Abstract
Over a third of patients with lung cancer will develop bone metastases during the course of their disease, resulting in symptoms of pain and immobility, and skeletal-related events (SREs) such as fracture, hypercalcaemia, surgery or radiotherapy to bones, and malignant spinal cord compression. These reduce quality of life and increase mortality. Preclinical research has identified the interactions between tumour cells and bone that are key to tumour cell survival and associated osteolysis. These data have led to the development of drugs to prevent osteoclast-mediated bone breakdown, such as zoledronic acid and denosumab, which are now licensed for use in patients with bone metastases from solid tumours. Both zoledronic acid and denosumab reduce the risk of SREs and increase time to first SRE, with minimal side effects. In addition, denosumab improved survival in patients with lung cancer compared with zoledronic acid. Ongoing trials are testing whether these drugs can prevent the development of bone metastases from lung cancer. New bone-targeted agents showing promise in breast and prostate cancer include radium-223, cabozantinib and Src inhibitors. These agents require further evaluation in patients with lung cancer.
Keywords: denosumab, skeletal-related events, survival outcomes, vicious cycle, zoledronic acid
Introduction
Lung cancer is the commonest cause of death from cancer worldwide. Over a third of patients with advanced lung cancer develop bone metastases during the course of their illness [Coleman, 1997], presenting a significant morbidity burden. These morbidities include bone pain, hypercalcaemia, pathological fractures, spinal cord compression and bone marrow infiltration.
Bone pain is the most common type of cancer-related pain [Mercadante, 1997] with both mechanical and neurogenic components, requiring a combination of pharmacological (nonsteroidal anti-inflammatory drugs, opioid analgesics, gamma-aminobutyric acid (GABA) analogues, corticosteroids) and nonpharmacological therapies (surgical stabilization, palliative radiotherapy, nerve blocks, transcutaneous electric nerve stimulation), in addition to systemic anticancer treatments.
Hypercalcaemia is related to direct induction of local osteolysis by tumour cells, as well as to chemical mediators including parathyroid-like related hormone, interleukin 1 and tumour necrosis factor, which promote the maturation of osteoclasts resulting in increased bone resorption. Hypercalcaemia leads to debilitating symptoms, including fatigue, nausea and constipation. It is a poor prognostic factor, with a life expectancy of a few months [Ralston et al. 1990; Clines and Guise, 2005].
Pathological fractures occur most commonly in weight-bearing bones (long bones, vertebrae), and are more likely if the bone metastases are large, lytic and involve the cortex [Coleman, 2006]. The sequelae of fractures include pain, reduced mobility, hospitalization, deterioration in quality of life, and requirement for interventions including surgery and radiotherapy.
Malignant spinal cord/cauda equina compression is a medical emergency, typically characterized by back pain, followed by weakness and, less commonly, sensory loss, with bladder and bowel dysfunction being late findings. Early diagnosis and prompt intervention with surgery, vertebroplasty or radiotherapy are crucial to avoiding irreversible neurological sequelae [Martenson et al. 1985; Kim et al. 1990].
Bone marrow infiltration can lead to pancytopenia, putting patients at risk of infection and bleeding. It can also compromise other anticancer treatments, such as cytotoxic chemotherapy.
The morbidities associated with bone metastases (often referred to as skeletal-related events, SREs) have major impacts on patients’ quality of life and present a significant economic burden. For patients with lung cancer and bone metastases, a retrospective analysis in the USA showed the lifetime SRE cost per patient to be US$12,000 [Delea et al. 2004]. Placebo treatment arms of clinical trials of antiresorptive agents in bone metastases have provided evidence of the frequency of SREs in non-small cell lung cancer (NSCLC) and other solid tumours, demonstrating that 48% of patients with bone metastases experience SREs and highlighting the need for preventive treatments (Table 1) [Rosen et al. 2004b; Coleman 2004]. Moreover, the development of bone metastases and SREs increases the risk of death and reduces median survival time (Table 2).
Table 1.
Frequency of SREs in 250 patients with NSCLC and other solid tumours (Rosen et al. 2004).
| Skeletal complication | Number of events (%) |
|---|---|
| Radiation to bone | 86 (34) |
| Pathological fracture | 55 (22) |
| Surgery to bone | 13 (5) |
| Spinal cord compression | 10 (4) |
| Hypercalcaemia | 9 (4) |
| Any SRE | 120 (48) |
NSCLC, non-small cell lung cancer; SRE, skeletal-related event.
Table 2.
Effects of bone metastases and SREs on survival in lung cancer.
| Author | Type of study | Population (n) and tumour type | Outcome |
|---|---|---|---|
| Bauml et al. [2013] | Single centre retrospective analysis | 376 NSCLC | Bone metastases were an independent adverse prognostic factor for survival. |
| Saad [2007] | Retrospective analysis of pooled data from RCTs of zoledronic acid | >3000 solid tumours including lung cancer | Pathological fracture increased death by 20% in all tumours except lung cancer* |
| Tsuya [2007] | Retrospective analysis | 259 NSCLC | ⩾1 SRE reduced median survival by half (366 days to 187 days) |
| Hirsh et al. [2008] | Retrospective analysis of a phase III study | 507 solid tumours including lung cancer | Development of SREs reduced median survival by half |
The reason for this exception was thought to be due to the short median survival of lung cancer patient. In addition, nearly 70% lung cancer patients already had an SRE at study entry.
