Abstract
Background:
Pancreatic cancer accounts for approximately 7% of all cancer deaths. More than half of all pancreatic cancers are stage IV at diagnosis, where systemic chemotherapy is used with the goal of life prolongation as well as palliation. The patient characteristics and health system factors that drive the use of systemic therapy are unknown.
Method:
This is a retrospective study of stage IV pancreatic cancer patients (n = 140,210) diagnosed between 2000 and 2011 in the NCDB. NCDB contains approximately 70% of new cancer diagnosis from more than 1500 accredited cancer programs in the United States and Puerto Rico. Chi-squared test was used to determine any differences in characteristics of patients who did or did not receive systemic therapy.
Results:
Our study demonstrated that only 49.1% of stage IV pancreatic cancer patients received systemic therapy. The use of systemic therapy is significantly lower in female, African American/Hispanic, patients older than 40 years, those without insurance or with Medicare and Medicaid, higher Charlson Comorbidity Score, poor economic and educational status and in nonacademic centers.
Conclusions:
This is the largest study to evaluate the determinants of systemic therapy use in stage IV pancreatic cancer. The use of systemic therapy was significantly lower in patients older than 40 years, lower educational status, nonprivate insurance and with higher Charlson Comorbidity Scores. In addition, the use of systemic therapy was lower with female sex, African Americans/Hispanic, and lower socio-economic status. Understanding the barriers in the use of systemic therapy as well as appropriate utilization of systemic therapy can both optimize cancer care.
Keywords: Systemic therapy, stage IV pancreatic cancer, disparities
Introduction
In the United States, pancreatic cancer is the fourth leading cause of cancer-related mortality and accounts for about 7% of all cancer-related deaths. In 2014, 46,420 new diagnoses and 39,590 deaths are expected from pancreatic cancer, reflecting the high public health burden of the disease. The 5-year overall survival (OS) of pancreatic cancer is 2% for stage IV disease [Siegel et al. 2014].
The only potentially curative therapy for pancreatic cancer is surgical resection. However, 53% cases of pancreatic cancer are stage IV at diagnosis, and hence not a candidate for curative resection [Siegel et al. 2014]. Even with surgical resection, the recurrence rate of stage II disease is around 40%, and that of stage III is up to 90% [Kayahara et al. 1993]. In metastatic pancreatic cancer, chemotherapy, compared with the best supportive care, improves the median OS [Sultana et al. 2007; Pelzer et al. 2011; Valsecchi et al. 2014; Ghosn et al. 2014] with risk of death reduced by as much as 36% [Sultana et al. 2007], and improves quality of life [Shore et al. 2003; Moinpour et al. 2010].
The use of chemotherapy in pancreatic cancer has improved significantly in last two decades. In 1997, a phase III clinical trial determined that gemcitabine confers a significant survival advantage (5.65 versus 4.41 months, p = 0.0025) and clinical benefit (23.8% versus 4.8%, p = 0.0022) over 5-fluorouracil, thus leading to its approval for improvement in symptoms [Burris et al. 1997]. Afterwards the use of chemotherapy gradually shifted from 5-fluorouracil to gemcitabine for advanced disease for next 10 years and subsequently gemcitabine became the comparator arm in newer trails [Oberstein et al. 2013]. In 2007, Moore and colleagues demonstrated that gemcitabine and erlotinib prolonged OS (median 6.24 months versus 5.91 months, p = 0.038) over gemcitabine alone [Moore et al. 2007]. However, the modest prolongation in survival prevented its widespread use.
Prior studies have demonstrated disparities in therapy and outcomes of different cancers based on demographic features such as age [Goodwin et al. 1993], race [Shavers and Brown, 2002; Murphy et al. 2009], education [Albano et al. 2007], socioeconomic status [Aarts et al. 2010] insurance status, hospital type [Bilimoria et al. 2009; Raigani et al. 2014] and year of treatment. Such studies on healthcare disparities have mainly focused on patients with nonmetastatic pancreatic cancer. There is a paucity of similar studies in stage IV pancreatic cancer. A prior study on this subject focused only on older patients and excluded patients, who died within 30 days of diagnosis [Oberstein et al. 2013]. Therefore, we utilized a large database of all stage IV pancreatic cancer patients to analyze any variation in the use of systemic therapy based on patients’ demographic and other characteristics.
