Abstract
Histamine, a biogenic amine, is a neurotransmitter in neurons and sensory receptors in invertebrates. Histamine has rarely been reported in bivalves. We used HPLC with pre-column derivatization using 2,3-naphthalenedicarboxaldehyde (NDA) as a fluorescent labeling agent to measure histamine in ganglia, and peripheral tissues of the oyster Crassostrea virginica. We also used Western Blot technique to look for the presence of a histamine receptor in the mantle rim. HPLC results found histamine present in ng amounts in both the cerebral and visceral ganglia, as well as the mantle rim and other peripheral tissues of C. virginica. The study confirms and quantifies histamine as an endogenous biogenic amine in C. virginica in the nervous system and innervated organs. Western Blot technique also identified a histamine H2-like receptor present in sensory tissue of the oyster's mantle rim.
Introduction
Histamine is a biogenic amine found in a wide variety of invertebrates, where it has been found to be involved in local immune responses as well as regulating physiological function in the gut. It also functions as a neurotransmitter, especially for sensory systems1. Histamine has been well studied in arthropods and gastropods, but has been rarely reported to be present or have a function in bivalves other than the limited reports identifying it in ganglia and nerve fibers of the Baltic clam, Macoma balthica2,3. In the gastropod Aplysia, histamine neurons are involved in feeding and respiration4-12. In insects and other arthropods, histamine neurons have been shown to be involved in photoreception13-15.
Bivalves, including the oyster, Crassostrea virginica, contain dopamine, serotonin and other biogenic amines in their nervous system and peripheral tissues. These biogenic amines serve as neurotransmitters and neurohormones and are important in the physiological functioning of the animals16-18. C. virginica has a reciprocal dopaminergic and serotonergic innervation of the lateral ciliated cells of the gill, originating in the cerebral and visceral ganglia, which slow down and speed up the beating rates of the cilia, respectively19. This neurophsyiological system is a useful model with which to study the actions of these and other biogenic amines. A preliminary physiology study in our lab indicates that histamine may be involved in a sensory-motor integrative response between the animal's sensory system in the mantle rim and beating of the gill lateral cell cilia. The pharmacology suggests the presence of a histamine H2-like receptor in the mantle rim tissue based on the responses to histamine receptor agonists and antagonists20.
In the present study we used HPLC to identify and quantify histamine in the nervous system and innervated organs of C. virginica, and immunoblotting to determine if histamine receptors are present in the sensory tissue of the mantle rim. Historically, methods to quantify histamine in tissue samples have had low accuracy and specificity. Recently a new HPLC analytical method, using pre-column derivatization with 2,3-naphthalene-dicarboxaldehyde (NDA) followed by fluorescence detection, showed improved accuracy and detection sensitivity for histamine21,22. We used this pre-column NDA derivatization technique in the present study.
Materials and Methods
Adult C. virginica of approximately 80 mm shell length were obtained from Blue Island Oyster Company, Sayville, NY, and maintained in the lab for up to two weeks in temperature regulated aquaria containing Instant Ocean® artificial sea water (ASW) at 16-18° C, specific gravity of 1.024 ± 0.001, 31.9 ppt salinity and pH of 7.8 ± 0.2. Animals that fully closed in response to tactile stimulation and required at least moderate hand pressure to being opened were used in each experiment.
NDA and histamine were obtained from Sigma-Aldrich (St. Louis, MO). Gemini 5μ C18 reverse phase HPLC columns were obtained from Phenomenex (Torrance, CA). NP-40 lysis buffer, Bradford reagent, Laemmli 2× loading buffer with β-mercaptoethenol (βME), Bio-Rad Mini-Protean TGX gels (10%), Bio-Rad Precision Plus Protein WesternC Standards, Tris/glycine SDS buffer and Bio-Rad Precision Protein StrepTactin-HRP conjugate were obtained from Bio-Rad.
Goat polyclonal anti-histamine H2 receptor 1° antibody (sc19773) and chicken anti-goat IgG-HRP 2° antibody (sc2953) were obtained from Santa Cruz Biotechnology. CN/DAB Substrate, Pierce Western Blot Signal Enhancer and all other reagents were obtained from Fisher Scientific.
