Abstract
Studies indicate that when abstinence is initiated, escalating reinforcement schedules maintain continuous abstinence longer than fixed reinforcement schedules. However, these studies were conducted for shorter durations than most clinical trials and also resulted in larger reinforcer value for escalating participants during the 1st week of the experiment. We tested whether escalating reinforcement schedules maintained abstinence longer than fixed reinforcement schedules in a 12-week clinical trial. Smokers (146) were randomized to an escalating reinforcement schedule, a fixed reinforcement schedule, or a control condition. Escalating reinforcement participants received $5.00 for their first breath carbon monoxide (CO) sample <3 ppm, with a $0.50 increase for each consecutive sample. Fixed reinforcement participants received $19.75 for each breath CO sample <3 ppm. Control participants received payments only for delivering a breath CO sample. Similar proportions of escalating and fixed reinforcement participants met the breath CO criterion at least once. Escalating reinforcement participants maintained criterion breath CO levels longer than fixed reinforcement and control participants. Similar to previous short-term studies, escalating reinforcement schedules maintained longer durations of abstinence than fixed reinforcement schedules during a clinical trial.
Keywords: contingency management, differential reinforcement, nicotine, schedule of reinforcement, tobacco, smoking cessation
Contingency management (CM) is a general approach to treatment in which reinforcement and punishment contingencies are applied to change behavior effectively (Higgins, Silverman, & Heil, 2008). For example, in a CM program for smoking cessation, a reinforcer is available only when a participant abstains from smoking for a certain period of time. Abstinence is verified by an objective test of smoking, such as breath carbon monoxide (CO). CM effectively reduces breath CO in smokers who are not seeking to quit (Lamb et al., 2007; Romanowich & Lamb, 2013; Stitzer, Rand, Bigelow, & Mead, 1986; Tidey, O’Neill, & Higgins, 2002; Volpp et al., 2009) and those who are seeking to quit smoking (Dallery, Meredith, & Glenn, 2008; Lamb, Kirby, Morral, Galbicka, & Iguchi, 2010; Meredith, Grabinski, & Dallery, 2011; Rand, Stitzer, Bigelow, & Mead, 1989).
Like any treatment, the efficacy of CM depends on the parameters of the treatment. Experiments have demonstrated the importance of both reinforcer value (Correia & Benson, 2006; Lamb, Morral, Kirby, Iguchi, & Galbicka, 2004; Paxton, 1981; Romanowich & Lamb, 2010; Stitzer & Bigelow, 1983, 1984) and how reinforcer value changes with changes in smoking (Roll & Higgins, 2000; Roll, Higgins, & Badger, 1996). Reinforcement schedules that increase the value of the reinforcer as the duration of continuous abstinence increases may differentially reinforce longer periods of abstinence. Such escalating reinforcement schedules are now a common element of most CM programs (e.g., Budney, Higgins, Radonovich, & Novy, 2000; Petry & Martin, 2002; Preston, Umbricht, & Epstein, 2000; Rawson et al., 2002; Shoptaw et al., 2002). These CM programs are consistently more effective than control conditions (Roll et al., 2006; Roll & Shoptaw, 2006). However, none of the control conditions have used an equal-valued abstinence contingency.
Roll and Higgins (2000) and Roll et al. (1996) conducted two analogue studies that investigated the contribution of escalating reinforcement schedules for decreasing breath CO levels in smokers who were not seeking to quit. Analogue studies are typically small-scale studies conducted for shorter periods of time than treatment studies and are focused on experimentally isolating one variable of interest, such as reinforcer schedule, immediacy, or value (Sigmon & Patrick, 2012). In the first study (Roll et al., 1996), participants who received equal-valued escalating and fixed reinforcement schedules were compared to a control group who received the average reinforcer value of the first 10 participants in the escalating group. During fixed reinforcement schedules, participants were paid a constant amount for each abstinent breath CO sample. For both escalating and fixed reinforcement participants, reinforcement was contingent on breath CO levels ≤ 11 ppm for 15 scheduled breath CO samples. The breath CO samples were taken during 5 consecutive weekdays, three times per day. Both contingent reinforcer groups had a significantly greater number of criterion breath CO samples than the noncontingent group. The total number of criterion breath CO samples was similar in the two contingent reinforcer groups. Eighteen of 20 escalating reinforcement participants and all 20 of the fixed reinforcement participants initiated abstinence as measured by three sequential breath CO samples ≤ 11 ppm, compared to only 11 of 20 control participants. Of the participants who initiated abstinence, 14 escalating reinforcement participants (78%) maintained abstinence for the remainder of the study, whereas only eight fixed reinforcement participants (40%) did so. These results provide evidence that escalating reinforcement schedules may promote continuous maintenance of abstinence.
Roll and Higgins (2000) compared the effects of escalating reinforcement schedules with or without a reset contingency to a fixed reinforcement schedule. A reset contingency temporarily decreases the next available reinforcer value back to an initial low level after a failed breath CO sample. Participants can typically regain the higher reinforcer value only by continuously meeting the breath CO criterion for a certain period of time (e.g., five consecutive criterion visits). The study used a within-subjects design, with each participant receiving each condition for 5 consecutive days (three breath CO samples per day) and a return-to-baseline washout period between conditions. For all three reinforcement schedules, mean breath CO levels were reduced relative to baseline. Likewise, a similar number of criterion (≤8 ppm) breath CO samples were delivered (10.4, 9.6, and 9.0 out of 15 possible) in each condition. However, participants in both escalating reinforcement conditions had a higher maximum number of sequential breath CO samples that met criterion than did those in the fixed reinforcement condition. There was no difference in the number of sequential criterion breath CO samples between the two escalating reinforcement conditions. However, in the escalating reinforcement schedule with a reset contingency, participants were more likely to maintain criterion breath CO levels during the entire 5-day period (nine of 18 participants who initiated abstinence) than in the escalating reinforcement schedule without a reset contingency (four of 18). Thus, this study provides evidence that escalating reinforcement schedules with a reset contingency may enhance maintenance of abstinence.
