Abstract
Sex hormone-binding globulin (SHBG) transports androgens and estrogens in blood and regulates their access to target tissues. Hepatic production of SHBG fluctuates throughout the life cycle and is influenced primarily by metabolic and hormonal factors. Genetic differences also contribute to interindividual variations in plasma SHBG levels. In addition to controlling the plasma distribution, metabolic clearance, and bioavailability of sex steroids, SHBG accumulates in the extravascular compartments of some tissues and in the cytoplasm of specific epithelial cells, where it exerts novel effects on androgen and estrogen action. In mammals, the gene-encoding SHBG is expressed primarily in the liver but also at low levels in other tissues, including the testis. In subprimate species, Shbg expression in Sertoli cells is under the control of follicle-stimulating hormone and produces the androgen-binding protein that influences androgen actions in the seminiferous tubules and epididymis. In humans, the SHBG gene is not expressed in Sertoli cells, but its expression in germ cells produces an SHBG isoform that accumulates in the acrosome. In fish, Shbg is produced by the liver but has a unique function in the gill as a portal for natural steroids and xenobiotics, including synthetic steroids. However, salmon have retained a second, poorly conserved Shbg gene that is expressed only in ovary, muscle, and gill and that likely exerts specialized functions in these tissues. The present review compares the production and functions of SHBG in different species and its diverse effects on reproduction.
Keywords: male reproductive tract, mechanisms of hormone action, sperm, steroid hormones/steroid hormone receptors, testis
This review compares the production and functions of sex hormone-binding globulin in different species and evaluates the diverse effects this has on reproduction.
INTRODUCTION
The sex steroids, testosterone and estradiol, control various aspects of sexual differentiation, gonadal development, as well as growth and functional maturation of reproductive tissues [1]. They also influence the maturation of other organ systems, including the lung [2] and kidney [3], during early development. The prenatal effects of androgens exert lifelong impact on the expression of genes in the liver [4] and have been associated with risk for metabolic syndrome and cardiovascular disease during later life [5–7]. In addition, androgens and estrogens modulate sexual behaviors that are critical determinants of reproductive success [8].
As classical hormones, sex steroids or their immediate precursors are produced in steroidogenic cells of the gonads, adrenal glands, and placenta, and they are transported in the blood to their target tissues by several steroid-binding proteins [9]. The most abundant plasma protein, albumin, binds all classes of steroids nonspecifically and with low affinity and functions as a reservoir that enhances the solubility of lipophilic molecules and prolongs their biological half-life [10]. By contrast, a plasma glycoprotein, known as sex hormone-binding globulin (SHBG), binds biologically active androgens and estrogens specifically, with an affinity four to five orders of magnitude greater than that of albumin, and is found in the blood of all classes of vertebrates with the exception of birds [9]. Because of its very high ligand-binding affinity, plasma SHBG is the major plasma transport protein for biologically active androgens and estrogens [11], and changes in the blood levels of SHBG influence their plasma distribution and access to target tissues and cells [12].
The steroid-binding specificity of SHBG varies between species, but androgens are generally the preferred SHBG ligand in mammals. In the past, SHBG has therefore also been widely referred to as the androgen-binding protein (ABP) by reproductive biologists, who have studied its production in the testis and how it might influence sperm maturation in the male reproductive tract [13]. The cloning of human SHBG and rat Abp cDNAs and their genes, however, revealed that these proteins are orthologs and the products of a single gene [14, 15], which is expressed at low levels in several tissues in addition to the liver and testis [13]. Recently, a second SHBG-related gene encoding an ancient paralog of SHBG has been identified in the Salmonidae order of teleosts and is referred to as salmonid shbgb [16]. Unlike the evolutionarily conserved shbg gene, the salmonid shbgb gene is expressed primarily in the ovary, gill, and muscle; it is not expressed in the liver or testis [16, 17].
The present review compares the expression and regulation of genes encoding SHBG in different species. It also evaluates the diverse effects SHBG may have on the reproductive strategies and fitness of those species.
EVOLUTION OF THE SHBG GENE AND ITS PROTEIN STRUCTURE
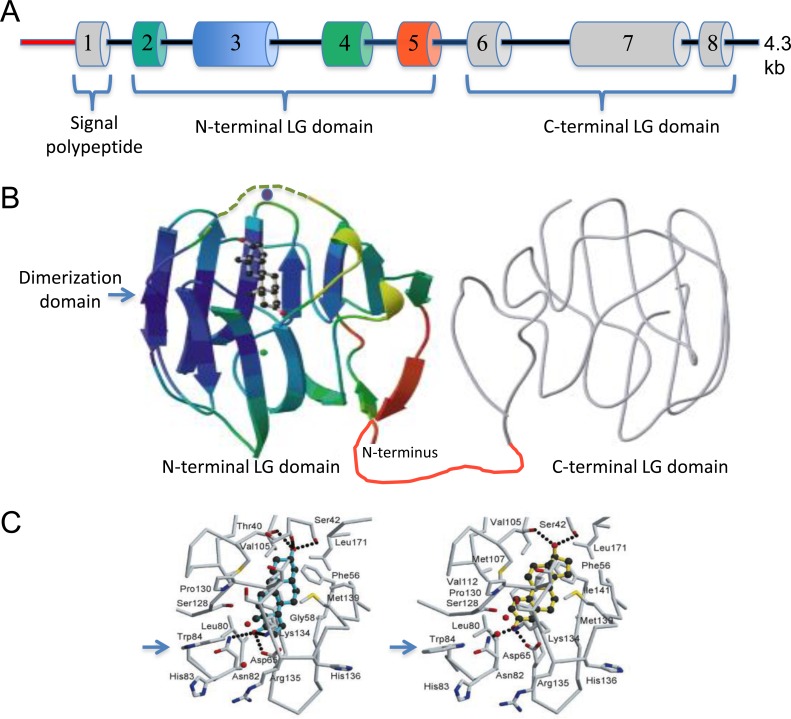
Phylogenetic analyses using gene orthology and paralogy prediction methods indicate that a gene encoding SHBG evolved with the appearance of the vertebrates some 500 million years ago (http://www.ensembl.org). How this gene originated is not known, but it encodes just two laminin G-like (LG) domains that are also found in the carboxy-terminal regions of two other, much larger secreted proteins (i.e., the vitamin K-dependent proteins, gas6 and protein S) [18]. The human SHBG gene is quite compact (Fig. 1A), comprising eight coding exons distributed over only approximately 4 kbp of genomic DNA [14]. The same Shbg gene structure is conserved across all vertebrate species, although the sizes of intronic sequences vary. Crystal structure studies [19] have shown that the steroid-binding site of SHBG is confined to its amino-terminal LG domain encoded by exons 2–5 (Fig. 1B). Within the amino-terminal LG domain, amino acids critical for steroid binding are highly conserved in SHBG molecules across vertebrate species, and they include a serine residue (Ser42 in human SHBG) located deep in the binding pocket. This serine residue appears to be essential for steroid binding (Fig. 1C), and hydrogen bonds with functional groups at the C3 position of the A ring of androgens [20] and the C-17 hydroxyl group of ring D of estrogens [20, 21]. This difference in orientation of androgens and estrogens within the SHBG steroid-binding site may be functionally important, because it alters the position of an exposed loop over the entrance to the steroid-binding site [22] and induces subtle changes in the orientation of surface exposed residues (e.g., Trp84) [20, 21] (Fig. 1C), which could influence interactions between SHBG and other proteins in a ligand-dependent manner [23]. These crystal structure studies not only revealed the location of calcium-binding sites in the human SHBG amino-terminal LG domain (Fig. 1B) but also demonstrated that SHBG is a zinc-binding protein [19, 24]. It had previously been shown that the presence of calcium is essential for maintaining homodimer stability and steroid-binding activity [25], but the presence of a zinc molecule was found to help orient the exposed loop over the entrance to the steroid-binding site (Fig. 1B) and to alter the binding affinity of estrogens versus androgens [24].
FIG. 1.
Organization of the human SHBG gene and tertiary structure of human SHBG showing the location and topography of the steroid-binding site. A) The 4.3-kb human SHBG transcription unit that is expressed in hepatocytes under the control of an approximately 800-bp promoter sequence (red). The positions and relative sizes of exons 1–8 that encode the signal polypeptide for secretion and the two LG domain structures that constitute the mature SHBG monomer are color-coded to match regions for which the tertiary structure has been resolved. Exon 1 includes a 60-bp, 5′ untranslated region and the translation start site for the SHBG precursor polypeptide sequence, which includes the signal polypeptide sequence that is removed during secretion of the mature polypeptide, as well as the 3 amino-terminal residues of the mature SHBG protein. B) Tertiary structure of the human SHBG monomer showing the amino-terminal LG domain that has been crystallized with steroid ligands in the single steroid-binding site joined by a linker region (red) to the carboxy-terminal LG domain (gray) that has been modeled from the amino-terminal LG domain structure. The position of the disordered sequence over the steroid-binding site that is influenced by occupancy of a zinc-binding site (zinc molecule shown as a purple ball) and steroid-ligand binding is shown (dashed green tracing). During synthesis and secretion, SHBG monomers dimerize spontaneously in a head-to-head manner to form a linear homodimer at a dimerization domain, the position of which is indicated, and this process is influenced by occupancy of a calcium-binding site (calcium molecule shown as a green ball). C) The topography of the SHBG steroid-binding site, showing how androgens (5α-androstane-3β,17β-diol) and estrogens (estradiol) are oriented differently in the steroid-binding site and how this induces subtle differences in the positioning of surface residues (e.g., Trp84 [arrow]).
