Abstract
Objective
To investigate the relationship between maternal vitamin D status and glucose intolerance, and its impact on pregnant women and their newborns.
Methods
A cohort of pregnant women were divided into three groups: women with gestational diabetes mellitus, ones with normal results both after the 50 gr and 100 gr OGTT (CG-1) and ones having a positive result after the 50 gr OGTT screening but negative results for gestational diabetes mellitus (GDM) after the 100 gr OGTT (CG-2)
Results
The newborn length in CG-1 was greater than in GDM and CG-2 (p= 0.002 and p= 0.02). Fasting blood glucose and insulin resistance (IR) were negatively correlated with length of the newborns (r=−0.3, p=0.03 and r=−0.3, p=0.01). The newborns of women with GDM had lower APGAR-1 and 5 scores than those of CG-1 and CG-2 (APGAR-1 p= 0.001 and p= 0.004, APGAR-5 p=0.005 and p=0.007, respectively). APGAR scores were correlated negatively with IR (APGAR-1 r=−0.32, p=0.01, APGAR-5 r=−0.3, p=0.03) and positively with 25OHD levels (APGAR-1 r=0.3, p=0.01, APGAR-5 r=0.3, p=0.02).
Conclusion
Vitamin D deficiency, gestational diabetes and insulin resistance are interrelated. Severe vitamin D deficiency during pregnancy is associated with poor pregnancy and neonatal outcome.
Keywords: Vitamin D deficiency, gestational diabetes, insulin resistance, pregnancy and neonatal outcome
Introduction
Vitamin D deficiency is common all around the world and has a prevalence of 26–98% in pregnancy, bringing concerns about its consequences and need for supplementation1–4. As vitamin D receptor is expressed widely in nucleated cells, it does not only have a role in calcium and bone homeostasis but also in various organs and system functions.
Vitamin D has a wide spectrum of extraskeletal effects and its deficiency has various consequences including muscle weakness, impairment of immune system, increase in cardiovascular disease and hypertension, disturbance of neuropsychiatric function and even increased mortality5–8. Taking this wide range of actions into consideration, it is not surprising that Vitamin D also has a role in insulin homeostasis and resistance. It may improve beta-cell activity and increase insulin sensitivity9. Poor Vitamin D status has also been linked with type-2 diabetes, obesity and other elements of metabolic syndrome10,11.
Pregnancy is another condition associated with insulin resistance and hyperinsulinemia. During pregnancy, the effects of diabetogenic hormones cannot be handled in women with insufficient pancreatic reserve, thus gestational diabetes occurs. Gestational diabetes is an important health issue since it has serious consequences involving increased risks of preeclampsia, macrosomia, cesarean delivery, and their associated morbidities.
There have been several reports that tried to ascertain the link between diabetes in pregnancy and maternal vitamin D status. To date, the connection between vitamin D status and gestational diabetes mellitus and/or insulin resistance is not clear. Given what is known in existing literature, the current study aimed to investigate the relationship between maternal vitamin D status with glucose intolerance and its consequences in pregnant women.
Materials and methods
A total of 21 consecutive women with gestational diabetes mellitus (GDM) were included in the study. Age and body mass index-matched 43 healthy pregnant subjects constituted the control group. There were no racial/ ethnic or socioeconomic differences between the groups.
The method used for diagnosing gestational diabetes had two parts. First, a 50 gr oral glucose tolerance test (OGTT) for screening was performed at 24 to 28 weeks of gestation. Plasma glucose, measured one hour later, was considered elevated if it was ≥ 7.8 mmol/L (140 mg/dL). Those with elevated levels were further evaluated with a 100 gr three-hour OGTT after a three-day 120 kcal/day carbohydrate-containing diet and 8 hours of fasting. GDM was diagnosed with at least two abnormal results during 100 gr OGTT: plasma glucose during fasting ≥ 5.8 mmol/L (105 mg/dL) or at one hour ≥ 10.5 mmol/L (190 mg/dL) or at two hour ≥ 9.2 mmol/L (165 mg/dL) or at three hour ≥ 8 mmol/L (145 mg/dL)12. Of the 21 cases with GDM, 6 (29%) were on the diet only whereas 15 (71%) cases were on insulin therapy.
