Abstract
Hepatic epithelioid hemangioendothelioma (HEHE) is a rare, often misdiagnosed vascular neoplasm with clinical behaviors that range from indolent to highly aggressive. Even when the appropriate diagnosis is achieved, the best treatment for HEHE has not been defined or standardized, further complicating the care of these patients. We present a diagnostically challenging case of HEHE where we utilized capecitabine and bevacizumab as another novel treatment option.
Keywords: metastatic, heptatic, epithelioid, hemangioendothelioma, capecitabine, bevacizumab
Introduction
First described in 1982, epithelioid hemangioendothelioma (EHE) is a rare vascular neoplasm of endothelial origin known to arise in various soft tissue and visceral organs. Common sites of disease include liver, lungs, lymph nodes, peritoneum and bones [Mistry et al. 2012]. The estimated incidence of hepatic epithelioid hemangioendothelioma (HEHE) is less than one in one million [Mehrabi et al. 2006]. The clinical course of HEHE can vary between benign hemangiomas to malignant angiosarcomas, thus rendering prognosis unpredictable. Mostly afflicting people in the third or fourth decade of life, there is no known etiology for HEHE. Cases reported in the literature have posited a causal relationship between use of oral contraceptives, liver disease, liver trauma, asbestos, vinyl chloride or thorotrast [Mistry et al. 2012; Mehrabi et al. 2006].
Diagnosing HEHE is challenging given the rarity of the disease and lack of clinically useful diagnostic tools. Misdiagnosis is common, occurring in up to 80% of cases [Mehrabi et al. 2006]. Up to 25% of patients are asymptomatic, and 15% have normal laboratory values including normal tumor markers at time of diagnosis [Mistry et al. 2012]. Imaging, including computerized tomography (CT) and magnetic resonance imaging (MRI), is also nonspecific [Mistry et al. 2012; Mehrabi et al. 2006; Amin et al. 2011]. A majority of patients presents with multifocal, bilobar hepatic lesions with peripheral enhancement (‘halo’ sign) and capsular retraction [Mistry et al. 2012; Mehrabi et al. 2006; Amin et al. 2011]. Definitive diagnosis requires histopathologic examination. HEHE appears in nest or cords of epithelioid endothelial cells that are positive for CD31, CD34 and factor VIII on immunohistochemistry [Mistry et al. 2012; Mehrabi et al. 2006]. Furthermore, vascular endothelial growth factor (VEGF) has been found to be overexpressed in HEHE [Emamaullee et al. 2010]. Importantly, podoplanin, a small mucin-like transmembrane protein involved in cell migration and invasion, is expressed in HEHE, distinguishing HEHE from other primary vascular tumors such as angiosarcoma or hemangiomas, which lack podoplanin expression [Mistry et al. 2012; Wicki and Christifori, 2007]. In 63% of cases, chromosomal translocation t(1;3)(p36.3;q25) causing gene fusion product WWTR1-CAMTA1 has been identified, which plays a role in oncogenesis [Mistry et al. 2012; Woelfel et al. 2011; Errani et al. 2011].
There is no consensus on standard of care or treatment strategies. Treatment experiences reported in the literature are limited to case reports or retrospective case series from single institutions [Mehrabi et al. 2006; Rodriguez et al. 2008; Cardinal et al. 2009]. The rarity of HEHE precludes prospective studies to elucidate optimal management. Treatment approaches vary, but include liver resection, liver transplantation, radioembolization/chemoembolization, radiotherapy, chemotherapy, combined-modality therapy and observation. In the proper candidate, liver transplant has 5-year survival rates ranging from 43 to 83%. Cumulative recurrence rate following liver transplant is about 30%, including recurrence in the allograft [Mistry et al. 2012; Mehrabi et al. 2006; Rodriquez et al. 2008; Cardinal et al. 2009]. In addition, extrahepatic HEHE at presentation can range from 37 to 48% [Mehrabi et al. 2006; Grotz et al. 2010; Thomas et al. 2014]. The presence of extrahepatic disease does not significantly affect overall survival (OS) after liver transplantation [Grotz et al. 2010].
