Abstract
Background:
There is some evidence to suggest a possible association between calcium channel blocker (CCB) use and a lower decline in cognitive function compared with use of other hypertensive treatments. In particular, there is an emerging interest in the potential for specific CCBs, particularly the dihydropyridine CCBs nitrendipine, nicardipine, cilnidipine, lercandipine, nimodipine, azelnidipine and nilvadipine. The aim of this review was to assess the evidence relating to these specific CCBs and incident cognitive decline or dementia in humans.
Methods:
A systematic review of the literature was carried out. The databases MEDLINE, Embase and PsychINFO were searched from 1980 to 18 April 2014. All abstracts were reviewed by two independent reviewers.
Results:
From 753 unique records, 16 full text articles were examined and three retained. The three articles reported data from two studies. A 12-week double-blind randomized controlled trial of nitrendipine compared with cilazapril and a longer and larger double-blind placebo-controlled trial also of nitrendipine, namely the Systolic Hypertension in Europe trial (SYST-EUR). Nitrendipine was associated with a reduction in incident dementia in the SYST-EUR trial. There was no association seen for cognitive outcomes in the smaller trial.
Conclusion:
At present there is limited evidence to suggest that nitrendipine may be associated with reduction in incident dementia. This association comes from a single trial and needs to be replicated. Furthermore, there is no high-quality evidence for any of the other potential candidate CCBs.
Keywords: calcium antagonists, calcium channel blockers, cognitive decline, dementia, hypertension
Introduction
Published evidence suggests a possible association between calcium channel blocker (CCB) use and less decline in cognitive function compared with use of other hypertensive treatments [Fournieret al. 2009; Shah et al. 2009; Peters et al. 2014]. Yet clear evidence for benefit from this antihypertensive class remains elusive. Possible reasons for this include the use of different populations under study, the lack of controlled trials and preponderance of observational studies, differing lengths of follow up, and use of differing CCB types. The last point is potentially the critical factor given the strong results from the Systolic Hypertension in Europe trial (SYST-EUR), namely a 50% reduction in incident dementia, when a nitrendipine-based CCB therapy was compared with matching placebo [Forette et al. 1998, 2002].
Most observational studies do not report results by type of CCB, although some have reported a possible greater benefit for the dihydropyridine CCBs [Yasar et al. 2005]. Furthermore, animal studies have demonstrated differing levels of amyloid production and clearance with different dihydropyridines. Other potential mechanisms include roles for CCBs in perturbed calcium homeostasis protecting against calcium influx and subsequent apoptosis [Fournier et al. 2009; Bachmeier et al. 2011; Paris et al. 2011].
The importance of preventing or delaying cognitive decline or dementia is clear. There are potential biological mechanisms and animal studies that have identified specific dihydropyridine CCBs (nitrendipine, nicardipine, cilnidipine, lercandipine, nimodipine, azelnidipine and nilvadipine) as possibly beneficial in this regard [Bachmeier et al. 2011; Paris et al. 2011; Nimmrich and Eckert, 2013]. It is timely to examine the evidence base for these specific CCBs in humans whilst acknowledging that they are not currently among the most commonly prescribed.
The aim of this systematic review was to assess the extant evidence relating to these specific CCBs (nitrendipine, nicardipine, cilnidipine, lercandipine, nimodipine, azelnidipine and nilvadipine) and incident cognitive decline or dementia in humans.
Materials and methods
The search strategy was developed based on that published in a previous review [Peters et al. 2014] alongside expertise from evidence based infor-mation practice, medicine for older adults and cognitive and dementia outcomes. See Box 1 for full details.
Box 1.
Search strategy.
