Abstract
Acquired drug resistance by Mycobacterium tuberculosis (MTB) may result in treatment failure and death. Bedaquiline was recently approved for the treatment of multidrug-resistant tuberculosis (MDR-TB). This report examines the available data on this novel drug for the treatment of MDR-TB. PubMed searches, last updated 18 February 2015, using the terms bedaquiline, TMC 207 and R207910 identified pertinent English citations. Citation reference lists were reviewed to identify other relevant reports. Pertinent MDR-TB treatment reports on the US Food and Drug Administration, Centers for Disease Control and Prevention (CDC), World Health Organization (WHO) and Cochrane websites were also evaluated. Bedaquiline is an adenosine triphosphate (ATP) synthase inhibitor specific for MTB and some nontuberculous mycobacteria. The early bactericidal activity (EBA) of bedaquiline is delayed until ATP stores are depleted but subsequently it is similar to the EBA of isoniazid and rifampin. Bedaquiline demonstrated excellent minimum inhibitory concentrations (MICs) against both drug-sensitive and MDR-TB. Adding it to the WHO-recommended MDR-TB regimen reduced the time for sputum culture conversion in pulmonary MDR-TB. Rifampin, other cytochrome oxidase 3A4 inducers or inhibitors alter its metabolism. Adverse effects are common with MDR-TB treatment regimens with or without bedaquiline. Nausea is more common with bedaquiline and it increases the QTcF interval. It is not recommended for children, pregnant or lactating women. More patients died in the bedaquiline-treatment arms despite better microbiological outcomes in two recent trials. The WHO and CDC published interim guidelines that recommend restricting its use to patients with MDR-TB or more complex drug resistance who cannot otherwise be treated with a minimum of three effective drugs. It should never be added to a regimen as a single drug nor should it be added to a failing regimen to prevent the emergence of bedaquiline-resistant strains.
Keywords: bedaquiline, diarylquinoline, extensively drug resistant, multidrug resistance, Mycobacterium tuberculosis, tuberculosis
Introduction
Acquired drug resistance by Mycobacterium tuberculosis (MTB) may result in treatment failure and death. Bedaquiline was recently approved for the treatment of multidrug-resistant tuberculosis (MDR-TB). This report examines the available data on this novel drug for the treatment of MDR-TB. PubMed searches, last updated 18 February 2015, using the terms bedaquiline, TMC 207 and R207910 identified pertinent English citations. Citation reference lists were reviewed to identify other relevant reports. Pertinent MDR-TB treatment reports on the US Food and Drug Administration (FDA), Centers for Disease Control and Prevention (CDC), World Health Organization (WHO), and Cochrane websites were also evaluated.
Individuals infected with drug-sensitive (DS) MTB strains can expect excellent outcomes if they are treated according to WHO guidelines [WHO, 2010b]. Treatment defaults occur for various reasons: costs of health care and medications, adverse drug effects, the inconvenience of 6-month directly observed therapy (DOT) programs, and the natural tendency for patients to discontinue treatment when they feel better. Failure to complete treatment may result in relapses, often with drug-resistant (DR) organisms [Burman et al. 1997]. MDR-TB strains, those resistant to rifampin and isoniazid and possibly to other medications, require extended treatment courses with less effective and harder to tolerate second-line medications that are associated with frequent side effects and poorer outcomes [Iseman, 1993]. Globally, 3.5% [95% confidence interval (CI) 2.2–4.7%] of new and 20.5% (CI 13.6–27.5%) of previously treated TB cases are MDR (Figures 1 and 2) [WHO, 2014a]. Rates of MDR-TB are considerably higher in countries with inadequate public health programs. In 2013, Belarus was one of the countries with concerning statistics; 35% of new and 55% of previously treated cases were due to MDR-TB [WHO, 2014a]. Since only a minority of TB isolates undergo drug susceptibility testing (DST) in most high prevalence countries, it is likely that the rates of MDR-TB are higher than reported.
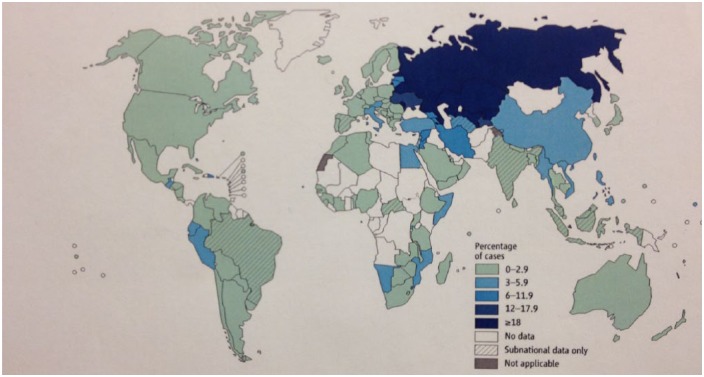
Figure 1.
Percentage of new tuberculosis cases with multidrug-resistant tuberculosis. Figures are based on the most recent year for which data have been reported, which varies among countries. Reproduced with permission from WHO [2013b, Figure 4.2, p. 47, chapter 4].
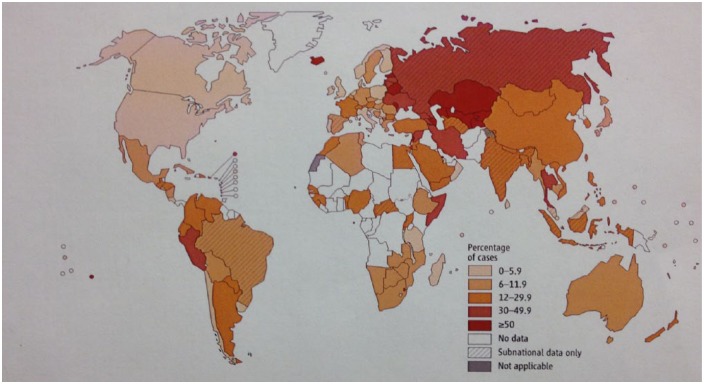
Figure 2.
Percentage of previously treated tuberculosis cases with multidrug-resistant tuberculosis. Figures are based on the most recent year for which data have been reported, which varies among countries. Reproduced with permission from WHO [2013b, Figure 4.3, p. 49, chapter 4].
There were an estimated 480,000 (CI 350,000–610,000) new MDR-TB cases worldwide and 210,000 (CI 130,000–290,000) died with MDR-TB in 2013 (Figure 3) [WHO, 2014a]. More than half were in China, India or the Russian Federation. WHO recommends that patients with MDR-TB be treated with five drugs for a minimum of 20 months [WHO, 2011]. These regimens are difficult to tolerate since virtually all patients experience adverse effects. Cure rates are lower, mortality is higher and care is significantly more expensive [WHO, 2010a]. Management of MDR-TB is resource intensive since medication courses are longer, drugs are more expensive, and treatment requires considerably more health care visits. MDR-TB cases comprised only 2.2% of the total in South Africa in 2011, yet accounted for 32% of the national TB management budget [Pooran et al. 2013]. Care costs per patient with MDR-TB are substantially more expensive in developed countries, estimated at $44,881 in the USA and 52,259 Є in Germany [Loddenkemper et al. 2012].
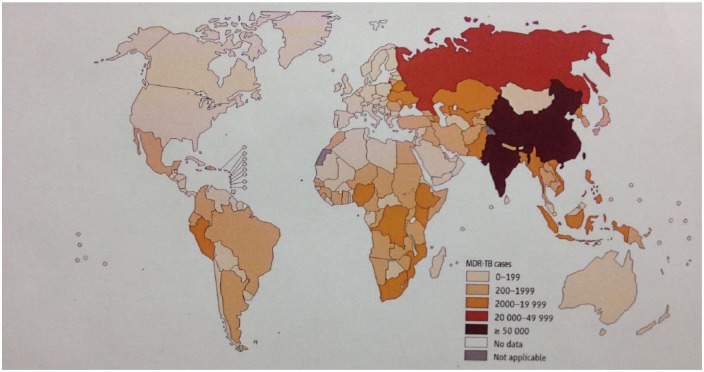
Figure 3.
