Abstract
Objective
We sought to determine the primary care-based prevalence of moderate-to-severe atopic eczema/dermatitis in children and to estimate what proportion had co-morbid aero-allergy and/or food allergy that was contributing to their atopic eczema/dermatitis.
Design
Multi-centre, cross-sectional study.
Participants
Infants, children and young people aged between 0–17 years.
Setting
Primary Care.
Methods
General practice electronic health records were interrogated to identify children (0–17 years) with current moderate-to-severe atopic eczema/dermatitis. Eligible children were assessed by an allergy specialist nurse, this involving a detailed allergy history, examination and, if appropriate, measurement of total IgE and specific IgE to relevant aero-allergens and/or food allergens.
Main outcome measures
Prevalence of atopic eczema, moderate to severe atopic eczema, IgE-mediated atopic eczema.
Results
We recruited eight practices, which together enrolled 16,877 children. Of these, 4331 (25.7%; 95% CI 25.0, 26.3) children had a recorded diagnosis of atopic eczema/dermatitis and 1316 (7.8%; 95% CI 7.4, 8.2) had treatment indicative of current moderate-to-severe atopic eczema/dermatitis. We recruited 159 children for clinical assessment, and complete data were available for 157. The clinical assessment revealed that 130/157 (82.8%) had no indication of IgE-mediated allergy contributing to their atopic eczema/dermatitis; the remaining 27/157 (17.2%; 95% CI 12.1, 23.9) were on clinical assessment considered to possibly have underlying IgE-mediated disease. Specific IgE tests were positive in 14/27 (51.9%; 95% CI 34.0, 69.3) children. Of the 14 children who tested positive, six (42.9%; 95% CI 21.4, 67.4) were positive to food allergens and six (42.9%; 95% CI 21.4, 67.4) to aero-allergens; the remaining two (14.3%; 95% CI 4.0, 40.0) were positive to both food and aero-allergens.
Conclusions
Although atopic eczema/dermatitis is a very common diagnosis in children in primary care, most appear to be relatively mild and/or transient. Only a small proportion of children had evidence of ongoing underlying IgE-mediated atopic eczema/dermatitis.
Keywords: Atopy, children , eczema, epidemiology, general practice
Introduction
The prevalence of allergic diseases has increased very considerably over recent decades in the Western world to the extent that recent estimates indicate 50% of children will be affected by an allergic condition at some point in their lifetime.1 In the UK, the majority of these children will be managed predominantly within a primary care setting, with only those with particularly severe and/or disabling disease being referred to specialist services.2 Access to allergists is known to be poor in many parts of the UK.3
Atopic eczema/dermatitis is one of the most common manifestations of allergy in early life. Although mild and transient in many children, in some individuals the disease follows a more aggressive course; the atopic eczema/dermatitis is in such children typically early in onset, difficult to manage with simple advice and emollients, and more persistent than the atopic eczema/dermatitis experienced by the majority of children, often heralding the onset of other allergic disorders.4 Filaggrin gene defects are an important precursor, which may increase the risk of allergic sensitisation.5,6 It is this group of children with moderate-to-severe atopic eczema/dermatitis in whom underlying allergy to foods (e.g. cow’s milk and hen’s eggs) and/or inhalant aero-allergens (e.g. house dust mite, pollens and pets) may be particularly important and who may therefore benefit from appropriate allergen avoidance advice and allergen-specific therapies in order to improve outcomes and reduce the risk of disease progression.7
A major evidence-gap in knowing how best to deliver the systematic assessment of those with more troublesome atopic eczema/dermatitis advocated by national guidelines is knowing what proportion of children seen in community settings with atopic eczema/dermatitis are likely to have underlying allergies.8 The current very high estimates, which indicate that approximately one-third of all children with moderate-to-severe atopic eczema/dermatitis have an underlying allergic trigger, are derived from specialist care settings raising the possibility that selection biases are likely to substantially inflate prevalence estimates.9–11 We, therefore, sought to estimate the prevalence of moderate-to-severe atopic eczema/dermatitis in children in primary care and estimate what proportion of these children had underlying aero- and/or food allergy.