| (1) ((cognitive decline or cognitive impairment or cognitive function or dementia or alzheimers disease or Alzheimers disease or alzheimer disease or Alzheimer disease or vascular dementia or dementia vascular or multi infarct dementia or dementia multi infarct)).af. |
| (2) ((Balodipine or Balotein or Baylotensin or Bayniroad or Bayotensin or Baypresol or Baypress or Caltren or Cardiazem or cardif or Cenipres or Cobatensin or Crivion or Ditrenil or Dosperopin or Downtensine or Ecatelisin or Ellenal or Eneas or Enit or Farnitran or Gericin or Grifonitren or Hiperdipina or Hishiromin or Issopres or Jutapress or Leonitren or Lisba or Lostradyl or Lusopress or Miniten or Nelconil or Nian or Nidrel or Nifecard or Nilzipin or Niprina or Nirapel or Nitensum or Nitopress or Nitotelocin or Nitregamma or Nitren or Nitren-acis or Nitrenal or Nitrencord Nitrend KSK or Nitrendil or Nitrendilat or Nitrendipin or Nitrendipin - 1 A Pharma or Nitrendipina or Nitrendipin-corax or Nitrendipin-CT or Nitrendipine or Nitrendipine - Pacific Pharm or Nitrendipine-Xinhua Pharm or Nitrendipino or Nitrendipin-ratio pharm or Nitrendipinum or Nitrendypina or Nitrensal or Nitrepin or Nitrepress or Nitre-Puren or Nitresan or Nitrezic or Nivitron or Potional or Pressodipin or Ravena or Shetlazorna or Shu Mai Te or Spidox or Tensogradal or Tepanil or Ufocard or Unipres or Unipress or Vastensium or Vipres or Nicardipine or Cardene or Cardene IV or Cardene SR or Cilnidipine or Cilacar or Cilnidipin or Cilnidipino or Atelec or Cinalong or Jiuyue or Xi Le Zhi Xin or Lercanidipine or Landip - 10 or Larpin or Lerez or Lerka or Lotensyl or Lercanidipin or Lercanidipino or Masnidipine or Anadip or Coripren or Lercanil or Lercapress or Lerpin or Zanadipine or Zanidip or Areta or Canider or Carbimen or Cardiapin or Cardiovasc or Carmen or Corifeo or Ercapin or Evipress or Karnidin or Konidip or Landip or Larcadip or Larcan or Lecadin or Lecadipine or Lecalpin or Lecalpine or Lecanipin or Lecard or Lecramed or Ledipin or Lenidipine or Lercadip or Lercamen or Lercan or Lercanidipina or Lercanidipin-HCl or Lercanidipino or Lercanil or Lercapin or Lercaprel or Lercapress or Lercapril or Lercaril or Lercaton or Lerdip or Lerka or Lerkamen or Lerpin or Lervasc or Lerzam or Licardipine or Lisitens or Locadine or Oktava or Pinox or Primacor or Renovia or Vasodip or Zaneril or Zanextra or Zan-Extra or Zanica or Zanicombo or Zanicor or Zanidip-Recordati or Zanipress or Zanipril or Zanitek or Zircol or Nimodipine or Nimotop or Nymalize or Modipin or Modipine or Nimocer or Nimodip or Nimotide or Vasotop or Azelnidipine or Azelnidipin or Azelnidipino or Beiqi or Calblock or Nilvadipin or Nilvadipino or Nivadipine or Nilvadipine or Escor or Naftdil or Nildilart or Nivadil or Tensan or Towajil)).af. |
| (3) (1) and (2) |
| (4) Limit (3) to (english language and humans and yr = ‘1980-Current’) |
The databases MEDLINE, Embase and PsychINFO were searched from 1980 to 18 April 2014. Details of the search strategies are given in Box 1. Reference lists of all papers identified were screened for other published papers. Searches were carried out for any ongoing relevant trials using the following sources:
Cochrane Controlled Trials Register (CENTRAL) from 1980 (in order to capture CCB use) to date of search.
ISRCTN Register - trials registered with a unique identifier.
ClinicalTrials.gov (http://www.ClinicalTrials.gov).
All identified abstracts, or titles for which abstracts were unavailable, were independently read and a list of potentially relevant papers compiled by each of the two analysts. The two lists of articles for potential inclusion was compared and any differences resolved by discussion. Full copies of candidate articles were independently read and assessed for relevance by both analysts. Any differences in agreement were resolved by discussion until the final set was agreed. A standard data extraction form was used, which included details of the patients, the interventions and the outcomes. Both analysts completed the data extraction.
Those articles identified as relevant were also independently assessed for quality and the data extracted by each analyst. A formal scoring scheme was not used to assess the quality of each paper in terms of its validity as existing scales are not sufficiently discriminatory. Instead the quality of reporting for each paper was assessed against the key factors given in the Quality of Reporting of Observational Longitudinal Research criteria [Tooth et al. 2005] and a table produced of the findings from this assessment. The Cochrane Risk of Bias methods (www.ncbi.nlm.nih.gov/pmc/articles/PMC3196245/pdf/bmj.d5928.pdf) and CONSORT (both for randomized controlled trials) and STROBE checklists (for observational studies) were used when appropriate. Finally, summary tables were prepared for quality assessment and outcomes data. The results of the identified studies were described and summarized using techniques of narrative synthesis, and subjected to further critique. An assessment for potential publication bias was to be performed using a funnel plot should the numbers of included studies make this feasible. Full inclusion and exclusion criteria are as follows.