Methods
This was a retrospective study of stage IV pancreatic cancer patients diagnosed between 2000 and 2011 in the National Cancer Data Base (NCDB). NCDB is a nationwide oncology database for more than 1500 Commission on Cancer-accredited cancer programs in the United States and Puerto Rico. Approximately 70% of all newly diagnosed cases of cancer in the United States are captured at the institutional level and reported to the NCDB [Bilimoria et al. 2008]. The NCDB, begun in 1989, now contains approximately 29 million records from hospital cancer registries across the United States. [American College of Surgeons (2014c)] The NCDB requires reporting of all new cancer diagnoses from the hospitals that are approved by the Commission on Cancer, and shares common data coding, collection, and accuracy assessment mechanisms with state and national cancer registries, including the Surveillance, Epidemiology, and End Results (SEER) database [Bilimoria et al. 2008].
Our study included newly diagnosed stage IV pancreatic cancer patients, and excluded those who had nonmetastatic disease at diagnosis and later developed metastasis. The Institutional Review Board waiver was obtained from the University of Nebraska Medical Center Institutional Review Board. Using NCDB aggregate hospital comparison benchmark reports, a total of 140,210 patients with stage IV pancreatic cancer were categorized into two groups: patients, who did versus did not receive systemic therapy (chemotherapy, hormone therapy, immunotherapy, or a combination of different therapies). The two groups were then compared in terms of age, race, sex, Charlson Comorbidity Score, distance traveled, hospital type, household income, insurance and educational status. NCDB uses Charlson Comorbidity Score to characterize the burden of comorbid conditions. Educational status in NCDB is recorded as an aggregate percentage of population without a high school degree residing in the zip code of the patient at the time of diagnosis, using US Census data from year 2000. [American College of Surgeons (2014b)] For the analysis, we classified hospital type into two main groups: academic centers (teaching/research hospitals associated with university medical schools or designated as National Cancer Institute Comprehensive Cancer Care Programs) and nonacademic centers (other hospitals including community cancer programs, comprehensive community cancer programs). [American College of Surgeons (2014a)]
Statistical analysis
Patient characteristics were computed using descriptive statistics. Pearson’s chi-squared test of independence was used to calculate any statistical difference in the distribution of different variables between these two groups. The level of statistical significance was set to a p-value of <0.01. Because the data were presorted into different categories by the NCDB, we were unable to conduct any patient-level multivariate analyses.
Results
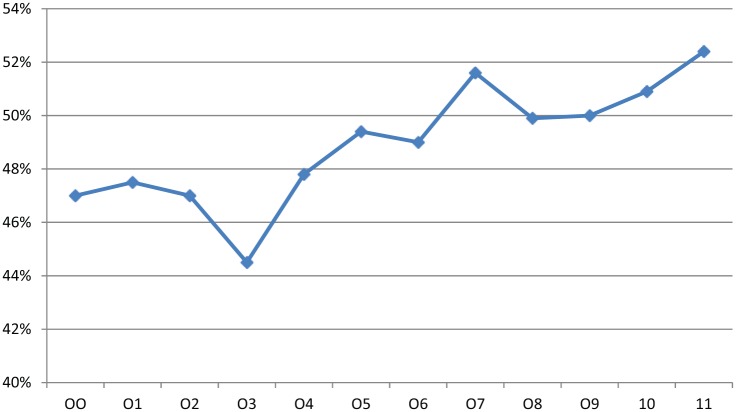
Of 303,534 total patients with pancreatic cancer reported to NCDB between 2000 and 2011, 46.2% (n = 140,210) of patients were diagnosed with stage IV pancreatic cancer. Patients with stage IV disease were predominantly elderly (74.6%; >60 years), White (79%), and male (53%). A total of 50% had Charlson Comorbidity Score of zero. Only 49.1% (n = 68,848) of stage IV pancreatic cancer patients received systemic therapy. The use of systemic therapy increased from 47% in 2000 to 52.4% in 2011 (Figure 1).
Figure 1.
Percentage of patients with stage IV pancreatic cancer, who received systemic therapy between the years 2000 and 2011.
