Gastrocutaneous fistulas are rare and difficult to treat complications with an incidence of 1.7–4% in patients who have undergone bariatric surgery, and are associated with high morbidity and mortality rates with difficult management problems [Nguyen et al. 2001]. They mostly occur after iatrogenic gastric injury, breakdown of a gastroenteric anastomosis, or disruption of stapler suture lines [Papavramidis et al. 2008]. Treatment is varied and dependent on the size of the fistula as well as its time course in relation to surgery, and on the patient’s underlying conditions.
Endoscopic closure of gastrocutaneous fistulas has been described using various techniques, including glue, stenting, band ligation, endoscopic clips, endoloop application, and combinations [Dişibeyaz et al. 2012; Binmoeller et al. 1993]. Endoscopic-clip application is most familiar to endoscopy specialists because of its many applications in the therapeutic endoscopy field. However, endoscopic-clip application is not an easy method to perform for closure of the orifice of a fistula when the orifice is wide or in the presence of accompanying fibrosis. Moreover, it also has limited effectiveness in its ability to entrap and hold the tissue.
In the past few years there have been several reports in the literature regarding the use of a novel device that is mainly used in cardiology practice but has begun to be used in the setting of bronchopleural fistula and gastrocolonic fistula using endobronchial and endoscopic approaches [Fruchter et al. 2011; Melmed et al. 2009]. Up to now, however, there have been no reports demonstrating the successful application of this device in gastrocutaneous fistula treatment. Herein, we would like to report a novel method of gastrocutaneous fistula closure using an Amplatzer Muscular VSD Occluder (AMPLATZERTM Muscular VSD Occluder, St Jude Medical, MN, USA), which is commonly used for transcatheter closure of ventricular septal defects.
A 35-year-old man underwent a laparoscopic sleeve gastrectomy operation because of morbid obesity in another hospital. After the operation, the patient experienced abdominal pain and tenderness with vomiting on postoperative day 4. Continuous drainage of the suction drain and a change in the drainage content raised a suspicion of gastrocutaneous fistula, and an upper gastrointestinal study demonstrated that a gastrocutaneous fistula had originated from the proximal edge of the anastomosis. The patient was referred to our tertiary care center for further treatment.
On admission to our hospital, physical examination revealed significant erythema around the upper left abdomen with pus discharging from a small hole measuring 0.6 mm × 0.7 mm. Abdominal computerized tomography demonstrated a soft tissue abnormality along the left upper abdomen wall communicating with the underlying greater curvature of the stomach (Figure 1). An upper gastrointestinal endoscopy revealed the presence of an orifice at the proximal edge of the anastomosis. The orifice of the fistula was wide, approximately 15 mm × 15 mm (Figure 2).
Figure 1.
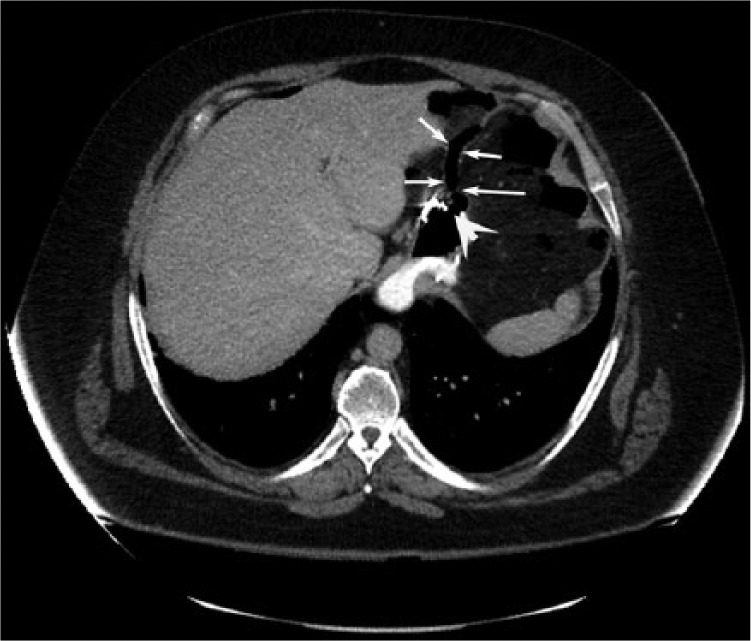
Axial computed tomography of the abdomen showing a large leak at the proximal part of the anastomosis (arrow: fistula tract; arrowhead: gastric orifice).