Inclusion criteria
Longitudinal studies or trials of antihypertensives that include analysis of one of the target CCB types identified in the literature as having a potentially beneficial impact on cognitive function (nitrendipine, nicardipine, cilnidipine, lercandipine, nimodipine, azelnidipine and nilvadipine) and a comparator group.
Some evidence, or a clear implication within the published report, that participants were free of cognitive decline or dementia at baseline assessment.
Use of formal assessment of cognitive function.
Report of cognitive decline (defined as any fall in performance on cognitive or neuropsychological tests) or dementia outcomes.
Adults (aged 18 or over).
Exclusion criteria
Cognitive assessment in postoperative, human immunodeficiency virus, electroconvulsive therapy, psychoactive drug user or cancer populations.
Non-English publications (there were no resources available for translation).
Results
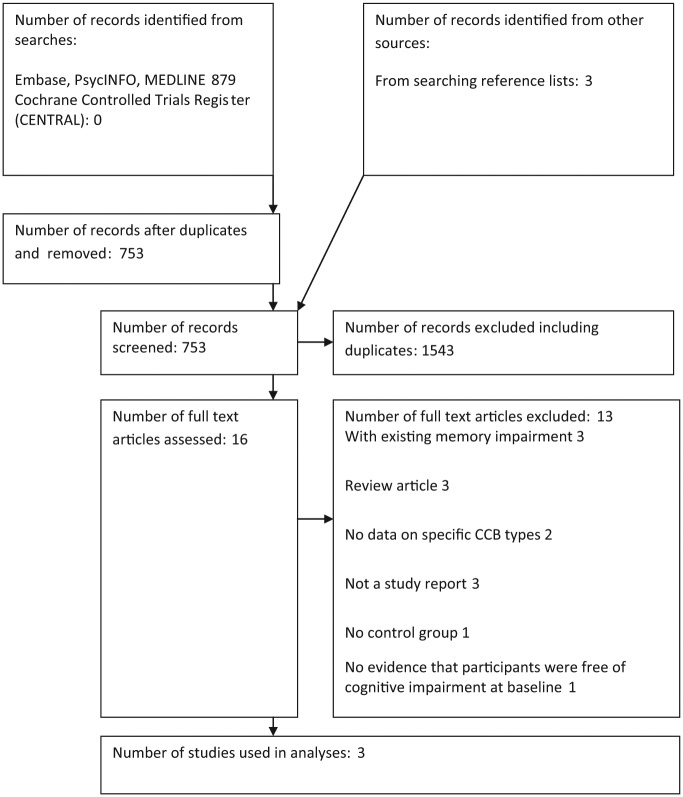
A total of 879 records were identified via searching Embase, PsycINFO and MEDLINE, and a further three were identified from reference lists. A total of 753 unique records were screened. Sixteen full text articles were examined. Only three articles were retained following comparison against the inclusion criteria. Articles were rejected at the full-text stage due to a variety of factors: three articles reported studies in those with existing memory impairment [Herrman et al. 1991; Jacobsen et al. 2001; Denolle et al. 2002], three were review articles [Frishman, 2002; Duron and Hanon, 2010, Chang-Quan et al. 2011], two provided no data on specific CCB types [Khactaturian et al. 2006; Trompet et al. 2008], three were not study reports (two reported on drug structure and one provided comment on mouse models) [Nimodipine, 1994, 1995; Van Der Stay et al. 1994], one had no control group [Tissaire-Sánchez et al. 2006], and in one there was no evidence that participants were free of cognitive impairment at baseline [Schrader et al. 2005]. See Figure 1 for details.
Figure 1.
Flow chart. CCB, calcium channel blocker.
Of the three articles that were retained, two related to the Systolic Hypertension in Europe (SYST-EUR) trial and one reported results of another clinical trial. SYST-EUR reported the results of a double-blind placebo-controlled trial [Forette et al. 1998] (SYST-EUR trial) and, extending this, an open-label follow-up cohort study [Forette et al. 2002] (SYST-EUR extension). Given differences in study design and for ease of understanding, the trial and its extension have been treated as separate studies. The other study was also a randomized double-blind trial and compared two actively treated arms [Leonetti et al. 1994].