Sample Preparation for HPLC Analysis
Right shells of animals were removed and mantle rim, mantle, heart, palps, posterior adductor muscle and gill were dissected, blotted dry, weighed. Approximately 1 g of each tissue was placed in eppendorf tubes containing 2 ml of 0.4M hydrochloric acid on ice. Cerebral ganglia and visceral ganglia were removed and pooled from 6 and 8 animals, respectively, and placed in eppendorf tubes containing 1 ml of hydrochloric acid on ice. Ganglia and tissues samples then were homogenized on ice with a Brinkman Polytron and centrifuged at 2,000 × g for 20 minutes at 3° C. The supernatants were re-centrifuge at 15,000 × g for 20 minutes. The resulting supernatants were vacuum filtered through 0.24 micron Millipore filters and the filtrates kept on ice for the derivatization reaction.
NDA Derivatization Reaction
Histamine standards and tissue fltrates were adjusted to pH 9.5 with NaOH. Aliquots (0.6 ml) of each standard or filtrate were derivatized at room temperature by adding in sequence: 0.2 ml borate buffer (20 mM, 10% v/v acetonitrile, pH 9.5), 0.2 ml potassium cyanide (20 mM) and 0.4 ml NDA (0.3 mM in methanol). After exactly 15 minutes of derivatization an aliquot of each derivitized sample was injected into the HPLC for separation and analysis.
HPLC Analysis and Sample Detection
Aliquots (20 μl) of derivatized samples were injected into a Beckman System Gold HPLC fitted with a Phenomenex-Gemini 5μ C18 reverse phase column and a guard column. The isocratic mobile phase (40/60 v/v acetonitrile /phosphate buffer, 50 mM, pH 6.8) had a flow rate of 2 ml/min. To detect and quantify derivatized histamine, the effluent from the HPLC column flowed through a Jasco FP 2020 Plus Spectrofluorometer fitted with a 16 μl flow cell set for 450 nm excitation and 484 nm emission. A histamine standard curve was generated and used to quantify histamine levels in the samples. Results are reported as ng/g wet weight for peripheral tissues and ng/ganglion for cerebral and visceral ganglia.
Fluorescence intensity of samples produce by NDA derivatization was time dependent. This method was very sensitive at quantifying histamine, but samples had to be derivatized one at a time to ensure that the reaction time was the same for each sample prior to HPLC injection. We found 15 minutes of derivatization was optimal and provided consistent results for both standards and tissue samples. If samples were allowed to derivatize longer than 30 minute before injection there was a marked decrease in fluorescence intensity.
Sample Preparation for Western Blot
Preparation of Tissue Lysate
Mantle rim tissue was dissected, rinsed well in ASW, blotted, cut into ½ inch segments and weighed. Each segment was placed into an Eppendorf tube with 2.5 mL of ice cold NP-40 lysis buffer (containing protease and phosphatase inhibitors), and sonicated on ice for 2-3, 5 sec bursts with a Brinkman Polytron. Sonicated samples were kept for 30 min on ice, then centrifuged at 10,000 × g for 20 min and pellets discarded. The lysate supernatants were pooled and aliquots were analyzed for protein concentration by Bradford assay. Aliquots of the remaining lysate were adjusted to a protein concentration of 4-5 mg/mL and stored at -80° C.
Preparation of Samples for Loading onto SDS-PAGE Gels
Lysate proteins were denatured by mixing aliquots in a 1:1 ratio with Laemmli 2× loading buffer containing βME and allowing the mixture to sit for one hour at room temperature. Laemmli-treated samples (20-40 μg total protein) were wet -loaded into wells of polyacrylamide gels (Bio-Rad Mini-Protean TGX gels, 10%), alongside pre-stained molecular weight markers (Bio-Rad Precision Plus Protein WesternC Standards), then electrophoresed in Tris/glycine SDS buffer (25 mM Tris, 190 mM glycine, 20% methanol, 0.1% SDS, pH 8.3), for approximately 1 hour at 150 v.