Men were more likely to receive systemic therapy compared with women (51% versus 47%, p < 0.01; Table 1). Utilization of systemic therapy was less with advancing age (61.8% in age group 50–59 versus 56.8% in 60–69, 45.7% in 70–79 and 25.7% in 80–89, p < 0.01). Patients with poor economic status were less likely to receive systemic therapy (42.2% with an annual income of less than US$28,000 versus 52.6% with an annual income of more than US$49,000, p < 0.01). Patients with private insurance (61.5%) were more likely to receive systemic therapy compared with those without insurance (43.5%), with Medicaid (48.7%) and with Medicare (42.2%) (p < 0.01). Patients were more likely to receive systemic therapy if they were White (50%) compared with African Americans (45.2%) and Hispanics (45.7%) (p < 0.01). Patients with higher educational status were more likely to receive systemic therapy (53.2% for patients living in an area with >88% with high school degree versus 43.8% for patients living in an area with <69% population with high school degree, p < 0.01). Patients were more likely to receive systemic therapy if they received treatment in academic center compared with nonacademic centers (52% versus 47%, p < 0.01). Patients with Charlson Comorbidity Score 0 were more likely to receive systemic therapy (52.9%) compared with those with a score of 1 (46.5%) and 2 or more (34.8%) (p < 0.01).
Table 1.
Systemic therapy use in stage IV pancreatic cancers.
| Variables | Systemic therapy n (%) | No systemic therapy n (%) |
|---|---|---|
| Age | ||
| Under 20 | 22 (71) | 9 (29) |
| 20–29 | 125 (64) | 70 (36) |
| 30–39 | 984 (69.4) | 433 (30.6) |
| 40–49 | 5613 (65.2) | 2998 (34.8) |
| 50–59 | 15,713 (61.8) | 9719 (38.2) |
| 60–69 | 21,701 (56.8) | 16,459 (43.2) |
| 70–79 | 18,692 (45.7) | 22,212 (54.3) |
| 80–89 | 5807 (25.7 ) | 16,794 (74.3) |
| 90 and above | 191 (6.6) | 2668 (93.4) |
| Sex | ||
| Male | 37,922 (51) | 36,464 (49) |
| Female | 30,926 (47) | 34,898 (53) |
| Race | ||
| White | 55,309 (50.1) | 55,251 (49.9) |
| Hispanic | 3087 (45.7) | 3661 (54.3) |
| African American | 7853 (45.2) | 9506 (54.8) |
| Charlson Comorbidity Score | ||
| None | 37,404 (52.9) | 33,355 (47.1) |
| 1 | 11,976 (46.5) | 13,783 (53.5) |
| 2 or more | 3210 (34.8) | 6016 (65.2) |
| Insurance | ||
| Private | 27,845 (61.5) | 17,422 (38.5) |
| Medicaid | 3249 (48.7) | 43,515 (51.3) |
| Not insured | 2099 (43.5) | 2722 (56.5) |
| Medicare | 31,836 (42.2) | 3417 (57.8) |
| Other govt. | 1458 (41.3) | 2074 (58.7) |
| Annual household income US$ | ||
| >49,000 | 23,350 (52.6) | 21,036 (47.4) |
| 39,000–48,999 | 15,598 (49.5) | 15,930 (50.5) |
| 33,000–38,999 | 11,836 (47.8) | 12,934 (52.2) |
| 28,000–32,999 | 8142 (46.5) | 9377 (53.5) |
| <28,000 | 5864 (42.2) | 8013 (57.8) |
| Educational status* | ||
| >88% | 18,519 (53.2) | 16,285 (46.8) |
| 82.1–88% | 16,644 (50.1) | 16,630 (49.9) |
| 77.1–82% | 10,807 (48.5) | 11,463 (51.5) |
| 69.1–77% | 10,626 (46.1) | 12,405 (53.9) |
| <69% | 8189 (43.8) | 10,500 (56.2) |
| Hospital type | ||
| Academic | 24,942 (51.9) | 23,146 (48.1) |
| Non-academic | 43,906 (47.7) | 48,216 (52.3) |
| Total | 68,848 (49.1) | 71,362 (50.9) |
(p < 0.01 for all comparisons.)
Aggregate percentage of individuals with a high school degree for patient’s zip code.