NSCLC, non-small cell lung cancer; RCT, randomized controlled trial; SRE, skeletal-related event.
The impact of bone metastases on morbidity and mortality has been increasingly recognized over the past 20 years and has led to interest in understanding the pathophysiology and pharmacological treatment of bone metastases from solid tumours. The remainder of this review will focus primarily on the two drugs that are licensed for use in America, the UK and Europe for patients with bone metastases from solid tumours: zoledronic acid and denosumab.
Bone-targeted agents in preclinical models
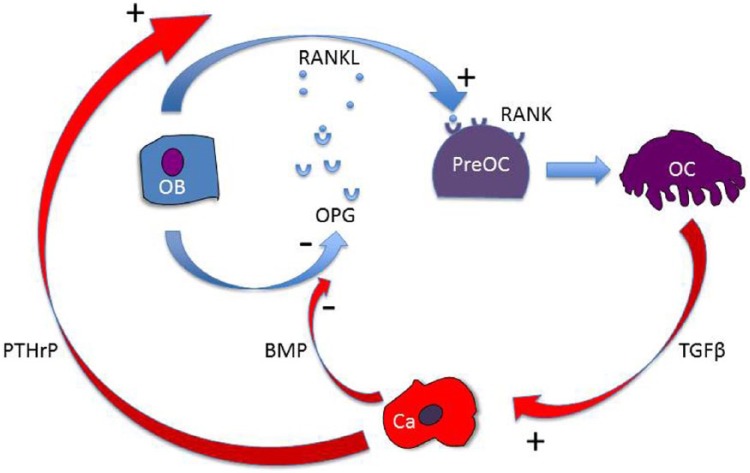
Bone is a dynamic organ that is constantly remodelled. The balance between bone formation by osteoblasts and resorption by osteoclasts is critical to prevent pathological excess of either process that could alter bone volume and architecture. One such coupling mechanism involves the receptor activator of nuclear factor κβ (RANK) and osteoprotegerin (OPG) (Figure 1). RANK ligand (RANKL) is secreted by osteoblasts and binds to RANK on the surface of preosteoclasts to promote differentiation into osteoclasts. This is prevented by osteoblast secretion of OPG, which binds and inactivates RANKL preventing it from interacting with RANK. The ratio between RANKL and OPG produced by osteoblasts controls RANKL-stimulated osteoclast activity [Dougall et al. 1999; Kong et al. 1999].
Figure 1.
The vicious cycle of bone metastases. Once tumour cells arrive in bone they interact with the bone forming osteoblasts (Ob) and bone-resorbing osteoclasts (Oc). Tumour cells secrete factors that stimulate Oc, that is, parathyroid-related protein (PTHrP). The increase in osteoclast bone resorption releases growth factors such as transforming growth factor β (TGFβ) which are stored in bone matrix which further increase tumour secretion of PTHrP. Other tumour-secreted factors such as bone morphogenetic proteins (BMPs) activate osteoblasts directly which subsequently release more receptor activator of nuclear factor κβ ligand (RANKL) and less osteoprotegerin (OPG), a RANKL decoy, to initiate osteoclast differentiation and hence further bone resorption.
When metastatic tumour cells arrive in bone, they localize to areas that are highly vascular, particularly trabecular bone [Holen, 2012], where they interact with osteoblasts and osteoclasts. The presence of tumour cells in bone disrupts the RANKL:OPG coupling mechanism, as osteoclasts are stimulated by tumour-secreted parathyroid-related protein (PTHrP), which increases local production of RANKL [Kingsley et al. 2007]. The increase in osteoclast bone resorption releases growth factors such as transforming growth factor β, which are stored in bone matrix and further increase tumour secretion of PTHrP [Mourskaia et al. 2009]. Other tumour-secreted factors such as bone morphogenetic proteins activate osteoblasts directly, which subsequently release more RANKL and less OPG to initiate osteoclast differentiation [Dai et al. 2005]. These interactions create what is termed ‘the vicious cycle’ of bone metastasis [Mundy, 1997], a tumour-induced self-propagating breakdown of bone (Figure 1).
Both zoledronic acid and denosumab have been shown in vitro and in vivo to disrupt this vicious cycle, primarily by their effect on osteoclast-mediated bone resorption. Zoledronic acid is an intravenous-administered nitrogen-containing bisphosphonate and is the most potent of the bisphosphonates [Russell et al. 2008], binding avidly to the bone surface, with a half life in serum of about 2 hours [Lin, 1996]. The drug is incorporated into the hydroxyapatite matrix and is taken up by osteoclasts during the resorption process. Zoledronic acid inhibits farnesyl pyrophosphate synthase [van Beek et al. 1999] preventing prenylation of small guanosine-5-triphosphatease (GTPases), which are responsible for many cellular functions in osteoclasts, thus inducing apoptosis and loss of cell membrane integrity [Coxon and Rogers, 2003]. In animal models of breast cancer metastases to bone, zoledronic acid has been shown to reduce tumour-induced osteolysis, and to reduce tumour burden outside bone in subcutaneous tumours from breast, lung and prostate [Holen and Coleman, 2010]. Denosumab is a subcutaneously administered human immunoglobulin G antibody that binds to and neutralizes RANKL in a similar way to OPG, with a serum half life of 46 days [Brown and Coleman, 2012]. In animal models denosumab reduces tumour-induced osteolysis and tumour progression in bone from breast, lung and prostate cancer, and it has been shown to decrease metastasis outside of bone [Dougall et al. 2014].
