Abstract
The pathophysiology of gestational hypertensive disorders is incompletely defined. T lymphocytes are implicated. Both T and natural killer (NK) cells express RAS and, in implantation sites, NK cells are highly enriched. We hypothesized that T cells and/or NK cells contribute to circulatory control during pregnancy. Using radiotelemetry of arterial pressure, heart rate, and activity, mice without T and B cells (genotypes BALB/c-Rag2−/− and NOD.scid) were examined at baseline and across pregnancy. These strains differ in NK cell competency, with Rag2−/− being normal and NOD.scid impaired. Circulatory features differed between these inbred strains. Rag2−/−; had blood pressure responses to pregnancy that did not differ from congenic normal mice. NOD.scid had higher midgestational blood pressure compared with normoglycemic NOD mice (3–5 mm Hg greater than NOD; P < 0.004). In comparison to controls, both T and B strains had much higher heart rates after first trimester that did not remit until parturition (>30 bpm greater than control; P < 0.0001). NOD.scid had additional anomalies, including 90% depletion of circulating NK cells and elevated (57%) proliferation of uterine NK cells within implantation sites. These data demonstrate immune control of midgestational heart rate and suggest NK cells contribute to midpregnancy regulation of mean arterial pressure.
Keywords: blood pressure monitoring, cardiovascular system, immunology, pregnancy, transgenic/knockout model
Both T and natural killer lymphocytes may have modulating roles on heart rate and blood pressure in murine pregnancy.
INTRODUCTION
Hypertension is a common complication of human pregnancy, typically manifesting as gestational hypertension or preeclampsia after the 20th wk of gestation. Though the pathophysiology of gestational hypertensive disorders is incompletely defined, these syndromes are associated with adverse maternal and fetal outcomes, including increased risk for later development of cardiovascular diseases [1, 2]. Strong evidence has linked gestational complications, cardiovascular disease, and immune perturbations [3, 4].
A unique lymphocyte subset, the uterine natural killer (uNK) cell, develops in abundance in decidualizing endometrium. These cells are terminally differentiated and generally accepted as necessary for normal human pregnancy [5]. Uterine NK cells secrete large amounts of cytokines and other molecules, including angiogenic factors, but are poorly cytotoxic. Chemokine receptors, cell adhesion molecules, and vascular addressins that promote uNK progenitor cell homing to decidualizing uterus have also been identified [6–9]. Normally, uNK cells are highly proliferative only within early decidua, reach peak numbers at midgestation (∼16–20 wk in humans [10]; Gestation Day (GD) 10 in mice [11]), then decline. Postulated functions of uNK cells are to promote decidual integrity, to monitor trophoblast invasion, and to participate in immune surveillance and in remodeling of maternal spiral arteries (SA), the principal vessels that feed into each placenta [11–13].
We hypothesized that NK cells contribute to circulatory regulation during mouse pregnancy and used mutant strains and continuous, chronic radiotelemetry to address gestational blood pressure regulation. Two inbred mouse models with absent adaptive immunity (i.e., T− and B−) were examined, BALB/c-Rag2−/− (Rag2−/−) and NOD.scid, and compared to their respective congenic control strains (BALB/c and NOD). Both strains have abnormalities in somatic recombination of the Variable, Diverse, and Joining segments required for normal production of T and B cells. The recombination of these gene segments in immature T and B cells is critical for cell maturation and antigenic specificity. The Rag2−/− mouse (knockout of the recombinase activating gene 2) does not have normal V(D)J rearrangement during T and B cell maturation. In these mice only immature, nonfunctional T and B cell precursor cells circulate [14, 15]. In contrast, scid is a spontaneous mutation in a DNA protein kinase that maps to chromosome 16 and promotes V(D)J recombination, but not to completion [16]. Scid mutants also lack functional T and B cells [16]. Rag2−/− mice have normal NK cell functions, whereas the NOD strain is characterized as having deficits in NK cell and macrophage functions and impaired complement activation [17–19]. Recently, Suwanai et al. [20] attributed the functional deficit in NOD NK cells to allelic underexpression (∼50%) of Il15, a cytokine critical to NK and uNK cell survival [21]. In our previous, midgestation study of NOD.scid females as nondiabetic controls for pregnant, diabetic (i.e., hyperglycemic) NOD mice, we reported that uNK cell numbers and SA modification in NOD.scid were equivalent to those in nondiabetic (i.e., normoglycemic) NOD mice [22]. However, SA remodeling was anomalous in both NOD background strains.
Using chronic radiotelemetry for data collection, we identified a dynamic, 5-phase pattern of gestational mean arterial pressure (MAP) in inbred (C57BL/6; BALB/c [23] and NOD, unpublished data) and randombred (CD1; Barrette and Croy, unpublished data) mice. During the preimplantation period (GD 0–5), there are no detectable hemodynamic alterations. Immediately postimplantation, MAP declines while heart rate (HR) increases until approximately midgestation (GD 9–10). MAP then increases to prepregnancy baseline levels at GD 14 and remains stable until term as HR declines back to baseline. This pattern appears to be linked to specific changes in placental development; however, the regulatory steps initiating the drop and subsequent gain in gestational MAP are not defined and could be due to maternal effects such as decidualization or decidual cytokine production [24–26]. In the current study, we hypothesized that the pregnant Rag2−/− females would have normal circulatory regulation due to normally functioning NK/uNK cells, whereas pregnant NOD.scid females would have increased blood pressure due to their deficit in NK cell function.
MATERIALS AND METHODS
Animals
C.129S6(B6)-Rag2tm1Fwa (Rag2−/−) and BALB/CAnNTac (BALB/c) mice were purchased from Taconic Farms (Germantown, NY). NOD/ShiLtJ (NOD) and NOD.CB17-Prkdcscid/J (NOD.scid) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in a specific pathogen-free facility, provided with autoclaved water and mouse chow ad libitum. All animals in these studies were bred and used as age and GD-matched. The morning of copulation plug detection was called GD 0. All procedures were approved by Queen's University Animal Care Committee.
Radiotelemetry
10-wk-old female mice were surgically implanted with TA11PA-C10 radiotransmitters (Data Sciences International [DSI], St Paul, MN) via the common carotid under isoflurane anesthesia as previously described [23]. Following 10 days of recovery and return to normal circadian rhythm, baseline hemodynamic recordings (minimum 3 days) were collected. Recording was stopped and females were mated with syngeneic males. On GD 0, males were removed and recording restarted. Data were collected every 4 min for 30 sec continuously throughout pregnancy, including MAP, systolic arterial pressure (SAP), diastolic arterial pressure (DAP), HR, pulse pressure (PP), and activity using Dataquest A.R.T. Acquisition System (DSI, version 4.1). Following parturition, neonates were removed and weighed. Recording was stopped, and females were rebred for morphometric study at GD 12.
