Abstract
Background
Vitamin D (vitD) supplementation may prolong remission in Crohn’s disease (CD); however, the clinical efficacy and mechanisms are unclear.
Aim
To determine changes in intestinal permeability (IP), antimicrobial peptide (AMP) concentrations and disease markers in CD, in response to vitD supplementation.
Methods
In a double-blind randomised placebo-controlled study, we assigned 27 CD patients in remission to 2000 IU/day vitD or placebo for 3 mos. We determined IP, plasma cathelicidin (LL-37 in ng/mL), human-beta-defensin-2 (hBD2 in pg/mL), disease activity (Crohn’s Disease Activity Index (CDAI)), C-reactive protein (CRP in mg/L), fecal calprotectin (µg/g), Quality of Life (QoL) and serum 25-hydroxyvitamin D (25(OH)D in nmol/L) at 0 and 3 mos.
Results
At 3 mos., 25(OH)D concentrations were significantly higher in those whom were treated (p < 0.001). Intra-group analysis showed increased LL-37 concentrations (p = 0.050) and maintenance of IP measures in the treated group. In contrast, in the placebo group, the small bowel (p = 0.018) and gastro-duodenal permeability (p = 0.030) increased from baseline. At 3 mos., patients with 25(OH)D ≥ 75 nmol/L had significantly lower CRP (p = 0.019), higher QoL (p = 0.037), higher LL-37 concentrations (p < 0.001) and non-significantly lower CDAI scores (p = 0.082), compared to those with levels <75 nmol/L.
Conclusion
Short-term treatment with 2000 IU/day vitD significantly increased 25(OH)D levels in CD patients in remission and it was associated with increased LL-37 concentrations and maintenance of IP. Achieving 25(OH)D ≥ 75 nmol/l was accompanied by higher circulating LL-37, higher QoL scores and reduced CRP. Registered at ClinicalTrials.gov (NCT01792388).
Keywords: Barrier function, calprotectin, cathelicidin, Crohn’s disease, human beta defensin 2, intestinal permeability, quality of life, vitamin D
Introduction
Crohn's disease (CD) is a lifelong chronic relapsing and remitting inflammatory condition, characterised by transmural patchy inflammation, which can involve any portion of the gastrointestinal tract. The aetiology is unknown; however, immune, genetic and environmental factors are thought to be involved.1,2 Maintaining adequate serum 25-hydroxyvitamin D (25(OH)D) status has an established role in CD for bone health;3 however vitamin D (vitD) insufficiency remains common.4,5,6 In Europe, CD incidence rates range from 0.7 to 9.8 cases per 100,000 person-years, with an evident ‘North-South gradient’ of incidence and prevalence that is well established.7,8 Implicated in this gradient, amongst other confounding factors, is vitD status,2,9 which decreases as one moves north. Relocation from southern to northern climates is similarly associated with an increased risk of developing CD.10 These association studies, with their inherent limitations, nonetheless support emerging animal and clinical data that suggest a role for vitD as a risk factor and a potential therapy for CD.
Several published studies in experimental models of inflammatory bowel disease (IBD) demonstrate the effect of vitD on inflammation;11,12 however, few have investigated the effects on intestinal permeability (IP). Altered IP and impaired barrier function have been implicated in the pathogenesis of CD, moreover, an increase in IP may predict and precede a clinical relapse.13,14 In experimental studies, Kong et al.15 report that activated vitD 1,25(OH)2D3 increases the tight junction (TJ) proteins zonula occludens and E-cadherin, and enhances healing following an injury in the intestinal epithelium;15 however, whether these effects on barrier integrity and IP clinically translate to CD has yet to be explored.
In addition to its direct effects on TJ proteins, vitD may preserve barrier integrity through immune mechanisms, as shown in experimental colitis models.16,17 Human cathelicidin (LL-37) and beta defensins are antimicrobial peptides (AMPs) of the innate immune system that are expressed by the gastrointestinal epithelium.18 AMPs work in synergy to protect against bacterial invasion19 and LL-37 promotes wound healing in intestinal epithelial cells20 and reduces intestinal inflammation in experimental colitis.21 In vitro, 1,25(OH)2D3 induces expression of hCAP18, the gene encoding LL-37,22 and in clinical tuberculosis (TB) studies, LL-37 concentrations increase in response to vitD supplementation;23 however, further work is required to examine if this effect is replicated in CD populations after vitD supplementation is initiated.
Newer research directions now focus on the role of vitD in autophagy. Genetic studies point clearly to defects in autophagy as a risk factor for CD pathogenesis.24,25 Autophagy, integral to the innate immune response to infection, is driven in part by NOD2 signalling and by intracellular LL-37 concentrations. Mutations in the NOD2/CARD15 locus represent one of the strongest genetic risk factors for CD.26,27 In addition, LL-37 is transcriptionally induced by 1,25(OH)2D3 in monocytes,28 and vitD signalling stimulates both autophagy and NOD2 gene expression.29 The emerging evidence suggests that vitD supplementation may be a potential therapy for treatment of active disease or prevention of relapse in CD.
