Abstract
Background
Although high-performance liquid chromatography (HPLC) is the commonly used method for the analysis of retinol in biological samples, simple and rapid test kits are available.
Objectives
This study compared a rapid test kit (ICHECK Fluoro®) to HPLC for the assessment of serum retinol concentrations.
Methods
For the analysis by HPLC, sample preparation included standard deproteinization and extraction phases. The analysis by ICHECK was performed by injecting serum into IEX reagent vials (n=89) and mixing manually for separation. After precipitation of the proteins, the vial was introduced into the chamber of the ICHECK Fluoro and analysed at 0 min (ICHECK0min) and 15 min later (ICHECK15min). Bland and Altman approach was applied to test the agreement between HPLC and ICHECK.
Results
Mean HPLC, ICHECK0min and ICHECK15min values were 421.2±106.0 µg/L, 423.1±118.3 µg/L and 413.2±107.6 µg/L, respectively. Retinol concentrations significantly decreased in the IEX solution over time (p<0.001). No significant proportional bias was observed between HPLC and ICHECK0min (r-0.038, p=0.73) and ICHECK15min (r=−0.024, p=0.82). Fixed biases (HPLC minus ICHECK) for ICHECK0min and ICHECK15min were respectively −1.9±23.1 µg/l (p=0.45) and 8.0±22.7 µg/l (p=0.002).
Conclusion
ICHECK Fluoro may offer a reliable mean for assessing serum retinol for measurements performed with no significant time delay.
Keywords: HPLC, ICHECK Fluoro, serum retinol, test kit, vitamin A status
Introduction
Vitamin A deficiency (VAD) is a major health issue worldwide with significant impact on the disease burden1–3. VAD is the leading cause for preventable sight-related diseases mainly xerophtalmia and blindness, and to the depletion of the immune function, increasing the risk of morbidity and mortality especially in children and pregnant women4,5. The prevalence of VAD has tremendously decreased worldwide, due to nutrition interventions including food fortification, mandatory supplementation, dietary diversification implementation, and nutritional education6,7. However, about 190 million children and 19.1 million pregnant women are still estimated as deficient in vitamin A, most of them are living in Africa and South-East Asia8.
The methods used to assess vitamin A status include clinical signs, serum retinol assessment, dose-response tests, and labelled isotopes; some of these methods even permit liver stores estimation9. At the population level, serum retinol is the most commonly recommended indicator to assess vitamin A status10,11 and high-performance liquid chromatography (HPLC) is the most widely used technique for measuring retinol concentrations in serum samples12. Thus, assessment of vitamin A status during and after nutritional interventions is routinely performed by measuring serum or plasma retinol levels.
ICHECK Fluoro ® is a recent kit that can be used for the analysis of vitamin A concentrations in both fortified foods and biological fluids13. The kit uses a portable analyzing module and appears as reliable and relatively affordable compared to laboratory-based methods, especially in developing countries. Analysis by ICHECK includes three major steps: injection of the sample into a reagent solution vial (IEX Mila ®), separation by vigorous manual mixing, and measurement in the portable device chamber.
The aim of our study was to evaluate the validity of the ICHECK for assessing serum retinol concentrations, comparatively to HPLC. Secondly, we hypothesized that working conditions in the field may result in some delay in the time of analysis. Therefore, we examined the evolution of retinol in the analysing solutions after a short period of time to assess the level of conservation of the retinol in the analysis solutions.
Methods
Serum samples were obtained from participants (n=89) of the PEN project, which is a one-year longitudinal study on vitamins A and D, iron and iodine deficiencies amongst school-age children (7 to 9 years) of a rural region in Morocco. Serum samples were stored in sealed 2.5 ml Eppendorf tubes at −80°C for less than 1 month before analysis. All the children and their parents had been clearly instructed about the protocol of the study and they provided a co-signed consent form. The PEN project was conducted under the ethical approval of the Ministry of Education of Morocco.