Hemodynamic data from each animal were averaged each 24 h. It has been previously determined that no hemodynamic changes are observed during the first 5 gestational days; a mean of GD 0–3 is calculated and subsequent data points are subtracted from this mean to obtain normalized data [23]. Also, to directly compare pregnant Rag2−/− and NOD.scid females to their wild-type strains and to each other, BALB/c and NOD gestational data (respectively) were normalized to zero, permitting analysis of statistical deviations above and below wild-type data.
Histology and Morphometry
The transmitter-implanted Rag2−/− (n = 3) and NOD.scid females (n = 3) were euthanized at GD 12 of second pregnancy for morphometric analysis. Rag2−/− and NOD.scid were anesthetized with tribromoethanol (250 mg/kg). Uteri were dissected and fixed in 4% (wt/vol) neutral buffered paraformaldehyde in 0.1 M phosphate buffer. Tissues were washed in 70% (vol/vol) ethanol and then processed into paraffin and embedded [27]. Implantation sites were serially sectioned at 7 μm and alternatively stained with hematoxylin and eosin or periodic acid-Schiff (PAS). Eight sections were scored from three implantation sites in each of three Rag2−/− and NOD.scid females for uNK cell numbers and for spiral arterial structure. Sections were from the center of each implantation site and 42 μm apart to prevent duplicate counting of uNK cells. The center of the implantation site was identified by the presence of mesentery containing radial branches from the uterine artery and of the mesometrial lymphoid aggregate of pregnancy (MLAp), a transient lymphoid structure that develops between the uterine wall muscles and is centered above each placenta.
PAS+ lymphoid cells with visible nuclei were enumerated as uNK cells. A minimum of 100 cells was counted per section in the MLAp and in the decidua basalis under 400× magnification using a 1-mm2 ocular grid [28]. All image analyses were performed using an AxioImager M.1 microscope and Axiovision software (Carl Zeiss, Oberkochen, Germany).
Three SA were measured per cross section. Lumen diameters (LD) were derived from the circumference (C) measurements of each vessel's cross section (LD = C/π). Wall thickness was then determined by (wall diameter − LD)/2. Measurements were made using ImagePro Software (Media Cybernetics, Inc., Bethesda, MD).
To evaluate uNK cell proliferation, sections from three viable implantation sites from each of three Rag2−/− and NOD.scid dams were reacted with mouse proliferating cell nuclear antigen (anti-PCNA; clone PC10; Abcam, Cambridge, MA) as previously described [29]. Slides were counterstained with PAS to identify uNK cells and counted as above. A proliferation index ([proliferating uNK cells/total uNK cells] × 100) was calculated.
Flow Cytometry, Organ Weight Measurements, and Ifng ELISA
Virgin and mated, noninstrumented female Rag2−/− (n = 3–5 per group) and NOD.scid mice (n = 3–5 per group) were used to assess peripheral blood leukocytes. Mice were anesthetized as above and euthanized via cardiac puncture, and their blood was immediately transferred into ACD anticoagulant (BD Biosciences, Mississauga, ON), mixed, and diluted 1:1 in sterile PBS. This was layered onto Lympholyte M (Cedarlane Laboratories, Burlington, ON), and lymphocytes were separated according to manufacturer's directions. Lymphocytes were counted and assessed for viability using trypan blue dye exclusion. Aliquots of lymphocytes in 2% (wt/vol) bovine serum albumin (BSA)/PBS were added to rat α-mouse FITC-NKp46 (CD335, clone 29A1.4; BD Pharmingen) and Armenian hamster α-mouse PE-Cy5-CD3ε (clone 145-2C11; eBioscience, San Diego, CA) and incubated on ice for 30 min. Rat α-mouse FITC-DX5 (CD49d) and rat α-mouse PE-CD122 (clone 5H4) were also substituted for NKp46 to confirm NK cell findings (eBioscience). Cells were washed in 1% (wt/vol) BSA/PBS and resuspended in buffer. Antibodies were previously titred using peripheral blood lymphocytes from immune-competent mice. Each acquisition included an unstained sample and appropriate isotype controls. Events (50 000 events per sample) were acquired on a Beckman Coulter FC500 Cytometer (Beckman Coulter, Mississauga, ON). Compensation and data analysis were completed using FlowJo software (Tree Star Inc., Ashland, OR).
Organs from the above, noninstrumented virgin and GD 12 Rag2−/− and NOD.scid females were dissected. Hearts were dissected, trimmed from adipose and connective tissue, and weighed. The atria and ventricles were separated and weighed. Kidneys and whole uterus (with ovaries and mesentery removed) were weighed. For pregnant females, placentas and deciduas were dissected from each implantation site and separately weighed.
Tissue homogenates were prepared from the uterus for quantification of interferon gamma (Ifng). For nonpregnant females, the mesometrial side of the uterus was collected. For GD 12 mice, MLAp and decidua basalis were dissected as separate tissues and pooled by litter. Samples were placed into Eppendorf tubes containing 100 μl RPMI medium supplemented with 10% (vol/vol) fetal bovine serum, immediately disrupted using a Kontes micropestle (Fisher Scientific, Mississauga, ON), then centrifuged at 800 × g (5 min, 4°C). The supernatant was collected and frozen at −20°C until assayed. The high-sensitivity Ifng mouse ELISA (eBioscience) was performed according to the manufacturer's directions. Samples were run in duplicate. The standard curve gave an r2 = 0.99.
Statistics
Data were analyzed using Prism 4.03 Statistical Software (GraphPad, San Diego, CA) and are presented as means ± SEM. Hemodynamic data (between and within group) were analyzed using two-way repeated measures ANOVA followed by Dunnett multiple comparison test. Baseline hemodynamics (between strain), flow cytometric data, and uNK cell proliferation were analyzed using one-way ANOVA followed by Bonferroni's post hoc test. All other data were compared between groups using unpaired two-tailed t-tests. When variances were unequal, Welch's correction was used. P < 0.05 was considered significant.
RESULTS
Hemodynamics in Virgin Rag2−/− and NOD.scid Females
Prepregnancy baseline hemodynamic data for the two T and B cell-deficient strains were compared with female data from their appropriate congenic strains [23] (Table 1). Rag2−/− females had a higher MAP than wild type (P < 0.02). This was not associated with a lower Rag2−/− HR; both BALB/c genotypes had equivalent HR, PP, and activity levels. NOD.scid females had significantly lower MAP and SAP (P < 0.01) compared with nondiabetic NOD females, whereas DAP, HR, PP, and activity levels were equivalent.
TABLE 1.