Western Blotting
Gels were removed from their plate, and before immunoblotting was started, the pre-stained WesternC standards were visualization to ensure that proteins migrated uniformly and evenly during electrophoresis. Gels were rinsed in transfer buffer (25 mM Tris, 190 mM glycine, 20% methanol, pH 8.3), and sandwiched onto nitrocellulose membranes. Wet-transfer was done in a Mini Trans-BlotR electrophoretic cell (Bio-Rad) under constant current at 20 v for 150 min in the presence of a cooling module to prevent excess heating. After transfer, membranes were rinsed with ddH2O, treated with a Western Blot Signal Enhancer (Pierce), rinsed 5× with ddH2O, and then blocked with 5% non-fat dry milk in TBS-T (tris buffered saline, 0.1% Tween-20) for 1 hour at room temperature. Membranes were treated with the 1° antibody (goat polyclonal anti-histamine H2 receptor) at 1:300 dilution (TBS-T and 2% blocker) for 24 hours at 4° C, followed by extensive washing with TBS-T. Membranes were then exposed to the 2° antibody (chicken anti-goat IgG-HRP) 1:7000 dilution for 60 min at room temperature. During this time, Precision Protein StrepTactin-HRP conjugate (Bio-Rad) was also present (1:5000 dilution) to resolve protein standards on the nitrocellulose membrane. Membranes were washed extensively with PBS-T (phosphate buffered saline with Tween), followed by chromogenic detection of HRP-conjugated standards and histamine H2 receptor protein using CN/DAB Substrate (Fisher Scientific). After membrane blots were chromogenically developed, images were captured using a Carestream Gl212 Pro Molecular Imaging System.