Methods
Overview of methods
We undertook a multi-centre, cross-sectional study in general practice which involved the interrogation of electronic health records and detailed clinical assessment of children (aged 0–17 years) with likely moderate-to-severe atopic eczema/dermatitis. This involved taking a detailed clinical history, physical examination and, if appropriate, specific IgE testing.
Ethical considerations
This work was undertaken with research ethics committee approval from South East Scotland Research Ethics Committee 1, and research management approvals were obtained from participating practices. Consent was obtained from all parents of participating children and accompanying assent of young people who the research nurse considered able to comprehend the nature of the study; this decision was made through discussion with parents of participating children.
Recruitment of practices
We wrote to general practices inviting them to participate in this study, and this was then followed up with a phone call and site visit to those who expressed an interest in participating. Practices were offered a modest honorarium (£250) to support their involvement in this study.
Recruitment of patients
Children aged ≤17 years with a clinician-recorded diagnosis of atopic eczema/dermatitis were initially identified through interrogation of GP records by practice staff using searches based on a sensitive set of READ codes. We then identified those with evidence indicative of active, moderate-to-severe atopic eczema/dermatitis; this being defined as those requiring topical corticosteroid use and/or immune-modulatory treatments in the preceding 12-month period. Those who were: aged ≥18 years; had atopic eczema/dermatitis which was no longer active or had mild atopic eczema/dermatitis controlled with emollients; unable to give informed parental/child consent/assent; or not willing for child/young person to have a blood test (if indicated) were ineligible for inclusion.
The parents of potentially eligible patients were sent a letter from their GP inviting them to participate in the study and an accompanying study information sheet and consent/assent form(s). Patients who confirmed that they met the eligibility criteria and were interested in participating were then seen by a trained allergy nurse in their local practice.
Patient assessment
A detailed allergy/clinical history and examination were undertaken in all recruited children by the study nurse; the findings being documented onto a structured study pro-forma (see Appendix 1). If the clinical assessment was suggestive of underlying IgE-mediated allergy to aero-allergens and/or foods contributing to the atopic eczema/dermatitis, then appropriate in vitro specific IgE testing (immunoCAP) was undertaken to the suspected allergens together with a measure of total IgE to aid interpretation. All samples were analysed by The Doctors Laboratory, London, UK (www.tdlpathology.com/). The results of any blood tests undertaken were subsequently communicated to the research team, the families of participating children and their GPs.
If the clinical picture was suggestive of non-IgE-mediated food allergy, then this was recorded and communicated to participating families and GPs, but no further investigations were undertaken.
If the clinical picture was not indicative of allergy-triggered atopic eczema/dermatitis, then this was communicated to the parent/patient and also to their GP.
Data handling and analysis
Data were entered into an Excel spreadsheet and then transferred to SPSS, and simple count and descriptive analyses were undertaken to provide prevalence estimates of the percentages and 95% confidence intervals (CIs) of children with moderate-to-severe atopic eczema/dermatitis, and the proportion of these children with evidence of IgE-mediated allergy to foods and/or aero-allergens.
Sample size considerations
No formal sample size calculations were undertaken for this prevalence study. We however a priori aimed to recruit 7–10 group general practices with a combined list size of 75,000–100,000 patients, as we estimated that this would give us the numbers needed to have reasonable precision in our prevalence estimates.
Results
We wrote to 39 general practices distributed throughout North Essex, England inviting them to participate in this study. Of these, eight were recruited with a combined list size of 85,164 patients. The main characteristics of these practices are summarised in Table 1.
Table 1.