Baseline hemodynamic parameters of BALB/c (n = 7), BALB/c-Rag2−/− (n = 9), normoglycemic NOD (n = 10) and NOD.scid (n = 9) mice.*
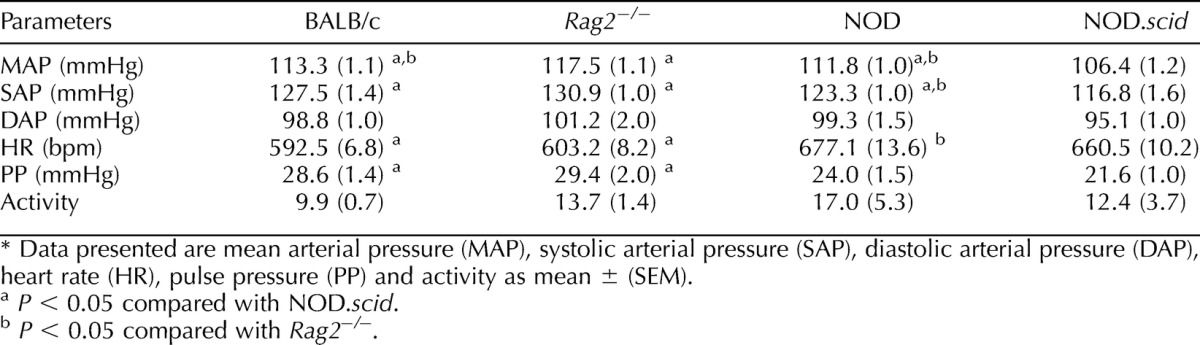
The impact of the combined T and B cell deficits was not equivalent for these two inbred backgrounds. Rag2−/− females had significantly higher pressures than NOD.scid females (MAP, SAP, and P; all P < 0.001) except for DAP (P = 0.06), and Rag2−/− HR was significantly lower (P < 0.005). The level of activity in Rag2−/− virgin females was similar to virgin NOD.scid females.
Hemodynamic Changes During Gestation
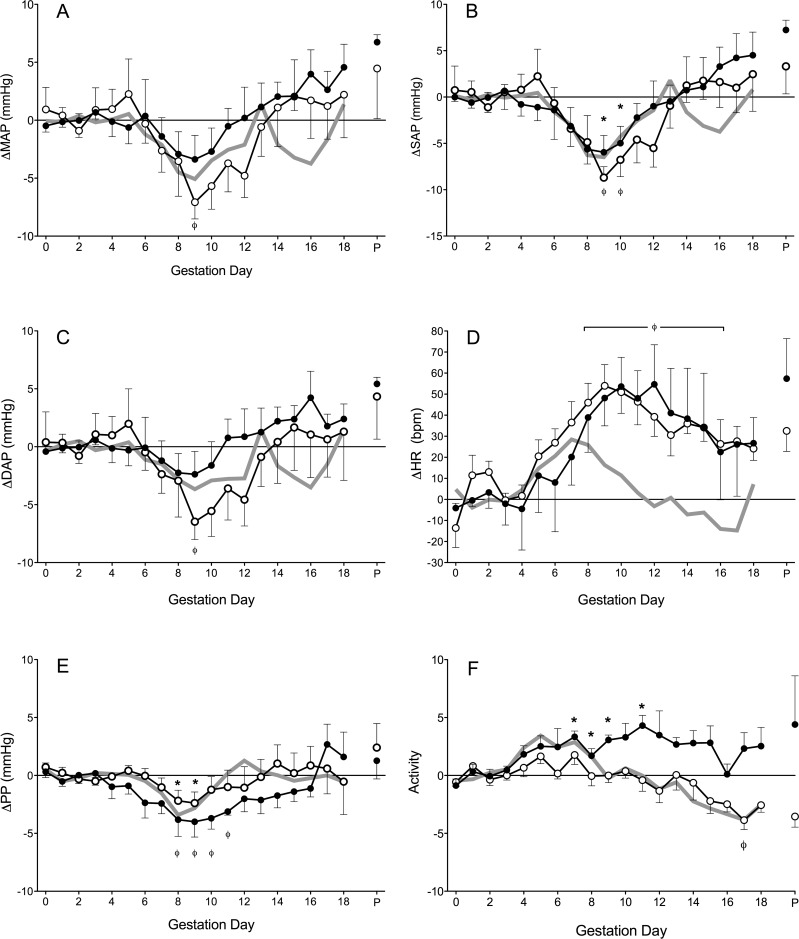
As previously reported, the baseline hemodynamic parameters of virgin mice are not altered significantly during preimplantation stages of pregnancy [23]. This finding was confirmed for both Rag2−/− and NOD.scid females. Thus, a GD 0–3 mean was calculated, and changes from this are illustrated over the remainder of pregnancy (Fig. 1). For pregnant Rag2−/− mice, MAP, SAP, and DAP followed the five-phase pattern we have observed for normal murine pregnancy (Fig. 1, A–C). Specifically, from GD 0–5 no changes were observed, whereas a significant decline in MAP, SAP, and DAP followed until GD 9. Subsequently, pressures increased to the Rag2−/− prepregnancy baseline at ∼GD 14, followed by small variations until parturition. There were no statistical differences between the Rag2−/− blood pressure patterns and BALB/c wild-type across gestation (Fig. 2, A–C).
FIG. 1.
Hemodynamics of Rag2−/− (n = 6, white circles), NOD.scid (n = 5, black circles), and wild-type (gray line, composite of previously published data) mice throughout pregnancy. A) Change in MAP (ΔMAP). B) Change in SAP (ΔSAP). C) Change in DAP (ΔDAP). D) Change in HR (ΔHR). E) Change in PP (ΔPP). F) Change in activity. Data is averaged over a 24-h period, normalized to GD 0–3 baseline and presented as mean ± SEM. *P < 0.05 compared to within strain baseline, ΦP < 0.01 compared to within strain baseline.