The structure of SHBG in relation to its function as a steroid-binding protein has been reviewed recently [23], but one aspect of its structure should be mentioned here in relation to potential functions of SHBG that are still poorly understood. The C-terminal regions of gas6 and protein S contain the same tandem laminin domain structure as SHBG, but they do not bind steroids. However, they do interact with the TAM (TYRO3, AXL, and MER) family of receptor protein tyrosine kinases, and this interaction occurs via their “SHBG-like” domains [26, 27]. Thus, like other proteins with LG domain structures, such as neurexin and laminin itself [28], they have the capacity to participate in macromolecular interactions through conserved protein-protein interaction domains. It is not surprising, therefore, that interactions have been reported between SHBG and plasma membrane-associated proteins [29, 30] and other extracellular proteins [31], which extend the role of SHBG beyond that of a simple steroid transport protein.
SHBG IN THE BLOOD
During Fetal Development
In mammals, production of plasma SHBG by the liver during fetal life [32] appears to be particularly important, because this is the only time at which the Shbg gene is expressed in the livers of mice and rats [33]. In support of this, hepatic Shbg expression, and the appearance of SHBG in the blood of fetal rats [33, 34], coincides with the appearance of the luteinizing hormone receptor and the key steroidogenic enzymes required for testosterone biosynthesis in the fetal testis [35]. Although less information is available about the ontogeny of fetal hepatic SHBG production and testicular steroidogenesis in other mammals, the window of maximum testosterone production by the fetal rabbit testis also occurs during the last third of gestation [1], coincident with a surge in hepatic SHBG production [36]. By contrast, the masculinization time window, during which the human fetal testis produces very high levels of testosterone, occurs between Weeks 10 and 20 of pregnancy [37], at a much earlier gestational age than in rodents or rabbits. This is interesting, both because SHBG is present in human fetal blood samples at this early stage of development [38] and because amniotic fluid levels of SHBG and testosterone correlate significantly in male fetuses during Gestational Weeks 13–20 [39], during the window of masculinization in the human male fetus [37].
It is not known if sex differences influence the hepatic expression of Shbg in fetal rabbits [36] or rodents [32, 33], but SHBG levels in male and female human fetal blood [38] and amniotic fluid [39] samples are similar. Although the overall function of SHBG in fetal blood has not been clearly defined, it may serve to protect female fetuses from exposure to androgens in utero in addition to regulating the action of androgens in terms of male sex differentiation. Some precedence exists for this, because alpha-fetoprotein (AFP) in rodents is also expressed in the developing liver at high levels during late fetal and early postnatal life and has the unusual characteristic of being a high-affinity estradiol-binding protein [40]. Physiologically, this appears to be important, both because Afp−/− mice do not ovulate as a result of an imbalance in the hypothalamo-pituitary-gonadal (HPG) axis [41, 42] and because female Afp−/− mice exhibit reproductive behavioral defects consistent with a defeminization and masculinization of the brain as a result of excess estrogen exposures during fetal and early postnatal life [40, 43]. In human fetuses, AFP does not bind estrogens, but human SHBG binds estradiol with relatively high affinity and, thereby, may provide a similar function in terms of modulating the actions of estrogen in human female fetuses and neonates.
During mid to late pregnancy, maternal plasma SHBG levels increase 5- to 10-fold in humans [44] and rabbits [36]. Why plasma SHBG increases so much during pregnancy in some species but not others is not fully understood, but it may be related to species differences in the magnitude of estrogen production by the placenta and/or its subsequent effects on hepatic Shbg expression. In support of this, maternal sheep plasma SHBG levels do not change during pregnancy and are not increased by exogenous estrogen treatment [45]. In species for which plasma SHBG increases during pregnancy, it has been thought that this protects the fetus from exposures to maternal sex steroids, but the opposite actually might be closer to reality. In other words, maternal SHBG might serve to protect mothers from sex steroids that originate from the fetus, particularly androgen. This has never been formally investigated. However, clinical studies of a woman with very low plasma SHBG levels, resulting from a combination of two rare single-nucleotide polymorphisms (SNPs) in each of her SHBG coding sequences, suffered from profound and transient virilization during pregnancy, whereas her twin daughters were born without any evidence of excessive androgen exposure [46]. A similar condition of transient maternal virilization is observed among patients with a fetus in which the P450 aromatase gene is defective, resulting in a spillover of fetal adrenal androgens into the maternal circulation and illustrating the importance of placental aromatase in protecting the mother from fetal adrenal androgens [47].
During Childhood and Puberty
At birth, plasma SHBG is virtually undetectable in rat pups, and the immunoreactive SHBG that reappears at low levels in the blood of male rats just before weaning originates from the Sertoli cells, before maturation of the blood-testis barrier [48]. That Shbg transcripts are not present in the livers of adult rats [49] and plasma SHBG is undetectable in their blood [48] presents an interesting conundrum, because it indicates that SHBG is not essential for transporting or regulating the actions of sex steroids in rodents after birth.
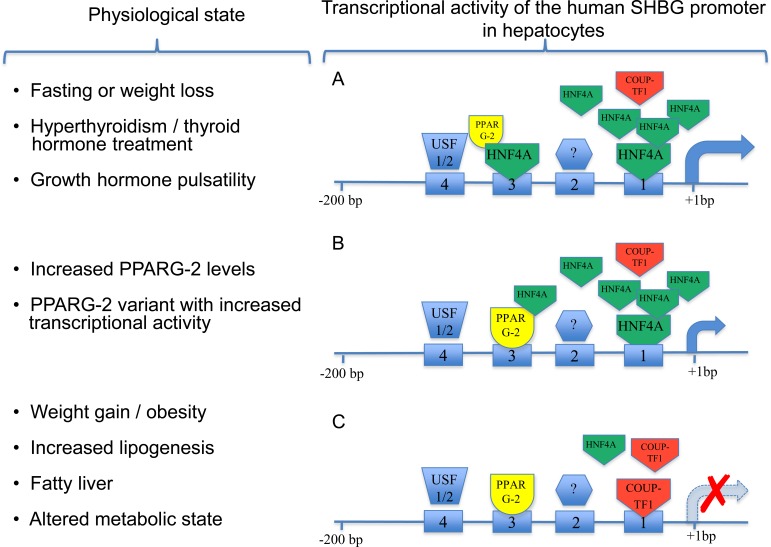
In human neonates, SHBG levels in cord blood are approximately 10-fold lower than those in maternal blood, then increase to relatively high levels (∼100 nM) in infants of both sexes until the onset of puberty, when they decline progressively [50, 51]. Some evidence indicates that this postnatal increase in plasma SHBG levels is brought about by maturation in the production and actions of thyroid hormones [52]. In this context, thyroid hormones act indirectly to increase human SHBG expression by increasing the hepatic levels of the transcription factor, hepatocyte nuclear factor 4 alpha (HNF4A), which has emerged in our understanding as the key regulator of SHBG transcription in the liver [53–55], as illustrated in Figure 2. The high plasma levels of SHBG during childhood will restrict the premature actions of sex steroids derived from the metabolism of adrenal androgens, and the progressive decline of plasma SHBG levels during puberty probably contributes indirectly to the maturation of the HPG axis [50, 51].
FIG. 2.
The 200-bp human SHBG proximal promoter sequence, showing occupancy of cis-elements by transcription factors in response to different physiological states and the corresponding transcriptional activity of the promoter. A) Under conditions when HNF4A levels in hepatocytes are high, the cis-element (1), which resembles a nuclear hormone DR1 response element, is occupied by HNF4A, resulting in maximal transcriptional activity that may be enhanced by differences in growth hormone pulsatility. HNF4A also binds to a second DR1 sequence (3), but this has a more modest effect on transcriptional activity. A binding-site USF1/2 (4) has little effect on the transcriptional activity of SHBG in liver cells. The identity of the transcription factor that binds to the cis-element (2) and its effect on transcription in the liver is not known. B) PPARG-2 competes with HNF4A for binding at the DR1 sequence (3) and preferential binding of a PPARG-2 variant with superior transcriptional activity to this site will repress SHBG promoter activity. C) Under conditions where HNF4A levels are reduced, the DR1 site closest to the transcription start site is occupied by COUP-TF, and this results in a marked reduction of SHBG transcription.