Results
HPLC
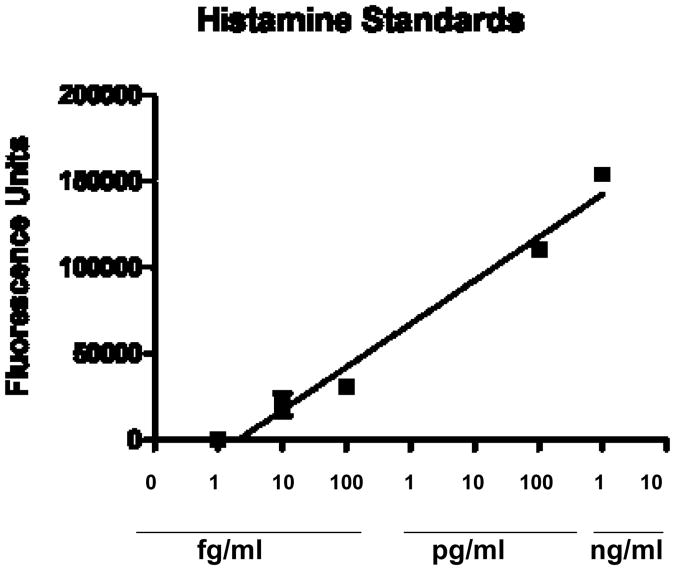
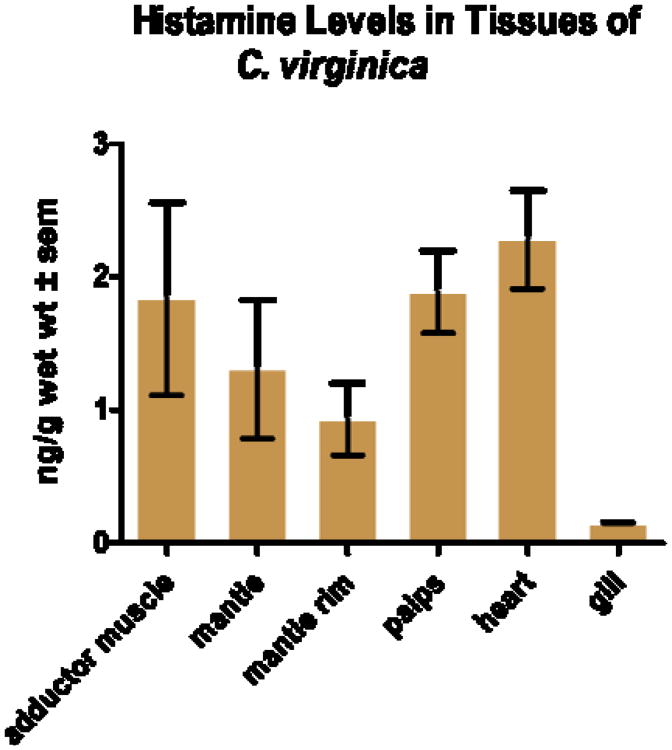
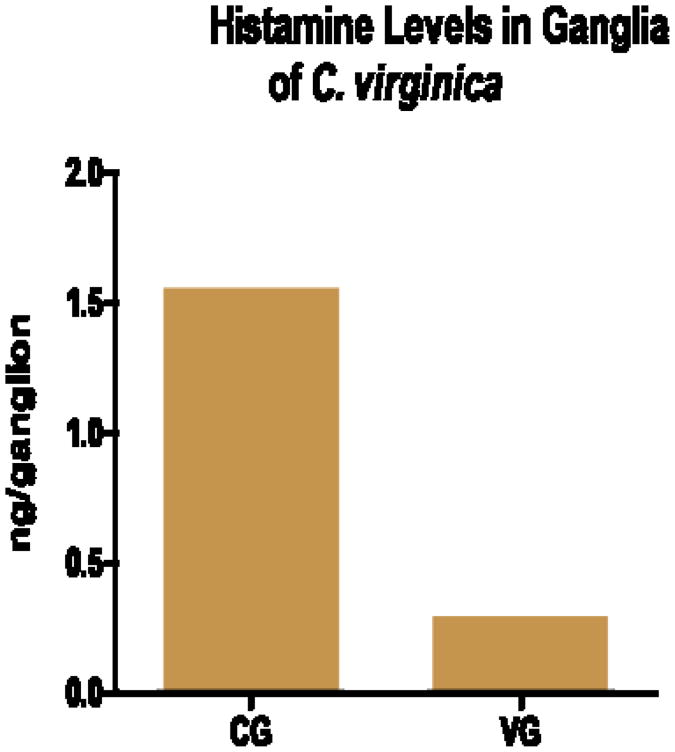
The HPLC parameters used were able to resolve NDA derivatized histamine in the standards and tissue samples as a strong peak with a retention time of 6.6 minutes (Fig. 2). The NDA derivatization reaction produced a fairly linear concentration response for histamine standards over the range of 1 femtogram to 1 nanogram (Fig. 3). HPLC analysis revealed histamine in peripheral tissues ranging from about 1.0 - 2.5 ng/g wet weight, with heart having the highest amounts and gill the least (Fig. 4). Histamine also was detectable in both cerebral and visceral ganglia with cerebral ganglia containing about 1.5 ng/ganglion and visceral ganglia about 0.25 ng/ ganglion (Fig. 5). Co-injecting histamine standards and tissue samples revealed a single derivatized histamine peak. Other amines tested with this NDA-derivatization procedure, including GABA, glutamine, dopamine, serotonin and histadine, did not have retention times similar to that of histamine.
Fig. 2.
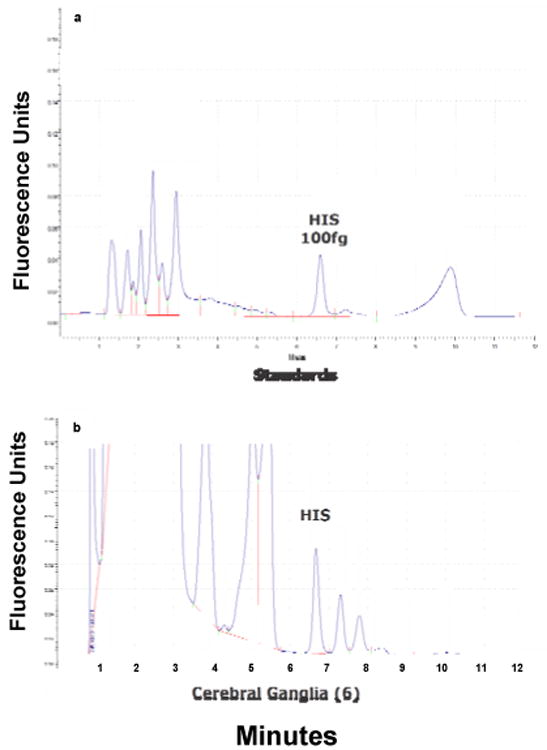
a. HPLC trace of 100 fg of histamine standard; b. HPLC trace of 6 (3 pairs) of pooled cerebral ganglia.
Fig. 3.
Standard curve for histamine using HPLC and fluorescence detection with pre-column derivatization with NDA.
Fig. 4.
Histamine levels (ng/g ± sem) in adductor muscle, mantle, mantle rim, palps, heart and gill of the oyster C. virginica detected by HPLC with fluorescence detection. N = 2.