When treated with chemotherapy/radiotherapy alone, 5-year OS is estimated to be about 30% [Mehrabi et al. 2006]. Multiple single agent and combination regimens have been used (Table 1). However, there are no case reports in the literature regarding the use of capecitabine in combination with bevacizumab in the treatment of metastatic HEHE. We present a case of metastatic HEHE currently being treated with capecitabine and bevacizumab.
Table 1.
Various chemotherapeutics used in the treatment of epithelioid hemangioendothelioma and their outcomes.
| Reference | Sites of disease | Age (years) | No. patient | Chemotherapy | Dose | Outcome | Duration of follow up |
|---|---|---|---|---|---|---|---|
| Belmont et al. [2008] | Lung | 41 | 1 | Carboplatin, paclitaxel, docetaxel, bevacizumab | 15 mg/kg, every 21 days (bevacizumab) | Partial Response | 13 months |
| Kim et al. [2010] | Lung, liver, bone | 44 | 1 | Carboplatin, paclitaxel, bevacizumab | 15 mg/kg, every 21 days (bevacizumab) | Progression | Not available |
| Mizota et al. [2011] | Lung, liver | 59 | 1 | Carboplatin, paclitaxel, bevacizumab | 15mg/kg, every 21 days (bevacizumab) | Progression | 3 month |
| Lazarus et al. [2011] | Lung | 42 | 1 | Paclitaxel, bevacizumab | Unknown | Progression | 1–2 months |
| Lazarus et al. [2011] | Lung | 42 | 1 | Carboplatin, etoposide, bevacizumab | Unknown | Progression | 2–3 months |
| Salech et al. [2010] | Lung, liver | 40 | 1 | Thalidomide | 300 mg daily | Partial response | 109 months |
| Raphael et al. [2010] | Lung, liver | 53 | 1 | Thalidomide | 400 mg daily | Stable disease | 84 months |
| Kassam and Mandel [2008] | Lung, liver | 13 | 1 | Thalidomide | 400 mg twice daily | Progressive disease | Not available |
| Bolke et al. [2006] | Liver, bone | 47 | 1 | Thalidomide | Unknown | Progressive disease/death | Not available |
| Mascarenhas et al. [2005] | Lung, liver | 52 | 1 | Thalidomide | Unknown | Partial response | Not available |
| Sumrall et al. [2010] | Brain, bone, liver, lung | 31 | 1 | Lenalidomide | 25 mg daily for 21/28 days | Stable disease | 48 months |
| Schilling et al. [2009] | Lung, liver, spleen | 33 | 1 | Lenalidomide | 30 mg daily for 21/28 days | Stable disease | 6 months |
| Radzikowska et al. [2008] | Lung | 62 | 1 | Interferon α-2a | 3 million units, 3 times/week | Stable disease | 3 months |
| Saleiro et al. [2008] | Lung | 39 | 1 | Interferon α-2a | Unknown | Progressive disease | 9 months |
| Calabro et al. [2007] | Lung, liver, spleen | 53 | 1 | Interferon α-2a | Unknown | Stable disease | Not available |
