Abstract
Clinical trial data have shown that oral pre-exposure prophylaxis (PrEP) is efficacious when taken as prescribed; however, PrEP adherence is complex and must be understood within the context of variable risk for HIV infection and use of other HIV prevention methods. Different levels of adherence may be needed in different populations to achieve HIV prevention, and the optimal methods for achieving the necessary adherence for both individual and public health benefits are unknown. Guidance for PrEP use must consider these questions to determine the success of PrEP-based HIV prevention programs. In this article, we propose a new paradigm for understanding and measuring PrEP adherence, termed prevention-effective adherence, which incorporates dynamic HIV acquisition risk behaviors and the use of HIV alternative prevention strategies. We discuss the need for daily PrEP use only during periods of risk for HIV exposure, describe key issues for measuring and understanding relevant behaviors, review lessons from another health prevention field (i.e., family planning), and provide guidance for prevention-effective PrEP use. Moreover, we challenge emerging calls for sustained, near perfect PrEP adherence regardless of risk exposure and offer a more practical and public health-focused vision for this prevention intervention.
Keywords: pre-exposure prophylaxis, PrEP, adherence
Introduction
To date, six clinical trials have explored the efficacy of pre-exposure prophylaxis (PrEP) against HIV infection using oral tenofovir and/or emtricitabine/tenofovir [1–6]. Efficacy ranged from 0 to 75% [7], the variation of which can largely be explained by differences in PrEP adherence [8]. Efficacy data directly correlate with the objective adherence measures in those studies, and efficacy estimates were between 90–100% when adherence was consistently high [2, 9, 10]. PrEP is clearly efficacious when taken as prescribed.
However, PrEP adherence is complex and must be understood within the context of variable risk for HIV infection and use of other HIV prevention methods. Different levels of adherence may be needed in different populations to achieve HIV prevention, and the optimal methods for achieving the necessary adherence for both individual and public health benefits are unknown. In moving beyond clinical trials toward demonstration projects (i.e., studies of PrEP delivery methods) and broader implementation in clinical care, guidance for PrEP use must consider these questions to determine if PrEP-based HIV prevention programs are successful at both the individual and public health level.
In this article, we propose a new paradigm for understanding and measuring PrEP adherence, termed prevention-effective adherence, which incorporates dynamic HIV acquisition risk behaviors and the use of HIV alternative prevention strategies. We focus on sexual transmission of HIV, as the role of PrEP in preventing HIV transmission via injection drug use is less well understood. We discuss the need for daily PrEP use only during periods of risk for HIV exposure, describe key issues for measuring and understanding relevant behaviors, review lessons from another health prevention field (i.e., family planning), and provide guidance for prevention-effective PrEP use. Moreover, we challenge emerging calls for sustained, near perfect PrEP adherence regardless of risk exposure and offer a more practical and public health-focused vision for this prevention intervention.
Understanding PrEP adherence
Sustained lifelong high adherence- the paradigm for antiretroviral therapy (ART)
The adherence message for antiretroviral treatment of HIV infection is simple: viral suppression and improved health require indefinite, consistently high adherence following ART initiation [11]. While early regimens required at least 95% adherence [12], contemporary regimens reliably suppress viral replication at somewhat lower adherence levels (e.g., 80%) [13, 14], depending on adherence patterns and prior duration of viral suppression [15, 16]. Nevertheless, lifelong, sustained adherence is required to halt disease progression. While scheduled intermittent dosing of ART (e.g., planned drug holidays) was suggested to ease the adherence burden, studies of prescribed intermittent ART and unplanned missed doses showed increased rates of viral rebound and worse clinical outcomes [17, 18].
Sustained high adherence for the duration of PrEP clinical trials
High levels of sustained adherence are also required for the evaluation of efficacy and safety of potential PrEP agents. Thus, in placebo-controlled randomized clinical trials, one could argue for selecting participants who are motivated to adhere, as well as providing intense adherence support to maintain consistently high adherence regardless of dynamic risk behaviors. This scenario, however, does not generalize to open label use and uptake of PrEP that is now known to be effective.
Prevention-effective adherence- a novel paradigm
Several differences between ART and PrEP (as used outside of clinical trials) are noted in Table 1, and the type of adherence needed for each setting is presented in Figure 1. The message for PrEP adherence is not simply 100% adherence for life or the duration of a research study. Because an individual’s risk for HIV acquisition changes over time and alternative prevention strategies may be used, the indication for PrEP also changes over time. PrEP use, for example, may not be indicated if sexual activity is restricted to a monogamous relationship with a known HIV-negative partner without other risk exposures, or if another effective HIV prevention tool (e.g., condoms) is consistently used. Perfect PrEP adherence in the absence of risk confers cost, side effects, and toxicity, but no benefit. Consistently high adherence during periods of exposure, however, is critical when PrEP is being used for effective HIV prevention.
Table 1.