Characteristics of recruited practices.
| Practice reference number | Practice postcode | Software system used | Number of partners (Whole time equivalent) | List size | Number of patients aged ≤17 years |
|---|---|---|---|---|---|
| 1 | CO2 9LA | SYSTEM1 | 3 | 5800 | 1867 |
| 2 | CO3 8NZ | EMIS PCS LAN | 3 | 5900 | 950 |
| 3 | CO9 1EX | SYSTEM1 | 10 | 15,887 | 2996 |
| 4 | CO11 1AA | EMIS WEB | 3 | 4925 | 871 |
| 5 | CO15 1DA | EMIS WEB | 8 | 13,651 | 2195 |
| 6 | CO4 5LE | SYSTEM1 | 8 | 11,614 | 2876 |
| 7 | CO3 4LN | SYSTEM1 | 8 | 15,101 | 2750 |
| 8 | CM3 3DX | VISION | 5 | 12,286 | 2372 |
Prevalence of atopic eczema/dermatitis and current moderate-to-severe atopic eczema/dermatitis
There were 16,877 children aged ≤17 years registered in the participating practices. Of these, 4331 (25.7%; 95% CI 25.0, 26.3) children had a recorded diagnosis of atopic eczema/dermatitis and 1316 (7.8%; 95% CI 7.4, 8.2) had treatment indicative of current moderate-to-severe atopic eczema/dermatitis, respectively.
Prevalence of underlying aero-allergy and/or food allergy triggered current moderate-to-severe atopic eczema/dermatitis
We recruited 159 of these children for clinical assessment, and complete data were available for 157 (see Figure 1 and Table 2). The clinical assessment revealed that 130/157 (82.8%) had no indication of IgE-mediated allergy contributing to their atopic eczema/dermatitis; the remaining 27/157 (17.2%, 95% CI 12.1, 23.9) were clinically assessed as having underlying IgE-mediated atopic eczema/dermatitis and appropriate tests were ordered to investigate this further.
Figure 1.
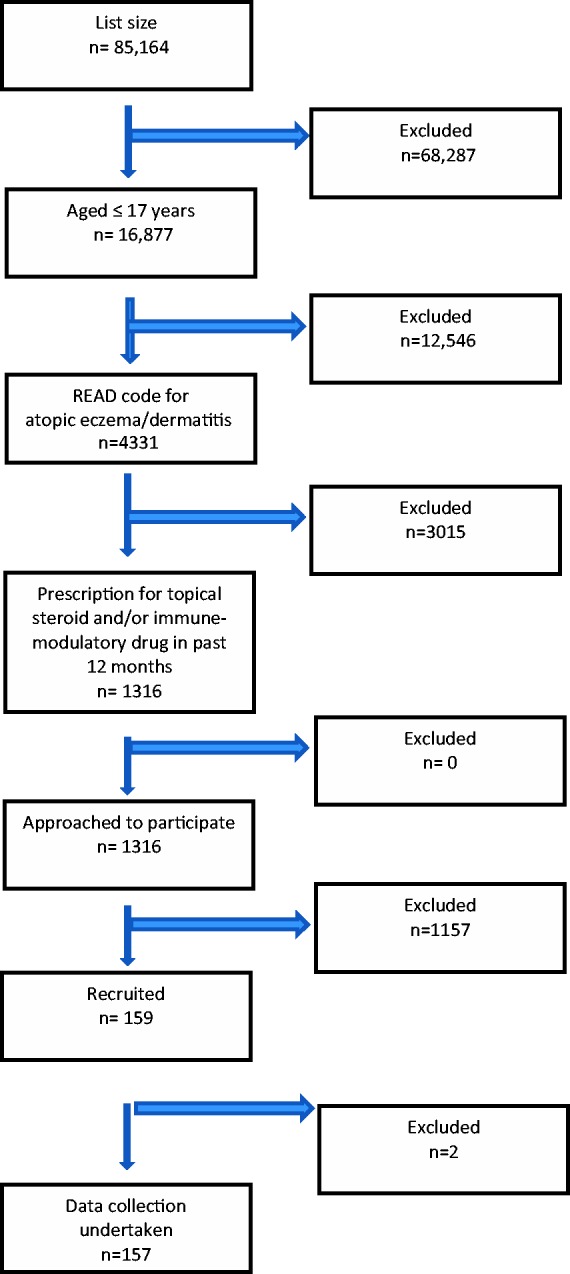
Flow diagram for identifying patients with current potential moderate-to-severe atopic eczema/dermatitis.
Table 3.