Discussion
Our study demonstrated that only 49% of stage IV pancreatic cancer patients received systemic therapy. The use of systemic therapy was lower among older patients, females and patients with higher Charlson Comorbidity Score and lower socio-economic status, which is consistent with prior studies [Goodwin et al. 1993; Albano et al. 2007; Bilimoria et al. 2009; Murphy et al. 2009; Aarts et al. 2010]. A large SEER Medicare database study of stage IV pancreatic cancer patients (n = 3231) demonstrated an increase in the utilization of chemotherapy from 53% in 1998–1999 to 57% in 2004–2005. Patients, who were older, female, black, unmarried, lived in suburban areas, or had lower socioeconomic status, poorly differentiated carcinomas and two or more comorbidities, were less likely to receive gemcitabine [Oberstein et al. 2013]. This study excluded patients, who died within 30 days, which may explain a higher receipt of chemotherapy than in our study.
The systemic therapy use in our study was significantly lower in patients >60 years old and with higher Charlson Comorbidity Score. Sehgal and colleagues demonstrated that stage I–IV pancreatic cancer patients >70 years old were less likely to receive chemotherapy; however, elderly patients derived similar benefits from chemotherapy as younger patients did [Sehgal et al. 2014]. Even in patients with resectable pancreatic cancer, patients with older age and higher comorbidities are less likely to be surgical candidates [Sener et al. 1999; Bilimoria et al. 2007].
Elderly patients (age >65 years) are often underrepresented in clinical trials because of the exclusion criteria related to age, comorbidities or performance status [Conroy et al. 2011; Von Hoff et al. 2013]. Several studies, however, have shown that there is no relationship of age with OS in patients receiving treatment [Moore et al. 2007; Marechal et al. 2013; Von Hoff et al. 2013], although elderly patients may have increased toxicity [Miyamoto et al. 2010]. Nakai and colleagues evaluated gemcitabine-based therapies in patients with advanced pancreatic cancer and demonstrated that comorbidities, rather than age, predicted poor outcomes [Nakai et al. 2011]. However, Vickers and colleagues analyzed the impact of comorbidities on patients receiving gemcitabine and erlotinib and found that OS was not affected by the presence of comorbidities [Vickers et al. 2012]. Although elderly patients seem to benefit from systemic therapy in pancreatic cancer [Hubbard et al. 2011], a fear of increased toxicity and decreased benefits may lead to less aggressive treatment [Lewis et al. 2003; Hubbard et al. 2011], which could explain lower utilization of systemic therapy with advancing age.
The major trials evaluating gemcitabine and erlonitib as well as gemcitabine and nab-paclitaxel did not show any difference in outcomes based on gender [Moore et al. 2007; Conroy et al. 2011; Von Hoff et al. 2013]. In fact, Moore and colleagues demonstrated an association between female sex and increased survival [Moore et al. 2007]. Hence, it is clear that female patients derive at least similar benefits from chemotherapy as men do. Despite this, in our study, women were less likely to receive systemic therapy compared with men (47% versus 51%, p < 0.01).
Similarly, patients were more likely to receive systemic therapy if they were White (50%) compared with African Americans (45.2%) and Hispanics (45.7%) (p < 0.01). In a SEER registry study of locally advanced pancreatic adenocarcinoma patients, African-Americans had lower rates of specialist consultation (p < 0.01), chemotherapy use (p < 0.01), and resection (p < 0.01) compared with Whites [Murphy et al. 2009]. It is unclear whether these gender and racial disparities are secondary to patients’ preferences, patient–provider interactions, socio-economic or educational differences or potential influences of gender or racial differences on providers’ decision-making.
In our study, a higher income status and the availability of a private insurance were associated with a higher receipt of systemic therapy. This is consistent with prior studies on pancreatic cancer [Oberstein et al. 2013] as well as metastatic gastric cancers [Sherman et al. 2013]. Although high cost associated with systemic therapy may explain this disparity, prior studies have also shown that cost consideration can influence oncologists’ recommendations. In a survey of 167 oncologists, Schrag and colleagues demonstrated that one in six oncologists admitted omitting treatment options based on their perception of patients’ ability to afford treatment. However, one-third of the oncologists were not comfortable discussing the economic impact of cancer treatment [Schrag and Hanger, 2007]. Prior studies have also shown that oncologists preferred patients to have access to effective cancer treatment only if the treatments are cost-effective, although the cost-effectiveness threshold varied among oncologists [Nadler et al. 2006; Berry et al. 2010].