Study characteristics
All three of the articles reported results for use of nitrendipine: the Leonetti trial (nitrendipine versus cilazapril) [Leonetti et al. 1994]; the SYST-EUR trial (nitrendipine versus placebo) [Forette et al. 1998]; and the SYST-EUR open follow up in which all participants were given access to open-label nitrendipine with analysis based on a comparison of the former placebo and former active groups [Forette et al. 2002]. See Table 1 for full details. Trial/study size and length of follow up differed across the studies. The SYST-EUR trial recruited 2418 participants across Europe and followed them for a median of 2 years with the open-label extension extending the follow-up time to an overall median of 3.9 years [Forette et al. 1998, 2002]; the Leonetti trial, based in Italy, recruited 114 participants from nine centres for a trial lasting 12 weeks [Leonetti et al. 1994]. The mean age of participants was similar across studies, ranging from 67 in the cilazapril arm of the Leonetti trial, 69 [Leonetti et al. 1994] and 69.9 [Forette et al. 1998] in the CCB-treated arms of the Leonetti trial and the SYST-EUR trial respectively and 69.9 in the SYST-EUR placebo group [Forette et al. 1998]. The SYST-EUR open-label follow-up study reported a median age of 68 [Forette et al. 2002]. Women were in the majority both in the SYST-EUR trial (65% in the placebo and 66% in the nitrendipine group) [Forette et al. 1998] and in the Leonetti trial (62% in the cilazapril and 59% in the nitrendipine group) [Leonetti et al. 1994].
Table 1.
Study populations.
| Author | Design | Antihypertensive drug | Source of subjects | Population | Age at baseline | Length of follow up | % female at baseline | Systolic/diastolic blood pressure (SBP/DBP) at baseline | |
|---|---|---|---|---|---|---|---|---|---|
| [Forette et al. 1998] | Randomized double-blind placebo-controlled trial. The Systolic Hypertension in Europe Trial (SYST-EUR) | Nitrendipine 10–40 mg/day combined with or replaced by enalapril 5–20 mg/day or hydrochlorothiazide 12.5–25 mg/day or both versus matching placebos | ⩾60 years with hypertension. | n = 2418 | n = 1861 in double-blind at final visit + 303 in open follow up | Placebo 69.9 (SD 6.2), active 69.9 (SD 6.5) | Median 2.0 years | Placebo 767 (65%), active 822 (66.4%) | Placebo SBP 173.4 (SD 10.1)/DBP 86.0 (SD 5.7) |
| Recruited from primary and secondary care centres in 19 European countries | Active SBP 173.5 (SD 10.1)/DBP 86.1(SD 5.6) | ||||||||
| [Forette et al. 2002] | Open-label extension of SYST-EUR | Nitrendipine 10–40 mg/day combined with or replaced by enalapril 5–20 mg/day or hydrochlorothiazide 12.5–25 mg/day or both | Primary and secondary care centres in 19 European countries | n = 2908 analysed1417 originally randomized to placebo | Number at final visit not reported | Median 68 (60–92) | Median (original trial and open-label extension reported together) 3.9 years (interquartile range 2.8–5.6) | Not reported at start of open label extension | Former placebo group 156.1 (SD 12)/82.5 (SD 6) |
| Former active group 149.1 (SD 9.7)/79.4 (SD 6.1) from final trial visit before extension | |||||||||
| [Leonetti et al. 1994] | Randomized double-blind trial | Nitrendipine 10–20m mg/day versus cilazapril 2.5–5 mg/day | ‘Moderately hypertensive elderly patients’ recruited from nine Italian centres | n = 114 | ~103 (not reported in detail) | Cilazapril 67 (SD 0.9), nitrendipine 69 (0.8)Overall mean 68 (range 60–84) | 12 weeks | Cilazapril 62%, nitrendipine 59% | Cilazapril 170 (SD 1.4)/100 (SD 0.6) |
| Nitrendipine 170 (SD 1.7)/101 (SD 0.6) | |||||||||
Assessment of cognitive function
All studies used standard measures of cognitive assessment. See Table 2. The SYST-EUR trial used a cognitive screening test, the Mini-Mental State Examination (MMSE), at annual intervals and collected information on diagnosis of dementia if either the MMSE score or other indications warranted this [Forette et al. 1998, 2002]. The Leonetti trial used a mixture of neuropsychological tests, including assessment of memory and attention and assessed participants at baseline, 4 and 12 weeks [Leonetti et al. 1994].
Table 2.