Figure 2.
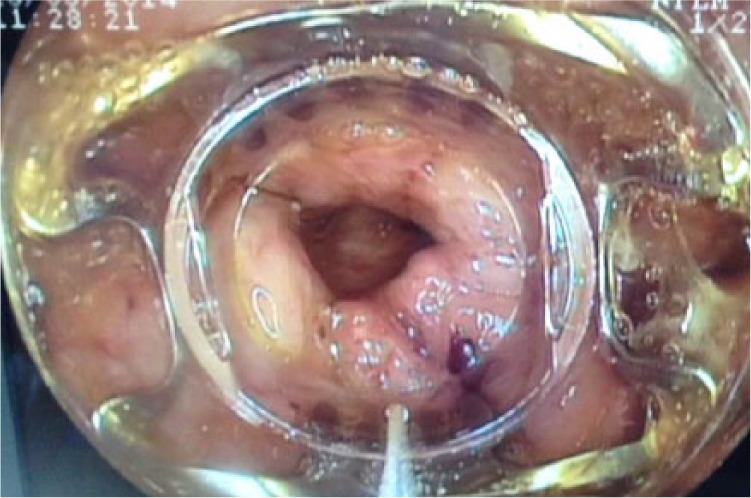
The gastric orifice of the gastrocutaneous fistula during endoscopic application of the over-the-scope-clip device.
The gastrocutaneous fistula was persistent after 4 weeks of conservative management with external drainage, gastric decompression with nasogastric catheter, intravenous broad-spectrum antibiotics, antisecretory drugs, and parenteral nutrition. Finally, endoscopic treatment was considered to be the preferred choice for this patient, with an initial attempt using an over-the-scope-clip (OTSC) system for closure of the gastric orifice. After two failed endoscopic repair attempts within a 5-day interval with OTSC application (nontraumatic type), we decided to use an Amplatzer Muscular VSD Occluder to close the fistula orifice, with the agreement of the patient.
The endoscope was advanced into the stomach adjacent to the fistula orifice and a 0.89 mm (0.035-inch) guidewire (VisiGlide, Olympus, Tokyo, Japan) was passed endoscopically and inserted through the fistula tract under fluoroscopic and endoscopic guidance. The endoscope was withdrawn with the guidewire still in the same place, and was subsequently reinserted adjacent to the guidewire. An 18 mm Amplatzer Muscular VSD Occluder (model 9-VSDMUSC-018) was delivered into the fistula via an oral route. The distal umbrella of the occluder pushed through the fistula to the distal end. Under tension of the whole assembly, the proximal umbrella unfolded in the stomach, while the waist of the device filled the fistula. The position was confirmed both fluoroscopically (Figure 3) and endoscopically (Figure 4). No side effects or complications were observed during or after the procedure. The patient remained asymptomatic for 6 months after the first presentation.
Figure 3.
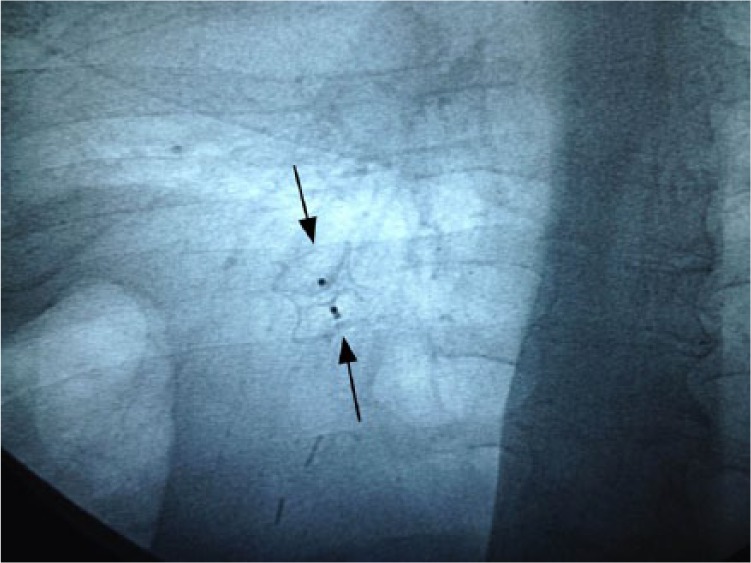
Fluoroscopy showing the Amplatzer Muscular VSD Occluder deployed across the gastrocutaneous fistula.