Clinical evidence for prevention of SREs with bone-targeted agents in lung cancer
The encouraging preclinical data demonstrating that both zoledronic acid and denosumab can modify the bone microenvironment and affect skeletal and extraskeletal tumour growth led to clinical trials that have provided the evidence for licensing in the USA, the UK and Europe for patients with bone metastases from solid tumours. The endpoints in clinical trials of bone-targeted agents vary and have included survival outcomes [Rosen et al. 2004b; Hirsh et al. 2008; Henry et al. 2011], SRE incidence, time to first SRE [Henry et al. 2011], changes in bone biomarkers [Lipton et al. 2008] or symptom outcomes including changes in reported pain or opioid use [Van Moos, 2010; Henry et al. 2011; Rosen et al. 2004b]. Bone bio-markers that have been studied include markers of bone formation such as bone alkaline phosphatase (BALP), type I procollagen and markers of bone resorption such as serum and urine N-terminal telopeptide (NTX), which have been shown to correlate with SREs and survival.
Zoledronic acid is the only bisphosphonate that has demonstrated long-term efficacy and safety in patients with lung cancer with bone metastases. Zoledronic acid has been shown to effectively reduce the risk of SREs in patients with metastatic prostate cancer [Lipton et al. 2002], multiple myeloma and breast cancer [Berenson et al. 2001; Rosen et al. 2001]. Rosen and colleagues analysed 773 patients with bone metastases from solid tumours (of whom nearly half had NSCLC) randomized to receive 4 mg or 8 mg of zoledronic acid or placebo. Due to concerns over renal toxicity, the 8 mg arm was later discontinued. Zoledronic acid significantly reduced the risk of all skeletal events (including hypercalcaemia) in the 4 mg group compared with placebo (38% versus 47%, p = 0.039) [Rosen et al. 2003]. This dose of zoledronic acid was also shown to increase the median time to first SRE by more than 2 months compared with placebo (p = 0.023). In an updated analysis after 21 months of treatment/follow up, these benefits were sustained [Rosen et al. 2004b].
In phase II and III trials in patients with breast cancer and bone metastases, denosumab was shown to have similar efficacy to intravenous bisphosphonates in reducing SREs but led to a greater reduction in bone turnover markers and significantly delayed the time to first on-study SRE [hazard ratio (HR) 0.77; 95% confidence interval (CI) 0.66–0.89; p = 0.001] [Stopeck et al. 2010]. In a phase III trial in patients with bone metastases from solid tumours (apart from breast and prostate) and multiple myeloma, eligible patients were randomized to receive denosumab or zoledronic acid. Of the 1776 patients recruited, 811 had lung cancer (702, 47% had NSCLC). Denosumab was found to be noninferior (trending to superiority) to zoledronic acid in terms of delaying time to first on-study SRE (HR 0.84; 95% CI 0.71–0.98; p = 0.0007), with a 4.3 month improvement in time to first SRE (20.6 months in the denosumab group versus 16.3 months in the zoledronic acid group) [Henry et al. 2011]. The authors also noted that the subcutaneous formulation was more convenient than the intravenous infusion required for zoledronic acid.
Clinical evidence for a survival benefit for bone-targeted agents in lung cancer
Survival outcomes with bone-targeted agents in lung cancer have been evaluated in clinical trials assessing the role of these agents in patients with and without bone metastases.
Bone metastases at trial entry
Zoledronic acid, when used as a single agent, has not been found to improve survival outcomes in patients with lung cancer and bone metastases. In a phase III study of zoledronic acid (4 mg) or placebo given 3 weekly for 9 months in 773 patients with solid tumour (n = 378 NSCLC), neither time to progression of bone lesions (145 days versus 109 days, p = 0.415) nor survival (202.5 days versus 183 days, p = 0.929) was significantly better in the zoledronic acid arm [Rosen et al. 2004b]. A subgroup analysis, restricted to patients with NSCLC patients, suggested that high levels of the biomarker urinary NTX at baseline predicted benefit from zoledronic acid and significantly decreased the relative risk of death 35% (relative risk 0.650, p = 0.024) [Hirsh et al. 2008]. These data suggest that NTX is a useful biomarker and the survival benefits of zoledronic acid are greatest in patients with a baseline high bone turnover, likely due to the increased risk of SREs in this group and subsequent prevention by antiresorptive therapy. However, predicting which individual patients will benefit from zoledronic acid is not currently possible.