During puberty, blood levels of SHBG decrease in girls and boys by approximately 2- and 4-fold, respectively [44]. It was originally thought that increased adrenal and gonadal production of androgens might account for these pubertal changes in SHBG levels in boys by directly influencing hepatic SHBG production. The reduction in plasma SHBG in boys serves to ensure a steady increase in the amounts of free testosterone in the blood required for the maturation of accessory sex organs and physical stature [44]. However, a direct effect of androgens on hepatic SHBG expression has never been substantiated in humans or any other species, and reason exists to believe that these effects are indirect. This was actually suggested many years ago [56] and is now supported by evidence that androgen exposures during early life are responsible for sex differences in the pulse frequency and amplitude of pituitary growth hormone release, and it is this that influences the sexual dimorphic expression of many genes in the liver [57]. The sex difference in growth hormone pulsatility that is established during puberty persists in adults until old age, and it involves the STAT5B signaling pathway in the liver [58, 59] together with HNF4A [58, 60] as key determinants of the sexual dimorphism of liver gene expression in mice [60]. In addition to being a sensor and key regulator of metabolic pathways in the liver [60, 61], HNF4A is the main transcriptional on-off switch for hepatic SHBG expression [53], as illustrated in Figure 2, and sex differences in growth hormone pulsatility might therefore contribute to sex differences in hepatic SHBG gene expression in human adults.
It is widely recognized that body mass in humans, and the relative amounts of adipose tissue versus lean muscle in particular, is one of the most important determinants of plasma SHBG levels [62]. In addition, awareness is increasing that obesity, although associated with early onset of menarche in girls, is less of a determinant of precocious puberty in boys [63] and that precocious puberty in boys is not as tightly linked to changes in body composition [64] or endocrine-metabolic abnormalities [65] as it is in girls. This makes sense from a teleological perspective, because it might be argued that changes in body composition during the transition to reproductive maturation are much more important for the reproductive success of females than of males. It is therefore conceivable that changes in body composition and metabolic state are primarily responsible for the reductions in plasma SHBG levels at puberty in females. This would be important, because reductions in plasma SHBG levels will cause substantial increases in the proportions of free androgens and estrogens in the blood and have a profound impact on the maturation of the HPG axis. In this regard, whereas childhood obesity is increasingly associated with precocious puberty in girls [63], young women who have a lean physique often present with delayed menarche and/or primary amenorrhea [66]. Moreover, plasma SHBG levels in women with anorexia are in the same range as those found in prepubertal girls but fall rapidly to levels seen in normal-weight, healthy women after only modest increases in body weight [67].
During Adult Life
Nutritional status is a prime determinant of reproductive fitness for all life forms. In recent times, humans have undergone unprecedented changes in nutrition and lifestyle that not only influence our ability to reproduce but have broader implications in terms of major health issues [68]. In this context, abnormally low plasma SHBG is one of the most reliable biomarkers of metabolic syndrome and its associated pathologies in obese individuals, including the polycystic ovarian syndrome (PCOS), type 2 diabetes, and cardiovascular disease [62]. Low plasma levels of SHBG are also observed in lean women with PCOS [69], and this presents the interesting dilemma of whether low plasma SHBG levels are a cause or an effect of disturbances in the HPG axis that characterize PCOS or even underpin the pathogenesis of metabolic syndrome. This question has never been formally addressed, but common SNPs within the human SHBG gene that are associated with increases or decreases in plasma SHBG levels have recently been linked to reduced or increased risk, respectively, for metabolic syndrome [70].
In human adults of normal body weight, plasma SHBG levels can also vary considerably. Some of these differences are attributed to variations in endocrine and metabolic state [62], but ample evidence suggests that interindividual differences in plasma SHBG levels are in part inherited [71]. This may involve individual differences in the levels or activities of key transcription factors that control SHBG expression in the liver. For instance, a genetic variant of PPARG-2 with superior transcriptional activity has been associated with reduced serum SHBG levels [72], and PPARG-2 appears to repress human SHBG transcription [73] by binding a DR1 nuclear hormone-response element (GGGTCAaGGGTCA) that is shared with HNF4A and NR2F1 (also known as COUP-TF1) in the SHBG promoter (Fig. 2). As indicated above, HNF4A represents the key on-off switch for SHBG expression in the liver, and it acts in this context by binding to another DR1-binding site located approximately 20 bp upstream of the transcriptional start site (Fig. 2). This is the position that the TATA-binding protein (TBP) normally binds in many gene promoters, and in the TATA-less SHBG promoter, HNF4A substitutes for TBP and actively recruits the transcriptional machinery to this site [53]. Because the COUP-TF1 also binds competitively with HNF4A for this site, the levels of HNF4A are critically important in this respect, and when COUP-TF1 binds in this location (Fig. 2), SHBG transcription is blocked [53]. It is therefore possible that genetic abnormalities in the production or function of HNF4A, which are linked to the risk for maturity-onset diabetes of the young [74], will also contribute to reduced SHBG expression in the liver.
Polymorphism within the human SHBG gene also contributes to individual differences in serum SHBG levels. For instance, a common SNP that results in a D327N substitution introduces an additional consensus site for N-glycosylation [75]. This SNP (rs6259) appears to increase the plasma half-life of SHBG [76] and to be associated with altered risk of reproductive tissue cancers [77, 78]. Other SNPs in the coding sequence are rare, but it has recently been found that another nonsynonymous SNP (rs6258), which results in a Ser156Pro substitution [46], actually reduces the affinity of SHBG for steroid ligands and contributes to reduced serum testosterone levels in men (Wu and Hammond, unpublished data). The frequency of rs6258 in white males is approximately 2% (National Center for Biotechnology Information SNP database), and further studies are required to determine its frequency in other ethnic populations and what impact this genetic variant of SHBG might have on androgen and estrogen levels and actions in women.
Several polymorphisms have been noted in the SHBG promoter, and these have also been associated with differences in serum SHBG levels [62, 79, 80]. The most well studied of these is a polymorphic TAAAA(n) penta-nucleotide repeat located at approximately 800 bp upstream of the SHBG transcription site in the liver [81], and it has been linked to abnormal plasma SHBG levels in women with PCOS, age at menarche, chronic heart disease, and semen quality in men [80]. So far, the identity of the transcription factors that might bind to this region of the SHBG promoter and information about how variations in this pentanucleotide repeat might influence transcriptional activity in an in vivo context remain elusive.
When considering the functions of SHBG in human adults, the substantial differences in occupancy of the SHBG steroid-binding site in men and women are often overlooked. Early studies that compared all the known SHBG ligands with respect to their relative binding affinities and serum concentrations in relation to the serum concentrations of SHBG in men and women [11] demonstrated that only approximately 18% of the SHBG steroid-binding sites are potentially occupied by endogenous steroids in women, versus approximately 56% in men. Sex differences in occupancy of the SHBG steroid-binding sites can be explained easily by the much higher testosterone and dihydrotestosterone levels in men than in women, coupled with the fact that SHBG levels are approximately 50% lower in men than in women. Nevertheless, this raises the question of what the unoccupied SHBG-binding sites are doing, especially in women. It is possible that this provides a means of sequestering active androgens more efficiently and thus limiting their access to target tissue or more effectively removing active androgen metabolites from some tissues in women. That women are clearly much more sensitive to increases in free androgens as a result of reduced serum SHBG levels, at least in terms of very obvious androgenic biomarkers, such as hirsutism, would tend to support this idea. Alternatively, unoccupied SHBG steroid-binding sites might be available for other ligands to bind, and evidence suggests that SHBG is capable of binding a wide range of xenobiotics [23]. What should also be noted is that plasma SHBG levels increase by at least 5-fold in women taking some oral contraceptive formulations [82], and in these women, the amount of unoccupied SHBG steroid-binding sites is further accentuated to the point at which more than 90% of the sites cannot possibly be occupied by steroid ligands. The overall effects of this are not well recognized, but oral contraceptives with these properties are frequently used to reduce symptoms of hyperandrogenism (e.g., hirsutism) in women [83]. However, a disproportionate amount of unoccupied SHBG steroid-binding sites could increase the bioaccumulation of potentially harmful xenobiotic ligands of SHBG, even if they bind with lower affinity than natural steroids [23].
As observed during pregnancy, serum SHBG levels increase by as much as 10-fold in women taking synthetic estrogens [44], and both natural and synthetic estrogens increase the production of SHBG by human liver cell lines [84]. However, 4.3- and 11-kbp human genomic fragments comprising the SHBG transcription unit that are expressed in the liver are not regulated by estrogen when introduced as transgenes into the mouse genome, despite being expressed in the appropriate tissues [85] and regulated appropriately by other metabolic and hormonal stimuli [54, 55, 73]. It is therefore not known how estrogens influence human SHBG expression in the liver, and this may involve long-range effects in regions that include more distal alternative promoters within the SHBG locus [86].