Assessment of cognitive function/dementia.
| Author | Assessment of cognitive function at baseline | Assessment of cognitive function at follow up |
|---|---|---|
| [Forette et al. 1998] | If participants scored⩽23 on the MMSE or the patient or relative reported appropriate symptoms or the local investigator observed clinical signs or if the MMSE was not administered then further examination was carried out | Annual MMSE assessments were carried out; if participants scored ⩽23 on the MMSE or the patient or relative reported appropriate symptoms or the local investigator observed clinical signs or if the MMSE was not administered then further examination was carried out and diagnoses made via: |
| DSMIIIR plus Modified Ischaemic Score. If CT scan imaging not available Hachinski score used | DSMIIIR plus Modified Ischaemic Score. If CT scan imaging not available Hachinski score used. All cases validated by a review board blinded to treatment allocation and all CT scans reviewed by two independent neuroradiologists | |
| All cases validated by a review board blinded to treatment allocation and all CT scans reviewed by two independent neuroradiologists | ||
| [Forette et al. 2002] | If participants scored ⩽23 on the MMSE or the patient or relative reported appropriate symptoms or the local investigator observed clinical signs or if the MMSE was not administered then further examination was carried out and diagnoses made via DSMIIIR plus Modified Ischaemic Score. If CT scan imaging not available Hachinski score was used. All cases validated by a review board blinded to treatment allocation and all CT scans reviewed by two independent neuroradiologists | |
| [Leonetti et al. 1994] | Toulouse Pieron (to assess attention) | Repeated assessments at 4 and 12 weeks |
| Rey* immediate and delayed recall (to assess memory) | ||
Not explicitly stated but most likely to be the Rey Auditory Verbal Learning Test.
CT, computed tomography; DSMIIIR, Diagnostic and Statistical Manual of Mental Disorder, third revised edition; MMSE, Mini-Mental State Examination.
Outcome
The SYST-EUR trial reported a reduction in incident dementia cases associated with nitrendipine-based active treatment with rates reported at 7.7/1000 patient years in the placebo and 3.8/1000 patient years in the actively treated group [Forette et al. 1998]. This finding was supported by analysis of the open-label extension which reported a hazard ratio of 0.43 (95% confidence interval 0.25–0.74) in favour of the previously treated group [Forette et al. 2002]. Leonetti and colleagues reported no difference between their randomized groups [Leonetti et al. 1994]. See Table 3 for details.
Table 3.
Analyses and adjustments from the included studies.
| Author | Main outcome | Adjusted results | Adjusted for |
|---|---|---|---|
| [Foretteet al. 1998] | Dementia | Placebo treatment 7.7/1000 and active treatment 3.8/1000 patient year | Not reported for main outcome, however sex, age at leaving school, systolic blood pressure, previous cardiovascular complications, previous antihypertensive treatment, atrial fibrillation, smoking and alcohol consumption were not identified as independent predictors of dementia |
| Stepwise Cox proportional hazard regression for incident dementia, active compared with placebo treatment | |||
| Intention to treat analysis HR 0.50 (95% CI 0.0–0.76) | |||
| Per protocol analysis HR 0.40 (95% CI 0.02–0.83) | |||
| [Foretteet al. 2002] | Dementia (results also given for AD and vascular/mixed dementia) | Cox proportional hazard regression former active versus former placebo group for incident dementia | Age or age as a time-dependent covariate, diastolic blood pressure, sex and level of education |
| HR 0.43 (95% CI 0.25–0.74) in favour of active treatment | |||
| For AD 12 cases in the former active group versus 29 cases in the former placebo group, total number of dementia cases was 21 in the former active and 43 in the former placebo group | |||
| Cox proportional hazard regression with nitrendipine as a time-dependent variable, comparison of former active and former placebo groups, for incident dementia | |||
| HR 0.38 (95% CI 0.23–0.64) in favour of active treatment | |||
| [Leonettiet al. 1994] | Change in cognitive test scores | Reports that the scores on the psychometric tests were normal at baseline and that no significant changes were seen during treatment | None reported |
AD, Alzheimer’s disease; HR, hazard ratio; CI, confidence interval.