Patient blood test results.
| Total no. of tests requested | Number of food allergen tests ordered | No. of positive food allergen tests | No. of aero-allergen tests ordered | No. of positive aero-allergen tests |
|---|---|---|---|---|
| 55 | 19 | 9 (47%) | 36 | 14 (39%) |
In the 27 children who had allergy testing undertaken, a total of 82 blood tests were ordered (i.e. 27 total IgE and 55 specific IgE tests), this equating to a mean of three tests per child. Of the specific IgE tests, 19 (34.5%) were performed for food allergens and 36 (65.5%) for aero-allergens.
Specific IgE tests were positive in 14/27 (51.9%; 95% CI 34.0, 69.3) children. Of the 14 children who tested positive, six (42.9%; 95% CI 21.4, 67.4) were positive to food allergens and six (42.9%; 95% CI 21.4, 67.4) were positive to aero-allergens; the remaining two (14.3%; 95% CI 4.0, 40.0) were positive to both food and aero-allergens (see Table 3).
Table 2.
Patient recruitment log.
| Practice identifier | Number of patients aged ≤17 years | Number of patients ≤17 years with READ code indicative of atopic eczema/dermatitis | Number of eligible patients invited to participate | Number of patients agreed to participate | Number of patients consented | Number of patients with complete dataset |
|---|---|---|---|---|---|---|
| 1 | 1867 | 360 | 127 | 23 | 23 | 22 |
| 2 | 950 | 410 | 116 | 14 | 14 | 14 |
| 3 | 2996 | 638 | 347 | 24 | 24 | 24 |
| 4 | 871 | 262 | 52 | 6 | 6 | 6 |
| 5 | 2195 | 598 | 140 | 15 | 15 | 13 |
| 6 | 2876 | 672 | 181 | 32 | 32 | 31 |
| 7 | 2750 | 707 | 214 | 25 | 25 | 25 |
| 8 | 2372 | 684 | 139 | 20 | 20 | 20 |
Discussion
Statement of principal findings
Based on a detailed clinical assessment, allergy was considered a contributory factor in approximately 20% of children with current moderate-to-severe atopic eczema/dermatitis and approximately half of these children tested positive to food and/or aero-allergens.
Strengths and limitations
This is one of the first studies undertaken to estimate the prevalence of underlying aero- and/or food allergy in children with atopic eczema/dermatitis in a primary care setting, where the majority of UK children with atopic eczema/dermatitis is seen and managed. We achieved our target recruitment figures for both practices and overall registered practice list size. Other key strengths include: that we had good precision in our estimates of atopic eczema/dermatitis, those with moderate-to-severe disease and the proportions of those with underlying IgE-mediated allergy; the combination of interrogating electronic health records and supplementing this with a detailed clinical assessment in children with suspected underlying allergy. We then sought to confirm this clinical suspicion with formal specific IgE testing to a range of potential candidate allergens. Blood tests were performed rather than skin prick testing as the former is in general more sensitive and the latter requires specialist training and resources that are not typically available in a GP surgery.12–14 Total IgE was measured in order to have a baseline to compare against as many allergic individuals will have greatly elevated levels making it difficult to interpret results accurately.13
The study population was diverse covering the North Essex region, and all GP practices in the region were invited to participate. However, given the relatively low rate of recruitment of practices and patients, care needs to be taken in interpreting the findings from this study. Although we employed a sensitive search strategy, which should have enabled us to identify the overwhelming majority of children with atopic eczema/dermatitis, there is always the possibility that some cases may have been misdiagnosed and miscoded by the GP and may, therefore, not have been detected. Given that atopic eczema/dermatitis is a very commonly seen condition in primary care, we consider it unlikely that this had a major bearing on the results. Non-IgE food allergy was not formally investigated in this study, but we identified at least 14 children who on clinical assessment were thought to have possible non-IgE-mediated food allergy contributing to their atopic eczema/dermatitis. Further investigation of these children using formal challenge testing was beyond the scope of this particular study, but this is clearly an area in which future research is needed.