The control group was further stratified by their OGTT results. Women with normal results after both the 50 gr and 100 gr OGTT comprised control group-1 (CG-1) whereas those with elevated glucose levels after the 50 gr OGTT but normal levels after the 100 gr OGTT constituted control group-2 (CG-2).
Demographic features and a medical history which included number of pregnancies, parity and abortus were obtained from all subjects. Height and weight were used to calculate the body mass index (BMI). Gestational week at birth, fetal status, weight and length of the newborn, and APGAR scores were also included. Newborns with weight below the 10th percentile for gestational age were considered small for gestational age (SGA) whereas newborns with birth weight greater than the 90th percentile for age were considered large for gestational age13. APGAR scores were evaluated at one and five minutes (APGAR-1 and 5) of age based on heart rate, respiratory effort, muscle tone, reflex irritability and color of the newborn14.
Fasting blood glucose (FBG), calcium (Ca), parathyroid hormone (PTH) and 25OHD levels of the cases were evaluated from their out-patient clinic registries when the 50 gr-OGTT was performed. Laboratory results were obtained during the study period and were concurrent with physical examination. Insulin resistance (IR) was calculated by the homeostasis model of assessment (HOMA) according to fasting blood glucose and insulin levels15. Cases with HOMA-IR >2.5 were considered to have insulin resistance16.
The study protocol was approved by the ethics committee of the Ministry of Health's antalya education and research hospital (26 December 2011/146). All the subjects read and signed the informed consent forms before enrolling in the study.
The data was statistically analyzed with the SPSS 15.0 package program. Chi-square test was used for categorical variables. Kruskal-Wallis test was used to compare the three groups. The variables with significance were then evaluated by Mann-Whitney U test to investigate difference between the groups. Independent variables affecting HOMA-IR, APGAR score and neonatal length were explored with multiple regression analysis. Spearman's correlation coefficient was used for the calculation of associations between variables. p <0.05 was considered as statistically significant.
Results
The mean age of the patients with GDM was 32.9 ± 4.7 years; it was 29.3 ± 4 in CG-1 cases and 30.9 ± 5.5 years in CG-2 cases (p = 0.09).The mean BMI of patients with GDM and in CG-1 and CG-2 cases were 27.2±4.9, 25.2±4.6 and 24.9±4.5 kg/m2, respectively (p=0.22).
Median numbers of pregnancy in GDM, CG-1 and CG-2 were 2.5 [IQR: 2–3.75], 2 [IQR: 1–3] and 2 [IQR: 1–2], respectively (p=0.1). Median numbers of parity in GDM, CG-1 and CG-2 were 1 [IQR: 1–2], 1 [IQR: 0–2] and 0 [IQR: 0–1.5], respectively (p=0.2). The incidence of abortus was 0 [IQR: 0–1] in the GDM group, 0 [IQR: 0–0] in CG-1 and 0 [IQR: 0–1] in CG-2 (p=0.4).
Median gestational week at birth was 38 [IQR: 36–38] weeks in GDM group, 38 [IQR: 37–40] in CG-1 and 38 [IQR: 38–39] in CG-2 (p=0.3). The mode of delivery and complications pertaining to the current pregnancy during the study period and the median birth weight, length and APGAR scores of the newborns in each group are summarized in (Table I).
Table 1.