To date, there are few randomised controlled intervention studies of vitD therapy in CD. Booth et al.30 show that CD patients (n = 15) treated with 10,000 IU/day vitD have significantly improved Crohn’s Disease Activity Index (CDAI) scores, compared to those treated with 1000 IU/day, after 26 weeks. A larger 12-month study in CD (n = 96) reports a reduction in relapse rates among those treated with 1200 IU/day vitD, compared to placebo.31 Emerging findings suggest the potential for vitD in reducing disease activity and inflammation,32 although the specific mechanisms of action remain unclear.
We propose that vitD therapy may alter IP and LL-37 concentrations in CD, which may be associated with changes in disease activity markers. The aim of the current study was to investigate the effect of 3 mos. vitD supplementation on IP, LL-37 and markers of disease activity, namely C-reactive protein (CRP), calprotectin, CDAI and quality of life (QoL).
Materials and methods
Study setting
The study was carried out at Tallaght Hospital, in Dublin, Ireland. The enrolment period was from October 2011 to December 2011. The study was approved by the St. James’s Hospital and the Adelaide & Meath Hospital, incorporating the National Children’s Hospital of Dublin’s Research Ethics Committee (reference 2011/11/04) and was registered at ClinicalTrials.gov (NCT 01792388). All participants provided informed, written consent.
Study design and intervention
This was a randomised, double-blind, placebo-controlled study, with patient data collected at baseline and at the 3-month follow-up. The active treatment consisted of 2000 IU of vitD3. The placebo capsule consisted of magnesium stearate and microcrystalline cellulose, organoleptically identical to the active treatment. The daily dose was one tablet, which patients were instructed to take with a meal. Compliance was evaluated by pill count at 3 mos. All packaging and tablets were identical. Patients prescribed vitD and calcium supplementation for bone health were instructed to continue taking their prescription, in line with ethical requirements. All other prescribed medication for CD was maintained for the study duration.
Inclusion and exclusion criteria
Patients enrolled had a confirmed diagnosis of CD, were in disease remission, were at least 18 years old and were able to provide informed, written consent. Remission was defined as a CDAI < 150, a CRP < 5 mg/L and stable CD therapy for a minimum of 3 mos. Exclusion criteria were as follows: extensive bowel resection, known hypersensitivity to vitD, history of hypercalcaemia (corrected serum calcium >2.66 mmol/L), supplemental intakes of vitD >1000 IU/d, antibiotic use within the 4 weeks prior to enrolment, renal impairment, diabetes mellitus, alcohol dependency, urinary tract infection, pregnancy, short-bowel syndrome and current use of bisphosphonates.
Randomisation and blinding
VitD and placebo were produced and randomized by Pharma Vinci (Denmark). The active vitD treatment and placebo were delivered in identical containers, which held a 3-mo supply of tablets in each. The containers were block-randomised into groups of 10, with a blinded randomisation list provided by the suppliers. In case of an adverse event(s), a sealed envelope for unblinding the study was kept securely at the Department of Medicine, Tallaght Hospital, Dublin.
Evaluation of intestinal permeability
Permeability was measured at baseline and 3 mos., by oral administration and subsequent 24-h urinary collection of sugar probes, which selectively characterise permeability from different regions of the gut. Subjects drank 7.5 mL lactulose (Abbott Healthcare Products Limited, Ireland), 100 g sucrose and 2 g mannitol (PCCA, Ontario, Canada) in 450 mL of water, following a 4-hour fast. Simultaneously, 2 g of sucralose (Polymed Therapeutics, Houston, TX, USA) contained within 9 gelatine capsules (Best Formulations, CA, USA) were consumed. Lactulose and mannitol were used as markers of small intestine permeability, sucrose as a marker of gastro-duodenal permeability, and sucralose as marker of combined small- and large-bowel permeability. Patients were instructed to avoid sugar substitutes, aspirin and alcohol over the 24-h collection period. Smoking status was reassessed at 3 mos., to ensure no change in habits. High-performance liquid chromatography (HPLC) was carried out to quantify the individual sugars. Values above the following cut-offs are considered as abnormal IP results:
lactulose/mannitol ratio > 0.025;33
sucralose excretion > 42.1 mg;34 and
sucrose excretion > 180 mg.
Vitamin D status
Serum 25(OH)D (nmol/L) concentration was measured by liquid chromatography-tandem mass spectrometry, at baseline and at 3 mos., by the Department of Biochemistry, St. James’s Hospital, Dublin (participating in a vitD external quality assessment scheme (DEQAS)). The study was performed during the winter months, which controlled for the effect of seasonality on circulating 25(OH)D concentrations. VitD deficiency was defined as 25(OH)D < 50 nmol/L (insufficiency, as 25(OH)D of 50–74 nmol/L; while ≥75 nmol/L is considered sufficient).35
Antimicrobial peptide measurements
LL-37 was measured in duplicate, in plasma, by Enzyme-Linked Immunosorbent Assay (ELISA) (Hycult Biotech, The Netherlands), as previously described.23 Serum hBD2 was measured by a commercial ELISA kit, according to the manufacturer's instructions (Phoenix Peptide, Germany). Samples were stored at –80℃ prior to analysis. Laboratory personnel were blinded to all clinical information associated with the samples.