Number of multidrug-resistant tuberculosis cases estimated to occur among notified pulmonary tuberculosis cases, 2012. Reproduced with permission from WHO [2013b] (Figure 4.6, p. 54, chapter 4).
Globally, only 48% of patients with MDR-TB were successfully treated in 2009 [WHO, 2013a]. Fifteen percent died and 28% were lost to follow up. Many leave their treatment program prematurely because of intolerable adverse drug effects [WHO, 2011]. More effective and less toxic medications are desperately needed to improve treatment outcomes in patients with MDR-TB. New oxazolidinones and several new classes of medications including diarylquinolines, nitroimidazopyrans, ethylenediamines and benzothiazinones are undergoing development and hopefully will provide more effective, better tolerated and shorter treatment regimens for DR-TB (Table 1) [Working Group on New TB Drugs, 2014].
Table 1.
Tuberculosis drugs in development.
| Drug class | Drug name | Clinical development | Target |
|---|---|---|---|
| Diarylquinolines | Bedaquiline | Phase III | ATP synthase inhibitor |
| Nitroimidazoles | Delamanid | Phase III | Mycolic acid synthesis |
| Pretonamid | Phase III | Mycolic acid synthesis | |
| Ethylenediamines | SQ-109 | Phase II | Unknown |
| Benzothiazinone | BTZ 043 | Early stage | Decaprenyl-phosphoribose epimerase |
| PBTZ169 | Early stage | Decaprenyl-phosphoribose epimerase | |
| Oxazolidinone | Linezolid | Phase II | Protein synthesis |
| AZD5847 | Phase II | Protein synthesis | |
| Sutezolid | Phase II | Protein synthesis | |
| Fluoroquinolone | Moxifloxacin | Phase III | DNA gyrase, topoisomerase |
| Gatifloxacin | Phase III | DNA gyrase, topoisomerase |
Derived from ‘Global TB drug pipeline’ from the Working Group on New TB Drugs, last updated August 2014 (http://www.newtbdrugs.org).
TB, tuberculosis.
Bedaquiline (Sirturo; Janssen Therapeutics, a subsidiary of Johnson & Johnson, New Brunswick, NJ), a diarylquinoline previously identified as R207910 and then TMC207, is the first drug in a novel class approved for the treatment of TB since rifampin was approved in 1971. Bedaquiline received accelerated approval by the FDA in December 2012 [Avorn, 2013]. Despite the obvious advantages of a drug with activity against MDR-TB, challenges to its use exist: QTc interval prolongation, important drug interactions with cytochrome oxidase (CYP) 3A4 inducers and inhibitors, including rifamycins and some antiretrovirals, and the emergence of resistant MTB strains.
Mechanism of action
Diarylquinolines are closely related to quinolones but do not inhibit DNA gyrase [Andries et al. 2005]. A series of molecules in this class were tested for their antimycobacterial activity against Mycobacterium smegmatis, a nonpathogenic surrogate for MTB, and bedaquiline was the most active compound [Andries et al. 2005]. It is an enantiomer with two chiral centers and has a molecular weight of 555.51 daltons [Andries et al. 2005]. Its chemical name is 1-(6-bromo-2methoxy-quinolin-3-yl)-4-dimethylamino-2-naphtalen-1-yl-1-phenyl-butan-2-ol.
Bedaquiline kills both dormant and actively replicating mycobacteria by inhibiting mycobacterial adenosine triphosphate (ATP) synthase, an essential membrane-bound enzyme, interfering with energy production and disrupting intracellular metabolism [Andries et al. 2005; Haagsma et al. 2011]. Evidence suggests that it acts by interfering with the proton transfer chain [de Jonge et al. 2007]. Inhibition of ATP synthase by bedaquiline is specific for mycobacteria. It demonstrated considerably higher minimum inhibitory concentrations (MIC) against gram positive and gram negative bacteria [Andries et al. 2005]. Bedaquiline does not interfere with mammalian ATP synthase activity. Human mitochondrial ATP synthase sensitivity to bedaquiline was 20,000-fold less than the mycobacterial enzyme [Haagsma et al. 2009].
Antimicrobial spectrum
This novel action on mycobacterial ATP synthase conveys activity both against DS-TB and DR-TB [Andries et al. 2005]. The MICs against DS strains ranged from 0.030 to 0.120 μg/ml, greater potency than either rifampin or isoniazid [Andries et al. 2005]. Bedaquiline demonstrated similar activity against clinical isolates resistant to the first-line drugs, including isoniazid, rifampin, pyrazinamide, ethambutol and streptomycin, and against moxifloxacin [Andries et al. 2005]. Bedaquiline showed similar activity against a number of MDR strains [Andries et al. 2005]. The MICs of MDR-TB strains, including an extensively drug-resistant (XDR) MTB strain, a MDR strain also resistant to injectables and fluoroquinolones, ranged from 0.004 to 0.13 μg/ml [Huitric et al. 2007].
The majority of nontuberculous mycobacteria (NTM), both rapidly and slowly growing, were susceptible to bedaquiline in vitro [Ji et al. 2006b; Huitric et al. 2007]. Phenotypic variations in ATP synthase provide some NTM with intrinsic resistance to bedaquiline [Andries et al. 2005]. In contrast to MTB, ATP synthase inhibition may be bacteriostatic rather than bactericidal against Mycobacterium avium [Lerat et al. 2014]. Bedaquiline was almost inactive against a murine model of Mycobacterium abscessus infection but was active against Mycobacterium leprae [Ji et al. 2006a; Lounis et al. 2009; Gelber et al. 2009].
Mechanisms of resistance
Ineffective or incomplete treatment can select resistant MTB strain mutants [WHO, 2014b]. Inadequate treatment regimens will eradicate DS organisms allowing DR ones to become predominant [WHO, 2014b]. If a MTB strain is only sensitive to a single drug in a regimen, the small number of organisms resistant to that particular drug will selectively propagate, eventually resulting in treatment failure and relapse [WHO, 2014b]. Spontaneous mutations with resistance to isoniazid and rifampin occur in 3.5 × 10−6 and 3.1 × 10−8 organisms, respectively [Chan et al. 2013]. Bedaquiline-resistant mutants arise at a rate of one in 108 organisms [Andries et al. 2005].
Resistance to ATP synthase is encoded by the atpE gene and mutations convey resistance by preventing bedaquiline from binding to its target, subunit C [Petrella et al. 2006; Koul et al. 2007; Segala et al. 2012]. Depletion of ATP kills both actively replicating and dormant MTB [Koul et al. 2008]. The slow growing NTM, Mycobacterium xenopi and Mycobacterium shimoidei, and the rapid grower, Mycobacterium novocastrense, are naturally resistant to bedaquiline because of atpE gene variants [Petrella et al. 2006; Segala et al. 2012].
There are other mechanisms that furnish mycobacterial resistance to bedaquiline. Fifteen of 53 bedaquiline-resistant mutants had demonstrable point mutations of the atpE gene. Mutations of the atpE gene were absent in the 38 other bedaquiline-resistant mutants [Huitric et al. 2010]. Mutations of the F0 or F1 ATP synthase operons were not present either [Huitric et al. 2010]. To investigate possible mechanisms of acquired resistance, MTB isolates with increased MICs from bedaquiline-treated patients were identified [Andries et al. 2014]. Mutations in the transcriptional gene repressor, Rv0678, endowed resistance against bedaquiline and cross resistance to clofazimine by enhancing drug efflux [Andries et al. 2014; Hartkoorn et al. 2014].
