Abstract
Background
In the present work we determined phenolic and flavonoids content of Eriobotrya japonica leaves extracts and fractions and their antioxidant and anti-inflammatory properties.
Objectives
To evaluate the inhibition of inflammatory PLA2 and antioxidant effects of extracts and fractions from Eriobotrya japonica leaves
Methods
Antioxidant activity was evaluated with DPPH radical scavenging assay and anti-inflammatory effect of fractions was measured by their inhibition potency on the human pro-inflammatory phospholipase A2 (group IIA).
Results
The EtOH/EtOAc 2:1 extract exhibited a potent inhibition of the hG-IIA with an IC50 values of 8 µg/ml. It also shows an antioxidant activity measured on DPPH with an IC50 of 42 µg/ml. Fractionation shows that CH2Cl2/MeOH 0:1 fraction was the rich one on flavonoids compounds (4.3 mg/g dry weight) and demonstrates a high antioxidant activity with an IC50 of 12 µg/ml. The anti-inflammatory evaluation demonstrates that the same fraction was the best one to inhibit the pro-inflammatory phospholipase A2 group IIA with an IC50 of 4 µg/ml.
Conclusion
Study conducted on Eriobotrya japonica shows that CH2Cl2/MeOH 0:1 fraction inhibits efficiently the hG-IIA phospholipase. which is considered as pro-inflammatory enzyme.
Keywords: Eriobotrya japonica, extraction, flavonoids, anti-inflammatory
Introduction
Eriobotrya japonica Lindl, also known as ‘loquat’, belongs to the Rosaceae family. This plant is an evergreen shrub or small tree with narrow leaves that are dark green on the upper surface and have a lighter color under surface. It is originated from south-eastern China and later became naturalized in Korea, Japan, India and many other countries.
Leaves of Eriobotrya japonica (LEJ) Lindl (Rosaceae) have been used as traditional medicines for lung and stomach diseases and have been found to be effective in chronic bronchitis, inflammation, asthma, low back pain and tumor.1–3,4 Studies have demonstrated that LEJ has anti-inflammatory activity in a 12-O-tetradecanoylphorbol-13-acetate induced inflammation model. These reports strongly suggest that LEJ can be used as an anti-inflammatory agent.
Various triterpenes, sesquiterpenes, flavonoids, tannins and megastigmane glycosides have been found in the LEJ and previous studies showed that some of these components have anti-tumor, antiviral, hypoglycemic, antioxidant and anti-inflammatory properties3,5–8.
During the inflammatory process, macrophages produce nitric oxide, cytokine and pro-inflammatory enzymes such as secreted phospholipase A2 (sPLA2)9,10 that catalyze the hydrolysis of membrane phospholipids to produce free arachidonic acid and lysophospholipids. Indeed, several studies showed that sPLA2 are the chief actors on the biosynthesis of lipid mediators in inflammatory cells11. sPLA2 enzymes are a heterogenic family that are divided on 11 groups (IB, IIA, IIC, IID, IIE, IIF, III, V, X, XIIA and XIIB)12–14. The sPLA2 group IIA was initially detected in synovial fluid of patients with rheumatoid arthritis15,16. Several studies demonstrated that the sPLA2 group IIA was involved in inflammatory process17–19 and many phospholipases A2 inhibitors have been discovered and their effectiveness have been proved as a treatment of inflammatory diseases20–22.
Because overproduction of these inflammatory mediators might cause inflammatory damage, we focused in the present study on the evaluation of the anti-inflammatory effect of LEJ extracts by measuring the inhibition of the pro-inflammatory sPLA2 group IIA as well as their antioxidant activity.
Material and methods
Plant material
Leaves of Eriobotrya Japonica (Rosaceae) (LEJ) were collected in the region from Sfax (Tunisia) in June 2010. The plant was identified by Pr. M. Chaieb (Faculty of Sciences, Sfax University, Tunisia) and a voucher specimen has been deposited in the Chemical Laboratory of Narural Products (Sfax, Tunisia: No. LCSN 108)
Extraction and fractionation of flavonoids
The dry leaves of plant sample were ground to fine powder in a mill, and 100 g of powder was extracted in 1 L of MeOH/H2O 7:3. After filtration, the methanol was removed by evaporation and 250 mL of n-butanol was added. The organic phase was evaporated and the extract was dissolved in 200 mL of EtOH/EtOAc 2:1. The issue sample was separated on four fractions using CH2Cl2/MeOH at 8:2, 7:3, 5:5 and 0:1 proportion, respectively.