Inflammatory and disease markers
Disease activity was determined at baseline and 3 mos., using the CDAI,36 where values < 150 represented clinical remission. A venous blood sample was collected at baseline and 3 mos., for measurement of CRP (in mg/L); faecal calprotectin was measured by sandwich immunoassay (Buhlmann, Switzerland). Concentrations ≤ 50 µg/g suggested no inflammation in the gastrointestinal tract and levels > 50 µg/g indicated gastrointestinal tract inflammation.37 QoL was assessed using the validated Inflammatory Bowel Disease Questionnaire (IBDQ).38
Statistical analysis
Parametric data were analysed by 2-tailed, non-paired t-tests. Nonparametric data were analysed by Mann-Whitney U-test. IP measures, AMPs (LL-37 and hBD2) and faecal calprotectin (µg/g) were expressed as median (IQR). We used SPSS Windows version 21.0 (SPSS, Chicago, IL, USA) for data analysis. Statistical significance was determined at p < 0.05. The primary outcome measures for this study were intra-group differences in IP measures and LL-37 concentrations at 3 mos., followed by analysis of potential accompanying changes in disease activity. Analysis of all variables (IP, LL-37, CRP, CDAI and QoL) at 3 mos. was performed based on the levels of 25(OH)D achieved (≥ or < 75 nmol/L). Analysis of covariance (ANCOVA) was used to control for baseline values when appropriate and intra-group analysis was performed using paired t-tests. A sample size of 30 was chosen to detect a clinically significant change of 30% in the lactulose-mannitol ratio from 0–12 weeks in the treated group, with a power of 80% and detection of 2-sided Type 1 significance at the 0.05 level. This estimate was derived from similar data examining the effects of a dietary intervention on small bowel permeability.34
Results
Study population and baseline characteristics
We randomised 14 subjects to the placebo and 13 were randomised to the active treatment. Three participants were excluded prior to randomisation, as they were not in disease remission by CDAI (Figure 1). Compliance with the assigned intervention was satisfactory (>95%). No adverse events, significant or otherwise, related to consumption of the oral permeability test solution or the study interventions were reported.
Figure 1.
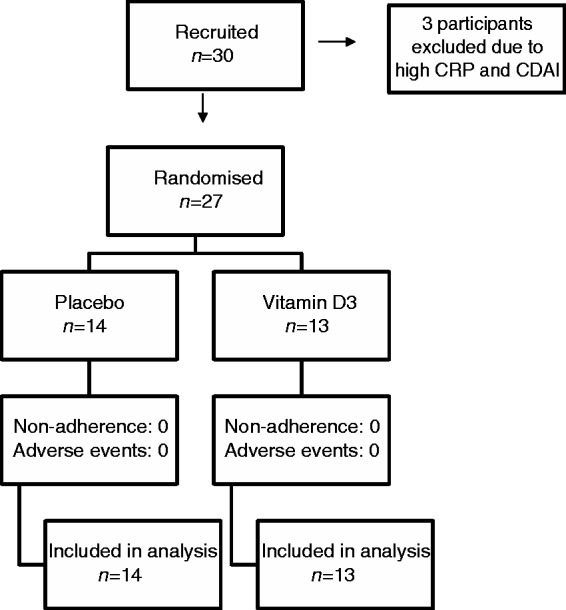
Consort study flow diagram. Three patients with clinical signs of active Crohn’s disease were excluded before randomisation.
CDAI: Crohn’s Disease Activity Index; CRP: C-reactive protein.
At baseline, the disease activity and inflammatory markers were consistent with clinical remission (Table 1). Medications for CD remained stable throughout the study period and no relapses occurred.
Table 1.