Although these studies provide evidence that escalating reinforcement schedules can improve maintenance of abstinence, they do not show that escalating reinforcement schedules will improve maintenance in participants who initiate abstinence in typical clinical CM interventions compared to fixed reinforcement schedules. Reinforcement value and time-scale differences between these analogue studies and clinical interventions create reason to question the generalizability of these analogue studies. In analogue studies, escalating reinforcement schedules were more effective than fixed reinforcement schedules only for participants who had at least three consecutive criterion breath CO samples. Thus, escalating reinforcement schedules were more effective than fixed reinforcement schedules only for participants who had experienced getting paid more (e.g., $14.00 vs. $9.80; Roll & Higgins, 2000) for achieving 1 day (three breath CO samples) of meeting the breath CO criterion. Basic behavior-analytic research shows that these trial-by-trial contingency differences can shape behavior (Galbicka, Kautz, & Jagers, 1993). By contrast, during clinical studies (e.g., Budney et al., 2000; Higgins, Wong, Badger, Ogden, & Dantona, 2000; Silverman et al., 1996), escalating reinforcement participants could earn more than fixed reinforcement participants only after 2 to 6 weeks of continuous abstinence. Even so, after 2 to 6 weeks of continuous abstinence, this reinforcement value differential would be small (4% to 5% greater). The reinforcement value differential would meet or exceed the 42% differential seen on the first day of abstinence in the aforementioned studies (Roll & Higgins, 2000; Roll et al., 1996), only after 7 to 10 weeks of continuous abstinence. In terms of time-scale differences, these two studies lasted only for 5 consecutive days. By contrast, clinical CM studies normally last 8 or more weeks. In those analogue studies during a 5-day period, participants could experience substantially larger (e.g., 42% to 104%) reinforcement value for maintaining smoking abstinence. In a clinical trial, experiencing five occasions with a 42% or greater reinforcement value differential would take more than 10 weeks of continuous abstinence.
A recent study directly compared escalating and fixed reinforcement schedules during CM treatments with opioid-dependent participants. In this study, pregnant, opioid-dependent women could receive vouchers contingent on drug-free urine samples (Hutchinson, Chisolm, Tuten, Leoutsakos, & Jones, 2012; Tuten, Svikis, Keyser-Marcus, O’Grady, & Jones, 2012). Patients were randomized into groups that received vouchers on either an escalating or fixed reinforcement schedule or to an attendance-only control group. The escalating reinforcement participants tended to have a greater number of consecutive drug-free urine samples than the fixed reinforcement participants, but this difference was not statistically significant. Neither contingent reinforcement group differed significantly on any measure of within-treatment abstinence from the control group. This is notable because in the analogue studies just described, the number of consecutive days of abstinence was greater in the escalating compared to the fixed group only for those participants who initiated abstinence. The lack of difference between the contingent groups and the control groups suggests that a substantial number of individuals did not initiate abstinence. This, combined with the relatively small sample size, may have reduced the power to detect differences between the two schedules in this study. It is important to note that in this study missing urine samples did not reset the value of the escalating reinforcement schedule. This likely decreased the expected effect-size difference between the two schedules, again reducing that study’s power to detect a differential effect. Still, as we hypothesize, escalating reinforcement schedules did not result in longer sustained abstinence in a typical clinical trial. However, this trial contained design and power issues that limited its ability to address the issue adequately.
The current experiment compared escalating and fixed reinforcement schedules for smoking abstinence over the course of a 12-week CM clinical trial in participants who sought to quit smoking. All participants were classified as early success (ES) from the results of a five-visit baseline fixed reinforcement period before randomization. We chose to enroll only ES participants because Roll and Higgins (2000) and Roll et al. (1996) found differences between escalating and fixed reinforcement schedules only for abstinence maintenance. Thus, examination of the effects of the two reinforcement schedules only in participants who could initiate abstinence increased our power to examine whether escalating reinforcement schedules were more effective at maintaining abstinence by eliminating the variance that would result from participants who could not initiate abstinence. Statistical power to compare escalating and fixed reinforcement in the current study, compared to that in Tuten et al. (2012), was further increased by assigning approximately 30% more subjects to these two conditions. In addition, we included a control condition, in which participants could earn payments contingent on attendance independent of their breath CO levels. Including this control condition allowed us to isolate the effect to the breath CO contingency. During the current experiment, initial abstinence reinforcement value was much greater for fixed reinforcement participants ($19.75) than for escalating reinforcement participants ($5.00). In addition, escalating reinforcement participants could earn as much as fixed reinforcement participants for a criterion breath CO sample only after 6 consecutive weeks of criterion breath CO samples. Thus, unlike previous analogue studies with escalating reinforcement schedules, the current study required escalating reinforcement participants to abstain initially for lower value reinforcers and maintain a longer period of smoking abstinence before reinforcement value was equal to or greater than that for fixed reinforcement participants. Due to these more extreme reinforcement-value and time-scale parameters, we hypothesized that there would be no difference in abstinence maintenance between escalating and fixed reinforcement participants, as was seen in another recent clinical trial (Hutchinson et al., 2012; Tuten et al., 2012).
METHOD
Participants
Participants were 146 volunteers who worked at the University of Texas Health Science Center at San Antonio, lived near that center, or both. Participants smoked at least 15 cigarettes per day, had smoked regularly for at least 1 year, and were planning to quit smoking within the next month. All participants were at least 18 years old at intake and produced an intake breath CO ≥15 ppm. Participants were expected to report to the research site and deliver a breath CO sample each workday (Monday through Friday), unless an absence was arranged ahead of time. Participants were paid $1.00 for each visit, regardless of breath CO level. This entire procedure usually took less than 5 min. Visits for most participants were scheduled in the morning between 7:30 a.m. and 10:00 a.m. Visits for workers in evening or night shifts coincided with the beginning of their shifts, so that these visits were equivalent to the morning for individuals who worked more typical shifts.
