–ORAI3 on SRCE and ER store refilling in human myometrial cells that may contribute to the regulation of myometrial Ca2+ dynamics. These findings have important implications for understanding the control of myometrial Ca2+ dynamics in relation to myometrial contractile function.
Keywords: calcium, endoplasmic reticulum calcium stores, intracellular calcium, myometrial cells, myometrium, ORAI, STIM1, TRPC1
TRPC1, STIM1, and ORAI1-3 influence signal-regulated intracellular and endoplasmic reticulum calcium dynamics in human myometrial cells and could play a role in the regulation of uterine activity.
INTRODUCTION
Intracellular Ca2+ signals play important roles in myometrium in the regulation of cellular function and contraction [1, 2]. The myometrium is an excitable tissue in which spontaneous depolarization and associated action potentials give rise to spontaneous contractions [3]. Increases in intracellular free Ca2+ ([Ca2+]i) are correlated with increases in contractile activity. Increases in [Ca2+]i in myometrium occur primarily as a result of the entry of extracellular Ca2+ through plasma membrane ion channels and release of Ca2+ from the endoplasmic reticulum (ER) via inositol 1,4,5-trisphosphate (IP3) receptors following G protein-coupled receptor (GPCR)-stimulated phospholipase C activation, or by inhibition of the ER Ca2+ ATPase (SERCA), or by passive leakage [2], but there is little contribution of Ca2+-induced Ca2+ release and no evidence of associated sparks in myometrial cells [1, 4, 5]. [Ca2+]i is lowered through the combined activities of SERCA, the plasma membrane Ca2+ ATPase, and Na+/Ca2+ exchangers [6, 7].
Influx of extracellular Ca2+ into cells occurs through voltage-dependent and signal-regulated (variously termed capacitative, store-operated, or receptor-operated) ion channels in the plasma membrane [8, 9]. The signal for store-operated Ca2+ entry has been attributed to ER Ca2+ depletion following SERCA inhibition and variously also to Ca2+ entry resulting from GPCR simulation and IP3 production. The term signal-regulated Ca2+ entry (SRCE) is operationally defined here as an increase in [Ca2+]i that is dependent on extracellular Ca2+ and a prior stimulus, such as GPCR stimulation or SERCA inhibition, regardless of mechanism.
The myometrial ER functions as an important intracellular Ca2+ store that contributes to both increases and decreases in [Ca2+]i. The concentration of ER luminal Ca2+ ([Ca2+]L) has been estimated to be submicromolar, in contrast to that of resting cytoplasmic [Ca2+]i, which is in the nanomolar range [7]. Simultaneous measurements of Ca2+ dynamics in myometrial cells by using the high- and low-affinity calcium indicators Fura-2 and Mag-fluo-4, respectively, revealed that there were no detectable changes in [Ca2+]L during spontaneous [Ca2+]i oscillations [10]. Moderate decreases in [Ca2+]L abolished agonist-induced [Ca2+]i transients, whereas increasing [Ca2+]L did not increase the size of agonist-induced [Ca2+]i transients [11].
Human myometrial cells express canonical transient receptor potential (TRPC) channels, with TRPC1, TRPC4, and TRPC6 mRNAs in highest relative abundance [12]. TRPC proteins form homo- or heterotetrameric ion channels with various properties and have been implicated in SRCE [8, 13, 14]. We have previously reported that knockdown of endogenous TRPC4 in myometrial cells specifically attenuates GPCR-stimulated but not thapsigargin- or diacylglycerol (OAG)-stimulated SRCE [15]. In contrast, TRPC6 knockdown specifically reduces the OAG-mediated increase in [Ca2+]i in a manner consistent with both an enhanced Na+ entry coupled to activation of voltage-dependent Ca2+ entry channels and a nifedipine-independent Ca2+ entry mechanism [16].
To assess the roles of TRPC1 alone and in relation to TRPC4 in myometrial SRCE, knockdown of TRPC1 mRNA as well as the combined knockdown of these two mRNAs was achieved by expressing tandem Short-hairpin RNA (shRNA) in a new adenoviral vector targeting TRPC1 alone or TRPC1 plus TRPC4 within a single adenovirus. This vector was modeled after the lentiviral vector created by Sun et al. [17] for expression of multi-microRNA hairpin constructs, effectively targeting knockdowns of either single or multiple mRNAs. A new multiple cloning site (MCS) inserted into the pAdTrack-CMV vector enables the potential targeting of single or multiple proteins through tandem shRNA expression and infection with a single adenoviral vector.
It has recently been recognized that the stromal interaction molecule (STIM) and calcium release-activated calcium modulator (ORAI) proteins constitute store-operated channels and are responsible for the highly selective Ca2+ release-activated Ca2+ (CRAC) channel in a number of cell types (see [18–21] for recent reviews). STIM proteins span the endoplasmic reticulum membrane and sense changes in ER Ca2+. In response to decreases in ER Ca2+, STIM1 protein oligomerizes and clusters into ER regions in close apposition to the plasma membrane. There STIM1 interacts with ORAI1 dimers and induces the formation of ORAI1 tetramers to produce the pore-forming unit of the CRAC channel. STIM1 and ORAI proteins have been shown to interact with TRPC proteins and have been implicated in GPCR-stimulated SRCE in some studies but not others [22]. Less work has been done on the functions of other STIM and ORAI isoforms.
To date, there have been no direct knockdown studies in myometrium that identify roles for TRPC1, STIM, and ORAI proteins in cytoplasmic or ER Ca2+store dynamics. In this study, we used channel inhibitors and viral shRNA delivery systems to examine the effects of TRPC1, STIM1, and ORAI1–ORAI3 mRNA knockdown on simultaneous [Ca2+]i and ER [Ca2+]L dynamics in response to GPCR activation and SERCA inhibition in human myometrial cells.
MATERIALS AND METHODS
Materials
Fura-2/acetoxymethylester (Fura-2/AM), Mag-fluo-4/AM and pluronic acid F127 were obtained from Invitrogen (Carlsbad, CA). KB-R7943 was obtained from Tocris Bioscience (Ellisville, MO). Thapsigargin, cyclopiazonic acid (CPA), nifedipine, mibefradil, gadolinium, oxytocin, and all other chemicals were obtained from Sigma (St. Louis, MO). Restriction enzymes were obtained from New England Biolabs Inc. (Beverly, MA) or Promega (Madison, WI). Cell culture medium and other tissue culture reagents were obtained from Invitrogen/GIBCO BRL (Carlsbad, CA). Oligonucleotides were purchased from Integrated DNA Technologies, Inc. (Coralville, IA).
Cell Culture
PHM1-41 immortalized myometrial cells derived from tissue collected from a nonlaboring pregnant woman at the time of cesarean section [23] were cultured in Dulbecco modified Eagle medium-high glucose with 10% fetal calf serum (FCS), 50 units/ml penicillin, 50 μg/ml streptomycin, and 2 mM l-glutamine and were used between passages 14 and 23. These cells retain many morphological and phenotypic responses in common with primary cells. Primary uterine smooth muscle cells (UtSMC) from nonpregnant human myometrium were purchased from Lonza (CC-2562, lot # 17590; Walkersville, MD).
Primary human myometrial cells (HMC) were isolated from myometrial tissue obtained at the time of cesarean section in uncomplicated pregnancies from 37–39-week pregnant women not in labor, with informed consent under approved protocols at both institutions, and cultured as described previously [16]. Cells were used for experiments at passages 3–9.
Adenoviral Construct Synthesis and Adenoviral Infection
STIMΔERM cDNA in pRK5/myc vector was obtained from Dr. P.F. Worley (The Johns Hopkins University School of Medicine, Baltimore, MD) and was cloned into pAdTrack-CMV vector using EcoRI and NotI restriction sites.