Mode of delivery, complications and fetal status in each group
| GDM (n=21) |
CG-1 (n=15) | CG-2 (n=28) | p | |
| Mode of delivery | 0.6 | |||
| Vaginal | 8 (38%) | 5 (33%) | 7 (25%) | |
| C/S | 13 (62%) | 10 (67%) | 21 (75%) | |
| Preeclampsia | 4 (19%) | 1 (7%) | 2 (7%) | 0.3 |
|
Early membrane rupture |
1 (4%) | 0 (0%) | 3 (10%) | 0.4 |
|
Preterm labor (spontaneous) |
8 (38%) | 1 (7%) | 5 (18%) | 0.06 |
|
Newborn-Birth weight (gr) † |
3180 [2700–3480] |
3370 [3150–3729] |
3230 [2960–3505] |
0.4 |
| Classification- | ||||
| Normal | 18 (85%) | 14 (93%) | 24 (86%) | |
| SGA | 1 (4%) | 0 (0%) | 3 (10%) | |
| LGA | 2 (10%) | 1 (7%) | 1 (4%) | |
|
Newborn-Length (cm) † |
50[48.5–51] | 52[51–52] | 50 [50–51] | 0.006* |
| Newborn-APGAR-1† | 7 [6–8] | 8 [8–8] | 8 [7–8] | 0.002* |
| Newborn-APGAR-5† | 8 [8–9] | 9 [9–9] | 9 [8–9] | 0.006* |
C /S cesarean section, IUGR intrauterine growth retardation, TTN transient tachypnea of newborn, SGA small for gestational age, LGA large for gestational age
The results are presented as median and interquartile range [IQR]
There was a tendency towards a difference between the three groups in terms of presence of preterm labor, although the difference was not statistically significant (p=0.06). The newborn length in CG-1 was higher than in GDM and CG-2 (p= 0.002 and p= 0.02, respectively). There was no difference between GDM and CG-2 in terms of median newborn length (p= 0.3). The newborns of the GDM group recorded lower of APGAR-1 and 5 scores than the newborns of CG-1 and CG-2 (for APGAR-1 p= 0.001 and p= 0.004, for APGAR-5 p=0.005 and p=0.007, respectively). The median scores of APGAR-1 and 5 were not different between the newborns of CG-1 and CG-2 (p=0.5 and p=0.9, respectively).
FBG levels were higher in cases with GDM than in CG-1 and CG-2 (p< 0.001 and p=0.004). Cases with higher glucose levels after the 50 gr OGTT (CG-2) also had higher FBG levels than the cases that had normal glucose levels after the 50 gr OGTT (CG-1) (p=0.001). HOMA-IR was significantly higher in patients with GDM compared to the cases in CG-1 (p=0.01). Cases in CG-2 did not have significantly different levels of HOMA-IR than the patients with GDM and the cases in CG-1 (p=0.2 and p=0.1, respectively) (Table II). 25OHD levels were not different between the three groups (p=0.6).
Table 2.
Comparison of HOMA-IR and laboratory values between the three groups
| GDM (n=21) | CG-1 (n=15) | CG-2 (n=28) | p | |
| FBG (mg/dl) | 95 [88.5–105] |
78 [75–87] |
87 [81–90] |
<0.001* |
| HOMA-IR | 2.3 [1.7–3.5] |
1.4 [1.2–1.9] |
1.8 [1.4–2.9] |
0.04* |
| Calcium (mg/dl) | 9.2 [8.9–9.6] |
9.2 [9–9.5] |
9.4 [9–9.5] |
0.8 |
| 25(OH) Dvit (ug/l) | 7.4 [6.1–9.9] |
11.71 [4.5–24.9] |
8.4 [5–15.8] |
0.6 |
| PTH (pg/ml) | 34 [27.9–49.4] |
34.6 [30.5–43.9] |
31 [28–40.9] |
0.4 |
FBG fasting blood glucose, PTH parathyroid hormone. Data was expressed as median and IQR.