Procedure
Vitalograph CO monitors were used to measure breath CO levels. Participants were required to take a deep breath, hold it for 20 s, and then to expire over 20 s into the disposable mouthpiece of the CO monitor. The peak breath CO reading was taken as the participant’s breath CO level. Breath CO levels increase with increasing cigarette consumption and decline with abstention (Henningfield, Stitzer, & Griffiths, 1980). We used an abstinence breath CO criterion of <3 ppm based on our previous smoking cessation studies. A cutoff of <3 ppm has been shown to have the highest true test accuracy, in which over 80% of the breath CO samples are correctly classified as either smoking abstinent or not for the previous 24-hr period (Javors, Hatch, & Lamb, 2005). During each visit, participants completed a form that inquired about use of medication to aid in smoking cessation in the past day and about how much they had smoked. Participants returned these forms after they had received any earned payments and were told that their answers to these questions in no way affected their payments.
As mentioned above, participants were all classified as ES based on their performance during a five-visit abstinence trial. During this trial, participants received $5.00 for each breath sample with <3 ppm CO. Participants who delivered at least one breath CO sample <3 ppm were classified as ES. Those participants who did not produce a breath CO sample <3 ppm were considered hard to treat and were enrolled in an alternative CM smoking-cessation study (Romanowich & Lamb, 2014). Immediately after the five-visit abstinence trial, ES participants were randomly assigned to one of three groups: escalating reinforcement schedule, fixed reinforcement schedule, or control. Random assignment to one of the three groups was accomplished by assigning two participants to the escalating reinforcement group, two to the fixed reinforcement group, and one to the control group from each group of five participants who completed the abstinence trial. This resulted in approximately 60 participants randomized to the escalating and fixed reinforcement groups and 30 participants randomized to the control group. Randomization was stratified by intent to use medication to help them stop smoking and by order of study entry. All 146 participants completed intake and were randomized after the five-visit abstinence trial. Table 1 shows demographic information for all 146 participants by group. We conducted one-way ANOVAs on continuous intake variables (e.g., age) and chi-square probability tests on proportional intake variables (e.g., proportion of women). Participants in the three groups were statistically similar on all intake measures. After randomization, participants were expected to submit a breath CO sample every workday (Monday through Friday) for 60 visits, which lasted approximately 12 weeks.
Table 1.
Participant Characteristics
| Escalating | Fixed | Control | |
|---|---|---|---|
| Number | 59 | 58 | 29 |
| Female (%) | 25 (42) | 26 (45) | 16 (55) |
| Mean age (SD) | 41.9 (13.0) | 41.3 (11.8) | 40.6 (10.0) |
| Caucasian (%) | 43 (73) | 39 (67) | 16 (55) |
| Marital status | |||
| Single (%) | 21 (36) | 22 (38) | 9 (31) |
| Married (%) | 14 (24) | 13 (22) | 9 (31) |
| Other (%) | 24 (40) | 23 (40) | 11 (38) |
| Income (US$) | |||
| <15,000 (%) | 26 (44) | 29 (50) | 8 (28) |
| 15 to 24,999 (%) | 12 (20) | 13 (22) | 7 (24) |
| 25 to 34,999 (%) | 6 (10) | 6 (10) | 7 (24) |
| ≥35,000 (%) | 15 (25) | 10 (17) | 7 (24) |
| Employment | |||
| Full time (%) | 26 (44) | 23 (40) | 15 (52) |
| Education | |||
| GED or high school (%) | 21 (36) | 23 (40) | 11 (38) |
| Vo tech or associate (%) | 21 (36) | 22 (38) | 12 (41) |
| Bachelors+ (%) | 17 (29) | 13 (22) | 6 (21) |
| Parents smoked | |||
| Yes (%) | 53 (90) | 48 (83) | 23 (79) |
| Mom only (%) | 11 (21) | 9 (19) | 4 (17) |
| Dad only (%) | 12 (23) | 10 (21) | 6 (26) |
| Both (%) | 30 (57) | 29 (60) | 13 (57) |
| Mean age of | |||
| First cigarette (SD) | 15.3 (4.3) | 15.7 (6.6) | 15.4 (2.8) |
| Regular smoker (SD) | 17.9 (5.8) | 17.6 (6.2) | 17.3 (3.1) |
| Lives with smoker (%) | 37 (63) | 32 (55) | 18 (62) |
| Tried to quit (%) | 54 (92) | 52 (90) | 24 (83) |
| Mean number of cigarettes per day (SD) | 21.7 (5.3) | 24.3 (6.9) | 21.9 (6.3) |
| Mean intake breath CO ppm (SD) | 24.0 (9.2) | 25.7 (11.7) | 22.1 (7.0) |
Participants in the escalating and fixed reinforcement groups both earned money by delivering breath CO samples <3 ppm. However, these two groups differed in how the value of the potential payment was determined. In the escalating reinforcement group, an escalating payment schedule with a reset contingency was used. Specifically, the value of the payment available started at $5.00 and increased by $0.50 with the delivery of each breath CO sample <3 ppm. The value of the payment reset to $5.00 with missed visits or delivery of a breath CO sample ≥3 ppm. Delivery of five consecutive breath CO samples <3 ppm reinstated the highest payment obtained by that individual.
Participants in the fixed reinforcement group also earned money for breath CO samples < 3 ppm. However, the value of the potential payment for these participants was always $19.75, regardless of how many consecutive criterion breath CO samples he or she had previously submitted. For both escalating and fixed reinforcement groups, the total payment amount possible was $1,185.00 over the 60-visit intervention period.
Participants in the control group had a two-in-three chance of receiving a payment on any visit (the probability for each visit was independent of other visits), regardless of their breath CO sample. Thus, monetary payments were contingent only on attendance and not for breath CO level. For control participants, the value of the payment potentially available on a given visit started at $5.00 and increased by $0.50 with each visit. Missed visits reset the value to $5.00, and five sequential visits reinstated the highest value earned. The value of the payment increased with all attended visits, despite the fact that no money was delivered on about 33% of these visits. For example, on the first visit a participant might not have earned a payment, but on the second sequential visit the participant could have earned $5.50.
A follow-up was conducted 6 months after study entry for all participants. At follow-up, participants submitted a breath CO sample and reported any smoking during the last day, last week, last month, and since the end of study participation. A salivary cotinine sample was also collected. Participants were paid $20.00 for completion of the follow-up assessment.