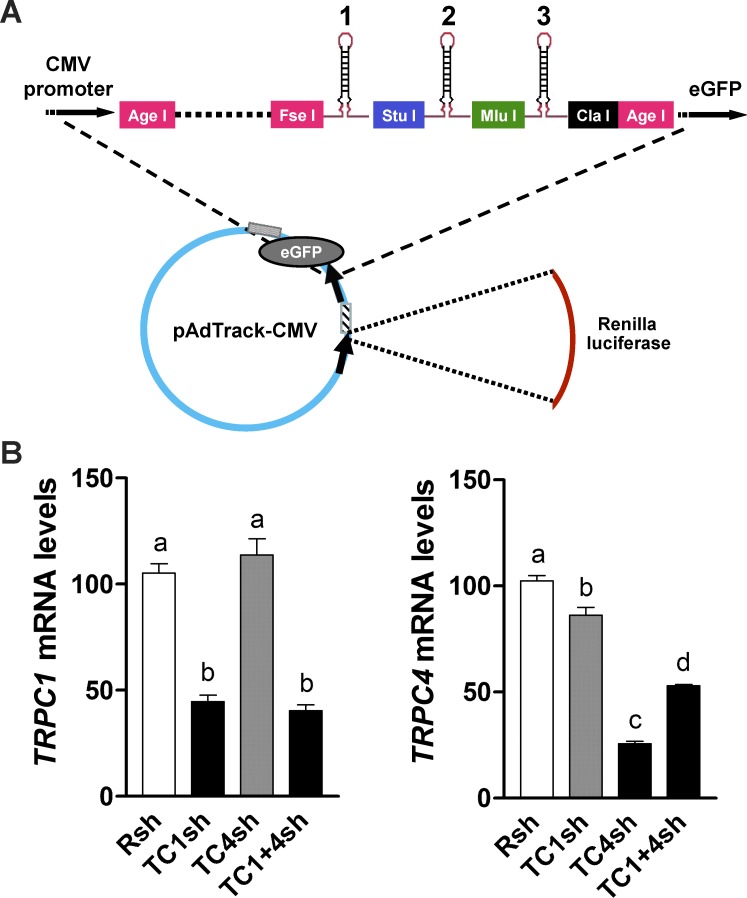
To generate a vector capable of accepting multiple shRNAs, the adenoviral vector pAdT-MCS was constructed by introduction of a new MCS (Table 1) between the CMV promoter in the pAdTrack-CMV vector (ATCC, Manassas, VA) and the sequence for green fluorescent protein (GFP) by using the AgeI restriction site (Fig. 1A). The MCS allows insertion of up to three shRNA hairpin sequences into the FseI, StuI, MluI, and ClaI sites. The Renilla luciferase cDNA sequence was excised from the psiCHECK-2 vector and cloned into this vector, using NheI and XhoI restriction sites, giving rise to pAdTrack-CMV/MCS/Renilla-luc (pAdT-CMR). The GFP reporter in this vector is expressed in cells, indicating infection, but fades with increasing numbers of shRNA constructs cloned upstream of the GFP transcription start site, presumably an indication of shRNA processing.
TABLE 1.
Short-hairpin RNA (shRNA) sequences for targeting ORAI1, ORAI2, ORAI3, STIM1, and TRPC1 mRNAs.a

FIG. 1.
A) Schematic representation of the pAdT-CMR vector construct shows the sites for tandem insertion of shRNAs at positions 1, 2, and 3. B) Specific TRPC mRNA knockdowns produced by adenovirus vector expressing tandem TRPC1 (TC1sh), TRPC4 (TC4sh), and TRPC1+TRPC4 (TC1+4sh) shRNAs in UtSMC cells are shown. Left: TRPC1 mRNA knockdown was achieved by tandem expression of TC1sh and TC1+4sh but not TC4sh, compared to effects of control shRNA (Rsh). Right: Tandem expression of TC4sh and TC1+4sh, but not TC1sh, induced a TRPC4 mRNA knockdown when compared to Rsh. Data represent means ± SEM (n = 4).
TRPC1, STIM1, ORAI1, ORAI2, ORAI3, and Renilla Luciferase shRNA sequences and the human pre-microRNA stem sequence (miR-30) used are shown in Table 1. Restriction sites used for insertion into the FseI, StuI, MluI, and ClaI restriction sites in the MCS were added to the shRNA oligonucleotides by PCR using adapter primers (sequences are available on request), and the resulting products were cloned into the pAdT-CMR vector in various combinations. TRPC1, STIM1, and Renilla control shRNA (Rsh) constructs in pAdT-CMR express three copies of the respective sequences. The TRPC1 plus TRPC4 (TRPC1+TRPC4) construct expresses one copy each of TRPC1 and TRPC4 shRNA, along with one Renilla shRNA sequence. The triple shRNA construct targeting ORAI1, ORAI2, and ORAI3 contains a single copy of each shRNA inserted into the FseI, StuI, and ClaI restrictions sites, respectively. Integrity was checked by DNA sequencing. The resulting pAdT-CMR clones were used to prepare adenovirus by recombination with the adenoviral backbone plasmid pAdEasy-1 (Stratagene, La Jolla, CA) as described previously [15]. Adenoviruses were crudely purified by passing the adenovirus-containing cell lysates through 0.45-μm polyvinylidene fluoride filters (Millipore, Billerica, MA) after three freeze-thaw cycles in a methanol-dry ice bath and titered by end-point dilution.
The day before infection, myometrial cells (0.4 × 105 to 0.7 × 105 cells) were plated on 35-mm dishes with glass inserts (MatTek, Ashland, MA) in 1 ml of culture medium. The next day, cells were infected in 1 ml of medium containing 2% FCS with adenovirus at a multiplicity of infection of 500 for UtSMC and HMC cells and 1000 for PHM1-41 cells. After 4–6 h, 1 ml of medium containing 18% FCS was added. Cells were used 72 h postinfection and exhibited morphology similar to that of uninfected cells, as determined by visual inspection. The effectiveness of infection under these conditions was 90%–95%.
Gene Expression Analysis by Quantitative Real-Time RT-PCR
Myometrial cell mRNA was prepared using the RNeasy mini-kit including the RNase-Free DNase step (QIAGEN, Valencia, CA). cDNA was synthesized using the qScript cDNA SuperMix synthesis kit (Quanta Biosciences, Gaithersburg, MD) with 1 μg of total RNA and the primers listed in Table1. Each PCR reaction was carried out in duplicate and contained 2 μl of cDNA template, 500 nM of forward and reverse primers for TRPC1 [12], STIM1, STIM2, ORAI1, ORAI2, ORAI3 (Table 1), and beta-glucuronidase (GUS) [15] and 10 μl of PerfeCta SYBR Green FastMix for the iQ mixture (Quanta Biosciences, Gaithersburg, MD). In some cases, 100 ng of mRNA was used with the iScript one-step reverse transcriptase (RT)-PCR kit with SYBR Green (Bio-Rad, Hercules, CA). Amplification was conducted using the iCycler iQ real-time PCR detection system (Bio-Rad, Hercules, CA). The PCR protocol was as follows: 15 sec denaturation at 95°C, 30 sec annealing at 60°C, and 30 sec elongation at 72°C for 40 cycles. Sequence integrity of RT-PCR products was verified by direct sequencing. Melting curves for all products showed single peaks. The relative target gene copy number was quantified by the ΔΔCt method, where a given RNA was first normalized to GUS in each sample and then expressed relative to the corresponding value in cells infected with control (Rsh) adenovirus for knockdown experiments or relative to TRPC1 activity in expression experiments.
Measurement of Ca2+ in Cytoplasm and ER
Cells were prepared for imaging experiments, and studies using only Fura-2 were carried out as previously described [15, 16]. To monitor changes in ER [Ca2+]L and cytoplasmic [Ca2+]i, respectively, cells were loaded with low-affinity Mag-fluo-4 (Kd = 22 μM) and high-affinity Fura-2 (Kd = 0.14 μM) indicators as described by Shmygol et al. [10, 11] with the following modifications. Cells were incubated in fluorescence buffer (FB; consisting of 145 mM NaCl, 5 mM KCl, 1 mM Na2HPO4, 0.5 mM MgCl2, 1 mM CaCl2, 10 mM HEPES, 5 mM glucose, pH 7.4) containing 4–5 μM Mag-fluo-4/AM and 0.05% Pluronic F127 at 37°C for 40 min, washed with FB, and then incubated in FB with 5 μM Fura-2 AM plus 0.05% Pluronic F127 at room temperature for 30 min. The cells were washed and incubated in FB at room temperature for an additional 30 min to allow de-esterification of the indicators and used immediately for measurements. Cells were placed in Ca2+-free buffer (Ca2+-free FB containing 100 μM EGTA) and alternately illuminated at 340 and 380 nm (Fura-2) and 465 nm (Mag-fluo-4) excitation and 510 nm emission, using a model 101 Photon Technology International fluorescent imaging system. Cells exposed to CPA were washed by perfusion with Ca2+-free FB for 10 min at 0.7–1 ml/min and incubated for another 5 min before SRCE was initiated by addition of 1 mM CaCl2. Choline chloride was substituted for Na+ in Ca2+-free FB where indicated. Changes in [Ca2+]i are reported as changes in the Fura-2 340:380 nm ratio. Relative changes in [Ca2+]L are reported as the Mag-fluo-4 signal normalized to its value at the beginning of each experiment (F/Fo).