Differences between antiretroviral therapy (ART) and pre-exposure prophylaxis (PrEP) as used outside of clinical trials.
| ART | PrEP | |
|---|---|---|
| Users | Everyone living with HIV needs or will need treatment at some point | Only those at high risk for HIV-infection are appropriate for PrEP |
| Regimen | ART must be taken every day on a fixed schedule to be effective | PrEP may still provide protection if taken less than daily, depending on adherence patterns and route and timing of HIV exposures |
| Motivation | ART treats a fatal infection; individual health benefits are clear | PrEP prevents a fatal infection |
| Duration | Treatment is presently taken for life | PrEP can be selected for periods of high risk and not used at other times |
| Alternatives | No alternative provides what treatment offers | PrEP users may choose other effective HIV prevention tools |
| Psychosocial factors | Relevant factors include stigma of being infected and depression | Relevant factors include a presumption of being HIV-infected and/or promiscuous |
| Access | ART is available in most settings, although cost and transportation may be barriers | PrEP is primarily available through studies and projects, although available in some clinical settings |
Figure 1. Adherence success by adherence paradigm.
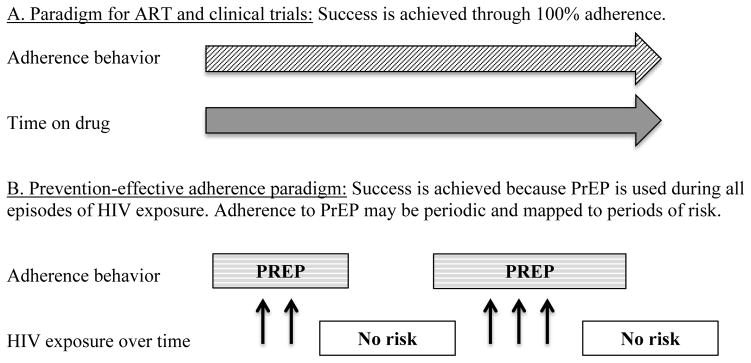
The paradigm of consistently high adherence for all individuals applies to ART and PrEP in clinical trials (panel A). The prevention-effective paradigm applies to PrEP in demonstration projects and wider implementation (panel B).
Prevention-effective PrEP adherence means the use of PrEP only during periods of risk exposure such that it leads to effective protection against HIV acquisition. Failure to understand this concept may result in missed opportunities for HIV prevention. For example, individuals who struggle to use PrEP at certain times in their lives (e.g., young women in high HIV prevalence areas) may be able to use it at others if they are guided through the process of understanding risk and choice of effective prevention options. Moreover, this concept can lead to efficient PrEP use that will limit adverse events and costs and potentially widen availability in settings of resource scarcity.
This paradigm is applicable to both PrEP demonstration project and wider implementation. Unlike in clinical trials, target participants in demonstration projects and implementation are those who are interested in PrEP when they are at risk for HIV exposure, but actual PrEP uptake and time-on-PrEP will vary considerably as needs change over time. Guidelines suggest PrEP should be used in populations at high risk for HIV acquisition [19, 20]. PrEP is unlikely to be a preferred or cost-effective HIV prevention tool for individuals with rare or unpredictable HIV exposure [21], for whom other prevention options may be more appropriate (e.g., post-exposure prophylaxis [PEP] for victims of rape, or condom use for individuals with infrequent sexual encounters who can use condoms effectively).
Measuring prevention-effective combination adherence
Prevention-effective adherence requires measurement of 1) HIV risk exposure, 2) use of PrEP, and 3) use of other effective HIV prevention tools. These factors are then combined in the calculation of adherence.
Risk exposure
The first step in measuring prevention-effective adherence is the assessment of risk for HIV exposure. Measurement of risk exposure is challenging and largely limited to self-report of behavior or perceived risk, which may be inaccurate due to recall and social desirability biases [22]. Accuracy, however, may increase via short recall periods [23, 24] and concurrent measurement with medication adherence (e.g., 24-hour recall by SMS surveys) [25]. Biomarkers of sexual intercourse (e.g., prostate specific antigen in vaginal secretions) can provide objective evidence of sex; however, they are difficult to obtain and impractical for clinical practice [26, 27]. They also do not comment on male sexual activity. Surrogate markers (e.g., incidence of other sexually transmitted infections and pregnancy) may provide some indication of potential HIV exposure, but are generally informative at the population, not individual, level. Risk assessments should involve periods of sustained risk, rather than day-to-day assessments of sexual activity. PrEP is unlikely to be a preferred HIV prevention option for individuals with infrequent sexual activity. Ongoing studies (i.e., IPERGAY, HPTN067, and HPTN 069) involve extensive data collection on sexual behavior and risk for HIV exposure, and may provide further insights into optimal measurement strategies.