| Kayler et al. [2002] | Liver, spleen, uterus, peritoneum | 21 | 1 | Interferon α-2a | 3 million units daily | Partial response | 4 months |
| Hassan et al. [2005] | Thyroid | 73 | 1 | Interferon α | 3 million units, 5 times/week | Progressive disease | 2 months |
| Marsh Rde et al. [2005] | Breast, lung, liver | 57 | 1 | Interferon α | 3 million units, 5 days/week for 1 year | Complete response | 84 months |
| Al-Shraim et al. [2005] | Lung, skin | 51 | 1 | Interferon α | 7 million units, 3 times/week | Progressive disease | 2 months |
| Agulnik et al. [2013] | Unknown | Unknown | 1 | Bevacizumab | 15 mg/kg, every 21 days | Partial response | Not available |
| Agulnik et al. [2013] | Unknown | Unknown | 1 | Bevacizumab | 15 mg/kg, every 21 days | Stable disease | Not available |
| Agulnik et al. [2013] | Unknown | Unknown | 1 | Bevacizumab | 15 mg/kg, every 21 days | Progression | Not available |
| Lakkis et al. [2013] | Lung, liver, spleen | 58 | 1 | Cyclophosphamide | 50 mg daily, continuous | Progression | 6 months |
| Lakkis et al. [2013] | Lung, liver, bone | 40 | 1 | Cyclophosphamide | 50 mg daily, continuous | Partial response | 24 months |
| Kelly and O’Neil [2005] | Liver, bone | 52 | 1 | Liposomal doxorubicin | 45 mg/m2, every 21 days | Partial response | 22 months |
| Grenader et al. [2011] | Lung, liver | 32 | 1 | Liposomal doxorubicin | 20 mg/m2, every 21 days | Partial response | 7 months |
| Pintoffl et al. [2009] | Lung, liver, bone | 32 | 1 | Gemcitabine | 1000 mg/m2, days 1, 8, and 15, every 28 days | Stable disease | 72 months |
| Sangro et al. [2012] | Lung, liver | 22 | 1 | Sorafinib | 200 mg every 36 hours | Partial response | 6 months |
| Trautmann et al. [2011] | Bone | 19 | 1 | Bevacizumab | 7.5 mg/m2 every 21 days | Stable disease | 16 months |
| Coppo et al. [2004] | Bone | 70 | 1 | Pamidronate | 90 mg, twice monthly | Complete response | 72 months |
Case presentation
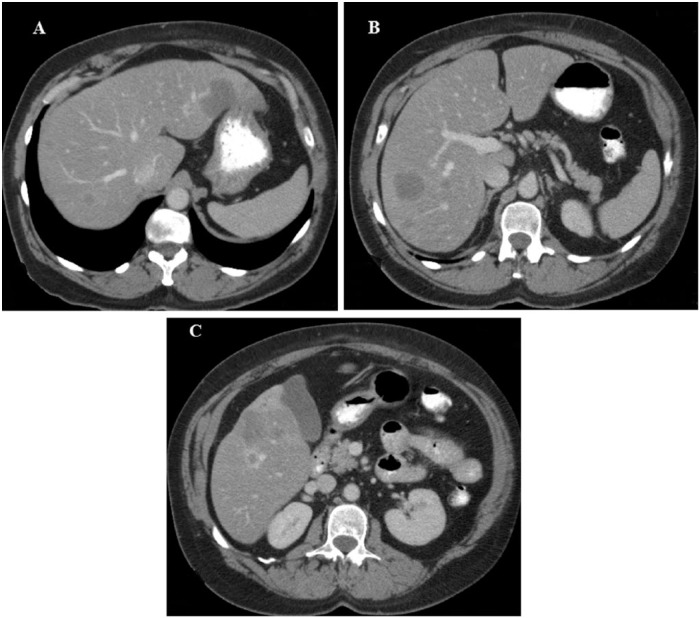
A 56-year-old woman presented in 2007 with worsening wheezing and a dry cough for approximately 10 months. Her past medical history included diabetes mellitus type II, allergic rhinitis and a 38-pack year history of tobacco use. A CT scan of the chest, abdomen and pelvis found multiple bilateral subcentimeter pulmonary nodules and multiple rim enhancing liver masses with liver capsular retraction (Figure 1a-c).
Figure 1.
Contrast enhanced axial computerized tomography images showing multiple attenuated lesions of hepatic epithelioid hemangioendothelioma. There are multiple lesions with varying size (A–C), the largest was 4.6 cm (A). Some lesions are rim enhancing and there is evidence of capsular retraction (C).