Two trained technicians implemented the breath CO contingencies over the five-visit abstinence trial, 60-visit intervention period, and follow-up. The technicians used a computer program that indicated when and how much reinforcement each participant could earn for a given breath CO level. The reinforcement procedures were implemented with 100% fidelity, and there were no delays in providing reinforcement for criterion breath CO samples.
Data Analysis
All participants randomly assigned to one of the study conditions were included in the analysis. All missing data points were counted as not meeting the breath CO criterion (i.e., positive) for that visit. We used one-way ANOVAs to estimate differences between the three groups on continuous variables (e.g., maximum number of consecutive criterion breath CO samples). Significant ANOVA (p<.05) results were followed up with Tukey post hoc honestly significant difference (HSD) tests between each group. We used chi-square probability tests to estimate differences between the groups on proportional variables (e.g., proportion of participants who met breath CO criterion at least once). Fisher’s exact tests were used if one of the cells contained less than five samples. We used Kaplan-Meier survival analysis to determine any group differences between the longest number of consecutive criterion breath CO visits. This was done to take into account participants who ended treatment with their longest consecutive criterion breath CO streak still ongoing.
RESULTS
Table 1 shows the intake demographics for all participants by payment schedule. Eighteen escalating reinforcement and 18 fixed reinforcement participants reported using a smoking-cessation aid for at least one visit. Eleven control participants also reported using a smoking-cessation aid. For those participants that reported using a smoking-cessation aid at least once, the mean number of visits using a smoking-cessation aid was 28.5 (SD = 20.5), 36.0 (20.7), and 13.1 (17.3) for escalating reinforcement, fixed reinforcement, and control participants, respectively. There was a group difference for the number of visits using a smoking-cessation aid, F(2) = 4.55, p <.02. Post hoc Tukey tests showed that control participants had a fewer number visits using smoking-cessation aids than did fixed reinforcement participants (p < .01). However, other comparisons were not statistically different.
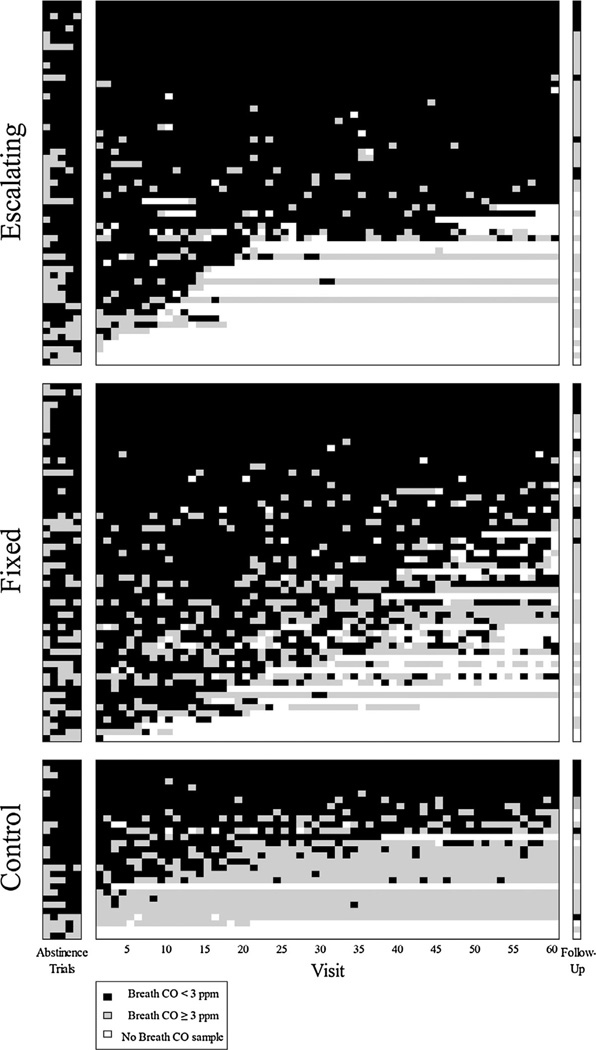
Figure 1 is an event record for all participants grouped by payment schedule. Each participant’s breath CO level was coded as either meeting criterion, not meeting criterion, or not being present over the course of the experiment. The mean number of criterion breath CO visits during baseline abstinence trials was 3.6 (SD = 1.5), 3.4 (1.5), and 3.7 (1.5) for participants in the escalating reinforcement, fixed reinforcement, and control groups, respectively. There was no difference for number of baseline criterion breath CO visits between groups, F(2)= 0.47, p = .63.
Figure 1.
Event records for escalating reinforcement, fixed reinforcement, and control participants. An individual participant constitutes one row on the ordinate. Visit number is shown on the abscissa. Black areas represent visits with breath CO samples <3 ppm. Gray areas represent visits with breath CO samples ≥3 ppm. White areas represent missed visits. Participants were first ordered by total number of criterion breath CO samples, then by number of consecutive breath CO samples, and finally by number of visits attended.
A similar proportion of escalating reinforcement (.93) and fixed reinforcement (1.0) participants met the breath CO criterion at least once (Fisher’s exact test; p > .05) during the 60-visit reinforcement period. A larger proportion of fixed reinforcement participants met the breath CO criterion at least once relative to control participants (.83; Fisher’s exact test; p < .01). There was no difference for meeting the breath CO criterion at least once between the participants in the escalating reinforcement and control groups (p > .05).