Use of PrEP
The next step in assessing prevention-effective adherence is to quantify PrEP use. No gold standard exists for measuring medication-taking behavior; all have strengths and weaknesses (see Table 2) [28, 29]. Because all adherence measures are imperfect, a context-appropriate set of more than one measure is needed to understand adherence behavior. Importantly, self-report did not correlate with drug levels in several PrEP clinical trials and was largely uninformative due to over-reporting of adherence [4, 6]. As noted above for self-reported risk behavior, self-reported adherence may be improved through SMS surveys of short recall periods and/or questions that have that been validated with objective PrEP adherence measures. The accuracy of self-report may also improve in the absence of consequences for reporting socially undesired behavior (e.g., burdensome intervention components when condomless sex or non-adherence is reported). Importantly, reporting of non-adherence is more likely to be accurate than reporting of adherence [30]. Pharmacy refill records provide an objective approach that has performed well in predicting viral suppression in ART programs [31] and may be feasible for routine clinical use with PrEP. While cost limits electronic adherence monitoring to research studies, it is the only method that provides dose-by-dose measurement for assessing adherence patterns, which are critical for understanding prevention-effective adherence [32]. Electronic monitoring in at least a subset of participants in demonstration projects should therefore be considered. Wireless electronic monitoring is also available and can provide data in real-time, reduce the risk of data loss, and decrease the human resources needed for data collection in geographically dispersed settings [33]. Drug levels were critical in providing objective documentation of drug ingestion in the PrEP clinical trials; however, they are also impractical for routine use. Stored samples for selective testing offer a less expensive approach that may be appropriate for demonstration projects.
Table 2.
Strengths and weaknesses of adherence measurement tools
| Measure | Strengths | Weaknesses |
|---|---|---|
| Subjective | ||
| Self-report |
|
|
| Objective | ||
| Clinic-based pill counts |
|
|
| Unannounced home or phone-based pill counts |
|
|
| Pharmacy refill |
|
|
| Electronic adherence monitoring |
|
|
| Drug levels |
|
|
Demonstration projects that involve multiple PrEP adherence measurements will help determine the best measures for prevention-effective PrEP adherence, which can then be compared to assess the most informative, accurate, and affordable measures for use in the less resourced context of implementation. For example, electronic monitoring or drug levels may be used to determine which types of self-report questions best identify individuals with adherence challenges.
Prevention-effective adherence also requires measurement of PrEP initiation and discontinuation. An objective approach is to determine when medication was picked up via pharmacy refill records. The timing of discontinuation, however, is difficult to know, as individuals may initiate and discontinue PrEP multiple times during a single refill period. Electronic monitors can provide more precise patterns of PrEP initiation and discontinuation. Self-report may be considered as a complementary tool to further define either of these objective measurements. For example, individuals can be asked whether periods of non-adherence were intentional or unintentional. The above-noted limitations of self-reported data, however, should be kept in mind.
A key distinction should be drawn between periodic and intermittent PrEP use. Periodic use involves voluntary starting and stopping of daily PrEP use depending on HIV prevention needs and choices (e.g., while trying to conceive a child within a serodiscordant couple [34]). Periodic use is based on the once daily dosing recommendation, and periods of time can be considered as on or off PrEP for as long as PrEP is part of an overall HIV-prevention approach. In contrast, intermittent PrEP refers to a prescription of less-than-daily PrEP, as was used in the International AIDS Vaccine Initiative (IAVI) pilot randomized controlled trial [35, 36] and is currently being evaluated in IPERGAY and HPTN 067. Preliminary data from IPERGAY are promising, although a full understanding of the data is pending [37]. Importantly, adherence to intermittent doses was lower than to fixed doses in the IAVI trial.
Use of other HIV prevention tools
While measuring use of the other HIV prevention tools is complex and beyond the scope of this article, it is an important concept in defining prevention-effective adherence. Briefly, condom use and behavior modification (e.g., partner reduction, knowledge of HIV status) typically rely on self-report. Medical male circumcision does not require ongoing adherence measurement; however, a 50% reduction in risk of HIV acquisition is arguably insufficient to recommend it as a sole prevention strategy. ART in the HIV-infected partner of a stable serodiscordant couple can prevent secondary transmission and adherence may be measured as described above for PrEP, as well as with HIV RNA levels. Other forms of PrEP beyond tablets (e.g., vaginal rings, gels, depo injections) may become available and individuals may choose different formulations at different times. Ongoing assessments of which HIV prevention tools are used when and how are therefore needed.