Chemicals
Ethanol, hexane, methanol, acetonitrile, retinol, retinyl acetate and butylated hydroxytoluene (BHT) of HPLC grade were purchased from Sigma-Aldrich (St. Louis, Mo, USA). IEX Mila extraction kit was purchased from Bioanalyt (Potsdam, Germany).
HPLC
The HPLC consisted of a Waters system (Waters, Milford, MA, USA), a 2695 separation module, equipped with a 2996 PDA detector, a precolumn and a column C18 Sunfire, 5µm, 4.6x250 mm. Serum samples were thawed at room temperature for 15 minutes. A volume of 1 ml of a solution of ethanol-BHT 0.1% (w/v) was added to an equal volume of serum and vortexed for 3 min to precipitate the proteins. Subsequently, 2 ml of hexane was added to the ethanol phase, followed by a cold centrifugation for 5 min at 3000 rpm, 4°C. Hexane in upper phase was recuperated and the extraction process was repeated. The recuperated hexane phases were combined, gently mixed and 1 ml was evaporated to dryness under nitrogen steam. The residue was reconstituted in 150 µL methanol and analyzed by HPLC. Retinol was detected at 325 nm. Mobile phase was constituted of methanol-acetonitrile (85%/15%, v/v). Retinyl acetate was used as internal standard and prepared on the day of analysis. Retinol was dissolved in ethanol and used as external standard. All the analysis was performed under yellow-orange light to prevent the degradation of retinoids.
ICHECK
The ICHECK Fluoro device was manufactured by Bioanalyt (Potsdam, Germany). A set of 96 analyzing IEX vials was used: 89 were analysed, 5 were used for control and 2 were discarded. Serum samples were allowed to thaw at room temperature for 15 minutes. Then, a volume of 500 µl of serum was added to the IEX solution vial using 1ml syringe. The IEX vial was held between the thumb and the index fingers and vigorously mixed for 10 seconds. After precipitation of the proteins, the IEX vial was introduced into the ICHECK chamber and assayed at time 0 (ICHECK0min) and after 15 min (ICHECK15min). Calibration of the ICHECK device was performed using a sealed calibration solution provided by the manufacturer.
Statistical analysis
Data was analysed using STATA 12 (StataCorp, College Station, TX, USA). Descriptive statistics were presented as mean±standard deviation (SD). The relation between HPLC and ICHECK measures was determined by the correlation of Pearson. Agreement between HPLC and ICHECK measures was examined using Bland and Altman analysis. Fixed biases (HPLC minus ICHECK values or ICHECK15min minus ICHECK0min) and limits of agreement (fixed bias±2SD) were calculated for each ICHECK measure. The significance of the fixed bias was assessed by the paired t-test. Proportional biases were assessed by the test of Pitman14. The non-significance of fixed and proportional biases was used as criteria for agreement. The statistical significance was considered at a p-value<0.05.
Results
Serum retinol levels measured by HPLC and ICHECK are presented in Table 1.
Table 1.