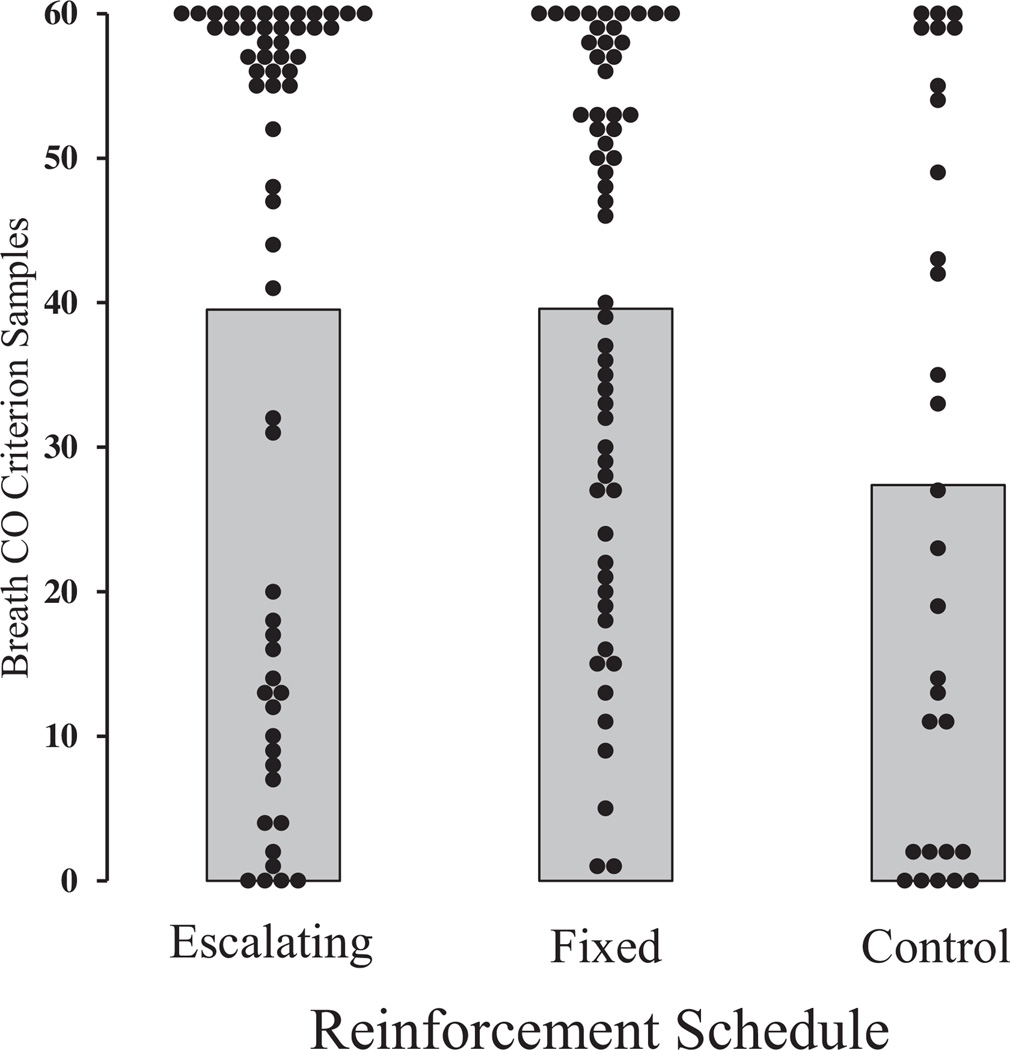
Figure 2 shows each participant’s total number of criterion breath CO samples across each group, along with group means. The mean total number of criterion samples for the control group was lower than both the escalating and fixed reinforcement groups. Consequently, the total number of criterion breath CO visits during the 60-visit reinforcement period was different between groups, F(2)=3.64, p =.03. Post hoc Tukey tests showed that control participants had fewer total criterion breath CO visits (µ = 27.4, SD = 23.9) than both escalating reinforcement (39.5, 23.6) and fixed reinforcement (39.6, 18.5) participants (p < .05). However, there was no difference in total criterion breath CO visits between escalating and fixed reinforcement participants (p > .05).
Figure 2.
Total number of breath CO samples <3 ppm across each group. Each filled circle represents one participant. Gray area represents the mean percentage of breath CO samples <3 ppm for each group.
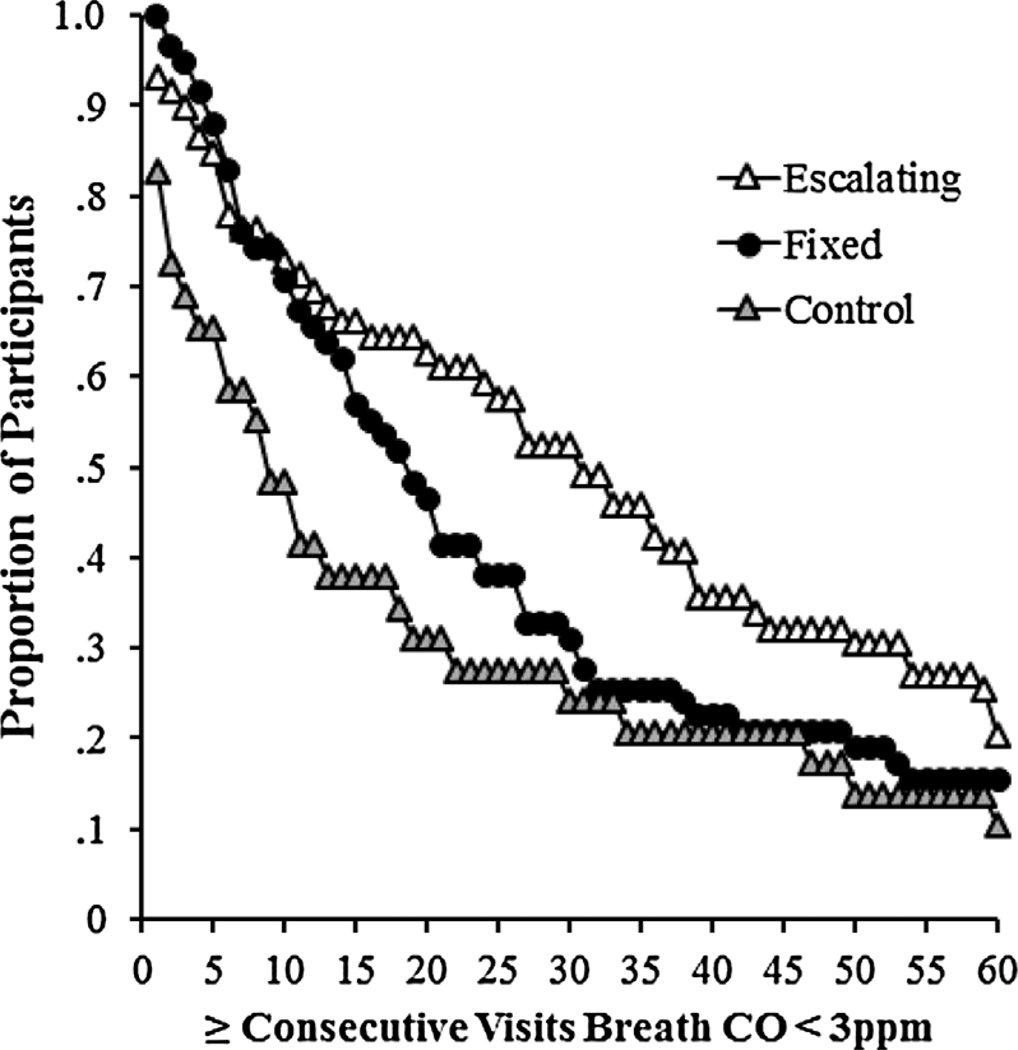
Figure 3 shows the proportion of participants who were able to maintain consecutive criterion breath CO samples for at least a given number of visits. On average, escalating reinforcement, fixed reinforcement, and control participants submitted 30.1 (SD = 22.3), 23.6 (19.6), and 16.6 (20.2) consecutive criterion breath CO samples, respectively. The largest number of consecutive criterion breath CO visits was different between groups, F(2) = 4.28, p = .02. A survival analysis showed that escalating reinforcement participants had a larger number of consecutive criterion breath CO samples than both fixed reinforcement (log-rank test, χ2 = 5.08, p < .05) and control participants (log-rank test, χ2 = 5.40, p < .05). There was no difference in the largest number of consecutive criterion breath CO samples between fixed reinforcement and control participants (log-rank test, χ2 = 0.56, p = .46).