Quality assessment
All three reports presented a clear hypothesis and reported appropriate, although not ideal, study design; two of the three cases used a randomized controlled trial design in order to understand the impact of a trial drug [Leonetti et al. 1994; Forette et al. 1998]. Both of the trials were double blind; one with matching placebos [Forette et al. 1998] and the other with an active drug comparator [Leonetti et al. 1994] and both gave details of drugs and doses. The remaining paper reported an open-label extension of the SYST-EUR study using a cohort study design. This report provided supplementary information for the main SYST-EUR results rather than standing alone as a robust observational study [Forette et al. 2002]. Selection of the population was less clear. Despite this, the mean/median age of patients within each study was similar and represented an externally valid group of older adults, that is, patients who can reasonably be expected to show some cognitive change. In addition, the entry blood pressure was high and matched across treatment groups for SYST-EUR and Leonetti and the percentage of women was similar [Leonetti et al. 1994; Forette et al. 1998, 2002]. Details of a priori power calculations are only given for the SYST-EUR trial [Forette et al. 1998, 2002]. Full details of study recruitment practices, numbers approached, who were eligible and consented were not given, although the number available at each visit is given for SYST-EUR [Forette et al. 1998, 2002]. Leonetti and colleagues report adherence to be high but do not comment on attrition [Leonetti et al. 1994]. The period of follow up varied; Leonetti had the shortest duration at 12 weeks [Leonetti et al. 1994], whereas SYST-EUR reported a median follow up of 2 years for the double-blind trial and a total median follow up of 3.9 years at the end of the open-label extension [Forette et al. 1998, 2002]. It should be noted that the double-blind phase of the SYST-EUR trial was stopped early due to a positive finding for cardiovascular outcomes. This meant that it was no longer possible to achieve further follow up [Forette et al. 2002]. Clearly the double-blind follow up was of sufficient duration to accrue some dementia cases. However, dementia cases were few and the same pattern was observed in those cases carried forward when the open-label extension was added into the analysis [Forette et al. 1998, 2002]. Leonetti and colleagues did not attempt to assess incident dementia but did assess cognitive change using standard assessment tools. It is unclear whether the short follow up, repeated measures and potential practice effects influenced the findings from Leonetti and colleagues. There is a lack of information relating to ways that these issues may have been handled or providing detail of the statistical tests used [Leonetti et al. 1994]. Furthermore Leonetti and colleagues do not report adjustment for potential confounding variables [Leonetti et al. 1994]. In contrast, the SYST-EUR trial does detail adjustment and reports the use of a Cox proportional hazard regression analysis [Forette et al. 1998, 2002]. This analysis takes time to event into account and is widely used to analyse clinical trials. However, the value of this test for an outcome such as dementia is questionable given the condition’s insidious onset over time. See Table 4 for details of quality assessment.
Table 4.
Assessment of study quality.
| Author | Clearly focused issue | Appropriate method to answer question | Recruitment bias | Exposure bias (intervention) | Bias in outcome measurement | Bias in assessment of confounders | Bias in follow up – patient loss | Bias in follow up – length | Randomization method | Concealment of allocation | Blinding allocation | Blinding of outcome assessment | Attrition bias | Selective outcome reporting bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [Forette et al. 1998] | Yes | Yes | Yes | No | No | No | Unknown | No | OK | Yes | Yes | Yes | Yes | Unknown |
| [Forette et al. 2002] | Yes | Yes | Yes | Unknown | No | No | Unknown | No | N/A not a clinical trials | |||||
| [Leonetti et al. 1994] | Yes | Yes | Yes | No | Unknown | Unknown | No | No | Unknown | Unknown | Yes | Unknown | Yes | Unknown |
N/A, not applicable.
Publication bias
This review identified too few studies to assess publication bias; however, the results reflect both positive and neutral findings.
Discussion
Overall the quality of the eligible studies can be described as adequate with only one study reporting a double-blind controlled trial, unavoidably stopped early having achieved a positive result for their primary cardiovascular outcome, resulting in very few secondary outcome (dementia) cases accrued. Although the studies fell short against recognized quality assessment standards, all three reports were published prior to current reporting requirements for clinical trials (CONSORT) (http://www.consort-statement.org/) and observational studies (STROBE) (http://www.strobe-statement.org/). Furthermore a focus on database sources of peer-reviewed journal articles may have meant that we overlooked results present in the grey literature, non-English language literature or published before 1980. Despite these limitations, 16 full text articles were examined and three identified as eligible and extracted. Logistic constraints meant that individual patient data were not requested.
Examination of the literature eligible for this systematic review and relating to the specific CCBs nitrendipine, nicardipine, cilnidipine, lercandipine, nimodipine, azelnidipine and nilvadipine reveals limited evidence in favour of nitrendipine. However, we were unable to identify evidence, either in favour or against, for any other of the specific CCB types. The evidence in favour of nitrendipine is dominated by the SYST-EUR trial and its open follow up with one other small open-label trial reporting no impact of nitrendipine versus cilazapril over a shorter 12-week follow up. Placing these results in the context of our previous review [Peters et al. 2014], which examined the class effect of CCB use and incident cognitive decline or dementia, looking at studies with at least one year of follow up, we find that this review adds only one additional study. Indeed this review further serves to highlight notable gaps in the literature. Animal studies suggest that several specific CCBs have the potential to make a positive impact on cognitive function but human studies looking at incident change are lacking.
