Abstract
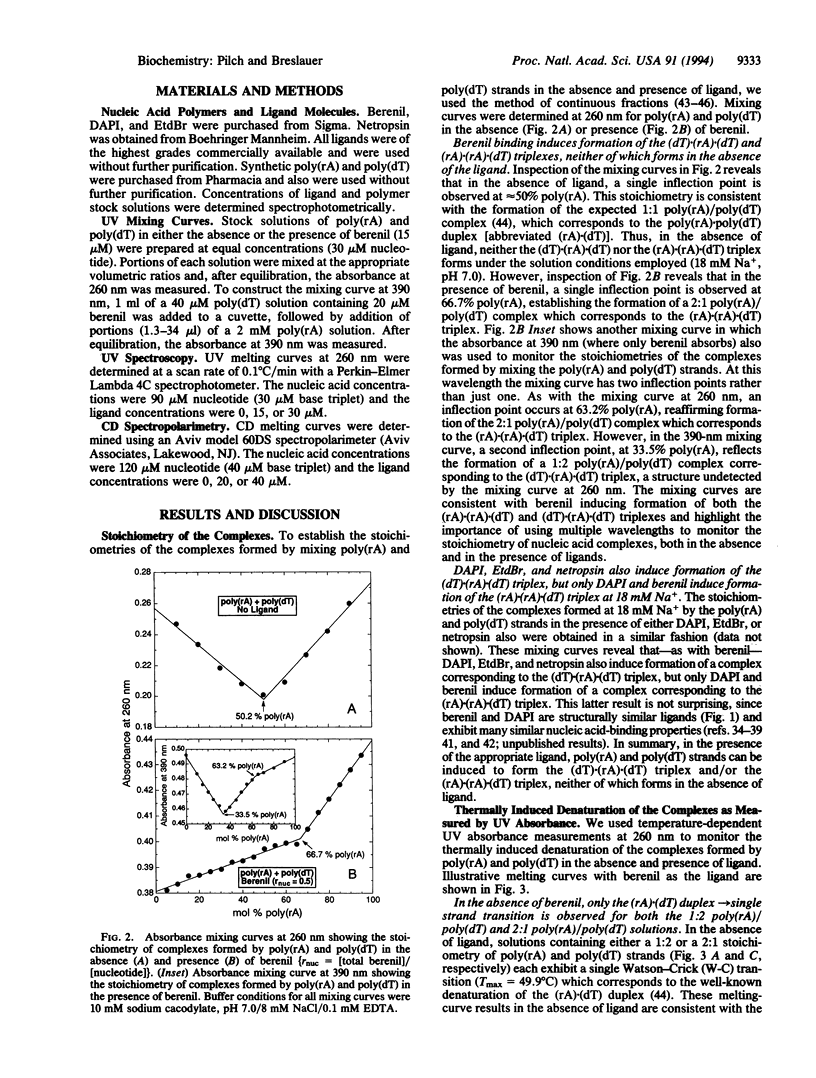
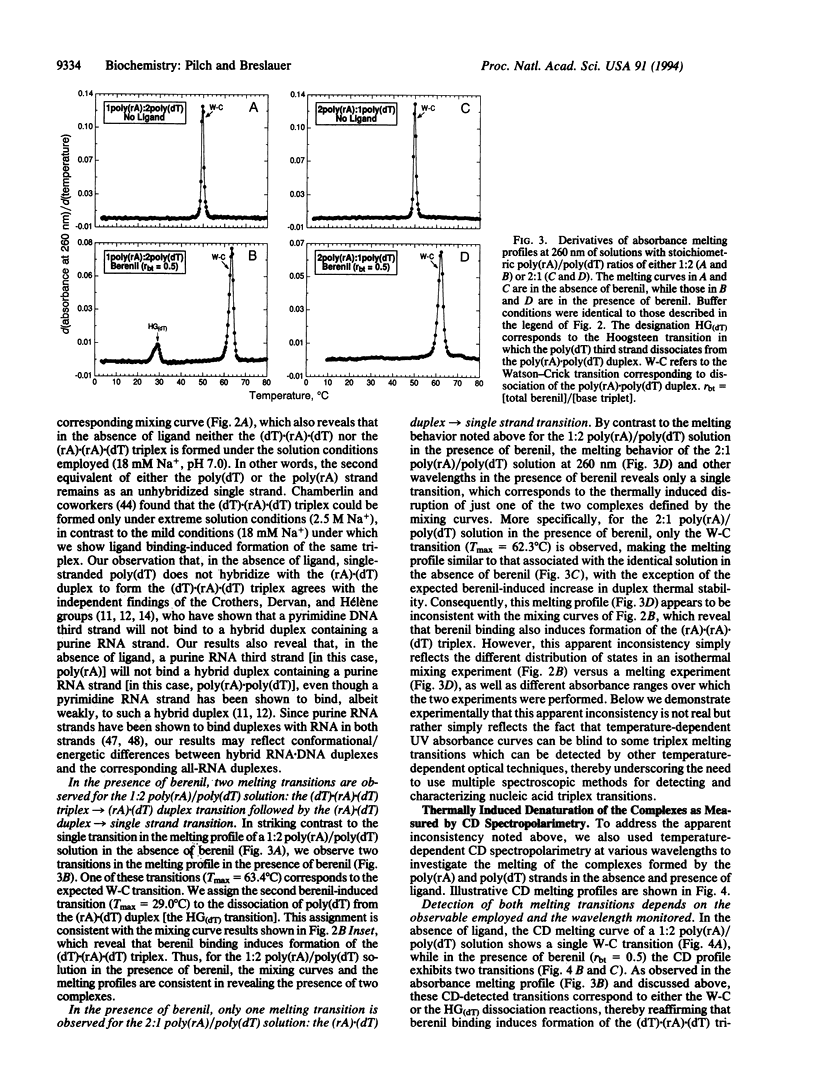
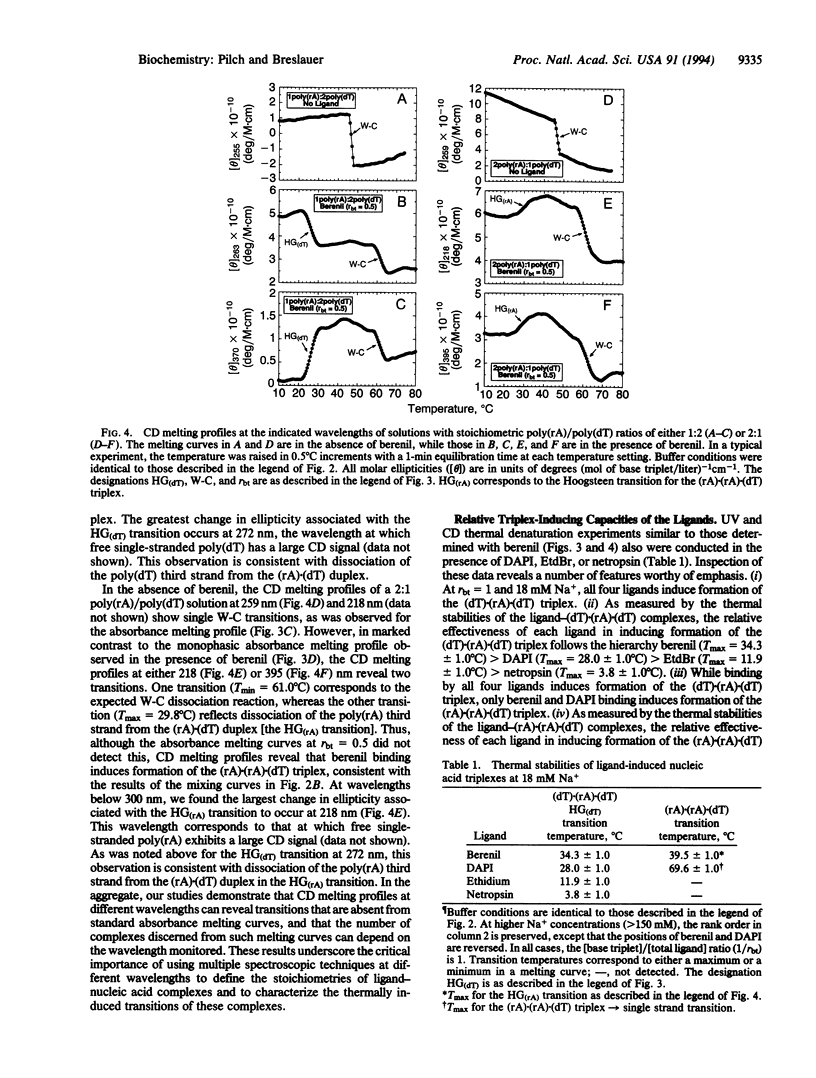
We demonstrate that ligand binding can be used to induce the formation of triplex structures that would not otherwise form. Specifically, we show that binding of berenil or 4',6-diamidino-2-phenylindole DAPI) induces formation of the poly(rA).poly(rA).poly(dT) triplex, providing an example of an RNA(purine).RNA(purine).DNA(pyrimidine) triplex. We also show that binding of berenil, DAPI, ethidium, or netropsin can induce formation of the poly(dT).poly(rA).poly(dT) triplex, thereby overcoming a practical limitation to the formation of DNA.RNA.DNA triplexes with a purine RNA strand. Based on the enhanced thermal stabilities of the drug-bound poly(dT).poly(rA).poly(dT) complexes at 18 mM Na+, we define the relative triplex-inducing efficiencies of these four ligands to be: berenil > DAPI > ethidium > netropsin. Our results demonstrate that ligand binding can be used to induce the formation of triplex structures that do not form in the absence of the ligand. This triplex-inducing capacity has potentially important implications in the design of novel antisense, antigene, and diagnostic strategies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braithwaite A. W., Baguley B. C. Existence of an extended series of antitumor compounds which bind to deoxyribonucleic acid by nonintercalative means. Biochemistry. 1980 Mar 18;19(6):1101–1106. doi: 10.1021/bi00547a009. [DOI] [PubMed] [Google Scholar]
- Bresloff J. L., Crothers D. M. Equilibrium studies of ethidium--polynucleotide interactions. Biochemistry. 1981 Jun 9;20(12):3547–3553. doi: 10.1021/bi00515a038. [DOI] [PubMed] [Google Scholar]
- Broitman S. L., Im D. D., Fresco J. R. Formation of the triple-stranded polynucleotide helix, poly(A.A.U). Proc Natl Acad Sci U S A. 1987 Aug;84(15):5120–5124. doi: 10.1073/pnas.84.15.5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. G., Sanderson M. R., Garman E., Neidle S. Crystal structure of a berenil-d(CGCAAATTTGCG) complex. An example of drug-DNA recognition based on sequence-dependent structural features. J Mol Biol. 1992 Jul 20;226(2):481–490. doi: 10.1016/0022-2836(92)90962-j. [DOI] [PubMed] [Google Scholar]