Figure 3.
Proportion of participants who achieved at least a given number of consecutive breath CO samples <3 ppm across each group. Open triangles, filled circles, and gray triangles represent escalating reinforcement, fixed reinforcement, and control participants, respectively.
Fixed reinforcement participants’ attendance appeared to be better than that for escalating reinforcement participants. On average, fixed reinforcement participants attended 48.8 (of 60) visits (SD = 16.6), whereas escalating reinforcement participants attended 44.2 visits (22.5). Control participants on average attended 51.2 scheduled visits (19.5). However, there was no statistically significant difference between groups, F(2) = 0.83, p =.44.
On average, participants in the escalating reinforcement, fixed reinforcement, and control groups earned $629.40 (SD = $475.27), $781.49 ($364.25), and $663.38 ($300.13), respectively during the 60-visit reinforcement period of the experiment. There was no difference in mean earnings between groups, F(2) = 2.21, p =.11. Two and four participants failed to earn a single monetary payment during the 60-visit reinforcement period in the control and escalating reinforcement groups, respectively. However, these participants either never attended any of the 60 visits or dropped out of the study after the first visit. All 58 fixed reinforcement participants earned at least one contingent payment.
A similar proportion of participants in the escalating reinforcement (.75), fixed reinforcement (.76), and control (.79) groups returned to complete the follow-up. There was no difference in mean breath CO levels between groups at follow-up, F(2) = 0.24, p =.79. In addition, a similar proportion of participants in each group had criterion breath CO levels at follow-up. The proportions were .14, .17, and .24 for the escalating reinforcement, fixed reinforcement, and control groups, respectively. However, to qualify as abstinent at follow-up, participants needed not only to submit a criterion breath CO sample but also to report no smoking in the past week and have a saliva cotinine level ≤ 20 ng/ml. Approximately .10, .12, and .14 participants met these additional criteria for the escalating reinforcement, fixed reinforcement, and control groups, respectively. The proportion of participants who met follow-up abstinence criteria and who also reported using smoking-cessation aids at least once was not different than participants who reported no use of smoking-cessation aids for each of the three groups (all chi-square tests p > .05).
DISCUSSION
Contrary to our hypothesis but similar to previous analogue studies (Roll & Higgins, 2000; Roll et al., 1996), escalating reinforcement participants were able to maintain longer continuous periods of smoking abstinence than both fixed reinforcement and control participants when contingent monetary payments were available during a 12-week CM clinical trial. This occurred for escalating reinforcement participants despite the reinforcement value being smaller during the initial 6 weeks of the 60-visit reinforcement period than it was for fixed reinforcement participants. Thus, the current findings replicate and extend the results of the analogue studies by Roll and Higgins (2000) and Roll et al. (1996). The differences in the mean number of criterion breath CO samples (Figure 2) between the control and contingent reinforcement groups also show that reinforcement must be contingent on reduced breath CO levels for CM treatments to increase the number of criterion breath CO samples delivered.
The current results are in contrast to a more recent study that compared escalating and fixed reinforcement schedules for opioid-dependent pregnant women (Hutchinson et al., 2012; Tuten et al., 2012). There may be at least two reasons why our results differed. First, Tuten et al. (2012) did not count missed visits as drug-positive tests. This is important for escalating schedule participants, because they could simply miss a visit if they had used drugs and avoid the reset contingency. In the current study, participants needed to provide at least 24 hr advance notice for that absence not to count as a failed breath CO test. Thus, in this study and the analogue studies by Roll and Higgins (2000) and Roll et al. (1996), participants experienced the reset contingency by either continuing to smoke or not delivering a sample. Therefore, the participants in these studies could not avoid the reset contingency by simply not delivering a sample. Second, in the current study we enrolled only those participants who could abstain from smoking at least once during the five-visit abstinence trial. No such measure was in place for the opioid-dependent participants. Thus, it is likely that a significant proportion of the opioid-dependent participants failed to contact the reinforcement contingency, making any schedule-induced differences less likely. As shown in Figure 1, all but two escalating schedule participants provided at least one criterion breath CO sample during the 60-visit reinforcement period. This likely increased our expected effect size and thus our power to detect a difference between the two schedules compared to the earlier studies in opioid-dependent pregnant women (Hutchinson et al., 2012; Tuten et al., 2012).