Demographic and clinical characteristics of the study population
| Demographics | Placebo, n = 14 | Treatment, n = 13 | P value+ |
|---|---|---|---|
| Age, years | 36.7 (12.1) | 36.5 (11.8) | 0.97 |
| Gender, M:F | 6:08 | 7:08 | 0.568 |
| Age at diagnosis, years | 28.4 (10.4) | 28.2 (9.4) | 0.906 |
| Duration of disease, years | 8.6 (6.2) | 8.9 (5.9) | 0.905 |
| Previous IBD-related surgery, n | 6 | 3 | 0.276 |
| Current medications | |||
| 5ASA, n | 8 | 6 | 0.427 |
| Immunosuppressants, nd | 8 | 10 | 0.276 |
| Anti-TNF therapy, n | 0 | 2 | 0.127 |
| Disease location, n | |||
| Small bowel | 7 | 1 | 0.087 c |
| Large bowel | 1 | 1 | |
| Small and large bowel | 5 | 11 | |
| Upper GI, small and large bowel | 1 | 0 | |
| Montreal classification, n | |||
| Inflammatory | 7 | 3 | 0.248 c |
| Penetrating | 0 | 2 | |
| Stricturing | 1 | 3 | |
| Penetrating and perianal disease | 5 | 3 | |
| Penetrating/stricturing disease | 1 | 2 | |
| Anthropometry | |||
| BMI, kg/m2 | 24.1 (2.7) | 25.1 (3.0) | 0.368 |
| Lifestyle | |||
| Current smokers, n | 8 | 4 | 0.168 c |
| Smoking, pack-yearsa | 8.9 (2.4) | 9.0 (3.4) | 0.979 |
| Alcohol, units per weeka | 8.8 (2.0) | 8.1 (2.5) | 0.842 |
| Vitamin D status | |||
| Vitamin D, nmol/L | 51.8 (20.7) | 69.2 (7.0) | 0.06 |
| Vitamin D, ≥ 75: < 75 nmol/L, n | 3:11 | 6:07 | 0.173 |
| Vitamin D, ≥ 50: < 50 nmol/L, n | 7:07 | 11:02 | 0.215 |
| Vitamin D, ≥ 25: < 25 nmol/L, n | 13:01 | 12:01 | 0.536 |
| Additional VitD intake | |||
| 800 IU/d (bone preparations), n | 5 | 6 | 0.702 |
| Baseline disease activity | |||
| CRP, mg/Lb | 1.4 (1.0–2.6) | 1.5 (1.0–2.9) | 0.339 |
| CDAI Scoreb | 77.0 (53.0–99.8) | 83.5 (47.0–145.8) | 0.607 |
| Calprotectin, µg/gb | 84.0 (26.0–179.5) | 202.0 (50.0–1060.0) | 0.164 |
| Intestinal permeability | |||
| LMRb | 0.013 (0.010–0.018) | 0.015 (0.113–0.025) | 0.127 |
| Sucrose (mg)b | 41.1 (37.1–49.8) | 63.6 (42.7–79.1) | 0.086 |
| Sucraloseb | 67.4 (41.3–83.4) | 56.9 (48.8–74.6) | 0.407 |
| Antimicrobial peptide concentrations | |||
| LL-37 (ng/mL)b | 105.3 (69.4–341.9) | 78.4 (59.4–239.5) | 0.506 |
| hBD2 (pg/mL)b | 2634.4 (896.4–3983.1) | 1866.0 (897.6–4353.0) | 0.886 |
Mean (SD)
Mean (SEM)
Median (IQR), +T- test; treatment versus placebo
Chi Square test
Immunosuppressants: Imuran, 6-MP, Salazopyrin
5ASA: 5-aminosalicylates; BMI: body mass index; CDAI: Crohn’s Disease Activity Index; CRP: C-reactive protein; F: female gender; GI: gastrointestinal; hBD2: human beta-defensin 2; IBD: irritable bowel disease; LL-37: plasma cathelicidin; LMR: lactulose:mannitol ratio; M: male gender; SEM: standard error of the mean; TNF: tumor necrosis factor; VitD: vitamin D.
25(OHD) levels and response to vitD supplementation
The study was performed during the winter season, a period when cutaneous vitD synthesis is reduced, which controlled for seasonality. At 3 mos. there was a significant increase in 25(OH)D concentrations within the treatment group and a significant decrease, within the placebo group. 25(OH)D concentrations were significantly higher in the vitD-treated group (91.6 (75.5–107.6) nmol/L), compared to the placebo group (40.4 (30.4–50.4) nmol/L (p < 0.001)) at 3 mos., with an overall mean difference of 51.2 nmol/L (–69.4, – 33.0) (p < 0.001). In the treatment group, four participants did not obtain serum 25(OH)D concentrations ≥75 nmol/L at 3 mos. All of those in the placebo group had 25(OH)D concentrations < 75 nmol/L at 3 mos.
Intestinal permeability in the vitD- and placebo-treated groups
Intra-group analysis for small bowel permeability showed that in the vitD-treated group, the lactulose: mannitol ration (LMR) was similar at 3 mos. and baseline (0 mos. was 0.015 (0.113–0.025) and 3 mos., 0.014 (0.010–0.020); p = 0.953)) (Figure 2(a)). In contrast, in the placebo group, there was a significant increase in LMR during the study period (0 mos. 0.013 (0.010–0.018) and 3 mos., 0.022 (0.014–0.026); p = 0.018).
Figure 2.