Of the CCBs determined by Paris and colleagues [Paris et al. 2011] and Bachmeier and colleagues [Bachmeier et al. 2011] as having a potentially positive impact on cognitive function in animal data, only nitrendipine has been examined appropriately in humans with regard to incident decline/dementia, primarily via the SYST-EUR trial [Forette et al. 1998, 2002]. Additional evidence from a Cochrane review favours nimodipine but only for the treatment of dementia [Birks et al. 2002]. Both the widely used amlodipine and nifedipine were not found to be beneficial in animal studies [Bachmeier et al. 2011; Paris et al. 2011] and so were not examined here. However, nifedipine at least has been associated with an increased risk of cognitive decline in the Canadian Study of Health and Ageing [Maxwell et al. 1999]. Furthermore, nifedipine performed worse than both placebo and atenolol in a small double-blind trial reported in 1992 [Skinner et al. 1992].
Given the limited published evidence available, any therapeutic benefit for specific dihydropyridine CCBs in preventing or delaying cognitive decline can be neither proved nor disproved. It would be incorrect to conclude that CCBs are unlikely to offer significant therapeutic benefit given that the large double-blind placebo-controlled SYST-EUR trial found a significant reduction in dementia cases despite stopping earlier than planned due to cardiovascular benefit. As yet the result from this single trial has not been tested further. Given animal data suggesting that nitrendipine may be one of a handful of CCBs that confer such benefit we feel that there is a clear justification for further studies examining the relationship between specific CCBs and cognitive decline.
Future studies must also ensure appropriate selection of CCBs as well as maintaining awareness of other important issues such as high-quality study design, blood pressure control, population selection and follow up of sufficient length to accrue appropriate outcomes.
Given the ageing population and associated rise in the number of cases of cognitive decline or dementia, it is clearly important to explore the possibilities for the repurposing of existing drugs, such as those used to manage hypertension, when such potential exists. For the present we have further established an important gap in the literature with regard to specific CCBs and their impact on cognitive outcomes.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors report no conflict of interest.
Contributor Information
Jean Peters, School of Health and Related Research (ScHARR). The University of Sheffield, Regent Court, Sheffield S1 4DA, UK.
Andrew Booth, School of Health and Related Research (ScHARR). The University of Sheffield, Regent Court, Sheffield S1 4DA, UK.
Ruth Peters, School of Public Health, Imperial College London, St Mary’s Campus, London W2 1PG, UK.
References
- Bachmeier C., Beaulieu-Abdelahad D., Mullan M., Paris D. (2011) Selective dihydropyiridine compounds facilitate the clearance of β-amyloid across the blood–brain barrier. Eur J Pharmacol 659: 124–129. [DOI] [PubMed] [Google Scholar]
- Birks J., López-Arrieta J. (2002) Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst Rev (3): CD000147. [DOI] [PubMed] [Google Scholar]
- Chang-Quan H., Hui W., Chao-min W., Zheng-Rong W., Jun-Wen G., Yong-Hong L., et al. (2011) The association of antihypertensive medication use with risk of cognitive decline and dementia: a meta-analysis of longitudinal studies. Int J Clin Pract 65: 1295–1305. [DOI] [PubMed] [Google Scholar]
- Denolle T., Sassano P., Allain H., Bentué-Ferrer D., Breton S., Cimarosti I., et al. (2002) Effects of nicardipine and clonidine on cognitive functions and electroencephalography. Fundam Clin Pharmacol 16: 527–535. [DOI] [PubMed] [Google Scholar]
- Duron E., Hanon O. (2010) Antihypertensive treatments, cognitive decline, and dementia.J Alzheimers Dis 20: 903–914. [DOI] [PubMed] [Google Scholar]
- Forette F., Seux M., Staessen J., Thijs L., Babarskiene M., Babeanu S., et al. (2002) The prevention of dementia with antihypertensive treatment. Arch Intern Med 162: 2046–2052. [DOI] [PubMed] [Google Scholar]
- Forette F., Seux M., Staessen J., Thijs L., Birkenhäger W., Babarskiene M., et al. (1998) Prevention of dementia in a randomised double blind placebo controlled systolic hypertension in Europe (Syst-Eur) trial. Lancet 352: 1347–1351. [DOI] [PubMed] [Google Scholar]