Our results and those of Roll et al. (1996) and Roll and Higgins (2000) clearly demonstrate the superiority of escalating reinforcement schedules with a reset contingency to facilitate abstinence maintenance relative to fixed reinforcement schedules. The results also generally show that contingent reinforcers can produce relatively high rates of smoking abstinence even for individuals with substantial smoking histories. Some individuals were able to maintain smoking abstinence after contingent reinforcement was discontinued. However, it should also be noted that the majority of participants resumed smoking after the reinforcement contingency was discontinued. Like all smoking-cessation treatments, CM treatment effects generally decline after treatment is withdrawn.
Although the current study does not address the specific mechanisms responsible for the greater continuous abstinence with the escalating reinforcement schedule, the value of early reinforcers does not appear to play a large role. If reinforcer value were critical, then fixed reinforcement participants should have initiated abstinence more quickly and maintained abstinence longer than escalating reinforcement participants during the first 6 weeks of the clinical trial when reinforcer value was much larger for fixed reinforcement participants. However, neither of those events occurred. In addition, fixed reinforcement participants did not maintain abstinence significantly longer than control participants. This suggests that either the escalating reinforcement schedule or the reset contingency was responsible for increasing abstinence maintenance during CM smoking-cessation trials, or both. The reset contingency is designed to act as a negative punisher. That is, monetary loss is directly contingent on continued smoking. However, the current study contained only an escalating reinforcement schedule with a reset contingency. Thus, this study cannot assess the role played by the reset contingency. The next logical step is to test the difference between escalating reinforcement schedules with and without a reset contingency in a CM clinical trial.
Although the goal of smoking-cessation treatments, including CM, is long-term abstinence, this study was not designed to detect differences in long-term abstinence between interventions. This design decision was based on the substantially larger sample that would be needed to detect such differences. Given that our hypothesis stated that there would be no within-treatment differences between the interventions, there was little reason to suspect posttreatment differences in intervention effectiveness. Whereas the current study provides little basis to suspect that there will be posttreatment differences between the interventions, a study properly designed to assess this issue is desirable. Early studies of CM provided little reason to suspect the long-term increases in abstinence that are readily apparent in studies designed to assess this (Volpp et al., 2009).
To date, the majority of smoking-cessation CM experiments have used analogue-type methodology (Sigmon & Patrick, 2012). Strong consistent results from these analogue experiments should lead to appropriately designed clinical trials that test both efficacy and generalizability to longer term treatments. Ultimately, not all aspects of CM analogue results will be successfully replicated through clinical trials. In terms of the current experiment, there was reason to believe that both an initial difference in reinforcement value between escalating and fixed schedules and a truncated experimental time frame may have been responsible for abstinence being maintained longer with escalating reinforcement schedules than with fixed reinforcement schedules. That is, the parameters used in typical clinical trials were different enough from the analogue experiments to formulate an alternative hypothesis for previous results (e.g., Roll & Higgins, 2000; Roll et al., 1996). However, these are ultimately empirical questions that demand rigorous experimental testing with both analogue and clinical trials. The current results generally supported continuing to use analogue experiments to test basic mechanisms responsible for CM treatment efficacy.
We chose to enroll participants who could abstain at least 1 day on the five-visit abstinence pretest to ensure large abstinence initiation rates during the 60-visit contingent reinforcement period. However, this limits the generalizability of the results to hard-to-treat smokers. Presumably, hard-to-treat participants would have infrequently contacted the reinforcement contingency, resulting in low levels of abstinence initiation and less of a difference in abstinence maintenance. In fact, it may be the case that the high initial reinforcement value for the fixed reinforcement schedule would facilitate higher rates of abstinence initiation than escalating reinforcement schedules would (Lamb, Kirby, Morral, Galbicka, & Iguchi, 2004; Romanowich & Lamb, 2010; Stitzer & Bigelow, 1983, 1984). Therefore, differences in abstinence maintenance between escalating and fixed reinforcement schedules may be useful only for smokers who can reliably abstain from smoking for at least 1 day.
Another limitation for the current study involves measuring breath CO levels only once per day As previously described, a once-daily breath CO sample accurately classifies smoking status over 80% of the time (Javors et al., 2005). However, this also means that approximately one in five samples may be misclassified as either positive (breath CO ≥ 3 ppm without smoking) or negative (breath CO < 3 ppm with smoking). This second possibility is especially problematic because it means that participants received payments without maintaining smoking abstinence. An alternative is to measure a participant’s breath CO levels multiple times per day, similar to Roll et al. (1996) and Roll and Higgins (2000). However, this can become burdensome. Recently, participants have been able to use mobile phones to send their breath CO results to the researchers (Meredith et al., 2014). Still, in this study, concerns about whether smoking abstinence was actually maintained may be tempered by the clear differential control of smoking behavior between the different reinforcement schedules (see Figure 1).
In sum, similar to previous analogue studies, escalating reinforcement schedules maintained abstinence longer than equal-valued fixed reinforcement schedules for ES smokers in a CM smoking-cessation clinical trial. Additional studies will be required both to replicate this difference in clinical settings and to elucidate the reasons why escalating reinforcement schedules with a reset contingency facilitate longer periods of abstinence than equal-valued fixed reinforcement schedules.
Acknowledgments
The research reported in this paper was supported by Grant DA013304 to R. J. Lamb.
Contributor Information
Paul Romanowich, University of Texas at San Antonio.