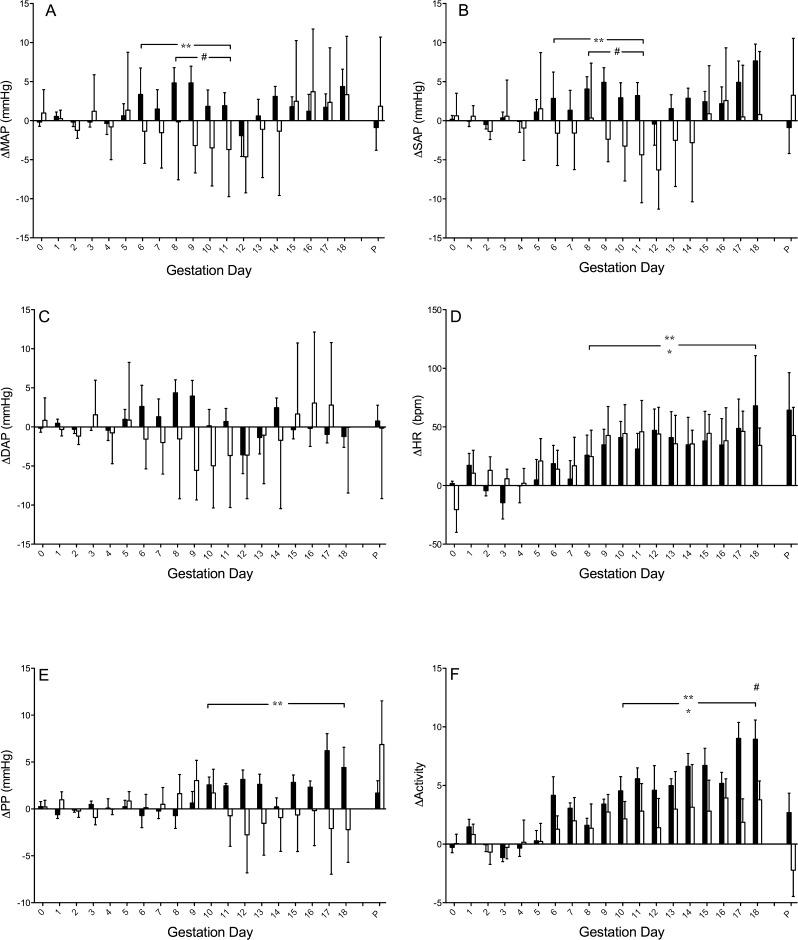
FIG. 2.
Gestational hemodynamics of Rag2−/− (n = 6, white bars) and NOD.scid (n = 5, black bars) relative to wild-type controls (BALB/c, n = 4; and NOD, n = 6; respectively). A) Change in MAP (ΔMAP). B) Change in SAP (ΔSAP). C) Change in DAP (ΔDAP). D) Change in HR (ΔHR). E) Change in PP (ΔPP). F) Change in activity. Data is averaged over a 24-h period, normalized to each strain per gestation day and presented as mean ± SEM. *P < 0.05 Rag2−/− compared to BALB/c, **P < 0.05 NOD.scid compared to NOD, #P < 0.01 Rag2−/− compared to NOD.scid.
In contrast, pregnant NOD.scid mice had no midgestational decline in blood pressure (Fig. 1, A–C) relative to GD 0–3. MAP and DAP did not decline in NOD.scid at midgestation, although a statistically significant decrease in SAP was detected at GD 8–9. The NOD.scid gestational blood pressure pattern differed significantly from that observed in NOD mice (Fig. 2, A–C). The pattern of the latter matched the patterns of BALB/c and now Rag2−/− mice. Comparisons with gestational blood pressures of NOD mice revealed that NOD.scid had significantly higher MAP and SAP at GD 8–10 (P < 0.01), whereas DAP was not different between the strains. Similarly, when comparing Rag2−/− and NOD.scid gestational blood pressures, NOD.scid mice had significantly higher MAP and SAP than Rag2−/− mice (P < 0.01), but no differences were detected in DAP.
In the congenic control strains (BALB/c and NOD), changes in HR patterns are similar across pregnancy. There is an initial rise in HR that peaks at GD 8, then HR declines until term. Pregnant Rag2−/− and NOD.scid females both deviated from this pattern; HR increased to GD 9 then remained above baseline until GD 15 (Fig. 1D). There were no HR differences between the two T− and B− strains. Both had significantly higher HR than their respective control strains from GD 8 until term (Fig. 2D; P < 0.05).
In pregnant Rag2−/− females, PP declined slightly but significantly at GD 8 and 9 (Fig. 1E). The gestational PP of Rag2−/− mice did not differ from BALB/c WT controls (Fig. 2E). Compared to their GD 0–3 baseline, PP in pregnant NOD.scid females was more variable throughout gestation. By GD 7, NOD.scid PP declined, reaching nadir at GD 9, and PP in NOD.scid mice remained lowered until GD 12, when it returned to baseline (Fig. 1E). The gestational PP of NOD.scid mice, relative to control NOD mice, was higher past midgestation (Fig. 2E; P < 0.004). Despite this, no significant gestational PP differences between T− and B− strains were detected.
In mice, levels of activity and HR typically have a positive correlation [23]. The characteristic pattern of activity we previously observed in mated mice (BALB/c and NOD) is an initial rise (which may be minor) followed by a steep decline to below basal activity levels after midgestation. Relative to GD 0–3 baseline, this activity pattern was also observed in pregnant Rag2−/− mice at GD 17, accompanied by a decline in HR (Fig. 1F; P < 0.01). Compared to BALB/c controls, similar to HR, activity level in pregnant Rag2−/− mice was significantly elevated past midgestation (Fig. 2F; P < 0.001). In NOD.scid mice, significant increases in activity were seen only on some days: specifically GD 7–9 and GD 11 compared to GD 0–3 (Fig. 1F; P < 0.01). In general, these times corresponded to periods of HR elevations. NOD.scid females did not exhibit the decline in activity or HR seen in most other strains during late gestation. During the latter half of gestation, the level of activity of NOD.scid mice was higher than in NOD mice (Fig. 2F; P < 0.0002). However, gestational activity level was not different between Rag2−/− and NOD.scid mice, with the exception of GD 17 (P < 0.005).