Total phenols determination
Total phenols determination of the fractions of Eriobotrya japonica leaves extracts was determined by colorimetric assay according to the method described by23. 1 ml of sample at 1 mg/ml was mixed with 1 ml of Folin-Ciocalteu reagent. After 3 min of incubation, 1 ml of saturated Na2CO3 solution was added and the volume was adjusted to 10 ml with distilled water. The reaction mixture was kept in the dark for 90 min, after which the absorbance was read at 725 nm. The total phenolic content was determined using gallic acid as a standard.
Determination of flavonoids content
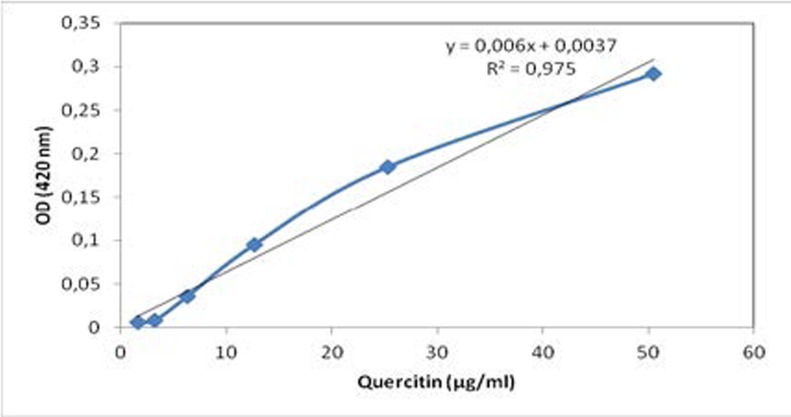
Total flavonoids were determined by following the procedure24. Briefly, 1 mL of aliquots of leaves extracts and fractions were placed in two test tubes, respectively. 7 mL of methanol were added to one tube. In the other one, 1 mL of 2 % ZrOCl2-8H2O and 6 mL of methanol were added. The solution was mixed again and placed into water bath at 30 °C for 1 h. The absorbance was measured at 420 nm and ΔOD was calculated. The amount of total flavonoids was calculated as a quercitin equivalent from the standard curve (figure 1), and expressed as mg quercitin/g dry leaves plant material (mg/g dry weight).
Figure 1.
Standard curve of quercitin
DPPH radical scavenging assay
The antioxidant activity of LEJ extract and fractions were measured as equivalent of hydrogen-donating or radical scavenging ability, using the DPPH method25–27 with some modifications. Briefly, 1.5 mL of DPPH solution at 10-5 M was incubated with 1.5 mL of extracts containing variable amounts of dry weight (between 0.01 and 1 mg). The reaction mixture was shaken and incubated in the dark for 30 min at room temperature. Control experiment was performed as described above without adding any LEJ extract. The OD of the solution was measured at 517 nm. The radical scavenging activity was calculated using the following equation: Scavenging effect (%) =
The extract concentration providing 50% inhibition (IC50) was calculated from the plot of the scavenging effect (percentage) against the extract concentration. BHT was used as standard.
Anti-inflammatory activity
The anti-inflammatory activity of extracts was followed by the inhibition of the human inflammatory phospholipase A2 group IIA (hG-IIA). The hG-IIA activity was measured as described by28. Briefly, the substrate consisted of 3.5 mM lecithin (Sigma Aldrich) in a mixture of 3 mM NaTDC, 100 mM NaCl, 10 mM CaCl2 and 0.055 mM red phenol as colorimetric indicator in 100 mL H2O. The pH of the reaction mixture was adjusted to 7.6. The hG-IIA or the pig pancreatic phospholipase A2 group IB (pG-IB) phospholipases were solubilized in 10% acetonitrile at a concentration of 0.02 and 0.002 µg/µl, respectively. A volume of 10 µl of these PLA2 solutions was incubated for 20 min at room temperature with 10 µl of each LEJ extracts and fractions. Then, 1 mL of the PLA2 substrate was injected in the medium, and the kinetic of hydrolysis was followed during 5 min by reading the decrease of OD at 558 nm. The inhibition percentage was calculated by comparison with a control experiment and the IC50 values were determined from the blot. The control experiment contained 10 µl of the enzyme (hG-IIA or pG-IB) and 10 µl of the corresponding organic solvent.