R. J. Lamb, University of Texas, Health Science Center at San Antonio
REFERENCES
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of Consulting and Clinical Psychology. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Correia CJ, Benson TA. The use of contingency management to reduce cigarette smoking among college students. Experimental and Clinical Psychopharmacology. 2006;14:171–179. doi: 10.1037/1064-1297.14.2.171. [DOI] [PubMed] [Google Scholar]
- Dallery J, Meredith S, Glenn IM. A deposit contract method to deliver abstinence reinforcement for cigarette smoking. Journal of Applied Behavior Analysis. 2008;41:609–615. doi: 10.1901/jaba.2008.41-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbicka G, Kautz MA, Jagers T. Response acquisition under targeted percentile schedules: A continuing quandary for molar models of operant behavior. Journal of the Experimental Analysis of Behavior. 1993;60:171–184. doi: 10.1901/jeab.1993.60-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Stitzer ML, Griffiths RR. Expired air carbon monoxide accumulation and elimination as a function of number of cigarettes smoked. Addictive Behaviors. 1980;5:265–272. doi: 10.1016/0306-4603(80)90049-0. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Silverman K, Heil SH, editors. Contingency management in substance abuse treatment. New York, NY: Guilford Press; 2008. [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. Journal of Consulting and Clinical Psychology. 2000;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Hutchinson ML, Chisolm MS, Tuten M, Leoutsakos JS, Jones HE. The efficacy of escalating and fixed contingency management reinforcement on illicit drug use in opioid-dependent pregnant women. Addictive Disorders and Their Treatment. 2012;11:150–153. doi: 10.1097/ADT.0b013e318264cf6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100:159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Kirby KC, Morral AR, Galbicka G, Iguchi MY. Improving contingency management programs for addiction. Addictive Behaviors. 2004;29:507–523. doi: 10.1016/j.addbeh.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Kirby KC, Morral AR, Galbicka G, Iguchi MY. Shaping smoking cessation in hard-to-treat smokers. Journal of Consulting and Clinical Psychology. 2010;78:62–71. doi: 10.1037/a0018323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Morral AR, Kirby KC, Iguchi MY, Galbicka G. Shaping smoking cessation using percentile schedules. Drug and Alcohol Dependence. 2004;76:247–259. doi: 10.1016/j.drugalcdep.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Morral AR, Kirby KC, Javors MA, Galbicka G, Iguchi M. Contingencies for change in complacent smokers. Experimental and Clinical Psychopharmacology. 2007;15:245–255. doi: 10.1037/1064-1297.15.3.245. [DOI] [PubMed] [Google Scholar]
- Meredith SE, Grabinski MJ, Dallery J. Internet-based group contingency management to promote abstinence from cigarette smoking: A feasibility study. Drug and Alcohol Dependence. 2011;118:23–30. doi: 10.1016/j.drugalcdep.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith SE, Robinson A, Erb P, Spieler CA, Klugman N, Dutta P, Dallery J. A mobile-phone-based breath carbon monoxide meter to detect cigarette smoking. Nicotine & Tobacco Research. 2014;16:766–773. doi: 10.1093/ntr/ntt275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton R. Deposit contracts with smokers: Varying frequency and amount of repayments. Behaviour Research and Therapy. 1981;19:117–123. doi: 10.1016/0005-7967(81)90035-8. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. Journal of Consulting and Clinical Psychology. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Epstein DH. Methadone dose increase and abstinence reinforcement for treatment of continued heroin use during methadone maintenance. Archives of General Psychiatry. 2000;57:395–404. doi: 10.1001/archpsyc.57.4.395. [DOI] [PubMed] [Google Scholar]
- Rand CS, Stitzer ML, Bigelow GE, Mead AM. The effects of contingent payment and frequent workplace monitoring on smoking abstinence. Addictive Behaviors. 1989;14:121–128. doi: 10.1016/0306-4603(89)90041-5. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber RA, Huber A, McCann M, Shoptaw S, Farabee D, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Archives of General Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug and Alcohol Dependence. 2000;58:103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis. 1996;29:495–505. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Huber A, Sodano R, Chudzynski JE, Moynier E, Shoptaw S. A comparison of five reinforcement schedules for use in contingency management-based treatment of methamphetamine abuse. The Psychological Record. 2006;56:67–81. [Google Scholar]
- Roll JM, Shoptaw S. Contingency management: Schedule effects. Psychiatry Research. 2006;144:91–93. doi: 10.1016/j.psychres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Romanowich P, Lamb RJ. Effects of escalating and descending schedules of incentives on cigarette smoking in smokers without plans to quit. Journal of Applied Behavior Analysis. 2010;43:357–367. doi: 10.1901/jaba.2010.43-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowich P, Lamb RJ. The effect of framing incentives as either losses or gains with contingency management for smoking cessation. Addictive Behaviors. 2013;38:2084–2088. doi: 10.1016/j.addbeh.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowich P, Lamb RJ. The effects of percentile versus fixed criterion schedules on smoking with equal incentive magnitude for initial abstinence. Experimental and Clinical Psychopharmacology. 2014;22:348–355. doi: 10.1037/a0036935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, Ling W. Smoking cessation in methadone maintenance. Addiction. 2002;97:1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Patrick ME. The use of financial incentives in promoting smoking cessation. Preventive Medicine. 2012;55:S24–S32. doi: 10.1016/j.ypmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR, Preston KL. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Archives of General Psychiatry. 1996;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent payment for carbon monoxide reduction: Effects of pay amount. Behavior Therapy. 1983;14:647–656. doi: 10.1901/jaba.1984.17-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent reinforcement for carbon monoxide reduction: Within-subject effects of pay amount. Journal of Applied Behavior Analysis. 1984;17:477–483. doi: 10.1901/jaba.1984.17-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer ML, Rand CS, Bigelow GE, Mead AM. Contingent payment procedures for smoking reduction and cessation. Journal of Applied Behavior Analysis. 1986;19:197–202. doi: 10.1901/jaba.1986.19-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, O’Neill SC, Higgins ST. Contingent monetary reinforcement of smoking reductions, with and without transdermal nicotine, in outpatients with schizophrenia. Experimental and Clinical Psychopharmacology. 2002;10:241–247. doi: 10.1037//1064-1297.10.3.241. [DOI] [PubMed] [Google Scholar]
- Tuten M, Svikis DS, Keyser-Marcus L, O’Grady KE, Jones HE. Lessons learned from a randomized trial of fixed and escalating contingency management schedules in opioid-dependent pregnant women. The American Journal of Drug and Alcohol Abuse. 2012;38:286–292. doi: 10.3109/00952990.2011.643977. [DOI] [PubMed] [Google Scholar]
- Volpp KG, Troxel AB, Pauly MV, Glick HA, Puig A, Asch DA, Audrain-McGovern J. A randomized, controlled trial of financial incentives for smoking cessation. New England Journal of Medicine. 2009;360:699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]