Serum retinol by HPLC and ICHECK
| Mean (µg/L. | SD | Range | |
| HPLC | 421.2 | 106.0 | 110 – 695 |
| ICHECK | |||
| ICHECK0min | 423.1 | 108.3 | 108 – 746 |
| ICHECK15min | 413.2 | 107.6 | 105 – 730 |
SD, standard deviation
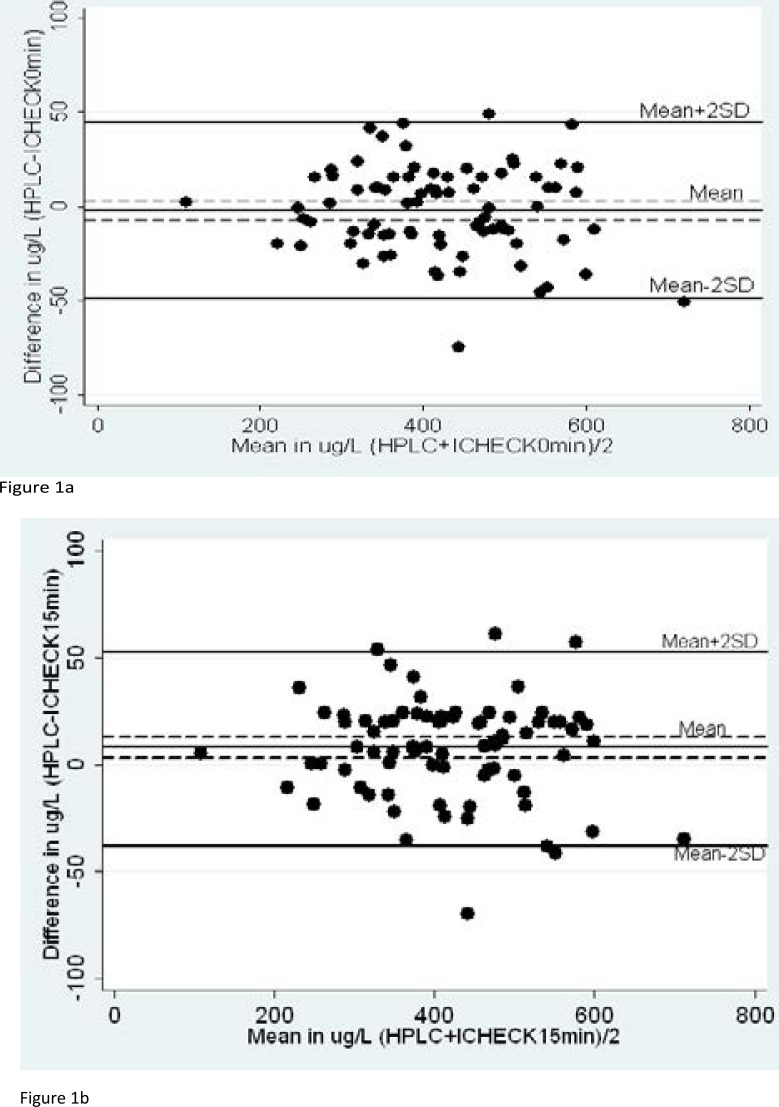
Agreement analysis between HPLC and ICHECK measures is presented in Table 2. The correlation coefficients between HPLC, ICHECK0min and ICHECK15min were significant. The fixed bias between HPLC and ICHECK0min was not significant (−1.9±23.2 µg/L, p=0.45) with a confidence interval (CI) at 95 % ranging from −7.0 µg/L to 3.1 µg/L whereas it was significant between HPLC and ICHECK15min (8.0±22.7 µg/L, p=0.002, CI : 3.1 to 13.4 µg/L).
Table 2.
Comparison between serum retinol determined by HPLC and ICHECK
| Correlationa | Fixed bias ± SDb |
p-valuec | Limits of agreementd |
Proportional biase | |
| HPLC -ICHECK0min | 0.977 | −1.9±23.2 | 0.45 | −48.2; 44.3 | −0.038 (p=0.73) |
| HPLC-ICHECK15min | 0.978 | 8.0±22.7 | 0.0020 | −37.5; 53.4 | −0.025 (p=0.83) |
| ICHECK15min-ICHECK0min | 0.995 | −9.9±10.5 | <0.001 | −30.9; 11.1 | −0.092 (p=0.40) |
SD, standard deviation, Vitamin A values are in µg/L
Pearson correlation coefficient
HPLC minus ICHECK value, or ICHECK15min minus ICHECK0min, a negative value reflects an overestimation
Student t-test value for the equality of the difference to 0
Mean±2SD
Pitman's test
There was a decrease of the concentration of retinol in the IEX analysing solution over time. This was showed by a significant fixed bias between ICHECK0min and ICHECK15min (−9.9±10.5 µg/L, p<0.001). None of the values of the ICHECK showed a significant proportional bias with HPLC. Bland and Altman plots between HPLC and ICHECK0min and ICHECK15min are presented respectively in Figure 1a and Figure 1b.
