Abstract
Objectives:
Many patients with Crohn’s disease on infliximab maintenance therapy have recurrent symptoms despite an initial clinical response. Therefore, concomitant therapies have been studied. We conducted a meta-analysis to assess the effect of specialized enteral nutrition therapy with infliximab versus infliximab monotherapy in patients with Crohn’s disease.
Methods:
A comprehensive search of multiple databases was performed. All studies of adult patients with Crohn’s disease comparing specialized enteral nutrition therapy (elemental or polymeric diet with low-fat or regular diet) with infliximab versus infliximab monotherapy without dietary restrictions were included. Meta-analysis was performed using the Mantel–Haenszel (fixed effect) model with odds ratio (OR) to assess for clinical remission.
Results:
Four studies (n = 342) met inclusion criteria. Specialized enteral nutrition therapy with infliximab resulted in 109 of 157 (69.4%) patients reaching clinical remission compared with 84 of 185 (45.4%) with infliximab monotherapy [OR 2.73; 95% confidence interval (CI): 1.73–4.31, p < 0.01]. Similarly, 79 of 106 (74.5%) patients receiving enteral nutrition therapy and infliximab remained in clinical remission after one year compared with 62 of 126 (49.2%) patients receiving infliximab monotherapy (OR 2.93; 95% CI: 1.66–5.17, p < 0.01). No publication bias or heterogeneity was noted for either outcome.
Conclusions:
The use of specialized enteral nutrition therapy in combination with infliximab appears to be more effective at inducing and maintaining clinical remission among patients with Crohn’s disease than infliximab monotherapy.
Keywords: Crohn’s disease, enteral nutrition, infliximab, meta-analysis
Introduction
Crohn’s disease (CD) is a chronic inflammatory disorder involving the entire gastrointestinal tract from oropharynx to anus. Although not fully defined, the pathogenesis for the development of CD is likely a result of environmental triggers resulting in chronic inflammation in the genetically susceptible patient. Until the advent of the first biologic therapy approved by the US Food and Drug Administration (FDA) (i.e. infliximab) in late 1998, patients with CD were usually treated with conventional therapy or a combination of therapies such as corticosteroids, 5-aminosalicylates, thiopurines and enteral nutrition. Specifically, several studies have demonstrated that in patients with mild CD, enteral nutrition monotherapy may be sufficient to induce and maintain clinical remission [Fell et al. 2000; Breese et al. 1995; Yamamoto et al. 1995]. Though these conventional therapies have shown to be effective at inducing and maintaining clinical remission in patients with mild to moderate disease, only infliximab has been consistently shown to be effective for patients with more aggressive perianal, internal penetrating, and fistulizing CD [Hanauer et al. 2002; Sands et al. 2004].
Specialized nutrition-based therapy for direct treatment of CD was first proposed in the 1970s. The exact role of specialized enteral nutrition in modulating the inflammatory process in CD is uncertain. Several mechanisms have been proposed including improving mucosal permeability, decreasing antigentic effects of regular food proteins, altering intestinal flora, improving cell-mediated immunity, and reducing production of inflammatory cytokines [Fell et al. 2000; Breese et al. 1995; Yamamoto et al. 1995]. Voitk and colleagues reported 13 patients treated with an elemental formulation orally over 22 days appeared to have improvement in inflammatory indices [Voitk et al. 1973]. Navarro and colleagues studied the efficacy of continuous exclusive enteral nutrition comprising peptides, monosaccharides and medium chain triglycerides through nasogastric tubes in pediatric patients over 4 months, which appeared to be effective in inducing remission in active CD [Navarro et al. 1982]. Despite early positive results, a recent Cochrane meta-analysis compared the efficacy of corticosteroids to exclusive specialized enteral nutrition in CD adults and found that steroids were superior to enteral nutrition monotherapy at inducing remission [Zachos et al. 2007]. A recent study by Takagi and colleagues examined 51 patients with CD in clinical remission who were assigned to either receive half of their daily calories from elemental formulation and regular diet versus receiving 100% of their daily caloric intake from a regular diet [Tagaki et al. 2009]. During a two-year follow up, patients on the half-elemental diet had a two-fold higher rate of clinical remission compared with the group receiving a regular diet, suggesting that there may be a role for enteral nutrition in maintenance of CD remission [Tagaki et al. 2009].