During reproductive aging in men and women, changes in plasma SHBG levels are modest and probably related to metabolic or endocrine changes. What is known is that plasma SHBG levels in women do not change appreciably after oophorectomy or the menopausal transition [87] and that orchiectomy has no influence on plasma SHBG levels [88]. It is therefore unlikely that modest increases in plasma SHBG levels in elderly men [44] are related to any decline in testicular testosterone production, and other age-related endocrine or metabolic changes may be responsible for this. Alternatively, evidence is increasing that elderly men with higher SHBG levels are less likely to suffer from pathologies associated with metabolic syndrome [89] and might therefore simply live longer.
SHBG IN THE TESTIS
The SHBG homolog (i.e., ABP) produced by Sertoli cells of most mammals is thought to influence the actions of androgens in the seminiferous tubules and the epididymis, and this has been reviewed in detail previously [13]. However, the precise role of SHBG/ABP in the testis and epididymis remains obscure, and whether it is absolutely essential for sperm maturation is not known. In rat seminiferous tubules, evidence suggests that the SHBG/ABP produced by Sertoli cells may be transferred to spermatogenic cells [90] and influence the dynamics of testicular germ cell proliferation [91]. The SHBG/ABP that is secreted into the seminiferous tubules and rete testis fluid migrates together with immature sperm to the caput epididymis, where it interacts with principal epithelial cells and is thought to promote testosterone access to these androgen-dependent cells [92, 93]. The observation that occupancy of the zinc-binding site with the amino-terminal LG domain of human SHBG specifically reduces its affinity for estradiol is of interest, because the preference of human SHBG for androgens over estrogens will be accentuated in male reproductive tract tissues that are particularly rich in zinc [24].
As in the rodent testis, the SHBG gene is expressed in the Sertoli cells of several other mammals, such as rabbits and sheep [9], but evidence that this occurs in the testis of humans and other primates is less convincing. Early studies indicated that whereas rat rete fluid contains substantial amounts of SHBG/ABP, it is undetectable in monkey rete testis fluid [94]. A subsequent study reported that human Sertoli cell cultures secrete SHBG [95]. However, the purity of these primary cultures in terms of the degree of germ cell contamination was not reported, and confirmation that the Sertoli cells of humans or other primates produce and secrete SHBG is lacking. However, SHBG transcripts in the human testis comprise a noncoding alternative exon 1 sequence and lack the coding sequence for a secretion signal polypeptide [96, 97] and therefore will not encode a protein that is secreted in the same way as SHBG from hepatocytes or SHBG/ABP from rat Sertoli cells.
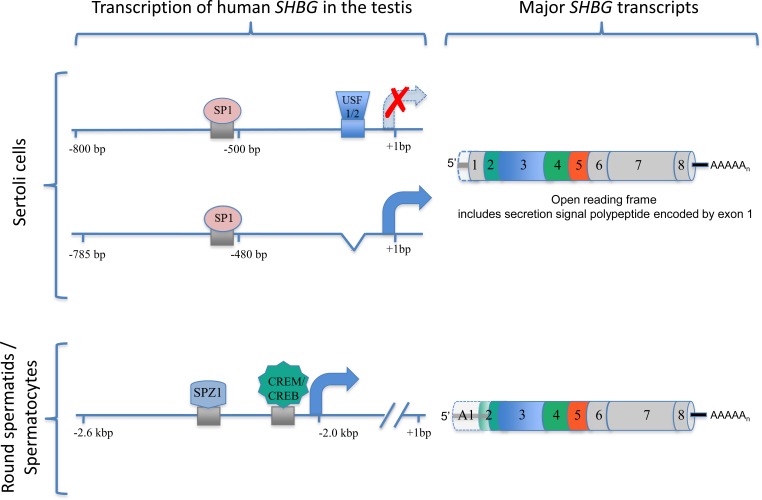
Cell-specific human SHBG expression in the testis has been studied in transgenic mice that harbor wild-type or mutated versions of human SHBG transcription units. When coupled with experiments in different testicular cell lines, the molecular basis for why human SHBG expression is confined to germ cells, rather than Sertoli cells, has become apparent [97]. The proximal promoter flanking the exon sequence that encodes the secretion signal polypeptide required to direct the secretion of SHBG from liver cells and SHBG/ABP from Sertoli cells differs between humans and other subprimate species that express Shbg in Sertoli cells in one important respect: A critical binding site for the upstream stimulatory factor (USF) transcription factors at approximately 110 bp from the transcription start site in the SHBG gene of humans (Fig. 3) is lacking in subprimate species. This cis-element specifically represses transcription of human SHBG in Sertoli cells, whereas its removal from the human SHBG promoter not only restores its ability to be expressed in Sertoli cells but also allows it to respond to follicle-stimulating hormone (FSH) and other hormones in a manner consistent with the way the rat Shbg gene is controlled and expressed in Sertoli cells [98].
FIG. 3.
Positions of key regulatory elements that control human SHBG transcription in different cell types in the testis. In Sertoli cells, the binding site of USF1/2 to the SHBG proximal promoter (see Fig. 2) blocks its transcription in Sertoli cells. Removal of this USF1/2-binding site from the human SHBG proximal promoter restores its activity in Sertoli cells in vivo in transgenic mice and in mouse Sertoli cells in culture. This modified human SHBG promoter also responds to hormones in the same way as the rat Shbg in Sertoli cells, producing a transcript encoding the SHBG precursor that can be secreted. A conserved SP1-binding site in the corresponding rat Shbg promoter plays a key role in its expression in Sertoli cells, and it probably acts in the same way in the human SHBG promoter that lacks the USF1/2-binding site. In round spermatids and spermatocytes, the SHBG promoter sequence that is active in hepatocytes is silent. Instead, a promoter sequence flanking an alternative exon 1 sequence located approximately 2 kbp upstream is active and under the control of at least two cis-elements for key transcription factors (SPZ1 and CREB/CREM) that influence the expression of genes in these cells.
The sequences in the rat Shbg promoter that are essential for its expression in Sertoli cells and response to FSH are not well defined, but they likely include a sequence (GGGAGG) that markedly enhances its activity in Sertoli cells [99]. This sequence is perfectly conserved in the corresponding position in the human SHBG promoter [53], and it is a binding site for the ubiquitously expressed transcription factor, SP1 [100]. Because it plays a key role in regulating numerous genes in Sertoli cells, including the inhibin subunits [101], SP1 or its related family members likely act through this site to enhance transcription of the rat Shbg promoter in Sertoli cells, and the human SHBG promoter therefore responds the same way in these cells if the downstream USF-binding site is removed (Fig. 3).
Although SHBG transcripts encoding the SHBG precursor polypeptide are not present in the human testis [14], SHBG transcripts comprising an alternative exon 1 sequence can be detected in testis RNA extracts by Northern blot analysis [14] or PCR-based methods [102]. The major SHBG transcript in the human testis contains an alternative exon sequence located approximately 2 kbp upstream of the transcription unit responsible for SHBG mRNA in the liver. This alternative human SHBG transcript accumulates in germ cells of transgenic mice in a highly regulated manner throughout the spermatogenic cycle [85]; starting in round spermatids (stages IV–V) and peaking in preleptotene spermatocytes (stages VII–VII), it appears to encode an amino-terminally truncated SHBG isoform that accumulates in the outer acrosomal space of sperm [102, 103]. This sperm SHBG isoform has been characterized biochemically and appears to be the product of translation that initiates from the codon for Met30 in the mature human SHBG (Fig. 3), which is flanked by strong Kozak consensus sequence for translation initiation [103]. The function of the human sperm-specific SHBG isoform is unknown. However, it has the capacity to bind steroids, and its abundance in sperm declines with age and is positively correlated with sperm motility [103]. Information about the regulation of the alternative human SHBG transcription unit that is expressed in male germ cells is limited, but evidence suggests that it is controlled by several germ cell-enriched transcription factors, including SPZ1 and CREB/CREM transcription factor family members (Fig. 3), which are enriched in male germ cells during early stages of spermatogenesis [97].
These species differences in the cell-type expression of SHBG genes in the testis force us to reconsider the role of SHBG/ABP in relation to sperm development. Clearly, much of the information related to the control and function of the testicular SHBG/ABP in subprimate species [13] may not apply to the human situation.