Pharmacokinetics
Orally administered bedaquiline is rapidly absorbed in mice [Andries et al. 2005]. Plasma concentrations are proportional to the administered dose between 8 and 64 mg/kg in a murine model of pulmonary TB [Rouan et al. 2012]. It is widely distributed throughout the tissues including the lungs and spleen [Andries et al. 2005]. Its principle metabolite, N-desmethyl bedaquiline, exhibits approximately 20% of the parent compound’s activity. Both bedaquiline and the N-desmethyl metabolite have half lives of 50–60 h in mice [Rouan et al. 2012]. Since the bactericidal activity of bedaquiline is concentration dependent, the time product of drug concentration [area under the curve (AUC)] is the main determinant of its activity [Rouan et al. 2012].
Bedaquiline is well absorbed in man, reaching a peak concentration in 5 h, and subsequently the concentration declines in a triexponential fashion [Andries et al. 2005; Diacon et al. 2013a]. In patients with MDR-TB, the peak, minimum and steady-state plasma concentrations were 3270 ± 1144, 956 ± 557, 1770 ± 701 and 1659 ± 722 ng/ml, 620 ± 466 ng/ml, 902 ng/ml after 2 weeks of 400 mg daily and after a further 6 weeks of treatment with 200 mg three times weekly, respectively [Diacon et al. 2009]. These values were above 600 ng/ml, the targeted steady-state plasma concentration [Diacon et al. 2009]. Administration with a meal containing 22 g of fat doubled its bioavailability relative to its ingestion on an empty stomach [Rustomjee et al. 2008; Janssen Therapeutics, 2012]. Bedaquiline is highly protein bound (>99.9%) [CDC, 2013]. It undergoes N-methylation primarily via hepatic CYP3A4 with contributions from CYP2C8 and CYP2C19 and it is primarily excreted fecally [Liu et al. 2014]. In another study, plasma concentrations of the parent drug and its N-monodesmethyl metabolite increased proportionately to the administered dose of bedaquiline [Rustomjee et al. 2008; Gupta et al. 2014b]. Peak plasma concentration occurred 4 hours after administration but steady state conditions were still not achieved after 7 days of treatment [Rustomjee et al. 2008].
Bedaquiline has a long terminal half life in man, best explained by its redistribution from tissue compartments [McLeay et al. 2014]. The mean terminal half lives for bedaquiline and its M2 metabolite were 164 (range 62–408) days and 159 (range 69–407) days, respectively [Diacon et al. 2012a]. Bedaquiline clearance was 52% greater in black patients than in other racial groups [CDC, 2013].
There were no significant pharmacokinetic interactions with isoniazid, pyrazinamide, ethambutol, kanamycin, ofloxacin or cycloserine [CDC, 2013]. Coadministration with nevirapine did not affect serum concentrations of bedaquiline or its M2 metabolite so dose adjustments will not be necessary when the drugs are dispensed together [Svensson et al. 2014a]. Rifampin, rifapentine and other inducers of CYP3A4 reduce exposure to bedaquiline and CYPA34 inhibitors, such as ketoconazole, increase exposure to bedaquiline [Lounis et al. 2008; Svensson et al. 2014b]. Lopinavir/ritonavir reduced clearance of bedaquiline and its M2 metabolite by 35% and 58%, respectively [Svensson et al. 2014a]. Dosing will require adjustment and careful monitoring when they are coadministered [Svensson et al. 2014a]. In healthy adult volunteers, a single dose of efavirenz only minimally affected bedaquiline pharmacokinetics [Dooley et al. 2012]. However, steady-state concentrations of bedaquiline were reduced by 52% with chronic administration in normal adult volunteers, suggesting that a simple dose adjustment will be necessary [Svensson et al. 2013].
Concomitant treatment with verapamil in vitro reduces the MIC of bedaquiline 8 to 16 fold by inhibiting mycobacterial efflux mechanisms [Gupta et al. 2014b; Adams et al. 2014; Andries et al. 2014]. Subinhibitory doses of bedaquiline had the same bactericidal effect with verapamil coadministration as the human bioequivalent dose in a murine model of TB [Gupta et al. 2014a]. However, verapamil did not reduce the MIC of bedaquiline in mice or alter efflux-based resistance [Andries et al. 2014].
Preclinical studies
Bedaquiline monotherapy
Bedaquiline MICs were superior to both those of rifampin and isoniazid when tested against a number of DS-MTB strains: 0.03–0.120 μg/ml versus 0.50 and 0.12 μg/ml, respectively [Andries et al. 2005]. Bedaquiline monotherapy was also superior to all currently available first-line drugs in a murine model of pulmonary TB with a high initial bacillary load [Ibrahim et al. 2007]. At 2 months, 20% of the bedaquiline-treated mice had negative cultures, whereas none treated with rifampin, moxifloxacin, isoniazid or pyrazinamide monotherapy were culture negative [Ibrahim et al. 2007]. Treatment with bedaquiline for 2 months was more effective than the combination of isoniazid, rifampin and pyrazinamide in mice infected with a DS-TB strain, H37Rv [Lounis et al. 2006]. Bedaquiline monotherapy for 8 weeks resulted in a greater bacillary load reduction than 8 weeks of rifapentine [Veziris et al. 2009]. In a guinea pig model of TB, 8 weeks of bedaquiline reduced the bacterial load in the lungs to undetectable levels [Shang et al. 2011]. In a murine model, bedaquiline for 4 months was as effective as standard 6-month first-line therapy [Ibrahim et al. 2009].
Bedaquiline combined with first-line drugs
Bedaquiline combined with any two of isoniazid, rifampin or pyrazinamide for 2 months rendered mice with an initial bacillary load of 6 log10 colony-forming units (CFUs) culture negative [Andries et al. 2005]. Bedaquiline, combined with pyrazinamide, pyrazinamide and isoniazid, pyrazinamide and rifampin, or pyrazinamide and moxifloxacin for 2 months resulted in 70–100% of mice infected with a high bacillary load converting to negative, whereas mice treated with isoniazid, rifampin and pyrazinamide or rifampin, pyrazinamide and moxifloxacin, remained culture positive [Ibrahim et al. 2007]. The addition of pyrazinamide to bedaquiline had a synergistic effect on its bactericidal activity [Ibrahim et al. 2007; Veziris et al. 2009]. In a murine TB model, bedaquiline combined with pyrazinamide and rifapentine for 3 months, or 5 months of bedaquiline, pyrazinamide and moxifloxacin were as effective as 6 months of rifampin, isoniazid and pyrazinamide [Andries et al. 2010].
Despite better early bacterial activity (EBA), the sterilizing ability of some regimens may be inferior to some with less robust EBA. Bedaquiline combined with pyrazinamide and rifapentine had a lower EBA but better and faster sterilizing activity than bedaquiline, pyrazinamide and moxifloxacin [Andries et al. 2010]. The sterilizing effects of bedaquiline and pyrazinamide were superior to isoniazid, rifampin and pyrazinamide. Clofazimine was the best third drug in combination with bedaquiline and pyrazinamide [Tasneen et al. 2011].
Bedaquiline and MDR-TB
Bedaquiline was active against MTB strains resistant to rifampin, isoniazid, streptomycin, ethambutol and pyrazinamide [Andries et al. 2005]. Bedaquiline, alone or in combination with various second-line agents, was more active than second-line regimens without it. Its addition to the WHO-recommended regimen for MDR-TB (BR) reduces the time required to sterilize TB. The relapse rate in a murine TB model treated with the BR and bedaquiline for 6 months was similar to 12 months of BR without bedaquiline [Veziris et al. 2011].
Combinations with other new drugs
In combination with SQ 109, an ethylenediamine, the bactericidal rate of bedaquiline against the H37Rv MTB strain increased four to eight fold in vitro [Reddy et al. 2010]. The combination of SQ 109 and bedaquiline was also effective when rifampin was included [Reddy et al. 2010].