The introduction of infliximab, a chimeric antitumor necrosis factor-α (TNR-α) antibody, has altered the treatment paradigm, aiming at not only reducing clinical symptoms but also inducing mucosal healing [Hanauer et al. 2002; Sands et al. 2004]. Despite its strong efficacy for the treatment of more severe CD, 25–60% of the patients with CD who showed a good initial clinical response will eventually lose response during maintenance therapy, occurring mostly within the first months of initiation of treatment [Gisbert and Panes, 2009; Ben-Horin and Chowers, 2011]. The loss of response to infliximab is thought, in part, to be due to low trough serum concentrations as well as neutralizing antibodies to infliximab produced by the patient’s immune system that occur during the maintenance phase [Colombel et al. 2010].
Concomitant use of immunomodulators has been associated with reduced risk of losing clinical response to infliximab [Colombel et al. 2010; Vermeire et al. 2007]. Several proposed mechanisms by which immunomodulators may improve efficacy include reduced risk for formation of antibodies to infliximab and additive immunosuppressive effects through inducing apoptosis of the lamina propria T lymphocytes and monocytes [Colombel et al. 2010; Vermeire et al. 2007]. Increasing doses of infliximab or shortening the interval of infliximab infusion have been employed as strategies to recapture patients who have lost clinical response [Schnitzler et al. 2009; Kopylov et al. 2011]. It is preferable, though, to prevent loss of response.
Though the concomitant use of immunomodulators is considered an effective option for increasing the long-term therapeutic effect of infliximab, there are serious complications associated with this approach such as an increased risk for hepato-splenic T-cell lymphoma [Mackey et al. 2009]. Therefore, several studies have been published evaluating the efficacy of the combination of specialized enteral nutrition and infliximab. The results, however, have varied. The goal of this meta-analysis is to further evaluate the overall efficacy of this therapeutic strategy in maintaining clinical remission in CD patients.
Materials and methods
Literature search
A comprehensive literature search of MEDLINE/PubMed, Embase, Cochrane databases, CINAHL, Scopus and Google Scholar was performed in August 2014. Search terms included “enteral nutrition and infliximab” and “elemental diet and infliximab”. Abstracts from major conferences – Digestive Disease Week (DDW) and American College of Gastroenterology (ACG) national meetings from 2003 to the present – were also searched. References of the retrieved articles and reviews were manually searched for any additional articles. If data were incomplete, missing or required clarification, the authors were contacted.
Data extraction
All studies of adult patients that compared infliximab monotherapy with specialized enteral nutrition therapy combination with infliximab during the maintenance of disease remission were included in the analysis. Two reviewers (M.L.B. and D.L.N.) independently assessed the trials and extracted the appropriate data to be included in the analysis. Any disagreements were evaluated and settled by consensus or a third party (L.B.P.).
Study quality assessment
The quality of studies was assessed using the Effective Public Health Practice Project model [Armijo-Olivo et al. 2010]. This scale assesses study quality as strong, moderate or weak based upon criteria ratings for selection bias, study design, confounders, blinding, data collection methods, withdrawal and dropout descriptions, intervention integrity and analysis. The quality of the study is based upon the number of weak ratings per category (⩾2 weak ratings = weak, 1 weak rating = moderate, 0 weak ratings = strong).
