Abstract
Purpose
To assess the association of positive post-radiotherapy (RT) biopsy with subsequent clinical outcomes in men with localized prostate cancer.
Methods
RTOG 94-08 analyzed 1979 men with stage T1b-T2b, PSA ≤ 20 prostate cancer testing whether 4 months of total androgen suppression (TAS) added to RT improved survival over RT alone. Patients randomized to TAS received flutamide with leutenizing hormone releasing hormone (LHRH) agonist. Per protocol, patients without evidence of clinical recurrence or initiation of additional endocrine therapy underwent repeat prostate biopsy 2 years following RT completion. Statistical analysis was performed to evaluate the impact of positive post-RT biopsies on clinical outcomes.
Results
831 patients underwent post-RT biopsy, 398 treated with RT alone and 433 RT + TAS. Patients with positive post-RT biopsies had higher rates of biochemical failure (BCF) [HR=1.7; 95% CI 1.3–2.1] and distant metastasis (DM) [HR=2.4; 95% CI 1.3–4.4] as well as inferior disease specific survival (DSS) [HR=3.8; 95% CI 1.9–7.5]. Positive biopsy remained predictive of such outcomes after correction for potential confounders such as Gleason score, tumor stage, and TAS administration. Prior TAS did not prevent elevated risk of adverse outcome in the setting of post-RT positive biopsy. Patients with Gleason score ≥ 7 with a positive biopsy additionally had inferior overall survival compared to those with a negative biopsy [HR=1.56; 95% CI 1.04–2.35].
Conclusions
Positive post-RT biopsy is associated with increased rates of DM and inferior DSS in patients treated with definitive RT and was associated with inferior OS for patients with high-grade tumors.
Introduction
Currently, there is no defined role for post-radiotherapy (RT) biopsy in the absence of clinical suspicion of treatment failure in the management of early stage prostate cancer.1 Single institution treatment protocols, and few randomized clinical trials performed in the past have, on occasion, included as part of the protocol a repeat prostate biopsy following the completion of RT. Results of these studies have generally focused on the rate of positive biopsy as a measure of effectiveness of a given treatment. What data are available correlating post-RT biopsy results with outcomes have suggested associations with increased rates of biochemical failure with limited demonstrable relationship with clinical findings such as distant metastases or survival.
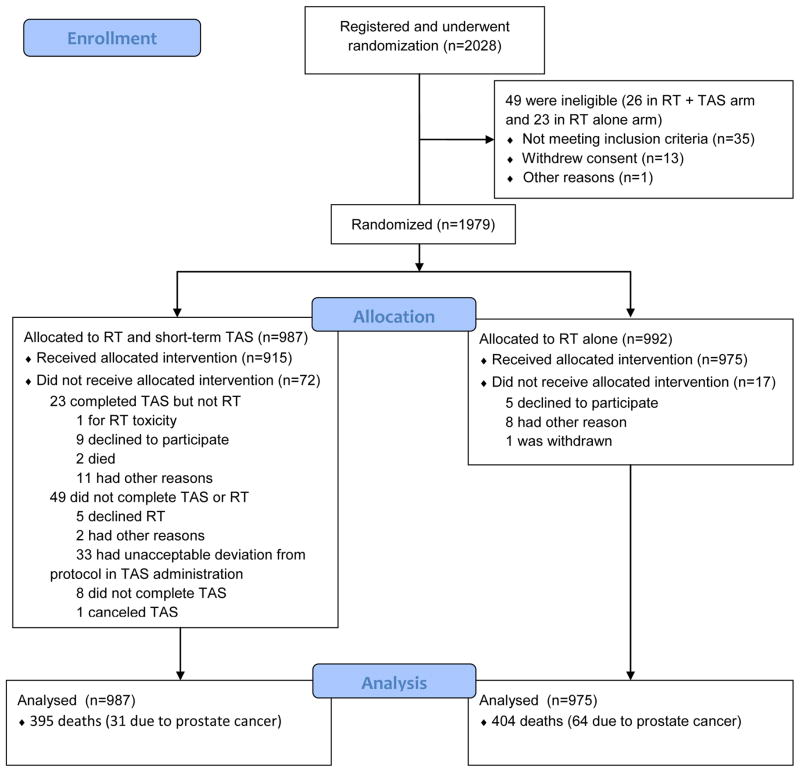
RTOG 9408 was a prospective randomized trial evaluating the use of short-term total androgen suppression (TAS) in the management of early stage prostate cancer. The study structure, enrollment, and treatment allocation is summarized in Figure 1, and outcomes have been reported previously.2 Randomization consisted of RT to a total dose of 66.6 Gy to the prostate gland with or without the addition of 4 months of TAS. Patients with no clinical or biochemical evidence of treatment failure, had not been started on additional androgen suppressive therapy, and who had no medical contraindication to such a procedure underwent repeat prostate biopsy 24 months following RT completion. The study was positive for its primary endpoint, demonstrating an overall survival benefit for those patients randomized to receive TAS together with RT. Clinical benefits of TAS additionally included improved rates of distant metastases (DM) and disease-specific survival (DSS). Finally, patients treated with RT alone were significantly more likely to have a positive post-RT biopsy than those receiving TAS.