More recently, denosumab has been shown to significantly improve survival in patients with lung cancer and bone metastases in a subgroup analysis (n = 811) [Scagliotti et al. 2012a] of a large international phase III study comparing denosumab (120 mg every 4 weeks) with zoledronic acid (4 mg every 4 weeks) in solid tumours (excluding breast and prostate) [Henry et al. 2011]. Denosumab increased overall survival compared with zoledronic acid (8.9 months versus 7.7 months, p = 0.01). In NSCLC, the greatest benefit from denosumab appeared to be in those patients with squamous cell carcinoma with an increased survival of 2.2 months compared with zoledronic acid (HR 0.68, p = 0.035); however, the trend to increased survival was seen in all histological subtypes, including small-cell lung cancer. Although a subgroup analysis, this represents a large prospective randomized trial in NSCLC. Following on from these data, denosumab was licensed for use in patients with established bone metastases from solid tumours in America (2010) and Europe (2011). Ongoing trials of both zoledronic acid and denosumab on survival outcomes in lung cancer will add to existing data (Table 3).
Table 3.
Ongoing trials of bone-targeted agents in lung cancer.
| Clinical Trial.gov identifier | Phase | N | Population | Design | Primary endpoint |
|---|---|---|---|---|---|
| NCT01951586 | II | 216 | NSCLC stage IV | 4-6 cycles of standard doublet platinum-based chemotherapy +/- denosumab 120 mg every 3–4 weeks | Overall survival |
| NCT02129699 (Splendour) | III | 1000 | NSCLC stage IV | 4-6 cycles of standard doublet platinum-based chemotherapy +/- denosumab 120 mg every 3–4 weeks | Overall survival |
| NCT01737216 | II | 100 | Aged >70 years, lung cancer | 4-6 cycles of standard doublet platinum-based chemotherapy +/- zoledronicacid 4 mg every 4 weeks/every 12 weeks until progression | Progression-free survival |
NSCLC, non-small cell lung cancer.
Absence of bone metastases at trial entry
A randomized trial in 437 patients with stage IIIA/B NSCLC without metastases evaluated the use of zoledronic acid administered every 3–4 weeks for 24 months after completion of primary therapy (including surgery, radiotherapy and chemotherapy) without progression [Scagliotti et al. 2012b]. No significant differences were seen between zoledronic acid treated patients and controls in progression-free survival (PFS) (9 months versus 11.3 months respectively, p = 0.096), risk of death (HR 1.17; 95% CI 0.86–1.59), or the number of patients who developed bone metastases (6.6% versus 9%). Similar results were seen in a smaller (n = 150) phase II study of zoledronic acid versus control in patients with stage IIIB/IV NSCLC after primary therapy with docetaxel and carboplatin, with no difference between treatment groups in time to progression or overall survival [Pandya et al. 2010]. These data suggest that zoledronic acid may decrease the formation of bone metastases, but as disease progression in patients with lung cancer is primarily in extraskeletal sites, there is no impact of the drug on survival outcomes. No data are currently available on the use of denosumab to prevent the formation of bone metastases specifically from lung cancer.
The ability of osteoclast inhibitors to prevent the formation of bone metastases and improve survival in patients with solid tumours has been demonstrated in a large meta-analysis (23,000 patients) of adjuvant trials of bisphosphonates in early breast cancer. There was a significant decrease in bone recurrence in the treated population compared with controls (6.9% versus 8.4%). In addition, there was a nonsignificant trend to a reduction in nonbone recurrence in postmenopausal women treated with zoledronic acid compared with controls (9%; 95% CI 5–19%), resulting in a significantly improved 10-year breast cancer mortality (15.2% versus 18.3%) [Coleman et al. 2013]. This suggests that there may be subpopulations of patients in whom bisphosphonates are more effective in preventing the formation of bone metastases, and in these populations they may potentially decrease the formation of extraskeletal metastases. How this preventive approach will translate to lung cancer, and how to identify these high-risk patients, is yet to be established and will need international collaborative trials. The SPLENDOUR (Survival Improvement in Lung Cancer Induced by Denosumab Therapy) trial will soon be open to recruitment. It is a large phase III trial in which patients with stage IV NSCLC will be randomized to receive standard cytotoxic chemotherapy alone or with denosumab, with the primary endpoint being overall survival. Patients will be stratified according to the presence or absence of bone metastases at trial entry, thus enabling an evaluation of the role of denosumab in preventing the formation of bone metastases [ClinicalTrials.gov identifier: NCT02129699].
Tolerability and toxicity of bone-targeted agents
Zoledronic acid is given as a short intravenous infusion, whereas denosumab is given by subcutaneous injection. Both are given with supplements of calcium and vitamin D to reduce the risk of hypocalcaemia. Because zoledronic acid is renally excreted and can cause renal toxicity, dose modifications have to be made in patients with renal impairment, an issue not applicable to denosumab; hence denosumab is easier to administer in an outpatient setting.