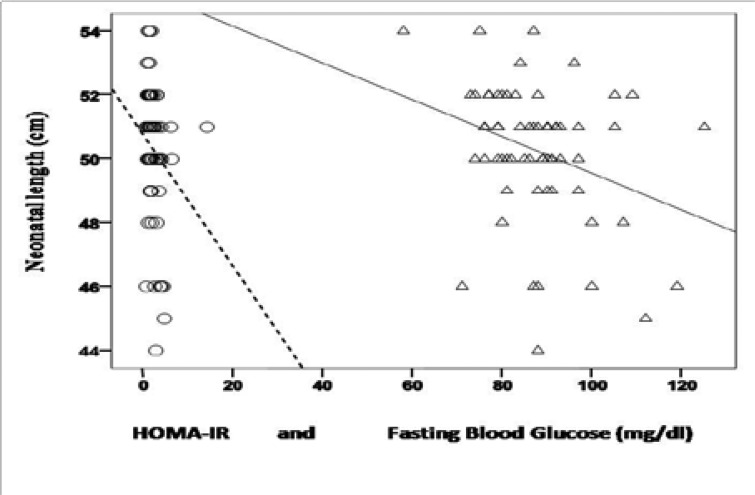
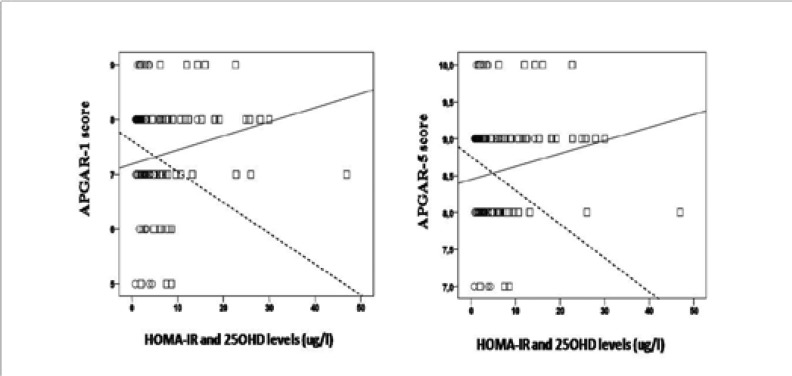
When the whole cohort was taken into consideration, FBG was positively correlated with age of the cases (r=0.3, p=0.02, n=60), BMI (r=0.4, p=0.001, n=61), HOMA-IR (r=0.5, p< 0.001, n=62), and was negatively correlated with length of the newborns (r=−0.3, p=0.03, n=62) (Figure 1). HOMA-IR was positively correlated with BMI (r=0.3, p=0.02, n=63) whereas it was negatively correlated with length of the newborns (r=−0.3, p=0.01, n=64), APGAR- 1 (r=−0.32, p=0.01, n=62) and APGAR-5 (r=−0.3, p=0.03, n=62) (Figure 1 and Figure 2).
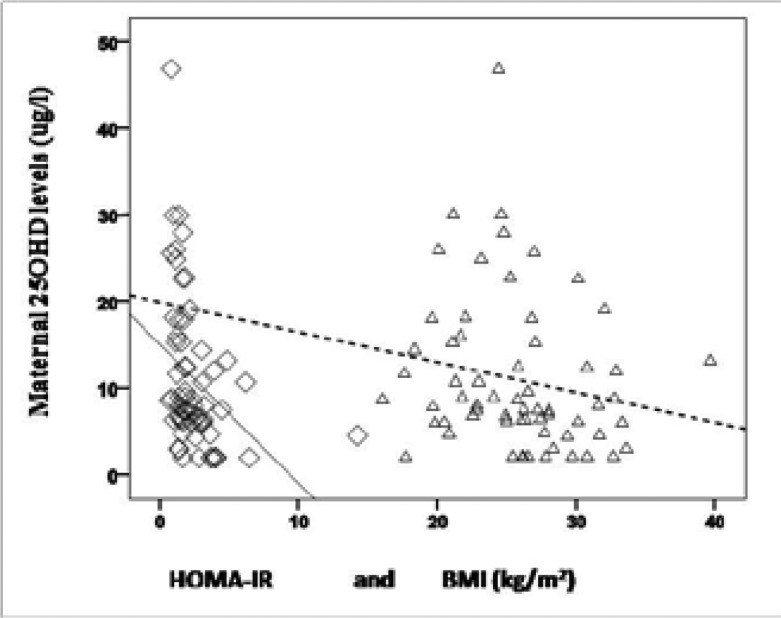
In addition, there was a negative correlation between HOMA-IR and Ca levels (r=−0.3, p=0.02, n=64). 25OHD levels were also negatively correlated with HOMA-IR and BMI (r=−0.5, p< 0.001, n=64 and r=−0.3, p=0.04, n= 63, respectively) (Figure 3).