Figure 1.
Enrollment, Randomization, and Follow-up of the Study Patients
Abbreviations: RT – radiotherapy; TAS – total androgen suppression
Despite a description of increased positive biopsy rates amongst patients treated with RT alone, data to this point remain limited regarding what independent prognostic value a positive post-RT biopsy confers. Thus, the hypothesis that a positive post-RT prostate biopsy is associated with inferior clinical outcomes was tested as a secondary analysis within the framework of a multi-institutional prospective, randomized trial and overcomes many of the limitations of previous attempts to define its value. Namely, patient numbers are large, treatment is standardized, and outcomes were recorded systematically in a prospective fashion under the auspices of an NCI-sponsored protocol.
Materials and Methods
Patients
Between October 1994 and April 2001, RTOG 9408 enrolled a total of 2028 patients with early stage prostate cancer. Eligibility requirements have been described previously but, briefly, were as follows: clinical stage T1b-T2b prostate adenocarcinoma with a PSA value ≤ 20 ng/dL, Karnofsky performance scores ≥ 70, no evidence of bone (bone scan required) or lymphatic (computed tomography, lymphoscintigraphy, lymphadenectomy) metastatic disease, and no prior local or systemic therapy administered for prostate cancer. Patients with prior invasive malignancy who had been disease-free for ≥ 5 years were considered eligible for enrollment as were patients with non-melanoma skin cancers who were disease-free for ≥ 2 years.
Study Design and Treatment
The original protocol was structured as follows: patients were stratified by PSA level (< 4 vs. ≥ 4 ng/dL), tumor grade (well- vs. moderately- vs. poorly-differentiated), and whether or not they underwent surgical staging of the pelvic lymph nodes (stage N0 vs. Nx). They were then randomized to undergo definitive RT alone or in conjunction with 4 months of TAS starting 2 months prior to and given concurrently with RT. RT consisted initially of a dose of 46.8 Gy to the prostate and pelvic lymph nodes followed by a prostate boost to a total cumulative dose of 66.6 Gy. Patients considered to have low risk disease (PSA ≤ 10 and Gleason score ≤ 5) or who underwent prior negative pelvic lymph node dissection underwent treatment to the prostate only. Post-treatment evaluation included repeat prostate biopsy at 2 years provided there was no clinical or biochemical evidence of disease recurrence/progression, no additional/salvage TAS was administered, and there were no existing medical contraindications to performing such a procedure. Both the initial diagnostic and post-treatment biopsies underwent central pathologic review. Positive post-treatment biopsies were graded according to Gleason score and assessed for the presence of therapy effect. For purposes of this analysis, any biopsy specimen with cancer present was regarded as positive, regardless of the degree of therapy effect identified. The current evaluation is a retrospective subset analysis evaluating the clinical outcomes of those patients who underwent planned post-RT biopsy.
Statistical Analysis
Outcomes were measured from the date of randomization to the date of failure or, if no failure, the date of last follow-up. Overall survival was defined as death due to any cause. Distant metastases were defined at the date of documented bone or visceral disease progression at sites other than the prostate or pelvic lymph nodes. Disease-specific survival (DSS) was defined at the point of death certified due to prostate cancer, death from any cause in the presence of active/progressive prostate cancer (clinical or biochemical), death due to cancer-related treatment complications (irrespective of disease status), or death from unknown causes in the setting of known prior clinical or biochemical disease relapse. For purposes of the current analysis, the Phoenix definition for biochemical failure was used, i.e. a PSA rise of 2 ng/mL above the nadir or a PSA rise sufficient to prompt the initiation of salvage hormonal therapy at a lesser value. The Kaplan-Meier method was used for actuarial overall survival estimates,3 and Cox regression was used to perform univariate and multivariate analyses.4 The cumulative incidence approach was used for yearly estimates of biochemical failure, DSS, and DM,5 and the Fine-Grey proportional hazards model was used to perform univariate and multivariate analyses,6 where competing events are deaths without specific failures of interest.
