Abstract
Background
Epidemiologic data indicates that rheumatoid arthritis is an independent risk factor for cardiovascular disease. Epicardial adipose tissue is a novel cardio-metabolic risk factor. Our aim was to evaluate epicardial fat thickness (EFT) using echocardiography in patients with rheumatoid arthritis compared to healthy control subjects. Secondly, we investigated relationship between epicardial fat thickness and clinical and echocardiographic parameters in patients with rheumatoid arthritis.
Method
The study population included 76 consecutive patients with rheumatoid arthritis (64 female; mean age, 53 ±11 years, median disease duration, 7.8 years) and 50 healthy subjects as controls (39 female; mean age, 52 ± 6 years). All patients underwent echocardiography to assess left ventricular diastolic dysfunction, left ventricular hypertrophy and EFT. All values were compared between groups.
Results
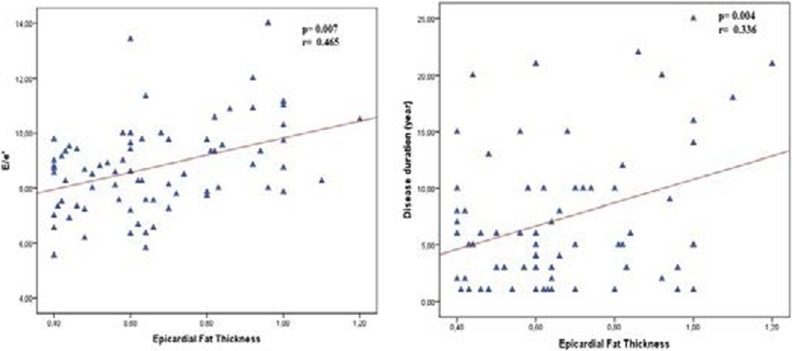
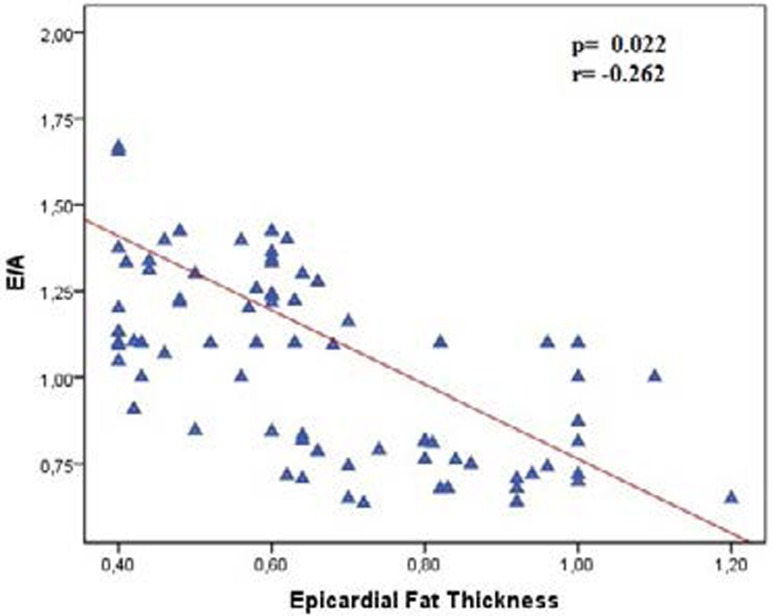
EFT was higher in rheumatoid arthritis patients than in healthy controls (0.66±0.20 vs. 0.54±0.18; p= 0.003). Thickness of Intra Ventricular Septum (IVS) (1.1±0.06 and 9.8±0.08; p=0.001) and posterior wall (PW) (0.98±0.05 and 0.93±0.08; p=0.015) was higher in patients with rheumatoid arthritis compared to healthy controls. Early diastolic myocardiac peak velocity or late diastolic mitral peak velocity (E/A) ratio was lower in rheumatoid arthritis patients compared to healthy patients (1.1 ±0.8 and 1.24±0.1 p=0.001) as well as, E/e' was higher in Rheumatoid arthritis (RA) patients than healthy patients. (E/e':8.7±1.6 and 8.0±1.4 p=0.020). In patients with rheumatoid arthritis, EFT was positively correlated with hypertension and duration of disease and E/e' (r: 0.10, p: 0.010, r: 0.306, p: 0.004 and r: 0.465 p: 0.007 respectively) and EFT was negatively correlated with E/A (r: −.262 p:0.022)
Conclusion
To our knowledge, this is the first report about epicardial adipose tissue in rheumatoid arthritis patients. Epicardial fat thickness as an indicator of cardiovascular involvement was higher in rheumatoid arthritis patients.