SHBG IN REPRODUCTIVE TISSUES
The liver is the major source of plasma SHBG in mammals, but genes encoding SHBG are expressed at lower levels in numerous other tissues, including the brain, kidney, uterus, and prostate [86, 104–107]. However, studies relying on methods that amplify partial transcripts, which have not been fully characterized, or alternatively are spliced in ways that disrupt the open reading frame for the SHBG precursor, are difficult to interpret. Most importantly, many human SHBG transcripts contain alternative exon 1 sequences [14, 86], which lack the signal polypeptide sequences for secretion, and evidence that they are translated into a functional steroid-binding protein is tenuous. Another limitation to these types of studies is that tissue samples are invariably contaminated by plasma SHBG in their capillary beds, and there is no guarantee that SHBG from plasma sources does not nonspecifically associate with isolated cell types. Studies in transgenic mice that overexpress human SHBG transgenes circumvent these issues, because antibodies against human SHBG do not cross-react with murine proteins in immunohistochemical experiments—in other words, the tissues of wild-type mice are completely negative when studied in this way [85]. However, these transgenic mice lack the additional alternative exon 1 sequences recently identified [86], and it is possible that SHBG transcripts containing novel alternative exon 1 sequences encode functional proteins that are not secreted, as in human testicular germ cells and the acrosome [102, 103].
One of the remarkable observations made during studies of mice expressing human SHBG transgenes was the substantial accumulations of SHBG in the stromal tissues of some reproductive tissues, such as the uterus and epididymis, in which human SHBG mRNA was undetectable [31, 105]. Moreover, this mechanism appears to involve a highly specific, ligand-dependent interaction between plasma SHBG and the carboxy-terminal domains of two members of the fibulin family of extracellular matrix (ECM)-associated proteins—namely, fibulin 1D and fibulin 2 [31]. The physiological significance of the accumulation of plasma SHBG within the stromal ECM remains to be determined, but in the mouse uterus, it fluctuates during the estrous cycle and is accentuated when plasma estradiol levels are at their peak [31], suggesting that it may somehow modulate the actions of estrogens in a more direct manner.
In other tissues of transgenic mice that express human SHBG transgenes, immunoreactive human SHBG was also seen to accumulate inside specific epithelial cell types, and this was most apparent in specific segments of the proximal convoluted tubules of the kidney [85]. Recent studies have shown that a substantial proportion of the SHBG produced by these cells is retained within them and that this markedly accentuates the androgen-dependent regulation of genes, especially under conditions of androgen withdrawal [108].
Interactions between SHBG and sex steroid target cells have been the subject of numerous studies over the past several decades, the results of which have been interpreted as providing evidence that SHBG either participates in targeted delivery of its steroid ligands through endocytotic mechanisms [30] or that SHBG itself acts as a ligand for a plasma receptor with signaling potential [29]. Apart from the fact that a specific receptor for SHBG has never been identified, studies of SHBG interactions with the plasma membranes of specific cell types have been conducted under conditions where other plasma or extracellular proteins are absent, and the same holds true for studies of SHBG internalization by the nonspecific endocytotic receptor, megalin [30]. This is a serious limitation, because the abundance of SHBG in plasma and other biological fluids is substantially lower than those of most other proteins. Moreover, SHBG is structurally related to a number of relatively abundant secreted proteins that interact with plasma membrane proteins having signaling potential, as noted above. Thus, until plasma membrane proteins with high affinity and specificity for SHBG can be identified, the question of whether SHBG acts directly on target cells to exert physiologically important actions will remain contentious.
INSIGHT REGARDING SHBG FUNCTION FROM OTHER SPECIES
Fish are the most ancient class of vertebrates, and their shbg coding sequences are poorly conserved, with only approximately 30% sequence identity at the protein level when compared to those in mammals. Several genomewide duplication events occurred during early vertebrate evolution, and fish species retain duplicated copies of many genes. Zebrafish and most other teleosts have a single shbg gene that is expressed predominantly in the liver [109, 110], but the salmonid genomes have retained a functional shbg paralog (shbgb) that encodes a protein with only approximately 25% sequence similarity to the Shbg in salmonids or other fish [16, 17]. Interestingly, the two salmonid Shbg paralogs are distinct both in terms of their steroid-binding properties and their sites of production, and this provides an opportunity to examine the potential functions of SHBG that have been conserved or lost during the evolution of vertebrates.
As in mammals, the steroid-binding specificity of Shbg in fish varies between species: For example, in zebrafish [109] and salmonids [17], it exhibits a preference for androgens over estrogens, whereas it binds estradiol with higher affinity than androgens in European sea bass [110]. However, fish Shbg differs from the mammalian ortholog in one important respect: It binds the androgen precursor, androstenedione, as strongly as testosterone, and with greater affinity than 11-ketotestosterone, which is an important, biologically active androgen in fish [109, 110]. It also binds several anthropogenic xenobiotics [111], and Shbg in all fish studied to date is characterized by a particularly high affinity for the synthetic estrogen, ethinylestradiol [110, 112], which is a potent endocrine disruptor in aquatic species [113]. These observations suggest that Shbg has a specialized function in fish that has been preserved throughout evolution and that its presence in the gills provides an extraordinarily efficient portal for the uptake of trace amounts of natural steroids and other ligands, like ethinylestradiol, from the aquatic environment [112]. In addition to playing an important role in the transport and regulation of steroid access to target tissues in fish, it therefore appears that Shbg acts to control the loss of steroids from the gills or to provide a mechanism for the uptake of trace amounts of steroids from water. This latter role has not yet been fully explored, but sex steroids and their precursors and metabolites, such as androstenedione, act as pheromones in some fish [114]. Therefore, it is possible that the rapid sequestration of pheromones by Shbg in the gills represents a conserved function that remains critical for spawning behaviors and reproductive success in some fish species. If this is correct, this efficient Shbg-mediated portal to sequester steroid ligands from the aquatic environment might be breached by anthropogenic compounds with the capacity to disrupt reproduction or other important biological processes in fish.
Fish Shbg is expressed in the liver from the embryo stage to adulthood [109], but little is known about changes in the expression of shbg genes in fish in relation to reproduction. In the European sea bass liver, the shbg gene appears to respond primarily to changes in feeding habits and metabolic state, and its expression decreases during sexual maturation [115]. By analogy with what is known about the changes in human SHBG gene expression during sexual maturation and in response to changes in metabolic state, these physiologically important changes appear to be conserved throughout evolution. In addition to the liver, the shbg gene is expressed in both the developing [109] and adult gut, and immunoreactive Shbg appears to accumulate at high levels in specific types of skeletal muscle, although it is not expressed in this tissue [112]. These observations in fish are interesting, because they provide additional evidence that the functions of Shbg extend beyond that of a simple steroid transport protein. In the salmonids for which the shbgb paralog is highly expressed in several tissues (ovary, gill, and muscle) other than the liver [16, 17], these additional functions may be even more complex, and studies of Shbg in relation to the life cycle of these fish may reveal some unexpected ways in which SHBG functions to control the actions of sex steroids during reproduction.
ACKNOWLEDGMENTS
I would like to thank my collaborators and members of my laboratory—past and present—for their contributions to the findings that have shaped the ideas I have expressed in this review, and I thank Laura Houghton for helping to prepare and edit the manuscript.
Footnotes
Supported by operating grants from the Canadian Institutes of Health Research (MOP-15261), the National Science and Engineering Research Council of Canada, and the Canada Foundation for Innovation. G.L.H. is holder of the Tier 1 Canada Research Chair in Reproductive Health.
