Abstract
The intracellular spatiotemporal organization of signaling events is critical for normal cellular function. In response to environmental stimuli, cells utilize highly organized signaling pathways that are subject to multiple layers of regulation. However, the molecular mechanisms that coordinate these complex processes remain an enigma. Scaffolding proteins (scaffolins) have emerged as critical regulators of signaling pathways, many of which have well-described functions in immune cells. IQGAP1, a highly conserved cytoplasmic scaffold protein, is able to curb, compartmentalize, and coordinate multiple signaling pathways in a variety of cell types. IQGAP1 plays a central role in cell-cell interaction, cell adherence, and movement via actin/tubulin-based cytoskeletal reorganization. Evidence also implicates IQGAP1 as an essential regulator of the MAPK and Wnt/β-catenin signaling pathways. Here, we summarize the recent advances on the cellular and molecular biology of IQGAP1. We also describe how this pleiotropic scaffolin acts as a true molecular puppeteer, and highlight the significance of future research regarding the role of IQGAP1 in immune cells.
Introduction
Cellular responses to environmental stimuli may result in growth, proliferation, trafficking, and a wide range of other cell-specific functions. Cells use a variety of receptors and signaling cascades to achieve these functions. However, the organization of signaling components that translate distinct extracellular stimuli into unique physiological responses is poorly understood. Scaffolding proteins (scaffolins) play an essential role in coordinating signaling events in all eukaryotic cells. Often highly conserved in their specific functions, scaffolins curb, compartmentalize, and coordinate signaling events by serving as dynamic platforms that regulate protein-protein interactions in a manner that is highly coordinated through space and time. In this way, scaffolins act as molecular puppeteers that guide and fine-tune cellular responses. Further, the spatiotemporal organization of signaling events coordinated by scaffolins provides an additional layer of regulation that has been previously underappreciated.
In cells of the immune system, scaffold proteins act as critical mediators in a wide variety of cytoskeletal and signaling complexes(Shaw and Filbert, 2009). By interacting with multiple positive and negative regulators of signaling complexes in specific subcellular compartments, scaffolds precisely orchestrate signaling events that influence leukocyte function. The evolutionarily conserved scaffold proteins, discs-large homologue 1 (DLG1) and kinase suppressor of Ras 1 (KSR1), are two well-known examples of scaffold proteins that regulate immune cell function.
In activated T cells, DLG1, a PDZ-domain-containing scaffold, is recruited to the immunological synapse and associates with essential components of the TCR signaling complex including CD3ζ, (ζ-chain-associated protein kinase 70 kDa (ZAP70), LCK, VAV1, and Casitas B-lineage lymphoma (CBL) (Xavier et al., 2004),(Round et al., 2005). DLG1 also associates with Wiskott-Aldrich syndrome protein (WASp), and siRNA-mediated knockdown of DLG1 resulted in reduced actin polymerization, TCR clustering, and cytokine production following TCR ligation (Round et al., 2005). Interestingly, the earliest study evaluating T cell development and function in DLG1-deficeint mice reported dissimilar observations and concluded that DLG1 functions as negative regulator of T cell proliferation (Stephenson et al., 2007). To address the discrepancies in the literature, Humphries et al(Humphries et al., 2012)., compared siRNA-mediated knockdown, germline and conditional deletion models of DLG1 and found that acute loss (siRNA-mediated knockdown) of DLG1 supported earlier findings by Round et al (Round et al., 2005). However, germline dlg1−/− T cells showed no defect in proliferation while siRNA-mediated knockdown, germline deletion, and conditional DLG1 knockout (dlg1flox/flox:CD4Cre) T cells were deficient in Th1 cytokine production(Humphries et al., 2012).
KSR1, the closest mammalian equivalent to the yeast mitogen-activated protein kinase (MAPK) scaffold Ste5, is a well-known positive regulator of the Ras-MAPK signaling pathway (Shaw and Filbert, 2009). KSR1 is highly expressed in the brain, thymus, and spleen and binds components of the extracellular signal-related kinase (Erk) signaling pathway (Nguyen et al., 2002b). Specifically, KSR1 binds Raf proto-oncogene serine/threonine-protein kinase (Raf) and mitogen-activated protein kinase 1 (Mek1) via its pseudokinase domain, and a serine/threonine rich region on KSR1 binds Erk (Claperon and Therrien, 2007). KSR1 also interacts with activated Ras (Ras-GTP) and contributes to the sequetional phosphorylation and activation of the Erk pathway (Ras→Raf→Mek1→Erk) (Shaw and Filbert, 2009). Erk activation was defective in KSR1-deficeint mice which resulted in decreased cytokine production and proliferation of activated T cells (Nguyen et al., 2002a). Although Erk is known to play a role in thymopoioesis (Fischer et al., 2005), T cell development is normal in KSR1-deficient mice (Nguyen et al., 2002a). KSR1 also regulates the pro-inflammatory cytokine response in macrophages as Erk phosphorylation in response to tumor necrosis factor (TNF), interleukin-1β (IL-1β), and lipopolysaccharide (LPS) is reduced in KSR1-deficient macrophages (Fusello et al., 2006). Interestingly, these pro-inflammatory stimuli activate Erk independent of Ras activation suggesting that KSR1 may couple Erk activation to MAPKKKs other that Raf (Fusello et al., 2006).
DLG1 and KSR1 exemplify the complex nature of scaffold proteins in the immune system. Clearly, the coordination of signaling complexes by scaffold proteins is important for leukocyte function(Shaw and Filbert, 2009); however, the list of scaffold proteins known to regulate immune cell physiology is incomplete. Further exportation into the molecular mechanisms by which other conserved scaffold proteins mediate signaling is critical in order to expand our understanding of leukocyte biology.
IQ motif-containing GTPase activating protein (IQGAP) 1 was first characterized in 1994 and has since been the most extensively studied of the IQGAP proteins (Weissbach et al., 1994a). In the past two decades, IQGAP1 has been featured in more than 120 peer-reviewed articles which highlight its involvement in a myriad of cellular functions (White et al., 2012), including its role in spatiotemporal signaling events (Malarkannan et al., 2012), as well as tumorigenesis (White et al., 2009; Johnson et al., 2009). Recent advances on the complex structure and functional diversity of the IQGAP1 scaffolin necessitate detailed investigation to better understand its role in cellular biology. Studies have shown that many of IQGAP1’s functions in mammalian cells are conserved from its homolog in yeast, Iqg1p, further exemplifying IQGAP1 as a critical regulator of basic cellular physiology. In fact, IQGAP1 is a well-known regulator of signaling events involved in cytoskeletal rearrangement, the mitogen activated protein kinase (MAPK) pathway, and β-catenin-mediated transcription. Although IQGAP1 is the major IQGAP family member in lymphocytes (Malarkannan et al, unpublished), little is known regarding the role of IQGAP1 in immune cell signaling and function. In this review, we summarize recent findings and provide novel mechanistic insights into the functions of the IQGAP1 scaffolin.
The IQGAPs: Origin of the IQGAP1 puppeteer
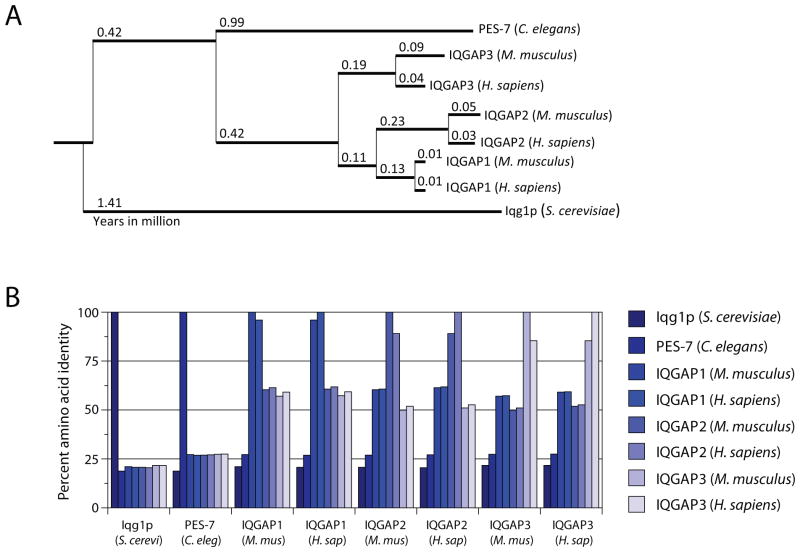
IQGAP1 is a 190 kDa protein that belongs to a conserved family of scaffolins. Of which, members have been identified in a variety of organisms, ranging from Saccharomyces cerevisiae and Caenorhabditis elegans to higher mammals such as Mus musculus and Homo sapiens. IQGAP1, encoded by Iqgap1, is located on chromosome 7 in mice and 15 in humans. Apart from IQGAP1, two other IQGAPs (IQGAP2 and IQGAP3) are also expressed in mammals. Mammalian IQGAPs share approximately 20% amino acid identity with their homolog in Saccharomyces cerevisiae, Iqg1p, and 27% with their homolog in Caenorhabditis elegans, PES-7 (Figure 1A and B). Divergence within the mammalian IQGAPs occurred unequally as IQGAP3 deviated from the uncharacterized common ancestor of IQGAP1 and IQGAP2 (Figure 1A). Interestingly, IQGAP1 proteins between mice and humans share the highest amino acid identity (96%) of the IQGAPs, compared to IQGAP2 (89%) and IQGAP3 (85%) (Figure 1B). The tissue distribution of the mammalian IQGAPs is also unequal. While IQGAP1 is ubiquitously expressed (Weissbach et al., 1994a), IQGAP2 (Wang et al., 2007; Schmidt et al., 2003; Cupit et al., 2004; Brill et al., 1996) and IQGAP3 (Wang et al., 2007; Nojima et al., 2008) are restricted to specific tissues. Global deletion of IQGAP1 in mice does not result in gross developmental or physiological defects(Li et al., 2000). Although Iqgap1−/− mice exhibit increased late-onset gastric hyperplasia, redundant functions between the IQGAPs or other scaffold proteins could possibly explain the mild phenotype. Iqgap2−/− mice develop age-dependent hepatocellular carcinoma characterized by increased expression of IQGAP1. Interestingly, this phenotype was not present in Iqgap1−/−Iqgap2−/− mice, implicating a tumor suppressive role for IQGAP2. This also suggests that IQGAP1 and IQGAP2 maintain non-redundant functions in the liver. Phenotypes of mice lacking IQGAP3 or targeted deletion of all three IQGAP genes have not been reported.