- Chastain M., Tinoco I., Jr Poly(rA) binds poly(rG).poly(rC) to form a triple helix. Nucleic Acids Res. 1992 Jan 25;20(2):315–318. doi: 10.1093/nar/20.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney M., Czernuszewicz G., Postel E. H., Flint S. J., Hogan M. E. Site-specific oligonucleotide binding represses transcription of the human c-myc gene in vitro. Science. 1988 Jul 22;241(4864):456–459. doi: 10.1126/science.3293213. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Waring M. J. Supercoiling of polyoma virus DNA measured by its interaction with ethidium bromide. J Mol Biol. 1967 Apr 14;25(1):23–30. doi: 10.1016/0022-2836(67)90276-8. [DOI] [PubMed] [Google Scholar]
- Durand M., Thuong N. T., Maurizot J. C. Binding of netropsin to a DNA triple helix. J Biol Chem. 1992 Dec 5;267(34):24394–24399. [PubMed] [Google Scholar]
- Duval-Valentin G., Thuong N. T., Hélène C. Specific inhibition of transcription by triple helix-forming oligonucleotides. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):504–508. doi: 10.1073/pnas.89.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudé C., François J. C., Sun J. S., Ott G., Sprinzl M., Garestier T., Hélène C. Stability of triple helices containing RNA and DNA strands: experimental and molecular modeling studies. Nucleic Acids Res. 1993 Dec 11;21(24):5547–5553. doi: 10.1093/nar/21.24.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Dervan P. B. Sequence-specific recognition of double helical RNA and RNA.DNA by triple helix formation. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3806–3810. doi: 10.1073/pnas.90.9.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C. The anti-gene strategy: control of gene expression by triplex-forming-oligonucleotides. Anticancer Drug Des. 1991 Dec;6(6):569–584. [PubMed] [Google Scholar]
- Kohlstaedt L. A., Wang J., Friedman J. M., Rice P. A., Steitz T. A. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992 Jun 26;256(5065):1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Lehrman E. A., Crothers D. M. An ethidium-induced double helix of poly(dA)-poly(rU). Nucleic Acids Res. 1977;4(5):1381–1392. doi: 10.1093/nar/4.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dervan P. B., Wold B. Analysis of promoter-specific repression by triple-helical DNA complexes in a eukaryotic cell-free transcription system. Biochemistry. 1992 Jan 14;31(1):70–81. doi: 10.1021/bi00116a012. [DOI] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Wold B., Dervan P. B. Inhibition of DNA binding proteins by oligonucleotide-directed triple helix formation. Science. 1989 Aug 18;245(4919):725–730. doi: 10.1126/science.2549631. [DOI] [PubMed] [Google Scholar]