Results
Patient Characteristics
As reported initially, 2028 patients were enrolled between October 1994 and April 2001. Forty-nine were found to be ineligible for analysis or withdrew consent leaving 1979 patients analyzed. Of these, 831 patients (42%) with no prior clinical or biochemical evidence of treatment failure underwent their planned post-treatment biopsy 24 months following the completion of RT. Characteristics of patients who underwent vs. having forgone post-RT biopsy are shown in Table 1. Median follow-up for the biopsied patients was 9 years (range 2.4–14.1 years). Baseline characteristics of repeat biopsy-positive vs. repeat biopsy-negative patients are shown in Table 2. Clinicopathologic factors associated with a higher positive biopsy risk were clinical stage T2 vs. T1 (34% vs. 24%), Gleason score ≥ 7 (36% vs. 27%), and treatment with RT alone (no TAS) (39% vs. 21%).
Table 1.
Baseline Patient Characteristics
| Repeat Biopsy Performed (n=831) | No Repeat Biopsy Performed * (n=924) | P-value | |
|---|---|---|---|
| Age(years) | p=0.85 | ||
| Mean | 69.6 | 69.7 | |
| Std. Dev. | 6.0 | 6.3 | |
| Median | 70 | 71 | |
| Min – Max | 50 – 88 | 47 – 91 | |
| Q1 – Q3 | 66 – 74 | 66 – 74 | |
| Gleason | p=0.02‡ | ||
| 2–6 | 524 (63.1%) | 568 (61.5%) | |
| 7 | 211 (25.4%) | 262 (28.4%) | |
| 8–10 | 68 (8.2%) | 82 (8.9%) | |
| Unknown | 28 (3.4%) | 12 (1.3%) | |
| PSA | p=0.35 | ||
| < 4 | 97 (11.7%) | 95 (10.3%) | |
| >= 4 | 734 (88.3%) | 829 (89.7%) | |
| T Stage | p=0.0004‡ | ||
| T1 | 380 (45.7%) | 501 (54.2%) | |
| T2 | 451 (54.3%) | 423 (45.8%) | |
| Assigned Treatment | p=0.23 | ||
| Hormone+RT | 433 (52.1%) | 455 (49.2%) | |
| RT Alone | 398 (47.9%) | 469 (50.8%) |
Indicates statistical significance at a significance level of 0.05.
• Patients without clinical or biochemical failure within 2 year and with a minimum 2 year survival.
Table 2.
Baseline Patient Characteristics by Repeat Biopsy Status
| Repeat Biopsy Negative (n=585) | Repeat Biopsy Positive (n=246) | P-value | |
|---|---|---|---|
| Age(years) | p=0.12 | ||
| Mean | 69.4 | 70.1 | |
| Std. Dev. | 5.9 | 6.3 | |
| Median | 70 | 72 | |
| Min – Max | 52 – 83 | 50 – 88 | |
| Q1 – Q3 | 66 – 74 | 66 – 74 | |
| Gleason | p=0.04‡ | ||
| 2–6 | 385 (65.8%) | 139 (56.5%) | |
| 7 | 138 (23.6%) | 73 (29.7%) | |
| 8–10 | 41 (7.0%) | 27 (11.0%) | |
| Unknown | 21 (3.6%) | 7 (2.8%) | |
| PSA | p=0.86 | ||
| < 4 | 69 (11.8%) | 28 (11.4%) | |
| >= 4 | 516 (88.2%) | 218 (88.6%) | |
| T Stage | p=0.001‡ | ||
| T1 | 289 (49.4%) | 91 (37.0%) | |
| T2 | 296 (50.6%) | 155 (63.0%) | |
| Assigned Treatment | p<0.0001‡ | ||
| Hormone+RT | 344 (58.8%) | 89 (36.2%) | |
| RT Alone | 241 (41.2%) | 157 (63.8%) |
Indicates statistical significance at a significance level of 0.05.