A core biopsy of a liver lesion revealed immunohistochemistry (IHC) positivity for cytokeratin 8+18, cytokeratin 7, cytokeratin 20 and carcinoembrionic antigen (CEA). Thyroid transcription factor 1 (TTF-1) and estrogen receptor (ER)/progesterone receptor (PR) were negative. Additionally, trichrome staining revealed fibrosis, favoring breast, lung or upper gastrointestinal malignancy. A subsequent positron emission tomography (PET) scan reported no abnormal uptake in the liver lesions.
Given her history, symptoms and pathology, she was treated with carboplatin and gemcitabine. CT imaging after two cycles reported stable liver lesions. The pulmonary nodules were not well visualized. Given minimal response, she then received two cycles of carboplatin and paclitaxel; subsequent imaging showed stable liver disease. Given stability, she opted for a treatment break which lasted 14 weeks. Restaging imaging revealed enlarged liver lesions. Her initial core liver biopsy was re-reviewed at another institution and the consensus was that her liver findings were the result of a bile duct carcinoma. This information prompted a change in regimen to single-agent oral capecitabine. She remained on capecitabine for 1 year with stable disease demonstrated with repeat serial imaging.
After a 4-month treatment break, subsequent restaging imaging revealed enlarging liver lesions. Another biopsy of a liver lesion was performed. IHC was positive for cytokeratins 7 and 8+18, consistent with adenocarcinoma. Results of molecular profiling of the liver lesion biopsy suggested hepatocholangioma. She was treated with sorafenib for 6 months at which point, she elected to stop given adverse side effects and stable disease demonstrated on imaging. After 4 years of observation, capecitabine was reinitiated after she complained of right upper quadrant pain and imaging revealed enlarging liver lesions. Another liver lesion biopsy was performed, which confirmed HEHE. IHC was positive for CD31 and CD34.
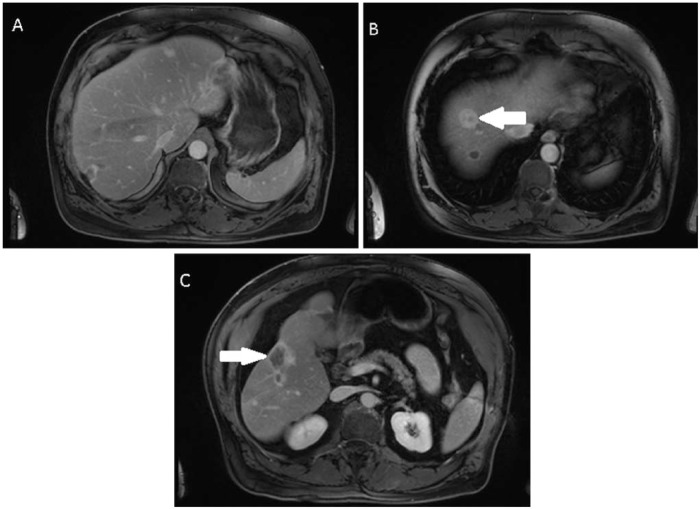
Bevacizumab was added to capecitabine given available data showing benefit [Grotz et al. 2010; O’Grady, 2000; Lazarus et al. 2011; Gaur et al. 2012; Salech et al. 2010; Agulnik et al. 2013]. After 6 months of treatment, the patient continued to show response to treatment (Figure 2a–c) as well as remaining asymptomatic. She is currently being evaluated for liver transplantation.
Figure 2.
Contrast enhanced axial magnetic resonance imaging (MRI) images while on capecitabine and bevacizumab. The initial hepatic lesion seen in Figure 1a is no longer visible (A). Index lesion at the junction of segment IV/VII (arrow) measures 2.6 cm × 2.2 cm (B). The index coalescent lesions seen in segments V/VI (arrow) measure approximately 5.3 cm × 3.4 cm (C).