Cardiac and Renal Changes with Pregnancy
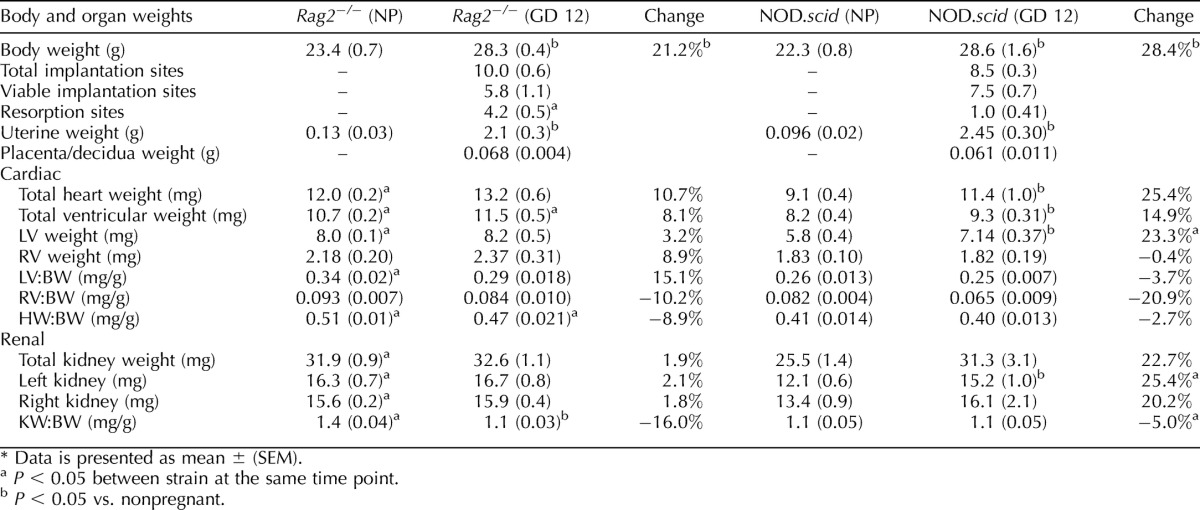
To better understand the differences in the gestational circulatory changes between Rag2−/− and NOD.scid, body and organ weights were compared for virgin and noninstrumented GD 12 females. Gestational Day 12 was selected because the major circulatory adaptations of pregnancy have occurred (i.e., increases in fluid volume and HR and decline in MAP), placentation is complete, and SA remodeling has occurred. Virgin Rag2−/− did not differ in body weight from age-matched virgin NOD.scid. However, Rag2−/− females had heavier organ weights (total heart weight, total ventricular weight, left ventricular weight, and kidney weight), and their ratios normalized to body weight were all significantly higher than for NOD.scid females (Table 2; P < 0.05). For GD 12 pregnant Rag2−/− females, the only weights that increased significantly over nonpregnant weights were body weight, uterine weight, and the kidney weight-body weight ratio (P < 0.05). Gestational Day 12 NOD.scid mice also had the expected increases in body and uterine weights compared to virgin NOD.scid (P < 0.05) but, in addition, had striking cardiac changes. Pregnant NOD.scid mice had increased total heart weight, total ventricular weight, and left ventricular weight, as well as left kidney weight, compared to virgin NOD.scid females (P < 0.01). The differences between the strains were reduced in pregnancy. Only ventricular weights and heart weight-body weight ratios differed (P < 0.02). In this set of noninstrumented animals, Rag2−/− dams had significantly higher numbers of resorption sites (4.2/10 implantation sites of smaller size and black color) than NOD.scid dams (1/8.5 implantation sites; P < 0.01) (Table 2). The neonatal outcomes of the first pregnancies in the transmitter-implanted mice did not show a difference in mean litter size between Rag2−/− (n = 9 litters, 6.7 pups per litter ± 0.8) and NOD.scid mice (n = 6 litters, 4.5 pups per litter ± 0.7; P = 0.067). However, neonates born to Rag2−/− were smaller (n = 9 litters, 1.48 g ± 0.023) than neonates born to NOD.scid (n = 6 litters, 1.74 g ± 0.53, P < 0.0001).
TABLE 2.
Body and organ weights of Rag2−/− (n = 5/group) and NOD.scid mice (n = 5/group) while nonpregnant (NP) and at Gestation Day (GD) 12.
Blood Lymphocytes
Blood lymphocytes were compared between virgin and GD 12 Rag2−/− and NOD.scid females. No CD3+ T cells were detected above isotype levels in any animal (data not shown). Virgin Rag2−/− (n = 3) and NOD.scid (n = 5) females had equivalent numbers of NKp46+ blood NK cells (11.40% ± 3.63% vs. 15.48% ± 1.55%; Fig. 3). At GD 12, Rag2−/− females (n = 5) had similar numbers of NKp46+ cells to virgin females (10.50% ± 2.03%), but NOD.scid (n = 3) females had a significant reduction (1.63% ± 0.27%; P < 0.001). This finding was confirmed using two other NK cell markers (not shown). Thus, pregnancy in NOD.scid mice depletes the blood of NK cells.
FIG. 3.
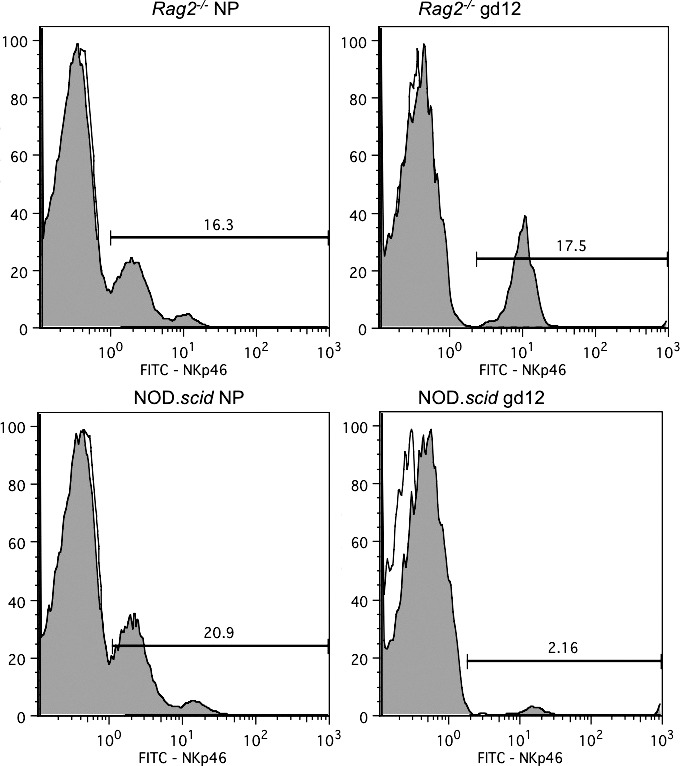
Representative histograms of NKp46 expression in peripheral blood lymphocytes from nonpregnant and GD 12 Rag2−/− (n = 3–5 per group) and NOD.scid mice (n = 3–5 per group). Lymphocytes were previously gated based on forward and side-scatter properties following lymphocyte isolation. Isotype controls were run in each series (unshaded).