Clinical Outcomes
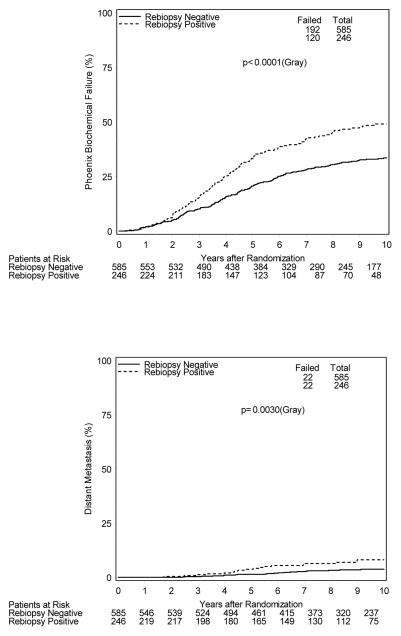
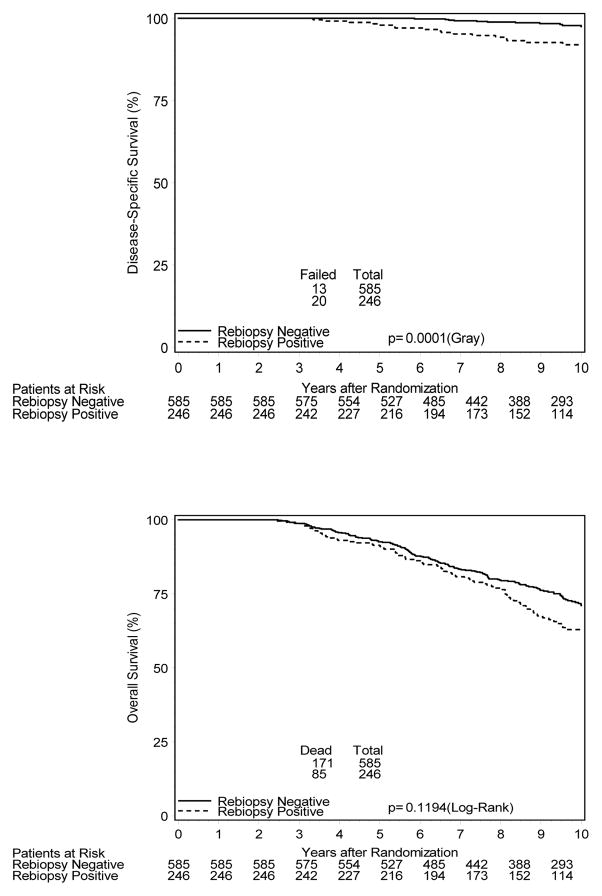
Figure 2 shows cumulative incidence estimates of evaluated clinical endpoints for all biopsied patients. 10-year estimates of biochemical failure (BCF) were 49% vs. 34% [HR=1.7; 95% CI 1.3–2.1], respectively, for patients with positive and negative post-treatment biopsies (p<0.001). Likewise, patients with a positive post-treatment biopsy had higher 10-year estimates of distant metastases (DM) with estimates of 8% vs. 4%, [HR=2.4; 95% CI 1.3–4.4] and inferior disease-specific survival (DSS), 92% vs. 98% [HR=3.8; 95% CI 1.9–7.5]. 10-year overall survival estimates were 63% and 71% [HR=1.23; 95% CI 0.95–1.6] for those with positive and negative biopsies, respectively (p=0.12).
Figure 2.
Cumulative Incidence Outcome Estimates (All Biopsied Patients)
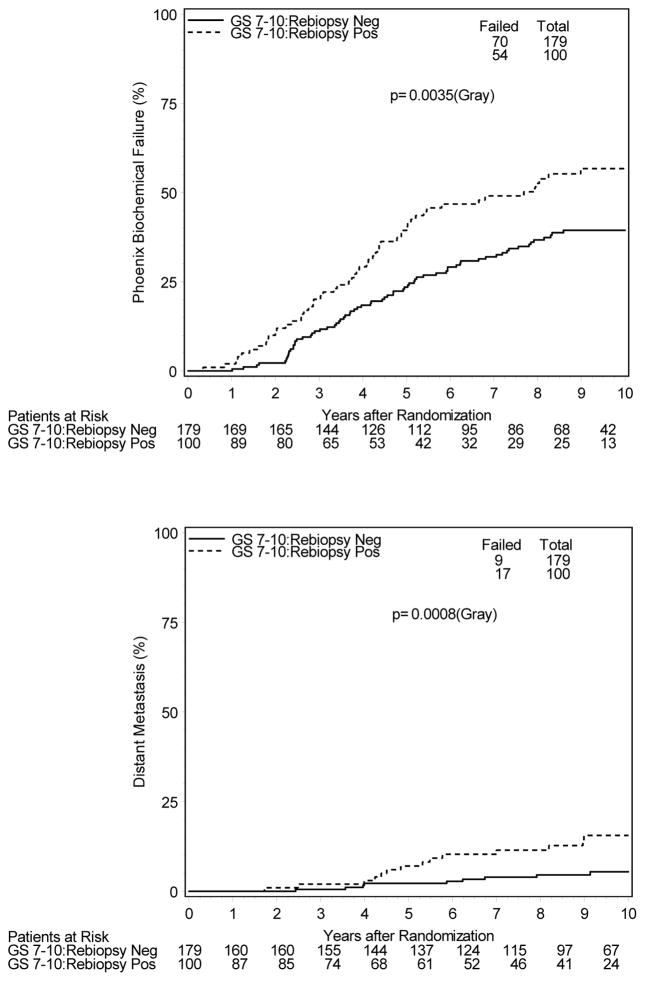
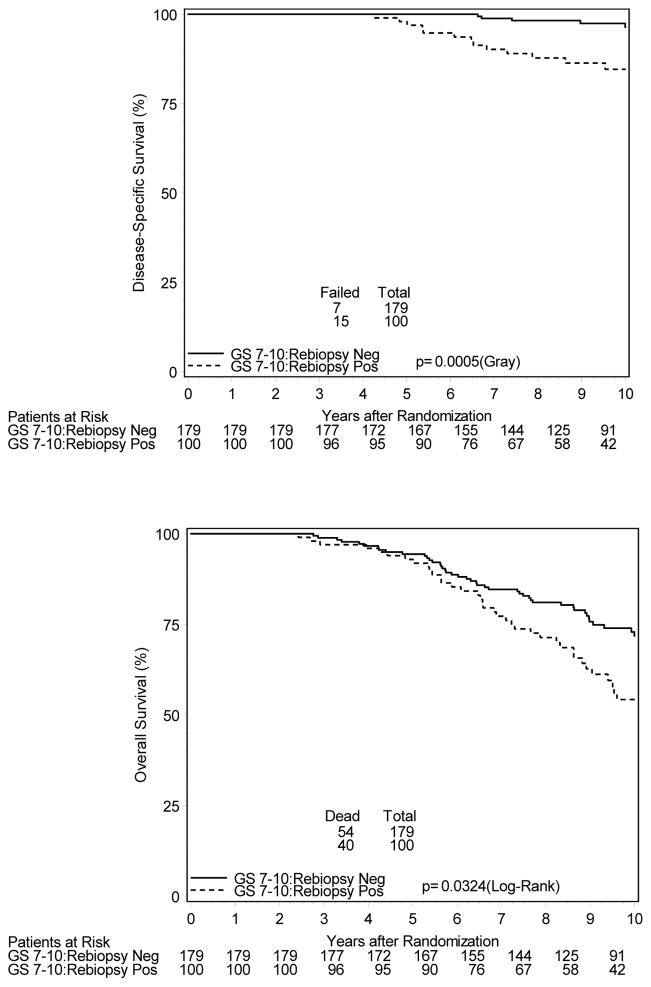
To evaluate potential confounding variable interaction, patients were analyzed separately based on TAS administration, tumor stage, and initial Gleason score, as these were the factors associated with a greater likelihood of a positive post-RT biopsy. Table 3 illustrates increased hazard ratios for biochemical and/or clinical failure regardless of initial presenting features (T1 vs. T2 disease, Gleason score < 7 vs. ≥ 7) or treatment allocation. Of the 433 patients randomized