Statistical analysis
The effects of infliximab compared with combination therapy of infliximab and specialized enteral nutrition were analyzed by calculating pooled estimates of maintaining short-term and long-term clinical remission. The results were reported using odds ratio (OR) with a Mantel–Haenszel fixed effect model (if no heterogeneity was present) or a DerSimonian and Laird random effects model (if heterogeneity was present). Heterogeneity was analyzed by calculating the I2 measure of inconsistency and was considered significant if p < 0.10 or I2 > 50%. If heterogeneity was statistically significant, a sensitivity analysis was utilized to examine for heterogeneity when certain studies were excluded from the analysis. Statistical analysis was performed using RevMan 5.1 (Review Manager, Version 5.1, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). Publication bias was assessed by funnel plots.
Results
Study selection
The initial literature search identified a total of 46 articles and abstracts (Figure 1). Of the 46 citations found, we excluded 36 duplicate articles, case reports, reviews, or letters to the editor. Among the 10 remaining articles identified, six were excluded because they did not address the primary clinical question. One study [Matsumoto et al. 2005] was excluded because it evaluated only effect of combination therapy of enteral nutrition and infliximab during the induction phase only. A total of four studies ultimately met inclusion criteria [Hirai et al. 2013; Sazuka et al. 2010; Tanaka et al. 2006; Yamamoto et al. 2010]. All of the studies included in the meta-analysis compared effectiveness of infliximab monotherapy to infliximab in combination with specialized enteral nutrition support at maintaining clinical remission in patients with CD.
Figure 1.
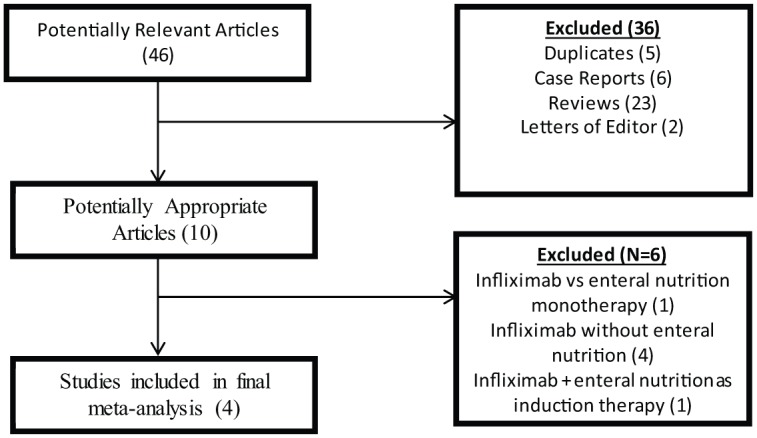
Algorithm demonstrating article search.
Study details
Details of the four included studies are summarized in Table 1. The mean length of follow up reported in the studies was 355 days (range: 112–712 days). The minimum amount of enteral nutrition support was reported to be ⩾600 kcal/day in the study by Sazuka and colleagues [Sazuka et al. 2012]. In all the studies, patients were allowed to receive an oral diet to supplement their daily caloric requirement, but only two studies reported requiring their patients to be on a low-fat diet while the other studies do not report the type of diets their patients ingested. The type of enteral formulation utilized in the included studies was predominantly elemental, but semi-elemental and polymeric formulations were also reported. Using the Effective Public Health Practice Project model [Armijo-Olivo et al. 2012], four of the five studies [Matsumoto et al. 2005; Hirai et al. 2013; Sazuka et al. 2010; Tanaka et al. 2006] were given an overall global grade of ‘moderate’, while the fifth study [Yamamoto et al. 2010] was given a score of ‘strong’ because of its prospective nature (Table 2).
Table 1.