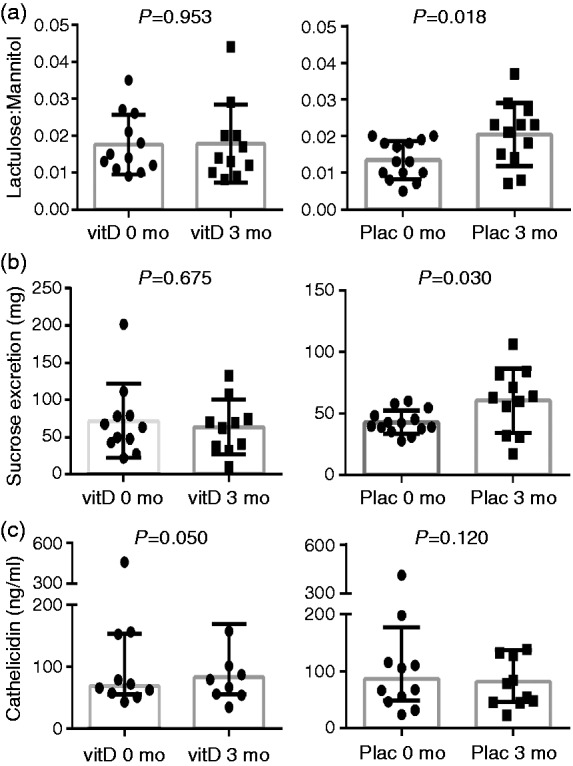
LMRs at 0 and 3 mos. in 13 vitamin D3-treated CD patients and 14 placebo-treated CD patients. Values are shown in absolute values (bars indicate medians). (a) The intra-group comparison of small bowel permeability; (b) the comparison of gastroduodenal permeability; and (c) the comparison of LL-37 concentrations, in the vitD and placebo groups.
CD: Crohn’s disease; LMR: lactulose:mannitol ratio; Plac: placebo; VitD: vitamin D.
Correspondingly, for gastro-duodenal permeability, mean sucrose excretion did not differ between baseline (63.6 (42.7–79.1)) and 3 mos. (65.9 (36.1–82.6); p = 0.742), in the vitD treated group (Figure 2(b)); whereas, in the placebo group, sucrose excretion increased significantly to 62.8 (31.7–81.4, p = 0.030) at 3 mos., from a baseline mean of 41.1 (37.1–49.8) (Figure 2(b)).
Small and large bowel permeability
Total sucralose excretion (mg), a measure of combined small and large bowel permeability, did not differ at 3 mos. from baseline in either the vitD- or placebo-treated groups (data not shown); however, at 3 mos. there was no significant difference in measures of small bowel (p = 0.524), gastroduodenal (p = 0.848) or small and large bowel permeability (p = 0.487) between the vitD-treated and placebo groups (data not shown).
Antimicrobial peptides concentrations in the vitD- and placebo-treated groups
In the vitD-treated group, LL-37 levels increased from baseline to 3 mos. (0 mos.: 78.4 (59.4–239.5); 3 mos.: 93.6 (58.3–192.1); p = 0.050; Figure 2(c)), while mean levels in the placebo group did not significantly change at 3 mos. (117.5 (68.2–208.0)) compared to baseline (105.3 (69.4–341.9); p = 0.120; Figure 1(c)). There were no significant differences in LL-37 concentrations between the two groups, at 3 mos. (p = 0.080; data not shown).
For hBD2 concentrations, no changes were observed for vitD nor placebo treatment, either by intra-group or analysis by comparison at 3 mos. (data not shown).
Changes in disease activity markers, in response to vitD supplementation
Intra-group analysis in the treatment group revealed no significant difference in disease activity markers, namely CDAI (p = 0.568), CRP (p = 0.304), QoL (p = 0.519) and FC (p = 0.706); at 3 mos. In the placebo group, neither CDAI (p = 0.258) nor CRP (p = 0.331); QoL (p = 0.364) nor FC (p = 0.964), changed within the study period. Consistent with this, no clinical relapses were documented during the 3-mo. study period. Comparison at 3 mos. showed no significant difference in CDAI, CRP, FC nor QoL between groups (p = 0.119, 0.624, 0.140 and 0.900, respectively).
Antimicrobial peptides, intestinal permeability and disease markers by levels of 25(OH)D achieved
As a secondary analysis, we analysed the data according to 25(OH)D concentrations achieved at 3 mos. (Figure 3). The subjects were split into two groups, those with 25(OH)D concentrations < 75 nmol/L (n = 18) or ≥ 75 nmol/L (n = 9). Those with levels ≥75 nmol/L had significantly higher concentrations of LL-37 and QoL scores, significantly lower CRP and non-significantly lower CDAI (Figure 3). Participants with 25(OH)D levels ≥ 100 nmol/L at 3 mos. (n = 4), had notably higher LL-37 concentrations (333.3 (56.9– 614.2 ng/ml)), compared to those with 25(OH)D levels ≤ 100 nmol/L (93.6 (67.7–199.0 ng/mL); p < 0.001). There was no difference in hBD2 concentrations, based on the levels of 25(OH)D achieved, nor IP measures.
Figure 3.