Figure 1. IQGAP proteins are evolutionarily conserved from yeast to mammals.
A) Phylogenic analysis from aligned amino acid sequences of the mammalian IQGAP proteins (IQGAP1, 2, and 3 in mice and humans) and their homologs, Iqg1p and PES-7, in Saccharomyces cerevisiae and Caenorhabditis elegans, respectively. Red numbers indicate evolutionary distance in millions of years and the mammalian IQGAP1 branch is shaded in grey. B) Graph of the percent identity matrix of IQGAP amino acid sequences. The phylogenic tree and percent identity matrix were generated using Clustal Omega alignment software using amino acid sequences retrieved from NCBI (accession numbers: AAB70827.1, CAB07179.2, NP_057930.2, NP_081987.1, NP_001028656.1, NP_003861.1, NP_006624.2, and AAP06954.1 for Iqg1p, PES-7, Mouse IQGAP1, 2, 3 and Human IQGAP1, 2, 3, respectively).
The domains of IQGAP1: the strings and the sticks
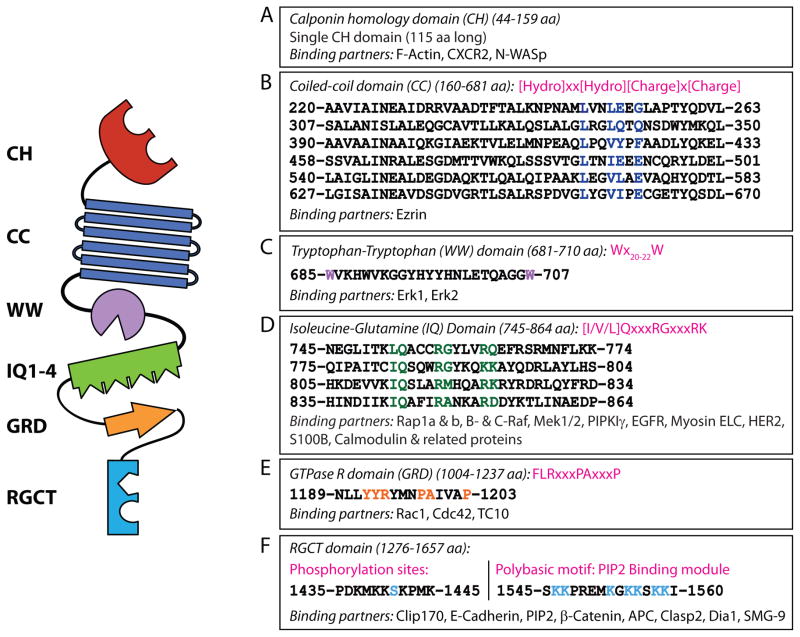
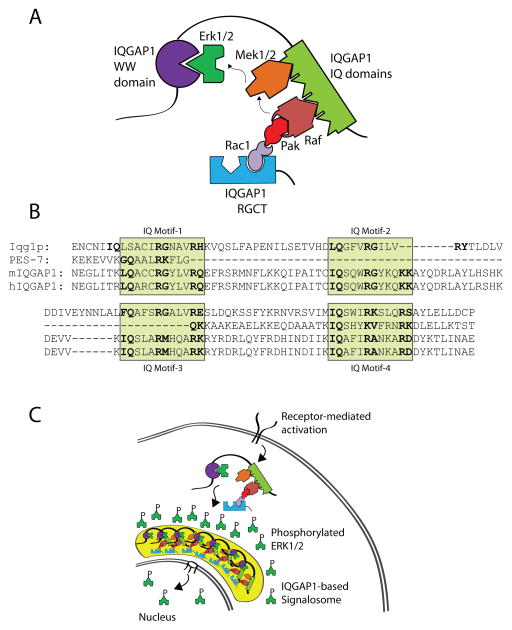
IQGAP1, originally named for containing isoleucine-glutamine (IQ) domains and a GTPase activating protein (GAP) homology domain, is one of the largest known scaffold proteins (Weissbach et al., 1994b). Its vast array of protein interactions (>50) (White et al., 2012) also makes IQGAP1 one of the most complex scaffolins in mammalian cells (Brown and Sacks, 2009). These multifarious interactions are mediated by clearly identifiable protein recognition-motifs present in the six domains IQGAP1 (Figure 2A–F).
Figure 2. Protein structure of and specific domain-interacting partners of IQGAP1.
IQGAP1 is a 190 kDa (1657 amino acid) protein that contains six distinct protein-interacting domains. The domains and known interacting proteins are shown as well as amino acid sequences (mouse). A) The calponin homology (CH) domain binds the chemokine receptor, CXCR2, and regulates the actin cytoskeleton by binding N-WASp and polymerized filamentous actin (F-actin). B) Six coiled-coil (CC) regions, comprised of highly conserved amino acid repeats, comprise the coiled-coil (CC) domain which binds Ezrin. C) Erk1/2 are recruited to the WW domain. D) The Isoleucine/glutamine-containing (IQ) domain is a binding domain for multiple proteins that include components of MAPK signaling (Rap1a, Rap1b, B-Raf, C-Raf, Mek1 and Mek2), phosphoinositide signaling (PIPKIγ), calcium signaling (S100B and Ca2+-independent interaction of calmodulin and its related proteins), as well as cytoskeletal components (myosin ELC) and cell surface receptors (EGFR and HER2). E) The Ras-GAP domain (GRD) does not function as a GTPase Activating Protein (GAP) but does interact with small GTPases Cdc42, Rac1, and TC10. F) The Ras-GAP C-terminus domain (RGCT) interacts with microtubule-binding proteins CLIP-170 and Clasp2, as well as membrane-resident proteins such as β-catenin, E-cadherin, and APC. The RGCT domain also contains two phosphorylation sites (Ser1441 and Ser1443) of IQGAP1 as well as a polybasic region which binds PIP2. Other proteins previously shown to interact with IQGAP1 (Akt, mTORC1, exorcist, Sec3/8, Lis1, Menin, SMG-2, and Disheveled) are not depicted.
The calponin homology (CH) domain (amino acids 44–159), present at the N-terminus of IQGAP1, has been shown to bind neuronal-WASp (N-WASP) (Le Clainche et al, JBC, 2007), chemokine C-X-C motif receptor 2 (CXCR2) (Neel, PLOS ONE, 2011), and filamentous actin (F-actin) (Ho et al., 1999; Mateer et al., 2002) (Figure 2A). Canonically, double CH domains are supposedly required for F-actin recruitment; however, all three mammalian IQGAPs, as well as yeast Iqg1p, contain only a single CH domain and have been shown to regulate actin polymerization. Although the role of IQGAP1 in actin polymerization is clearly identified, the molecular mechanism(s) are not completely understood.
A coiled-coil (CC) domain (amino acids 160–681) follows the CH domain of IQGAP1 (Figure 2B). CC domains, also known as heptad domains, display a conserved repetition of hydrophobic (h) and charged (c) amino acids (hxxhcxc). Recently, these repeats were shown to facilitate the binding of IQGAP1 to the N-terminal FERM domain of Ezrin, a protein that connects components of the plasma membrane to the actin cytoskeleton (Liu et al., 2014). In addition to forming amphipathic structures, the α-helical configuration of the CC domains shares significant sequence homology to the myosin family of actin-binding motor proteins which may provide vital clues for additional functions of IQGAP1 (Ho et al., 1999).