to receive TAS who subsequently underwent post-treatment biopsy, 89 (21%) were found to be positive. These patients had significantly increased 10-year estimates of BCF (44% vs. 29%) and DM (9.0% vs. 3.1%), as well as inferior DSS (89.9% vs. 98.2%). For 398 patients undergoing post-treatment biopsy randomized to RT alone, no statistically significant differences in clinical endpoints were appreciated for positive vs. negative re-biopsied patients. However, BCF estimates were higher for those with a positive biopsy (51.7% vs. 39.9%). Evaluated separately, 451 patients with T2a–T2b disease underwent biopsy with 155 (34%) being positive. Patients with positive post-treatment biopsy had increased rates of BCF (48.7% vs. 32.8%) with a trend toward increased DM (6.7% vs. 4.1%), and DSS was inferior (89.6% vs. 97.3%). Similar adverse findings were noted for the 100 of 279 patients (36%) with Gleason score ≥ 7 who had a positive post-RT biopsy. DM rates at 10 years were 15.6% vs. 5.5% and DSS 84.6% vs. 96.4% for those who were biopsy positive vs. negative, respectively. Additionally, for this subset of patients, 10-year overall survival rates were significantly lower for those with a positive post-RT biopsy at 54% vs. 72% (Figure 3).
Table 3.
10-Year Clinical Outcomes Hazard Ratios for Re-Biopsy Positive Patients [95% Confidence Interval] by Patient Subset; Referenced to Re-Biopsy Negative Patients.