Combining bedaquiline with sutezolid and SQ 109 provided fully additive bactericidal activity in whole blood culture with comparable results to first-line therapy. Bedaquiline was evaluated in various three drug combinations with PA-824, a nitroimidazole recently renamed pretonamid, pyrazinamide, moxifloxacin and rifapentine. The combinations of bedaquiline, pyrazinamide and either moxifloxacin or rifapentine were the most effective [Tasneen et al. 2011]. The addition of pretonamid to bedaquiline was less effective and possibly had an antagonistic effect on bactericidal activity in whole blood culture [Wallis et al. 2012].
The benzothiazinones act by inhibiting decaprenyl-phosphoribose epimerase, a heterodimeric enzyme essential for arabinan biosynthesis [Lechartier et al. 2012; Makarov et al. 2014]. Arabinans are mycobacterial cell wall polysaccharides necessary to maintain cell wall integrity. The lead molecule of this new drug class, BTZ043, appears to act synergistically with bedaquiline. Bedaquiline also exhibited synergy when combined with another benzothiazinone, PBTZ169, in mouse and zebrafish TB models [Makarov et al. 2014].
The sterilizing potentials of bedaquiline, pretonamid, sutezolid, clofazimine and rifapentine were investigated in mice [Williams et al. 2012]. Bedaquiline and sutezolid was the most effective pair in the two-drug study. The combination of bedaquiline and pyrazinamide was also studied in three- and four-drug regimens. The addition of rifapentine and clofazimine to the pair was the most effective regimen followed by clofazimine, rifapentine and sutezolid, in order of decreasing effectiveness.
The ability to rapidly kill actively replicating organisms is reflected by the EBA but dormant intracellular mycobacteria must also be eradicated to cure TB [Mitchison, 2000]. Drug regimens with good EBA are not necessarily effective at sterilization, that is, eradicating dormant or latent TB infection (LTBI) [Andries et al. 2010]. Although the EBA of isoniazid is superior, inclusion of rifampin, with its greater activity against LTBI, reduces the required treatment duration [Mitchison, 2000]. Drugs that inhibit mycolic acid synthesis, including isoniazid, may not be active against LTBI whereas bedaquiline, which interferes with ATP synthase, is bactericidal even when bacilli are dormant [Zhang et al. 2012]. In a mouse model of LTBI, the sterilizing activity of bedaquiline was similar to rifampin with or without isoniazid but was inferior to rifapentine with or without isoniazid [Zhang et al. 2011]. Moreover, the addition of bedaquiline to rifapentine did not improve its sterilizing activity. In a paucibacillary model of LTBI, bedaquiline- and sutezolid-containing regimens were at least as effective as rifampin [Lanoix et al. 2014].
The bactericidal activity of bedaquiline is only evident 3–4 days after initial exposure to the drug. Inhibition of ATP synthase is bacteriostatic within hours but induction of the MTB dormancy regulon maintains ATP synthesis by shifting to glycolytic metabolism, temporarily prolonging bacterial survival, and reduces ATP requirements by downregulating protein, mycolic acid and nucleic acid synthesis [Koul et al. 2014]. In the extracellular milieu, bedaquiline is bacteriostatic during the first 7–14 days, and bactericidal activity only begins subsequently. Its bactericidal activity is evident earlier in murine peritoneal macrophages and accelerates over the first 5–7 days [Dhillon et al. 2010].
Clinical drug trials
Studies in patients with pulmonary DS-TB
To study the EBA of bedaquiline in man, 75 treatment-naive patients with smear-positive pulmonary TB were randomized to receive one of 25, 100 or 400 mg of bedaquiline, rifampin 600 mg or isoniazid 300 mg daily for 7 days [Rustomjee et al. 2008]. Mean decreases in log10 CFU counts were 0.04, 0.26 and 0.77 for 25, 100 and 400 mg of bedaquiline, respectively, compared with 1.70 for rifampin and 1.88 for isoniazid. Similar to pyrazinamide, none of the bedaquiline doses demonstrated an obvious effect initially but after a delay of 4 days the EBA of bedaquiline, 400 mg daily, was similar to the EBA of isoniazid and rifampin over the same period [Zhang and Mitchison, 2003; Rustomjee et al. 2008]. Although ATP synthesis is disrupted by bedaquiline, the bactericidal effect is postponed until intracellular ATP stores are depleted.
In a 14-day, dose-ranging study in treatment-naïve individuals with newly diagnosed smear-positive pulmonary DS-TB, EBA increased linearly with increasing daily doses of bedaquiline between 100 and 400 mg [Diacon et al. 2013a]. Pyrazinamide enhances the bactericidal activity in man as well as in mice [Diacon et al. 2012a]. The protocol included loading doses of bedaquiline for the first 2 days to partially overcome the delay in EBA [Diacon et al. 2013b]. The 14-day bactericidal activity of combinations of pretonamid, bedaquiline, pyrazinamide and moxifloxacin were tested in hospitalized patients with pulmonary DS-TB [Diacon et al. 2012a]. The administered dose of bedaquiline was 700 mg on the first day, 500 mg on the second day, and 400 mg daily for the rest of the trial. The 14-day EBA of the combination of pretonamid, moxifloxacin and pyrazinamide was better than bedaquiline monotherapy, bedaquiline and pyrazinamide, or bedaquiline and pretonamid, but not better than the combination of pretonamid and pyrazinamide, and was comparable to standard first-line therapy. All treatment combinations were well tolerated and safe, although one patient treated with pretonamid, moxifloxacin and pyrazinamide was withdrawn from the study because of QTc interval prolongation.
Studies in patients with pulmonary MDR-TB
In a phase II study, newly diagnosed patients with sputum-smear-positive MDR-TB, between the ages of 18 and 65 years, were treated with BR consisting of a standard five-drug, second-line MDR-TB regimen after a 1-week screening period during which treatment with first-line drugs was stopped. All subjects were randomized to receive bedaquiline 400 mg daily for 2 weeks, followed by 200 mg three times weekly for 6 weeks or placebo [Diacon et al. 2009]. Following the 8-week trial period, patients continued their BR for a further 96 weeks. The preferred BR, which most received, was kanamycin, ofloxacin, ethionamide, pyrazinamide and either cycloserine or terizidone. Patients were stratified by the extent of lung cavitation. Patients were excluded if the infecting organism was resistant to aminoglycosides or to fluoroquinolones, if they had previously been treated for MDR-TB, had severe extrapulmonary infection, were human immunodeficiency virus (HIV) positive with a CD4-positive count less than 300/ml or had significant cardiac arrhythmias [Diacon et al. 2009].
Forty-seven patients were recruited and 23 were randomized to receive bedaquiline. Twenty of the 23 who received bedaquiline plus BR and 21 of the 24 treated with placebo and BR completed the 8-week study. Two of the six withdrawals, one in each study arm, had XDR-TB and one was withdrawn because sputum remained culture negative throughout the study. Demographics of the two groups were similar: 55% were black race, 74% were male, mean body mass index was 18.3, 87% were HIV negative, median age was 33 years, 85% had cavitary lung disease, and one third were infected with TB strains also resistant to pyrazinamide [Diacon et al. 2009].
At 4 weeks and at 8 weeks the proportions of subjects with negative sputum smears were 77% and 84% for the bedaquiline plus BR group and 57% and 68% for the placebo plus BR group, respectively [Diacon et al. 2009]. Sputum culture CFUs declined more rapidly in the bedaquiline-treated group. Addition of bedaquiline to BR reduced the time to sputum culture conversion [hazard ratio (HR) 11.8; CI 2.3–61.3; p = 0.003] and increased the proportion of patients whose sputum cultures converted to negative, 48% (10 of 21) versus 9% (2 of 23) at 8 weeks, p = 0.003 [Diacon et al. 2009]. The time required for 50% of the patients to achieve sputum culture conversion was 78 days with bedaquiline versus 129 days in the placebo arm [Diacon et al. 2012b].