Statistical analysis study
Experimental results were given as mean value ± SD of three separate experiments. Statistical analysis was conducted using Microsoft Excel software using the Duncan test performed after analysis of variance (ANOVA).
Results
Extraction yields of plant material
Dried and powdered LEJ were extracted with MeOH/ H2O 7:3 and then fractionated after that with butanol, EtOH/EtOAc 2:1 and CH2Cl2/MeOH at different percentage. Table 1 summarizes the extraction yield of LEJ.
Table 1.
Extraction yields of LEJ
| Solvents | Yields (g/100 g dry weight) |
| Methanol/water (70/30) | 15 |
| Butanol | 12 |
| Ethanol-Ethyl acetate (2/1) | 9 |
| CH2Cl2-MeOH (8/2) | 1.2 |
| CH2Cl2-MeOH (7/3) | 2.4 |
| CH2Cl2-MeOH (5/5) | 3.1 |
| Methanol | 2.3 |
Total phenolic and flavonoids content
Total phenolic content, expressed as mg GAEs/g DW and flavonoids content, expressed as mg quercitin/g DW of LEJ extracts were presented in Table 2. Results show that phenolic and flavonoids content in EtOH/EtOAc 2:1 extract were about 28 mg GAEs/g DW and 7 mg EQ/g DW, respectively. These concentrations were lower than those from E. japonica cv. Zaozhong No. 6 and E. japonica Lindl29. These studies show that the two species contain 47.5 and 54.9 mg GAEs/g DW as phenolic content and 109.3 and 119 mg QE/g DW as flavonoids content, respectively. Therefore, the EtOH/ EtOAc 2:1 extract of LEJ was further fractionated into CH2Cl2/MeOH (8:2, 7:3, 5:5 and 0:1) soluble fractions. Results reported in Table 2 show that CH2Cl2/MeOH (0:1) extract was the richest on phenolic and flavonoids compound with 13 mg GAEs/g DW and 4.3 mg QE/g DW, respectively.
Table 2.
Phenolic and Flavonoids content in each fraction and their antioxidant activity.
| Component (mg/g dry weight) | |||
| Fractions | Phenolic | Flavonoids | IC50 on DPPH radical (µg/mL) |
| Ethanol-ethyl acetate (2/1) | 28 ± 1.3 | 7 ± 0.52 | 42 ± 2.1 |
| CH2Cl2-MeOH (8/2) | 2 ± 0.04 | 0.4 ± 0.03 | 83 ± 3.0 |
| CH2Cl2-MeOH (7/3) | 5 ± 0.07 | 0.8 ± 0.03 | 67 ± 2.4 |
| CH2Cl2-MeOH (5/5) | 8 ± 0.09 | 1.4 ± 0.08 | 35 ± 1.7 |
| CH2Cl2-MeOH (0/1) | 13 ± 0.4 | 4.3 ± 0.1 | 12 ± 0.8 |
| BHT | - | - | 69 ± 3.2 |
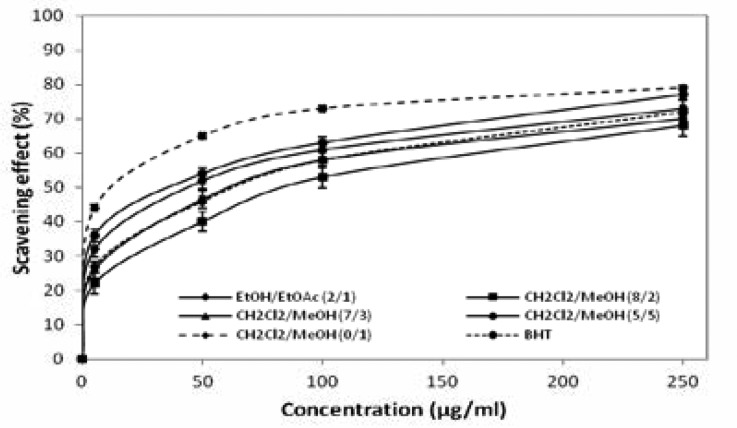
DPPH radical scavenging activity
The antiradical activities of the extracts were determined using the DPPH free radical assay (figure 2) and the radical scavenging activities were expressed as the mean of the IC50 values (µg/mL). IC50 values and BHT were reported in Table 2. Our results show that the EtOH/EtOAc 2:1 extracts exhibit a capacity to reduce the DPPH with an IC50 of 42 µg/mL. Using this extract, the most potent fraction obtained with CH2Cl2/MeOH 0:1 shows an IC50 value about 12 µg/ mL, being 3.5 times more active than the initial extract. This result shows that there is correlation between the enrichment of phenolic and flavonoids compounds and the antiradical activity. Consequently, we can hypothesize that phenolic or flavonoids compounds might be responsible for the antiradical activity.