Our study also demonstrated a positive correlation between educational status and systemic therapy use. Higher education is associated with better utilization of screening modalities, less exposure to risk factors and better access to healthcare services [Mouw et al. 2008]. Furthermore, Albano and colleagues in their analysis of 137,708 cancer deaths demonstrated that educational status was inversely associated with cancer-related mortality [Albano et al. 2007]. These findings may suggest that education in general as well as patient education may improve utilization of systemic therapy.
In our study, there was comparatively less utilization of systemic therapy in stage IV pancreatic cancer in nonacademic hospitals than academic hospitals which is consistent with prior studies that have analyzed the surgical management of pancreatic cancer [Bilimoria et al. 2007; Raigani et al. 2014]. This may be related to quality of patient counseling [Koedoot et al. 2004] and the differences in experience and availability of resources between the two settings.
Our study has certain limitations, which include retrospective study design, utilization of a large secondary database with a potential for miscoding, missing data and lack of patient-level data for multivariate analysis. Although the use of systemic therapy differed by race and other socio-economic factors, minority status and low socioeconomic status frequently overlap. Hence, these factors may not necessarily be the separate drivers of the observed disparity. The differences between some of the groups are statistically significant but the actual difference is small. Such results may be due to the large sample size of our study. Patients, who initially presented with early stage pancreatic cancer and later on developed metastasis, were excluded from the study. NCDB does not include patients seeking care in non-Commission on Cancer-approved hospitals, which are usually smaller, located away from urban locations and have less cancer-related services available to patients. Patients diagnosed with stage IV pancreatic cancer between 2000 and 2011 were selected for this study. Hence, it is unlikely that many patients receiving newer therapy options such as FOLFIRINOX or gemcitabine and nab-paclitaxel were included in our study. However, disparities in receipt of systemic therapy may get worse with the use of more intensive and expensive therapies such as oxaliplatin, irinotecan, fluorouracil, and leucovorin (FOLFIRINOX) [Conroy et al. 2011] and gemcitabine plus nab-paclitaxel [Von Hoff et al. 2013]. These newer regimens has been shown to further improve survival, hence it becomes even more important to understand these disparities in cancer treatment.
A few prior studies have evaluated the receipt of systemic therapy in stage IV pancreatic cancer, however, these studies were small and evaluated fewer variables. To the best of the authors’ knowledge, our study is the largest study to analyze different factors that influence the utilization of systemic therapy in stage IV pancreatic cancer. We included all age groups and evaluated the receipt of systemic therapy based on several variables including types of insurance, educational status, and hospital type.
Conclusions
This is the largest study to evaluate the determinants of systemic therapy use in stage IV pancreatic cancer. Only 49% of stage IV pancreatic cancer patients received systemic therapy. The use of systemic therapy was significantly lower in older patients, females, African Americans and Hispanics, nonacademic hospitals, uninsured patients and patients with nonprivate insurance, lower economic or educational status, and higher Charlson Comorbidity Score. Future studies should focus on identifying the cause for lower systemic therapy use in these patient populations. Disparities in receipt of systemic therapy in pancreatic cancer may get worse with the use of improved but more intensive and expensive therapies such as FOLFIRINOX and gemcitabine plus nab-paclitaxel . This highlights a need to understand the barriers in the use of systemic therapy that can improve the OS of the stage IV pancreatic cancer.
Acknowledgments
The study was published as an abstract form in the proceedings of the American Society of Clinical Oncology 50th annual meeting, 31 May 2014.
Footnotes
Conflict of interest statement: Peter T Silberstein reports receiving payment for lectures from Bristol Myers and Celgene in the past. There are no conflicts of interest for any other authors.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Nabin Khanal, Department of Internal Medicine, Creighton University Medical Center, 601 N. 30th Street Suite 5850, Omaha, NE 68131, USA.
Smrity Upadhyay, Department of Internal Medicine, Creighton University Medical Center, Omaha, NE, USA.
Sumit Dahal, Department of Internal Medicine, Interfaith Medical Center, Brooklyn, NY, USA.
Vijaya Raj Bhatt, Department of Internal Medicine, Division of Hematology-Oncology, University of Nebraska Medical Center, Omaha, NE, USA.