Side effects with both zoledronic acid and denosumab in metastatic disease are usually mild and easily managed, provided the drugs are used at recommended dosing and schedule [Wilson et al. 2013]. Side effects reported more commonly with zoledronic acid than denosumab include acute phase reactions and renal toxicity. The acute phase reaction is characterized by fever, arthralgia and myalgia, and is thought to be due to an increase in γ/δ lymphocytes and release of interleukin 6 and tumour necrosis factor α [Dicuonzo et al. 2003]. It typically occurs with the first cycle with an incidence in metastatic breast cancer and myeloma trials of 16–38% [Rosen et al. 2004a; Body et al. 2007]. Renal toxicity is dose and schedule dependent, but with the standard 4 mg dose infused over 15 min the incidence is about 10–15%, necessitating an evaluation of renal function in patients before administration of zoledronic acid [Gartrell et al. 2014]. Side effects reported with similar frequency in both denosumab and zoledronic acid include osteonecrosis of the jaw (ONJ). ONJ is a painful exposure of bone in the mandible and maxilla, commonly occurring after tooth extraction and exacerbated by poor dental hygiene. In trials comparing zoledronic acid with denosumab, ONJ rates were similar, with an incidence of 1–2% over 2–3 years of use [Stopeck et al. 2010; Henry et al. 2011]. Side effects more commonly associated with denosumab include hypocalcaemia, although this is often subclinical and prevented by adequate vitamin D and calcium replacement. In a large randomized trial comparing denosumab with zoledronic acid in the treatment of bone metastases from solid tumours (excluding breast and prostate), the incidence of hypocalcaemia was 10% with denosumab and 5.8% with zoledronic acid [Henry et al. 2011].
Other bone-targeted agents
Several novel bone-targeted agents are currently in clinical trials evaluating their efficacy in reducing skeletal complications, improving quality of life and improving survival outcomes.
Radiopharmaceuticals
Radium-223 is a first-in-class α-particle emitting radioisotope that homes to areas of high bone turnover, making it ideal in targeting metastatic bone disease [Nilsson et al. 2012]. It has a half life of 11.4 days and achieves its cytotoxic effect by causing irreparable double-strand DNA breaks. Given its extremely short range, 60–100 µm, marrow irradiation is spared, hence limiting its myelotoxicity [Hobbs et al. 2012].
The results of the phase III ALSYMPCA trial provided the main evidence for its clinical effectiveness and safety, and led to its approval by the US Food and Drugs Administration (May 2013) and the European Medicines Agency (November 2013) for use in patients with castration-resistant prostate cancer (with bone metastases and no visceral metastases). In this trial, 921 patients with castration-resistant prostate cancer and symptomatic bone metastases (but no visceral metastases), were randomized in a 2:1 ratio to receive radium-223 (six intravenous injections every 4 weeks at a dose of 50 kBq/kg body weight) or placebo. An updated analysis showed a median overall survival of 14.9 months in the radium-223 group and 11.3 months in the placebo group (HR 0.70; 95% CI 0.58–0.83; p < 0.001) [Parker et al. 2013]. In addition, time to first symptomatic skeletal event was 15.6 months in the radium-223 group and 9.8 months in the placebo group (HR 0.66; 95% CI 0.52–0.83; p < 0.001) [Sartor et al. 2014]. No significant difference was seen in frequency of grade 3 or 4 toxicities, while there was a significant improvement in quality of life in the radium-223 group.
An open-label phase II study of radium-223 in patients with breast cancer (n = 23) with bone-dominant disease and previous bisphosphonate treatment used four treatments of 50 kBq/kg every 4 weeks and evaluated the effect on urine NTX and serum BALP. NTX fell with treatment [median change from baseline −4.1; interquartile range (IQR) −17 to 1.7], with the greatest decline in NTX seen in patients with a high baseline levels. BALP also fell (median change from baseline −3.4; IQR −16 to 1.2). A secondary endpoint analysis was the effect of radiun-223 on bone pain showing a consistent reduction in Brief Pain Inventory scores during treatment. These data show that radium-223 can further decrease bone turnover in a breast cancer population already treated with bisphosphonates [Coleman et al. 2014]. The role of radium-223 in patients with lung cancer is yet to be tested.
Strontium-89 and Samarium-153 are β-emitting radionuclides which have been shown to have a role in the palliation of pain associated with bone metastases [Lewington et al. 1991; Serafini et al. 1998; Sartor et al. 2004; Tian et al. 1999]. Neither agent has been shown to have a survival benefit. Strontium-89 has a half life of 50.5 days, a mean range of 6.7 mm and is approved for use in painful bone metastases in prostate cancer with bone metastases; while samarium-153, with a half life of 1.95 days, and mean range of 3.4 mm, can be used for osteoblastic metastases of other cancer types. The mechanism behind their palliative effect is not fully understood, but is likely related to cell signalling changes induced by radiation damage to cancer cells and surrounding tissues, resulting in alterations in the way pain signals are received and conducted. The main toxicities associated with these agents are myelotoxicity, which is related to the greater range of these agents (compared with the α-emitting radium-223), and pain flare.
Targeted agents
Cabozantinib is an oral tyrosine kinase inhibitor which blocks tyrosine kinase (MET), vascular endothelial growth factor receptor 2 and proto-oncogene (RET). MET is known to be overexpressed in bone metastases from solid tumours, that is, prostate cancer [Knudsen et al. 2002] and is involved in the proliferation, differentiation and migration of osteoblasts and osteoclasts [Grano et al. 1996]. A phase II randomized trial of cabozantinib 100 mg daily in prostate cancer showed 68% of patients had improvement of bone disease on bone scan, including a complete response in 12%. BALP and serum CTX also decreased by at least 50% in 57% of patients, with improvements in bone pain in 67% of patients [Smith et al. 2013]. The most common adverse events were fatigue, gastrointestinal toxicities and plantar palmar erythrodysesthesia. It is not known whether similar effects will be seen in patients with bone metastases from other tumour types.