Figure 2.
Radical scavenging activities of LEJ extracts 1 and fractions measured 2 on DPPH.
Evaluation of the anti-inflammatory effect
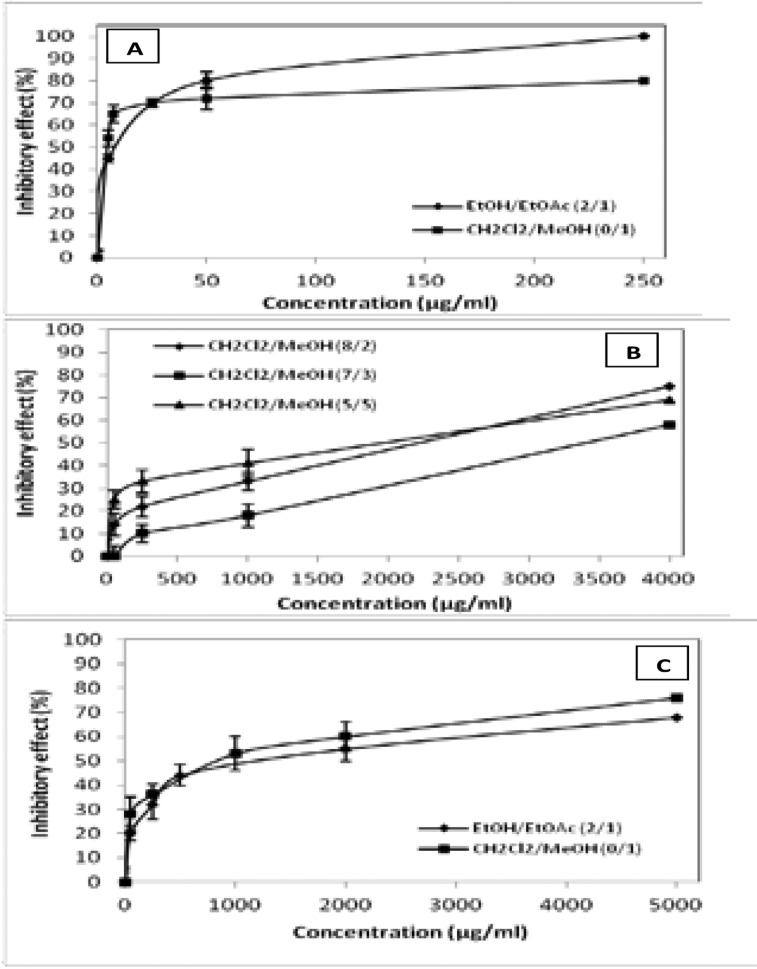
To evaluate the anti-inflammatory effect, we measured the ability of these extracts and fractions to inhibit the inflammatory hG-IIA (figure 3A, 3B) and the digestive pG-IB (figure 3C) phospholipases A2.
Figure 3.
Inhibitory effect of LEJ extracts and fractions on PLA2. A and B: pro2 inflammatory PLA2 (hG-IIA), C: digestive PLA2 (pG-IB).
Results show that the EtOH/EtOAc 2:1 extract inhibits the hG-IIA PLA2 and the pG-IB with an IC50 of 8 µg/mL and 1200 µg/mL, respectively (Table 3).
Table 3.
Inhibitory effect of LEJ extracts on hG-IIA and pG-IB phospholipases.
| Fractions | IC50 values on hG-IIA (µg/mL) |
IC50 values on pG-IB (µg/mL) |
Inhibition specificity (IC50 pG-IB /IC50 hG-IIA) |
| Ethanol-ethyl acetate (2/1) | 8 ± 0.4 | 1200 ± 50 | 150 |
| CH2Cl2-MeOH (8/2) | 2300 ± 100 | > 5000 | > 2.17 |
| CH2Cl2-MeOH (7/3) | 3500 ± 120 | > 5000 | > 1.42 |
| CH2Cl2-MeOH (5/5) | 1000 ± 40 | > 5000 | > 5 |
| CH2Cl2-MeOH (0/1) | 4 ± 0.3 | 800 ± 20 | 200 |
This finding proves that this extract inhibits preferentially hG-IIA with a relative specificity inhibition factor of about 150. Fractions from this extract were tested for their ability to inhibit these two PLA2 and results revealed that CH2Cl2/MeOH 0:1 fraction is the most interesting one (Table 3).