- Mergny J. L., Collier D., Rougée M., Montenay-Garestier T., Hélène C. Intercalation of ethidium bromide into a triple-stranded oligonucleotide. Nucleic Acids Res. 1991 Apr 11;19(7):1521–1526. doi: 10.1093/nar/19.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergny J. L., Duval-Valentin G., Nguyen C. H., Perrouault L., Faucon B., Rougée M., Montenay-Garestier T., Bisagni E., Hélène C. Triple helix-specific ligands. Science. 1992 Jun 19;256(5064):1681–1684. doi: 10.1126/science.256.5064.1681. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Nelson J. W., Tinoco I., Jr Intercalation of ethidium ion into DNA and RNA oligonucleotides. Biopolymers. 1984 Feb;23(2):213–233. doi: 10.1002/bip.360230205. [DOI] [PubMed] [Google Scholar]
- Paoletti J., Le Pecq J. B. Resonance energy transfer between ethidium bromide molecules bound to nucleic acids. Does intercalation wind or unwind the DNA helix? J Mol Biol. 1971 Jul 14;59(1):43–62. doi: 10.1016/0022-2836(71)90412-8. [DOI] [PubMed] [Google Scholar]
- Park Y. W., Breslauer K. J. Drug binding to higher ordered DNA structures: netropsin complexation with a nucleic acid triple helix. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6653–6657. doi: 10.1073/pnas.89.14.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrouault L., Asseline U., Rivalle C., Thuong N. T., Bisagni E., Giovannangeli C., Le Doan T., Hélène C. Sequence-specific artificial photo-induced endonucleases based on triple helix-forming oligonucleotides. Nature. 1990 Mar 22;344(6264):358–360. doi: 10.1038/344358a0. [DOI] [PubMed] [Google Scholar]
- Pilch D. S., Levenson C., Shafer R. H. Structural analysis of the (dA)10.2(dT)10 triple helix. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1942–1946. doi: 10.1073/pnas.87.5.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch D. S., Waring M. J., Sun J. S., Rougée M., Nguyen C. H., Bisagni E., Garestier T., Hélène C. Characterization of a triple helix-specific ligand. BePI (3-methoxy-7H-8-methyl-11- [(3'-amino)propylamino]-benzo[e]pyrido[4,3-b]indole) intercalates into both double-helical and triple-helical DNA. J Mol Biol. 1993 Aug 5;232(3):926–946. doi: 10.1006/jmbi.1993.1440. [DOI] [PubMed] [Google Scholar]