The WW domain of IQGAP1 (amino acids 685–710) contains two highly conserved tryptophans, positioned 20–22 amino acids apart (Wx20–22W) (Figure 2C). Historically, WW domains function as an interaction module for proline-rich ligands (Macias et al., 2002). Erk1/2 is the only identified ligand for the WW domain on IQGAP1 and binds via a polyproline (APPPxxPY) motif (Roy et al., 2004). The interaction between IQGAP1 and Erk1/2 is critical in tumor formation as competing for Erk1/2 binding with a peptide encompassing the WW domain inhibits Ras and Raf-driven tumorgenesis (Jameson et al., 2013).
Four tandem isoleucine-glutamine (IQ) domains (amino acids 745–864), each 25 amino acids long, contain the [I/V/L]Qxxx[RG]xxx[RK] motif (Figure 2D). These IQ domains mediate interactions with Ras-related protein 1 (Rap1) (Jeong et al., 2007), B-Raf, c-Raf (Raf-1) (Ren et al., 2007), Mek1/2 (Roy et al., 2005), myosin essential light chains (Weissbach et al., 1998), S100B (Mbele et al., 2002), a Zn2+ and Ca2+-binding protein, phosphatidlyinositol 4-phosphate 5-kinase (PIPKγ), a phosphatidylinositol 4,5 bisphosphate (PIP2) generating enzyme (Choi et al.), calmodulin (Ho et al., 1999; Joyal et al., 1997; Hart et al., 1996) and calmodulin-related proteins (Briggs and Sacks, 2003). Among these four IQ domains, the first and the fourth interact with myosin light chain and the myosin essential light chain, Mlc1p, while the first also recruits Mlc1sa (Pathmanathan et al., 2008). The first and second IQ domains interact with S100B. This domain also interacts with the cell surface receptors, epidermal growth factor receptor (EGFR) (McNulty et al., 2011) and human epidermal growth factor receptor 2 (HER2) (White et al., 2011).
A primitive GTPase-activation-related domain (GRD) is present in IQGAP1 (amino acids 1025–1237) and is responsible for its nomenclature (Figure 2E). However, it lacks GTPase-activating protein (GAP) activity and, therefore, is unable to execute GTP hydrolysis. Although small GTPases such as cell division cycle 42 (Cdc42) (Joyal et al., 1997; Hart et al., 1996), Ras-related C3 botulinum toxin substrate 1 (Rac1) (Hart et al., 1996), and Rho family GTPase (TC10) (Neudauer et al., 1998) interact with this domain, the crystal structure of the GRD reveals the presence of a threonine (threonine1046) in place of the catalytic ‘arginine finger’ essential for GTP hydrolysis (Kurella et al., 2009). Further, deviation from the conserved Ras-GAP motif FLRXXXPAXXXP to YYRTMNPAIVAP in mammalian IQGAPs and YYGYKDSNVQKN in yeast Iqg1p may also prevent GTPase function as a substitution of the leucine with isoleucine in this motif abrogates GAP function (Adachi et al., 1997). Interestingly, the two consecutive tyrosine residues are highly conserved among the IQGAPs and are involved in the formation of a π-helix (Kurella et al., 2009), which may be critical for other IQGAP functions that have yet to be described.
The Ras GAP C-terminus (RGCT) domain of IQGAP1 (amino acids 1276–1657) engages multiple protein partners including the nonsense-mediated mRNA decay factor (SMG-9) (Takeda et al., 2011), E-cadherin (Kuroda et al., 1998), β-catenin (Fukata et al., 1999), the microtubule plus-end-trafficking protein (Clip170) (Fukata et al., 2002), cytoplasmic linker associated protein 2 (Clasp2) (Watanabe et al., 2004), and adenomatous polyposis coli (APC) protein (Watanabe et al., 2004) (Figure 2F). The RGCT domain also functions as a docking center for AKAP79, a regulator of calcium flux via protein kinase A (PKA) (Nauert et al., 2003; Logue et al., 2011). Further, the RGCT domain is the site of two serine residues (1441 and 1443) that have been shown to be minor and major phosphorylation sites on human IQGAP1, respectively (Li et al., 2005). Phosphorylation of serine1443 may regulate a dynamic interaction between IQGAP1 and Cdc42 (Grohmanova et al., 2004) (Wang et al., 2009). Interestingly, these serine residues are absent in yeast Iqg1p and mouse IQGAP1 contains only the serine1441 residue, which may indicate divergent mechanisms of Cdc42 regulation from yeast to human. Aside from regulating small GTPases near the leading edge, this domain is also responsible for anchoring IQGAP1 to the membrane. PIP2 has been shown to bind the RGCT domain of IQGAP1 through a polybasic motif and serves as the anchoring mechanism (Choi et al., 2013).
IQGAP1 and the cytoskeleton: Pulling the strings
The membrane proximal polymerized actin mesh provides structural integrity for cell shape and size, a skeletal frame work for signal transduction, and a controllable conduit for exocytosis of effector and messenger proteins. Similar to DLG1 in T cells (Round et al., 2005), IQGAP1 also links signaling components to cytokeletal regulators (Smith et al., 2015). In fact, in a study using Iqgap1−/− T cells, IQGAP1 was shown to act as a negative regulator of TCR-mediated signaling and F-actin dynamics (Gorman et al., 2012). Two functional abilities of IQGAP1 qualify it as an essential regulator of the cytoskeleton. First, the CH domain of IQGAP1 has been shown to directly recruit and bind polymerized F-actin with high affinity (~ 47μM) (Mateer et al., 2004) (Le et al., 2007; Watanabe et al., 2004) (Figure 3A). Second, the ability of IQGAP1 to interact with numerous cytoskeletal effector proteins such as Cdc42 and Rac1 (Hart et al., 1996; Fukata et al., 1997; Bashour et al., 1997), APC (Tirnauer, 2004), CLIP-170 (Gundersen, 2002), Clasp2 (Watanabe et al., 2009), and microtubule-end binding protein 1 (EB1) (Zhang et al., 2009a) further facilitates its role in cytoskeletal remodeling.
Figure 3. IQGAP1 plays a critical role in actin polymerization.
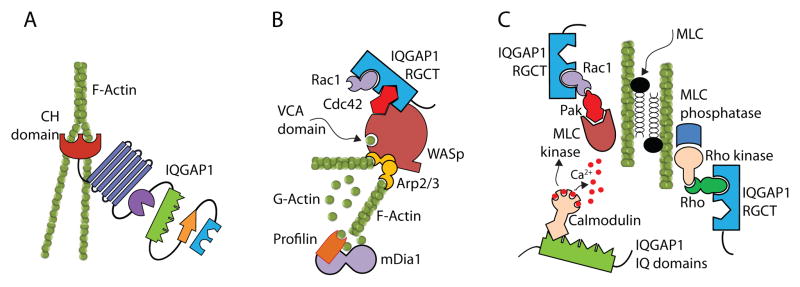
IQGAP1 can regulate actin polymerization by at least three different mechanisms. A) RGCT domain of IQGAP1 can Cdc42 or Rac1 is recruited by autoinhibited WASp through ‘Cdc42-interactive-binding’ (CRIB) motif. This causes a conformational change in the C-terminal verprolin homology domain and central acidic (VCA) region of WASp allowing globular actin to bind through an electrostatic steering mechanism. In addition, IQGAP1 transiently protects Cdc42-GTP in its activated state, reducing its GTP hydrolysis. By delaying the hydrolysis of WASp-bound Cdc42-GTP, IQGAP1 stabilizes the VCA domain of WASp. This sustains the interaction with Arp2/3 and globular actin, promoting the polymerization and branching of actin. B) The CH domain of IQGAP1 interacts with polymerized F-actin. a single N-terminal CH domain facilitates actin polymerization by cross linking filaments into interconnected bundles. C) RGCT domain of IQGAP1 can regulate polymerized actin filament through myosin light chain (MLC) in a co-ordinated effort of MLC kinase and MLC phosphatase by PAK and Rho, respectively. In addition, MLC kinase function can be regulated by IQGAP1-bound calmodulin.