Keywords: Rheumatoid arthritis, epicardial fat thickness, cardiac involvement
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disease characterized by chronic symmetric and erosive synovitis that preferentially affects peripheral joints, with prevalence of 0.5– 1% in the population1. Epidemiologic data indicates that RA is an independent risk factor for cardiovascular disease (CVD)2,3. Emerging epidemiological evidence showed that CVDs account for approximately 50% of all RA associated deaths4.
Epicardial adipose tissue (EAT), a type of visceral adipose tissue, is considered to play a pivotal role in the pathogenesis of coronary artery disease (CAD). Recently, several studies have demonstrated that epicardial fat is associated with insulin resistance5, increased cardio-metabolic risk6, inflammatory markers7,8 and coronary artery disease9,10. The echocardiographic measurement of EAT, is an objective, noninvasive, readily available, and less expensive measure than magnetic resonance imaging or computed tomography.
Our aim was to evaluate epicardial fat thickness (EFT) using echocardiography in patients with rheumatoid arthritis compared to healthy control subjects. Secondly, we investigated the relationship between EFT and clinical and echocardiographic parameters in patients with rheumatoid arthritis.
Methods
Study Population
The study population included 76 consecutive patients with rheumatoid arthritis (64 female; mean age, 53 ±11 years, and median disease duration, 7.8 years) and 50 healthy subjects as controls (39 female; mean age, 52± 6 years). All patients met the American College of Rheumatology's grading criteria for a diagnosis of RA11. A detailed history and analysis of patients was performed. The inclusion criteria for the study groups were: age ≥18 years, patient's informed consent, absence of any acute disease. Patients with any of the following features were excluded from participation: patients with valvular heart disease; chronic obstructive pulmonary disease, any other significant systemic disease, obstructive coronary artery disease, history of heart failure, hepatic failure, hypertension, serum creatinine >1.4 mg/dL, patients with a history of diabetes mellitus (or fasting blood glucose >125 mg/dL), pregnant women, patients with hypo or hyperthyroidism, patients with a BMI >25 kg/m2, and patients who wished to consume alcohol during the study period. Also, we excluded patients with : heart failure, significant valvular heart disease, pacemaker implantation, atrial flutter or fibrillation, frequent ventricular pre-excitation and atrioventricular conduction abnormalities, renal failure, previous myocardial infarction, or cerebrovascular accident and poor echocardiographic imaging.
This study was conducted in accordance with the Declaration of Helsinki and was approved by our local ethics committee. Informed consent for the procedure was obtained from each patient.