Data Collection and Analysis
In most of the studies, we present data obtained in both PHM1-41 immortalized and primary myometrial cells in order to minimize preparation artifacts. In each dish, 10–40 individual myometrial cells were examined. Because the results after viral infection were somewhat variable, in the studies using infected cells, we determined the mean of the responses from all the cells measured in a given dish and expressed the data as means ± SEM of these values for n dishes in order not to introduce selection bias into interpretation of the data. In single-labeling experiments, changes in [Ca2+]i were analyzed using numerical analyses software (CalciumComp; K. J. Bois, Fort Collins, CO) [15]. In dual-labeling experiments, the area of the [Ca2+]i response was determined using features in Kaleidagraph software (Synergy Software, Reading, PA). The initial rate of ER Ca2+ store refilling was determined by linear regression analysis with Excel software (Microsoft, Seattle, WA), and the ER store refilling:ER store depletion ratio was determined from mean responses by using the equation, fraction of ER refilling = [(F/Fo)t − (F/Fo)min]/[1 − (F/Fo)min], where F/Fo is the 465-nm fluorescence relative to time, t, zero, (F/Fo)t is relative fluorescence at time t, and (F/F0)min is relative fluorescence at the point of maximal store depletion.
Data were analyzed by one-way ANOVA, and post hoc comparison of means was performed using Tukey multiple comparison tests with Prism (GraphPad Software Inc., San Diego, CA) or Kaleidagraph software or by Student t-test for unpaired samples using Kaleidagraph software. P values of ≤0.05 were considered significant and are indicated with different lowercase letters or an asterisk, as appropriate.
RESULTS
TRPC1 Is Specifically Involved in GPCR-Stimulated [Ca2+]i Increases in Myometrial Cells
Infection of myometrial UtSMC with an adenoviral vector expressing three copies of TRPC1 shRNA under the control of the cytomegalovirus (CMV) promoter produced a 57% TRPC1 mRNA knockdown compared to cells infected with control vector (Rsh) without affecting TRPC4 mRNA levels, whereas infection with a virus expressing three copies of TRPC4 shRNA produced a 75% TRPC4 mRNA knockdown without affecting TRPC1 mRNA (Fig. 1B). TRPC6 expression was not changed in either case (data not shown). The TRPC1+TRPC4 shRNA tandem construct induced a knockdown of both TRPC1 and TRPC4 mRNA (61% and 48%, respectively). Similar results were obtained in PHM1-41 cells (data not shown). Hence, the tandem approach allows the knockdown of several mRNAs by using a single adenovirus, thus eliminating the ambiguity of multiple infections of the same cells, and is especially useful when working with myometrial cells that are difficult to transfect.
Expression of TRPC1 shRNA attenuated oxytocin (OT)-stimulated SRCE in UtSMC (Fig. 2A, left panel) and PHM1-41 cells (Fig. 2A, middle panel), with an average of 56% and 50% inhibition of the [Ca2+]i transient peak height and integrated area, respectively (Fig. 2A, right panel). Similar to our previous results using the U6-promoter virus [15], expression of TRPC4 shRNA in the pAdT-CMR vector inhibited OT-stimulated SRCE (Fig. 2A). Simultaneous knockdown of both TRPC1 and TRPC4 mRNAs by using the tandem shRNA construct induced a decrease in OT-stimulated SRCE that was not significantly greater than the decrease obtained after knockdown of either TRPC1 or TRPC4 alone (Fig. 2A). Thapsigargin-stimulated SRCE was not significantly affected by TRPC1, TRPC4, or TRPC1 plus TRPC4 mRNA knockdown in either UtSMC or PHM1-41 cells (Fig. 2B). Similarly, none of these shRNA combinations had any effect on OAG-stimulated SRCE (data not shown). Therefore, TRPC1 mRNA knockdown, like TRPC4 knockdown [15], resulted in specific attenuation of GPCR-mediated SRCE.
FIG. 2.
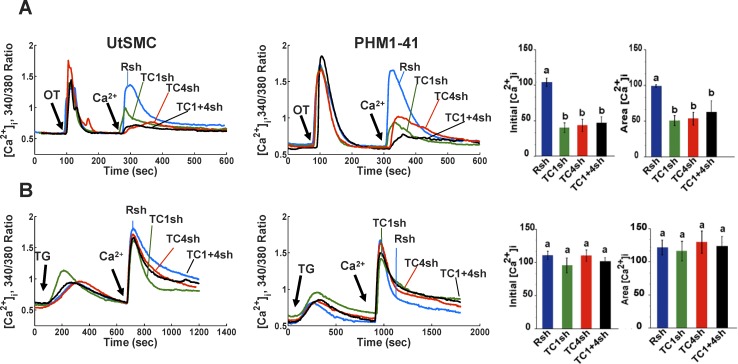
TRPC1, TRPC4, and TRPC1 plus TRPC4 mRNA knockdown induces specific inhibition of OT-stimulated SRCE in UtSMC (left panels) and PHM1-41 (middle panels) cells. A) Attenuation of SRCE induced by 100 nM OT in cells infected with a control shRNA targeting Rsh (Rsh, blue lines) or adenovirus expressing TRPC1 (TC1sh, green lines), TRPC4 (TC4sh, red lines), or TRPC1 plus TRPC4 shRNAs (TC1+4sh, black lines) is shown. The addition of 1 mM Ca2+ that initiates SRCE is indicated. Traces represent the mean responses of 10–35 cells. B) No effect of these shRNAs was observed on thapsigargin (TG, 100 nM)-stimulated SRCE. Right panels: Mean changes in [Ca2+]i (A and B), calculated as peak height (initial [Ca2+]i) and integrated area under the curve ([Ca2+]i area), are shown. As no significant differences were observed in responses from UtSMC and PHM1 cells, data from these sources were pooled for this analysis. Data are presented as means ± SEM (n = 6–8).
Calcium Responses to GPCR Stimulation and SERCA Inhibition Are Consistent with Fura-2 and Mag-fluo-4 Measuring Changes in Myometrial Cell [Ca2+]i and [Ca2+]L, Respectively
Differential loading of Mag-fluo-4 and Fura-2 has previously been reported to produce changes in [Ca2+]L and [Ca2+]i, respectively, in pregnant rat uterine myocytes [10, 11]. Our results, obtained with human myometrial cells, are consistent with those observations and further validate the use of this approach. OT elicited a rapid but transient increase in [Ca2+]i in PHM1-41 cells and a decrease in [Ca2+]L in the absence of extracellular Ca2+ (Fig. 3A). Subsequent addition of 1 mM Ca2+ resulted in an increase in [Ca2+]i (SRCE), as previously reported [24, 25]. This was accompanied by a return of [Ca2+]L to basal levels, reflecting refilling of the ER store. Neither event occurred if Ca2+-free buffer (0 Ca) was added instead, indicating that the changes were completely dependent on extracellular Ca2+. Thapsigargin, which irreversibly inhibits SERCA pumps and elicits ER Ca2+ store depletion, increased [Ca2+]i and produced a greater decline in [Ca2+]L than OT (Fig. 3B). The addition of 1 mM extracellular Ca2+ after thapsigargin resulted in an increase in [Ca2+]i (SRCE), but, consistent with the inhibition of SERCA, there was only a small increase in [Ca2+]L. This small increase in [Ca2+]L was not eliminated by the use of a higher concentration of thapsigargin (1 μM) and was observed in cells exposed to an equivalent amount of vehicle (0.1% DMSO) (data not shown).
FIG. 3.
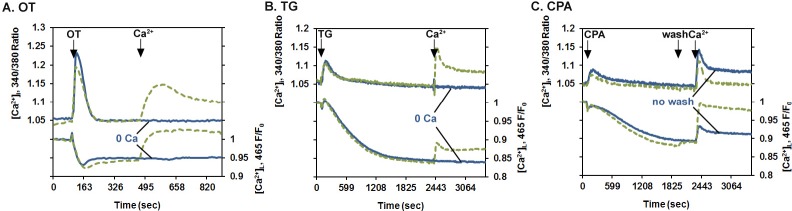
Changes in Fura-2 and Mag-fluo-4 fluorescence are consistent with the expected changes in PHM1-41 myometrial cell ([Ca2+]i) and ([Ca2+]L), respectively. Cells loaded with the dyes were exposed to (A) OT (100 nM), (B) thapsigargin (TG, 100 nM), or (C) CPA (10 μM) in the absence of extracellular Ca2+. After Ca2+ changes stabilized, cells were exposed to Ca2+-free buffer (0 Ca, blue line) or 1 mM extracellular Ca2+ (green line) in A and B. C) CPA was added as described for thapsigargin and was then removed from one group of cells by washing with calcium-free buffer (wash) at the time indicated. Both the washed (green line) and unwashed (blue line) cells were then exposed to 1 mM extracellular Ca2+. Additions of OT, CPA, wash, and Ca2+ are indicated by arrows. Each line represents an average of the responses of 35–40 cells in one of three similar experiments.