Fig. 5.
Histamine levels (ng/ganglion) in the cerebral ganglia (CG) and visceral ganglia (VG) of the oyster C. virginica detected by HPLC with fluorescence detection. For the CG, ganglia from 3 animals were pooled for analysis. For the VG, ganglia from 4 animals were pooled.
Western Blot
Western Blot analysis of C. virginica mantle rim proteins revealed the presence of histamine H2-like receptors. A protein band of 70 kDa, corresponding to histamine H2 receptors is shown in Fig. 6.
Fig. 6.
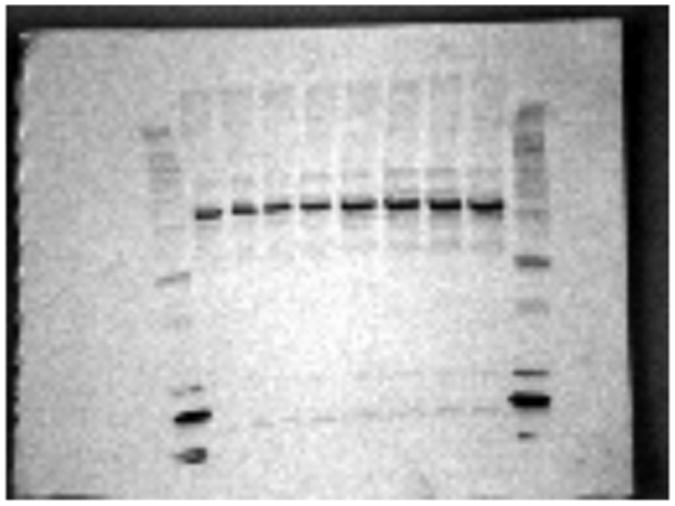
Western Blot of mantle rim showing a protein band of approximately 70 kDa indicating the presence of an H2 histamine receptor.
Discussion
Histamine is an important bioactive compound, serving as a neurotransmitter and neurohormone in invertebrates, but has rarely been studied in bivalves. Histamine is considered to be a universal neurotransmitter of arthropod photoreceptors23. Histamine is a neurotransmitter of statocyst hair cells and involved in graviception In gastropods, and it has been suggested that histaminergic involvement in graviception may be a general feature of many molluscs24.
Preliminary work with C. virginica in our lab showed histamine to be involved in a sensory response of the mantle rim that effects gill lateral cilia beating rates25. These findings prompted us to look for histamine in various tissues and ganglia of C. virginica using a recently developed HPLC method with NDA pre-column derivatization. In the present study we found histamine in small amounts in oyster peripheral tissues (ng/g amounts) and ganglia (ng/ganglion). For comparison, other biogenic amines present in C. virginica such as serotonin and dopamine are about 100 - 200× higher in peripheral tissues and about 10× higher in ganglia26.
In this study we also were able to use immunoblotting to demonstrate the presence of a strong band at 70 kDa correlating with histamine H2 receptors. Our Western Blot results match well with that of Matsuda et al. who found a 69 kDa band identified as histamine H2 receptor proteins in rat and human tissues27.
The mantle rim of bivalves is a sensory structure containing various sensory receptors. The involvement of histamine in sensory systems of invertebrates, particularly gastropods, coupled with our preliminary physiology research, strongly suggest histamine to be a sensory neurotransmitter in the mantle rim of C. virginica. Future work will involve a full pharmacological study of the physiological effects of histamine at the mantle rim, as well as immunohistochemical studies of histamine and histamine receptor types in mantle and other innervated tissues of C. virginica.
Fig. 1.