- Fournier A., Oprisiu-Fournier R., Serot J., Godefroy O., Achard J., Faure S., et al. (2009) Prevention of dementia by antihypertensive drugs: how AT1-receptor-blockers and dihydropyridines better prevent dementia in hypertensive patients than thiazides and ACE-inhibitors. Expert Rev Neurother 9: 1413–1431. [DOI] [PubMed] [Google Scholar]
- Frishman W. (2002) Are antihypertensive agents protective against dementia? A review of clinical and preclinical data. Heart Dis 4: 380–386. [DOI] [PubMed] [Google Scholar]
- Herrman W., Stephan K. (1991) Efficacy and clinical relevance of cognition enhancers. Alzheimer Dis Assoc Disord 5(Suppl. 1): S7–S12. [DOI] [PubMed] [Google Scholar]
- Jacobsen E., Salehmoghaddam S., Dorman J., Land S., Back C., Barrio J. (2001) P-79: the effect of blood pressure control on cognitive function (the Focus study). Am J Hypertens 14: 55A. [Google Scholar]
- Khactaturian A., Zandi P., Lyketsos C., Hayden K., Skoog I., Norton M., et al. (2006) Antihypertensive medication use and incident Alzheimer disease. Arch Neurol 63: 686–692. [DOI] [PubMed] [Google Scholar]
- Leonetti G., Salvetti A. on behalf of participating centres (1994) Effects of cilazapril and nitrendipine on blood pressure, mood, sleep, and cognitive function in elderly hypertensive patients: an Italian multicenter study. J Cardiovasc Pharmacol 24(Suppl. 3): S73–S77. [PubMed] [Google Scholar]
- Maxwell C., Hogan D., Ebly E. (1999) Calcium-channel blockers and cognitive function in elderly people: results from the Canadian study of health and aging. CMAJ 161: 501–506. [PMC free article] [PubMed] [Google Scholar]
- Nimmrich V., Eckert A. (2013) Calciumchannel blockers and dementia. Br J Pharmacol 169: 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimodipine (1994) Drugs Fut 1: 193–194. [Google Scholar]
- Nimodipine (1995) Drugs Fut 20: 203–205. [Google Scholar]
- Paris D., Bachmeier C., Patel N., Quadros A., Volmar C., Laporte V., et al. (2011) Selective antihypertensive dihydropyridines lower Aβ accumulation by targeting both the production and the clearance of Aβ across the blood brain barrier. Mol Med 17: 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Booth A., Peters J. (2014) A systematic review of calcium channel blocker use and cognitive decline/dementia in the elderly. J Hypertens 32: 1945–1958. [DOI] [PubMed] [Google Scholar]
- Schrader J., Lüders S., Kulschewski A., Hammersen F., Plate K., Berger J., et al. (2005) Morbidity and mortality after stroke, eprosartin compared with nitrendipine for secondary prevention: principal results of a prospective randomised controlled study. Stroke 36: 1218–1224. [DOI] [PubMed] [Google Scholar]
- Shah K., Qureshi S., Johnson M., Parikh N., Schulz P., Kunik M. (2009) Does use of antihypertensive drugs affect the incidence or progression of dementia? A systematic review. Am J Geriatr Pharmacother 7: v250–v261. [DOI] [PubMed] [Google Scholar]
- Skinner M., Futterman A., Morrissette D., Thompson L., Hoffman B., Blaschke T. (1992) Atenolol compared with nifedipine: effect on cognitive function and mood in elderly hypertensive patients. Ann Intern Med 116: 615–623. [DOI] [PubMed] [Google Scholar]
- Tissaire-Sánchez J., Roma J., Camacho-Azcargorta I., Bueno-Gómez J., Mora-Maciá J., Navarro A. (2006) Assessment of cognitive function in patients with essential hypertension treated with lercanidipine. Vasc Health Risk Manag 2: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooth L., Ware R., Bain C., Purdie D., Dobson A. (2005) Quality of reporting of observational longitudinal research. Am J Epidemiol 161: 280–288. [DOI] [PubMed] [Google Scholar]
- Trompet S., Westendorp R., Kamper A., Craen A. (2008) Use of calcium antagonists and cognitive decline in old age. The Leiden 85-plus study. Neurobiol Aging 29: 306–308. [DOI] [PubMed] [Google Scholar]
- Van Der Stay F., Freund W., Gebert I. (1994) Cognition enhancing antidegenerative and neuroprotective effects of nimodipine. Behav Pharmacol 5: 46. [Google Scholar]
- Yasar S., Corrada M., Brookmeyer R., Kawas C. (2005) Calcium channel blockers and risk of AD: the Baltimore longitudinal study of aging. Neurobiol Aging 26: 157–163. [DOI] [PubMed] [Google Scholar]