Echocardiography
All patients underwent echocardiography. Following a resting period of 15 min, all the patients underwent two-dimensional and Doppler echocardiographic evaluation, including tissue Doppler imaging (TDI) with the echocardiogram device using a 3.5-MHz transducer. Echocardiograms of all patients were recorded as standard parasternal and apical images with the patients lying in the left lateral position. The measurement and recordings were carried out as normal inspiratory and end-expiratory. Doppler records of M-mode, pulse and continuous waves were obtained for each case. All the measurements were performed based on the standards of the American Society of Echocardiography by the same cardiologist. Left atrial diameter (LAD), left ventricular end-diastolic diameter (LVEDD), ejection fraction (EF), intraventricular septum thickness (IVS), posterior wall thickness (PW) values were defined from the recordings obtained with the conventional echocardiography. In the pulsed-wave echocardiographic transmittal flow screenings, early diastolic mitral peak velocity (E), late diastolic mitral peak velocity (A), were measured based on the reference images of the apical 4 chamber. On TDI, early diastolic myocardial peak velocity (é) was recorded with apical 4-chamber images using a sampling volume of 5 mm in the septal and lateral mitral annular regions. All Doppler measurements were carried out manually E/A, and E/é.
Epicardial fat thickness was evaluated on the free wall of the right ventricle from the parasternal long-axis view, using the aortic annulus as an anatomic reference. Epicardial fat thickness, identified as an echo-free space between the myocardium and visceral pericardium on two-dimensional echocardiography, was measured perpendicularly, ahead of the right ventricular free wall, at the end of diastole, for three cardiac cycles12.
Laboratory
Blood samples were drawn by venipuncture to measure routine blood chemistry parameters after fasting for at least eight hours. Fasting blood glucose, serum creatinine, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglyceride levels were recorded. Glucose, creatinine, and lipid profile were determined using standard methods.
Statistical analysis
The statistical analyis was performed using software (SPSS 18.0). Parametric values were given as mean ± standard deviation and non-parametric values were given as a percentage. To compare parametric continuous variables, the Student's t-test was used; to compare nonparametric continuous variables, the Mann-Whitney U-test was used. Categorical data was compared by the Chi-square distribution. Correlation analysis was performed to determine the relationship between epicardial fat tissue and other cardio-metabolic risk factor. Two-tailed P-values of less than 0.05 were considered to indicate statistical significance.
Results
Clinical Features
According to the basic clinical and demographic characteristics, both groups of the study were similar with regard to age, body mass index, fasting glucose, hypertension, diabetes mellitus, and smoking status (table 1).
Table 1.
Basal characteristic of patients
| Rheumatoid arthritis (n=76) |
Healthy controls (n=50) |
P value | |
| Age | 53 ± 11 | 52 ± 6 | 0.430 |
| Gender (Female) | 64 84% | 39 78% | 0.257 |
| Diabetes mellitus, % | 8 10% | 6 11% | 0.105 |
| Hypertension, % | 35 47% | 21 43% | 0.401 |
| Smoking, % | 7 9.2% | 6 12.2% | 0.416 |
| Rheumatoid factor | 23.7 (1.0–200) | - | |
| Disease duration, years | 7.84 (2.4–30) | - | |
| Steroid use, n | 29 (38%) | - | |
| C-reactive protein, mg/dl | 19.2 ± 46.3 | 1.7 ± 3.7 | 0.007 |
| Body mass Index | 32 ± 6 | 34 ± 8 | 0.122 |
| Waist circumstance | 101.12±13.62 | 103.18±14.53 | 0.424 |
| Glucose, mg/dl | 93.18±16.83 | 98.7333±13.26 | 0.770 |
| Triglyceride, mg/dl | 137.75±52.29 | 149.41±60.99 | 0.360 |
| High density lipoprotein, mg/dl | 42.87±6.98 | 41.81±7.0 | 0.494 |
| Total cholesterol, mg/dl | 203.70±53.70 | 211.31±47.38 | 0.529 |
| Low density lipoprotein, mg/dl | 126.62±34.28 | 122.16±33.384 | 0.553 |
Echocardiographic data
Comparison of the baseline echocardiographic values among rheumatoid arthritis patients and healthy controls are shown in table 2. Thickness of IVS (1.1±0.06 and 9.8±0.08; p=0.001) and PW (0.98±0.05 and 0.93±0.08; p=0.015) were higher in patients with rheumatoid arthritis compared to healthy controls.
Table 2.