The Src family of nonreceptor tyrosine kinases regulate a number of cell-signalling pathways and inhibition of the Src family has been shown in preclinical studies to interrupt the development of osteoclasts from precursors, hence inhibiting the progression of bone metastases [Zhang et al. 2009]. Lung cancers express Src and a phase II study of saracatinib in patients with NSCLC who had attained at least stable disease with platinum chemotherapy (n = 37) showed a soft tissue response rate of 5.5% (95% CI 2–13%) and stabilization of disease in a further 17% (95% CI 4–29%), indicating some single-agent activity in second-line therapy [Laurie et al. 2014]. Adverse events included fatigue, gastrointestinal toxicity and skin rashes. Specific effects on bone metastases from NSCLC were not reported. Ongoing studies evaluating the effects of saracatinib in bone metastases from solid tumours will provide more evidence for their role in NSCLC, including the phase II SarCaBon study evaluating the effect of saracatinib in reducing pain associated with bone metastases [ClinicalTrials.gov identifier: NCT01605227].
Conclusion
Zoledronic acid and denosumab have improved outcomes for patients with lung cancer with bone metastases by reducing SREs. In addition, denosumab has been shown to improve survival. However, the ability of either drug to prevent the formation of bone or extraskeletal metastases is not well defined and trials such as SPLENDOUR will assist in defining their role in this context. Denosumab appears to be at least equivalent to zoledronic acid in SRE prevention and superior in terms of overall survival, it does not affect renal function and is well tolerated. These factors may make it popular amongst lung cancer physicians, however the cost of each treatment should be considered. Whether denosumab or zoledronic acid is utilized in patients with lung cancer is likely to be driven by decisions of local health establishments. However, it is important that a bone-targeted agent is prescribed to prevent morbidity and mortality from the high incidence of SREs in this patient group.
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Shobha C. Silva, Weston Park Hospital, Sheffield, UK
Caroline Wilson, Weston Park Hospital, Sheffield, UK.
Penella J. Woll, Academic Unit of Clinical Oncology, Weston Park Hospital, Whitham Road, Sheffield S10 2SJ, UK
References
- Bauml J., Mick R., Zhang Y, et al. (2013) Determinants of survival in advanced non-small-cell lung cancer in the era of targeted therapies. Clin Lung Cancer 14: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson J., Rosen L., Howell A., Porter L., Coleman R., Morley W., et al. (2001) Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 91: 1191–1200. [DOI] [PubMed] [Google Scholar]
- Body J., Lichinitser M., Tjulandin S., Garnero P., Bergstrom B. (2007) Oral ibandronate is as active as intravenous zoledronic acid for reducing bone turnover markers in women with breast cancer and bone metastases. Ann Oncol 18: 1165–1171. [DOI] [PubMed] [Google Scholar]
- Brown J., Coleman R. (2012) Denosumab in patients with cancer-a surgical strike against the osteoclast. Nat Rev Clin Oncol 9: 110–118. [DOI] [PubMed] [Google Scholar]
- Clines G., Guise T. (2005) Hypercalcaemia of malignancy and basic research on mechanisms responsible for osteolytic and osteoblastic metastasis to bone. Endocr Relat Cancer 12: 549–583. [DOI] [PubMed] [Google Scholar]
- Coleman R. (1997) Skeletal complications of malignancy. Cancer 80(8 Suppl.): 1588–1594. [DOI] [PubMed] [Google Scholar]
- Coleman R. (2004) Bisphosphonates: clinical experience. The Oncologist 9(Suppl. 4): 14–27. [DOI] [PubMed] [Google Scholar]
- Coleman R. (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12: 6243s–6249s. [DOI] [PubMed] [Google Scholar]
- Coleman R., Aksnes A., Naume B., Garcia C., Jerusalem G., Piccart M., et al. (2014) A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Breast Cancer Res Treat 145: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Gnant M., Paterson A.et al. (2013) Effects of bisphosphonate treatment on recurrence and cause-specific mortality in women with early breast cancer: a meta-analysis of individual patient data from randomized trials. San Antonio Breast Cancer Symposium 2013, 12 December, San Antonio, TX; Abstr S4–07. [Google Scholar]
- Coxon F., Rogers M. (2003) The role of prenylated small GTP-binding proteins in the regulation of osteoclast function. Calcif Tissue Int 72: 80–84. [DOI] [PubMed] [Google Scholar]
- Dai J., Keller J., Zhang J., Lu Y., Yao Z., Keller E. (2005) Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res 65: 8274–8285. [DOI] [PubMed] [Google Scholar]
- Delea T., Langer C., McKiernan J., Liss M., Edelsberg J., Brandman J., et al. (2004) The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology 67: 390–396. [DOI] [PubMed] [Google Scholar]