Maternal levels of 25OHD showed a positive correlation with APGAR-1 and APGAR-5 scores of the newborns (r=0.3, p=0.01, n=62 and r=0.3, p=0.02, n=62) (Figure 2).
Multiple regression analysis was used to test if BMI, 252Dvit levels and FBG predicted HOMA-IR, APGAR score and neonatal length. In the regression analysis for HOMA-IR (adjusted R2= 0.20, F(3,57)=4.71, p=0.005), it was found that 25OHDvit levels significantly predicted HOMA-IR (β=−0.3, p=0.02). In the regression analysis for APGAR score (adjusted R2= 0.10, F(3,55)=3.2, p=0.03) APGAR-1 was associated with FBG (β=−0.35, p=0.01). Neonatal length was also associated with FBG (β=−0.35,p=0.01, adjusted R2= 0.09, F(3,57)=3.012, p=0.03).
Of the cases in GDM group 16 (76%), in CG-1 7(47%) and in CG-2 17 (61%) had severe vitamin D deficiency (25OHDvit levels below 10 ug/l) (p=0.2). When the entire cohort was stratified by levels of 25OHDvit, with a cut-off 10 ug/l, those with levels below 10 ug/l (n=40) had increased HOMAIR compared to the cases who had higher 25OHDvit levels (n=24) ( 2.4 [IQR: 1.5–3.5] and 1.6 [IQR: 1.1–2.1], respectively, p=0.02). Both APGAR-1 and 5 scores of the newborns were lower in case of decreased maternal 25OHDvit. APGAR-1 scores of the newborns with maternal levels below and above 10 ug/l were 7 [IQR: 7–8] and 8 [IQR: 8–8], respectively (p=0.001). APGAR-5 scores of the newborns were 8 [IQR: 8–9] in those with maternal 25OHDvit levels below 10 ug/l and 9 [IQR: 9–9] in those with maternal 25OHDvit levels above 10 ug/l (p=0.002). Number of cases who had preterm labor were higher in women with 25OHDvit levels below 10 ug/l than the women with higher levels (n=12 (30%) and n=2 (8%), respectively, p=0.04). Also preeclampsia was seen more frequently in cases with lower 25OHDvit levels in comparison to the cases with levels above 10 ug/l (n=7 (18%) and n=0 (0%), respectively, p=0.03).
Discussion
The current study shows that low vitamin D levels are associated with increased maternal insulin resistance and body mass index. Decrease in maternal vitamin D levels is also correlated with lower APGAR scores of the newborn. Severe vitamin D deficiency was also associated with increased frequency of preterm labor and preeclampsia. Additionally, increased maternal fasting blood glucose, presence of gestational diabetes and insulin resistance contributed to lower APGAR scores. These results indicate that metabolic condition of the mother is closely associated with health of the newborn and vitamin D deficiency not only contributes to metabolic impairment in the mother but also is related with birth related complications and poor health of the newborn. Both fasting blood glucose and insulin resistance had a negative impact on length of the newborn. Women with gestational diabetes also tended to have preterm labor more frequently than the women without diabetes, although the difference was not statistically significant.