Two-year outcomes were reported separately [Diacon et al. 2012b]. Study withdrawal occurred in 54% of the placebo patients and 44% in the bedaquiline arm before completion of the 2-year follow up. The efficacy at 24 weeks, defined as the time to culture conversion, was significantly better in the bedaquiline-treated patients (HR 2.25; CI 1.08–4.71; p = 0.031). At 24 weeks, 81% in the bedaquiline arm and 65% receiving placebo were sputum culture negative. Treatment success rates were similar at 104 weeks, 52.4% and 47.8% in the bedaquiline and placebo arms, respectively. At study initiation, 70% of the 43 baseline isolates had a bedaquiline MIC of 0.03 μg/ml or less and the MIC was 0.12 or less in 95%. In the few samples obtained at 2 years, there were no significant changes in the bedaquiline MICs.
Nausea was the only adverse effect more common in the bedaquiline arm; 26% versus none in the placebo arm [Diacon et al. 2012b]. Other common adverse effects were unilateral deafness, arthralgias, hemoptysis, hyperuricemia, rash, extremity and chest pain. Most adverse events were considered mild or moderate. Only one patient treated with bedaquiline acquired resistance to a companion drug compared with five receiving placebo (p = 0.18). The QTc increased more in the bedaquiline patients, 10.8 versus 1.0 ms (p = nonsignificant) [Diacon et al. 2009].
In another double-blind, placebo-controlled, phase IIb trial, 160 patients with newly diagnosed sputum-smear-positive MDR-TB, treated with BR, were randomized to receive bedaquiline 400 mg daily for 2 weeks followed by 200 mg thrice weekly for 22 weeks, or placebo [Gras, 2013; Diacon et al. 2014]. After completing the 24-week experimental period, patients were instructed to continue BR for a further 96 weeks. The primary objective of the trial was the time to sputum culture conversion, defined as two consecutive negative cultures in liquid medium at least 25 days apart not followed by a positive sputum culture. Drug susceptibility testing was done at 8, 24 and 72 weeks and in all patients who relapsed; that is, became culture positive after converting to negative.
Patients were stratified by study site and by severity of cavitation. The preferred five-drug BR was pyrazinamide, ethionamide, ofloxacin, kanamycin and cycloserine. The study was carried out in eight countries with relatively high burdens of MDR-TB. Inclusion criteria included being 18–65 years with newly diagnosed, sputum-smear-positive pulmonary MDR-TB. Important exclusion criteria were previous treatment for MDR-TB or previous treatment with bedaquiline, HIV positivity with a CD4-positive count less than 300/ml, complicated or severe extrapulmonary disease, an electrocardiographic QTcF (QTc interval corrected with Fridericia’s formula, i.e. QTc interval divided by the cube root of the RR interval (RR is the time interval between the R waves, part of the electrocardiographic pattern, of two consecutive heart beats)) greater than 450 ms, cardiac arrhythmias requiring medication, concomitant serious illness, pregnancy or lactation, or alcohol or drug abuse. Concomitant use of moxifloxacin, gatifloxacin, CYP3A4 inhibitors or enhancers was prohibited.
Separate analyses of the intent-to-treat (ITT) group, those receiving at least one dose of study medication (160 patients), and on the modified ITT group (132 patients, 66 in each group) excluding those with negative pretreatment sputum cultures, those without documented resistance to isoniazid and rifampin, those with pre-extensive drug resistance, that is MDR-TB and also resistance to any second-line injectable medication or to a fluoroquinolone (pre XDR), and those not assessed after baseline were censored at the last assessment and were considered to have not responded [Gras, 2013].
Sixty of the 160 patients in the ITT group discontinued the trial prematurely. Reasons for discontinuation were similar in both study arms, mostly consent withdrawal or adverse events. In the modified ITT group the demographics of the bedaquiline and placebo groups were similar except that there were more with pyrazinamide resistance and pre-XDR-TB in the bedaquiline group and more HIV-positive patients and patients with low serum albumins in the placebo arm. The median overall treatment phase was longer in the placebo group. At least one more drug was added to the BR of 58% of the placebo patients and to 47% of the bedaquiline patients.
Bedaquiline reduced the median time to sputum culture conversion from 125 to 83 days in the modified ITT population. The HR adjusted for cavitation was 2.44 (CI 1.57–3.80, p < 0.001). The results were similar in the ITT cohort. The culture conversion rates were 79% versus 58%, p = 0.008, 71% versus 56%, p = 0.069, and 62% versus 44%, p = 0.04, at 24, 72 and 120 weeks, in the bedaquiline and placebo patients, respectively [Diacon et al. 2014]. There were no differences in the bedaquiline AUC curves between the patients who converted or did not convert to negative. Cure rates for MDR-TB, as defined by WHO, were 58% in the bedaquiline arm and 32% in the placebo arm at 120 weeks (p = 0.003). At 120 weeks, sputum culture conversion occurred in 69% versus 43% and 60% versus 42% of the patients with resistance to isoniazid and rifampin only, and with pre-XDR-TB, respectively, favoring bedaquiline.
New resistance to at least one TB drug developed in two subjects treated with bedaquiline and in 16 of the placebo group. Treatment failure occurred in one of the two bedaquiline group patients and in 9 of the 16 placebo arm patients who developed additional drug resistance. In the ITT population there were as many adverse events, treatment-related adverse events and adverse events leading to study discontinuation in the two study arms. In a pooled analysis of the data from the two phase IIb trials, 96.1% of the patients in the bedaquiline arms and 95.2% in the placebo arms experienced at least one adverse effect [WHO, 2013]. The most frequent adverse events were nausea (35%), arthralgias (29%), headache (24%), hyperuricemia (23%) and vomiting (21%) [Diacon et al. 2014]. Headache (24% versus 11%), nausea (35% versus 26%) and arthralgias (29% versus 20%) were more common in the bedaquiline-treated patients [Diacon et al. 2014]. In the pooled analysis of the two phase IIb studies, there were 10 (13%) deaths in the bedaquiline group, including 5 from progressive TB, versus 2 (2%) in the placebo group [Gras, 2013]. One death in the bedaquiline arm was due to a motor vehicle accident 130 weeks after bedaquiline was discontinued [Cox and Laessig, 2014]. Nine of 10 deaths in the bedaquiline arm occurred after 24 weeks and bedaquiline had already been discontinued for a median of 49 weeks at the time of death (range 12.3–130.1 weeks). There was no common cause of death among the four other cases, making it difficult to attribute them to bedaquiline [Cox and Laessig, 2014]. Despite the concerns about excess deaths, the alternatives to bedaquiline are less effective, very difficult to tolerate and toxic medications. Moreover, its successful and reasonably safe use in MDR-TB and XDR-TB in a cohort treated on a compassionate basis is reassuring [Guglielmetti et al. 2015].
The QTcF increased a mean of 15.4 ms in the bedaquiline arm versus 3.3 ms in the control arm and the QTcF returned to normal after discontinuation of bedaquiline. There was no association between deaths and bedaquiline plasma levels or with QTcF prolongation.