Echocardiographic features of patients
| Rheumatoid arthritis (n=76) |
Healthy controls (n=50) |
P value | |
| Epicardial fat thickness,cm | 0.66 ± 0.20 | 0.54 ± 0.18 | 0.003 |
| Ejection fraction, % | 63 ± 6 | 62 ± 4 | 0.733 |
| LVEDD,cm | 4.5±0.31 | 4.4±0.27 | 0.536 |
| LAD,cm | 3.43±0.42 | 3.46±0.52 | 0.647 |
| IVS,cm | 1.1±0.06 | 0.98±0.08 | 0.010 |
| PW, cm | 0.98±0.05 | 0.93±0.08 | 0.015 |
| Diastolic function | |||
| E cm/s | 74±10 | 86±12 | 0.001 |
| A cm/s | 76±14 | 73±9 | 0.140 |
| E/A | 1.1±0.8 | 1.24±0.1 | 0.001 |
| e' cm/s | 8.6±1.5 | 11±1.6 | 0.001 |
| E/e' | 8.7 ±1.6 | 8.0 ± 1.4 | 0.020 |
E wave and E/A ratio was lower in rheumatoid arthritis patients compared to healthy patients (E: 74±10 and 86±12 p=0.01 vs E/A: 1.1 ±0.8 and 1.24±0.1 p=0.001) as well as, E/e' was higher in RA patients than non-rheumatoid arthritis patients. (E/e':8.7±1.6 and 8.0±1.4 p=0.020). EFT was higher in rheumatoid arthritis patients compared to healthy controls (0.66±0.20 vs. 0.54±0.18; p:0.003). In patients with rheumatoid arthritis, EFT was positively correlated with hypertension and duration of disease and E/e' (r: 0.10 p:0.010, r:0.306 p: 0.004 and r:0.465 p: 0.007 respectively) (Figure 1) and EFT was negatively correlated with e/a (r: −.262 p:0.022 and) (Figure 2).
Figure 1.
Figure 2.
Left ventricular diastolic dysfunction was detected in 30 (39%) of 75 patients with rheumatoid arthritis; 24 patients presented with diastolic dysfunction I and 6 patients presented with diastolic dysfunction II.
Discussion
In our study, we showed that EFT was higher in patients with rheumatoid arthritis than in the healthy control. Secondly, we demonstrated that left ventricular wall thickness and diastolic dysfunction were higher and EFT was well correlated with diastolic dysfunction and disease duration in patients with rheumatoid arthritis. These findings may be associated with cardiovascular involvement in patients with rheumatoid arthritis.
Rheumatoid arthritis is linked with an increase in mortality because of stimulation of coronary and cerebrovascular atherosclerosis.13 Emerging epidemiological evidence showed that CVDs account for approximately 50% of all RA associated deaths4. EAT, a type of visceral adipose tissue, is thought to play a pivotal role in the pathogenesis of coronary artery disease (CAD). EAT releases a wide range of biologically active molecules that modulate vascular smooth-muscle contraction. Their paracrine effects might be attributable to their location being close to the adventitia and extravascular bed14–16 Gastaldelli et al.16 reported the existence of a link between EAT and hypertension, atherosclerosis, and coronary heart disease. Nakanishi and colleagues17 reported that increased epicardial fat volume measured by CT is associated with greater progression of coronary artery calcification. Transthoracic echocardiography provides non-invasive assessment of EFT6, 12. Several studies have emphasized the link between EFT and the severity of coronary artery disease (CAD)19–21. EFT has an important role in the inflammatory process within the atherosclerotic plaque9. In our study, we demonstrated that EFT was higher in patients with rheumatoid arthritis. In addition , we showed that EFT was associated with duration of disease, hypertension, and diastolic dysfunction in patients with rheumatoid arthritis. These findings indicate that rheumatoid arthritis patients may be having an underlying risk of cardiovascular disease.