Implantation Site Assessment
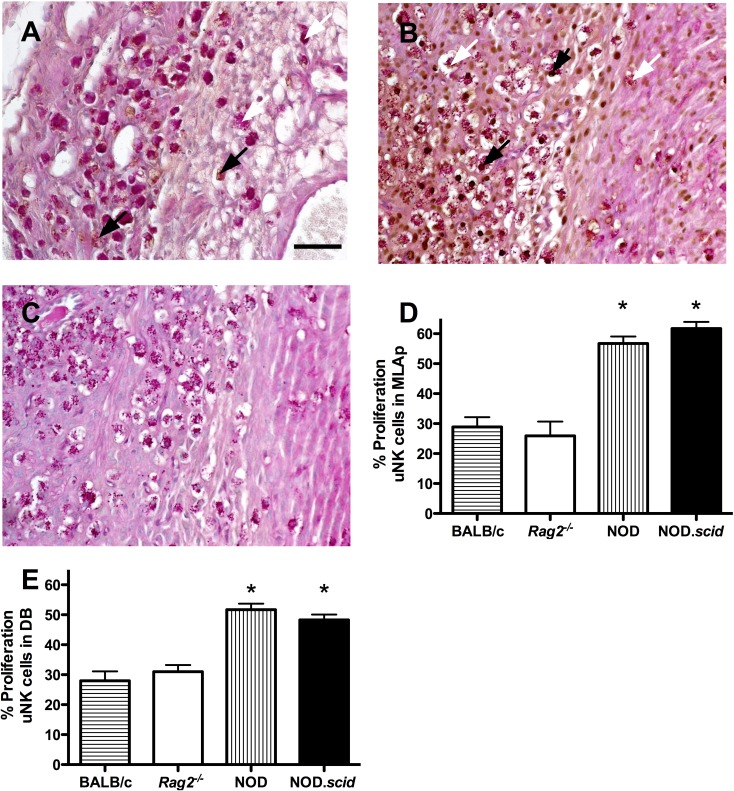
Uterine NK cells were enumerated in the MLAp and decidua basalis of Rag2−/− and NOD.scid at GD 12. In the MLAp, no differences were found in PAS+ uNK cell numbers between Rag2−/− and NOD.scid (98.58 ± 3.27 vs. 90.50 ± 10.10; P = 0.49). Similarly, no differences in uNK cell numbers were found in decidua basalis (Rag2−/−, 38.15 ± 0.96 vs. NOD.scid, 38.25 ± 1.37; P = 0.95). NOD.scid uNK cell numbers were similar to those we reported for GD 10 implantation sites [22], suggesting uNK cell numbers peak in NOD.scid females between GD 10–12. The proliferative status of uNK cells was assessed in the T− and B− strains and in BALB/c and NOD females at GD 12 (Fig. 4, A–E). The proliferation index of uNK cells within Rag2−/− MLAp (25.9% ± 4.8%) and decidua basalis (31.0% ± 2.3%) did not differ from BALB/c (28.9% ± 3.2%, 28.8 ± 3.2; Fig. 4, D and E). However, the NOD.scid and NOD uNK cell proliferation indices of 61.7% ± 2.2% and 56.7% ± 2.4% for the MLAp and 48.3% ± 1.8% and 51.7% ± 2.0% for the decidua basalis were significantly elevated compared to both BALB/c genotypes (Fig. 4, D and E; P < 0.001). Uterine NK cell proliferation between the NOD strains was not different.
FIG. 4.
Uterine NK proliferation assessed by PCNA immunohistochemistry in GD 12 implantation sites. A) MLAp (left side) and decidua basalis (DB; right side) of Rag2−/− implantation site. B) Similar micrograph of NOD.scid implantation site with increased uNK cell PCNA staining. C) Isotype control of NOD.scid implantation site with PAS counterstaining. Proliferation indices of (D) MLAp and (E) DB from control BALB/c and normoglycemic NOD compared with T- and B-cell deficient Rag2−/− and NOD.scid mice. n = 3 viable implantation sites scored per dam; three dams per strain. White arrows, nonproliferating uNK cells; black arrows, proliferating uNK cells. Bar = 50 μm. *P < 0.002 compared with other strains.
GD 12 Rag2−/− SA were modified. They had thin smooth muscle walls (10.2 ± 0.4 μm) and dilated lumens (108.7 ± 0.3 μm). SA of NOD.scid dams had significantly thicker walls (24.6 ± 1.1 μm; P < 0.0001) that incorporated extracellular matrix deposits and uNK cells. NOD.scid SA diameters were also significantly shorter than Rag2−/− (69.0 ± 2.7 μm; P < 0.001). Rag2−/− dams had significantly lower wall-lumen ratios than NOD.scid dams (P < 0.001), indicating more extensive SA remodeling.
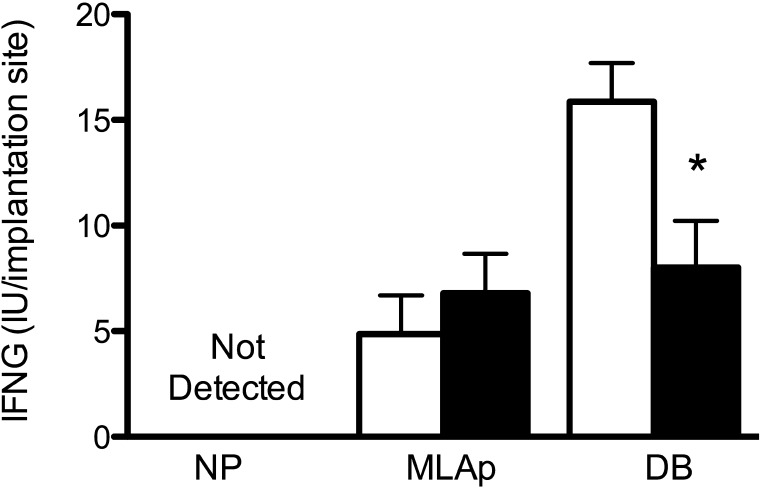
Because uNK cell numbers were equivalent in Rag2−/− and NOD.scid implantation sites but the extent of SA remodeling differed, Ifng, a product largely derived from uNK cells in normal implantation sites, was quantified. All samples of nonpregnant mesometrial uterus lacked detectable Ifng, consistent with previous studies [21]. At GD 12, Ifng concentrations within the MLAp did not differ between the strains. However, within decidua basalis, Rag2−/− implantation sites had higher concentrations of Ifng than NOD.scid (Fig. 5; P < 0.02).
FIG. 5.
Quantification of interferon gamma (IFNG) within nonpregnant uteri (n = 5 per genotype) GD 12 implantation sites of Rag2−/− (white bars) and NOD.scid (black bars) mice (n = 5 per genotype) by ELISA. Tissue samples from pregnant mice were assayed separately as MLAp and DB. Data are normalized to number of implantation sites assayed, expressed as mean ± SEM of IU of murine IFNG. *P < 0.02.
DISCUSSION
To advance understanding of the regulatory influences of NK and T cells on gestational hemodynamics, this study compared systemic and local features of pregnancy in two inbred T and B cell-deficient mouse strains. Despite reported similar immune phenotypes, the two strains differed significantly in their circulatory and uterine adaptations to pregnancy. Rag2−/− females had slightly higher baseline blood pressures than other strains we have studied, including congenic females of wild-type or alymphoid genotypes. Despite this, the Rag2−/− pattern of blood pressure change over gestation did not differ from that in congenic mice. No cardiac or renal hypertrophy was detected in pregnant compared to nonpregnant Rag2−/− females, nor was there a detectable change in numbers of circulating NK cells. Rag2−/− females had normal numbers of uNK cells in both the MLAp and decidua basalis, SA remodeling was completed, and although some midgestational fetal losses and smaller neonatal weights were seen, the overall neonatal outcomes were considered to be normal, given the small number of litters examined. Thus, on the BALB/c background, genetic depletion of T cells or T cells and NK cells has no influence on the blood pressure or the local circulatory adaptations to pregnancy. These adaptations must therefore be due to fetal-placental, hormonal, or decidual stromal regulation.