| Biochemical Failure | Distant Metastases | Disease-Specific Survival | Overall Survival | |
|---|---|---|---|---|
| Stage T1 | 1.62 [1.14–2.28] | 2.88 [1.18–7.03] | 1.92 [0.45–8.04] | 1.13 [0.74–1.73] |
| Stage T2a–T2b | 1.76 [1.30–2.39] | 2.14 [0.98–4.71] | 4.31 [1.89–9.84] | 1.27 [0.91–1.78] |
| Gleason 2–6 | 1.63 [1.20–2.21] | 1.11 [0.38–3.21] | 2.33 [0.69–7.24] | 1.06 [0.74–1.51] |
| Gleason 7–10 | 1.68 [1.18–2.39] | 3.67 [1.64–8.21] | 4.43 [1.84–10.7] | 1.56 [1.04–2.35] |
| RT Alone | 1.49 [1.11–2.00] | 1.87 [0.84–4.16] | 2.04 [0.81–5.11] | 1.22 [0.85–1.75] |
| RT + TAS | 1.66 [1.14–2.42] | 3.22 [1.34–7.76] | 7.57 [2.58–22.2] | 1.28 [0.87–1.89] |
RT – radiotherapy; TAS – total androgen suppression
Figure 3.
Cumulative Incidence Estimates of Clinical Outcomes for Gleason score 7–10 Patients Undergoing Post-RT Biopsy
Therapy Effect
All post-treatment biopsies were assessed for presence/degree of therapy effect according to the technique of Dhom and Degro.7 532 of the 831 post-RT biopsy cases had questionnaires answered with respect to the presence vs. absence of therapy effect, and 512 reported some degree of therapy effect present. Only 4 reported absence of therapy effect, and 7 were felt to be inevaluable for this feature. Unfortunately, only 105 cases completed the questionnaire regarding the degree of therapy effect appreciated. Of these, 28 reported minimal, 50 moderate, and 27 marked therapy effect. Given the small patient numbers, no conclusions regarding this finding’s association with clinical outcomes could be evaluated.
Discussion
Repeat transrectal biopsy of the prostate is not routinely performed following the completion of RT. Prostate biopsies are invasive and have associated complication risks including pain, rectal bleeding, urinary tract infection, urinary retention, and, in rare instances, sepsis and death.8–10 The risk of major complications is small and accepted in the setting of initial workup as 12-core systematic sampling is the definitive procedure to diagnose and characterize prostate cancer. However, once a patient has received definitive, local, nonsurgical treatment for prostate cancer, there has yet to be a defined value as a tradeoff for the invasiveness and risks associated with such a procedure. Namely, biopsies are not routinely performed until there is biochemical failure or clinical suspicion of treatment failure/local recurrence.
There have been previous prostate radiotherapy studies that have included as part of the protocol a planned post-treatment biopsy. Crook et al. describe outcomes of 205 men who underwent post-radiotherapy prostate biopsy between 24–30 months post-treatment in the context of a randomized trial evaluating 3 months vs. 8 months of androgen suppression. Only 12% of biopsies were positive with little or no therapy effect, but a significant difference in progression free survival was nonetheless detected between those patients with positive and negative biopsies. This included an increased risk of developing distant metastases and inferior prostate cancer specific mortality.11 Similarly, Zelefsky et al. demonstrated inferior biochemical control and inferior distant metastasis free survival in patients with a positive post-RT biopsy.12 Additional studies examining the clinical implications of a positive post-RT biopsy have shown similar outcomes with respect to biochemical control, but have not demonstrated inferior outcomes with respect to the relevant clinical endpoints of distant metastases and survival.13,14
The large number of patients undergoing post-RT biopsy on RTOG 9408 has enabled a more comprehensive analysis of clinical outcomes than has been done in any prior study. Namely, there were more positive post-treatment biopsies on this study than there were total performed biopsies in nearly all of the other analyses looking at this issue. The number of positive biopsies reported was more than double that of any prior series. As such, not only could clear clinical consequences of a positive post-RT biopsy be defined, but subset analyses could be performed to perhaps identify which patients may be most likely to clinically benefit from such a procedure in the future. While increased rates of distant metastases and inferior cause-specific survival was appreciated for the group as a whole, the relatively low rates of these events may not necessarily warrant the risks/invasiveness of post-RT biopsy on a routine basis. However, for the 279 patients with Gleason score ≥ 7, the estimate of distant metastases jumps to nearly 16% at 10 years and cause-specific survival falls to just below 85%. These numbers could certainly be improved, whether through local salvage therapies or additional androgen suppression, and the high-grade disease subset of patients likely represents the ideal group within which to investigate this issue further.