Isolated diastolic dysfunction is related to prominent increase in all-cause mortality in the general population22,23. Left ventricular diastolic dysfunction (LVDD) is frequently related to common structural abnormalities, such as hypertrophy or interstitial fibrosis, and impaired myocyte relaxation due to ischemia24. Previous studies showed the existence of LVDD in patients with RA without clinically prominent cardiac disease.25–27. Liang et al. investigated the prevalence of LVDD in patients with RA. They found that patients with RA have a higher prevalence of LVDD than those healthy controls and RA duration is also independently associated with LVDD28. In our study, LVDD was detected in 30 (39%) of 75 patients with rheumatoid arthritis; 24 patients presented with diastolic dysfunction I and 6 patients presented with diastolic dysfunction II. Rudominer et al. showed that left ventricular hypertrophy is higher compared to healthy patients3. LV hypertrophy predicts cardiovascular outcomes independent of traditional risk factors30–32. In our study, we found that left ventricular wall thickness was increased in patients with rheumatoid arthritis.
The use of the Doppler echocardiography technique to evaluate left ventricular filling by trans-mitral flow is considered a reliable method.2 The relation between trans-mitral flow variation and disease duration in rheumatoid arthritis indicate a subclinical myocardial involvement.25 In our study, we showed that diastolic function in patients with rheumatoid arthritis was impaired compared to those healthy patients. In addition, we demonstrated that disease duration in patients with rheumatoid arthritis was associated with diastolic dysfunction: consistent with previous studies.
Limitations
Some limitations of this study are evident. The primary limitation of our study was the small sample size. A small sample size has low statistical power and, thus, may yield false-negative results. The other limitation of our study is its cross-sectional design. The results cannot be generalized to the general population. Neither can we apply our results to the general population due to the numerous exclusion criteria. Despite this, we believe that our findings provide a valuable contribution to the EFT and RA. Future prospective much larger multicenter studies are required to confirm our results.
Conclusion
Epicardial fat thickness as an indicator of cardiovascular involvement was higher in patients with rheumatoid arthritis. Also, we showed that diastolic dysfunction and left ventricular hypertrophy were higher in rheumatoid arthritis patients. These findings suggest that subclinical cardiac involvement in patients with RA and those patients may be underlying risk factors for development of cardiovascular disease.
Conflict of Interest: None
References
- 1.Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27(2):269–281. doi: 10.1016/s0889-857x(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 2.Meune C, Touze E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology. 2009;48(10):1309–1313. doi: 10.1093/rheumatology/kep252. [DOI] [PubMed] [Google Scholar]
- 3.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59(12):1690. doi: 10.1002/art.24092. PubMed -7. [DOI] [PubMed] [Google Scholar]
- 4.Yazmalar L, Ediz L, Alpayci M, Hiz O, Toprak M, Tekeoglu I. Seasonal disease activity and serum vitamin D levels in rheumatoid arthritis, ankylosing spondylitis and osteoarthritis. Afr Health Sci. 2013 Mar;13(1):47–55. doi: 10.4314/ahs.v13i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cengel A. Epicardial adipose tissue, metabolic syndrome, inflammation, and cardiovascular risk. Turk Kardiyol Dern Ars. 2012 Dec;40(8):696–698. doi: 10.5543/tkda.2012.60669. [DOI] [PubMed] [Google Scholar]