Figure 4.
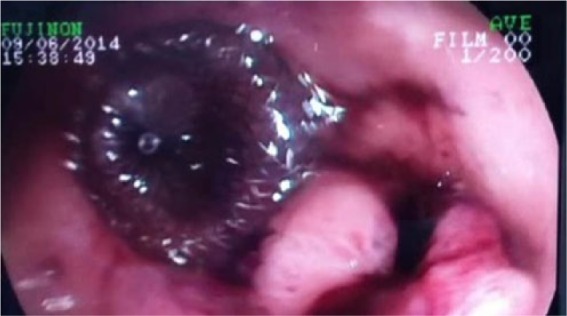
Endoscopic view of the Amplatzer Muscular VSD Occluder after closure.
In this report, we demonstrated for the first time a new application method of an Amplatzer Muscular VSD Occluder in a patient who had developed a persistent gastrocutaneous fistula. The reported fistula was a complication that developed after a laparoscopic sleeve gastrectomy operation with increasing problems induced by uncontrolled chronic fluid leakage. This spontaneously developed complication was permitting the passage of gastric fluids and secretions from the stomach to the outside body surface and represented an important problem that was difficult to treat. Although no major infection complication was observed, a therapeutic intervention to close the chronic fistula and thus avoid future complications seemed essential.
Conservative, nonsurgical management of gastrocutaneous fistulas includes medications aimed at increasing gastric emptying with promotility agents and increasing gastric pH with proton pump inhibitors, fibrin sealant glues, and parenteral nutrition [Alkhiari et al. 2013]. Unfortunately, we failed with our initial conservative management strategy with the patient due to a persistent and mature fistula tract. Moreover, being aware that surgical closure was the standard treatment approach for fistulas, our patient was considered to be a poor surgical candidate secondary to underlying comorbid conditions. For closing the orifice, an endoscopic approach was therefore considered to be the preferred choice for this patient. Based on the large size of the defect (> 10 mm), OTSC was our initial choice for closing this orifice. One of the most important steps of the OTSC procedure is ideally to ensure a steady full-thickness closure of the whole defect, which means that the fistula’s edges must be brought against the clipping device before firing the instrument [Mercky et al. 2014]. Although the existence of vital tissue is also one of the most important prerequisites to achieve a successful outcome for OTSC application, in this case the grasper could not capture enough tissue to close the defect, probably due to adjacent inflammatory changes with tissue fibrosis. After failure of the OTSC application, with the agreement of the patient we decided to use the Amplatzer Muscular VSD Occluder to close the fistula orifice.
The Amplatzer Muscular VSD Occluder is a self-expanding, double umbrella-like round disk made from nitinol wire mesh. The two disks are linked together by a short connecting mesh tube, which acts to stent the defect [Gulkarov et al. 2009]. It is widely used to treat congenital heart defects [Butera et al. 2007]. Owing to the large range of device sizes, they can be appropriately matched to the diameter and length of the lesion, and provide immediate effective closure of the defect. The Amplatzer Muscular VSD Occluder can be easily deployed, retrieved, and repositioned prior to detachment, which is an important advantage for accurate positioning and coverage of the fistula. Although the use of an Amplatzer Muscular VSD Occluder to close a gastrocutaneous fistula as described herein is novel, the use of this device was reported in the setting of a bronchopleural fistula via an endobronchial approach, and gastrocolonic fistula and biliodigestive fistula between the bulbus and common bile duct via an endoscopic approach [Fruchter et al. 2011; Melmed et al. 2009].