The strengths of this study rest obviously in the number of patients included, which has enabled the clear demonstration of inferior clinical endpoints for patients with a positive post-RT biopsy. This illustrates the importance of local therapy and that failure to control the local disease within the prostate at an early stage predisposes patients to developing distant metastases and death from cancer. There are, however, several shortcomings that must be considered, especially with respect to the application of this data to contemporary patients undergoing RT for prostate cancer. First, RTOG 9408 did not include patients with locally advanced tumors or very high PSA values. As such, there are certainly subsets of patients to whom this data cannot be applied, subsets that may actually have a greater chance of persistent local disease following RT. Additionally, current standard practice for the 154 high-risk patients that were included in this protocol (Gleason score ≥ 8) would typically include a duration of androgen suppression considerably longer (typically 24–36 months) than that administered in this study.15,16 It remains unclear what impact this would have on the positive biopsy rate. Additionally, radiotherapy was administered using lower doses and what would generally be considered suboptimal techniques by today’s standards [no intensity modulated or image guided radiotherapy (IMRT, IGRT)]. While the clinical benefits of IMRT/IGRT have not been formally demonstrated in prospective, randomized fashion, these are techniques that potentiate safe RT dose escalation, and it is known that dose escalation well beyond what was administered in RTOG 9408 is of biochemical and clinical benefit to patients undergoing prostate RT.17–20 Dose escalation has additionally been shown directly by previous authors to decrease the probability of a positive post-RT biopsy.12,14 Should such techniques significantly reduce the positive post-RT biopsy rate, any clinical benefits to patients based on detection of post-RT residual disease may be more difficult to demonstrate.
An additional interesting finding of this analysis was that of the association between an increased rate of DM and inferior DSS in patients receiving initial TAS + RT while only BCF was inferior for patients treated initially with RT alone. This was true despite an overall lower positive re-biopsy rate for the patients receiving initial TAS. The exact reasons for this finding remain unclear but could potentially be related to a degree of resistance to TAS as a salvage measure in patients exposed to such treatment as initial therapy. This will require corroboration in additional patient series.
Finally, the initial quantification of therapy effect in positive biopsies in this study was suboptimal, incomplete, and done on too few patients to allow for meaningful evaluation of its significance. To have incorporated this data would have required comprehensive pathologic review of the re-biopsy specimens and was beyond the scope and resources available for this analysis. Patients with positive biopsies with severe therapy effect have, historically, been shown to exhibit clinical behavior similar to those patients with negative post-RT biopsies.11,14 For purposes of this analysis, patients exhibiting any degree of therapy effect (including severe) were included in the positive biopsy cohort. Based on this prior evidence, this would only serve to decrease the incidence of adverse clinical outcomes in such patients and would be highly unlikely to alter the findings of this study, specifically, that patients with significant, residual viable cancer following RT are at significantly increased risk for adverse clinical outcomes. This contention is supported by the estimate that less than half (49%) of patients with a positive post-RT biopsy would experience biochemical failure at 10 years, suggesting that a significant percentage of those with “positive” biopsies harbored clinically indolent or even insignificant disease.
Finally, the findings of this analysis beg the question of what could be done for patients found to have residual/recurrent prostate cancer in the context of positive post-treatment biopsy. Data do exist suggesting a clinical advantage for the administration of early vs. delayed salvage treatment in the context of androgen suppressive therapy,21,22 and repeat prostate biopsy has the potential, in appropriately selected patients, to augment that advantage. Local salvage therapies remain less well evaluated but may play a role in such patients with residual disease post-RT. The Radiation Therapy Oncology Group recently completed a study of salvage brachytherapy for men with locally recurrent disease following external beam RT (RTOG 0526). Results are pending, but as local salvage therapies become increasingly investigated, the value of post-RT biopsy could certainly be increased as well.
In summary, patients with a positive post-RT biopsy are more likely to experience adverse clinical outcomes compared to patients with a negative biopsy, illustrating the critical importance of successful local therapy in men with early stage prostate cancer. This was true even after controlling for potential confounding variables such as androgen suppression, presenting T-stage, and Gleason score. While a positive post-RT biopsy was less common in patients randomized to receive TAS, this finding remained predictive of distant metastases and inferior cancer-specific survival in these patients.
To be clear, the purpose of this analysis was not to contend that post-RT biopsy should be performed as routine in the management of early stage prostate cancer, but rather to confirm, in a large, prospectively treated cohort, that failure to control local disease portends inferior clinical outcomes, especially in men with high grade (Gleason score ≥ 7) disease. Future consideration should be given to studies confirming patient subsets most likely to derive benefit from routine post-RT prostate biopsy.
Brief Summary.
831 of the 1979 patients analyzed in prospective, randomized trial RTOG 9408 who had no prior evidence of clinical or biochemical failure underwent repeat prostate biopsy 2 years following completion of radiotherapy (+/− androgen suppression). Those patients with evidence of recurrent/persistent disease on post-radiotherapy biopsy had increased rates of biochemical and distant failure as well as inferior disease-specific survival, emphasizing the importance of local control in the management of early-stage prostate cancer.