- 6.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88(11):5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 7.Malavazos AE, Ermetici F, Cereda E, Coman C, Locati M, Morricone L, et al. Epicardial fat thickness: relationship with plasma visfatin and plasminogen activator inhibitor-1 levels in visceral obesity. Nutr Metab Cardiovasc Dis. 2008;18(8):523–530. doi: 10.1016/j.numecd.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Kremen J, Dolinkova M, Krajickova J, Blaha J, Anderlova K, Lacinova Z, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91(11):4620–4627. doi: 10.1210/jc.2006-1044. [DOI] [PubMed] [Google Scholar]
- 9.şengül C, Özveren O. Epicardial adipose tissue: a review of physiology, pathophysiology, and clinical applications. Anadolu Kardiyol Derg. 2013 May;13(3):261–265. doi: 10.5152/akd.2013.075. [DOI] [PubMed] [Google Scholar]
- 10.Iacobellis G, Lonn E, Lamy A, Singh N, Sharma AM. Epicardial fat thickness and coronary artery disease correlate independently of obesity. Int J Cardiol. 2011;146(3):452. doi: 10.1016/j.ijcard.2010.10.117. PubMed -4. [DOI] [PubMed] [Google Scholar]
- 11.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315. doi: 10.1002/art.1780310302. PubMed -24. [DOI] [PubMed] [Google Scholar]
- 12.Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11(2):304. doi: 10.1038/oby.2003.45. PubMed -10. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan MJ. Cardiovascular disease in rheumatoid arthritis. Curr Opin Rheumatol. 2006;18(3):289. doi: 10.1097/01.bor.0000218951.65601.bf. PubMed -97. [DOI] [PubMed] [Google Scholar]
- 14.Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci (Lond) 2012;122(1):1–12. doi: 10.1042/CS20110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachar GN, Dicker D, Kornowski R, Atar E. Epicardial adipose tissue as a predictor of coronary artery disease in asymptomatic subjects. Am J Cardiol. 2012;110(4):534. doi: 10.1016/j.amjcard.2012.04.024. PubMed -8. [DOI] [PubMed] [Google Scholar]
- 16.Gastaldelli A, Basta G. Ectopic fat and cardiovascular disease: what is the link? Nutr Metab Cardiovasc Dis. 2010;20(7):481–490. doi: 10.1016/j.numecd.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi R, Rajani R, Cheng VY, Gransar H, Nakazato R, Shmilovich H, et al. Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: a serial study using non-contrast cardiac CT. Atherosclerosis. 2011;218(2):363. doi: 10.1016/j.atherosclerosis.2011.07.093. PubMed -8. [DOI] [PubMed] [Google Scholar]
- 18.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaowalit N, Somers VK, Pellikka PA, Rihal CS, Lopez-Jimenez F. Subepicardial adipose tissue and the presence and severity of coronary artery disease. Atherosclerosis. 2006;186(2):354. doi: 10.1016/j.atherosclerosis.2005.08.004. PubMed -9. [DOI] [PubMed] [Google Scholar]
- 20.Jeong JW, Jeong MH, Yun KH, Oh SK, Park EM, Kim YK, et al. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J. 2007;71(4):536. doi: 10.1253/circj.71.536. PubMed -9. [DOI] [PubMed] [Google Scholar]
- 21.Ahn SG, Lim HS, Joe DY, Kang SJ, Choi BJ, Choi SY, et al. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008;94(3) doi: 10.1136/hrt.2007.118471. PubMed e7. [DOI] [PubMed] [Google Scholar]
- 22.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194. doi: 10.1001/jama.289.2.194. PubMed -202. [DOI] [PubMed] [Google Scholar]
- 23.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296(18):2209. doi: 10.1001/jama.296.18.2209. PubMed -16. [DOI] [PubMed] [Google Scholar]
- 24.Udayakumar N, Venkatesan S, Rajendiran C. Diastolic function abnormalities in rheumatoid arthritis: relation with duration of disease. Singapore Med J. 2007;48(6):537. PubMed -42. [PubMed] [Google Scholar]
- 25.Di Franco M, Paradiso M, Mammarella A, Paoletti V, Labbadia G, Coppotelli L, et al. Diastolic function abnormalities in rheumatoid arthritis. Evaluation By echo Doppler transmitral flow and pulmonary venous flow: relation with duration of disease. Ann Rheum Dis. 2000;59(3):227. doi: 10.1136/ard.59.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]