Similar to the effects of thapsigargin, the addition of 1 mM extracellular Ca2+ after exposure to CPA, a reversible SERCA inhibitor, produced an increase in [Ca2+]i but only a small increase in [Ca2+]L (Fig. 3C). However, when CPA was washed out before the addition of 1 mM extracellular Ca2+, in addition to the increase in [Ca2+]i, significant ER store refilling also occurred. These data are consistent with prior reports [10, 11] that Fura-2 and Mag-fluo-4 are simultaneously measuring changes in [Ca2+]i and [Ca2+]L, respectively, and show that increases in both compartments occur following introduction of Ca2+ into the extracellular medium subsequent to stimulation of human myometrial cells as described.
SRCE and ER Ca2+ Store Refilling Are Not Inhibited by Inhibitors of L- or T-Type Channels or Reverse Mode Na+/Ca2+ Exchanger Activity But Are Attenuated by Gadolinium
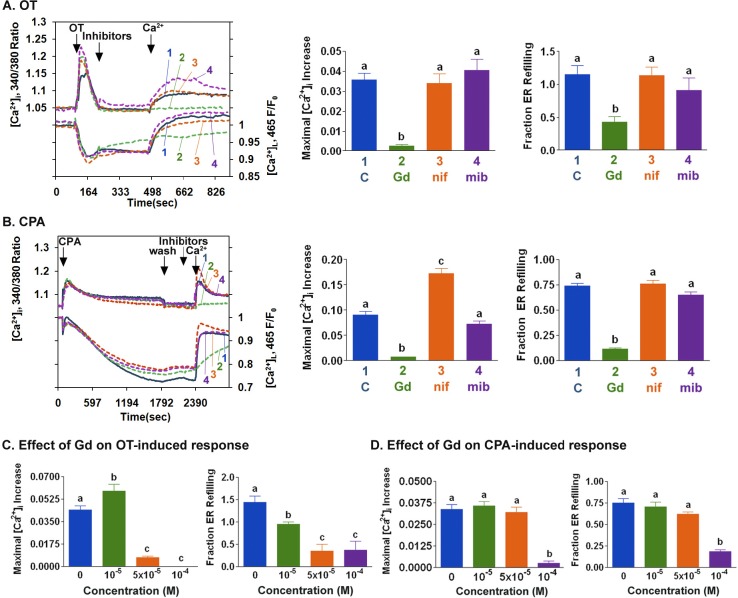
Inhibitors were used to assess the contribution of different types of Ca2+ entry mechanisms to myometrial cell ER store refilling after decreases in [Ca2+]L. Gadolinium (10−4 M) inhibited OT-induced SRCE and slowed ER store refilling (Fig. 4A). The effect of gadolinium was concentration-dependent and was statistically different from that of control at 5 × 10−5 M and 10−5 M for OT-stimulated SRCE and ER store refilling, respectively (Fig. 4C). In contrast, OT-induced SRCE and ER refilling were not inhibited by 10−7 M nifedipine, a concentration of the L-type channel blocker that effectively inhibits OAG-stimulated increases in [Ca2+]i in myometrial cells [16], or by 10−6 M mibefradil, a T-type calcium channel blocker that inhibits T-type electrical activity and contractions in human myometrium (Fig. 4A) [26]. CPA-induced SRCE was significantly inhibited, and ER store refilling was slowed by 10−4 M gadolinium (Fig. 4, B and D) but not by 10−7 M nifedipine or 10−6 M mibefradil (Fig. 4B). No effects on OT or CPA responses were observed at nifedipine concentrations of up to 10−6 M (data not shown). Similar effects of these compounds on CPA-stimulated SRCE and ER store refilling were also seen in HMC cells; modest inhibition of OT-stimulated SRCE by nifedipine and mibefradil was observed (Supplemental Fig. S1, available online at www.biolreprod.org).
FIG. 4.
A) After stimulation with OT (100 nM) in the absence of extracellular Ca2+, PHM1-41 cells were exposed to buffer (blue line, 1), 10−4 M gadolinium (green line, 2), 10−7 M nifedipine (orange line, 3), or 10−6 M mibefradil (purple line, 4) for 4 min before the addition of 1 mM Ca2+. The traces represent the mean of responses in 35–40 cells in one of three experiments. Bar graphs summarize the effect of SRCE inhibitors on OT-stimulated responses, expressed as maximal [Ca2+]i increase and fraction of ER store refilling at 1 min. Data represent the means ± SEM of responses from 90–120 cells. B) After stimulation with CPA (10 μM) in the absence of extracellular Ca2+, PHM1-41 cells were washed with Ca2+-free buffer for 10 min (wash) and then exposed for 4 min to the same compounds as shown in A before addition of 1 mM Ca2+. Each line represents an average of the responses of 35–40 cells in one of three similar experiments. Bar graphs represent the means ± SEM of responses from 90–120 cells. A and B) Additions of OT, CPA, wash, and Ca2+ are indicated by arrows. Concentration dependence of the effect of gadolinium (Gd) on OT-induced (C) and CPA-induced (D) responses (means ± SEM, n = 35–40 cells) is shown.
Some TRPC channels appear to activate L-type Ca2+ channels as a consequence of facilitating Na+ entry and associated membrane depolarization [27, 28]. Neither OT- nor CPA-stimulated SRCE or ER store refilling following addition of 1 mM Ca2+ were significantly affected by the absence of extracellular Na+ or the presence of the Na+/Ca2+ exchanger reverse mode inhibitor KB-R7943 in PHM1-41 cells (Fig. 5), suggesting that these mechanisms do not pertain.
FIG. 5.
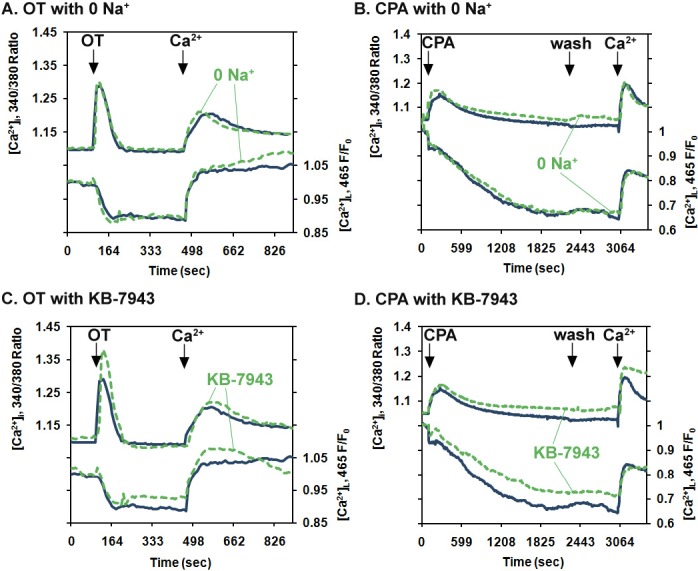
Removing extracellular Na+ or exposing PHM1-41 myometrial cells to the Na/Ca2+ exchanger inhibitor KB-R7943 had no effect on SRCE and ER store depletion stimulated by oxytocin or CPA or the refilling of the ER stores following addition of 1 mM extracellular Ca2+. Cells in medium in which choline chloride was substituted for NaCl (green line) were exposed to 100 nM OT (A) or 10 μM CPA (B) as described in the legend to Figure 4. Cells in normal FB were exposed to 10 μM KB-R7943 (green line) and then treated with OT (C) or with CPA (D). Each line represents an average of the responses of 35–40 cells in one of three similar experiments.