An open-label, phase II, single-arm trial, C209, in patients with newly diagnosed MDR-TB or in those who previously failed treatment for MDR-TB to assess safety, tolerability and efficacy of bedaquiline in combination with individualized regimens to treat sputum-smear-positive MDR-TB patients has been completed [US FDA, 2012]. HIV-positive patients could participate if their antiretroviral regimen could be switched to a triple nucleotide reverse transcriptase inhibitor regimen, zidovudine/lamivudine, epivir/abacavir, or if antiretroviral therapy could be discontinued. This trial included patients with additional drug resistance. Patient profiles were more like the typical patient with MDR-TB than the participants in the C208 trials [Diacon et al. 2009; Gras, 2013]. Patients with pre-XDR-TB or XDR-TB could participate provided that their regimen included at least three drugs that the TB isolate was likely to be susceptible to. Patients with sputum-smear-positive MDR-TB received bedaquiline for 24 weeks along with an individualized background regimen according to national TB program treatment guidelines [US FDA, 2012]. Bedaquiline treatment consisted of 400 mg once daily for 2 weeks followed by 200 mg three times weekly for 22 weeks. The 24-week bedaquiline treatment period had been completed but the 96-week follow-up period was still ongoing at the time of the FDA report [UD FDA, 2012].
Bedaquiline was provided to 35 French patients with MDR-TB on a compassionate basis [Guglielmetti et al. 2015]. Nineteen had XDR-TB and 14 had pre-XDR-TB. They received a median of four (two to five) drugs, including linezolid in 33 patients. After 6 months of bedaquiline, sputum culture conversion occurred in 28 of 29 patients who were culture positive initially and the median time to sputum culture conversion was 85 (8–235) days. The QTc interval increased by 60 ms or more in seven patients, requiring discontinuation in two. One death occurred but was not felt to be related to TB or to drug treatment.
Indications
Both WHO and CDC published provisional recommendations for the appropriate use of bedaquiline in the treatment of MDR-TB in 2013 [WHO, 2013; CDC, 2013]. Bedaquiline should be reserved for patients with MDR-TB when an effective regimen with pyrazinamide and four second-line drugs, as recommended by WHO, cannot otherwise be designed. It is also recommended for the treatment of MDR-TB with documented resistance to any fluoroquinolone. The recommended dose is 400 mg daily for 2 weeks followed by 200 mg three times per week for a further 22 weeks. It should be administered with food to maximize absorption. WHO recommended that it not be given for more than 6 months whereas the CDC suggested that treatment for longer than 24 weeks could be considered on a case-by-case basis when an effective regimen cannot otherwise be provided.
Bedaquiline should be used in combination with a minimum of four drugs provided to adults closely supervised in a DOT program for patients with pulmonary MDR-TB. Bedaquiline should not be added as a single drug to companion drugs known or believed to be ineffective or to a failing regimen [WHO, 2013]. In the absence of a specific bedaquiline susceptibility test, resistance should be monitored by serial assessment of MICs [WHO, 2013]; a fourfold MIC increase suggests acquired resistance [CDC, 2013]. Since bedaquiline has a long terminal half life, it should be discontinued 4–5 months before other drugs are stopped to prevent extended exposure of MTB to low levels of bedaquiline monotherapy which could increase the risk of resistance [CDC, 2013].
Both organizations emphasized that the current recommendations were limited to adults with pulmonary MDR-TB between the ages of 18 and 65 years. Caution should be exercised in the use of bedaquiline in older patients and in those living with HIV. WHO recommended against the use of bedaquiline in pregnant women and children less than 18 years of age, whereas the CDC recommended that it could be considered in these populations and in patients with extrapulmonary disease on a case-by-case basis. It is considered a pregnancy category B drug by the FDA [CDC, 2013]. It is not known whether bedaquiline and its metabolites are excreted in human breast milk, although it is concentrated in breast milk in rats [CDC, 2013]. Both organizations raised concerns and highlighted the need for monitoring in patients with certain comorbidities, including liver disease, cardiac disease, and alcohol and drug abuse.
The Republic of South Africa created a clinical access program for bedaquiline [Conradie et al. 2014]. Its goals are to provide it to patients with pre-XDR-TB or XDR-TB. Bedaquiline should never be added as a single drug to a failing drug regimen. It should be reserved for infections that can be treated with a BR that includes at least three drugs proven effective by DST within the previous 6 months. The recommended bedaquiline regimen is the same as that recommended by WHO and CDC; 400 mg daily for 2 weeks followed by 200 mg three times per week for a further 22 weeks. The BR should be continued after the course of bedaquiline is completed to reduce the risk of resistance to bedaquiline developing [Conradie et al. 2014].
The CDC, FDA and the South African program all raised concerns about QTcF prolongation and the higher mortality in the bedaquiline-treated groups but endorsed its use when an MDR regimen with four second-line drugs could not otherwise be provided, particularly in cases with a fluoroquinolone-resistant TB strain. All three organizations raised concerns about its use with other medications known to prolong the QTcF, including moxifloxacin, macrolides and clofazimine.
Possible future roles for bedaquiline?
Bedaquiline should be reserved for patients with MDR-TB who cannot otherwise be successfully treated but it may have other indications in the future. There is no consensus about the treatment of LTBI in contacts of MDR-TB and XDR-TB cases [European Centre for Disease Prevention and Control, 2012]. The European Centre for Disease Prevention and Control suggests respiratory fluoroquinolones as a treatment option but clinical data are lacking. Bedaquiline could potentially be an alternative for MDR-TB contacts.
A recent small case series explored the role of bedaquiline as salvage therapy in patients with macrolide-resistant M. avium and M. abscessus lung disease [Philley et al. 2015]. Side effects were common but six of the ten patients had a positive microbiological response.
Although the addition of bedaquiline to standard therapy shortened the time to sterilize murine DS-TB, careful consideration and appropriate trials to test efficacy and safety will be essential before endorsing its use to treat DS-TB. Bedaquiline and the other new drugs must be preserved for DR-TB that cannot otherwise be effectively treated. The very long half life of bedaquiline due to slow tissue release could expose MTB after other medications are stopped to the development of bedaquiline resistance [Andries et al. 2005]. Cost and adverse drug effects will obviously limit its widespread use.
The development of new drug classes directed at novel mycobacterial targets will provide effective treatments against MDR-TB and XDR-TB. Hopefully, trials of various combinations of new drugs, including bedaquiline, will be undertaken to identify the best way to treat these seriously ill patients.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author declares no conflicts of interest in preparing this article.