GDM is the onset or first recognition of impaired glucose tolerance during pregnancy17. Since insulin resistance is a major contributing factor for GDM, in the present study, as expected, HOMA-IR was found to be significantly higher in cases with GDM. There are several well-known risk factors for developing GDM, including increased maternal age and body mass index18. In the current study FBG increased with advanced age. Women with GDM tended to be older, but this was not statistically significant.
GDM has various adverse outcomes which tend to increase with higher maternal FBG levels and these outcomes also influence the newborn19–21. Rates of preterm labor increase when pre-gestational diabetes is present22. Likewise, in the present study, women with GDM tended to have higher incidences of preterm labor, but did not reach a statistically significant level of association. In addition, newborns of the women with GDM had lower scores of APGAR in comparison to newborns of women without diabetes. Nevertheless, the median scores were at levels calling for urgent medical attention. Moreover, increased levels of maternal FBG and insulin resistance were associated with lower APGAR scores. Since APGAR reflects general well-being of the newborn, these results suggest that maternal glucose metabolism may influence health status of the newborn.
Interestingly, the median length of the newborns of women without diabetes was significantly higher than those of women with positive results only after the 50 gr OGTT and GDM. The reason why infants of women with diabetes had lower length is inconclusive. However, in the whole cohort, both maternal FBG and HOMA-IR were negatively correlated with length of the newborns. In regression analysis FBG was a reliable predictive factor for decreased newborn length. Impaired maternal glucose metabolism and insulin resistance may be seen as contributing factors. Previous reports indicate that 10–20% of the infants of mothers with diabetes have hypocalcemia and this proportion may reach up to 50%23.
In our study, maternal HOMA-IR was negatively correlated with maternal Ca levels. Thus, increase in insulin resistance was also associated with a decrease in Ca levels. Theoretically, maternal serum Ca levels may have some impact on fetal bone maturation and fetal growth. Accordingly, increased glucose levels and insulin resistance in pregnant women may have indirectly affected length of the newborns. Although statistically insignificant, lower levels of 25OHD in women with GDM may be responsible for lower newborn length in the present study since vitamin D inadequacy may alter fetal skeletal development24,25. However, further studies are necessary for a certain explanation.
The relationship between Vitamin D deficiency and GDM is controversial. While a number of previous studies have found an association between decreased levels of 25OHD and GDM, others have failed to show such an association26–30. In the current study, 25OHD levels were 36.7% and 28% lower in the groups of GDM and CG-2, respectively. However, this decrease was not statistically significant. A distinctive characteristic and one of the limitations of our study was the overwhelmingly lower levels 25OHD than found in previous studies. Ethnicity may be a major contributing factor since 25OHD levels may vary between different ethnic groups27,29. All cases in our cohort were of the same ethnic origin. In the whole cohort, lower levels of 25OHD were associated with higher HOMA-IR and increased BMI, suggesting vitamin D deficiency during pregnancy may be related to the components of metabolic syndrome, namely insulin resistance and higher BMI. Also in regression analysis vitamin D levels were predictive of insulin resistance. Additionally, both human and animal studies show maternal Vitamin D deficiency also has adverse perinatal outcomes31,32. Although the exact mechanism has not been found, in the light of this data, it was not surprising that in our study, lower levels of 25OHD were associated with lower APGAR scores.
Most striking examples for impact of low maternal vitamin D levels on maternal and fetal outcomes were revealed when the whole cohort was grouped based on presence and absence of severe vitamin D deficiency, with a cut-off of 10 ug/l. Severe vitamin D deficiency was associated with maternal insulin resistance, increased frequencies of preterm labor and preeclampsia, and with decreased APGAR scores of the newborns. This means extra caution both for the mother and the newborn should be taken when physicians deal with pregnancies especially in the presence of severe vitamin D deficiency.