- Plum G. E., Park Y. W., Singleton S. F., Dervan P. B., Breslauer K. J. Thermodynamic characterization of the stability and the melting behavior of a DNA triplex: a spectroscopic and calorimetric study. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9436–9440. doi: 10.1073/pnas.87.23.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal J., Waring M. J. Antibiotics which can alter the rotational orientation of nucleosome core DNA. Nucleic Acids Res. 1986 Nov 25;14(22):8735–8754. doi: 10.1093/nar/14.22.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M., Maling B. Physical and chemical characterization of two- and three-stranded adenine-thymine and adenine-uracil homopolymer complexes. J Mol Biol. 1966 Sep;20(2):359–389. doi: 10.1016/0022-2836(66)90069-6. [DOI] [PubMed] [Google Scholar]
- Roberts R. W., Crothers D. M. Stability and properties of double and triple helices: dramatic effects of RNA or DNA backbone composition. Science. 1992 Nov 27;258(5087):1463–1466. doi: 10.1126/science.1279808. [DOI] [PubMed] [Google Scholar]
- Scaria P. V., Shafer R. H. Binding of ethidium bromide to a DNA triple helix. Evidence for intercalation. J Biol Chem. 1991 Mar 25;266(9):5417–5423. [PubMed] [Google Scholar]
- Schmitz H. U., Hübner W. A thermodynamic and spectroscopic study on the binding of berenil to poly d(AT) and to poly (dA) x poly (dT). Biophys Chem. 1993 Nov;48(1):61–74. doi: 10.1016/0301-4622(93)80042-h. [DOI] [PubMed] [Google Scholar]
- Skoog J. U., Maher L. J., 3rd Repression of bacteriophage promoters by DNA and RNA oligonucleotides. Nucleic Acids Res. 1993 May 11;21(9):2131–2138. doi: 10.1093/nar/21.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]
- Sun J. S., François J. C., Montenay-Garestier T., Saison-Behmoaras T., Roig V., Thuong N. T., Hélène C. Sequence-specific intercalating agents: intercalation at specific sequences on duplex DNA via major groove recognition by oligonucleotide-intercalator conjugates. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9198–9202. doi: 10.1073/pnas.86.23.9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. S., Lavery R., Chomilier J., Zakrzewska K., Montenay-Garestier T., Hélène C. Theoretical study of ethidium intercalation in triple-stranded DNA and at triplex-duplex junctions. J Biomol Struct Dyn. 1991 Dec;9(3):425–436. doi: 10.1080/07391102.1991.10507926. [DOI] [PubMed] [Google Scholar]