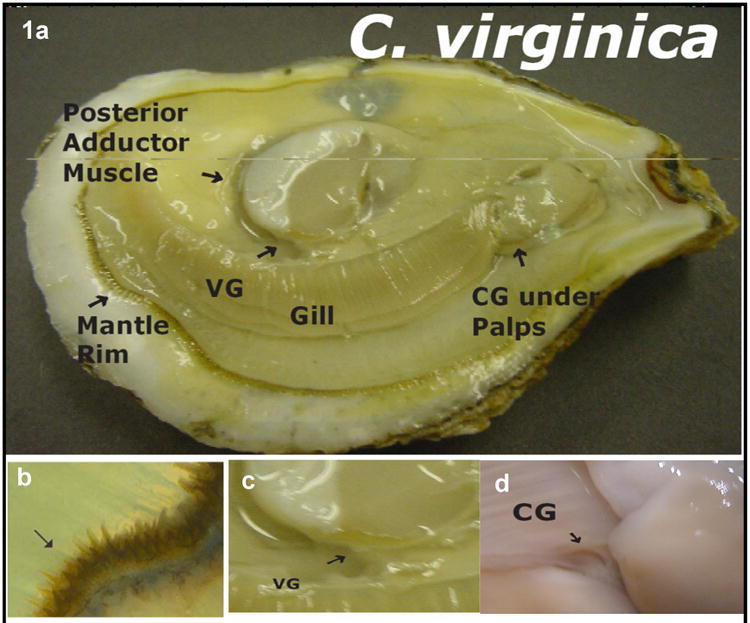
a - oyster with right shell removed showing tissue; b - enlargement showing sensory tentacles of the mantle rim (arrow), c - enlargement show visceral ganglia (VG), d– enlargement showing cerebral ganglia (CG) after removal of palps.
Acknowledgments
This work was supported in part by grants 2R25GM06003 of the Bridge Program of NIGMS and 0516041071 of NYSDOE.
References
- 1.Haas HL, Sergeeva OA, Selbach O. Histamine in the Nervous System. Physiological Reviews. 2008;88(3):1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 2.Karhunen T, Panula P. Histamine in the nervous system of Macoma balthica (Bivalvia) Agents and Actions. 1991;33:116–118. doi: 10.1007/BF01993142. [DOI] [PubMed] [Google Scholar]
- 3.Karhunen T, Airaksinen SM, Tuomisto L, Panula P. Neurotransmitters in the Nervous System of Macoma balthica (Bivalvia) Journal of Comparative Neurology. 1993;334:477–488. doi: 10.1002/cne.903340311. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter DO, Gaubatz GL. H1 and H2 histamine receptors on Aplysia neurones. Nature. 1975;254:343–344. doi: 10.1038/254343a0. [DOI] [PubMed] [Google Scholar]
- 5.Weinreich D. Synaptic responses mediated by identified histamine-containing neurones. Nature. 1977;267:854–856. doi: 10.1038/267854a0. [DOI] [PubMed] [Google Scholar]
- 6.Duch DS, Bowers SW, Nichol CA. Elevation of brain histamine levels by diaminopyrimidine inhibitors of histamine N-methyltransferase. Biochemical Pharmacology. 1978;27:1507–1509. doi: 10.1016/0006-2952(78)90109-0. [DOI] [PubMed] [Google Scholar]
- 7.Bailey CH, Chen MC, Weiss KR, Kupfermann I. 1982. Ultrastructure of a histaminergic synapses in Aplysia. Brain Research. 1982;238:205–210. doi: 10.1016/0006-8993(82)90784-3. [DOI] [PubMed] [Google Scholar]
- 8.McCaman RE, Weinreich D. Histaminergic synaptic transmission in the cerebral ganglion of Aplysia. Journal of Neurophysiology. 1985;53:1016–1037. doi: 10.1152/jn.1985.53.4.1016. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz JH, Elste A, Shapiro E, Gotoh H. Biochemical and morphological correlates of transmitter type in C2, an identified histaminergic neuron in Aplysia. Journal of Comparative Neurology. 1986;245:401–421. doi: 10.1002/cne.902450308. [DOI] [PubMed] [Google Scholar]
- 10.Weiss KR, Shapiro E, Kupfermann I. Modulatory synaptic actions of an identified histaminergic neuron on the serotonergic metacerebral cell of Aplysia. Journal of Neuroscience. 1986;6:2393–2402. doi: 10.1523/JNEUROSCI.06-08-02393.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiel HJ, Weiss KR, Kupfermann I. Multiple roles of a histaminergic afferent neuron in the feeding behavior of Aplysia. Trends in Neuroscience. 1990;13:223–227. doi: 10.1016/0166-2236(90)90164-6. [DOI] [PubMed] [Google Scholar]
- 12.Elste A, Koester J, Shapiro E, Panula P, Schwartz JH. Identification of histaminergic neurons in Aplysia. Journal of Neurophysiology. 1990;64:736–744. doi: 10.1152/jn.1990.64.3.736. [DOI] [PubMed] [Google Scholar]
- 13.Pirvola U, Tuomisto L, Yamatodani A, Panula P. Distribution of histamine in the cockroach brain and visual system: an immune-cytochemical and biochemical study. Journal of Comparative Neurology. 1998;276:514–526. doi: 10.1002/cne.902760406. [DOI] [PubMed] [Google Scholar]