The ability of the GRD domain of IQGAP1 to directly recruit small GTPases such as Cdc42 (Joyal et al., 1997; Hart et al., 1996), Rac1 (Hart et al., 1996) or TC10 (Neudauer et al., 1998) strongly demonstrates the crucial role played by IQGAP1 in cytoskeletal remodeling (Fukata et al., 2002; Mataraza et al., 2003). In particular both Cdc42 and Rac1 bind to IQGAP1 with relatively high affinities; however, they use different but partially overlapping topological determinants in the RGCT domain (Owen et al., 2008). The activated GTP form of Cdc42 preferentially binds to the IQGAP1 GRD demonstrating an activation-dependent association between these two proteins (McCallum et al., 1996). Cdc42 or Rac1 is recruited by autoinhibited WASp through a Cdc42-interactive-binding (CRIB) motif. This causes a conformational change in the C-terminal verprolin homology domain and central acidic (VCA) region of WASp allowing globular actin to bind through an electrostatic steering mechanism. In addition, IQGAP1 transiently protects Cdc42-GTP in its activated state (Zhang et al., 1997), reducing its GTP hydrolysis. By delaying the hydrolysis of WASp-bound Cdc42-GTP, IQGAP1 stabilizes the VCA domain of WASP. This sustains the interaction with actin-related protein 2 and 3 (Arp2/3) and globular actin, promoting the polymerization and branching of actin (Hemsath et al., 2005). Thus, IQGAP1 could add multiple layers of structural complexity to the actin meshwork through not only by the direct binding of actin to the CH domain but also by interacting with the WASp/Arp2/3/Cdc42 complex (Le et al., 2007) (Figure 3B). Studies have further shown that the C-terminal half of IQGAP1, activated N-WASp by interacting with the BR-CRIB domain (Le et al., 2007). Additionally, in contrast to its C-terminus, the N-terminal half of IQGAP1 has the ability to antagonize its interaction with N-WASp. Therefore, conformational intramolecular changes in IQGAP1 can function as a switch to regulate Arp2/3-dependent actin assembly through two-way interaction with N-WASp (Le et al., 2007).
Additional studies have shown that the interaction between Cdc42 and IQGAP1 is regulated by calmodulin (Joyal et al., 1997) (Figure 3C). Exogenous calmodulin partially inhibits binding of IQGAP1 to F-actin, and is more effective in the absence, rather than in the presence of calcium (Bashour et al., 1997). Thus, during the active recognition of a target cells by effector lymphocytes, calcium influx or calmodulin activation could disrupt the interactions between Cdc42 and IQGAP1, thereby preventing the reorganization of cytoskeleton and transiently freezing the effector cells. In contrast, non-migrant epithelial cells may use Cdc42/Rac1/IQGAP1-mediated actin polymerization to successfully form non-permeable tight junctions (Kuroda et al., 1998) or intercellular adherence junctions. These molecular zippers require E-cadherin, which is critical for morphogenesis and tissue architecture. It is also interesting to note that E-cadherin is one of the few extracellular membrane proteins that can recruit IQGAP1 directly to their cytoplasmic tails (Kuroda et al., 1998). Under these conditions, Cdc42 and Rac1 interaction with IQGAP1 could lead to a continuous polymerization of F-actin, providing a lasting skeletal support for tight or adherence junctions. Based on other studies (Watanabe et al., 2005), this probably occurs through the continuous oligomerization of IQGAP1 facilitated by Cdc42-GTP.
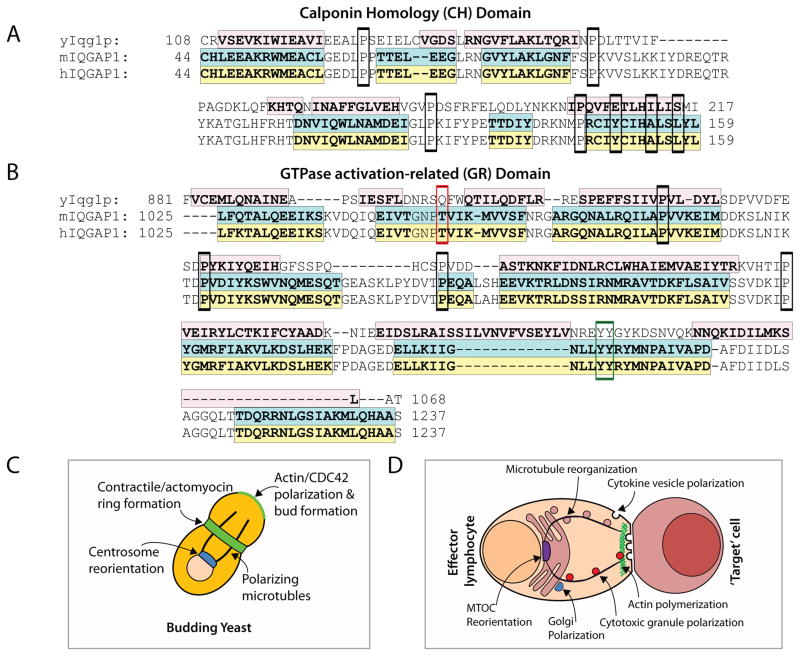
The role of IQGAPs in actin polymerization has been extensively investigated for IQGAP1 and previous studies with Iqg1p in yeast suggest that this function is highly conserved. Along with considerable homology in secondary structure to IQGAP1 (Figure 4A), the CH domain of Iqg1p has also been reported to mediate actin-based cellular functions and (Shannon and Li, 1999; Osman and Cerione, 1998). Iqg1p is also critical for the formation and contraction of the actomyocin ring in dividing yeast cells (Figure 4B) (Shannon and Li, 1999), and Iqg1p-deficient cells exhibit a defect in cytokinesis (Epp and Chant, 1997). Iqg1p has been shown to physically interact with Cdc42 by yeast two-hybrid assay (Osman and Cerione, 1998) and, in line with mammalian IQGAP1, can localize to the periphery and mediate cell polarization and actin polymerization (Osman et al., 2002) (Figure 4B&C). The GRD of IQGAP1 binds Cdc42 and this domain is also conserved in amino acid composition and secondary structure (Figure 4D).
Figure 4. Cytoskeletal remodeling is a conserved function of IQGAP1.
The CH and GR domains of IQGAP1 are critical for orchestrating cytoskeletal remodeling and are conserved from yeast Iqg1p. A) Amino acid sequence alignment of the CH domains of yeast Iqg1p (yIqg1p), mouse IQGAP1 (mIQGAP1), and human IQGAP1 (hIQGAP1). Conserved proline residues are outlined in black. Shaded regions indicate alpha-helices previously determined for the CH domain of IQGAP1 (Umemoto et al., 2010) and predicted for Iqg1p using PSIPRED analysis software (UCL Bioinformatics). B) Diagram of Iqg1p-mediated functions involved in cytoskeletal remodeling in yeast mirrors what has been shown for C) IQGAP1 in mammalian cells such as lymphocytes. D) Amino acid sequence alignment of the GR domain. Shaded regions and conserved proline residues are indicated as in (A). Alpha-helical structures were previously determined for the GRD of IQGAP1 (Kurella et al., 2009) and predicted for Iqg1p using PSEPRED analysis software (UCL Bioinformatics). The threonine1046 residue thought to be related to the absence of GAP function is outlined in red. Amino acids involved in the π-helix structure (Kurella et al., 2009), outlined in blue, are located within the variant Ras-GAP motif and contain one of the two conserved tyrosine residues outlined in green.
Recent studies have demonstrated that Rac1 and Cdc42 recruit other downstream effectors of IQGAP1 involved in cytoskeletal reorganizations (Watanabe et al., 2005). Neuronal dendrites require Clip-170 and IQGAP1 to maintain their shape and morphology (Swiech et al., 2011). The recruitment of Clip-170 to the IQGAP1 scaffold can be augmented by Cdc42 and Rac1. In addition, a direct interaction of the mechanistic target of rapamycin (mTOR) with Clip-170 is required for the formation of the Clip-170/IQGAP1 complex (Swiech et al., 2011), which is capable of regulating the actin/tubulin cytoskeletons. Thus, IQGAP1, along with Clip-170, functions as a focal point for feedback interactions between the actin and microtubule cytoskeletal systems at the leading edges and filopodia. In addition, Cdc42 and Rac1 activation results in the increased recruitment of APC to the IQGAP1 scaffold. With Clip-170, APC forms a tripartite complex that helps link the cortical actin meshwork to the microtubule cytoskeleton, aiding cell polarization and migration (Tirnauer, 2004; Watanabe et al., 2004). In particular, membrane ruffling and filopodia at the leading edges depend on interaction between actin and tubulin, which is facilitated by multiple molecules including the CLIP-associating protein, Clasp2. Clasp2 can bind to both the growing tips and the lattices of the microtubule, using IQGAP1 as a scaffolding link between the actin meshwork and the microtubule network. In fact, siRNA-mediated knockdown of IQGAP1 severely impairs actin and tubulin polymerization in the filopodia of the leading edges of migrating fibroblasts (Watanabe et al., 2004). Clasp2 accumulates near the plus-ends of microtubules at the leading edges of migrating cells to control microtubule dynamics and cytoskeletal coupling (Watanabe et al., 2009). This asymmetric subcellular distribution of Clasp2 and its ability to link actin and microtubules is regulated by glycogen synthase kinase-3β (GSK-3β)-mediated phosphorylation (Watanabe et al., 2009). GSK-3β phosphorylation of the serine533 and serine537 residues of Clasp2 is critical for binding to the region between amino acids 1503 and 1667 of IQGAP1 (Watanabe et al., 2009). This phosphorylation (probably through a conformational change) results in the release of Clasp2 from IQGAP1/EB1/microtubule/actin complexes at the leading edges of cells, restricting their movement (Watanabe et al., 2009). Further, IQGAP1 is known to interact with Lis1, a dynein motor complex-interacting protein. Lis1 regulates calcium-dependent neuronal stem cell migration through Cdc42, Rac1 and RhoA and aids in the co-localization of IQGAP1 and CLIP-170 at the perimembrane region tethering the microtubule plus-ends to the cortical actin meshwork (Kholmanskikh et al., 2006). Interestingly, defects in the human Lis1 gene results in severe human brain malformation (lissencephaly) (Dujardin et al., 2003; Vallee et al., 2001; Tai et al., 2002); however, the precise role of IQGAP1 in this disease is still unknown.