In fact, this fraction inhibits preferentially the hG-IIA enzyme with an IC50 of 4 µg/mL versus 800 µg/mL measured on pG-IB PLA2. To highlight the specificity inhibition of hG-IIA versus pG-IB, we calculate the specificity factor
which is around 200. This value indicates that the inhibitory potency of this fraction toward hG-IIA is 200 times higher than its toward pG-IB. This fraction was likely able to inhibit preferentially the inflammatory PLA2 (hG-IIA) and not the digestive one (pG-IB). Moreover, we can strongly suggest that phenolic or flavonoid compounds in CH2Cl2/MeOH 0:1 were responsible for the hG-IIA inhibition.
Discussion
In this study, we targeted the extraction of phenolic and flavonoids compounds present in LEJ. In fact, several previous works described the importance of the biological functions of these molecules such as antioxidant24,30–32, anti-inflammatory33,34, anti-atherosclerotic35,36, anticancer35,37,38 and antimicrobial activities39,40. Indeed, the ethanol-ethyl acetate (2/1) extract contains 28 mg GAE/g DW of phenolic compounds and 7 mg EQ/g DW of flavonoid contents and show an important antioxidant activity measured on DPPH with an IC50 of 42 µg/mL. These results are in agreement with those obtained by41 and24 who reported that there is a close relationship between phenolic and flavonoid content and the antioxidant activity in Eriobotrya japonica extracts.
On the purpose to identify natural anti-inflammatory compounds, several studies were performed using Eriobotrya japonica due to its well known potent anti- inflammatory effects42 and these have demonstrated that leaf of Eriobotrya japonica was able to suppress LPS-induced cytokine production in a dose dependent manner. Moreover,8 they have proved that water extract of Eriobotrya japonica leaves regulates production of pro-inflammatory cytokines such as TNFα, IL6 and IL8 in mast cells. We also reported in this study that the ethanol-ethyl acetate (2/1) extract of Eriobotrya japonica inhibits the pro-inflammatory PLA2 (hG-IIA) with an IC50 of 8 µg/mL. The selective inhibition was performed using the digestive PLA2 (pG-IB) and our results reveal that the EtOH/EtOAc 2:1 extract inhibits the pancreatic enzyme with an IC50 of 1200 µg/mL.
This result confirms that the extract inhibits preferentially the pro-inflammatory PLA2 with a relative selectivity factor of 150. These results have encouraged us to split over this extract. On this purpose, liquid-liquid extraction was performed using CH2Cl2/MeOH at various percentages. Obtained fractions were evaluated for their phenolic and flavonoids content and their ability to possess antioxidant and anti-inflammatory activities. Results presented in Table 2 show that CH2Cl2/MeOH 0:1 fraction was the richest on phenolic and flavonoids content with values of 13mg EAG/g DW and 4.3 mg EQ/g DW, respectively, and with the most antioxidant effect (IC50 = 12 µg/mL). In the same way, this fraction has demonstrated the best capacity to inhibit hG-IIA versus pG-IB with IC50 values of 4 µg/mL and 800 µg/mL, respectively. These results suggest that the phenolic and flavonoids compounds in CH2Cl2/MeOH 0:1 are responsible for preferential inhibition of hG-IIA compared to the digestive pG-IB one.
Several studies investigated medicinal plants for their potent natural therapeutic virtues and only few of them were described for their capacity to inhibit the inflammatory PLA2 enzyme. The ethanol extract of the stem of Sinomenium acutum, Spatholobus suberectus and Trachelosermum jasminoide show IC50 values of 112, 54 and 33 µg/mL, respectively43. Compared to these works, the fractions that we obtained are more efficient to inhibit the pro-inflammatory PLA2 with an IC50 of 4 µg/ml.