Summary of studies included in meta-analysis.
| Author | Study type | Mean length of follow up | Location | No. of patients | Type of enteral formula | Total mean daily calorie from formula | Diet while not on enteral formula | Definition of remission |
|---|---|---|---|---|---|---|---|---|
| Hirai et al. [2013] | Retrospective | 544 days | Japan | 102 | Elemental or Semi-Elemental | 1,233 kcal/day | Not reported | No CD-related hospitalization, escalation of treatment, shortening interval of infliximab, and subsequent rebound in CRP |
| Sazuka et al. [2012] | Retrospective | 712 days | Japan | 74 | Elemental or Polymeric | ⩾600 kcal/day | Not reported | Reduction in CDAI by >70 or CDAI <150 |
| Tanaka et al. [2006] | Retrospective | 112 days | Japan | 119 | Elemental | 3762 KJ/day (equivalent to 899 kcal/day) | Low fat diet | Reduction in HBI by 3 points or HBI < 4 |
| Yamamoto et al. [2010] | Prospect | 392 days | Japan | 56 | Elemental | 17.5-20 kcal/kg/day (ideal body weight) | Low fat diet | CDAI <150 at Week 56 |
CDAI, Crohn’s Disease Activity Index; CRP, C-reactive protein; HBI, Harvey–Bradshaw index.
Table 2.
Quality assessment of studies included in meta-analysis.
| Study | Study design | Selection bias | Confounders | Blinding | Data collection methods | Withdrawals and dropouts | Intervention integrity | Analyses | Overall quality assessment grade |
|---|---|---|---|---|---|---|---|---|---|
| Hirai et al. [2013] | Retrospective cohort | Limited | Moderate | None | Moderate | None | Strong | Strong | Moderate |
| Sazuka et al. [2012] | Retrospective cohort | Limited | Moderate | None | Moderate | None | Strong | Strong | Moderate |
| Tanaka et al. [2006] | Retrospective cohort | Limited | Moderate | None | Moderate | None | Strong | Strong | Moderate |
| Yamamoto et al. [2010] | Prospective cohort | Limited | Limited | None | Strong | None | Strong | Strong | Strong |
Overall clinical remission
The overall rate of clinical remission was assessed in four studies [Hirai et al. 2013; Sazuka et al. 2010; Tanaka et al. 2006; Yamamoto et al. 2010]. Specialized enteral nutrition therapy with infliximab resulted in 109 of 157 (69.4%) patients reaching clinical remission compared with 84 of 185 (45.4%) with infliximab monotherapy. The use of combination therapy of infliximab with specialized enteral nutrition therapy resulted in a statistically significant higher odds of maintaining overall clinical remission compared with infliximab alone [OR 2.73; 95% confidence interval (CI): 1.73–4.31, p < 0.01] (Figure 2). The number needed-to-treat with combination therapy of infliximab and specialized enteral nutrition to maintain clinical remission is four patients. No publication bias or heterogeneity was noted (I2 = 0%, p = 0.76).
Figure 2.
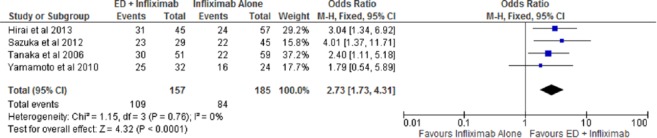
Forest plot of overall clinical remission among patients on combination therapy with infliximab and enteral nutrition compared with infliximab monotherapy.
CI, confidence interval; df, degrees of freedom; ED, elemental diet; M-H, Mantel–Haenszel.
Long-term clinical remission
Of the four included studies, three studies provided long-term clinical remission rates beyond 1 year [Hirai et al. 2013; Sazuka et al. 2010; Yamamoto et al. 2010]. Specialized enteral nutrition therapy with infliximab resulted in 79 of 106 (74.5%) patients remaining in clinical remission after 1 year compared with 62 of 126 (49.2%) patients receiving infliximab monotherapy. The use of specialized nutrition therapy with infliximab demonstrated a statistically significant higher odds of long-term clinical remission compared with infliximab alone (OR 2.93; 95% CI: 1.66–5.17, p < 0.01) (Figure 3). The number needed-to-treat with combination therapy of infliximab and enteral nutrition to maintain clinical remission remained at four patients. No publication bias or heterogeneity was noted (I2 = 0%, p = 0.61).
Figure 3.