Figure 1.
Bland and Altman plots between HPLC and ICHECK
Figure 1a and Figure 1b represent Bland and Altman plots between HPLC and ICHECK0min and ICHECK15min, respectively. Solid lines represent fixed bias and limits of agreement, while dashed lines represent 95% CI of the biases.
Discussion
The determination of vitamin A status through the assessment of liver reserves, which is considered the gold standard method, is difficult to apply when samples are large15. Thus, serum retinol measurement represents a valuable alternative. However, intra- and inter-individual variations of serum retinol suggest that the interpretation of serum retinol levels is only valid in populations, and is useless at individual levels16. Moreover, vitamin A is sensitive to light, heat and oxidation, and losses can occur during sample collection, centrifugation, transport and storage.
ICHECK Fluoro is a fluorometry-based portable kit, easy-to-use and not requiring highly trained technicians. The ICHECK briefcase includes a digital mini-scale for solid samples, a multiple charger adapted to remote areas, a calibration solution, additionally to IEX Mila solutions and the ICHECK device. We were concerned about the stability of retinol in the IEX solution over a period of time. We assumed that 15 minutes might represent the average interval of time during which an overloaded technician would keep the samples before analysis.
Our result showed that ICHECK measurement provided good correlations with the HPLC for serum retinol concentrations. Nevertheless, our study showed that the concentration of retinol decreased in the IEX solution over time. As expected, assay performed directly after separation of the IEX-serum complex provided better agreement with HPLC. The degradation of retinol in organic solvents offers a plausible explanation for the decrease of retinol concentrations over time. Several authors reported that the decrease of retinol concentrations started immediately after addition of organic solvents to serum even at ice temperature17,18. Previous studies reported that addition of an antioxidant, especially ascorbic acid19, butylated hydroxyanisole (BHA) and BHT20, to solvents during extraction slowed the degradation of retinol.
The protocol of our study did not include the addition of an antioxidant to the serum or to the IEX kit to test the hypothesis of conservation. Thus, this can be considered as a limitation of the study. Another limitation of this study was the low prevalence of vitamin A deficiency among participants (only two participants). However, the non-significance of the proportional bias showed that the device may be suitable to provide appropriate measurement of retinol concentrations at both lower and higher concentrations.
The concentration of serum retinol in IEX reagent solution decreased over time, therefore the complex IEX-serum sample should be analysed immediately after the separation of the supernatant. Otherwise, further studies might test the effect of the addition of appropriate antioxidants in the IEX solution. Overall, based on the simplicity of the analysis procedure, ICHECK device should be recommended for field and epidemiologic studies, especially in developing countries.
Acknowledgements
We thank Sight and Life for providing graciously the ICHECK device and for their ongoing support in the fight against micronutrient deficiencies. The PEN study was sponsored by Fondation Centrale Laitière pour la Nutrition de l'Enfant in partnership with Ministries of Health and Education of Morocco.
Conflict of interest
None of the authors declared a conflict of interest.