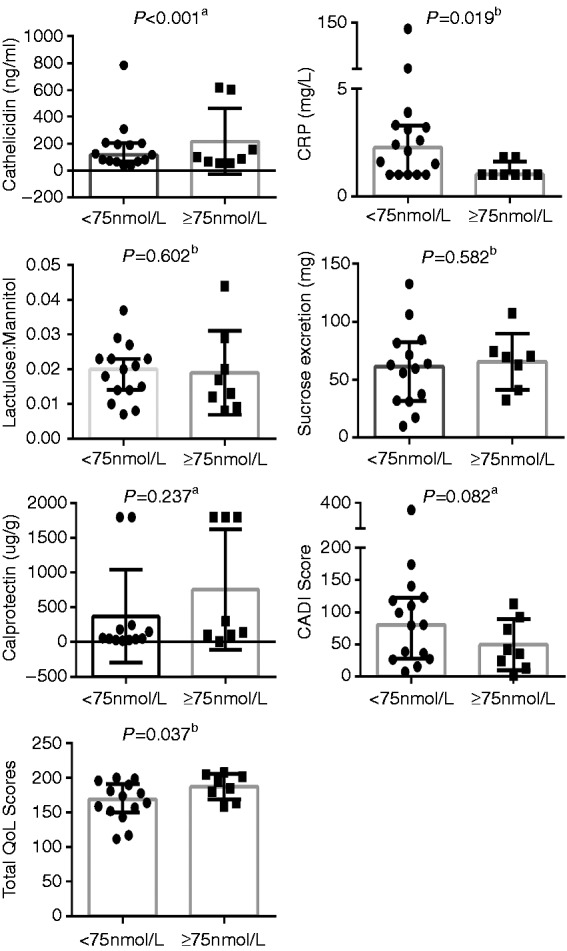
Inflammatory, immune and disease markers: Difference in CDAI, CRP, calprotectin, LL-37 and QoL based on serum 25(OH)D ≥ or < 75 nmol/L at 3 mos.
aMean (SD).
bMedian (IQR).
CDAI: Crohn’s Disease Activity Index; CRP: C-reactive protein; LL-37: plasma cathelicidin; QoL: quality of life.
Discussion
Oral vitD supplementation has been proposed as a potential adjunctive therapy in CD; however to date, few randomised controlled intervention studies31 have investigated its clinical efficacy or mechanisms of action. In this randomised double-blind placebo-controlled pilot study, we aimed to investigate any changes in IP, LL-37 and disease markers (CDAI, CRP, FC and IBDQ scores) in CD patients in remission, in response to 3 mos. of vitD supplementation. A secondary analysis investigating differences in these parameters and based on serum 25(OH)D concentration achieved, was also performed, as newer data suggests that a higher 25(OH)D level may be important for changes in disease activity or inflammation.1,39
First, we observed that 2000 IU vitD/day taken for 3 mos. was sufficient to raise the 25(OH)D concentrations to ≥ 75 nmol/L in 8/12 participants of the treated group, in winter. We were interested in determining if supplementation would alter IP measures, as permeability is reported to precede clinical relapse in CD13,14 and therefore, may reflect an early change in disease activity.15 In the placebo group, IP increased significantly over time and it was stable instead, in the treated group. These findings are the first preliminary clinical data suggesting that vitD may maintain IP in CD, and appeared to support emerging evidence from experimental studies17,40 that report similar effects; however, despite the intra-group changes, there were no significant differences between the vitD-treated and placebo groups, in terms of IP measures at 3 mos.; moreover, IP measures in the treated group did not improve at 3 mo. This may be due to the relatively short nature or study size of this pilot study, or perhaps vitD may have a more prominent role in IP maintenance, rather than reversal. Future work should aim to perform studies of longer duration, achieve higher 25(OH)D concentrations, and that measure TJ proteins at the tissue level.
To date, no published data has explored vitD and LL-37 in CD. We found that within the treated group, mean LL-37 concentrations increased from baseline (p = 0.050); and they decreased within the placebo group. Analysis by levels revealed that those with 25(OH)D levels ≥75 nmol/L at 3 mos. had significantly higher LL-37 concentrations, compared to those with 25(OH)D levels < 75 nmol/L. Interestingly, the difference in LL-37 concentrations was more pronounced when ≥ 100 nmol/L was used as a cut-off; this may support arguments that 25(OH)D levels need to reach a level of 100–175 nmol/L to exert immune effects.41 In other disease states such as TB42 and renal disease,22 results on the relationship between LL-37 and 25(OH)D are inconsistent. Some studies show no associations, while others report significant associations in the healthy and sick,43 where vitD levels were ≥ 75 nmol/L. From a CD perspective, altering LL-37 through vitD therapy is interesting, considering newer evidence implies that this peptide may promote wound healing in intestinal epithelial cells20 and reduce inflammation in experimental colitis.21 Further work is required to more fully explore the vitD effects, if any, on LL-37; including potential effects at the mucosal level.
Few studies to date have examined the effect of vitD supplementation on disease activity markers in CD in randomised controlled trials.31 In this study, we observed no significant changes in markers of disease activity, namely CDAI, CRP, FC or QoL, in response to vitD; however, when analysed according to the level of 25(OH)D achieved at 3 mos., we found significantly lower CRP, correspondingly higher self-reported QoL and a non-significant reduction in CDAI (p = 0.082). Yang et al.39 conducted a study that aimed to achieve a circulating concentration of 100 nmol/L in CD patients with mild-to-moderate disease. The authors report a significant reduction in CDAI (p < 0.0001) and significant improvement in QoL scores (p < 0.0004) after 24 weeks of supplementation, suggesting that the levels attained may be important to induce changes in disease activity and correspondingly, in QoL.39 In an observational study, Jorgensen et al.31 also report that 25(OH)D levels are inversely associated with CDAI (p < 0.01) and by CRP (p < 0.05).