REFERENCES
- Wilson JD, Griffin JE, George FW. Sexual differentiation: early hormone synthesis and action. Biol Reprod 1980; 22: 9 17. [DOI] [PubMed] [Google Scholar]
- Ballard PL. Hormonal control of lung maturation. Baillieres Clin Endocrinol Metab 1989; 3: 723 753. [DOI] [PubMed] [Google Scholar]
- Bardin CW, Catterall JF. Testosterone: a major determinant of extragenital sexual dimorphism. Science 1981; 211: 1285 1294. [DOI] [PubMed] [Google Scholar]
- Nada SE, Thompson RC, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone excess on insulin target tissues. Endocrinology 2010; 151: 5165 5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott DH, Bruns CR, Barnett DK, Dunaif A, Goodfriend TL, Dumesic DA, Tarantal AF. Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring. Am J Physiol Endocrinol Metab 2010; 299: E741 E751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev 2003; 24: 313 340. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, Abbott DH, Recabarren SE, Herkimer C. Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology 2010; 151: 595 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K, Hassett JM. Sexual differentiation of behavior in monkeys: role of prenatal hormones. J Neuroendocrinol 2009; 21: 421 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal U. Steroid-protein interactions II. Monogr Endocrinol 1986; 27: 198 301. [PubMed] [Google Scholar]
- Baker ME. Albumin, steroid hormones and the origin of vertebrates. J Endocrinol 2002; 175: 121 127. [DOI] [PubMed] [Google Scholar]
- Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab 1981; 53: 58 68. [DOI] [PubMed] [Google Scholar]
- Hammond GL. Access of reproductive steroids to target tissues. Obstet Gynecol Clin North Am 2002; 29: 411 423. [DOI] [PubMed] [Google Scholar]
- Joseph DR. Structure, function, and regulation of androgen-binding protein/sex hormone-binding globulin. Vitam Horm 1994; 49: 197 280. [DOI] [PubMed] [Google Scholar]
- Hammond GL, Underhill DA, Rykse HM, Smith CL. The human sex hormone-binding globulin gene contains exons for androgen-binding protein and two other testicular messenger RNAs. Mol Endocrinol 1989; 3: 1869 1876. [DOI] [PubMed] [Google Scholar]
- Joseph DR, Hall SH, Conti M, French FS. The gene structure of rat androgen-binding protein: identification of potential regulatory deoxyribonucleic acid elements of a follicle-stimulating hormone-regulated protein. Mol Endocrinol 1988; 2: 3 13. [DOI] [PubMed] [Google Scholar]
- Bobe J, Mahe S, Nguyen T, Rime H, Vizziano D, Fostier A, Guiguen Y. A novel, functional, and highly divergent sex hormone-binding globulin that may participate in the local control of ovarian functions in salmonids. Endocrinology 2008; 149: 2980 2989. [DOI] [PubMed] [Google Scholar]
- Miguel-Queralt S, Underhill C, Devlin RH, Hammond GL. Characterization and measurement of the plasma alpha- and beta-sex hormone-binding globulin paralogs in salmon. Endocrinology 2009; 150: 366 375. [DOI] [PubMed] [Google Scholar]
- Joseph DR, Baker ME. Sex hormone-binding globulin, androgen-binding protein, and vitamin K-dependent protein S are homologous to laminin A, merosin, and Drosophila crumbs protein. FASEB J 1992; 6: 2477 2481. [DOI] [PubMed] [Google Scholar]
- Grishkovskaya I, Avvakumov GV, Sklenar G, Dales D, Hammond GL, Muller YA. Crystal structure of human sex hormone-binding globulin: steroid transport by a laminin G-like domain. EMBO J 2000; 19: 504 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishkovskaya I, Avvakumov GV, Hammond GL, Catalano MG, Muller YA. Steroid ligands bind human sex hormone-binding globulin in specific orientations and produce distinct changes in protein conformation. J Biol Chem 2002; 277: 32086 32093. [DOI] [PubMed] [Google Scholar]
- Avvakumov GV, Grishkovskaya I, Muller YA, Hammond GL. Crystal structure of human sex hormone-binding globulin in complex with 2-methoxyestradiol reveals the molecular basis for high affinity interactions with C-2 derivatives of estradiol. J Biol Chem 2002; 277: 45219 45225. [DOI] [PubMed] [Google Scholar]
- Grishkovskaya I, Avvakumov GV, Hammond GL, Muller YA. Resolution of a disordered region at the entrance of the human sex hormone-binding globulin steroid-binding site. J Mol Biol 2002; 318: 621 626. [DOI] [PubMed] [Google Scholar]
- Avvakumov GV, Cherkasov A, Muller YA, Hammond GL. Structural analyses of sex hormone-binding globulin reveal novel ligands and function. Mol Cell Endocrinol 2010; 316: 13 23. [DOI] [PubMed] [Google Scholar]
- Avvakumov GV, Muller YA, Hammond GL. Steroid-binding specificity of human sex hormone-binding globulin is influenced by occupancy of a zinc-binding site. J Biol Chem 2000; 275: 25920 25925. [DOI] [PubMed] [Google Scholar]
- Bocchinfuso WP, Hammond GL. Steroid-binding and dimerization domains of human sex hormone-binding globulin partially overlap: steroids and Ca2+ stabilize dimer formation. Biochemistry 1994; 33: 10622 10629. [DOI] [PubMed] [Google Scholar]
- Evenas P, Dahlback B, Garcia de Frutos P. The first laminin G-type domain in the SHBG-like region of protein S contains residues essential for activation of the receptor tyrosine kinase sky. Biol Chem 2000; 381: 199 209. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Nagata K, Ohashi K, Nakano T, Arita H, Mizuno K. Roles of gamma-carboxylation and a sex hormone-binding globulin-like domain in receptor-binding and in biological activities of Gas6. FEBS Lett 1997; 408: 306 310. [DOI] [PubMed] [Google Scholar]
- Rudenko G, Hohenester E, Muller YA. LG/LNS domains: multiple functions—one business end? Trends Biochem Sci 2001; 26: 363 368. [DOI] [PubMed] [Google Scholar]
- Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA. Sex hormone-binding globulin mediates steroid hormone signal transduction at the plasma membrane. J Steroid Biochem Mol Biol 1999; 69: 481 485. [DOI] [PubMed] [Google Scholar]
- Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, Metzger J, Schweigert FJ, Luppa PB, Nykjaer A, Willnow TE. Role of endocytosis in cellular uptake of sex steroids. Cell 2005; 122: 751 762. [DOI] [PubMed] [Google Scholar]
- Ng KM, Catalano MG, Pinos T, Selva DM, Avvakumov GV, Munell F, Hammond GL. Evidence that fibulin family members contribute to the steroid-dependent extravascular sequestration of sex hormone-binding globulin. J Biol Chem 2006; 281: 15853 15861. [DOI] [PubMed] [Google Scholar]
- Jänne M, Hogeveen KN, Deol HK, Hammond GL. Expression and regulation of human sex hormone-binding globulin transgenes in mice during development. Endocrinology 1999; 140: 4166 4174. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Petrusz P, Szpirer C, Joseph DR. Alternative processing of androgen-binding protein RNA transcripts in fetal rat liver. Identification of a transcript formed by trans splicing. J Biol Chem 1991; 266: 143 154. [PubMed] [Google Scholar]
- Becchis M, Sullivan PM, Ordronneau P, Petrusz P, Joseph DR. Distribution of immunoreactive androgen-binding protein/sex hormone-binding globulin in tissues of the fetal rat. Steroids 1996; 61: 392 400. [DOI] [PubMed] [Google Scholar]
- Majdic G, Saunders PT, Teerds KJ. Immunoexpression of the steroidogenic enzymes 3-beta hydroxysteroid dehydrogenase and 17alpha-hydroxylase, C17,20-lyase and the receptor for luteinizing hormone (LH) in the fetal rat testis suggests that the onset of Leydig cell steroid production is independent of LH action. Biol Reprod 1998; 58: 520 525. [DOI] [PubMed] [Google Scholar]
- Ng KM, So MT, Lee WM. Expression of rabbit sex hormone-binding globulin during pregnancy and prenatal development and identification of a novel isoform. Endocrinology 2005; 146: 1965 1972. [DOI] [PubMed] [Google Scholar]
- Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev 2009; 30: 883 925. [DOI] [PubMed] [Google Scholar]
- Abramovich DR, Towler CM, Bohn H. The binding of sex steroids in human maternal and fetal blood at different stages of gestation. J Steroid Biochem 1978; 9: 791 794. [DOI] [PubMed] [Google Scholar]
- Hammond GL, Leinonen P, Bolton NJ, Vihko R. Measurement of sex hormone binding globulin in human amniotic fluid: its relationship to protein and testosterone concentrations, and fetal sex. Clin Endocrinol (Oxf) 1983; 18: 377 384. [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci 2006; 9: 220 226. [DOI] [PubMed] [Google Scholar]