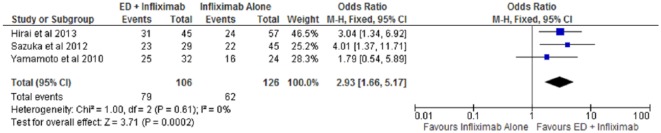
Forest plot of long-term clinical remission among patients on combination therapy with infliximab and enteral nutrition compared with infliximab monotherapy.
CI, confidence interval; df, degrees of freedom; ED, elemental diet; M-H, Mantel–Haenszel.
Discussion
In this meta-analysis, there appears to be over a two-fold increase in the odds of achieving clinical remission among patients on combination therapy with specialized enteral nutrition and infliximab compared with infliximab monotherapy. Furthermore, the probability of maintaining clinical remission on combination therapy appears to extend beyond 1 year. It is difficult to conclude from our meta-analysis whether or not the type of enteral formula (elemental versus polymeric) made a clinical difference in achieving clinical remission in patients on infliximab. All studies evaluated patients who were on elemental formulation, but two of the five studies also included patients on polymeric formulation. In a prior meta-analysis evaluating the clinical difference between elemental compared with polymeric formulas as primary therapies for inducing and maintain remission in CD patients, the efficacy of the two formulations were similar [Zachos et al. 2007]. Therefore, the effect of enteral nutrition therapy among CD patients on infliximab may be independent of the type of enteral formulation utilized.
The included studies in our meta-analysis varied in the amount of enteral formulation given. The daily caloric intake from enteral formulation ranged from 600 kcal to 1500 kcal/day. In previous studies evaluating enteral nutrition as monotherapy in maintaining clinical remission CD patients, it appears that higher amounts of formula (⩾1200 kcal/day) were associated with higher clinical remission rates [Eskai et al. 2006; Koga et al. 1993]. However, this strategy of higher enteral nutrition support is probably only applicable when enteral nutrition is monotherapy for maintaining clinical remission in CD patients. Based on the data included here, it appears that ⩾600 kcal/day of enteral formula [Sazuka et al. 2012] may be sufficient to augment the therapeutic effect of infliximab therapy.
Among CD patients on infliximab therapy, the number needed-to-treat using enteral nutrition to maintain long-term clinical remission beyond a year is four patients. However, a major limitation of the studies to date is that they only assessed the efficacy of this combination strategy based on the patient’s clinical response as measured by clinical indices. Only one study used an objective endpoint [C-reactive protein (CRP)] to define remission and they do not address whether combination therapy improves the more rigorous treatment endpoints of mucosal and histologic remission as was seen in a study of enteral nutrition therapy versus corticosteroid therapy in pediatric CD [Borrelli et al. 2006]. It is unclear by which mechanisms enteral nutrition may be augmenting the clinical effect of infliximab therapy. Previous studies on monotherapy of enteral nutrition and CD have demonstrated that, mechanistically, enteral nutrition appears to improve mucosal permeability, decrease antigenic effects of food proteins, improve cell-mediated immunity, reduce production of inflammatory cytokines and alter the intestinal microbiome [Fell et al. 2000; Breese et al. 1995; Yamamoto et al. 1995]. Furthermore, it is not fully understood if enteral nutrition actually increases the serum drug levels of infliximab or decreases immunogenicity through the reduction of anti-infliximab antibody formation, similar to the mechanism of immunomodulators [Colombel et al. 2010]. Finally, it is unclear how long this strategy of combination therapy needs to be continued to maximize the efficacy of infliximab and whether this strategy can be employed with other anti-TNF α or biologic agents.
There are several additional limitations to the trials included in this meta-analysis that should be noted. All of the studies were conducted in Japan and it is unclear if the results can be generalized to the Western population. Additionally, four of the five studies are retrospective in nature. The included studies do not fully document patient’s compliance with the prescribed enteral nutrition formulation, nor do they define the optimal diet regimen (low-fat diet versus unrestricted diet) to make up the remaining daily required caloric intake. Additionally, there are no studies to date that evaluate the role of enteral nutrition in combination with other approved biologics in the treatment of CD including adalimumab, certilizumab pegol and vedolizumab.