Our study, taken in context with others, demonstrated that increasing 25(OH)D ≥ 75 nmol/L is an important consideration for the design of future randomised controlled trials. Different patients may require different dosages of vitD and different durations of supplementation in order to bring them over the study threshold; therefore, dosages may need to be titrated individually for each participant.
A strength of our study was its randomised, double-blind, placebo-controlled design, comprehensive measures and gold-standard analysis of serum 25(OH)D using an external quality assessment scheme. There are, however, a number of limitations to consider, including the relatively small sample size and study duration. Nevertheless, this study was a pilot study that set out to investigate potential changes in novel markers, such as LL-37 and IP, in response to vitD supplementation in CD. Based on our findings, a number of important considerations for future studies have emerged. Apart from a larger sample size, future randomised controlled trials should consider the dose of vitD supplemented, duration of treatment, as well as the levels of 25(OH)D achieved. The tentative changes in disease activity markers here were observed only at levels ≥ 75 nmol/L, which may suggest that CD participants have a minimum 25(OH)D threshold level, likely ≥ 75 nmol/L, in order to exert immuno-modulatory and anti-inflammatory effects. Equally, we did not observe any clinical relapses for CD. Follow-up periods > 3 mos. may be warranted to observe clinical effects (e.g. relapse, changes in FC and maintenance of remission), as it may be the case that biochemical changes precede clinical changes, as suggested in the current study.
In conclusion, our study demonstrated that 2000 IU/day vitD was sufficient to raise 25(OH)D concentrations to ≥ 75 nmol/L in most study participants, after a 3-mo. treatment. Inter-group analysis in the treated group revealed an apparent maintenance of small bowel and gastroduodenal permeability and an increase in circulating LL-37. This is the first reporting of vitD, IP and LL-37 measures in a CD cohort, and while the data requires confirmation, it broadly supported emerging experimental evidence that suggests a role for vitD in maintaining intestinal barrier integrity. Furthermore, the findings from this pilot study suggested that future randomised controlled trials consider factors such as dosage, duration and attainment of sufficiently raised 25(OH)D concentrations for possibly longer disease durations, to fully explore and capture the possible immune-modulatory and anti-inflammatory effects of vitD therapy in CD.
Funding
This work was supported by the Irish Research Council (scholarship to Tara Raftery).
Conflict of interest
None declared.
References
- 1.Raftery T, O'Morain CA, O'Sullivan M. Vitamin D: New roles and therapeutic potential in inflammatory bowel disease. Curr Drug Metab 2012; 13: 1294–1302. [DOI] [PubMed] [Google Scholar]
- 2.Ananthakrishnan AN, Khalili H, Higuchi LM, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn's disease. Gastroenterology 2012; 142: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011; 60: 571–607. [DOI] [PubMed] [Google Scholar]
- 4.Kelly P, Suibhne TN, O'Morain C, O'Sullivan M. Vitamin D status and cytokine levels in patients with Crohn's disease. Int J Vitam Nutr Res 2011; 81: 205–210. [DOI] [PubMed] [Google Scholar]
- 5.Joseph AJ, George B, Pulimood AB, et al. 25(OH) vitamin D level in Crohn's disease: Association with sun exposure and disease activity. Indian J Med Res 2009; 130: 133–137. [PubMed] [Google Scholar]
- 6.Ulitsky A, Ananthakrishnan AN, Naik A, et al. Vitamin D deficiency in patients with inflammatory bowel disease: Association with disease activity and quality of life. J Parenter Enteral Nutr 2011; 35: 308–316. [DOI] [PubMed] [Google Scholar]
- 7.Loftus EV, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am 2002; 31: 1–20. [DOI] [PubMed] [Google Scholar]
- 8.Schultz M, Butt AG. Is the North-to-South gradient in inflammatory bowel disease a global phenomenon? Expert Rev Gastroenterol Hepatol 2012; 6: 445–447. [DOI] [PubMed] [Google Scholar]
- 9.Jantchou P, Clavel-Chapelon F, Racine A, et al. High residential sun exposure is associated with a low risk of incident Crohn's disease in the prospective E3N cohort. Inflamm Bowel Dis 2014; 20: 75–81. [DOI] [PubMed] [Google Scholar]
- 10.Sewell JL, Yee HF, Inadomi JM. Hospitalizations are increasing among minority patients with Crohn's disease and ulcerative colitis. Inflamm Bowel Dis 2010; 16: 204–207. [DOI] [PubMed] [Google Scholar]
- 11.Froicu M, Weaver V, Wynn TA, et al. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol 2003; 17: 2386–2392. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Mahon BD, Froicu M, et al. Calcium and 1 alpha 25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol 2005; 35: 217–224. [DOI] [PubMed] [Google Scholar]