TRPC1 Knockdown Specifically Attenuates OT-Stimulated SRCE But Does Not Significantly Affect Myometrial ER Store Refilling
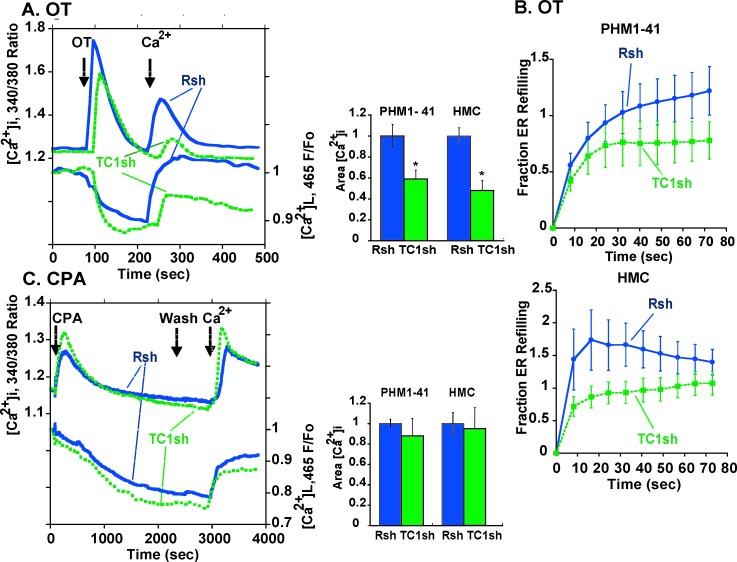
In PHM1-41 cells loaded with both Fura-2 and Mag Fluo-4, adenoviral-mediated reduction in TRPC1 shRNA attenuated OT-stimulated SRCE (Fig. 6A, left panel). SRCE was reduced by 41% (P < 0.001) in PHM1-41 cells and by 52% in HMC cells (P < 0.01) (Fig. 6A, right panel). Because the amount of ER store depletion was relatively small and there was some store refilling in the absence of extracellular Ca2+, the sensitivity of our system did not permit accurate assessment of initial rates of ER store refilling following OT stimulation. Nonetheless, as shown in Figure 6B, there appeared to be a trend toward slower store refilling in PHM1-41 (Fig. 6B, upper graph) and HMC (Fig. 6B, lower graph) cells expressing TRPC1 shRNA than in cells infected with control virus.
FIG. 6.
Effects of TRPC1 knockdown on SRCE and ER store depletion and refilling following treatment of myometrial cells with OT and CPA, as described in the legend to Figure 4, are shown. A) Tracings in the left panel represents the mean responses of 10–15 PHM1-41 cells infected with control virus (Rsh, blue lines) or adenovirus expressing TRPC1 shRNA (TC1sh, green lines). The middle panel presents the mean changes in integrated SRCE area in PHM1-41 and HMC cells (n = 10–11). B) The fraction of ER refilling after OT stimulation and Ca2+ addition in cells infected with control (Rsh, blue line) or TRPC1 (TC1sh, green line) shRNAs in PHM1 cells (upper graph) and HMC cells (lower graph) (n = 9–11). C) Effects of TRPC1 mRNA knockdown on CPA-stimulated responses. Data are presented as described in the legend to A (n = 4–6).
In contrast to the inhibitory effects on OT-stimulated SRCE, TRPC1 knockdown did not significantly affect CPA-stimulated SRCE in PHM1-41 or HMC cells (Fig. 6C) and did not inhibit ER store refilling (data not shown). No effects of expression of TRPC1 shRNA on the ability of OT or CPA to produce the initial increase in [Ca2+]i in the absence of extracellular [Ca2+] were apparent in either cell type.
STIM1 and ORAI1–ORAI3 Influence Myometrial SRCE and ER Store Refilling
In a number of other systems, STIM1 and ORAI1 proteins have been implicated in store depletion-mediated Ca2+ entry mechanisms. In order to design shRNAs to target the most abundant forms, we determined the relative expression of STIM and ORAI mRNA isoforms in myometrial cells. Figure 7A shows that STIM2 mRNA is significantly less abundant than STIM1 mRNA in myometrial cells. Although ORAI2 and ORAI3 mRNAs were less abundant than ORAI1 mRNA in PHM1-41 cells, the differences were less apparent in HMC and UtSMC cells. Based on these data, we created STIM1 and ORAI1–ORAI3 shRNA tandem viruses expressing three copies of STIM1 shRNA or one single copy each of ORAI1, ORAI2, and ORAI3 shRNAs. The STIM1 shRNA vector achieved an average of 61% and 64% knockdown of STIM1 mRNA in PHM1-41 and HMC cells, respectively. The tandem ORAI1–ORAI3 shRNA vector produced knockdowns in ORAI1, ORAI2, and ORAI3 mRNAs of 94%, 55%, and 31%, respectively, in PHM1-41 cells and 93%, 37%, and 45%, respectively, in HMC cells. STIM1 and ORAI1–ORAI3 mRNA knockdowns did not affect the concentrations of TRPC1, TRPC4, or TRPC6 mRNA (data not shown). In addition to these constructs, we generated a recombinant adenovirus expressing STIMΔERM, a dominant negative STIM1 form that interferes with the interaction between STIM1 and ORAI1 proteins [29].
FIG. 7.
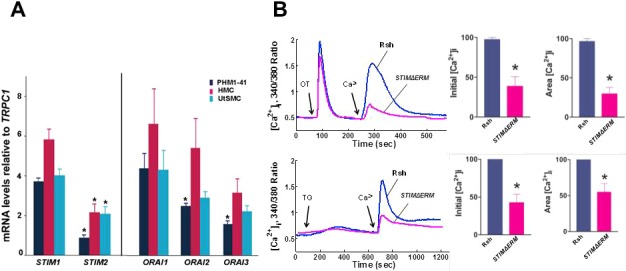
A) Relative expression of STIM and ORAI mRNA isoforms are shown in PHM1-41, HMC, and UtSMC myometrial cells, each compared to STIM1 and ORAI1 mRNA for that cell type (n = 3–4). B) STIM1ΔERM significantly inhibits OT and thapsigargin SRCE in UtSMC cells. Representative tracing (left) of mean SRCE induced by 100 nM OT or 100 nM thapsigargin (TG) in 10–15 cells infected with either control (Rsh, solid line) or adenovirus expressing STIMΔERM (dotted line) is shown. Mean changes in initial [Ca2+]i peak height (middle) and integrated SRCE area (right) compared to control (n = 5–7).
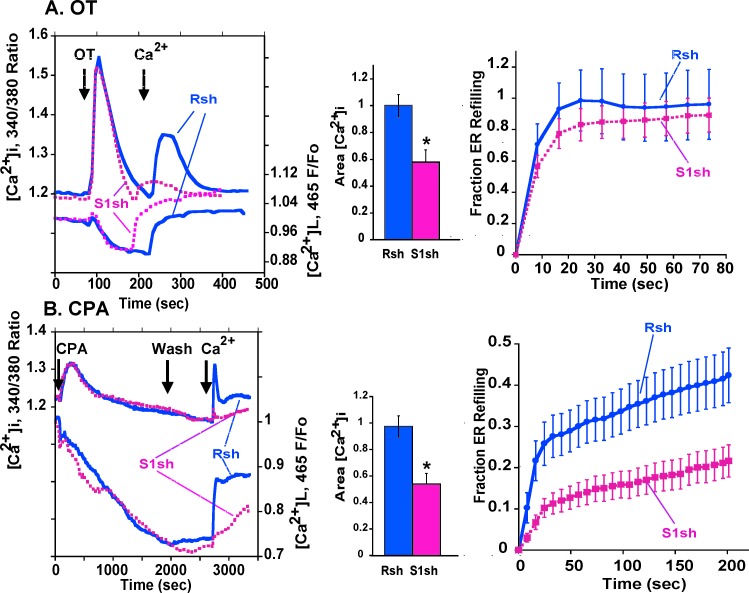
Infection with virus expressing STIMΔERM attenuated both OT- and thapsigargin-stimulated SRCE (Fig. 7B). Expression of STIM1 shRNA attenuated CPA-stimulated SRCE and the rate of ER store refilling compared to control in PHM1-41 cells (Fig. 8B). Mean initial rates were 2.1 ± 0.6 versus 0.7 ± 0.2 arbitrary units/sec for control and STIM1 shRNA, respectively (P < 0.05, n = 25 and 29). STIM1 mRNA knockdown also inhibited OT-stimulated SRCE but had no significant effect on ER store refilling in PHM1-41 cells (Fig. 8A). In HMC cells, STIM1 shRNA knockdown also significantly attenuated CPA-stimulated SRCE (Supplemental Fig. S2B). While there was a trend toward decline in the rate of ER store refilling, neither the initial rate nor the values at selected time points were significantly different from those of control. STIM1 knockdown attenuated OT-stimulated SRCE in HMC cells, and there was a trend toward a slowing of ER store refilling (Supplemental Fig. S2A).