In conclusion, among CD patients with moderate to severe disease requiring infliximab therapy, supplemental specialized enteral nutrition of at least 600 kcal/day appears to be an effective strategy at enhancing both short-term and long-term clinical remission rates. Pooling the current studies, it still remains unclear if the type of enteral formulations (i.e. elemental versus semi-elemental versus polymeric) makes a difference in achieving long-term clinical remission. However, Zachos and colleagues clearly demonstrated that the type of enteral formulation does not appear to make a difference with regard to the induction or maintenance of clinical remission among adult CD patients [Zachos et al. 2007]. Given the limitations of the existing trials, further randomized placebo controlled studies are needed to evaluate the effect of specialized enteral nutrition on infliximab drug levels, the optimal amount of daily calories needed from enteral nutrition therapy, and the effect of adjunctive specialized enteral nutrition on mucosal and histologic healing. Future studies are also necessarily to confirm the findings of Matsumoto and colleagues [Matsumoto et al. 2005] if this combination therapy of enteral nutrition and infliximab is also effective at induction of clinical remission among CD patients.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest in preparing this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Douglas L. Nguyen, Department of Medicine, University of California Irvine, Irvine, CA, USA
Lena B. Palmer, Department of Medicine, Loyola University, Chicago, IL, USA
Emily T. Nguyen, Department of Pharmacy, University of California – Irvine, CA, USA
Stephen A. McClave, Department of Medicine, University of Louisville, Louisville, KY, USA
Robert G. Martindale, Department of Surgery, Oregon Health & Science University, Portland, OR, USA
Matthew L. Bechtold, Division of Gastroenterology & Hepatology, University of Missouri Health Sciences Center, CE405, DC 043.00, Five Hospital Drive, Columbia, MO 65212, USA
References
- Armijo-Olivo S., Stiles C., Hagen N., Biondo P., Cummings G. (2012) Assessment of study quality for systematic reviews. A comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract 18: 12–18. [DOI] [PubMed] [Google Scholar]
- Ben-Horin S., Chowers Y. (2011) Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther 33: 987–995. [DOI] [PubMed] [Google Scholar]
- Borrelli O., Cordischi L., Cirulli M., Paganelli M., Labalestra V., Uccini S., et al. (2006) Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol 4: 744–753. [DOI] [PubMed] [Google Scholar]
- Breese E., Michie C., Nicholls S., Williams C., Domizio P., Walker-Smith J., et al. (1995) The effect of treatment on lymphokine-secreting cells in the intestinal mucosa of children with Crohn’s disease. Aliment Pharmacol Ther 9: 547–552. [DOI] [PubMed] [Google Scholar]
- Colombel J., Sandborn W., Reinisch W., Mantzaris G., Kornbluth A., Rachmilewitz D., et al. (2010) Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 362: 1383–1395. [DOI] [PubMed] [Google Scholar]
- Esaki M., Matsumoto T., Nakamura S., Yada S., Fujisawa K., Jo Y., et al. (2006) Factors affecting recurrence in patients with Crohn’s disease under nutritional therapy. Dis Colon Rectum 49: S68–S74. [DOI] [PubMed] [Google Scholar]
- Fell J., Paintin M., Arnaud-Battandier F., Beattie R., Hollis A., Kitching P., et al. (2000) Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharmacol Ther 14: 281–289. [DOI] [PubMed] [Google Scholar]
- Gisbert J., Panes J. (2009) Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol 104: 760–767. [DOI] [PubMed] [Google Scholar]
- Hanauer S., Feagan B., Lichtenstein G., Mayer L., Schreiber S., Colombel J., et al. (2002) Maintenance infliximab for Crohn’s disease: the ACCENT I randomized trial. Lancet 359: 1541–1549. [DOI] [PubMed] [Google Scholar]