Peter T. Silberstein, Department of Internal Medicine, Division of Hematology-Oncology, Creighton University Medical Center, Omaha, NE, USA
References
- American College of Surgeons (2014a) NCDB Public Benchmark Reports. Available from http://cromwell.facs.org/bmarks/bmpub/ver10/help/hcr_09_hosp_typesys.cfm (accessed on 1 April 2014)
- American College of Surgeons (2014b) NCDB Public Benchmark Reports. Available from http://cromwell.facs.org/bmarks/bmpub/ver10/help/hcr_09_ptdemog.cfm (accessed on 1 April 2014).
- American College of Surgeons (2014c) NCDB Public Benchmark Reports. Available from http://www.facs.org/cancer/ncdb/index.html (accessed on 1 April 2014).
- Aarts M., Lemmens V., Louwman M., Kunst A., Coebergh J. (2010) Socioeconomic status and changing inequalities in colorectal cancer? A review of the associations with risk, treatment and outcome. Eur J Cancer 46: 2681–2695. [DOI] [PubMed] [Google Scholar]
- Albano J., Ward E., Jemal A., Anderson R., Cokkinides V., Murray T., et al. (2007) Cancer mortality in the United States by education level and race. J Natl Cancer Inst 99: 1384–1394. [DOI] [PubMed] [Google Scholar]
- Berry S., Bell C., Ubel P., Evans W., Nadler E., Strevel E., et al. (2010) Continental divide? The attitudes of US and Canadian oncologists on the costs, cost-effectiveness, and health policies associated with new cancer drugs. J Clin Oncol 28: 4149–4153. [DOI] [PubMed] [Google Scholar]
- Bilimoria K., Balch C., Wayne J., Chang D., Palis B., Dy S., et al. (2009) Health care system and socioeconomic factors associated with variance in use of sentinel lymph node biopsy for melanoma in the United States. J Clin Oncol 27: 1857–1863. [DOI] [PubMed] [Google Scholar]
- Bilimoria K., Bentrem D., Ko C., Stewart A., Winchester D., Talamonti M. (2007) National failure to operate on early stage pancreatic cancer. Ann Surg 246: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilimoria K., Stewart A., Winchester D., Ko C. (2008) The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 15: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris H., III, Moore M., Andersen J., Green M., Rothenberg M., Modiano M., et al. (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–2413. [DOI] [PubMed] [Google Scholar]
- Conroy T., Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., et al. (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- Ghosn M., Kourie H., El Karak F., Hanna C., Antoun J., Nasr D. (2014) Optimum chemotherapy in the management of metastatic pancreatic cancer. World J Gastroenterol 20: 2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J., Hunt W., Samet J. (1993) Determinants of cancer therapy in elderly patients. Cancer 72: 594–601. [DOI] [PubMed] [Google Scholar]
- Hubbard J., Grothey A., Sargent D. (2011) Systemic therapy for elderly patients with gastrointestinal cancer. Clin Med Insights Oncol 5: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayahara M., Nagakawa T., Ueno K., Ohta T., Takeda T., Miyazaki I. (1993) An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging. Cancer 72: 2118–2123. [DOI] [PubMed] [Google Scholar]
- Koedoot C., Oort F., De Haan R., Bakker P., De Graeff A., De Haes J. (2004) The content and amount of information given by medical oncologists when telling patients with advanced cancer what their treatment options are: palliative chemotherapy and watchful-waiting. Eur J Cancer 40: 225–235. [DOI] [PubMed] [Google Scholar]
- Lewis J., Kilgore M., Goldman D., Trimble E., Kaplan R., Montello M., et al. (2003) Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 21: 1383–1389. [DOI] [PubMed] [Google Scholar]
- Marechal R., Demols A., Van Laethem J. (2013) Adjuvant pharmacotherapy in the management of elderly patients with pancreatic cancer. Drugs Aging 30: 155–165. [DOI] [PubMed] [Google Scholar]
- Miyamoto D., Mamon H., Ryan D., Willett C., Ancukiewicz M., Kobayashi W., et al. (2010) Outcomes and tolerability of chemoradiation therapy for pancreatic cancer patients aged 75 years or older. Int J Radiat Oncol Biol Phys 77:1171–1177. [DOI] [PubMed] [Google Scholar]