Conclusion
The aim of the present study was to evaluate the anti-inflammatory and the antioxidant activities of phenolic and flavonoids content in Eriobotrya japonica leaves. To that end, we performed fractionation of EtOH/EtOAc 2:1 using CH2Cl2/MeOH in different proportions. The evaluation of these fractions shows that a correlation may exist between phenolic and flavonoids compounds and the anti-inflammatory and the antioxidant activities.
So far we are using extract from LEJ and its fraction; the compound responsible for the preferential inhibition of the hG-IIA PLA2 is still not identified. The efforts in purification and identification of active components from LEJ are ongoing.
Acknowledgements
This research was supported by “Ministère de l'enseignement supérieur et de la recherche scientifique-Tunisia” through a grand to “Laboratoire de Biochimie et de Génie Enzymatique des Lipases-ENIS” (Tunisia).
Abbreviations
- IC50
inhibitory concentration at 50 %
- sPLA2
secreted phospholipase A2
- hG-IIA
human secreted phospholipase A2 group IIA
- pG-IB
pig secreted phospholipase A2 group IB
- LEJ
leaves of Eriobotrya japonica
- DPPH
2,2-diphényl 1-picrylhydrazyl
- CH2Cl2
dichloromethane
- MeOH
methanol
- DW
dry weight
- GAE
gallic acid equivalent
- QE
quercitin equivalent
- NaTDC
sodium taurodeoxycholate
References
- 1.Kimura T, But PPH, Guo J-X, Sung CK, editors. International Collation of Traditional and Folk Medicine: Northeast Asia. World Scientific Pub Co Inc; 1996. [Google Scholar]
- 2.Zhu Y-P. Chinese Materia Medica: Chemistry, Pharmacology and Applications. Harwood Acad Publ; 1998. [Google Scholar]
- 3.Ito H, Kobayashi E, Takamatsu Y, Li SH, Hatano T, Sakagami H, Kusama K, Satoh K, Sugita D, Shimura S, Itoh Y, Yoshida T. Polyphenols from Eriobotrya japonica japonica and their cytotoxicity against human oral tumor cell lines. Chem Pharm Bull. 2000 May;48:687–693. doi: 10.1248/cpb.48.687. [DOI] [PubMed] [Google Scholar]
- 4.Banno N, Akihisa T, Tokuda H, Yasukawa K, Taguchi Y, Akazawa H, Ukiya M, Kimura Y, Suzuki T, Nishino H. Anti-inflammatory and antitumor-promoting effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol Pharm Bull. 2005 Oct;28:1995–1999. doi: 10.1248/bpb.28.1995. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu M, Fukumura H, Tsuji H, Tanaami S, Hayashi T, Morita N. Anti-inflammatory constituents of topically applied crude drugs. I. Constituents and anti- inflammatory effect of Eriobotrya japonica LINDL. Chem Pharm Bull. 1986 Jun;34:2614–2617. doi: 10.1248/cpb.34.2614. [DOI] [PubMed] [Google Scholar]
- 6.De Tommasi N, De Simone F, Cirino G, Cicala C, Pizza C. Hypoglycemic effects of sesquiterpene glycosides and polyhydroxylated triterpenoids of Eriobotrya japonica. Planta Med. 1991 Oct;57:414–416. doi: 10.1055/s-2006-960137. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi S, Imayoshi Y, Kobayashi E, Takamatsu Y, Ito H, Hatano T, Sakagami H, Tokuda H, Nishino H, Sugita D, Shimura S, Yoshida T. Production of bioactive triterpenes by Eriobotrya japonica calli. Phytochemistry. 2002 Feb;59:315–323. doi: 10.1016/s0031-9422(01)00455-1. [DOI] [PubMed] [Google Scholar]
- 8.Kim S-H, Shin T-Y. Anti-inflammatory effect of leaves of Eriobotrya japonica correlating with attenuation of p38 MAPK, ERK, and NF-kappaB activation in mast cells. Toxicol In Vitro. 2009 Oct;23:1215–1219. doi: 10.1016/j.tiv.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 9.Tsukahara Y, Morisaki T, Horita Y, Torisu M, Tanaka M. Phospholipase A2 mediates nitric oxide production by alveolar macrophages and acute lung injury in pancreatitis. Ann Surg. 1999 Mar;229:385–392. doi: 10.1097/00000658-199903000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granata F, Frattini A, Loffredo S, Del Prete A, Sozzani S, Marone G, Triggiani M. Signaling events involved in cytokine and chemokine production induced by secretory phospholipase A2 in human lung macrophages. European Journal of Immunology. 2006;36:1938–1950. doi: 10.1002/eji.200535567. [DOI] [PubMed] [Google Scholar]