Execution and persistence of prevention-effective PrEP adherence
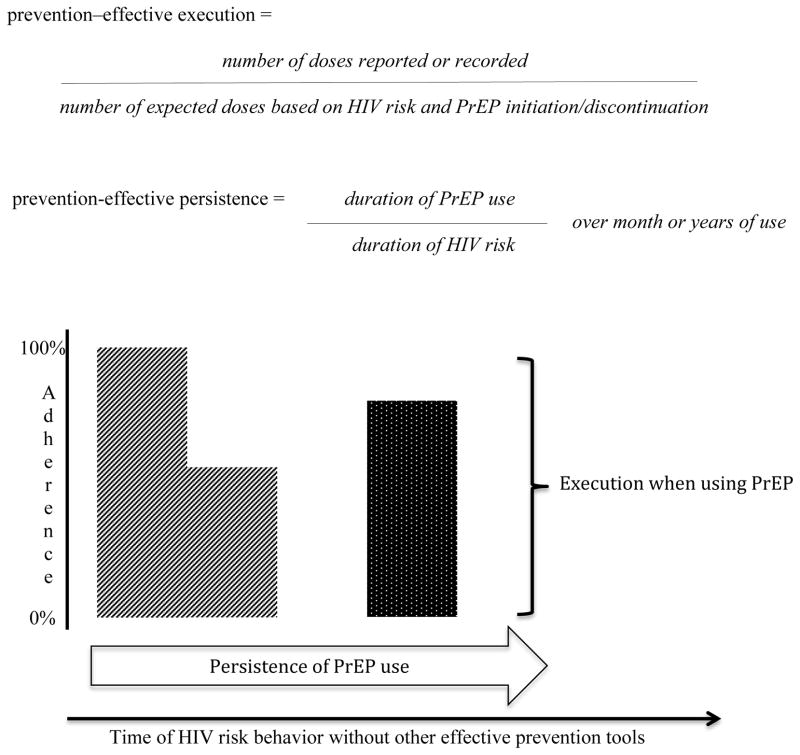
The above three factors are all required to calculate two types of adherence: execution and persistence. First, execution of prevention-effective PrEP adherence refers to adherence during the time an individual relies upon PrEP for protection against HIV acquisition (Figure 2). It depends on periods of HIV risk as defined by behavior and/or use of other effective prevention tools, as well as initiation/discontinuation of PrEP. It is not simply lifelong as with ART or for the duration of a clinical trial. It should include dosing to achieve effective drug concentrations (discussed below), but should not include periods without risk exposure.
Figure 2. Prevention-effective execution and persistence of adherence.
Execution refers to adherence during the time that an individual is at risk for HIV acquisition and is intending to rely upon it for protection. Persistence describes the duration of PrEP use during periods of HIV risk and should reference the absolute time of use. As an example for individuals taking PrEP during periods of HIV risk, the striped shape represents 100% executed adherence for the first three months of PrEP use, followed by 50% adherence for the next three months of use, with a persistence of 100%. The dotted shape represents 80% executed adherence with a persistence of three months.
For example, a sex worker may engage in unprotected sex for six months in an urban setting, but then return home to a rural area for the following six months where she does not engage in any sex. If she took PrEP daily for all but the final two weeks of the six months spent doing sex work, her executed adherence would be 92%. If she stopped sex work a month early, her executed adherence would be 100%. Conversely, if she continued daily PrEP for the full year without sex work in the final six months, her executed adherence would be 200% (thus, a potential misallocation of resources and unnecessary risk for side effects). Both PrEP use and the number of expected dosing events should be censored when HIV transmission risk approaches zero.
Persistence of prevention-effective PrEP adherence describes the duration of PrEP use before an individual stops it (either temporarily or permanently). End of use should be defined by self-reported intentional cessation or a clear break in use (e.g., 28 days non-use), as consistent with the concept of periodic PrEP use. Missing a few doses because of temporary lapses still constitutes persistence, albeit with imperfectly executed adherence. With ART, persistence is desired for life, so no denominator is needed. With prevention-effective PrEP adherence, persistence should be considered over the duration of risk based on behavior and/or use alternative HIV prevention tools. Importantly, the length of the denominator should be noted to reflect the extent of risk.
For example, an individual using PrEP for the first five of six months with HIV risk behavior and no other effective prevention tools has a persistence of 83% over six months. Another individual using PrEP for the first ten of 12 months with risk and no alternative effective prevention tools also has a persistence of 83% over twelve months. Although the percent of persistence is the same, the overall risk for the latter individual is higher given the potential for exposure during one month more than the former individual. This time-dependent nature of risk is important in considering the overall effectiveness of a given level of persistence. Additionally, it is important to note that someone may have high persistence and still not achieve prevention-effective adherence, if the execution of adherence during that time is inadequate.
HIV testing
The effectiveness aspect of prevention-effective adherence requires HIV testing. Current guidelines recommend testing prior to initiation of PrEP and periodic testing during use [19, 20], although the optimal schedule for periodic testing is not clear. Both clinic-based and home-based approaches are being explored in demonstration projects [38]. Given the lack of evidence on the optimal interval for retesting, adherence to HIV testing at a given interval should not preclude PrEP use. However, testing prior to re-initiation of PrEP after a period of non-use is critical to avoid drug resistance due to ongoing use during acute infection. Under-appreciation of risk could result in exposure without PrEP use, making such testing particularly important when targeting prevention-effective adherence.
Interpretation PrEP adherence by route of infection
When putting PrEP adherence in the context of risk exposure, two additional factors must be considered to discern if a given PrEP adherence pattern will provide the anticipated effectiveness: steady state drug concentration and drug level at the time of exposure. These factors differ with the route of exposure and are critical for knowing how much PrEP is enough PrEP.