- 13.Wyatt J, Vogelsang H, Hübl W, et al. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 1993; 341: 1437–1439. [DOI] [PubMed] [Google Scholar]
- 14.D'Incà R, Di Leo V, Corrao G, et al. Intestinal permeability test as a predictor of clinical course in Crohn's disease. Am J Gastroenterol 1999; 94: 2956–2960. [DOI] [PubMed] [Google Scholar]
- 15.Kong J, Zhang Z, Musch MW, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 2008; 294: G208–G216. [DOI] [PubMed] [Google Scholar]
- 16.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol 2007; 8: 5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao H, Zhang H, Wu H, et al. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol 2012; 12: 57–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jäger S, Stange EF, Wehkamp J. Inflammatory bowel disease: An impaired barrier disease. Langenbecks Arch Surg 2013; 398: 1–12. [DOI] [PubMed] [Google Scholar]
- 19.Tollin M, Bergman P, Svenberg T, et al. Antimicrobial peptides in the first-line defence of human colon mucosa. Peptides 2003; 24: 523–530. [DOI] [PubMed] [Google Scholar]
- 20.Otte JM, Zdebik AE, Brand S, et al. Effects of the cathelicidin LL-37 on intestinal epithelial barrier integrity. Regul Pept 2009; 156: 104–117. [DOI] [PubMed] [Google Scholar]
- 21.Koon HW, Shih DQ, Chen J, et al. Cathelicidin signaling via the Toll-like receptor protects against colitis in mice. Gastroenterology 2011; 141: 1852–1863, e1851–e1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 2005; 19: 1067–1077. [DOI] [PubMed] [Google Scholar]
- 23.Martineau AR, Wilkinson KA, Newton SM, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: The role of cathelicidin LL-37. J Immunol 2007; 178: 7190–7198. [DOI] [PubMed] [Google Scholar]
- 24.Hampe J, Franke A, Rosenstiel P, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet 2007; 39: 207–211. [DOI] [PubMed] [Google Scholar]
- 25.Rioux JD, Xavier RJ, Taylor KD, et al. Genome-wide association study identifies new susceptibility loci for Crohn’s disease and implicates autophagy in disease pathogenesis. Nat Genet 2007; 39: 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Limbergen J, Wilson DC, Satsangi J. The genetics of Crohn's disease. Annu Rev Genom Hum Genet 2009; 10: 89–116. [DOI] [PubMed] [Google Scholar]
- 27.Barrett JC, Hansoul S, Nicolae DL, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet 2008; 40: 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang TT, Tavera-Mendoza LE, Laperriere D, et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol 2005; 19: 2685–2695. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010; 376: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Booth DL, Lakehomer H, Jacob V, et al. High-dose vitamin D improves clinical activity in Crohn's disease, Washington DC: American College of Gastroenterology, 2011, 2011. [Google Scholar]
- 31.Jørgensen SP, Agnholt J, Glerup H, et al. Clinical trial: Vitamin D3 treatment in Crohn's disease. A randomized double-blind placebo-controlled study. Aliment Pharmacol Ther 2010; 32: 377–383. [DOI] [PubMed] [Google Scholar]
- 32.Bartels LE, Jørgensen SP, Agnholt J, et al. 1,25-dihydroxyvitamin D3 and dexamethasone increase interleukin-10 production in CD4+ T cells from patients with Crohn's disease. Int Immunopharmacol 2007; 7: 1755–1764. [DOI] [PubMed] [Google Scholar]
- 33.Smecuol E, Bai JC, Vazquez H, et al. Gastrointestinal permeability in celiac disease. Gastroenterology 1997; 112: 1129–1136. [DOI] [PubMed] [Google Scholar]
- 34.Zeng J, Li YQ, Zuo XL, et al. Clinical trial: Effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2008; 28: 994–1002. [DOI] [PubMed] [Google Scholar]
- 35.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96(7): 1911–1930. [DOI] [PubMed] [Google Scholar]
- 36.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 1976; 70: 439–444. [PubMed] [Google Scholar]
- 37.Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis 2006; 12: 524–534. [DOI] [PubMed] [Google Scholar]
- 38.Irvine EJ, Feagan B, Rochon J, et al. Quality of life: A valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn's Relapse Prevention Trial Study Group. Gastroenterology 1994; 106: 287–296. [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Weaver V, Smith JP, et al. Therapeutic effect of vitamin D supplementation in a pilot study of Crohn's patients. Clin Transl Gastroenterol 2013; 4: e33–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assa A, Vong L, Pinnell LJ, et al. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J Infect Dis 2014; 210: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 41.Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Altern Med Rev 2008; 13: 6–20. [PubMed] [Google Scholar]
- 42.Yamshchikov AV, Kurbatova EV, Kumari M, et al. Vitamin D status and antimicrobial peptide cathelicidin (LL-37) concentrations in patients with active pulmonary tuberculosis. Am J Clin Nutr 2010; 92: 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med 2009; 7: 28–28. [DOI] [PMC free article] [PubMed] [Google Scholar]