- Gabant P, Forrester L, Nichols J, Van Reeth T, De Mees C, Pajack B, Watt A, Smitz J, Alexandre H, Szpirer C, Szpirer J. Alpha-fetoprotein, the major fetal serum protein, is not essential for embryonic development but is required for female fertility. Proc Natl Acad Sci U S A 2002; 99: 12865 12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Martinez D, De Mees C, Douhard Q, Szpirer C, Bakker J. Absence of gonadotropin-releasing hormone 1 and Kiss1 activation in alpha-fetoprotein knockout mice: prenatal estrogens defeminize the potential to show preovulatory luteinizing hormone surges. Endocrinology 2008; 149: 2333 2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Pawluski JL, Brock O, Douhard Q, Bakker J. The alpha-fetoprotein knock-out mouse model suggests that parental behavior is sexually differentiated under the influence of prenatal estradiol. Horm Behav 2010; 57: 434 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DC. Sex-hormone-binding globulin. Clin Endocrinol (Oxf) 1974; 3: 69 96. [DOI] [PubMed] [Google Scholar]
- Kouretas D, Laliotis V, Taitzoglou I, Georgellis A, Tsantarliotou M, Mougios V, Amiridis G, Antonoglou O. Sex-hormone binding globulin from sheep serum: purification and effects of pregnancy and treatment with exogenous estradiol. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 1999; 123: 233 239. [DOI] [PubMed] [Google Scholar]
- Hogeveen KN, Cousin P, Pugeat M, Dewailly D, Soudan B, Hammond GL. Human sex hormone-binding globulin variants associated with hyperandrogenism and ovarian dysfunction. J Clin Invest 2002; 109: 973 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Boon WC, McInnes K, Maffei L, Carani C, Simpson ER. Recognizing rare disorders: aromatase deficiency. Nat Clin Pract Endocrinol Metab 2007; 3: 414 421. [DOI] [PubMed] [Google Scholar]
- Gunsalus GL, Musto NA, Bardin W. Immunoassay of androgen binding protein in blood: a new approach for study of the seminiferous tubule. Science 1978; 200: 65 66. [DOI] [PubMed] [Google Scholar]
- Joseph DR, Hall SH, French FS. Rat androgen-binding protein: evidence for identical subunits and amino acid sequence homology with human sex hormone-binding globulin. Proc Natl Acad Sci U S A 1987; 84: 339 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgorosky A, Rivarola MA. Progressive decrease in serum sex hormone-binding globulin from infancy to late prepuberty in boys. J Clin Endocrinol Metab 1986; 63: 510 512. [DOI] [PubMed] [Google Scholar]
- Belgorosky A, Rivarola MA. Progressive increase in nonsex hormone-binding globulin-bound testosterone and estradiol from infancy to late prepuberty in girls. J Clin Endocrinol Metab 1988; 67: 234 237. [DOI] [PubMed] [Google Scholar]
- Leger J, Forest MG, Czernichow P. Thyroid hormones influences sex steroid binding protein levels in infancy: study in congenital hypothyroidism. J Clin Endocrinol Metab 1990; 71: 1147 1150. [DOI] [PubMed] [Google Scholar]
- Jänne M, Hammond GL. Hepatocyte nuclear factor-4 controls transcription from a TATA-less human sex hormone-binding globulin gene promoter. J Biol Chem 1998; 273: 34105 34114. [DOI] [PubMed] [Google Scholar]
- Selva DM, Hogeveen KN, Innis SM, Hammond GL. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest 2007; 117: 3979 3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selva DM, Hammond GL. Thyroid hormones act indirectly to increase sex hormone-binding globulin production by liver via hepatocyte nuclear factor-4alpha. J Mol Endocrinol 2009; 43: 19 27. [DOI] [PubMed] [Google Scholar]
- von Schoultz B, Carlstrom K. On the regulation of sex-hormone-binding globulin—a challenge of an old dogma and outlines of an alternative mechanism. J Steroid Biochem 1989; 32: 327 334. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, O'Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 2006; 20: 2613 2629. [DOI] [PubMed] [Google Scholar]
- Lichanska AM, Waters MJ. How growth hormone controls growth, obesity and sexual dimorphism. Trends Genet 2008; 24: 41 47. [DOI] [PubMed] [Google Scholar]
- Park SH, Wiwi CA, Waxman DJ. Signaling cross-talk between hepatocyte nuclear factor 4alpha and growth-hormone-activated STAT5b. Biochem J 2006; 397: 159 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiwi CA, Gupte M, Waxman DJ. Sexually dimorphic P450 gene expression in liver-specific hepatocyte nuclear factor 4alpha-deficient mice. Mol Endocrinol 2004; 18: 1975 1987. [DOI] [PubMed] [Google Scholar]
- Jover R, Moya M, Gomez-Lechon MJ. Transcriptional regulation of cytochrome p450 genes by the nuclear receptor hepatocyte nuclear factor 4-alpha. Curr Drug Metab 2009; 10: 508 519. [DOI] [PubMed] [Google Scholar]
- Pugeat M, Nader N, Hogeveen K, Raverot G, Dechaud H, Grenot C. Sex hormone-binding globulin gene expression in the liver: drugs and the metabolic syndrome. Mol Cell Endocrinol 2010; 316: 53 59. [DOI] [PubMed] [Google Scholar]
- Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction 2010; 140: 399 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics 2008; 121 (suppl 3): S208 S217. [DOI] [PubMed] [Google Scholar]
- Potau N, Ibanez L, Rique S, Sanchez-Ufarte C, de Zegher F. Pronounced adrenarche and precocious pubarche in boys. Horm Res 1999; 51: 238 241. [DOI] [PubMed] [Google Scholar]
- Frisch RE. The right weight: body fat, menarche and ovulation. Baillieres Clin Obstet Gynaecol 1990; 4: 419 439. [DOI] [PubMed] [Google Scholar]
- Estour B, Pugeat M, Lang F, Dechaud H, Pellet J, Rousset H. Sex hormone binding globulin in women with anorexia nervosa. Clin Endocrinol (Oxf) 1986; 24: 571 576. [DOI] [PubMed] [Google Scholar]
- Pasquali R. Obesity, fat distribution and infertility. Maturitas 2006; 54: 363 371. [DOI] [PubMed] [Google Scholar]
- Grimmichova T, Vrbikova J, Matucha P, Vondra K, Veldhuis PP, Johnson ML. Fasting insulin pulsatile secretion in lean women with polycystic ovary syndrome. Physiol Res 2008; 57 (suppl 1): S91 S98. [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, Buring JE, Gaziano JM, Liu S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 2009; 361: 1152 1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Folkerd E, Doody D, Schroen C, Treloar SA, Giles GG, Pike MC, English DR, Southey MC, Hopper JL, Dowsett M. Familial correlations in postmenopausal serum concentrations of sex steroid hormones and other mitogens: a twins and sisters study. J Clin Endocrinol Metab 2009; 94: 4793 4800. [DOI] [PubMed] [Google Scholar]
- Mousavinasab F, Tahtinen T, Jokelainen J, Koskela P, Vanhala M, Oikarinen J, Laakso M, Keinanen-Kiukaanniemi S. The Pro12Ala polymorphism of the PPAR gamma 2 gene influences sex hormone-binding globulin level and its relationship to the development of the metabolic syndrome in young Finnish men. Endocrine 2006; 30: 185 190. [DOI] [PubMed] [Google Scholar]
- Selva DM, Hammond GL. Peroxisome-proliferator receptor gamma represses hepatic sex hormone-binding globulin expression. Endocrinology 2009; 150: 2183 2189. [DOI] [PubMed] [Google Scholar]
- Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med 2001; 345: 971 980. [DOI] [PubMed] [Google Scholar]
- Power SG, Bocchinfuso WP, Pallesen M, Warmels-Rodenhiser S, Van Baelen H, Hammond GL. Molecular analyses of a human sex hormone-binding globulin variant: evidence for an additional carbohydrate chain. J Clin Endocrinol Metab 1992; 75: 1066 1070. [DOI] [PubMed] [Google Scholar]
- Cousin P, Dechaud H, Grenot C, Lejeune H, Pugeat M. Human variant sex hormone-binding globulin (SHBG) with an additional carbohydrate chain has a reduced clearance rate in rabbit. J Clin Endocrinol Metab 1998; 83: 235 240. [DOI] [PubMed] [Google Scholar]
- Cui Y, Shu XO, Cai Q, Jin F, Cheng JR, Cai H, Gao YT, Zheng W. Association of breast cancer risk with a common functional polymorphism (Asp327Asn) in the sex hormone-binding globulin gene. Cancer Epidemiol Biomarkers Prev 2005; 14: 1096 1101. [DOI] [PubMed] [Google Scholar]
- Ahn J, Schumacher FR, Berndt SI, Pfeiffer R, Albanes D, Andriole GL, Ardanaz E, Boeing H, Bueno-de-Mesquita B, Chanock SJ, Clavel-Chapelon F, Diver WR, et al. Quantitative trait loci predicting circulating sex steroid hormones in men from the NCI-Breast and Prostate Cancer Cohort Consortium (BPC3). Hum Mol Genet 2009; 18: 3749 3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson AL, Lorentzon M, Mellstrom D, Vandenput L, Swanson C, Andersson N, Hammond GL, Jakobsson J, Rane A, Orwoll ES, Ljunggren O, Johnell O, et al. SHBG gene promoter polymorphisms in men are associated with serum sex hormone-binding globulin, androgen and androgen metabolite levels, and hip bone mineral density. J Clin Endocrinol Metab 2006; 91: 5029 5037. [DOI] [PubMed] [Google Scholar]