- 11.Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins & Other Lipid Mediators. 2002 Aug;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 12.Valentin E, Lambeau G. Increasing molecular diversity of secreted phospholipases A2 and their receptors and binding proteins. Biochimica et Biophysica Acta (BBA)- Molecular and Cell Biology of Lipids. 2000 Oct;1488:59–70. doi: 10.1016/s1388-1981(00)00110-4. [DOI] [PubMed] [Google Scholar]
- 13.Murakami M, Kudo I. Advances in Immunology [Internet] Academic Press; 2001. Diversity and regulatory functions of mammalian secretory phospholipase A2s; pp. 163–194. [cited 2013 Feb 13] Available from: http://www.sciencedirect.com/science/article/pii/S0065277601770174. [DOI] [PubMed] [Google Scholar]
- 14.Lambeau G, Gelb MH. Biochemistry and physiology physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 15.Dennis EA. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends in Biochemical Sciences. 1997 Jan;22:1–2. doi: 10.1016/s0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- 16.Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead MV, Tischfield JA, Kudo I. The functions of five distinct mammalian phospholipase A2S in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2S are functionally redundant and act in concert with cytosolic phospholipase A2. J Biol Chem. 1998 Jun;273:14411–14423. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- 17.Pruzanski W, Albin-Cook K, Laxer RM, MacMillan J, Stefanski E, Vadas P, Silverman ED. Phospholipase A2 in juvenile rheumatoid arthritis: correlation to disease type and activity. J Rheumatol. 1994 Oct;21:1951–1954. [PubMed] [Google Scholar]
- 18.Kitsiouli E, Nakos G, Lekka ME. Phospholipase A2 subclasses in acute respiratory distress syndrome. Biochim Biophys Acta. 2009 Oct;1792:941–953. doi: 10.1016/j.bbadis.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Granata F, Frattini A, Loffredo S, Staiano RI, Petraroli A, Ribatti D, Oslund R, Gelb MH, Lambeau G, Marone G, Triggiani M. Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. J Immunol. 2010 May;184:5232–5241. doi: 10.4049/jimmunol.0902501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snyder DW, Bach NJ, Dillard RD, Draheim SE, Carlson DG, Fox N, Roehm NW, Armstrong CT, Chang CH, Hartley LW, Johnson LM, Roman CR, Smith AC, Song M, Fleisch JH. Pharmacology of LY315920/S-5920, [[3-(aminooxoacetyl)-2-ethyl-1- (phenylmethyl)-1H-indol- 4-yl]oxy] acetate, a potent and selective secretory phospholipase A2 inhibitor: A new class of anti-inflammatory drugs, SPI. J Pharmacol Exp Ther. 1999 Mar;288:1117–1124. [PubMed] [Google Scholar]
- 21.Reid RC. Inhibitors of secretory phospholipase A2 group IIA. Curr Med Chem. 2005;12:3011–3026. doi: 10.2174/092986705774462860. [DOI] [PubMed] [Google Scholar]
- 22.Rosenson RS. Future role for selective phospholipase A2 inhibitors in the prevention of atherosclerotic cardiovascular disease. Cardiovasc Drugs Ther. 2009 Feb;23:93–101. doi: 10.1007/s10557-008-6148-1. [DOI] [PubMed] [Google Scholar]
- 23.Singleton VL, Rossi JA. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am J Enol Vitic. 1965 Jan;16:144–158. [Google Scholar]
- 24.Zhou C, Sun C, Chen K, Li X. Flavonoids, Phenolics, and Antioxidant Capacity in the Flower of Eriobotrya japonica Lindl. Int J Mol Sci. 2011;12:2935–2945. doi: 10.3390/ijms12052935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology. 1995;28:25–30. [Google Scholar]
- 26.Chen Y, Wang M, Rosen RT, Ho CT. 2,2-Diphenyl- 1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum thunb. J Agric Food Chem. 1999 Jun;47:2226–2228. doi: 10.1021/jf990092f. [DOI] [PubMed] [Google Scholar]