Discussion
Early therapeutic options other than surgery have not been clearly defined or agreed upon for HEHE. Despite poorer survival outcomes with the use of chemotherapy alone, numerous regimens have been used with varying results [Mistry et al. 2012; Mehrabi et al. 2006; Thomas et al. 2014]. Neoadjuvant biotherapy, specifically interferon-α, for HEHE prior to liver transplant has been described; however, there is no standard neoadjuvant chemotherapy regimen known prior to surgery. There were two cases where patients were free of disease at follow up following orthotropic liver transplant after progressing with chemotherapy or embolization [Thomas et al. 2014]. Additionally, there have been limited case reports using adjuvant chemotherapy with interferon-α with mixed results [Mehrabi et al. 2006; Makhlouf et al. 1999]. Our case report describes the use of capecitabine and bevacizumab, which may prove to be a treatment option other than surgery, especially in situations when surgical resection is impossible. Furthermore, combination capecitabine and bevacizumab has the potential to be a reasonable neoadjuvant option, with the aim of converting patients to resection candidates.
Although capecitabine was utilized in this case before the accurate diagnosis was made, there is evidence to support the use of 5-fluorouracil based therapy in patients with HEHE. Hepatic intra-arterial 5-fluorouracil was used successfully in HEHE to prolong survival [Makhlouf et al. 1999; Holley and Cuschieri, 1989; Lauffer et al. 1996]. The use of systemic 5-fluorouracil in combination with other chemotherapy was also attempted in a patient with HEHE who was initially diagnosed with cholangiocarcinoma, thus making 5-fluorouracil an option for systemic therapy [Mehrabi et al. 2006; O’Grady, 2000].
Given VEGF expression in HEHE, there is the rationale to use anti-VEGF agents such as bevacizumab either alone or in combination with other chemotherapy in the treatment of pulmonary and metastatic EHE. In particular, bevacizumab has been used with carboplatin, paclitaxel, docetaxel and/or etoposide [Grotz et al. 2010; O’Grady, 2000; Lazarus et al. 2011; Gaur et al. 2012]. Most commonly, bevacizumab is used in combination with paclitaxel. Regardless of the chemotherapy bevacizumab is paired with, progression-free survival (PFS) and OS varies from months to years [Grotz et al. 2010; Lazarus et al. 2011; Gaur et al. 2012; Salech et al. 2010]. A single-arm, multicenter, phase II trial utilizing single agent bevacizumab for unresectable EHE reported PFS and OS of 39 weeks and 143 weeks, respectively, when bevacizumab was given at 15 mg/kg every 3 weeks [Agulnik et al. 2013].
Other therapies reported include combination or single-agent regimens of the following: interferon-α, sunitinib, sorafenib, thalidomide, lenalidomide, vinorelbine, dacarbazine, cisplatin, ifosfamide, etoposide, docetaxel, paclitaxel, nab-paclitaxel, liposomal doxorubicin, gemcitabine, pamidronate, cyclophosphamide and nonsteroidal anti-inflammatory drugs [Cardinal et al. 2009; Gaur et al. 2012; Sangro et al. 2012; Raphael et al. 2010; Sumrall et al. 2010; Schilling et al. 2009; Sharif et al. 2004; Marquez-Medina et al. 2004; Belmont et al. 2008; Cronin and Arenberg, 2004; Schattenberg et al. 2008; Grenader et al. 2011; Kelly and O’Neil, 2005; Pintoffl et al. 2009; Coppo et al. 2004; Lakkis et al. 2013](see Table 1).
Capecitabine and bevacizumab have not been previously described to treat HEHE. This proved to be an effective, tolerable regimen for a patient with metastatic hepatic hemangioendothelioma. Although liver transplantation or resection may present the best options for improved survival, clinicians should consider the use of capecitabine with bevacizumab in the treatment of HEHE, especially in those awaiting liver transplantation or nonsurgical candidates.
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Augustine Lau, Yuma Regional Medical Center, Yuma Regional Cancer Center, 2375 S Ridgeview Dr, Yuma, AZ 85364.
Steve Malangone, University of Arizona Cancer Center, Department of Medicine, Division of Hematology/Oncology, Tucson, AZ, USA.
Myke Green, University of Arizona Cancer Center, Department of Medicine, Division of Hematology/Oncology, Tucson, AZ, USA.