References
- Adams K., Szumowski J., Ramakrishnan L. (2014) Verapamil and its metabolite norverapamil, inhibit macrophage-induced, bacterial efflux pump-mediated tolerance to multiple anti-tubercular drugs. J Infect Dis 210: 456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andries K., Gevers T., Lounis N. (2010) Bactericidal potencies in a murine model of tuberculosis. Antimicrob Agents Chemother 54:4540–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andries K., Verhasselt P., Guillemont J., Gohlmann H., Neefs J., Winkler H., et al. (2005) A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307: 223–227. [DOI] [PubMed] [Google Scholar]
- Andries K., Villellas C., Coeck N., Thys K., Gevers T., Vranckx L., et al. (2014) Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 9: e102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avorn J. (2013) Approval of a tuberculosis drug based on a paradoxical surrogate measure. JAMA 309: 1349–1350. [DOI] [PubMed] [Google Scholar]
- Burman W., Cohn D., Rietmeijer C., Judson F., Sbarbaro J., Reves R. (1997) Noncompliance with directly observed therapy for tuberculosis: epidemiology and effect on the outcome of treatment. Chest 111: 1168–1173. [DOI] [PubMed] [Google Scholar]
- CDC (2013) Provisional CDC guidelines for the use and safety monitoring of bedaquiline fumarate (Sirturo) for the treatment of multidrug-resistant tuberculosis. MMWR Recomm Rep 62 (RR-090: 1–12). [PubMed] [Google Scholar]
- Chan B., Khadem T., Brown J. (2013) A review of tuberculosis: focus on bedaquiline. Am J Health-Syst Pharm 70: 1984–1994. [DOI] [PubMed] [Google Scholar]
- Conradie F., Meintjes G., Hughes J., Maartens G., Ferreira H., Siwendu S., et al. (2014) Clinical access to bedaquiline programme for the treatment of drug-resistant tuberculosis. S Afr Med J 104: 164–166. [DOI] [PubMed] [Google Scholar]
- Cox E., Laessig K. (2014) FDA approval of bedaquiline – the benefit–risk balance for drug-resistant tuberculosis. New Engl J Med 371: 689–691. [DOI] [PubMed] [Google Scholar]
- de Jonge M., Koymans L., Guillemont J., Koul A., Andries K. (2007) A computational model of the inhibition of Mycobacterium tuberculosis ATPase by a new drug candidate R207910. Proteins 67: 971–980. [DOI] [PubMed] [Google Scholar]
- Dhillon J., Andries K., Phillips P., Mitchison D. (2010) Bactericidal activity of the diarylquinoline TMC207 against Mycobacterium tuberculosis outside and within cells. Tuberculosis (Edinb) 90: 301–305. [DOI] [PubMed] [Google Scholar]
- Diacon A., Dawson R., von Groote-Bidlingmaier F., Symons G., Venter A., Donald P., et al. (2012a) 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomized trial. Lancet 380(9846): 986–993. [DOI] [PubMed] [Google Scholar]
- Diacon A., Dawson R., von Groote-Bidlingmaier F., Symons G., Venter A., Donald P., et al. (2013a) Randomized dose-ranging study of the 14-day early bactericidal activity of bedaquiline (TMC207) in patients with sputum microscopy smear-positive pulmonary tuberculosis. Antimicrob Agents Chemother 57: 2199–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacon A., Donald P., Pym A., Grobusch M., Patientia R., Mahanyele R., et al. (2012b) Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother 56: 3271–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacon A., Grosset J., Ammerman N. (2013b) Dose-ranging activity of the newly registered antituberculosis drug bedaquiline (TMC207). Expert Rev Anti Infect Ther 11: 649–651. [DOI] [PubMed] [Google Scholar]
- Diacon A., Pym A., Grobusch M., Patientia R., Rustomjee R., Page-Shipp L., et al. (2009) The diarylquinoline TMC207 for multidrug-resistant tuberculosis. New Engl J Med 360: 2397–2405. [DOI] [PubMed] [Google Scholar]
- Diacon A., Pym A., Grobusch M., de los Rios J., Gotuzzo E., Vasilyeva I., et al. (2014) Multidrug-resistant tuberculosis and culture conversion with bedaquiline. New Engl J Med 371: 723–732. [DOI] [PubMed] [Google Scholar]
- Dooley K., Park J., Swindells S., Allen R., Haas D., Cramer Y., et al. (2012) Safety, tolerability, and pharmacokinetic interactions of the antituberculosis agent TMC 207(bedaquiline) with efavirenz in healthy volunteers: AIDS clinical trials group. J AIDS 59: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (2012) Management of Contacts of MDR TB and XDR TB Patients. Stockholm: ECDC. [Google Scholar]
- Gelber R., Andries K., Paredes R., Andaya C., Burgos J. (2009) The diarylquinoline R207910 is bactericidal against Mycobacterium leprae in mice at low dose and administered intermittently. Antimicrob Agents Chemother 53: 3989–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras J. (2013) Bedaquiline for the treatment of pulmonary, multidrug-resistant tuberculosis in adults. Drugs Today (BARC) 49: 353–361. [DOI] [PubMed] [Google Scholar]
- Guglielmetti L., Le Du D., Jachym M., Henry B., Martin D., Caumes E., et al. (2015) Compassionate use of bedaquiline for the treatment of MDR- and XDR-tuberculosis: an interim analysis of a French cohort. Clin Infect Dis 60: 188–194. [DOI] [PubMed] [Google Scholar]
- Gupta S., Cohen K., Winglee K., Maiga M., Diarra B., Bishai W. (2014a) Efflux inhibition with verapamil potentiates bedaquiline in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58: 574–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Tyagi S., Bishai W. (2014b) Verampamil increases the bactericidal activity of bedaquiline against Mycobacterium tuberculosis in the mouse model. Anitmicrob Agents Chemother 59: 673–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagsma A., Abdilla-Ibrahim R., Wagner M., Krab K., Vergauwen K, Guillemont J., et al. (2009) Selectivity of TMC207 towards mycobacterial ATP synthase compared with that towards the eukaryotic homologue. Antimicrob Agents Chemother 53:1290–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagsma A., Podasca I., Koul A., Andries K., Guillemont J., Lill H., et al. (2011) Probing the interaction of the diarylquinoline TMC207 with its target mycobacterial ATP synthase. PLoS One 6: e23575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartkoorn R., Uplekar S., Cole S. (2014) Cross-resistance between clofazimine and bedaquiline through upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:2979–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitric E., Verhasselt P., Andries K., Hoffner S. (2007) In vitro antimycobacterial spectrum of a diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother 51: 4202–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitric E., Verhasselt P., Koul A., Andries K., Hoffner S., Andersson D. (2010) Rates and mechanisms of resistance development in Mycobacterium tuberculosis to a novel diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother 54: 1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M., Andries K., Lounis N., Chaffour A., Truffot-Pernot C., Jarlier V., et al. (2007) Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob Agents Chemother 51: 1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M., Truffot-Pernot C., Andries K., Jarlier V., Veziris N. (2009) Sterilizing activity of R207910 (TMC207)-containing regimens in the murine model of tuberculosis. Am J Respir Crit Care Med 180: 553–557. [DOI] [PubMed] [Google Scholar]
- Iseman M. (1993) Treatment of multidrug-resistant tuberculosis. New Engl J Med 329: 784–794. [DOI] [PubMed] [Google Scholar]
- Janssen Therapeutics (2012) Sirturo (bedaquiline) product information. Available at: http://www.sirturo.com/sites/default/files/pdf/SIRTURO-product-guide.pdf (accessed 14 April 2015).