- Xita N, Tsatsoulis A. Genetic variants of sex hormone-binding globulin and their biological consequences. Mol Cell Endocrinol 2010; 316: 60 65. [DOI] [PubMed] [Google Scholar]
- Hogeveen KN, Talikka M, Hammond GL. Human sex hormone-binding globulin promoter activity is influenced by a (TAAAA)n repeat element within an Alu sequence. J Biol Chem 2001; 276: 36383 36390. [DOI] [PubMed] [Google Scholar]
- Hammond GL, Langley MS, Robinson PA, Nummi S, Lund L. Serum steroid binding protein concentrations, distribution of progestogens, and bioavailability of testosterone during treatment with contraceptives containing desogestrel or levonorgestrel. Fertil Steril 1984; 42: 44 51. [DOI] [PubMed] [Google Scholar]
- Wiegratz I, Kuhl H. Managing cutaneous manifestations of hyperandrogenic disorders: the role of oral contraceptives. Treat Endocrinol 2002; 1: 372 386. [PubMed] [Google Scholar]
- Hammond GL, Hogeveen KN, Visser M. Coelingh Bennink HJ. Estetrol does not bind sex hormone binding globulin or increase its production by human HepG2 cells. Climacteric 2008; 11 (suppl 1): 41 46. [DOI] [PubMed] [Google Scholar]
- Jänne M, Deol HK, Power SG, Yee SP, Hammond GL. Human sex hormone-binding globulin gene expression in transgenic mice. Mol Endocrinol 1998; 12: 123 136. [DOI] [PubMed] [Google Scholar]
- Pinos T, Barbosa-Desongles A, Hurtado A, Santamaria-Martinez A, de Torres I, Morote J, Reventos J, Munell F. Identification, characterization and expression of novel sex hormone binding globulin alternative first exons in the human prostate. BMC Mol Biol 2009; 10: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 2005; 90: 3847 3853. [DOI] [PubMed] [Google Scholar]
- Carlstrom K, Eriksson A, Gustafsson SA, Henriksson P, Pousette A, Stege R, von Schoultz B. Influence of orchidectomy or estrogen treatment on serum levels of pregnancy associated alpha 2-glycoprotein and sex hormone binding globulin in patients with prostatic cancer. Int J Androl 1985; 8: 21 27. [DOI] [PubMed] [Google Scholar]
- Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab 2005; 90: 2618 2623. [DOI] [PubMed] [Google Scholar]
- Gerard H, Gerard A. En Nya A, Felden F, Gueant JL. Spermatogenic cells do internalize Sertoli androgen-binding protein: a transmission electron microscopy autoradiographic study in the rat. Endocrinology 1994; 134: 1515 1527. [DOI] [PubMed] [Google Scholar]
- Jeyaraj DA, Grossman G, Weaver C, Petrusz P. Dynamics of testicular germ cell proliferation in normal mice and transgenic mice overexpressing rat androgen-binding protein: a flow cytometric evaluation. Biol Reprod 2002; 66: 877 885. [DOI] [PubMed] [Google Scholar]
- Pelliniemi LJ, Dym M, Gunsalus GL, Musto NA, Bardin CW, Fawcett DW. Immunocytochemical localization of androgen-binding protein in the male rat reproductive tract. Endocrinology 1981; 108: 925 931. [DOI] [PubMed] [Google Scholar]
- Hermo L, Barin K, Oko R. Androgen binding protein secretion and endocytosis by principal cells in the adult rat epididymis and during postnatal development. J Androl 1998; 19: 527 541. [PubMed] [Google Scholar]
- Vigersky RA, Loriaux DL, Howards SS, Hodgen GB, Lipsett MB, Chrambach A. Androgen binding proteins of testis, epididymis, and plasma in man and monkey. J Clin Invest 1976; 58: 1061 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiemma V, Rosati P, Guerzoni C, Mariani S, Beligotti F, Magnanti M, Garufi G, Galoni T, Fabbrini A. Human Sertoli cells in vitro: morphological features and androgen-binding protein secretion. J Steroid Biochem Mol Biol 1992; 43: 423 429. [DOI] [PubMed] [Google Scholar]
- Hammond GL, Bocchinfuso WP. Sex hormone-binding globulin: gene organization and structure/function analyses. Horm Res 1996; 45: 197 201. [DOI] [PubMed] [Google Scholar]
- Selva DM, Hammond GL. Human sex hormone-binding globulin is expressed in testicular germ cells and not in Sertoli cells. Horm Metab Res 2006; 38: 230 235. [DOI] [PubMed] [Google Scholar]
- Selva DM, Hogeveen KN, Hammond GL. Repression of the human sex hormone-binding globulin gene in Sertoli cells by upstream stimulatory transcription factors. J Biol Chem 2005; 280: 4462 4468. [DOI] [PubMed] [Google Scholar]
- Fenstermacher DA, Joseph DR. Analysis of promoter and androgen regulatory sequences required for optimal transcription of the rat androgen-binding protein gene. J Androl 1998; 19: 81 91. [PubMed] [Google Scholar]
- Jänne M. Regulation of human sex hormone-binding globulin gene (shbg) expression. Saarijärvi, Finland: University of Helsinki; 2000. Dissertation. [Google Scholar]
- Walton KL, Makanji Y, Robertson DM, Harrison CA. The synthesis and secretion of inhibins. Vitam Horm 2011; 85: 149 184. [DOI] [PubMed] [Google Scholar]
- Selva DM, Hogeveen KN, Seguchi K, Tekpetey F, Hammond GL. A human sex hormone-binding globulin isoform accumulates in the acrosome during spermatogenesis. J Biol Chem 2002; 277: 45291 45298. [DOI] [PubMed] [Google Scholar]
- Selva DM, Bassas L, Munell F, Mata A, Tekpetey F, Lewis JG, Hammond GL. Human sperm sex hormone-binding globulin isoform: characterization and measurement by time-resolved fluorescence immunoassay. J Clin Endocrinol Metab 2005; 90: 6275 6282. [DOI] [PubMed] [Google Scholar]
- Hryb DJ, Nakhla AM, Kahn SM, St George J, Levy NC, Romas NA, Rosner W. Sex hormone-binding globulin in the human prostate is locally synthesized and may act as an autocrine/paracrine effector. J Biol Chem 2002; 277: 26618 26622. [DOI] [PubMed] [Google Scholar]
- Joseph DR, Power SG, Petrusz P. Expression and distribution of androgen-binding protein/sex hormone-binding globulin in the female rodent reproductive system. Biol Reprod 1997; 56: 14 20. [DOI] [PubMed] [Google Scholar]
- Misao R, Nakanishi Y, Fujimoto J, Tamaya T. Expression of sex hormone-binding globulin exon VII splicing variant messenger ribonucleic acid in human ovarian endometriosis. Fertil Steril 1998; 69: 324 328. [DOI] [PubMed] [Google Scholar]
- Wang YM, Bayliss DA, Millhorn DE, Petrusz P, Joseph DR. The androgen-binding protein gene is expressed in male and female rat brain. Endocrinology 1990; 127: 3124 3130. [DOI] [PubMed] [Google Scholar]
- Hong EJ, Sahu B, Jänne OA, Hammond GL. Cytoplasmic accumulation of incompletely glycosylated SHBG enhances androgen action in proximal tubule epithelial cells. Mol Endocrinol 2011; 25: 269 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Queralt S, Knowlton M, Avvakumov GV, Al-Nouno R, Kelly GM, Hammond GL. Molecular and functional characterization of sex hormone binding globulin in zebrafish. Endocrinology 2004; 145: 5221 5230. [DOI] [PubMed] [Google Scholar]
- Miguel-Queralt S, Avvakumov GV, Blazquez M, Piferrer F, Hammond GL. Sea bass (Dicentrarchus labrax) sex hormone binding globulin: molecular and biochemical properties and phylogenetic comparison of its orthologues in multiple fish species. Mol Cell Endocrinol 2005; 229: 21 29. [DOI] [PubMed] [Google Scholar]
- Thorsteinson N, Ban F, Santos-Filho O, Tabaei SM, Miguel-Queralt S, Underhill C, Cherkasov A, Hammond GL. In silico identification of anthropogenic chemicals as ligands of zebrafish sex hormone binding globulin. Toxicol Appl Pharmacol 2009; 234: 47 57. [DOI] [PubMed] [Google Scholar]
- Miguel-Queralt S, Hammond GL. Sex hormone-binding globulin in fish gills is a portal for sex steroids breached by xenobiotics. Endocrinology 2008; 149: 4269 4275. [DOI] [PubMed] [Google Scholar]
- Sumpter JP. Xenoendocrine disrupters—environmental impacts. Toxicol Lett 1998; 102–103: 337 342. [DOI] [PubMed] [Google Scholar]
- Sorensen PW, Pinillos M, Scott AP. Sexually mature male goldfish release large quantities of androstenedione into the water where it functions as a pheromone. Gen Comp Endocrinol 2005; 140: 164 175. [DOI] [PubMed] [Google Scholar]
- Miguel-Queralt S, Blazquez M, Piferrer F, Hammond GL. Sex hormone-binding globulin expression in sea bass (Dicentrarchus labrax L.) throughout development and the reproductive season. Mol Cell Endocrinol 2007; 276: 55 62. [DOI] [PubMed] [Google Scholar]