Steady state drug concentration
Efficacy in most oral PrEP trials has been associated with blood concentrations of tenofovir [1–3, 5]. Most estimates indicate that approximately seven daily oral doses will result in steady state drug concentrations in blood mononuclear cells [39, 40]. Fewer data are available to guide when steady state concentrations are achieved in vaginal and rectal tissue [41], but data generally parallel concentrations in blood [42], thus providing a reasonable evidence-based recommendation for seven days of PrEP use prior to achieving protection. The impact of missed doses on efficacy may have more serious implications when steady state has not yet been achieved (i.e., there is likely less “forgiveness” for missed doses). Given that PrEP use may be periodic, these factors must be considered throughout PrEP use, not just at initiation.
Drug level at the time of exposure
The adherence pattern must result in a drug level at time of risk exposure that is adequate to prevent HIV replication in order to provide protection. In modeling studies based on men who have sex with men (MSM), two doses per week on average may provide 76% efficacy, four doses per week 96% protection, and seven doses per week 99% efficacy [39]. However, tenofovir concentrations are much higher in rectal compared to vaginal tissue; therefore, vaginal tissue may require more frequent oral dosing to maintain drug levels in a protective range [41, 43].
Additionally, the precise timing as to when risk from an exposure has passed is unclear because it is unknown how long HIV takes to be completely cleared from the body. Practical guidance can be drawn from PEP, for which recommendations indicate 28 days of continued use after exposure [44]. PrEP differs from PEP in that early replication is presumably blocked by PrEP from ongoing use, and 28 days of post-exposure dosing without further risk may be challenging for some individuals. However, 28 days of continued dosing allows ample time for adopting alternative HIV prevention tools. Additional research in this area is needed.
Guidance in achieving prevention-effective adherence for individual and public health benefits
The paradigm of prevention-effective adherence will be new for at risk individuals, as well as researchers, clinicians, and policy makers. In efficacy trials, at risk individuals are typically given one or more HIV prevention tools and are expected to consistently adhere to all prevention strategies regardless of need or preference. In demonstration projects and implementation, a menu of prevention options is more likely to reflect how individuals will achieve personal HIV prevention. Specific guidance is needed in the provision of HIV prevention options and associated counseling messages.
Lessons from family planning
While conception and HIV infection are clearly very different conditions, both occur via sex and multiple strategies exist for prevention; lessons learned from family planning may therefore be useful for PrEP [45]. Studies have shown that women frequently move among contraceptive options [46]. A woman may use oral contraceptive pills (OCPs) for some time, but stop when she no longer has a sexual partner. She may choose depo injection, OCPs, or some other female-controlled method if her partner does not want to reliably use condoms. After missing several OCPs, she may switch to condoms as an effective alternative for contraception with future exposures. Women can adapt and change their choice of contraception to fit their needs, perceptions, and preferences. A similar approach could be used for HIV prevention, and measurement of adherence to each method will then determine if the individual has achieved prevention-effective adherence. Of note, even after long-acting injectables or rings are available for PrEP, pill-based options will still make sense for some people at certain times in their lives.
Despite the many existing contraceptive options, unwanted pregnancies still occur. Correspondingly, HIV infections will still likely occur despite the availability of PrEP and other prevention tools. Nevertheless, more HIV will be prevented with these tools than without them, just as family planning programs implemented in real world settings globally have prevented many unplanned pregnancies, in spite of imperfect risk assessment and use of contraception. Further research on understanding and supporting prevention-effective adherence is therefore needed to optimize outcomes.
Counseling for prevention-effective PrEP adherence
To understand prevention-effective PrEP adherence, individuals will need guidance in making decisions about prevention options. Considerations include their preferences, abilities, risks, and behaviors, as well as the timing of potential exposures [47]. Key adherence information for PrEP users is listed in Table 3; additional information for general PrEP use and adherence should be obtained through other sources, such as governmental and other guidelines and may need targeting the specific needs of each population [19, 20]. For instance, daily PrEP may be useful as a short-acting bridge while an HIV-infected partner accesses and becomes virally suppressed on ART, as is being investigated in the Partners Demonstration Project. Frequent use of PEP may suggest the need for the more proactive strategy of PrEP. If depo injections of long-acting PrEP become available, daily PrEP may be a good option initially to see if side effects develop. Adherence to longer-acting agents will require unique messages directed at infrequent events (e.g., getting depo injections), which has been shown to be challenging for multistage vaccines [48]. Both execution and persistence of adherence can help individuals determine if PrEP will be an effective prevention tool for them. Those who vacillate between PrEP and condoms, for example, are unlikely to achieve prevention-effective adherence with PrEP and should consider focusing on condoms alone or another strategy. Adherence counseling should also address the current recommendation for seven daily doses to achieve protective levels of drug, as well as risk for infection after an exposure, as discussed above.
Table 3. Key information for PrEP users specific to prevention-effective adherence.