Ambuga Badari, Yuma Regional Medical Center Yuma Regional Cancer Center, Yuma, AZ, USA.
Kathryn Clarke, University of Arizona Cancer Center, Department of Medicine, Division of Hematology/Oncology, Tucson, AZ, USA.
Emad Elquza, University of Arizona, College of Medicine and University of Arizona Cancer Center, Department of Medicine, Division of Hematology/Oncology, Tucson, AZ, USA.
References
- Agulnik M., Yarber J., Okuno S., von Mehren M., Jovanovic B., Brockstein B., et al. (2013) An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol 24: 257–263. [DOI] [PubMed] [Google Scholar]
- Al-Shraim M., Mahboub B., Neligan P., Chamberlain D., Ghazarian D. (2005) Primary pleural epithelioid haemangioendothelioma with metastases to the skin. A case report and literature review. J Clin Pathol 58: 107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin S., Chung H., Jha R. (2011) Hepatic epithelioid hemangioendothelioma: MR imaging findings. Abdom Imaging 36: 407–414. [DOI] [PubMed] [Google Scholar]
- Belmont L., Zemoura L., Couderc L. (2008) Pulmonary epithelioid haemangioendothelioma and bevacizumab. J Thorac Oncol 3: 557–558. [DOI] [PubMed] [Google Scholar]
- Bolke E., Gripp S., Peiper M., Budach W., Schwartz A., Orth K., et al. (2006) Multifocal epithelioid hemangioendothelioma: case report of a clinical chamaeleon. Eur J Med Res 11: 462–466. [PubMed] [Google Scholar]
- Calabro L., Di Giacomo A., Altomonte M., Fonsatti E., Mazzel M., Volterrani L., et al. (2007) Primary hepatic epithelioid hemangioendothelioma progressively responsive to interferon-alpha: is there room for novel anti-angiogenetic treatments? J Exp Clin Cancer Res 26: 145–150. [PubMed] [Google Scholar]
- Cardinal J., de Vera M., March J., Steel J., Geller D., Fontes P., et al. (2009) Treatment of hepatic epithelioid hemangioendothelioma: a single-institution experience with 25 cases. Arch Surg 144: 1035–1039. [DOI] [PubMed] [Google Scholar]
- Coppo P., Lassoued S., Billey T., Lassoued K. (2005) Successful treatment of osteolytic epithelioid hemangioendothelioma with pamidronate. Clin Exp Rheumatol 23: 400–401. [PubMed] [Google Scholar]
- Cronin P., Arenberg D. (2004) Pulmonary Epithelioid Hemangioendothelioma: an unusual case and a review of the literature. Chest 125: 789–793. [DOI] [PubMed] [Google Scholar]
- Emamaullee J., Edgar R., Toso C., Thiesen A., Bain V., Bigam D., et al. (2010) Vascular endothelial growth factor expression in hepatic epithelioid hemangioendothelioma: Implications for treatment and surgical management. Liver Transpl 16: 191–197. [DOI] [PubMed] [Google Scholar]
- Errani C., Zhang L., Sung Y., Hajdu M., Singer S., Maki R., et al. (2011) A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer 50: 644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur S., Torabi A., O’Neill T. (2012) Activity of angiogenesis inhibitors in metastatic epithelioid hemangioendothelioma: a case report. Cancer Biol Med 9: 133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenader T., Vernea F., Reinus C., Gabizon A. (2011) Malignant epithelioid hemangioendothelioma of the liver successfully treated with pegylated liposomal doxorubicin. J Clin Oncol 29: e722–e724. [DOI] [PubMed] [Google Scholar]