- Dicuonzo G., Vincenzi B., Santini D., Avvisati G., Rocci L., Battistoni F., et al. (2003) Fever after zoledronic acid administration is due to increase in TNF-alpha and IL-6. J Interferon Cytokine Res 23: 649–654. [DOI] [PubMed] [Google Scholar]
- Dougall W., Glaccum M., Charrier K., Rohrbach K., Brasel K., De Smedt T., et al. (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13: 2412–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall W., Holen I., Gonzalez Suarez E. (2014) Targeting RANKL in metastasis. BoneKEy Rep 3: 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartrell B., Coleman R., Fizazi K., Miller K., Saad F., Sternberg C. (2014) Toxicities following treatment with bisphosphonates and receptor activator of nuclear factor-kappaB ligand inhibitors in patients with advanced prostate cancer. Eur Urol 65: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grano M., Galimi F., Zambonin G., Colucci S., Cottone E., Zallone A., et al. (1996) Hepatocyte growth factor is a coupling factor for osteoclasts and osteoblasts in vitro. Proc Natl Acad Sci USA 93: 7644–7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry D, Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J., et al. (2011) Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 29: 1125–1132. [DOI] [PubMed] [Google Scholar]
- Hirsh V., Major P., Lipton A., Cook R., Langer C., Smith M., Brown J., Coleman R. (2008) Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity. J Thorac Oncol 3: 228–236. [DOI] [PubMed] [Google Scholar]
- Hobbs R., Song H., Watchman C., Bolch W., Aksnes A., Ramdahl T., et al. (2012) A bone marrow toxicity model for (2)(2)(3)Ra alpha-emitter radiopharmaceutical therapy. Phys Med Biol 57: 3207–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holen I. (2012) Pathophysiology of bone metastases. In: Coleman R., Abrahamsson P., Hadji P. (eds), Handbook of Cancer-Related Bone Disease, 2nd edn. Bristol, UK: Bioscientifica, pp. 33–52. [Google Scholar]
- Holen I., Coleman R. (2010) Anti-tumour activity of bisphosphonates in preclinical models of breast cancer. Breast Cancer Res 12: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R., Spencer S., Meredith R., Weppelmann B., Lee J., Smith J., et al. (1990) Extradural spinal cord compression: analysis of factors determining functional prognosis – prospective study. Radiology 176: 279–282. [DOI] [PubMed] [Google Scholar]
- Kingsley L., Fournier P., Chirgwin J., Guise T. (2007) Molecular biology of bone metastasis. Molecular cancer therapeutics 6: 2609–2617. [DOI] [PubMed] [Google Scholar]
- Knudsen B., Gmyrek G., Inra J., Scherr D., Vaughan E., Nanus D. (2002) High expression of the Met receptor in prostate cancer metastasis to bone. Urology 60: 1113–1117. [DOI] [PubMed] [Google Scholar]
- Kong Y., Yoshida H., Sarosi I., Tan H., Timms E., Capparelli C., et al. (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397: 315–323. [DOI] [PubMed] [Google Scholar]
- Laurie S., Goss G., Shepherd F., Reaume M., Nicholas G., Philip L.et al. 2014. A phase II trial of saracatinib, an inhibitor of Src kinases, in previously-treated advanced non-small-cell lung cancer: the Princess Margaret Hospital Phase II Consortium. Clin Lung Cancer 15: 52–57. [DOI] [PubMed] [Google Scholar]
- Lewington V., McEwan A., Ackery D., Bayly R., Keeling D., Macleod P., et al. (1991) A prospective, randomised double-blind crossover study to examine the efficacy of strontium-89 in pain palliation in patients with advanced prostate cancer metastatic to bone. Eur J Cancer 27: 954–958. [DOI] [PubMed] [Google Scholar]
- Lin J. (1996) Bisphosphonates: a review of their pharmacokinetic properties. Bone 18: 75–85. [DOI] [PubMed] [Google Scholar]
- Lipton A., Cook R., Saad F., Major P., Garnero P., Terpos E., et al. (2008) Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer 113: 193–201. [DOI] [PubMed] [Google Scholar]
- Lipton A., Small E., Saad F., Gleason D., Gordon D., Smith M., et al. (2002) The new bisphosphonate, Zometa (zoledronic acid), decreases skeletal complications in both osteolytic and osteoblastic lesions: a comparison to pamidronate. Cancer Invest 20(Suppl. 2): 45–54. [DOI] [PubMed] [Google Scholar]
- Martenson J., Jr, Evans R., Lie M., Ilstrup D., Dinapoli R., Ebersold M., et al. (1985) Treatment outcome and complications in patients treated for malignant epidural spinal cord compression (SCC).J Neurooncol 3: 77–84. [DOI] [PubMed] [Google Scholar]
- Mercadante S. (1997) Malignant bone pain: pathophysiology and treatment. Pain 69: 1–18. [DOI] [PubMed] [Google Scholar]
- Mourskaia A., Dong Z., Ng S., Banville M., Zwaagstra J., O’Connor-McCourt M., et al. (2009) Transforming growth factor-beta1 is the predominant isoform required for breast cancer cell outgrowth in bone. Oncogene 28: 1005–1015. [DOI] [PubMed] [Google Scholar]