- Hirai F., Ishihara H., Yada S., Esaki M., Ohwan T., Nozaki R., et al. (2013) Effectiveness of concomitant enteral nutrition therapy and infliximab for maintenance treatment of Crohn’s disease in adults. Dig Dis Sci 58: 1329–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H., Iida M., Aoyagi K., Matsui T., Fujishima M. (1993) Long-term efficacy of low residue diet for the maintenance of remission in patients with Crohn’s disease. Nihon Shokakibyo Gakkai Zasshi 90: 2882–2888. [PubMed] [Google Scholar]
- Kopylov U., Mantzaris G., Kansanos K., Reenaers C., Ellul P., Rahier J., et al. (2011) The efficacy of shortening the dosing interval to once every six weeks in Crohn’s patients losing response to maintenance dose of infliximab. Aliment Pharmacol Ther 33: 349–357. [DOI] [PubMed] [Google Scholar]
- Mackey A., Green L., Leptak C., Avigan M. (2009) Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease: update. J Pediatr Gastroenterol Nutr 48: 386–388. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Iida M., Kohgo Y., Imamura A., Kusugami K., Nakano H., et al. (2005) Therapeutic efficacy of infliximab on active Crohn’s disease under nutritional therapy. Scand J Gastroenterol 40: 1423–1430. [DOI] [PubMed] [Google Scholar]
- Navarro J., Vargas J., Cezard J., Charritat J., Polonovski C. (1082) Prolonged constant rate elemental enteral nutrition in Crohn’s disease.J Pediatr Gastroenterol Nutr 1: 541–546. [DOI] [PubMed] [Google Scholar]
- Sands B., Anderson F., Bernstein C., Chey W., Feagan B., Fedorak R., et al. (2004) Infliximab maintenance therapy in inflammatory bowel disease. N Engl J Med 350: 876–885. [DOI] [PubMed] [Google Scholar]
- Sazuka S., Katsuno T., Nakagawa T., Saito M., Saito K., Matsumura T., et al. (2012) Concomitant use of enteral nutrition therapy is associated with sustained response to infliximab in patients with Crohn’s disease. Eur J Clin Nutr 66: 1219–1223. [DOI] [PubMed] [Google Scholar]
- Schnitzler F., Fidder H., Ferrante M., Noman M., Arijs I., Van Assche G., et al. (2009) Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease : results from a single-centre cohort. Gut 58: 492–500. [DOI] [PubMed] [Google Scholar]
- Takagi S., Utsumoniya K., Kuriyama S., Yokoyama H., Takahashi S., Umemura K., et al. (2009) Quality of life of patients and medical cost of “half-elemental diet” as maintenance therapy for Crohn’s disease: secondary outcomes of a randomized control trial. Dig Liver Dis 41: 390–394. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Takahama K., Kimura T., Mizuno T., Nagasaka M., Iwata K., et al. (2006) Effect of concurrent elemental diet on infliximab treatment for Crohn’s disease. J Gastroenterol Hepatol 21: 1143–1149. [DOI] [PubMed] [Google Scholar]
- Vermeire S., Noman M., Van Assche G., Baert F., D’Haens G., Rutgeerts P. (2007) Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 56: 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voitk A., Echave V., Feller J., Brown R., Gurd F. (1973) Experience with elemental diet in the treatment of inflammatory bowel disease. Is this primary therapy? Arch Surg 107: 329–333. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nakahigashi M., Umegae S., Kitagawa T., Matsumoto K. (2005) The impact of elemental diet on mucosal inflammation in patients with active Crohn’s disease: cytokine production and endoscopic and histological findings. Inflamm Bowel Dis 11: 580–588. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nakahigashi M., Umegae S., Matsumoto K. (2010) Prospective clinical trial: enteral nutrition during maintenance infliximab in Crohn’s disease. J Gastroenterol 45: 24–29. [DOI] [PubMed] [Google Scholar]
- Zachos M., Tondeur M., Griffiths A. (2007) Enteral nutrition therapy for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 1: CD000542. [DOI] [PubMed] [Google Scholar]