- Grotz T., Nagorney D., Donohue J., Que F., Kendrick M., Farnell M., et al. (2010) Hepatic epithelioid haemangioendothelioma: is transplantation the only treatment option? HPB 12: 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan I., Barth P., Celik I., Hoffmann S., Langer P., Ramaswamy A., et al. (2005) An authentic malignant epithelioid hemangioendothelioma of the thyroid: a case report and review of the literature. Thyroid 15: 1377–1381. [DOI] [PubMed] [Google Scholar]
- Holley M., Cuschieri A. (1989) Epithelioid haemangioendothelioma of the liver: objective response to hepatic intra-arterial 5-FU. Eur J Surg Oncol 15: 73–78. [PubMed] [Google Scholar]
- Kassam A., Mandel K. (2008) Metastatic hepatic epithelioid hemangioendothelioma in a teenage girl. J Pediatr Hematol Oncol 30: 550–552. [DOI] [PubMed] [Google Scholar]
- Kayler L., Merion R., Arenas J., Magee J., Campbell D., Rudich S., et al. (2002) Epithelioid hemangioendothelioma of the liver disseminated to the peritoneum treated with liver transplantation and interferon alpha-2B. Transplantation 74: 128–130. [DOI] [PubMed] [Google Scholar]
- Kelly H., O’Neil B. (2005) Response of epithelioid haemangioendothelioma to liposomal doxorubicin. Lancet Oncol 6: 813–815. [DOI] [PubMed] [Google Scholar]
- Kim Y., Mishima M., Miyagawa-Hayashino A. (2010) Treatment of pulmonary epithelioid hemangioendothelioma with bevacizumab. J Thorac Oncol 5: 1107–1108. [DOI] [PubMed] [Google Scholar]
- Lakkis Z., Kim S., Delabrousse E., Jary M., Nguyen T., Mantion G., et al. (2013) Metronomic cyclophosphamide: an alternative treatment for hepatic epithelioid hemangioendothelioma. J Hepatol 58: 1254–1257. [DOI] [PubMed] [Google Scholar]
- Lauffer J., Zimmermann A., Krahenbuhl L., Triller J., Baer H. (1996) Epithelioid hemangioendothelioma of the liver: a rare hepatic tumor. Cancer 78: 2318–2327. [DOI] [PubMed] [Google Scholar]
- Lazarus A., Fuhrer G., Malekiani C., McKay S., Thurber J. (2011) Primary pleural epithelioid hemangioendothelioma (EHE) - two cases and review of the literature. Clin Respir J 5: e1–e5. [DOI] [PubMed] [Google Scholar]
- Makhlouf H., Ishak K., Goodman Z. (1999) Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer 85: 562–582. [DOI] [PubMed] [Google Scholar]
- Marquez-Medina D., Samme-Perez-Vargas J., Tuset-DerAbrain N., Montero-Fernanadez A., Taberner-Bonastre T, Porcel J. (2011) Pleural epithelioid hemangioendothelioma in an elderly patient. A case report and review of the literature. Lung Cancer 73: 116–119. [DOI] [PubMed] [Google Scholar]
- Marsh Rde W., Walker M., Jacob G., Liu C. (2005) Breast implants as a possible etiology of epithelioid hemangioendothelioma and successful therapy with interferon-α2. Breast J 11: 257–261. [DOI] [PubMed] [Google Scholar]
- Mascarenhas R., Sanghvi A., Friedlander L., Geyer S., Beasley H., Van Thiel D. (2005) Thalidomide inhibits the growth and progression of hepatic epithelioid hemangioendothelioma. Oncology 67: 471–475. [DOI] [PubMed] [Google Scholar]
- Mehrabi A., Kashfi A., Fonouni H., Schemmer P., Schmied B., Hallscheidt P., et al. (2006) Primary Malignant Hepatic Epithelioid Hemangioendothelioma. Cancer 107: 2108–2121. [DOI] [PubMed] [Google Scholar]
- Mistry A., Gorden D., Busler J., Coogan A., Kelly B. (2012) Diagnostic and Therapeutic Challenges in Hepatic Epithelioid Hemangioendothelioma. J Gastrointest Canc 43: 521–525. [DOI] [PubMed] [Google Scholar]
- Mizota A., Shitara K., Fukui T. (2011) Bevacizumab chemotherapy for pulmonary epithelioid hemangioendothelioma with severe dyspnea. J Thorac Oncol 6: 651–652. [DOI] [PubMed] [Google Scholar]