Interpretation in the context of wider literature
Our estimates of the prevalence of moderate-to-severe atopic eczema/dermatitis and underlying allergic disease were substantially lower than suggested by the previous literature on this subject. Prior estimates have, however, been derived predominantly from academic hospital-based specialist dermatology/allergy clinic settings and these are, therefore, unlikely to be representative of more population-based settings.9–11 The findings from the present study are, however, likely to be much more representative of the true picture in the general population. This suggests, therefore, that the vast majority of children with atopic eczema/dermatitis seen in general practice settings has relatively mild disease in which allergy is unlikely to be a major contributory factor. Allergy should, however, be more routinely considered by GPs and their teams in the small subset of patients with relatively moderate-to-severe atopic eczema/dermatitis and in this group a detailed allergy assessment is likely to identify individuals with underlying allergy who may have the potential to benefit from allergen avoidance advice and, in some cases, immunotherapy.15–17
Implications for policy, practice and research
This is the first study to estimate the prevalence of allergy in children with atopic eczema/dermatitis in general practice settings and it, therefore, provides the first population-based estimates of aero- and/or food allergy in children with atopic eczema/dermatitis. Scaling these data up to a population level, the findings suggest that approximately 25% of UK children have atopic eczema/dermatitis, 8% have current moderate-to-severe atopic eczema/dermatitis, 2% require allergy testing and 1% have underlying IgE-mediated allergy, which has the potential to be incorporated into the workload of GPs and their teams. Guidelines on the management of atopic eczema/dermatitis correctly highlight the possibility of food allergy as a possible trigger;18,19 future iterations of these guidelines will, however, need to be cognisant of these primary care-based prevalence estimates in order to provide realistic estimates of the likely scale of the problem. Notwithstanding this, there is a need for additional educational training and support for GPs and other hospital-based general paediatricians in order to enable them to correctly diagnose and manage this important sub-group of children with atopic eczema/dermatitis.20
Future research needs to confirm these estimates through additional population-based studies in other parts of the UK and elsewhere.21 There is in addition, a need for associated research to assess whether intervening in children with confirmed IgE-mediated atopic eczema/dermatitis improves outcomes in these children. Trials are, therefore, needed of allergen avoidance measures and specific immunotherapy and other potential immuno-modulatory therapies.12,17
Conclusions
atopic eczema/dermatitis is a very common diagnosis in primary care with approximately one in three children being diagnosed within the first 17 years of life. Approximately one in three of these children had current moderate-to-severe atopic eczema/dermatitis. Based on a detailed clinical assessment, allergy was considered a contributory factor in approximately 20% of children with current moderate-to-severe atopic eczema/dermatitis and approximately half of these children tested positive to food and/or aero-allergens. Such work once conducted has the potential to inform guidelines on the optimum management of children with atopic eczema/dermatitis seen in primary care settings.8,22
Appendix 1. Patient history, examination and investigation template
Patient initials:
DOB:
Current medication:
Study inclusion criteria met: Yes No
Personal History
Birth history: CS NVD
Feeding: Breast Bottle Details
Age weaned onto solids?
Is there a personal history of eczema?
Yes No Details
What was the age of onset?
What was the situation of onset?
Was it associated with any foods
Purchase of a new pet
Moving house, from town to country , mould, etc.
How widespread is the eczema?
Skin Clear Mild Moderate Severe
How severe is the eczema and what regular medication is used and how often:
How often does he/she get skin infections?
How effective is the treatment? Very Moderate Poor
Please give details:
Is there a family history of eczema/asthma/atopy?
Yes No Details
Impact on quality of life
Is sleep regularly disturbed? Yes No Details
Are activities of daily living affected? Yes No Details
Any other social or psychological effects (including on family/carers)?
Yes No Details
Can any triggers be identified?
Skin products, bubble bath, soap, washing powder, etc. Yes No
Skin infections
Yes No
Contact allergens
Yes No
Food allergens
Yes No
Aeroallergens
Yes No
Pets
Yes No
What symptoms are triggered?