The prerequisites for placement of this system could be achieved using advanced endoscopic techniques for localization and guidewire cannulation of the fistula. In our case, we obtained correct positioning and orientation under fluoroscopic and endoscopic guidance. Thereafter, we were able to reposition the Amplatzer Muscular VSD Occluder and the fistula was closed completely after spontaneous retraction of the occluder through the gastric wall and expansion in this position. The presence of two discs, one on either side of the lesion, led to greater coverage and increased the likelihood of closure in our patient. Moreover, the procedure was well tolerated by the patient with no side effects or complications.
In conclusion, we describe a novel endoscopic technique for gastrocutaneous fistula closure using an Amplatzer Muscular VSD Occluder. Although a number of different techniques can be used for this purpose, our case successfully demonstrated that this technique is safe and reliable for large gastrocutaneous fistulas that develop after gastric surgery. Furthermore, the ease of implantation of this device with only conscious sedation in an endoscopy room adds this novel technique to the current armamentarium of minimally invasive modalities for the treatment of gastrointestinal fistulas. Therefore, we expect there will be future clinical trials using this device for treatment of different congenital or acquired defects of the gastrointestinal tract.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest for this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Bulent Odemis, Department of Gastroenterology, Turkiye Yuksek Ihtisas Hospital, Ankara, Turkey.
Yavuz Beyazit, Department of Gastroenterology, Canakkale State Hospital, Canakkale, 17100, Turkey.
Serkan Torun, Department of Gastroenterology, Turkiye Yuksek Ihtisas Hospital, Ankara, Turkey.
Ertugrul Kayacetin, Department of Gastroenterology, Turkiye Yuksek Ihtisas Hospital, Ankara, Turkey.
References
- Alkhiari R., Jacob D., Kassam Z., Abu Zaghlan O., Tse F. (2013) Closure of a percutaneous endoscopic gastrostomy-associated nonhealing gastrocutaneous fistula using endoscopic hemoclips. Can J Gastroenterol 27: 501–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binmoeller K., Grimm H., Soehendra N. (1993) Endoscopic closure of a perforation using metallic clips after snare excision of a gastric leiomyoma. Gastrointest Endosc 39: 172–174. [DOI] [PubMed] [Google Scholar]
- Butera G., Chessa M., Carminati M. (2007) Percutaneous closure of ventricular septal defects. Cardiol Young 17: 243–253. [DOI] [PubMed] [Google Scholar]
- Dişibeyaz S., Köksal A., Parlak E., Torun S., şaşmaz N. (2012) Endoscopic closure of gastrointestinal defects with an over-the-scope clip device. A case series and review of the literature. Clin Res Hepatol Gastroenterol 36: 614–621. [DOI] [PubMed] [Google Scholar]
- Fruchter O., Kramer M., Dagan T., Raviv Y., Abdel-Rahman N., Saute M., et al. (2011) Endobronchial closure of bronchopleural fistulae using amplatzer devices: our experience and literature review. Chest 139: 682–687. [DOI] [PubMed] [Google Scholar]
- Gulkarov I., Paul S., Altorki N., Lee P. (2009) Use of Amplatzer device for endobronchial closure of bronchopleural fistulas. Interact Cardiovasc Thorac Surg 9: 901–902. [DOI] [PubMed] [Google Scholar]
- Melmed G., Kar S., Geft I., Lo S. (2009) A new method for endoscopic closure of gastrocolonic fistula: novel application of a cardiac septal defect closure device (with video). Gastrointest Endosc 70: 542–545. [DOI] [PubMed] [Google Scholar]
- Mercky P., Gonzalez J., Aimore Bonin E., Emungania O., Brunet J., Grimaud J., et al. (2014) Usefulness of over-the-scope clipping system for closing digestive fistulas. Dig Endosc 10.1111/den.12295. [DOI] [PubMed] [Google Scholar]
- Nguyen N., Goldman C., Rosenquist C., Arango A., Cole C., Lee S., et al. (2001) Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg 234: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavramidis T., Kotzampassi K., Kotidis E., Eleftheriadis E., Papavramidis S. (2008) Endoscopic fibrin sealing of gastrocutaneous fistulas after sleeve gastrectomy and biliopancreatic diversion with duodenal switch. J Gastroenterol Hepatol 23: 1802–1805. [DOI] [PubMed] [Google Scholar]