Pathogenic bacteria require polymerized F-actin pedestals for their successful entry into host cells. Salmonella typhimurium-derived SopE functions as a guanine nucleotide exchange factor (GEF) that when injected by the bacterium into the host cell, can activate both Cdc42 and Rac1 (Fu and Galan, 1999). Recent studies have shown that IQGAP1 is necessary for the cytoskeletal alteration of actin that allows the entry of Salmonella (Brown et al., 2007; Brown et al., 2008). These studies provide exciting possibilities that IQGAP1 could also play an important role in the uptake, processing, and presentation of antigens by professional antigen-presenting cells. In fact, the mechanism by which mTOR/CLIP-170/IQGAP1 complex regulates dendritic morphology in synaptic neurons may be applied to professional antigen-presenting cells such as dendritic cells and macrophages (Swiech et al., 2011). Further, the interaction between IQGAP1 and the Diaphanous-related formin (Dia1), a nucleator of the actin cytoskeleton, is required for phagocytosis in macrophages (Brandt et al., 2007; Brandt and Grosse, 2007). Future studies are required to determine the dynamic roles of Cdc42 or Rac1 activation and IQGAP1 oligomerization and function during supra-molecular activation cluster (SMAC) formation in T cells or Natural Killer (NK) cell immunological synapse (NKIS) formation.
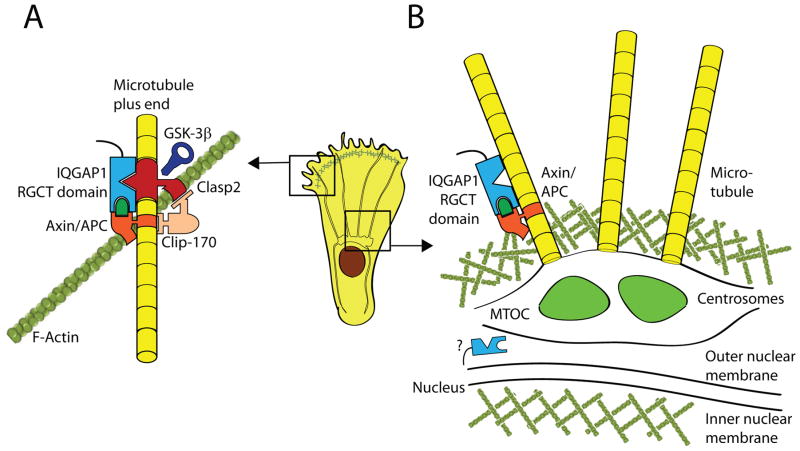
The microtubule organizing center (MTOC), the anchoring center for microtubules, regulates the intracellular trafficking of secretory vesicles, including those containing lytic granules (Gundersen, 2002). Together, microtubules and MTOC determine the shape, size and directional movements of cells. MTOC is made up of two centrioles that act as the core microtubule nucleation site (Basto et al., 2008). A number of studies have strongly demonstrated the central roles played by IQGAP1 in the microtubule-mediated cytoskeletal cellular reorganization (Figure 5A) (Le et al., 2007; Bielak-Zmijewska et al., 2008; Suzuki and Takahashi, 2008; Nakhaei-Nejad et al., 2009; Wickstrom et al., 2010; Logue et al., 2011; Swiech et al., 2011; Watanabe et al., 2004). MTOC reorientation is independent of Cdc42-induced changes in actin and microtubule stabilization in a dynein-dependent manner (Palazzo et al., 2001). IQGAP1 has also been found localized to the cytoplasmic face of the nuclear envelope, potentially promoting cell cycle-associated assembly and nuclear envelope disruption (Johnson and Henderson, 2012) (Figure 5B).
Figure 5. An IQGAP1 complex tethers F-actin to microtubules.
IQGAP1 plays an important role in linking polymerized actin filaments (F-actin) with microtubules. A) The RGCT domain of IQGAP1 links F-actin to plus-end microtubules at the leading edge membrane via a complex including Axin, APC, CLIP170 and Clasp2. GSK-3β acts as a negative regulator of this complex by phosphorylating Clasp2 which causes it to disassociate from IQGAP1 leading to a reduction in cell movement. B) Although the role of IQGAP1 in the formation and function of the MTOC is not well understood, its interaction with Axin and APC may facilitate the organization and multimerization of γ-tubulin molecules that form the core of the centrosome and MTOC. IQGAP1 has also been to localize to the cytoplasmic face of the nuclear envelope, potentially promoting cell cycle-associated assembly and disruption of nuclear envelope
The size and shape of MTOC could be regulated by IQGAP1 (Figure 5B) (Awasthi et al., 2010) via its direct interaction with the small GTPase, Rap1b (Jeong et al., 2007). Lack of Rap1b resulted in multiple NK and B cell dysfunctions (Chu et al., 2008; Awasthi et al., 2010); Although, it did not affect the formation of MTOC, the size, height, and the length of the MTOCs were abnormally large in Rap1b−/− NK cells (Awasthi et al., 2010). These NK cells also displayed multiple, non-complete MTOCs. Phosphorylated-Erk2 localizes to the microtubules and regulates the polarization and directional translocation of MTOC to the T cell SMAC or in NK cell NKIS (Chen et al., 2006). In line with these findings, Erk1/2 phosphorylation in NK cells that lack Rap1b is considerably reduced due to an impairment in the sequential phosphorylation of B-Raf/C-Raf→Mek1/2→Erk1/2 signaling pathway that utilizes the scaffolding function of IQGAP1(Awasthi et al., 2010). Since this pathway requires IQGAP1 for scaffolding, knockdown of IQGAP1 impaired MTOC reorientation in the NK cell line YTS; however, the interaction between the NK and target cells and the ability to form conjugates was not affected (Kanwar and Wilkins, 2011). This study further demonstrates that IQGAP1 played an obligatory role for the perigranular polarization and an accumulation of F-actin meshwork proximal to NKIS (Kanwar and Wilkins, 2011).
IQGAP1 and cell surface receptors: A puppeteer extraordinaire
Many cell surface receptors have been described to directly recruit and utilize IQGAP1-containing complexes to mediate signaling. Research on receptor-mediated recruitment of IQGAP1 has been conducted in a variety of cell types. Receptors involved in immune cell trafficking, such as CD13, CXCR2, and CD44, have been shown to associate with IQGAP1. Further, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in the central nervous system (CNS), growth factor receptors, and Ca2+-dependent E- and N-cadherins have revealed novel functions of IQGAP1 in mediating cell surface receptor signaling.
In immune cells, IQGAP1 was found to be constitutively associated with CD13, a homing molecule and a cell surface peptidase expressed on monocytes and activated endothelial cells (Subramani et al., 2013). Further, CXCR2, a G-protein-coupled receptor (GPCR) involved in immune cell chemotaxis, directly binds IQGAP1 at the leading edge of human neutrophils (Neel et al., 2011). CXCR2 binds the CH domain of IQGAP1 and stimulation of CXCR2 with its ligand, CXCL8, enhances the association of IQGAP1 with Cdc42. Further, competition experiments using the CXCR2-interacting domain of IQGAP1 revealed that IQGAP1 is essential for CXCR2-meidated chemotaxis (Neel et al., 2011). CD44 is expressed in both immune and non-immune cells and is used as a critical marker of developmental stages in thymic T (Heyzer-Williams and Davis, 1995) and NK cells (Ballas and Rasmussen, 1990). Earlier studies (Bourguignon et al., 2005) and recent proteomic searches (Skandalis et al., 2010) have indicated that CD44 can interact with IQGAP1 after binding to its cognitive ligand, hyaluronan (HA). Recruitment of IQGAP1 and small GTPase Cdc42 to the cytoplasmic tail of CD44 is critical for the polarized polymerization of F-actin and phosphorylation of Erk2 that lead to the activation of the ETS domain-containing protein, Elk-1, and estrogen receptor-α (ER-α) (Bourguignon et al., 2005). Irrespective of these observations, the functional relevance of IQGAP1 and CD44 interaction in immune development and function is yet unclear (Isacke and Yarwood, 2002; Johnson and Ruffell, 2009; Sackstein, 2011).