NOD.scid mice had lower MAP and SAP than congenic NOD females before mating. Further, the gestational hemodynamics of NOD.scid mice were atypical; there was no nadir in MAP or DAP at GD 9, but SAP was lower at midgestation than the pregestational baseline, suggesting differential changes in cardiac function. Relative to NOD controls, pregnant NOD.scid mice had significantly higher MAP and SAP at midgestation, which has not been observed in wild-type or immune-compromised mice. Between NOD.scid and NOD mice, several gestational circulatory differences were observed, although both strains had similarly impaired SA remodeling, normal uNK cell counts, and unremarkable fetal outcomes. Gestational MAP, SAP, HR, PP, and activity were generally higher in NOD.scid than NOD females. From these data, we postulate that NK cells have pressor ability during gestation and, in normal strains, may interact with T cells. The dysfunctional NK cells in NOD mice may be compensated for by T cell crosstalk, accounting for the observed normal gestational blood pressure profile in NOD mice. This crosstalk and the normal pattern of circulatory control are absent in NOD.scid mice.
Cardiac hypertrophy was present by midgestation in NOD.scid females, accompanied by an increase in renal mass. These findings were accompanied by a profound, pregnancy-induced decrease in circulating NK cell numbers. While we did not exclude marginalization of flowing NK cells and their adhesion to vascular endothelium as an explanation for this loss, we did show that normal uNK cell numbers were present in both the MLAp and decidua basalis. Pregnancy-induced redistribution of an entire lymphocyte subset is a novel observation. This finding implies tremendous chemotactic signaling from the decidualizing uterus. While the absence of systemic NK cells during gestation may represent a delay in repopulation from progenitors, it indicates the pregnant uterus is a preferential niche requiring uNK cell function. Despite normal numbers of uNK cells in NOD.scid implantation sites, this population of cells was abnormal; uNK cells were both hyperproliferative and hypofunctional. In all other strains studied, uNK cell proliferation has decreased significantly by GD 12 [30–32]. Delayed macrophage maturation combined with suboptimal production of Il15 [20], the essential growth factor for uNK cells [33], likely account for continued late midgestational division of uNK cells. The uNK cells might also be delayed in their maturation or have functional limits within implantation sites that could account for the poor SA remodeling seen in implantation sites of mice on the NOD background [22]. The remarkable number of proliferative uNK cells within the implantation sites of both NOD.scid and NOD females may also represent inadequate regulatory feedback on NK cells. To date, the only apoptotic signal known to regulate the uNK cell population is autocrine Ifng [34]. A deficit in this pathway is consistent with the low concentration of decidual Ifng and the lack of arterial modification found in midgestation NOD.scid mice. In spite of these differences, neonatal outcomes were normal and display the effective compensatory mechanisms available in mice to promote gestational success.
Examination of different circulatory parameters during gestation provides insight into the interrelationships between heart function, circulation, and the immune system. Typically, in strains we have studied, the gestational PP pattern has been the one observed in Rag2−/− females, with a PP decline at midgestation. This pattern was observed in pregnant NOD.scid females as well; however, relative to NOD controls, PP was significantly higher. Significant PP differences suggest that NOD.scid females adapt to gestational challenges differently than WT or Rag2−/− females. The systemic vasculature in NOD.scid mice may alter its physical properties (such as increasing compliance) during mid- to late gestation, as measured by increasing PP, in part, indicative of an increased cardiac afterload and resulting in the observed left ventricular hypertrophy and relative increase in SAP. Whether this cardiac response to pregnancy is strain-specific or due to immune impairment is yet to be resolved.
The major circulatory difference detected during gestation between wild-type and both of the T- and B-deficient strains was HR. In mice and humans, HR change is one of the earliest alterations of pregnancy [23, 35]. This is likely a compensatory mechanism to maintain higher cardiac output in a hyperdynamic circulation, with volume loading and decreased vascular resistance. We previously reported, in mice, that HR rises early in gestation and normalizes prior to term [23]; this is similar to that observed in human gestations [35, 36]. In both Rag2−/− and NOD.scid, HR remains elevated until term and is not directly attributable to activity level. It is unlikely that lack of T and B cells is a direct cause of increased HR during the physiological stress of pregnancy; however, the marked increase in gestational HR observed in these T− and B− strains implies significant increases to cardiac output without gains in stroke volume, as MAP does not become elevated. Based on this concept, these adaptations would be reflected by a substantial decline in total peripheral resistance, likely in the uterine vasculature. Sustained elevation of HR and high cardiac output may allow appropriate placental perfusion. As a consequence, HR variability or arrhythmias may develop, which were not assessed in the current study. De novo and worsening arrhythmias are common in pregnant women and can negatively impact maternal and fetal health [37].
The maintenance of MAP observed in NOD.scid mice is consistent with adequate perfusion of the placenta, despite incomplete remodeling of SA as a consequence of the increased cardiac output. However, these subtle circulatory changes in NOD.scid during pregnancy cause cardiac hypertrophy, which can also be a feature of human gestational complications [38]. Whether cardiac and vascular changes persist postpartum in this strain requires further investigation. The cardiac and circulatory adaptations to pregnancy, particularly in reference to immune function, are highly relevant to clinical studies. Hypertensive disorders of pregnancy have a poorly understood pathogenesis; fundamental knowledge can be gained from circulatory adaptation in animal models.
This study has demonstrated that in mice lacking adaptive immunity (T and B lymphocytes), gestational HR elevation is observed relative to T- and B-sufficient mice. HR elevation likely acts to maintain high cardiac output in the presence of potentially low peripheral resistance in T− and B− pregnant mice. While HR was similar, blood pressure between Rag2−/− and NOD.scid females differed. While Rag2−/− females showed a pattern of gestational blood pressure change typical of normal pregnancy, NOD.scid mice did not. NOD.scid mice, with significant genetic impairment of NK cell and macrophage functions, had significantly higher midgestational blood pressure phenotype relative to controls. This study establishes a modulating role for T cells and NK cells in control of the circulation during pregnancy.
ACKNOWLEDGMENTS
We thank David Beseau and Jennifer Ahn for technical assistance.
Footnotes
Supported by the Canadian Institutes for Health Research (77519 to B.A.C. and M.A.A.) and Frederick Banting and Charles Best Canada Graduate Scholarships–Doctoral Award to S.D.B., by the Canada Research Chairs Program (B.A.C.) and by the Leaders Opportunity Fund of the Canada Foundation for Innovation (B.A.C. and M.A.A.); Canadian Foundation for Women's Health (B.A.C., S.D.B.); and Natural Sciences and Engineering Research Council of Canada (to B.A.C. and A.L.C.).