Additional information for general PrEP use and adherence should be obtained through other sources, such as governmental and other guidelines [19,20].
| Prevention-effective PrEP adherence messages |
| PrEP is not effective immediately. Based on current information, you are unlikely to have full protection with PrEP until you have taken one dose a day for about seven days. Use of another HIV prevention method (like condoms, if possible, or post-exposure prophylaxis) is recommended during this period. |
| PrEP works best when it is taken daily. People who use PrEP daily get very high levels of protection from HIV. |
| PrEP should be taken when you are at risk for HIV infection. Your risk depends on your behavior (like having sex), the presence of HIV in your sexual network (like having a sexual partner who has HIV regardless of whether you or the partner is aware of it), and use of other prevention tools (like condoms). |
| Determining your risk for HIV can be difficult and your risk may change over time. PrEP is recommended if you have ongoing risk for weeks or months at a time. Do not adjust your use of PrEP based on your HIV risk from one day to the next. Similarly, determining which HIV prevention tool or tools make sense for you at different times in your life can also be difficult. For guidance, consult with your clinic. |
| Stopping PrEP for several days in a row will decrease your protection against HIV infection. If you have sex during a time when you missed several days or more of PrEP, check in with your provider before starting up daily PrEP again. If you were exposed to HIV and there was not enough PrEP to effectively prevent it, getting back on PrEP could limit your HIV treatment options. You should get tested for HIV before starting up again. |
| Don’t stop taking PrEP if you might have been recently exposed to HIV, even if you think you are entering a time of no risk and don’t need it for future risk. You should keep taking PrEP after a possible HIV exposure for at least four weeks because it may take that long for HIV to be cleared from your body. You should also get HIV testing. |
| If you are unable to take PrEP regularly, you should rely on other prevention methods (like condoms). The current information available is for daily PrEP. PrEP is highly effective in preventing HIV when taken daily. |
| Assessing prevention-effective PrEP adherence in practice |
| Everyone will struggle to take a daily medication for any condition from time to time. Since you were here last, how has it gone with trying to take PrEP? How well are you doing with taking it every day? |
| Have you taken any breaks from PrEP? Tell me about that. Do you want to re-start? Did you already re-start? |
| For those having trouble with near daily adherence- What are the things you do now to help you to take PrEP about daily? What has worked and what has not? When you have struggled with taking PrEP, what have those situations been like? Can you think of different ways to try to take PrEP in those situations? |
| What would you say your current risks for getting HIV are? What, if anything, are you doing now or even thinking of doing to manage HIV risk besides PrEP? Given all that, does PrEP still feel like a good strategy for you? |
Using prevention-effective adherence to define programmatic success
Success for HIV prevention programs should not depend on consistently high PrEP adherence every day for life, as is the standard for ART programs and clinical trials under the old adherence paradigm. Rather, success may be better defined as providing access to multiple prevention tools for the people most at risk for HIV infection with high adherence to at least one tool at any given time. This approach may be conceptualized as prevention coverage. To what extent is the population covered? To what extent does PrEP increase that coverage and at what cost? Within the iPrEx study, men using PrEP were not the same individuals who were using condoms at baseline [49]; PrEP filled a prevention gap.
Conclusions
Adherence is critical for PrEP effectiveness. Rather than viewing PrEP as a daily pill taken indefinitely, however, it should be seen through the lens of prevention-effective adherence, which takes into account multiple prevention strategies and dynamic risk for HIV acquisition. Measurement of PrEP adherence must include an individual’s choice of tools, the timing of those choices, and the pattern of risk behaviors. While further research is needed on the best measures for both PrEP use and HIV risk, the concept of prevention-effective adherence should be consider as programs explore PrEP for HIV prevention. Discontinuation of PrEP does not necessarily equal failure. Rather, it may be a strategic, effective, and efficient choice for a given individual. HIV prevention programs may be able to learn from other prevention efforts, such as family planning, so they can supply the access and support needed to successfully guide individuals through a pathway of HIV prevention.
Acknowledgments
The authors would like to thank the World Health Organization and Bill and Melinda Gates Foundation for convening a meeting of the authors to discuss key issues in PrEP adherence in June 2014.
Funding
Bill and Melinda Gates Foundation (OPP1056051), US National Institutes of Health (R01MH098744, R01MH095507, U01AI106499), World Health Organization, and the President’s Emergency Plan for AIDS Relief
Footnotes
Contributions of authors
All authors contributed to the development of the ideas contained in this manuscript. JEH wrote the first draft of the manuscript, which all other authors edited significantly.