- in IgE-mediated allergy look for
- involvement of cutaneous symptoms such as urticaria, angioedema and itchiness
- GI symptoms such as oral pruritus, vomiting or diarrhoea
- involvement of the respiratory system and less commonly the cardiovascular system, indicates anaphylaxis
- in non-IgE-mediated allergy look for
- persistent symptoms involving mainly the skin and GI system such as eczema, gastro-oesophageal reflux, loose stools, pallor and tiredness, faltering growth plus one or more GI symptom
- especially those symptoms that do not respond to first-line treatment
What is the time course between exposure and the onset of symptoms?
IgE-mediated reactions are more acute in onset and rapidly progressive
non-IgE-mediated reactions are more likely to cause chronic symptoms
Examination:
General appearance:
Height: Weight:
Eyes: look for allergic shiners, chemosis, watery,
Nose: blockage, irritation, allergic salute (crease on nasal bridge), rhinnorhoea, sneezing
Chest:
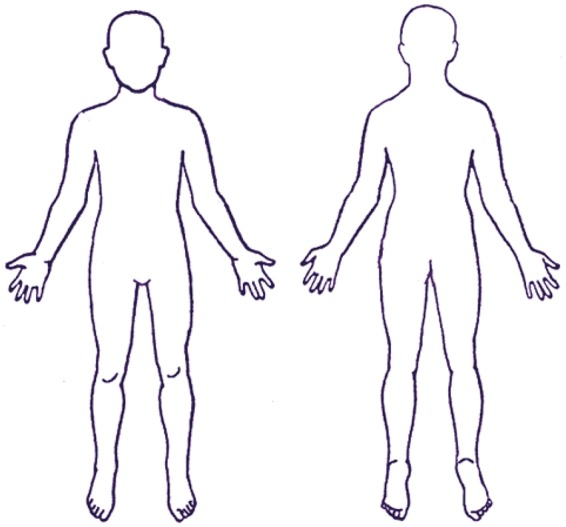
SCORAD Eczema score:
Area
To determine extent, the sites affected by eczema are shaded on a drawing of a body. The rule of 9 is used to calculate the affected area (A) as a percentage of the whole body.
Head and neck 9%
Upper limbs 9% each
Lower limbs 18% each
Anterior trunk 18%
Back 18%
1% each for genitals, each palm and the back of each hand.
The score for each area is added up. The total area is ‘A', which has a possible maximum of 100%.
Intensity
A representative area of eczema is selected. In this area, the intensity of each of the following signs is assessed as none (0), mild (1), moderate (2) or severe (3).
Redness
Swelling
Oozing / crusting
Scratch marks
Skin thickening (lichenification)
Dryness (this is assessed in an area where there is no inflammation)
The intensity scores are added together to give ‘B' (maximum 18).
Diagnosis:
Eczema –no allergic triggers identified
Eczema – allergic , most likely non-IgE mediated
Other diagnosis
Eczema-allergic, most likely IgE mediated
If 1, 2 or 3, thank parent/carer/child for participation and explain to them that they are no longer needed for the study. If 4, then proceed to investigations:
Take a 1 mL sample of blood and test for clinically relevant allergens:
Food: please specify which
Other foods
Aero-allergens – please specify which
Results:
Review of results with patient/parent:
Declarations
Competing interests
SD and AS have served as consultants to Thermo Fisher Scientific.
Funding
This project was funded by a research grant from Thermo Fisher Scientific. The funders had no influence on the design, execution or decision to publish the findings from this work.
Ethical approval
Ethics approval was obtained from South East Scotland Research Ethics Committee 02.
Guarantor
SD
Contributorship
AS and SD jointly conceived the idea for this study. SD was the PI for this work and led the drafting of the manuscript, which was revised in the light of critical comments from AS. SD is guarantor for this work.
Acknowledgements
We would like to express our thanks to National Services for Health Improvement for their help in undertaking this study. In particular, we thank the research nurses Terri Chandler and Margaret Hobbs who recruited families and undertook clinical assessments of the children. We also wish to record our appreciation to the participating practices, parents and children, without whose involvement this study would not have been possible.