FIG. 8.
Expression of STIM1 shRNA attenuated OT- and CPA-stimulated SRCE in PHM1-41 cells is shown. A) Tracings (left panel) represent the mean responses to OT stimulation and Ca2+ addition of 10–15 cells infected with control virus (Rsh, blue lines) or adenovirus expressing STIM1 shRNA (S1sh, pink lines). The middle panel presents the mean changes in integrated SRCE area (n = 16–17). The fraction of ER refilling in cells infected with control (Rsh, blue line) or STIM1 (S1sh, pink line) shRNA is shown in the right panel (n = 16–17). B) Effects of STIM1 mRNA knockdown on CPA-stimulated responses are shown. Data are presented as described in the legend to A (n = 24–29 dishes).
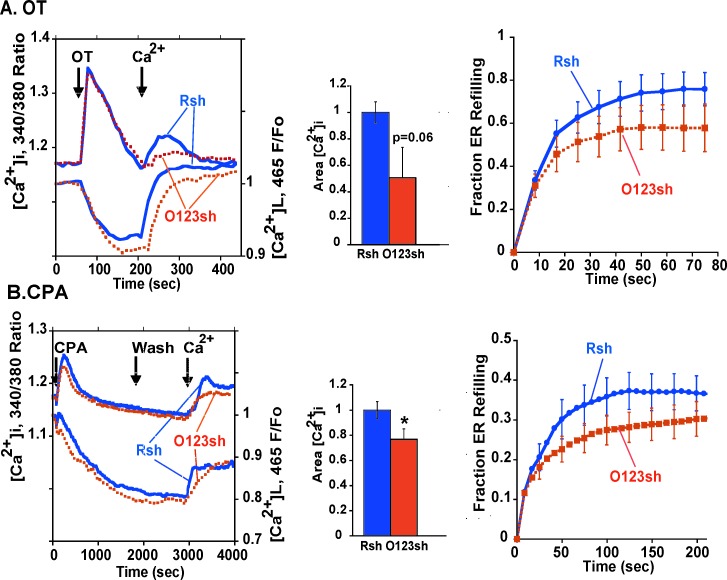
Knockdown of ORAI1, ORAI2, and ORAI3 mRNAs suppressed CPA-stimulated SRCE, and, whereas there was no significant effect on initial rate or at selected time points, there was a trend toward a slowing of ER store refilling in PHM1-41 cells (Fig. 9B). ORAI1–ORAI3 suppression attenuated OT-stimulated SRCE but had no significant effect on ER store refilling (Fig. 9A). In HMC cells, knockdown of ORAI1–ORAI3 mRNAs attenuated CPA-stimulated SRCE and significantly slowed store refilling (initial rates of 2.7 ± 0.5 versus 0.9 ± 0.2 arbitrary units/sec for control ORAI1–ORAI3 shRNA, respectively; n = 13) (Supplemental Fig. S3B) and attenuated OT-stimulated SRCE but had no significant effect on ER store refilling. No consistent effects of STIM1 or ORAI1–ORAI3 mRNA knockdowns on OT- or CPA-stimulated increases in [Ca2+]i in the absence of extracellular [Ca2+] were observed in either cell type.
FIG. 9.
Effects of ORAI1, ORAI2, and ORAI 3 tandem shRNA expression on OT- and CPA-stimulated SRCE and ER refilling in PHM1-41 cells are shown. A) Effects of ORAI1–ORAI3 mRNA knockdown on OT-stimulated responses. Data are presented as described in the legend to Figure 8 (control adenovirus (Rsh, blue lines); ORAI1–ORAI3 shRNA (O123sh, orange lines); n = 10–11). B) Effects of ORAI1, ORAI2, and ORAI3 mRNA knockdown on CPA-stimulated responses are shown. Data are presented as described in the legend to A (n = 16–17).
DISCUSSION
Data presented here provide strong evidence for the involvement of TRPC1, STIM1, and ORAI1–ORAI3 proteins in OT-stimulated SRCE and of STIM1 and ORAI1–ORAI3 in CPA-stimulated SRCE, thus reinforcing a distinction in human myometrium between receptor-operated and classical store-operated SRCE mechanisms [15] while identifying some commonalities in the regulation of cytoplasmic intracellular Ca2+. Furthermore, the kinetic measurements presented here suggest that STIM1 or ORAI1–ORAI3 mRNA knockdowns slow the rate of ER store replenishment following removal of SERCA inhibition.
TRPC channels have been implicated in both GPCR-stimulated and store depletion-stimulated increases in [Ca2+]i in response to addition of extracellular Ca2+ [8, 13, 14]. TRPC1 expression plays an important role in the formation of heterotetramers with other TRPCs and may contribute to the unique characteristics of these channels in a given cellular setting. The effect of TRPC1 knockdown in human myometrial cells specifically on OT-stimulated SRCE is similar to the effect of TRPC4 knockdown [15]. The combined knockdown of TRPC1 plus TRPC4 was no more effective in inhibiting OT-stimulated SRCE than responses obtained from single TRPC1 or TRPC4 knockdowns, suggesting that both proteins may be contributing to the same GPCR-mediated SRCE response, either together or separately. In agreement with these results, knockdown of either TRPC1 or TRPC4 had no effect on thapsigargin-stimulated [Ca2+]i increases or on CRAC currents in endothelial cells [30], and single and combined TRPC1, TRPC4, or TRPC6 knockdowns had no effect on thapsigargin-stimulated [Ca2+]i increases in vascular SMCs [31]. In contrast, in a number of other cell types, shRNAs or antisense nucleotides targeted against TRPC1 and/or TRPC1 plus TRPC4 decreased thapsigargin-induced membrane currents and [Ca2+]i increases [32–36]. These apparently contradictory results in different cell types may be due to differences in the relative abundance of TRPC isoforms expressed and hence the nature of the TRPC channels formed, as well as to differences in regulatory coupling and modulation of activity.
The ER functions as an intracellular Ca2+ store that plays complex roles in the regulation of myometrial Ca2+ dynamics. In response to an increase in [Ca2+]i, SERCA contributes to the sequestration of a portion of this Ca2+ and, along with the plasma membrane pump and Na+/Ca2+ exchanger, is responsible for the decline in [Ca2+]i [1, 6, 7, 10]. Depending on the circumstances, the ER can refill its Ca2+ store and/or deliver Ca2+ to the plasma membrane pumps and exchangers for efflux, thus protecting the cell from the dangers of elevated [Ca2+]i and dampening contractile activity.
Fura-2 and Mag-fluo-4 are effective for measuring relative changes in cytoplasmic and ER Ca2+, respectively, both because of their differential affinities for Ca2+ and the buffering capacities that exist in both compartments [11, 37]. In the present study, the responses to agents known to elicit a decrease in [Ca2+]L followed the expected dependence on extracellular Ca2+ for both rises in [Ca2+]i and ER Ca2+ store refilling, demonstrating the usefulness of this approach for use in human myometrial cells. Thus, OT elicited a transient increase in [Ca2+]i and a decrease in [Ca2+]L in the absence of extracellular Ca2+, as expected from its ability to stimulate phospholipase C activity and generate IP3 [2]. The refilling of ER Ca2+ stores was partially dependent on addition of extracellular Ca2+, similar to what has been reported for ATP-stimulated store depletion in rat myometrial cells [11]. The irreversible SERCA inhibitor thapsigargin produced a sustained decrease in [Ca2+]L that was not reversed by addition of extracellular Ca2+, whereas the reversible SERCA inhibitor CPA elicited a similar decrease in [Ca2+]L, but the store was rapidly refilled following addition of extracellular Ca2+ after CPA washout.