In the CNS, the AMPA receptors regulate excitatory synaptic transmission and plasticity of neurons (Nakagawa, 2010). AMPA receptors are heterotetrameric proteins composed of the glutamate-gated ion channels GluR1, GluR2, GluR3 and GluR4. Recent studies have revealed that GluR4 recruits both α-actinin-1 and IQGAP1 with its C-terminus cytoplasmic tail to regulate downstream signaling events (Nuriya et al., 2005). Although α-actinin-1 and IQGAP1 bind GluR4 at the same region, phosphorylation of serine842 within this region inhibited the GluR4-α-actinin-1 interaction while the GluR4-IQGAP1 interaction remained intact (Nuriya et al., 2005).
The cytoplasmic region of EGFR has been shown to require IQGAP1 for B-Raf/Mek1/2-mediated Erk1/2 phosphorylation (McNulty et al., 2011). Both EGFR and IQGAP1 co-localize in epithelial cells and co-precipitate in biochemical assays (Takahashi and Suzuki, 2006). EGFR directly interacts with the IQ domain of IQGAP1 and silencing of IQGAP1 results in mitotic spindle misorientation in a Madin-Darby Canine Kidney (MDCK) cell system (Banon-Rodriguez et al., 2014). In addition, EGFR has been shown to facilitate the phosphorylation of serine1443 of IQGAP1 by PKC-α (McNulty et al., 2011). Vascular endothelial growth factor type 2 receptor (VGFR2), which requires the presence of IQGAP1 for successful interactions between endothelial cells for capillary tube formation, has also been shown to phosphorylate Akt in an IQGAP1-dependent manner following activation with VEGF (Yamaoka-Tojo et al., 2004).
Also utilizing the IQGAP1 scaffolin is the Cadherin family which consists of multiple cell surface proteins important for morphogenesis and tissue integrity. The Ca2+-dependent E- and N-cadherins recruit IQGAP1 in order to maintain their trans interactions in tight and adherens junctions (Kuroda et al., 1998). E- and N-cadherin-mediated cell-cell adhesion requires the presence and association of IQGAP1/Cdc42/Rac1/α-catenin/β-catenin complexes (Kuroda et al., 1999; Kaibuchi et al., 1999; Fukata et al., 2001). Additionally, a few members of the cadherin family play important roles in transendothelial migration or diapedesis of lymphocytes. Lack of IQGAP1 significantly reduced the ability of interendothelial cell junctions to allow for lymphocyte extravasation and lack of IQGAP1 destabilizes the microtubule tethering to the cytoplasmic domain of cadherins in endothelial cells (Nakhaei-Nejad et al., 2010). Menin, encoded by the multiple endocrine neoplasia type 1 (MEN1) gene, is associated with a dominantly inherited tumor syndrome. Interestingly, Menin has been shown to modify the activity of IQGAP1 as this interaction reduced IQGAP1’s ability to bind Rac1-GTP while increasing the association of E-cadherin/β-catenin complexes with IQGAP1 (Yan et al., 2009).
IQGAP1-based signalosome: Setting the stage to curb, compartmentalize, and coordinate
Development and survival of organisms and communal harmony of cells depend on their ability to continuously sense their microenvironment and process complex information. Mitogen-activated protein kinases (MAPKs) play an essential role in cell survival, proliferation, and differentiation. Lymphocytes are a common model used to study MAPK signaling and IQGAP1 has been shown to regulate the Rap1b-GTP→Vav1→Cdc42→Pak→B-Raf/C-Raf→Mek1/2→Erk1/2 signaling pathway in NK cells (Figure 6A) (Awasthi et al., 2010).
Figure 6. IQGAP1 is a MAPK scaffold that regulates Erk1/2 phosphorylation and facilitates signalosome formation.
A) IQGAP1 acts as a scaffold for sequential activation of the Cdc42-Pak1-B/C-Raf-Mek1/2-Erk1/2 MAPK pathway. Receptor-mediated activation of Cdc42, recruited to the GRD domain of IQGAP1, results in the recruitment and activation of Pak1 which binds and activates B-Raf and C-Raf on the IQ domains of IQGAP1. Activated Rafs phosphorylate Mek1/2, also associated with the IQ domains, which in turn phosphorylate Erk1/2 that are recruited to the WW domains of IQGAP1. B) Amino acid sequence alignment of the IQ domain in yeast (yIqg1p), mouse IQGAP1 (mIQGAP1), and human IQGAP1 (hIQGAP1). Shaded regions indicate four individual IQ domains containing the [I/V/L]Qxxx[RG]xxx[RK] motif. C) IQGAP1 facilitates the formation of a MAPK signalosome. Upon receptor-mediated activation and IQGAP1 oligomerization, the IQGAP1-mediated signalosome localizes to the periphery of the nucleus and may promote nuclear translocation of phosphorylated Erk1/2.
Similar to what has been shown for KSR1 (Shaw and Filbert, 2009), IQGAP1 can directly recruit and sequentially activate B-Raf (Ren et al., 2007), Mek1/2 (Roy et al., 2005) and Erk1/2 (Roy et al., 2004). At the start of this sequence, receptor-mediated signaling activates Fyn, a Src family PTK, to phosphorylate Vav1 (Huang et al., 2000). Activated Vav1 is one of the major GEFs required for Cdc42 or Rac1 activation, resulting in the conversion of GDP into GTP forms. The active Cdc42-GTP uses its second β-strand and a region of a peptide loop between the first α-helix and switch I region to bind the PBD46 motif of the serine/threonine p21-activating kinase (Pak) with very high affinity (Guo et al., 1998). Studies have indicated that Cdc42 exhibits differential binding patterns to Pak1, WASp and IQGAP1 (Li et al., 1999). Activated Cdc42-GTP uses its ‘insert region’ (Zong et al., 2001) and a part of the switch I domain (McCallum et al., 1996) to interact with IQGAP1. Specifically, the switch I domain (amino acids 29–55) serves as the binding site for Pak1, while the determinants outside this region (amino acids 84–120 and 157–191) are required for the binding of IQGAP1 and WASp. Synthetic peptide analogs from the PBD46 motif of Pak partly prevented the ability of Cdc42 binding to IQGAP1. This demonstrates that IQGAP1, Pak and WASp may form complexes that interact with Cdc42 in a synchronized fashion (McCallum et al., 1996). Then, Pak1 directly binds to Rafs to induce conformational changes and phosphorylate serine338 of Raf-1 or serine445 of B-Raf (King et al., 1998; Zang et al., 2002). Under resting conditions, both Raf-1 and B-Raf contain an N-terminal autoinhibitory domain that interacts with their respective catalytic domains (Tran and Frost, 2003; Tran et al., 2005). However, upon activation, H-Ras (Tran et al., 2005) or another Ras family member, such as Rap1b (Awasthi et al., 2010), can directly interact with the Rafs and relieve this autoinhibition. However, on the IQGAP1 scaffold, it is unclear how the Cdc42/Pak1 complex transfers Pak1 to B- or Raf-1 or how the small GTPases mitigate their autoinhibition. B- or Raf-1 phosphorylate Mek1/2, which is next recruited to the conserved IQ motifs in the IQGAP1 scaffold (Figure 6C) (Roy et al., 2005) and lack of IQGAP1 reduces EGF-mediated Raf phosphorylation of Mek1/2 (Ren et al., 2007; Ren et al., 2008).
Activation via CD44 by hyaluronan in human ovarian tumor cells results in the preferential association of Erk2 but not Erk1 with IQGAP1 (Bourguignon et al., 2005). Active Erk1/2 phosphorylates cytosolic and nuclear targets, resulting in cell growth and proliferation. For example, activation via CD44 increases phosphorylation of both Elk-1 and ER-α, resulting in transcriptional regulation. IQGAP1 can function as a signal integrator by modulating Cdc42 cytoskeletal function and mediating Elk-1-specific transcriptional activation. Thus, cross-talk can occur between a membrane receptor (CD44) and a nuclear hormone receptor (ER-α) signaling pathway during ovarian cancer progression. Recently, activation of NK cells via NKG2D receptor utilized IQGAP1 to initiate Rap1b-GTP→Vav1→Cdc42→Pak→B-Raf/C-Raf→Mek1/2→Erk1/2 signaling cascade (Awasthi et al., 2010). This study also demonstrated that IQGAP1 was capable of forming a master signalosome in the periphery of the nucleus to transiently sustain Erk1/2 phosphorylation (Awasthi et al., 2010) (Figure 6B). These studies provide a molecular mechanism by which IQGAP1 functions as a scaffold to facilitate the coupling of kinases in the Ras→Raf→Mek→Erk pathway (Schrick et al., 2007; Sbroggio et al., 2011; Heil et al., 2011; Roy et al., 2004). Over-expression of IQGAP1 resulted in non-functional binary complexes of IQGAP1, with only one of the components of the MAP kinase cascade, for example Mek1/2 or Erk1/2 and thereby preventing optimum signaling (McNulty et al., 2011). Also, similar to CD44, EGFR can directly recruit IQGAP1 (McNulty et al., 2011). Stimulation of epithelial cells with EGF promotes interaction of IQGAP1 with Mek1/2, leading to Erk1/2 activation (Roy et al., 2004). This suggests that IQGAP1 preferentially activates the Mek1 pathway. It has been suggested that Mek1 promotes proliferation, whereas Mek2 promotes differentiation (Briggs et al., 2002). Together, these studies provide strong evidence that a coordinated sequential Erk1/2 activation can be regulated and compartmentalized by IQGAP1.