References
- 1.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marazzo J, Ramjee G, Nair G, Palanee T, Mkhize B, Nakabiito Taljaard M, et al. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE study (MTN 003). 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. March 2013. Oral Abstract 26LB. [Google Scholar]
- 5.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 6.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amico KR, Stirratt MJ. Adherence to preexposure prophylaxis: current, emerging, and anticipated bases of evidence. Clin Infect Dis. 2014;59 (Suppl 1):S55–60. doi: 10.1093/cid/ciu266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26:F13–19. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 9.Grant R, Anderson P, McMahan V, Liu A, Amico KR, Mehrotra M, et al. for the iPrEx study team. Results of the iPrEx open-label extension (iPrEx OLE) in men and transgender women who have sex with men: PrEP uptake, sexual practices, and HIV incidence. Lancet Infect Dis. 2014;14:820–9. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haberer JE, Baeten JM, Campbell J, Wangisi J, Katabira E, Ronald A, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10:e1001511. doi: 10.1371/journal.pmed.1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376:49–62. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]
- 12.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 13.Shuter J, Sarlo JA, Kanmaz TJ, Rode RA, Zingman BS. HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95% J Acquir Immune Defic Syndr. 2007;45:4–8. doi: 10.1097/QAI.0b013e318050d8c2. [DOI] [PubMed] [Google Scholar]
- 14.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43:939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 15.Lima VD, Bangsberg DR, Harrigan PR, Deeks SG, Yip B, Hogg RS, et al. Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. J Acquir Immune Defic Syndr. 2010;55:460–465. doi: 10.1097/QAI.0b013e3181f2ac87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenblum M, Deeks SG, van der Laan M, Bangsberg DR. The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS One. 2009;4:e7196. doi: 10.1371/journal.pone.0007196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogg RS, Heath K, Bangsberg D, Yip B, Press N, O’Shaughnessy MV, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 18.Dybul M, Nies-Kraske E, Daucher M, Hertogs K, Hallahan CW, Csako G, et al. Long-cycle structured intermittent versus continuous highly active antiretroviral therapy for the treatment of chronic infection with human immunodeficiency virus: effects on drug toxicity and on immunologic and virologic parameters. J Infect Dis. 2003;188:388–396. doi: 10.1086/376535. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Guidance on oral pre-exposure prophylaxis (PrEP) for serodiscordant couples, men and transgender women who have sex with men at high risk of HIV: Recommendations for use in the context of demonstration projects. 2012 Jul; Available at: http://www.who.int/hiv/pub/guidance_prep/en/ [PubMed]
- 20.U.S. Public Health Service. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States - 2014; A Clinical Practice Guideline. 2014 May; Available at: www.cdc.gov/hiv/pdf/prepguidelines2014.pdf.
- 21.Gomez GB, Borquez A, Case KK, Wheelock A, Vassall A, Hankins C. The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of cost-effectiveness modelling studies. PLoS Med. 2013;10:e1001401. doi: 10.1371/journal.pmed.1001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corneli AL, McKenna K, Headley J, Ahmed K, Odhiambo J, Skhosana J, et al. A descriptive analysis of perceptions of HIV risk and worry about acquiring HIV among FEM-PrEP participants who seroconverted in Bondo, Kenya, and Pretoria, South Africa. J Int AIDS Soc. 2014;17:19152. doi: 10.7448/IAS.17.3.19152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson IB, Carter AE, Berg KM. Improving the self-report of HIV antiretroviral medication adherence: is the glass half full or half empty? Curr HIV/AIDS Rep. 2009;6:177–186. doi: 10.1007/s11904-009-0024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu M, Safren SA, Skolnik PR, Rogers WH, Coady W, Hardy H, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav. 2008;12:86–94. doi: 10.1007/s10461-007-9261-4. [DOI] [PubMed] [Google Scholar]
- 25.Haberer JE, Baeten J, Heffron R, Mugo N, Katabira E, Ngure K, et al. Self-reported Adherence to Pre-Exposure Prophylaxis (PrEP) and Sexual Behavior by Text Messaging: Preliminary Findings from the Partners Mobile Adherence to PrEP Study. 9th International Conference on HIV Treatment and Prevention Adherence; Miami, FL. June 2014. Poster 316. [Google Scholar]
- 26.Minnis AM, Steiner MJ, Gallo MF, Warner L, Hobbs MM, van der Straten A, et al. Biomarker validation of reports of recent sexual activity: results of a randomized controlled study in Zimbabwe. Am J Epidemiol. 2009;170:918–924. doi: 10.1093/aje/kwp219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallo MF, Behets FM, Steiner MJ, Hobbs MM, Hoke TH, Van Damme K, et al. Prostate-specific antigen to ascertain reliability of self-reported coital exposure to semen. Sex Transm Dis. 2006;33:476–479. doi: 10.1097/01.olq.0000231960.92850.75. [DOI] [PubMed] [Google Scholar]