- Ji B., Chauffour A., Andries K., Jarlier V. (2006a) Bactericidal activities of R207910 and other newer antimicrobial agents against Mycobacterium leprae in mice. Antimicrob Agents Chemother 50: 1558–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B., Lefrancois S., Robert J., Chauffour A., Truffot C., Jarlier V. (2006b) In vitro and in vivo activities of rifampin, streptomycin, amikacin, moxifloxacin, R207910, linezolid, and PA-824 against Mycobacterium ulcerans. Antimicrob Agents Chemother 50: 1921–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul A., Dendouga N., Vergauwen K., Molenberghs B., Vranckx L., Willebrords R., et al. (2007) Diarylquinolines target subunit C of mycobacterial synthase. Nat Chem Biol 3: 323–324. [DOI] [PubMed] [Google Scholar]
- Koul A., Vranckx L., Dendouga N., Balemans W., van den Wyngart I., Vergauwen K., et al. (2008) Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J Biol Chem 283: 25273–25280. [DOI] [PubMed] [Google Scholar]
- Koul A., Vranckx L., Dhar N., Gohlmann H., Ozdemir E., Neefs J., et al. (2014) Delayed bactericidal response of Mycobacterium tuberculosis to bedaquiline involves remodelling of bacterial metabolism. Nat Commun 5: 3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoix J., Betoudji F., Nuermberger E. (2014) Novel regimens identified in mice for treatment of latent tuberculosis infection in contacts of patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother 58: 2316–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechartier B., Hartkoorn R., Cole S. (2012) In vitro combination studies of the benzothiazinone lead compound BTZ043 against Mycobacterium tuberculosis. Antimicrob Agents Chemother 56: 5790–5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerat I., Cambau E., Roth dit Bettoni R., Jarlier V., Truffot C., Veziris N. (2014) In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J Infect Dis 209: 905–912. [DOI] [PubMed] [Google Scholar]
- Liu K., Li F., Lu J., Liu S., Dorko K., Xie W., et al. (2014) Bedaquiline metabolism: enzymes and novel metabolites. Drug Metab Dispos 42:863–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddenkemper R., Sotgiu G., Mitnick C. (2012) Editorial. Cost of tuberculosis in the era of multidrug resistance: will it become unaffordable? Eur Respir J 40: 9–11. [DOI] [PubMed] [Google Scholar]
- Lounis N., Gevers T., van den Berg J., Andries K. (2008) Impact of the interaction of R207910 with rifampin on the treatment of tuberculosis studied in the mouse model. Antimicrob Agents Chemother 52: 3568–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounis N., Gevers T., van den Berg J., Vranckx L., Andries K. (2009) ATP synthase inhibition of Mycobacterium avium is not bactericidal. Antimicrob Agents Chemother 53: 4927–4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounis N., Veziris N., Chauffour A., Truffot-Pernot C., Andries K., Jarlier V. (2006) Combinations of R207910 with drugs used to treat multidrug-resistant tuberculosis have the potential to shorten treatment duration. Antimicrob Agents Chemother 50: 3543–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov K., Lechartier B., Zhang M., Neres J., van der Sar A., Raadsen S., et al. (2014) Towards a new combination therapy for tuberculosis with next generation benzothiazinones. EMBO Mol Med 6: 372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeay S., Vis P., van Heeswijk R., van Heeswijk R., Green B. (2014) Population pharmacokinetics of bedaquiline (TMC207), a novel antituberculosis drug. Antimicrob Agents Chemother 58: 5315–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison D. (2000) Role of individual drugs in the chemotherapy of tuberculosis. Int J Tuberc Lung Dis 4: 796–806. [PubMed] [Google Scholar]
- Petrella S., Cambau E., Chaffour A., Andries K., Jarlier V., Sougakoff W. (2006) Genetic basis for natural and acquired resistance to the diarylquinoline R207910 in mycobacteria. Antimicrob Agents Chemother 50: 2853–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philley J., Wallace R., Jr, Benwill J., Taskar V., Brown-Elliott B., Thakkar F., et al. (2015) Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest On line 12 February 2015. (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooran A., Pieterson E., Davids M., Theron G., Dheda K. (2013) What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PLoS One 8: e54587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V., Einck L., Andries K., Nacy C. (2010) In vitro interactions between new antitubercular drug candidates SQ109 and TMC207. Antimicrob Agents Chemother 54: 2840–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouan M., Lounis N., Gevers T., Dillen L., Gilissen R., Raoof A., et al. (2012) Pharmacokinetics and pharmacodynamics of TMC207 and its N-desmethyl metabolite in a murine model of tuberculosis. Antimicrob Agents Chemother 56: 1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustomjee R., Diacon A., Allen J., Venter A., Reddy C., Patientia R., et al. (2008) Early bactericidal activity and pharmacokinetics of the diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob Agents Chemother 52: 2831–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segala E., Sougakoff W., Nevejans-Chaffour A., Jarlier V., Petrella S. (2012) New mutations in the mycobacterial ATP synthase: new insights into the binding of the diarylquinoline TMC207 to the ATP synthase C-ring structure. Antimicrob Agents Chemother 56: 2326–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang S., Shanley C., Caraway M., Orme E., Henao-Tamayo M., Hascall-Dove L., et al. (2011) Activities of TNC 207, rifampin, and pyrazinamide against Mycobacterium tuberculosis infection in guinea pigs. Antimicrob Agents Chemother 55: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson E., Aweeka F., Park J., Marzan F., Dooley K., Karlsson M. (2013) Model-based estimates of the effects of efavirenz on bedaquiline pharmacokinetics and suggested dose adjustments for patients co-infected with HIV and tuberculosis. Antimicrob Agents Chemother 57: 2780–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson E., Dooley K., Karlsson M. (2014a) Impact of lopinavir/ritonavir or nevirapine on bedaquiline exposures: potential implications for patients with TB/HIV coinfection. Antimicrob Agents Chemother 11 August 2014. (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson E., Murray S., Karlsson M., Dooley K. (2014b) Rifampicin and rifapentine significantly reduce concentrations of bedaquiline, a new anti-TB drug. J Antimicrob Chemother 21 December 2014. (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasneen R., Li S., Peloquin C., Taylor D., Williams K., Andries K., et al. (2011) Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother 55: 5485–5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US FDA (2012) Center for drug evaluation and research. Application number: 204384Orig1S000. Clinical review bedaquiline. Available at: www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=search.label_approvalhistory#apphist (accessed 20 October 2014).
- Veziris N., Ibrahim M., Lounis N., Andries K., Jarlier V. (2011) Sterilizing activity of second-line regimens containing TMC 207 in a murine model of tuberculosis. PLoS One 6: e17556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veziris N., Ibrahim M., Lounis N., Chaffour A., Truffot-Pernot C., Andries K., et al. (2009) A once-weekly R207910-containing regimen exceeds activity of the standard daily regimen in murine tuberculosis. Am J Respir Crit Care Med 179: 75–79. [DOI] [PubMed] [Google Scholar]
- Wallis R., Jakubiec W., Mitton-Fry M., Ladutko L., Campbell S., Paige D., et al. (2012) Rapid evaluation in whole blood culture of regimens for XDR-TB containing PNU-100480 (sutezolid), TMC207, PA-824, SQ109, and pyrazinamide. PLoS One 7: e30479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2010a) Treatment of Tuberculosis: Guidelines (4th edition). WHO/HTM/TB/2009.420. Available at: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf?ua=1 (accessed 14 April 2015).
- WHO (2010b) Media Centre. Drug-resistant Tuberculosis Now at Record Levels. Geneva, March 18, 2010. Available at: http://www.who.int/mediacentre/news/releases/2010/drug_resistant_tb (accessed 20 October 2014). [Google Scholar]
- WHO (2011) Guidelines for the Programmatic Management of Drug-resistant Tuberculosis. Available at: http://www.who.int/tb/publications/2011/en/index.html (accessed 14 April 2015).
- WHO (2013a) The Use of Bedaquiline in that Treatment of Multidrug-resistant Tuberculosis. Interim Policy Guidance. Available at: http://apps.who.int/iris/bitstream/10665/75146/1/9789241548441_eng.pdf (accessed 14 April 2015). [PubMed]
- WHO (2013b) Global Tuberculosis Report, 2013. Available at: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf (accessed 21 September 2014).
- WHO (2014a) Global Tuberculosis Report 2014. Available at: http://www.who.int/tb/publications/global_report/gtbr14_main_text.pdf?ua=11 (accessed 22 October 2014).
- WHO (2014b) Companion Handbook to the WHO Guidelines for the Programmic Management of Drug-resistant Tuberculosis. Available at: http://apps.who.int/iris/bitstream/10665/130918/1/97892241548809_eng.pdf?ua=1 (accessed 2 October 2014). [PubMed]
- Williams K., Minkowski A., Amoabeng O., Peloquin C., Taylor D., Andries K., et al. (2012) Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother 56: 3114–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Working Group on New TB Drugs (2014) TB Drug Pipeline, August 2014. Available at: http://www.newtbdrugs.org/downloads/pipeline-slide/WGND_Global_TB_Drug_and_Discovery_Pipelines-AUG2014.ppt (accessed 31 October 2014).
- Zhang M., Sala C., Hartkoorn R., Dhar N., Mendoza-Losana A., Cole S. (2012) Streptomycin-starved Mycobacterium tuberculosis 18b, a drug discovery tool for latent tuberculosis. Antimicrob Agents Chemother 56: 5782–5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Li S., Williams K., Andries K., Nuermberger E. (2011) Short-course chemotherapy with TMC207 and rifapentine in a murine model of latent tuberculosis infection. Am J Respir Crit Care Med 184: 732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Mitchison D. (2003) The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis 7: 6–21. [PubMed] [Google Scholar]