The IQGAP1 scaffolin: An essential puppeteer for β-catenin-mediated gene transcription
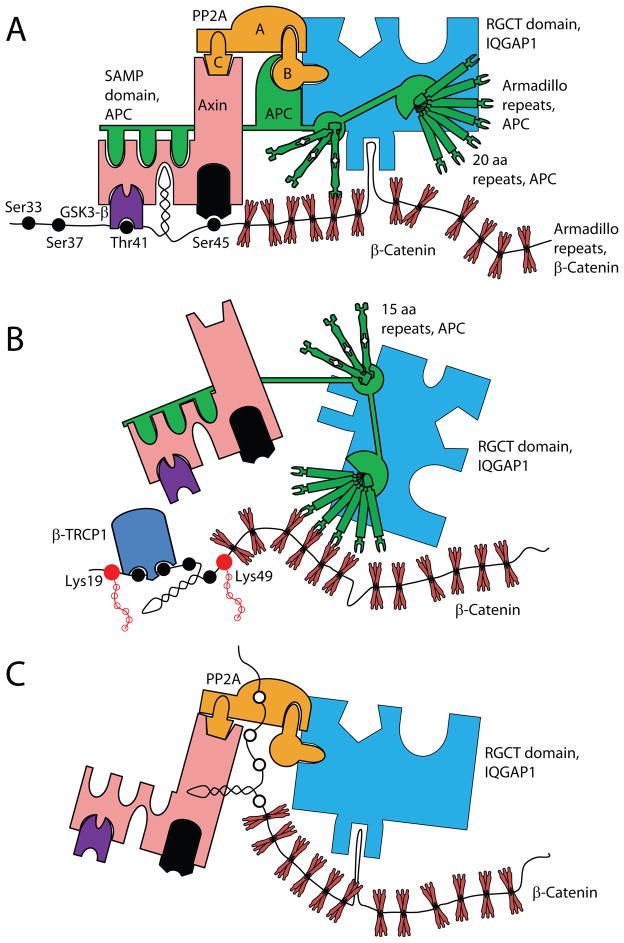
The Wnt-mediated β-catenin activation pathway controls significant aspects of multicellular heterotrophic eukaryote development (Angers and Moon, 2009). β-catenin forms complexes with TCF and LEF, which are two major transcription factors that regulate a multitude of developmental processes and effector functions in lymphocytes (Staal and Clevers, 2000). Defects in β-catenin signaling can lead to tumor transformation and severe developmental and immunological defects (Staal et al., 2008). Wnt signaling has been well characterized to determine cell and tissue fate decisions (Staal and Luis, 2010). In this context, it is imperative to note that IQGAP1 functions as a regulator of β-catenin-mediated gene transcriptions (Fukata et al., 1999; Wang et al., 2008; Briggs et al., 2002). Direct binding of APC (Watanabe et al., 2004; Aoki and Taketo, 2007) or protein phosphatase 2A (PP2A) (Suzuki et al., 2005) and an indirect interaction of GSK-3β (Watanabe et al., 2009) signifies the biological relevance of IQGAP1 in Wnt signaling (Figure 7A). Most importantly, a region between the IQ domains and the GRD of IQGAP1 was shown to bind the C-terminus of Dishevelled (DVL) (Goto et al., 2013b) and direct binding of β-catenin itself to the RGCT domain (Fukata et al., 1999) confirms the role of IQGAP1 in the activation and degradation of β-catenin. Signaling downstream of the Frizzled receptor by Wnt isoforms results in the activation of DVL which leads to the inhibition of GSK-3β that would otherwise phosphorylate and target β-catenin to the APC/Axin/SCF-β-TrCP/CK1 degradation complex (Su et al., 2008). Once the constitutive phosphorylation cycle of GSK-3β is transiently interrupted, β-catenin is ‘stabilized’ through dephosphorylation by PR55α, a subunit of the protein phosphatase, PP2A, and translocated into the nucleus where it binds to the TCF and LEF family of transcription factors (Zhang et al., 2009b). Genes encoding cell cycle regulators such as c-myc (He et al., 1998) and cyclin D1 (Tetsu and McCormick, 1999) are two major targets of β-catenin/TCF/LEF transcription complexes. Thus, the IQGAP1 scaffold can bring together both the ‘degradation’ and the ‘activation’ complexes of β-catenin.
Figure 7. Potential mechanism by which IQGAP1 regulates β-catenin-mediated gene transcription.
IQGAP1 organizes the N-terminal phosphorylation of β-catenin. A) The RGCT domain of IQGAP1 directly interacts with β-catenin. Under steady state, β-catenin constitutively associates with IQGAP1-RGCT domain-associated APC and Axin as part of the degradation complex. APC associates with Axin via its SAMP domain and facilitates the recruitment of CK1 and GSK3β. APC using its third 15 amino acid repeat grasps the 5th armadillo repeat of β-catenin to facilitate the phosphorylation of β-catenin by CK1 (Ser45) and GSK3β (Thr41, Ser37, Ser33). B) Phosphorylation of these residues leads to the ubiquitination of β-catenin by β-TrCP. At this time, one of the 20 amino acid repeat of APC help to stabilize the association of β-TrCP to β-catenin by associating with the armadillo repeats of β-catenin. C) After activation (such as Wnt3a), β-catenin is rescued from degradation through IQGAP1-RGCT domain-associated PP2A-mediated dephosphorylation and allowed to interact with the TCF/LEF family of transcription factors. β-catenin/TCF/LEF complex is translocated into the nucleus that promotes specific gene transcriptions.
These functional abilities of IQGAP1 form the basis for new paradigm testing. First, the spatiotemporal kinetics and the compartmental localization of these complexes inside the cells have yet to be understood. The direct interaction of β-catenin and APC and indirect recruitment of the Axin/GSK3β/CK1/SCF-β-TrCP complex to the IQGAP1 scaffold demonstrate that under steady state, Casein kinase 1 (CK1) phosphorylates GSK3β, which in turn constitutively phosphorylates β-catenin and directs it for degradation. SCF-β-TrCP is an F-box/WD40-repeat E3 ubiquitin ligase that ubiquitinates only the phosphorylated β-catenin. Under steady state, association of APC to this complex prevents the dephosphorylation of β-catenin that eventually results in its degradation by the proteasome (Su et al., 2008). When activated by Wnt (or other mechanisms), β-catenin is not protected by APC, which exposes the N-terminal phosphorylated serine/threonine residues of β-catenin to PP2A (Su et al., 2008). This dephosphorylation by PP2A eliminates the SCF-β-TrCP recognition site in β-catenin, thereby stopping its ubiquitination and degradation. Thus, while APC functions to destabilize, PP2A plays a critical role in activating β-catenin. APC and PP2A can interact with IQGAP1; however, the independent role of IQGAP1 in the activation and degradation of β-catenin or in the compartmentalization of these two processes is yet to be explored (Figure 7 B&C). Interestingly, TCF1/β-catenin activation is independent of Cdc42 or Rac1 interaction with IQGAP1 as a mutation in the GRD domain abrogates the ability of IQGAP1 to interact with Cdc42 and Rac1 without impairing the interactions with β-catenin (Wang et al., 2008).