L-type Ca2+ channel blockers inhibit Ca2+ entry following myometrial cell membrane depolarization and have marked inhibitory effects on spontaneous and agonist-induced uterine contractile activity [1, 26, 38–40]. T-type Ca2+ channels have been implicated in the initiation of action potentials and in spontaneous contractile activity in myometrium [26, 40]. With time in culture, myometrial cells such as those used in this study tend to lose robust responses to iso-osmotic KCl-dependent depolarization, indicative of dampened voltage-dependent responses. Nonetheless, we have observed that these cells express mRNA for the α subunit of Cav1.2 (our unpublished observations). Our previous finding of a TRPC6-mediated mechanism that is inhibited by removing extracellular Na+ and by nifedipine [16], consistent with a previous report linking TRPC6 to Na+ entry and L-type channel activation [27], is the only evidence to date that L-type channels are still functional to some extent in PHM1-41 and primary myometrial cells in culture. In the present study, CPA- stimulated increases in [Ca2+]i and ER store refilling in PHM1-41 or primary myometrial cells were not inhibited by nifedipine or mibefradil, suggesting that voltage-activated channels do not play a direct role in either GPCR- or store depletion-stimulated SRCE or the subsequent ER store refilling following addition of extracellular Ca2+ in these cells. Similar effects were found in the primary cells, apart from a modest inhibition of OT-stimulated SRCE by nifedipine and mibefradil, which may be consistent with effects of these compounds on SRCE mechanisms under some conditions [41]. The responses in zero Na+ and with KB-R7943 also rule out reverse-mode operation of Na+/Ca2+ exchangers in these mechanisms. It remains to be determined, however, whether these conclusions also pertain in acutely isolated human cells displaying robust L-type and/or T-type currents.
Lanthanides in μM concentrations are considered relatively specific inhibitors of store-operated channels, but TRPC4 and TRPC5 proteins are potentiated by μM and inhibited by mM concentrations of gadolinium [42]. Cells with TRPC3, TRPC5, or TRPC6 but not TRPC1 overexpression exhibited carbachol-stimulated SRCE in the presence of 5 μM gadolinium, which was used to suppress endogenous store-operated channels [22]. In the present study, both OT- and CPA-stimulated SRCE and ER store refilling were attenuated by gadolinium, but it is not possible to infer with certainty which specific channels are affected, from these observations.
Thapsigargin- and CPA-stimulated SRCE in human myometrial cells is sensitive to reduction of STIM1 and ORAI1–ORAI3 mRNAs but is not attenuated by TRPC1, TRPC4, or TRPC6 [16] mRNA knockdown. This finding is consistent with the identification of STIM and ORAI proteins as comprising store-operated channels that give rise to the CRAC current and are activated by SERCA inhibitors in other systems [18–21]. The attenuation of OT-stimulated SRCE by STIM1 and by ORAI1–ORAI3, as well as by TRPC1 and TRPC4, mRNA knockdowns is consistent with emerging evidence suggestive of potential interactions among STIM1, ORAI1, and TRPC [18, 19, 21, 33, 36, 43]. Interestingly, STIM1 uses different interaction domains to activate ORAI1 and TRPCs, and both STIM-dependent and STIM1-independent modes of TRPC function have been described [18, 19, 44, 45]. TRPC channels are organized into microdomains, and this can affect their assembly with STIM1 and ORAI1 [33, 36, 43]. These assemblies may depend on cell-specific properties and signals and remain to be defined in myometrium.
To our knowledge, there is only one study of the effects of STIM1 knockdown on the rate of ER store refilling in any cell type and no study of the effects of ORAI on this parameter. Jousset et al. [46] reported an inhibitory effect of STIM1 knockdown on both GPCR- and thapsigargin-mediated SRCE in HeLa cells. Using transfected reporters to measure [Ca2+]i and [Ca2+]L simultaneously, they found that STIM1 knockdown slowed the rate of ER refilling following histamine stimulation but that the ER store eventually refilled even though there was no detectable increase in [Ca2+]i. Overall, our data also support the concept that the ER stores in myometrial cells can refill, albeit at a slower rate, when STIM1 or ORAI mRNA concentrations are reduced. Our findings and those of Jousset et al. [46] are consistent with the observation that in response to decreases in [Ca2+]L, STIM1 and ORAI1 form punctae indicative of close apposition of plasma membrane and ER membranes, making it possible to refill ER Ca2+ stores via channel-mediated Ca2+ influx through these microdomains, without significant increases [Ca2+]i detectable by Fura-2.
Because of the marked dependence of prolonged myometrial spontaneous and hormone-stimulated activity on extracellular Ca2+ and L-type channel activity, a physiological role for capacitative Ca2+ entry in the myometrium has been questioned [1]. Nonetheless, a preliminary report of CPA-stimulated SRCE and increase in basal force that is nifedipine-insensitive but inhibited by SKF96365 in pregnant rat myometrium, slightly different responses in nonpregnant rat myometrium, and reference to unpublished effects of OT on voltage-independent calcium transients inhibited by CPA depletion of ER stores [5] suggest functionality of CPA- and GPCR-mediated capacitative mechanisms in rat myometrium. Furthermore, Shimamura et al. [47] reported that OT elicited a long-lasting nonselective cation current in late pregnant rat myometrium. Therefore, the evidence in favor of a physiological role for SRCE in myometrium is growing.
Our studies defining components of the SRCE mechanism in myometrium were carried out in primary and immortalized human myometrial cells to facilitate evaluation of individual cell responses. Assessing the effect of these same knockdowns on human myometrial tissue function is logistically more difficult and will take additional time to accomplish. Nonetheless, it interesting to speculate on the potential significance of these findings. Uterine contractants such as OT increase [Ca2+]i by releasing ER Ca2+ and stimulating Ca2+ entry through SRCE mechanisms involving TPRC1, TRPC4, STIM1, and ORAI1–ORAI3. While these mechanisms are independent of L-type channel involvement, they also generate local OAG that could potentially stimulate TRPC6 and L-type channels through protein kinase C activation. STIM1 has also recently been shown to inhibit Cav1.2 L-type Ca2+ channels [48, 49], suggesting that GPCRs might stimulate the formation of complexes containing some combination of TRPC, STIM, and ORAI in microdomains where subtle temporal regulation of other proteins such as Cav1.2 could occur. In the myometrium such TRPC complexes in specialized subcellular environments might locally influence the pattern of [Ca2+]i and, in turn, the pattern of contractions. Interestingly, the study by Shimamura et al. [47] reported an OT-stimulated nonselective cation current and also found that OT partially inhibited L-type currents . There are few clues in the literature as to what might be the physiological equivalent of chemical inhibition of SERCA. In this regard, Gehrig-Burger et al. [50] reported that high progesterone concentrations inhibit OT-stimulated uterine contractions and deplete intracellular ER Ca2+ stores in HEK293 cells, and they speculate that this action of progesterone may contribute to uterine quiescence during pregnancy. Clearly, there is still much to be learned about the interactions among and influence of the many components that regulate [Ca2+]i and ER Ca2+ in the myometrium. Because of their ubiquitous nature, we consider it unlikely that targeting ORAI or STIM1 would produce myometrial-specific effects on Ca2+ dynamics. On the other hand, the species- and tissue-specific patterns of TRPC protein expression and the distinctive effects of TRPC1, TRPC4, and TRPC6 knockdowns on human myometrial cells suggest that they might be potential targets for tocolytic intervention if specific inhibitors can be developed.
ACKNOWLEDGMENTS
The authors thank Dr. P.W. Worley (The Johns Hopkins University School of Medicine, Baltimore, MD) for the STIMΔERM clone and Dr. R.A. Bowen (Colorado State University, Fort Collins, CO) and Dr. K. Bois (Fort Collins, CO) for assistance with data analysis.
Footnotes
These authors contributed equally to this work and are considered equal first authors.
Supported by NIH- HD38970 (to B.M.S.), March of Dimes grant no. 6-FY05-77 (to B.M.S.), and NIHF31-HD051037 (to A.U.). Some of this work was completed by A.U. in partial fulfillment of Ph.D. degree requirements.