Provenance
Not commissioned; peer-reviewed by Liz Angier
References
- 1.Punekar YS, Sheikh A. Establishing the incidence and prevalence of clinician-diagnosed allergic conditions in children and adolescents using routinely collected data from general practices. Clin Exp Allergy 2009; 39: 1209–1216. [DOI] [PubMed] [Google Scholar]
- 2.Levy ML, Sheikh A, Walker S, Woods A. Should UK allergy services focus on primary care? BMJ 2006; 332: 1347–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hazeldine M, Worth A, Levy ML, Sheikh A. Follow-up survey of general practitioners' perceptions of UK allergy services. Prim Care Respir J 2010; 19: 84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Punekar YS, Sheikh A. Establishing the sequential progression of multiple allergic diagnoses in a UK birth cohort using the General Practice Research Database. Clin Exp Allergy 2009; 39: 1889–1895. [DOI] [PubMed] [Google Scholar]
- 5.van den Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ 2009; 339: b2433–b2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worth A, Sheikh A. Food allergy and atopic eczema. Curr Opin Allergy Clin Immunol 2010; 10: 226–230. [DOI] [PubMed] [Google Scholar]
- 7.Gustafsson D, Sjöberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis--a prospective follow-up to 7 years of age. Allergy 2000; 55: 240–245. [DOI] [PubMed] [Google Scholar]
- 8.NICE. Food allergy in children and young people (CG116). See http://guidance.nice.org.uk/CG116 (last accessed 2 November 2014).
- 9.Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA and Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics 1998; 101: E8. [DOI] [PubMed]
- 10.Eigenmann PA, Calza AM. Diagnosis of IgE-mediated food allergy among Swiss children with atopic dermatitis. Pediatr Allergy Immunol 2000; 11: 95–100. [DOI] [PubMed] [Google Scholar]
- 11.Greenhawt M. The role of food allergy in atopic dermatitis. Allergy Asthma Proc 2010; 31: 392–397. [DOI] [PubMed] [Google Scholar]
- 12.Soares-Weiser K, Takwoingi Y, Panesar SS, et al. The diagnosis of food allergy: a systematic review and meta-analysis. Allergy 2014; 69: 76–86. [DOI] [PubMed] [Google Scholar]
- 13.Muraro A, Werfel T, Hoffmann-Sommergruber K, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy 2014; 69: 1008–1025. [DOI] [PubMed] [Google Scholar]
- 14.Sheikh A, Levy ML. Costs are a barrier to GPs performing skin prick testing. Br J Gen Pract 1999; 49: 67–67. [PMC free article] [PubMed] [Google Scholar]
- 15.Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess 2000; 4: 1–191. [PMC free article] [PubMed] [Google Scholar]
- 16.Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol 2013; 132: 110–117. [DOI] [PubMed] [Google Scholar]
- 17.Compalati E, Rogkakou A, Passalacqua G, Canonica GW. Evidences of efficacy of allergen immunotherapy in atopic dermatitis: an updated review. Curr Opin Allergy Clin Immunol 2012; 12: 427–433. [DOI] [PubMed] [Google Scholar]
- 18.Scottish Intercollegiate Guidelines Network. Management of atopic eczema in primary care: a national clinical guideline. See http://www.sign.ac.uk/pdf/sign125.pdf (last checked 2 November 2014).
- 19.NICE. Atopic eczema in children: management of atopic eczema in children from birth up to the age of 12 years. See http://www.nice.org.uk/guidance/cg57 (last checked 2 November 2014).
- 20.BSACI. Primary care training courses in allergy and immunology. See http://www.bsaci.org/meetings-and-events/primary-care-training (last checked 2 November 2014).
- 21.de Benedictis FM, Franceschini F, Hill D, et al. The allergic sensitization in infants with atopic eczema from different countries. Allergy 2009; 64: 295–303. [DOI] [PubMed] [Google Scholar]
- 22.Werfel T, Ballmer-Weber B, Eigenmann PA, et al. Eczematous reactions to food in atopic eczema: position paper of the EAACI and GA2LEN. Allergy 2007; 62: 723–728. [DOI] [PubMed] [Google Scholar]