REFERENCES
- Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension 2010; 56: 166 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GN, Walker MC, Liu A, Wen SW, Swansburg M, Ramshaw H, White RR, Roddy M, Hladunewich M. Pre-Eclampsia New Emerging Team (PE-NET). A history of preeclampsia identifies women who have underlying cardiovascular risk factors. Am J Obstet Gynecol 2009; 200:58.e1 58.e8. [DOI] [PubMed]
- Blumenstein M, McMaster MT, Black MA, Wu S, Prakash R, Cooney J, McCowan LM, Cooper GJ, North RA. A proteomic approach identifies early pregnancy biomarkers for preeclampsia: novel linkages between a predisposition to preeclampsia and cardiovascular disease. Proteomics 2009; 9: 2929 2945. [DOI] [PubMed] [Google Scholar]
- Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost 2009; 7: 375 384. [DOI] [PubMed] [Google Scholar]
- Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, et al. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One 2010; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, Evans SS, Croy BA. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol 2002; 168: 22 28. [DOI] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006; 12: 1065 1074. [DOI] [PubMed] [Google Scholar]
- Kruse A, Merchant MJ, Hallmann R, Butcher EC. Evidence of specialized leukocyte-vascular homing interactions at the maternal/fetal interface. Eur J Immunol 1999; 29: 1116 1126. [DOI] [PubMed] [Google Scholar]
- Peralta CG, Han VK, Horrocks J, Croy BA, van den Heuvel MJ. CD56bright cells increase expression of {alpha}4 integrin at ovulation in fertile cycles. J Leukoc Biol 2008; 84: 1065 1074. [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Lash GE. Human uterine natural killer cells: a reappraisal. Mol Immunol 2005; 42: 511 521. [DOI] [PubMed] [Google Scholar]
- Croy BA, van den Heuvel MJ, Borzychowski AM, Tayade C. Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev 2006; 214: 161 185. [DOI] [PubMed] [Google Scholar]
- Yadi H, Burke S, Madeja Z, Hemberger M, Moffett A, Colucci F. Unique receptor repertoire in mouse uterine NK cells. J Immunol 2008; 181: 6140 6147. [DOI] [PubMed] [Google Scholar]
- Wang C, Tanaka T, Nakamura H, Umesaki N, Hirai K, Ishiko O, Ogita S, Kaneda K. Granulated metrial gland cells in the murine uterus: localization, kinetics, and the functional role in angiogenesis during pregnancy. Microsc Res Tech 2003; 60: 420 429. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 1992; 68: 855 867. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 1992; 68: 869 877. [DOI] [PubMed] [Google Scholar]
- Bosma MJ, Carroll AM. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol 1991; 9: 323 350. [DOI] [PubMed] [Google Scholar]
- Serreze DV, Gaedeke JW, Leiter EH. Hematopoietic stem-cell defects underlying abnormal macrophage development and maturation in NOD/Lt mice: defective regulation of cytokine receptors and protein kinase C. Proc Natl Acad Sci U S A 1993; 90: 9625 9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol 1995; 154: 180 191. [PubMed] [Google Scholar]
- Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, Pertel T, Carnaud C, Bluestone JA, Lanier LL. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity 2003; 18: 41 51. [DOI] [PubMed] [Google Scholar]
- Suwanai H, Wilcox MA, Mathis D, Benoist C. A defective Il15 allele underlies the deficiency in natural killer cell activity in nonobese diabetic mice. Proc Natl Acad Sci U S A 2010; 107: 9305 9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkar AA, Croy BA. Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod 1999; 61: 493 502. [DOI] [PubMed] [Google Scholar]
- Burke SD, Dong H, Hazan AD, Croy BA. Aberrant endometrial features of pregnancy in diabetic NOD mice. Diabetes 2007; 56: 2919 2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SD, Barrette VF, Bianco J, Thorne JG, Yamada AT, Pang SC, Adams MA, Croy BA. Spiral arterial remodeling is not essential for normal blood pressure regulation in pregnant mice. Hypertension 2010; 55: 729 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley JH, South S, Chiasson VL, Mitchell BM. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol 2010; 298: R713 R719. [DOI] [PubMed] [Google Scholar]
- Baker CH, Abel FL. Macro- and microcirculatory effects of IL-15. Shock 1995; 4: 307 310. [DOI] [PubMed] [Google Scholar]
- Nomoto T, Okada T, Shimazaki K, Yoshioka T, Nonaka-Sarukawa M, Ito T, Takeuchi K, Katsura KI, Mizukami H, Kume A, Ookawara S, Ikeda U, et al. Systemic delivery of IL-10 by an AAV vector prevents vascular remodeling and end-organ damage in stroke-prone spontaneously hypertensive rat. Gene Ther 2009; 16: 383 391. [DOI] [PubMed] [Google Scholar]
- Prophet EB, Mills B, Arrington JB, Sobin LH. Armed Forces Institute of Pathology Laboratory Methods in Histotechnology. Washington, DC: American Registry of Pathology; 1992: 39 44. [Google Scholar]
- Xie X, He H, Colonna M, Seya T, Takai T, Croy BA. Pathways participating in activation of mouse uterine natural killer cells during pregnancy. Biol Reprod 2005; 73: 510 518. [DOI] [PubMed] [Google Scholar]
- Dong H, Burke SD, Croy BA. Vascular addressins in the uterus and pancreas of type 1 diabetic mice in early pregnancy. Placenta 2008; 29: 201 209. [DOI] [PubMed] [Google Scholar]
- Peel S. Granulated metrial gland cells. Adv Anat Embryol Cell Biol 1989; 115: 1 112. [DOI] [PubMed] [Google Scholar]
- Paffaro VA, Jr, , Bizinotto MC, Joazeiro PP, Yamada AT. Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta 2003; 24: 479 488. [DOI] [PubMed] [Google Scholar]
- Delgado SR, McBey BA, Yamashiro S, Fujita J, Kiso Y, Croy BA. Accounting for the peripartum loss of granulated metrial gland cells, a natural killer cell population, from the pregnant mouse uterus. J Leukoc Biol 1996; 59: 262 269. [PubMed] [Google Scholar]
- Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, Croy BA. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol 2003; 171: 2937 2944. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Croy BA. Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin Immunol 2001; 13: 235 241. [DOI] [PubMed] [Google Scholar]
- Carlin A, Alfirevic Z. Physiological changes of pregnancy and monitoring. Best Pract Res Clin Obstet Gynaecol 2008; 22: 801 823. [DOI] [PubMed] [Google Scholar]
- Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG, Dahms T, Schrier RW. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 1998; 54: 2056 2063. [DOI] [PubMed] [Google Scholar]
- Ferrero S, Colombo BM, Ragni N. Maternal arrhythmias during pregnancy. Arch Gynecol Obstet 2004; 269: 244 253. [DOI] [PubMed] [Google Scholar]
- Vasapollo B, Novelli GP, Valensise H. Total vascular resistance and left ventricular morphology as screening tools for complications in pregnancy. Hypertension 2008; 51: 1020 1026. [DOI] [PubMed] [Google Scholar]