- 14.Nassel DR. Histamine in the brain of insects: a review. Microscopic Research Techniques. 1999;44:121–136. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<121::AID-JEMT6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Stuart AE, Borycz J, Meinertzhagen IA. The dynamics of signaling at the histaminergic photoreceptor synapse of arthropods. Progress in Neurobiology. 2007;82:202–227. doi: 10.1016/j.pneurobio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Aiello EL. Identification of the cilioexcitatory substance present in the gill of the mussel Mytilus edulis. Journal of Cellular and Comparative Physiology. 1962;60:17–21. doi: 10.1002/jcp.1030600103. [DOI] [PubMed] [Google Scholar]
- 17.Stefano GB, Aiello E. Histofluorescent localization of serotonin and dopamine in the nervous system and gill of Mytilus edulis (Bivalvia) Biological Bulletin. 1975;148:141–156. doi: 10.2307/1540655. [DOI] [PubMed] [Google Scholar]
- 18.Stefano GB, Catapane EJ. Seasonal monoamine changes in the central nervous system of Mytilus edulis (Bivalvia) Experientia. 1977;33:1341–1342. [Google Scholar]
- 19.Carroll MA, Catapane EJ. The nervous system control of lateral ciliary activity of the gill of the bivalve mollusc, Crassostrea virginica. Comparative Biochemistry and Physiology. 2007;148A(2):445–450. doi: 10.1016/j.cbpa.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akande P, Duncan J, Catapane EJ, Carroll MA. Role of histamine in the sensory motor integration of gill lateral cell cilia in the bivalve mollusc, Crassostrea virginica. Annals of the 2014 Experimental Biology Conference; San Diego. 2014. Abstract 14-506-EB. [Google Scholar]
- 21.Zhang L, Sun M. Determination of histamine and histidine by capillary zone electrophoresis with pre-column naphthalene-2,3-dicarbox-aldehyde derivatization and fluorescence detection. Journal of Chromatography A. 2004;1040:133–140. doi: 10.1016/j.chroma.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Shin IS, Lee YK, Ho HJ, Ban SJ. Improved HPLC Method Using 2,3-naphthalenedicarboxaldehyde as Fluorescent Labeling Agent for Quantification of Histamine in Human Immunoglobulin Preparations. Public Health Research Perspectives. 2011;2(2):127–134. doi: 10.1016/j.phrp.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sombke A, Harzsch S. Immunolocalization of histamine in the optic neuropils of Scutigera coleoptrata (Myriapoda: Chilopoda) reveals the basal organization of visual systems in Mandibulata. Neuroscience Letters. 2015;594:111–116. doi: 10.1016/j.neulet.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 24.Braubach OR, Croll RP. Evidence that hisatimine acts as a neurotransmitter in statocyst hair cells in the snail, Lymnaea stagnalis. J Grav Physiol. 2004;11(3):57–66. [Google Scholar]
- 25.Williams P, Akande P, Catapane EJ, Carroll MA. Further studies on the sensory motor integration of gill lateral cilia in the bivalve mollusc Crassostrea virginica. Annals of the 2013 Experimental Biology Conference. 2013 abstract 262. [Google Scholar]
- 26.King C, Myrthil M, Carroll MA, Catapane EJ. Effects of p-Aminosalicylic acid on the Neurotoxicity of Manganese and Levels of Dopamine and Serotonin in the Nervous System and Innervated Organs of Crassostrea virginica. In Vivo. 2008;29(3):26–34. [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda N, Jesmin S, Takahashi Y, Hatta E, Kobayashi M, Matsuyama K, Kawakami N, Sakuma I, Gando S, Fukui H, Hattori Y, Levi R. Histamine H1 and H2 Receptor Gene and Protein Levels Are Differentially Expressed in the Hearts of Rodents and Humans. Journal of Pharmacology and Experimental Therapeutics. 2004;309(2):786–795. doi: 10.1124/jpet.103.063065. [DOI] [PubMed] [Google Scholar]