- 28.Williams AB, Amico KR, Bova C, Womack JA. A proposal for quality standards for measuring medication adherence in research. AIDS Behav. 2013;17:284–297. doi: 10.1007/s10461-012-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baeten JM, Haberer JE, Liu AY, Sista N. Preexposure prophylaxis for HIV prevention: where have we been and where are we going? J Acquir Immune Defic Syndr. 2013;63 (Suppl 2):S122–129. doi: 10.1097/QAI.0b013e3182986f69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amico KR, Marcus JL, McMahan V, Liu A, Koester KA, Goicochea P, et al. Study product adherence measurement in the iPrEx placebo-controlled trial: concordance with drug detection. J Acquir Immune Defic Syndr. 2014;66:530–537. doi: 10.1097/QAI.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bisson GP, Gross R, Bellamy S, Chittams J, Hislop M, Regensberg L, et al. Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Med. 2008;5:e109. doi: 10.1371/journal.pmed.0050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haberer JE, Kahane J, Kigozi I, Emenyonu N, Hunt P, Martin J, et al. Real-time adherence monitoring for HIV antiretroviral therapy. AIDS Behav. 2010;14:1340–1346. doi: 10.1007/s10461-010-9799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haberer JE, Kiwanuka J, Nansera D, Muzoora C, Hunt PW, So J, et al. Realtime adherence monitoring of antiretroviral therapy among HIV-infected adults and children in rural Uganda. AIDS. 2013;27:2166–2168. doi: 10.1097/QAD.0b013e328363b53f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthews LT, Baeten JM, Celum C, Bangsberg DR. Periconception pre-exposure prophylaxis to prevent HIV transmission: benefits, risks, and challenges to implementation. AIDS. 2010;24:1975–1982. doi: 10.1097/QAD.0b013e32833bedeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kibengo FM, Ruzagira E, Katende D, Bwanika AN, Bahemuka U, Haberer JE, et al. Safety, adherence and acceptability of intermittent tenofovir/emtricitabine as HIV pre-exposure prophylaxis (PrEP) among HIV-uninfected Ugandan volunteers living in HIV-serodiscordant relationships: a randomized, clinical trial. PLoS One. 2013;8:e74314. doi: 10.1371/journal.pone.0074314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mutua G, Sanders E, Mugo P, Anzala O, Haberer JE, Bangsberg D, et al. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One. 2012;7:e33103. doi: 10.1371/journal.pone.0033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ANRS Press Release. A drug taken at the time of sexual intercourse effectively reduces the risk of infection. 2014 Oct; Available at: http://www.ipergay.fr.
- 38.Ngure KHR, Mugo N, Irungu E, Njambi Njuguna N, Mwaniki L, Thompson K, Celum C, Baeten JM. Uptake of HIV Self-testing among People Receiving PrEP in Kenya. HIV Research for Prevention; Cape Town, South Africa. October 2014. Oral abstract OA27.02. [Google Scholar]
- 39.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hendrix CW, Andrade A, Kashuba AD, Marzinke M, Anderson PL, Moore A, et al. Tenofovir-Emtricitabine Directly Observed Dosing: 100% Adherence Concentrations (HPTN 066). Conference on Retorviruses and Opportunistic Infections; Boston, MA. March 2014. Abstract 014. [Google Scholar]
- 41.Louissaint NA, Cao YJ, Skipper PL, Liberman RG, Tannenbaum SR, Nimmagadda S, et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses. 2013;29:1443–1450. doi: 10.1089/aid.2013.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson PL, Meditz A, Zheng JH, Predhomme J, Klein B, Guida LA, et al. Cellular pharmacology of tenofovir and emtricitabine in blood, rectal, and cervical cells from HIV-negative volunteers. Abstracts of the Nineteenth Conference on Retroviruses and Opportunistic Infections; Seattle, WA. Alexandria, VA, USA: Foundation for Retrovirology and Human Health; 2012. p. Abstract 587. [Google Scholar]
- 43.Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112re114. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.U.S. Public Health Service Working Group. Kuhar DT, Henderson DK, Struble KA, Heneine W, Thomas V, Cheever LW, et al. Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HIV and Recommendations for Postexposure Prophylaxis. US Public Health Service Guidelines for the Management of Occupational Exposures to HIV. 2013 Sep; doi: 10.1086/672271. Available at: http://stacks.cdc.gov/view/cdc/20711. [DOI] [PubMed]
- 45.Myers JE, Sepkowitz KA. A pill for HIV prevention: deja vu all over again? Clin Infect Dis. 2013;56:1604–1612. doi: 10.1093/cid/cit085. [DOI] [PubMed] [Google Scholar]
- 46.Matteson PS, Hawkins JW. Women’s patterns of contraceptive use. Health Care Women Int. 1997;18:455–466. doi: 10.1080/07399339709516300. [DOI] [PubMed] [Google Scholar]
- 47.Amico KR, McMahan V, Marcus J, Goicochea P, Vargas L, Grant R, et al. Integrated Next Step Counseling (iNSC): A discussion based sexual health promotion conversation to support men who have sex with men using PrEP in the iPrEx open label extension. 7th Annual HIV Treatment and Prevention Adherence Conference of the International Association of Providers in AIDS Care; Miami, FL. June 2012.p. Abstract 80467. [Google Scholar]
- 48.Katz IT, Ware NC, Gray G, Haberer JE, Mellins CA, Bangsberg DR. Scaling up human papillomavirus vaccination: a conceptual framework of vaccine adherence. Sex Health. 2010;7:279–286. doi: 10.1071/SH09130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buchbinder SP, Glidden DV, Liu AY, McMahan V, Guanira JV, Mayer KH, et al. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect Dis. 2014;14:468–475. doi: 10.1016/S1473-3099(14)70025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]