References
- 1.Arlappa N. Vitamin A deficiency is still a public health problem in India. Indian Pediatr. 2011;48(11):853–854. doi: 10.1007/s13312-011-0133-7. [DOI] [PubMed] [Google Scholar]
- 2.Samba C, Tchibindat F, Gourmel B, Houzé P, Malvy D. Prevalence of vitamin A deficiency in pregnant and lactating women in the Republic of Congo. J Health Popul Nutr. 2013;31(1):28–36. doi: 10.3329/jhpn.v31i1.14746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azevedo MM, Cabral PC, Diniz Ada S, Fisberg M, Fisberg RM, Arruda IK. Vitamin A deficiency in preschool children of Recife, Northeast of Brazil. Arch Latinoam Nutr. 2010 Mar;60(1):36–41. [PubMed] [Google Scholar]
- 4.Sommer A, Vyas KS. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr. 2012;96(5):1204S–1206S. doi: 10.3945/ajcn.112.034868. [DOI] [PubMed] [Google Scholar]
- 5.Sherwin JC, Reacher MH, Dean WH, Ngondi J. Epidemiology of vitamin A deficiency and xerophthalmia in at-risk populations. Trans R Soc Trop Med Hyg. 2012;106(4):205–214. doi: 10.1016/j.trstmh.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ. 2011;343:d5094. doi: 10.1136/bmj.d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dary O, Mora JO; International Vitamin A Consultative Group. Food fortification to reduce vitamin A deficiency: International Vitamin A Consultative Group recommendations. J Nutr. 2002;132(9 Suppl.):2927S–2933S. doi: 10.1093/jn/132.9.2927S. [DOI] [PubMed] [Google Scholar]
- 8.WHO, author. WHO Global Database on Vitamin A Deficiency. Geneva: World Health Organization; 2009. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. [Google Scholar]
- 9.Tanumihardjo SA. Assessing vitamin A status: past, present and future. J Nutr. 2004;134(1):290S–293S. doi: 10.1093/jn/134.1.290S. [DOI] [PubMed] [Google Scholar]
- 10.Palmer AC, West KP, Jr, Dalmiya N, Schultink W. The use and interpretation of serum retinol distributions in evaluating the public health impact of vitamin A programmes. Public Health Nutr. 2012;15(7):1201–1215. doi: 10.1017/S1368980012000560. [DOI] [PubMed] [Google Scholar]
- 11.de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr. 2002;132(9 Suppl.):2895S–2901S. doi: 10.1093/jn/132.9.2895S. [DOI] [PubMed] [Google Scholar]
- 12.WHO, author. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. (WHO/NMH/NHD/MNM/11.3. http://www.who.int/vmnis/indicators/retinol.pdf. [Google Scholar]
- 13.Schweigert FJ, Frey SK, Mothes R, Dary O, Juarez P, Lascano V. A new test kit's potential for the rapid analysis of vitamin A in human and cow milk. Sight and Life. 2011;25(3):18–22. [Google Scholar]
- 14.Ludbrook J. Statistical techniques for comparing measurers and methods of measurement: a critical review. Clin Exp Pharmacol Physiol. 2002;29:527–536. doi: 10.1046/j.1440-1681.2002.03686.x. [DOI] [PubMed] [Google Scholar]
- 15.Tanumihardjo SA. World Health Organization. Report: Priorities in the assessment of vitamin A and iron status in populations. Panama City, Panama, 15–17 September 2010. Geneva: World Health Organization; 2012. Biomarkers of vitamin A status: what do they mean? [Google Scholar]
- 16.Gillespie C, Ballew C, Bowman AB, Donehoo R, Serdula MK. Intraindividual variation in serum retinol concentrations among participants in the third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2004;79:625–632. doi: 10.1093/ajcn/79.4.625. [DOI] [PubMed] [Google Scholar]
- 17.Driskell WJ, Bashor MM, Neese JW. Loss of vitamin A in long-term stored, frozen sera. Clin Chim Acta. 1985 Mar 30;147(1):25–30. doi: 10.1016/0009-8981(85)90006-3. [DOI] [PubMed] [Google Scholar]
- 18.Barreto-Lins MH, Campos FA, Azevedo MC, Flores H. A re-examination of the stability of retinol in blood and serum, and effects of a standardized meal. Clin Chem. 1988;34(11):2308–2310. [PubMed] [Google Scholar]
- 19.Driskell WJ, Lackey AD, Hewett JS, Bashor MM. Stability of vitamin A in frozen sera. Clin Chem. 1985 Jun;31(6):871–872. [PubMed] [Google Scholar]
- 20.Furr HC, Barua AB, van Breemen RB, Olson JA. In: Vitamin A and Carotenoids, in Modern Chromatographic Analysis of Vitamins, Revised and Expanded. Lambert Willy E, De Leenheer Andre P, Van Bocxlaer Jan F., editors. CRC Press; 2000. pp. 13–16. [Google Scholar]