- Tanious F. A., Veal J. M., Buczak H., Ratmeyer L. S., Wilson W. D. DAPI (4',6-diamidino-2-phenylindole) binds differently to DNA and RNA: minor-groove binding at AT sites and intercalation at AU sites. Biochemistry. 1992 Mar 31;31(12):3103–3112. doi: 10.1021/bi00127a010. [DOI] [PubMed] [Google Scholar]
- Waring M. J. Complex formation between ethidium bromide and nucleic acids. J Mol Biol. 1965 Aug;13(1):269–282. doi: 10.1016/s0022-2836(65)80096-1. [DOI] [PubMed] [Google Scholar]
- Waring M. J. Stablilzation of two-standard ribohomopolymer helices and destabilzation of a three-stranded helix by ethidium bromide. Biochem J. 1974 Nov;143(2):483–486. doi: 10.1042/bj1430483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Collier D. A., Hanvey J. C., Shimizu M., Wohlrab F. The chemistry and biology of unusual DNA structures adopted by oligopurine.oligopyrimidine sequences. FASEB J. 1988 Nov;2(14):2939–2949. [PubMed] [Google Scholar]
- Wilson W. D., Tanious F. A., Barton H. J., Jones R. L., Fox K., Wydra R. L., Strekowski L. DNA sequence dependent binding modes of 4',6-diamidino-2-phenylindole (DAPI). Biochemistry. 1990 Sep 11;29(36):8452–8461. doi: 10.1021/bi00488a036. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Tanious F. A., Mizan S., Yao S., Kiselyov A. S., Zon G., Strekowski L. DNA triple-helix specific intercalators as antigene enhancers: unfused aromatic cations. Biochemistry. 1993 Oct 12;32(40):10614–10621. doi: 10.1021/bi00091a011. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Banville D. L., Shafer R. H. Structural analysis of d(GCAATTGC)2 and its complex with berenil by nuclear magnetic resonance spectroscopy. Biochemistry. 1990 Jul 17;29(28):6585–6592. doi: 10.1021/bi00480a006. [DOI] [PubMed] [Google Scholar]
- Young S. L., Krawczyk S. H., Matteucci M. D., Toole J. J. Triple helix formation inhibits transcription elongation in vitro. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10023–10026. doi: 10.1073/pnas.88.22.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C., Wähnert U. Nonintercalating DNA-binding ligands: specificity of the interaction and their use as tools in biophysical, biochemical and biological investigations of the genetic material. Prog Biophys Mol Biol. 1986;47(1):31–112. doi: 10.1016/0079-6107(86)90005-2. [DOI] [PubMed] [Google Scholar]