Acknowledgments
Grant Acknowledgement: This project was supported by U10CA21661, U10CA180868, U10CA180822 from the National Cancer Institute (NCI). This project is funded, in part, under a grant with the Pennsylvania Department of Health. The Department specifically declaims responsibility for any analyses, interpretations or conclusions.
Footnotes
Original Presentation: American Society of Therapeutic Radiology and Oncology (ASTRO) Annual Meeting: Atlanta, GA; September, 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cox JD, Gallagher MJ, Hammond EH, et al. Consensus statements on radiation therapy of prostate cancer: guidelines for prostate re-biopsy after radiation and for radiation therapy with rising prostate-specific antigen levels after radical prostatectomy. J Clin Oncol. 1999;17:1155–1163. doi: 10.1200/JCO.1999.17.4.1155. [DOI] [PubMed] [Google Scholar]
- 2.Jones C, Hunt D, McGowan D, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. NEJM. 2011;365:107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:447–457. [Google Scholar]
- 4.Cox DR. Regression models and life tables. J Royal Statistics Soc. 1972;34:187–220. [Google Scholar]
- 5.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annual Statistics. 1988;16:1141–1143. [Google Scholar]
- 6.Fine J, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 7.Dhom G, Degro S. Therapy of prostate cancer and histopathologic follow up. Prostate. 1982;3:531–542. doi: 10.1002/pros.2990030602. [DOI] [PubMed] [Google Scholar]
- 8.Djavan B, Waldert M, Zlotta A, et al. Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: results of a prospective European prostate cancer detection study. J Urol. 2001;166:856–60. [PubMed] [Google Scholar]
- 9.Collins G, Lloyd S, Hehir M, et al. Multiple transrectal unltrashound-guided prostatic biopsies – true morbidity and patient acceptance. Br J Urol. 1993;71:460. doi: 10.1111/j.1464-410x.1993.tb15993.x. [DOI] [PubMed] [Google Scholar]
- 10.Aus G, Ahlgren G, Bergdahl S, et al. Infection after transrectal core biopsies of the prostate – risk factors and antibiotic prophylaxis. Br J Urol. 1996;77:851. doi: 10.1046/j.1464-410x.1996.01014.x. [DOI] [PubMed] [Google Scholar]
- 11.Crook J, Malone S, Perry G, et al. Twenty-four month postradiation prostate biopsies are strongly predictive of 7-year disease-free survival. Cancer. 2009;115:673–679. doi: 10.1002/cncr.24020. [DOI] [PubMed] [Google Scholar]
- 12.Zelefsky M, Reuter V, Fuks Z, et al. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J Urol. 2008;179:1368–1373. doi: 10.1016/j.juro.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vance W, Tucker S, De Crevoisier R, et al. The predictive value of 2-year posttreatment biopsy after prostate cancer radiotherapy for eventual biochemical outcome. Int J Radiat Oncol Biol Phys. 2007;67:828–833. doi: 10.1016/j.ijrobp.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 14.Kestin L, Goldstein N, Vicini F, et al. Pathologic evidence of dose-response and dose-volume relationships for prostate cancer treated with combined external beam radiotherapy and high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2002;54:107–118. doi: 10.1016/s0360-3016(02)02925-5. [DOI] [PubMed] [Google Scholar]
- 15.Bolla M, de Reijke T, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. NEJM. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 16.Hanks G, Pajak T, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group protocol 92-02. J Clin Oncol. 2003;21:3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Kuban D, Tucker S, Dong L, et al. Long-term results of the MD Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 18.Heemsbergen WD, Al-Mamgani A, Slot A, Dielwart MFH, Lebesque JV. Long-term results of the Dutch randomized prostate cancer trial: Impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol. 2014;110:104–109. doi: 10.1016/j.radonc.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Zeitman A, DeSilvio M, Slater J, et al. Comparison of conventional-dose vs. high-dose conformal radiation therapy in localized adenocarcinoma of the prostate. JAMA. 2005;294:1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 20.Peeters S, Heemsbergen W, Coper P, et al. Dose response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 21.Souhami L, Bae K, Pilepich M, Sandler H. Timing of salvage hormonal therapy in prostate cancer patients with unfavorable prognosis treated with radiotherapy: a secondary analysis of Radiation Therapy Oncology Group 85-31. Int J Radiat Oncol Biol Phys. 2010;78:1301–1306. doi: 10.1016/j.ijrobp.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Shipley WU, Desilvio M, Pilepich MV, et al. Early initiation of salvage hormone therapy influences survival in patients who failed initial radiation for locally advanced prostate cancer: A secondary analysis of RTOG protocol 86-10. Int J Radiat Oncol Biol Phys. 2006;64:1162–1167. doi: 10.1016/j.ijrobp.2005.09.039. [DOI] [PubMed] [Google Scholar]