Limitations
This study was not without certain limitations. First the method for screenin GDM is still controversial. Although certain organizations recommend one step aprroach with 75-gram OGTT others recommend two step approach or any of them15,33–36. With one step approach more women are labeled with GDM and it is uncertain whether there will be any benefit from treating them all, rendering this approach not cost effective.15 Therefore we preferred to use the two-step approach. Moreover in addition to extremely low levels of 25OHD, other limitations include the measurement of the levels once during whole pregnancy and uncertainty of the contributing factors to the low levels including diet, lifestyle and medications. Further studies are needed to demonstrate the relation between maternal serum 25OHD concentration and fetal development and perinatal outcome.
Conclusion
Lower maternal serum vitamin D is related with both maternal insulin resistance and adverse perinatal outcomes of newborns. Particularly severe vitamin D deficiency during pregnancy may have detrimental effects. Infants of the women with vitamin D deficiency and/or gestational diabetes mellitus should be carefully evaluated. During pregnancy, insulin resistance and vitamin D status are interrelated, so while dealing with one of them the other should also be taken into consideration.
Acknowledgements
None
Declaration of interest
All the authors declare that they have no conflict of interest.
Funding
This study did not receive any specific grant from any funding agency in the public commercial, or not-for-profit sector.
References
- 1.Hamilton SA, McNeil R, Hollis BW, Davis DJ, Winkler J, Cook C, et al. Profound Vitamin D Deficiency in a Diverse Group of Women during Pregnancy Living in a Sun-Rich Environment at Latitude 32 degrees. N Int J Endocrinol. 2010;2010:917428. doi: 10.1155/2010/917428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D deficiency and insufficiency is common during pregnancy. Am J Perinatol. 2011;28(1):7–12. doi: 10.1055/s-0030-1262505. [DOI] [PubMed] [Google Scholar]
- 3.Karim SA, Nusrat U, Aziz S. Vitamin D deficiency in pregnant women and their newborns as seen at a tertiary-care center in Karachi, Pakistan. Int J Gynaecol Obstet. 2011;112(1):59–62. doi: 10.1016/j.ijgo.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 4.Teale GR, Cunningham CE. Vitamin D deficiency is common among pregnant women in rural Victoria. Aust N Z J Obstet Gynaecol. 2010;50(3):259–261. doi: 10.1111/j.1479-828X.2010.01147.x. [DOI] [PubMed] [Google Scholar]
- 5.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88(12):5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 6.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 7.Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, et al. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 2012;79(13):1397–1405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schottker B, Ball D, Gellert C, Brenner H. Serum 25-hydroxyvitamin D levels and overall mortality. A systematic review and meta-analysis of prospective cohort studies. Ageing Res Rev. 2013;12(2):708–718. doi: 10.1016/j.arr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Snijder M, van Dam R, Visser M, Deeg D, Seidell J, Lips P. To: Mathieu C, Gysemans C, Giulietti A, Bouillon R, (2005) Vitamin D and diabetes. Diabetologia 48:1247–1257. Diabetologia. 2006;49(1):217–218. doi: 10.1007/s00125-005-0047-9. [DOI] [PubMed] [Google Scholar]
- 10.Kayaniyil S, Vieth R, Retnakaran R, Knight JA, Qi Y, Gerstein HC, et al. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care. 2010;33(6):1379–1381. doi: 10.2337/dc09-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59(1):242–248. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28(12):1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 13.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 14.Apgar V. A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg. 1953;32(4):260–267. [PubMed] [Google Scholar]
- 15.Vandorsten JP, Dodson WC, Espeland MA, Grobman WA, Guise JM, Mercer BM, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29(1):1–31. [PubMed] [Google Scholar]