REFERENCES
- Wray S. Insights into the uterus. Exp Physiol 2007; 92: 621 631. [DOI] [PubMed] [Google Scholar]
- Sanborn B. Hormonal signaling and signal pathway crosstalk in the control of myometrial calcium dynamics. Semin Cell Dev Biol 2007; 18: 305 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield RE, Maner WL. Physiology and electrical activity of uterine contractions. Semin Cell Dev Biol 2007; 18: 289 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S, Shmygol A. Role of the calcium store in uterine contractility. Semin Cell Dev Biol 2007; 18: 315 320. [DOI] [PubMed] [Google Scholar]
- Noble K, Matthew A, Burdyga T, Wray S. A review of recent insights into the role of the sarcoplasmic reticulum and Ca entry in uterine smooth muscle. Eur J Obstet Gynecol Reprod Biol 2009; 144 (suppl 1): S11 S19. [DOI] [PubMed] [Google Scholar]
- Shmigol A, Eisner D, Wray S. The role of the sarcoplasmic reticulum as a Ca2+ sink in rat uterine smooth muscle cells. J Physiol 1999; 520: 153 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmygol A, Wray S. Functional architecture of the SR calcium store in uterine smooth muscle. Cell Calcium 2004; 35: 501 508. [DOI] [PubMed] [Google Scholar]
- Ramsey S, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol 2006; 68: 619 647. [DOI] [PubMed] [Google Scholar]
- Putney JW. New molecular players in capacitative Ca2+ entry. J Cell Sci 2007; 120: 1959 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmigol AV, Eisner DA, Wray S. Simultaneous measurements of changes in sarcoplasmic reticulum and cytosolic. J Physiol 2001; 531: 707 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmygol A, Wray S. Modulation of agonist-induced Ca2+ release by SR Ca2+ load: direct SR and cytosolic Ca2+ measurements in rat uterine myocytes. Cell Calcium 2005; 37: 215 223. [DOI] [PubMed] [Google Scholar]
- Ku C, Babich L, Word R, Zhong M, Ulloa A, Monga M, Sanborn BM. Expression of transient receptor channel proteins in human fundal myometrium in pregnancy. J Soc Gynecol Invest 2006; 13: 217 225. [DOI] [PubMed] [Google Scholar]
- Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol 2010; 2: a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Sweet TB, Clapham DE. International union of basic and clinical pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev 2010; 62: 381 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa A, Gonzales AL, Zhong M, Kim YS, Cantlon J, Clay C, Ku CY, Earley S, Sanborn BM. Reduction in TRPC4 expression specifically attenuates G-protein coupled receptor-stimulated increases in intracellular calcium in human myometrial cells. Cell Calcium 2009; 46: 73 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D, Kim YS, Phillips JN, Ulloa A, Ku CY, Galan HL, Sanborn BM. Attenuation of canonical transient receptor potential-like channel 6 expression specifically reduces the diacylglycerol-mediated increase in intracellular calcium in human myometrial cells. Endocrinology 2010; 151: 406 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Melegari M, Sridhar S, Rogler CE, Zhu L. Multi-miRNA hairpin method that improves gene knockdown efficiency and provides linked multi-gene knockdown. Biotechniques 2006; 41: 59 63. [DOI] [PubMed] [Google Scholar]
- Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol 2009; 11: 669 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Wang Y, Zhou Y, Soboloff J, Gill DL. STIM. and Orai: dynamic intermembrane coupling to control cellular calcium signals. J Biol Chem 2009; 284: 22501 22505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JT, Hwang SY, Tomita T, DeHaven WI, Mercer JC, Putney JW. Activation and regulation of store-operated calcium entry. J Cell Mol Med 2010; 14: 2337 2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Yuan JP, Hong JH, So I, Worley PF, Muallem S. An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS Lett 2010; 584: 2022 2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaven WI, Jones BF, Petranka JG, Smyth JT, Tomita T, Bird GS, Putney JW., Jr TRPC channels function independently of STIM1 and Orai1. J Physiol 2009; 587: 2275 2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga M, Campbell DF, Sanborn BM. Oxytocin-stimulated capacitative calcium entry in human myometrial cells. Am J Obstet Gynecol 1999; 181: 424 429. [DOI] [PubMed] [Google Scholar]
- Yang M, Gupta A, Shlykov SG, Corrigan R, Tsujimoto S, Sanborn BM. Multiple Trp isoforms implicated in capacitative calcium entry are expressed in human pregnant myometrium and myometrial cells. Biol Reprod 2002; 67: 988 994. [DOI] [PubMed] [Google Scholar]
- Shlykov SG, Yang M, Alcorn JL, Sanborn BM. Capacitative cation entry in human myometrial cells and augmentation by hTrpC3 overexpression. Biol Reprod 2003; 69: 647 655. [DOI] [PubMed] [Google Scholar]
- Young R, Zhang P. Inhibition of in vitro contractions of human myometrium by mibefradil, a T-type calcium channel blocker: support for a model using excitation-contraction coupling, and autocrine and paracrine signaling mechanisms. J Soc Gynecol Invest 2005; 12: e7 e12. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova M, Xu W, He L, Cuesta N, Gill D. Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J Biol Chem 2005; 280: 39786 39794. [DOI] [PubMed] [Google Scholar]
- Lemos VS, Poburko D, Liao CH, Cole WC, van Breemen C. Na+ entry via TRPC6 causes Ca2+ entry via NCX reversal in ATP stimulated smooth muscle cells. Biochem Biophys Res Commun 2007; 352: 130 134. [DOI] [PubMed] [Google Scholar]
- Huang G, Zeng W, Kim J, Yuan J, Han L, Muallem S, Worley P. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol 2006; 8: 1003 1010. [DOI] [PubMed] [Google Scholar]
- Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res 2008; 103: 1289 1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J 2009; 23: 2425 2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagranichnaya T, Wu X, Villereal M. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem 2005; 280: 29559 29569. [DOI] [PubMed] [Google Scholar]
- Ambudkar I, Ong H, Liu X, Bandyopadhyay B, Cheng K. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium 2007; 42: 213 223. [DOI] [PubMed] [Google Scholar]
- Ong H, Cheng K, Liu X, Bandyopadhyay B, Paria B, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh B, Gill D, Ambudkar I. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem 2007; 282: 9105 9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sours-Brothers S, Ding M, Graham S, Ma R. Interaction between TRPC1/TRPC4 assembly and STIM1 contributes to store-operated Ca2+ entry in mesangial cells. Exp Biol Med 2009; 234: 673 682. [DOI] [PubMed] [Google Scholar]
- Pani B, Ong HL, Brazer SC, Liu X, Rauser K, Singh BB, Ambudkar IS. Activation of TRPC1 by STIM1 in ER-PM microdomains involves release of the channel from its scaffold caveolin-1. Proc Natl Acad Sci U S A 2009; 106: 20087 20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S, Burdyga T. Sarcoplasmic reticulum function in smooth muscle. Physiol Rev 2010; 90: 113 178. [DOI] [PubMed] [Google Scholar]
- Shmigol A, Eisner D, Wray S. Properties of voltage-activated [Ca2+]i transients in single smooth muscle cells isolated from pregnant rat uterus. J Physiol 1998; 511: 803 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo M, Jain V, Vedernikov Y, Hankins G, Garfield R, Saade G. Effects of L-type Ca(2+)-channel blockade, K(+)(ATP)-channel opening and nitric oxide on human uterine contractility in relation to gestational age and labour. Hum Reprod 2003; 9: 159 164. [DOI] [PubMed] [Google Scholar]
- Lee SE, Ahn DS, Lee YH. Role of T-type Ca channels in the spontaneous phasic contraction of pregnant rat uterine smooth muscle. Korean J Physiol Pharmacol 2009; 13: 241 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Schumann R, Zhang P. Nifedipine block of capacitative calcium entry in cultured human uterine smooth-muscle cells. J Soc Gynecol Invest 2001; 8: 210 215. [DOI] [PubMed] [Google Scholar]
- Jung S, Muhle A, Schaefer M, Strotmann R, Schultz G, Plant TD. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem 2003; 278: 3562 3571. [DOI] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong D, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A 2007; 104: 4682 4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci U S A 2009; 106: 3202 3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Yuan JP, So I, Worley PF, Muallem S. STIM1-dependent and STIM1-independent function of transient receptor potential canonical (TRPC) channels tunes their store-operated mode. J Biol Chem 2010; 285: 38666 38673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousset H, Frieden M, Demaurex N. STIM1 knockdown reveals that store-operated Ca2+ channels located close to sarco/endoplasmic Ca2+ ATPases (SERCA) pumps silently refill the endoplasmic reticulum. J Biol Chem 2007; 282: 11456 11464. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Kusaka M, Sperelakis N. Oxytocin induces an inward current in pregnant rat myometrial cells. Can J Physiol Pharmacol 1994; 72: 759 763. [DOI] [PubMed] [Google Scholar]
- Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science 2010; 330: 105 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science 2010; 330: 101 105. [DOI] [PubMed] [Google Scholar]
- Gehrig-Burger K, Slaninova J, Gimpl G. Depletion of calcium stores contributes to progesterone-induced attenuation of calcium signaling of G protein-coupled receptors. Cell Mol Life Sci 2010; 67: 2815 2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiani RK, Abdullaev IF, Trebak M. A novel native store-operated calcium channel encoded by Orai3: selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J Biol Chem 2010; 285: 19173 19183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BF, Boyles RR, Hwang SY, Bird GS, Putney JW. Calcium influx mechanisms underlying calcium oscillations in rat hepatocytes. Hepatology 2008; 48: 1273 1281. [DOI] [PMC free article] [PubMed] [Google Scholar]