- 27.Naik GH, Priyadarsini KI, Satav JG, Banavalikar MM, Sohoni DP, Biyani MK, Mohan H. Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry. 2003 May;63:97–104. doi: 10.1016/s0031-9422(02)00754-9. [DOI] [PubMed] [Google Scholar]
- 28.Lôbo de Araújo A, Radvanyi F. Determination of phospholipase A2 activity by a colorimetric assay using a pH indicator. Toxicon. 1987;25:1181–1188. doi: 10.1016/0041-0101(87)90136-x. [DOI] [PubMed] [Google Scholar]
- 29.Hong Y, Lin S, Jiang Y, Ashraf M. Variation in contents of total phenolics and flavonoids and antioxidant activities in the leaves of 11 Eriobotrya species. Plant Foods Hum Nutr. 2008 Dec;63:200–204. doi: 10.1007/s11130-008-0088-6. [DOI] [PubMed] [Google Scholar]
- 30.Burda S, Oleszek W. Antioxidant and antiradical activities of flavonoids. J Agric Food Chem. 2001 Jun;49:2774–2779. doi: 10.1021/jf001413m. [DOI] [PubMed] [Google Scholar]
- 31.Majo DD, Giammanco M, Guardia ML, Tripoli E, Giammanco S, Finotti E. Flavanones in Citrus fruit: Structure-antioxidant activity relationships. Food Research International. 2005 Dec;38:1161–1166. [Google Scholar]
- 32.Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manthey JA, Grohmann K, Guthrie N. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr Med Chem. 2001 Feb;8:135–153. doi: 10.2174/0929867013373723. [DOI] [PubMed] [Google Scholar]
- 34.Ravipati AS, Zhang L, Koyyalamudi SR, Jeong SC, Reddy N, Bartlett J, Smith PT, Shanmugam K, Munch G, Wu MJ, Satyanarayanan M, Vysetti B. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement Altern Med. 2012 Oct;12:173. doi: 10.1186/1472-6882-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993 Oct;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 36.Lee M-K, Moon S-S, Lee S-E, Bok S-H, Jeong T-S, Park YB, Choi M-S. Naringenin 7-O-cetyl ether as inhibitor of HMG-CoA reductase and modulator of plasma and hepatic lipids in high cholesterol-fed rats. Bioorg Med Chem. 2003 Feb;11:393–398. doi: 10.1016/s0968-0896(02)00441-8. [DOI] [PubMed] [Google Scholar]
- 37.Elangovan V, Sekar N, Govindasamy S. Chemopreventive potential of dietary bioflavonoids against 20-methylcholanthrene-induced tumorigenesis. Cancer Lett. 1994 Nov;87:107–113. doi: 10.1016/0304-3835(94)90416-2. [DOI] [PubMed] [Google Scholar]
- 38.Ogasawara M, Matsunaga T, Suzuki H. Differential effects of antioxidants on the in vitro invasion, growth and lung metastasis of murine colon cancer cells. Biol Pharm Bull. 2007 Jan;30:200–204. doi: 10.1248/bpb.30.200. [DOI] [PubMed] [Google Scholar]
- 39.Rauha JP, Remes S, Heinonen M, Hopia A, Kähkönen M, Kujala T, Pihlaja K, Vuorela H, Vuorela P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int J Food Microbiol. 2000 May;56:3–12. doi: 10.1016/s0168-1605(00)00218-x. [DOI] [PubMed] [Google Scholar]
- 40.Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents. 2005 Nov;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song F-L, Gan R-Y, Zhang Y, Xiao Q, Kuang L, Li H-B. Total phenolic contents and antioxidant capacities of selected chinese medicinal plants. Int J Mol Sci. 2010;11:2362–2372. doi: 10.3390/ijms11062362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee C-H, Wu S-L, Chen J-C, Li C-C, Lo H-Y, Cheng W-Y, Lin J-G, Chang Y-H, Hsiang C-Y, Ho T-Y. Eriobotrya japonica leaf and its triterpenes inhibited lipopolysaccharide- induced cytokines and inducible enzyme production via the nuclear factor-kappaB signaling pathway in lung epithelial cells. Am J Chin Med. 2008;36:1185–1198. doi: 10.1142/S0192415X0800651X. [DOI] [PubMed] [Google Scholar]
- 43.Li RW, David Lin G, Myers SP, Leach DN. Anti-inflammatory activity of Chinese medicinal vine plants. J Ethnopharmacol. 2003 Mar;85:61–67. doi: 10.1016/s0378-8741(02)00339-2. [DOI] [PubMed] [Google Scholar]