- 16.Bastard JP, Grimaldi A, Jardel C, Porquet D, Bruckert E, Hainque B. A simple index of insulin resistance. Diabetes Metab. 1997;23(1):87–88. [PubMed] [Google Scholar]
- 17.Committee opinion no. 504: screening and diagnosis of gestational diabetes mellitus. Obstet Gynecol. 2011;118(3):751–753. doi: 10.1097/AOG.0b013e3182310cc3. [DOI] [PubMed] [Google Scholar]
- 18.Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278(13):1078–1083. [PubMed] [Google Scholar]
- 19.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 20.Landon MB, Mele L, Spong CY, Carpenter MW, Ramin SM, Casey B, et al. The relationship between maternal glycemia and perinatal outcome. Obstet Gynecol. 2011;117(2 Pt 1):218–224. doi: 10.1097/aog.0b013e318203ebe0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suevo DM. The infant of the diabetic mother. Neonatal Netw. 1997;16(5):25–33. [PubMed] [Google Scholar]
- 22.Sibai BM, Caritis SN, Hauth JC, MacPherson C, VanDorsten JP, Klebanoff M, et al. Preterm delivery in women with pregestational diabetes mellitus or chronic hypertension relative to women with uncomplicated pregnancies. The National institute of Child health and Human Development Maternal- Fetal Medicine Units Network. Am J Obstet Gynecol. 2000;183(6):1520–1524. doi: 10.1067/mob.2000.107621. [DOI] [PubMed] [Google Scholar]
- 23.Rosenn B, Miodovnik M, Tsang R. Common clinical manifestations of maternal diabetes in newborn infants: implications for the practicing pediatrician. Pediatr Ann. 1996;25(4):215–222. doi: 10.3928/0090-4481-19960401-09. [DOI] [PubMed] [Google Scholar]
- 24.Dror DK. Vitamin D status during pregnancy: maternal, fetal, and postnatal outcomes. Curr Opin Obstet Gynecol. 2011;23(6):422–426. doi: 10.1097/GCO.0b013e32834cb791. [DOI] [PubMed] [Google Scholar]
- 25.Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, et al. Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res. 2010;25(1):14–19. doi: 10.1359/jbmr.090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maghbooli Z, Hossein-Nezhad A, Karimi F, Shafaei AR, Larijani B. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes Metab Res Rev. 2008;24(1):27–32. doi: 10.1002/dmrr.737. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, Qiu C, Hu FB, David RM, van Dam RM, Bralley A, et al. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS One. 2008;3(11):e3753. doi: 10.1371/journal.pone.0003753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parlea L, Bromberg IL, Feig DS, Vieth R, Merman E, Lipscombe LL. Association between serum 25-hydroxyvitamin D in early pregnancy and risk of gestational diabetes mellitus. Diabet Med. 2012;29(7):e25–e32. doi: 10.1111/j.1464-5491.2011.03550.x. [DOI] [PubMed] [Google Scholar]
- 29.Clifton-Bligh RJ, McElduff P, McElduff A. Maternal vitamin D deficiency, ethnicity and gestational diabetes. Diabet Med. 2008;25(6):678–684. doi: 10.1111/j.1464-5491.2008.02422.x. [DOI] [PubMed] [Google Scholar]
- 30.Baker AM, Haeri S, Camargo CA, Jr, Stuebe AM, Boggess KA. First-trimester maternal vitamin D status and risk for gestational diabetes (GDM) a nested case-control study. Diabetes Metab Res Rev. 2012;28(2):164–168. doi: 10.1002/dmrr.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leffelaar ER, Vrijkotte TG, van Eijsden M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam Born Children and their Development cohort. Br J Nutr. 2010;104(1):108–117. doi: 10.1017/S000711451000022X. [DOI] [PubMed] [Google Scholar]
- 32.Becker A, Eyles DW, McGrath JJ, Grecksch G. Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behav Brain Res. 2005;161(2):306–312. doi: 10.1016/j.bbr.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Blumer I, Hadar E, Hadden DR, Jovanovic L, Mestman JH, Murad MH, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227–4249. doi: 10.1210/jc.2013-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol. 2013;122(2 Pt 1):406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 35.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 36.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]