- O’Grady J. (2000) Treatment options for other hepatic malignancies. Liver Transpl. 6:S23-29. [DOI] [PubMed] [Google Scholar]
- Pintoffle J., Meisinger I., Mayer F., Horger M., von Weyham C., Kanz L., et al. (2009) Long-term disease stabilization during second-line gemcitabine in a refractory metastatic haemangioendothelioma. Anticancer Drugs 20: 73–74. [DOI] [PubMed] [Google Scholar]
- Radzikowska E., Szczepulska-Wojcik E., Chabowski M., Oniszh K., Langfort R., Roszkowski K. (2008) Pulmonary epithelioid haemangioendothelioma-interferon 2-alpha treatment-case report. Pneumonol Alergol Pol 76: 281–285. [PubMed] [Google Scholar]
- Raphael C., Hudson E., Williams L., Lester J., Savage P. (2010) Successful treatment of metastatic hepatic epithelioid hemangioendothelioma with thalidomide: a case report. J Med Case Rep 4: 413–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J., Becker N., O’Mahony C., Gross J., Atoia T. (2008) Long-term outcomes following liver transplantation for hepatic hemangioendothelioma: the UNOS experience from 1987 to 2005. J Gastrointest Surg 12: 110–116. [DOI] [PubMed] [Google Scholar]
- Salech F., Valderrama S., Nervi B., Rodriguez J., Oksenberg D., Koch A., et al. (2010) Thalidomide for the treatment of hepatic epithelioid hemangioendothelioma: a case report with a long term follow-up. Ann Hepatol 10: 99–102. [PubMed] [Google Scholar]
- Saleiro S., Barbosa M., Souto Moura C., Almeida J., Ferreira S. (2008) Epithelioid hemangioendothelioma–a rare pulmonary tumor. Rev Port Pneumol 14: 421–425. [PubMed] [Google Scholar]
- Sangro B., Inarrairaegui M., Fernandez-Ros N. (2012) Malignant epithelioid hemangioendothelioma of the liver successfully treated with Sorafenib. Rare Tumors 4: 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattenberg T., Kam R., Klopp M., Herpel E., Schnabel P., Mechtersheimer G., et al. (2008) Pulmonary epithelioid hemangioendothelioma: report of three cases. Surg Today 38: 844–849. [DOI] [PubMed] [Google Scholar]
- Schilling G., Schuch G., Panse J., Sterneck C. (2009) Activity of lenalidomide in metastatic hepatic epithelioid hemangioendothelioma (HEH): a case report. J Clin Oncol (Meeting Abstracts) 27(Suppl.): abstract 15S. [Google Scholar]
- Sharif K., English M., Ramani P., Alberti D., Otte J., McKiernan P., et al. (2004) Management of hepatic epithelioid haemangio-endothelioma in children: what option? B J Cancer 90: 1498–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumrall A., Fredericks R., Berthold A., Shumaker G. (2010) Lenalidomide stops progression of multifocal epithelioid hemangioendothelioma including intracranial disease. J Neurooncol 97: 275–277. [DOI] [PubMed] [Google Scholar]
- Thomas R., Aloia T., Truty M., Tseng W., Choi E., Curley S., et al. (2014) Treatment sequencing strategy for hepatic epithelioid hemangioendothelioma. HPB 16: 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann K., Bethke A., Ehninger G., Folprecht G. (2011) Bevacizumab for recurrent hemangioendothelioma. Acta Oncol 50: 153–154. [DOI] [PubMed] [Google Scholar]
- Wicki A., Christifori G. (2007) The potential role of podoplanin in tumour invasion. Br J Cancer 96: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelfel C., Liehr T., Weise A., Langrehr J., Kotb W., Pancyna-Gengelbach M., et al. (2011) Molecular cytogenetic characterization of epithelioid hemangioendothelioma. Br J Cancer 204: 671–676. [DOI] [PubMed] [Google Scholar]