- Mundy G. (1997) Mechanisms of bone metastasis. Cancer 80(8 Suppl.): 1546–1556. [DOI] [PubMed] [Google Scholar]
- Nilsson S., Strang P., Aksnes A., Franzen L., Olivier P., Pecking A., et al. (2012) A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer 48: 678–686. [DOI] [PubMed] [Google Scholar]
- Pandya K., Gajra A., Warsi G., Argonza-Aviles E., Ericson S., Wozniak A. (2010) Multicenter, randomized, phase 2 study of zoledronic acid in combination with docetaxel and carboplatin in patients with unresectable stage IIIB or stage IV non-small cell lung cancer. Lung Cancer 67: 330–338. [DOI] [PubMed] [Google Scholar]
- Parker C., Pascoe S., Chodacki A., O’Sullivan J., Germa J., O’Bryan-Tear C., et al. (2013) A randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur Urol 63: 189–197. [DOI] [PubMed] [Google Scholar]
- Ralston S., Gallacher S., Patel U., Campbell J., Boyle I. (1990) Cancer-associated hypercalcemia: morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med 112: 499–504. [DOI] [PubMed] [Google Scholar]
- Rosen L., Gordon D., Dugan W., Jr, Major P., Eisenberg P., Provencher L., et al. (2004a) Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 100: 36–43. [DOI] [PubMed] [Google Scholar]
- Rosen L., Gordon D., Kaminski M., Howell A., Belch A., Mackey J., et al. (2001) Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J 7: 377–387. [PubMed] [Google Scholar]
- Rosen L., Gordon D., Tchekmedyian S., Yanagihara R., Hirsh V., Krzakowski M., et al. (2003) Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial – the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol 21: 3150–3157. [DOI] [PubMed] [Google Scholar]
- Rosen L., Gordon D., Tchekmedyian N., Yanagihara R., Hirsh V., Krzakowski M., et al. (2004b) Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, phase III, double-blind, placebo-controlled trial. Cancer 100: 2613–2621. [DOI] [PubMed] [Google Scholar]
- Russell R., Watts N., Ebetino F., Rogers M. (2008) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporosis Int 19: 733–759. [DOI] [PubMed] [Google Scholar]
- Saad F., Lipton A., Cook R., Chen Y. M., Smith M., Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 110(8): 1860–1867. [DOI] [PubMed] [Google Scholar]
- Sartor O., Coleman R., Nilsson S., Heinrich D., Helle S., O’Sullivan J., et al. (2014) Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 15: 738–746. [DOI] [PubMed] [Google Scholar]
- Sartor O., Reid R., Hoskin P., Quick D., Ell P., Coleman R. (2004) Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology 63: 940–945. [DOI] [PubMed] [Google Scholar]
- Scagliotti G., Hirsh V., Siena S., Henry D., Woll P., Manegold C., et al. (2012a) Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J Thorac Oncol 7: 1823–1829. [DOI] [PubMed] [Google Scholar]
- Scagliotti G., Kosmidis P., de Marinis F., Schreurs A., Albert I., Engel-Riedel W. (2012b) Zoledronic acid in patients with stage IIIA/B NSCLC: resultsof a randomized, phase III study. Ann Oncol 23: 2082–2087. [DOI] [PubMed] [Google Scholar]
- Serafini A., Houston S., Resche I., Quick D., Grund F., Ell P., et al. (1998) Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J Clin Oncol 16:1574–1581. [DOI] [PubMed] [Google Scholar]
- Smith D., Smith M., Sweeney C., Elfiky A., Logothetis C., Corn P., et al. (2013) Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol 31: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopeck A., Lipton A., Body J., Steger G., Tonkin K., de Boer R., et al. (2010) Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 28: 5132–5139. [DOI] [PubMed] [Google Scholar]
- Tian J., Zhang J., Hou Q., Oyang Q., Wang J., Luan Z., et al. (1999) Multicentre trial on the efficacy and toxicity of single-dose samarium-153-ethylene diamine tetramethylene phosphonate as a palliative treatment for painful skeletal metastases in China. Eur J Nucl Med 26: 2–7. [DOI] [PubMed] [Google Scholar]
- Tsuya A., Kurata T., Tamura K., Fukuoka M. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer 57(2): 229–232. [DOI] [PubMed] [Google Scholar]
- van Beek E., Pieterman E., Cohen L., Lowik C., Papapoulos S. (1999) Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun 264: 108–111. [DOI] [PubMed] [Google Scholar]
- Van Moos R., Patrick D., Fallowfield L., Cleeland C., De Boer R., Stegger G., et al. (2010) Effects of denosumab versus zoledronic acid (ZA) on pain in patients (pts) with advanced cancer (excluding breast and prostate) or multiple myeloma (MM): results from a randomised phase III trial. J Clin Oncol 7(Suppl.). [Google Scholar]
- Wilson C., Taylor F., Coleman R. (2013) Toxicity of bone targeted agents in malignancy. In: Dicato M. (ed.), Side Effects of Medical Cancer Therapy. London: Springer, pp. 531–569. [Google Scholar]
- Zhang X., Wang Q., Gerald W., Hudis C., Norton L., Smid M., et al. (2009) Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 16: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]