- Moinpour C., Vaught N., Goldman B., Redman M., Philip P., Millwood B., et al. (2010) Pain and emotional well-being outcomes in Southwest Oncology Group–directed intergroup trial S0205: a phase III study comparing gemcitabine plus cetuximab versus gemcitabine as first-line therapy in patients with advanced pancreas cancer. J Clin Oncol 28: 3611–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M., Goldstein D., Hamm J., Figer A., Hecht J., Gallinger S., et al. (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:1960–1966. [DOI] [PubMed] [Google Scholar]
- Mouw T., Koster A., Wright M., Blank M., Moore S., Hollenbeck A., et al. (2008) Education and risk of cancer in a large cohort of men and women in the United States. PLoS One 3: e3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M., Simons J., Ng S., Mcdade T., Smith J., Shah S., et al. (2009) Racial differences in cancer specialist consultation, treatment, and outcomes for locoregional pancreatic adenocarcinoma. Ann Surg Oncol 16: 2968–2977. [DOI] [PubMed] [Google Scholar]
- Nadler E., Eckert B., Neumann P. (2006) Do oncologists believe new cancer drugs offer good value? Oncologist 11: 90–95. [DOI] [PubMed] [Google Scholar]
- Nakai Y., Isayama H., Sasaki T., Sasahira N., Tsujino T., Kogure H., et al. (2011) Comorbidity, not age, is prognostic in patients with advanced pancreatic cancer receiving gemcitabine-based chemotherapy. Crit Rev Oncol Hematol 78:252–259. [DOI] [PubMed] [Google Scholar]
- Oberstein P., Hershman D., Khanna L., Chabot J., Insel B., Neugut A. (2013) Uptake and patterns of use of gemcitabine for metastatic pancreatic cancer: a population-based study. Cancer Invest 31: 316–322. [DOI] [PubMed] [Google Scholar]
- Pelzer U., Schwaner I., Stieler J., Adler M., Seraphin J., Dörken B., et al. (2011) Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-Study Group. Eur J Cancer 47: 1676–1681. [DOI] [PubMed] [Google Scholar]
- Raigani S., Ammori J., Kim J., Hardacre J. (2014) Trends in the treatment of resectable pancreatic adenocarcinoma. J Gastrointest Surg 18: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag D., Hanger M. (2007) Medical oncologists’ views on communicating with patients about chemotherapy costs: a pilot survey. J Clin Oncol 25: 233–237. [DOI] [PubMed] [Google Scholar]
- Sehgal R., Alsharedi M., Larck C., Edwards P., Gress T. (2014) Pancreatic cancer survival in elderly patients treated with chemotherapy. Pancreas 43: 306–310. [DOI] [PubMed] [Google Scholar]
- Sener S., Fremgen A., Menck H., Winchester D. (1999) Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg 189: 1–7. [DOI] [PubMed] [Google Scholar]
- Shavers V., Brown M. (2002) Racial and ethnic disparities in the receipt of cancer treatment.J Natl Cancer Inst 94: 334–357. [DOI] [PubMed] [Google Scholar]
- Sherman K., Merkow R., Shah A., Wang C., Bilimoria K., Bentrem D. (2013) Assessment of advanced gastric cancer management in the United States. Ann Surg Oncol 20: 2124–2131. [DOI] [PubMed] [Google Scholar]
- Shore S., Raraty M., Ghaneh P., Neoptolemos J. (2003) Review Article: Chemotherapy for pancreatic cancer. Aliment Pharmacol Ther 18: 1049–1069. [DOI] [PubMed] [Google Scholar]
- Siegel R., Ma J., Zou Z., Jemal A. (2014) Cancer Statistics, 2014. CA 64: 9–29. [DOI] [PubMed] [Google Scholar]
- Sultana A., Smith C., Cunningham D., Starling N., Neoptolemos J., Ghaneh P. (2007) Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol 25:2607–2615. [DOI] [PubMed] [Google Scholar]
- Valsecchi M., Díaz-Cantón E., De La Vega M., Littman S. (2014) Recent treatment advances and novel therapies in pancreas cancer: a review.J Gastrointest Cancer 45: 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers M., Powell E., Asmis T., Jonker D., Hilton J., O’Callaghan C., et al. (2012) Comorbidity, age and overall survival in patients with advanced pancreatic cancer–results from NCIC CTG PA. 3: a phase III trial of gemcitabine plus erlotinib or placebo. Eur J Cancer 48: 1434–1442. [DOI] [PubMed] [Google Scholar]
- Von Hoff D., Ervin T., Arena F., Chiorean E., Infante J., Moore M., et al. (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]