Concluding remarks and future challenges
Mechanistic insights on how IQGAP1 functions as a molecular puppeteer in cytoskeletal rearrangement, MAPK activation, and β-catenin-mediated gene transcription are fundamental in understanding these essential signaling processes. More importantly, understanding the transient spatiotemporal organization of the signaling events by IQGAP1 will provide novel insights that will help to develop additional cellular paradigms. The nuclear localization of IQGAP1 and its involvement regulation cell cycle progression and nuclear import of proteins involved in Wnt signaling has recently been appreciated (Johnson et al., 2011; Johnson et al., 2013; Goto et al., 2013a; Goto et al., 2013b); however, further studies are needed to define the precise physiological role of IQGAP1 in these processes. The dephosphorlyation and nuclear translocation of nuclear factor of activated T cells (NFAT), a calcium-regulated transcription factor, is regulated by IQGAP1 in CD8+ T cells (Sharma et al., 2011). The exact mechanism by which IQGAP1 regulates NFAT is unknown; however, experiments using Iqgap1−/− CD8+ T cells suggests that the presence of IQGAP1 in a complex containing the noncoding RNA, noncoding repressor of NFAT (NRON), and NFAT associated kinases is required to control NFAT-associated cytokine production (Sharma et al., 2011). This is an intriguing example of a noncoding RNA-protein complex that modulates immune cell function. Further, the potential role for IQGAP1 in regulating metabolic programming of cells via mTOR-Akt-MAPK signaling (Tekletsadik et al., 2012; Wang et al., 2009; Osman et al., 2013) has yet to be extensively investigated. Another critical feature of IQGAP1 is its ability to oligomerize to form macromolecular structures (Ren et al., 2005). The self-association region is mapped to amino acids 763–863 of IQGAP1 that contain the four IQ domains. Since IQGAP1 can bind to B-Raf (Ren et al., 2007), Mek1/2 (Sacks, 2006) and Erk1/2 (Roy et al., 2004), a structured signalosome is indispensable for the generation of transient but optimal functions of kinases. Such an ordered regulation of kinases is an essential and integral part of the IQGAP1-mediated signalosome. These IQGAP1-based signalosomes are exciting molecular structures, and irrespective of recent studies focusing on its formation and possible functions, much of the biochemical basis and broader functional relevance of these signaling structures remain unknown. Therefore, the precise spatiotemporal organization and recruitment of distinct signaling molecules to the IQGAP1 scaffold must be examined in further detail. Research regarding the role of IQGAP1, specifically in immune cells, is limited and also warrants further investigation. Given the role of IQGAP proteins in tumorigenesis (White et al., 2009), novel tumor therapies may be created by pharmacologically targeting components of IQGAP1-mediated signaling complexes. Overall, studies aimed at elucidating the molecular mechanisms by which IQGAP1 orchestrates cellular physiology may lead to better understanding and, perhaps, paradigm-changing insights into the control of these basic biological processes.
Highlights.
Cellular responses to environmental stimuli may result in growth, proliferation, trafficking, and a wide range of other cell-specific functions. Cells use a variety of receptors and signaling cascades to achieve these functions. However, the organization of signaling components that translate distinct extracellular stimuli into unique physiological responses is poorly understood. Scaffolding proteins (scaffolins) play an essential role in coordinating signaling events in all eukaryotic cells. Often highly conserved in their specific functions, scaffolins curb, compartmentalize, and coordinate signaling events by serving as dynamic platforms on which a continuum of proteins interact to connect extracellular signals to the genome in a manner that is highly coordinated through space and time. Most importantly, the spatiotemporal organization of signaling events coordinated by scaffolins provides an additional layer of regulation that has been previously underappreciated. In this way, scaffolins act as molecular puppeteers that guide and fine-tune cellular responses.
IQ motif-containing GTPase activating protein (IQGAP) 1 was first characterized in 1994 and has since been the most extensively studied of the IQGAP proteins. In the past two decades, IQGAP1 has been featured in more than 120 peer-reviewed articles which highlight its involvement in a myriad of cellular functions, including its role in spatiotemporal signaling events 3, as well as tumorigenesis. Recent advances on the complex structure and functional diversity of the IQGAP1 scaffolin necessitate detailed investigation to better understand its role in cellular biology. Many of IQGAP1’s known functions are conserved from its homolog in yeast, Iqg1p, further exemplifying IQGAP1 as a critical regulator of basic cellular physiology. In fact, IQGAP1 is a well-known regulator of signaling events involved in cytoskeletal rearrangement, the mitogen activated protein kinase (MAPK) pathway, and β-catenin-mediated transcription. In this review, we summarize recent findings and provide novel mechanistic insights into these important functions of the IQGAP1 scaffolin.
Acknowledgments
We thank Lucia Sammarco and her Lulu’s Lemonade Stand for inspiration, motivation and support. This work was supported in part by NIH R01 AI064826, NIH R01 AI102893 and NCI R01 CA179363 (S.M.); NHLBI-HL087951 (S.R.); NIH-CA151893-K08 (M.R.); Alex Lemonade Stand Foundation (S.M.); HRHM Program of MACC Fund (S.M.; S.R.), Nicholas Family Foundation (S.M.); Gardetto Family Chair (S.M.); Hyundai Scholars Program (M.S.T.); Hyundai Hope on Wheels (S.R.); Pavlove Foundation (M.S.T.); Rebecca Jean Slye Endowment (M.S.T.); MCW-Cancer Center-Large Seed Grant (S.M. & M.S.T.; S.R.); MACC Fund (M.S.T. and S.M.); American Cancer Society Grants (S.M. & M.S.T; S.R.; M.R.); and by the Clinical & Translational Science Institute of Southeastern Wisconsin (NIH UL1RR031973) (M.S.T. and S.M.). Children’s Research Institute, MCW (S.R.); Kathy Dyffy Fogerty Award (M.R.); We thank Kate Dixon for the critical reading of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adachi H, Takahashi Y, Hasebe T, Shirouzu M, Yokoyama S, Sutoh K. Dictyostelium IQGAP-related protein specifically involved in the completion of cytokinesis. J Cell Biol. 1997;137:891–898. doi: 10.1083/jcb.137.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci. 2007;120:3327–3335. doi: 10.1242/jcs.03485. [DOI] [PubMed] [Google Scholar]
- Awasthi A, Samarakoon A, Chu H, Kamalakannan R, Quilliam LA, Chrzanowska-Wodnicka M, White GC, Malarkannan S. Rap1b facilitates NK cell functions via IQGAP1-mediated signalosomes. J Exp Med. 2010;207:1923–1938. doi: 10.1084/jem.20100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas ZK, Rasmussen W. NK1.1+ thymocytes. Adult murine CD4-, CD8-thymocytes contain an NK1.1+, CD3+, CD5hi, CD44hi, TCR-V beta 8+ subset. J Immunol. 1990;145:1039–1045. [PubMed] [Google Scholar]
- Banon-Rodriguez I, Galvez-Santisteban M, Vergarajauregui S, Bosch M, Borreguero-Pascual A, Martin-Belmonte F. EGFR controls IQGAP basolateral membrane localization and mitotic spindle orientation during epithelial morphogenesis. EMBO J. 2014;33:129–145. doi: 10.1002/embj.201385946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashour AM, Fullerton AT, Hart MJ, Bloom GS. IQGAP1, a Rac- and Cdc42-binding protein, directly binds and cross-links microfilaments. J Cell Biol. 1997;137:1555–1566. doi: 10.1083/jcb.137.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basto R, Brunk K, Vinadogrova T, Peel N, Franz A, Khodjakov A, Raff JW. Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008;133:1032–1042. doi: 10.1016/j.cell.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak-Zmijewska A, Kolano A, Szczepanska K, Maleszewski M, Borsuk E. Cdc42 protein acts upstream of IQGAP1 and regulates cytokinesis in mouse oocytes and embryos. Dev Biol. 2008;322:21–32. doi: 10.1016/j.ydbio.2008.06.039. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Gilad E, Rothman K, Peyrollier K. Hyaluronan-CD44 interaction with IQGAP1 promotes Cdc42 and ERK signaling, leading to actin binding, Elk-1/estrogen receptor transcriptional activation, and ovarian cancer progression. J Biol Chem. 2005;280:11961–11972. doi: 10.1074/jbc.M411985200. [DOI] [PubMed] [Google Scholar]
- Brandt DT, Grosse R. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 2007;8:1019–1023. doi: 10.1038/sj.embor.7401089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt DT, Marion S, Griffiths G, Watanabe T, Kaibuchi K, Grosse R. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J Cell Biol. 2007;178:193–200. doi: 10.1083/jcb.200612071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs MW, Li Z, Sacks DB. IQGAP1-mediated stimulation of transcriptional co-activation by beta-catenin is modulated by calmodulin. J Biol Chem. 2002;277:7453–7465. doi: 10.1074/jbc.M104315200. [DOI] [PubMed] [Google Scholar]
- Briggs MW, Sacks DB. IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep. 2003;4:571–574. doi: 10.1038/sj.embor.embor867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill S, Li S, Lyman CW, Church DM, Wasmuth JJ, Weissbach L, Bernards A, Snijders AJ. The Ras GTPase-activating-protein-related human protein IQGAP2 harbors a potential actin binding domain and interacts with calmodulin and Rho family GTPases. Mol Cell Biol. 1996;16:4869–4878. doi: 10.1128/mcb.16.9.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MD, Bry L, Li Z, Sacks DB. IQGAP1 regulates Salmonella invasion through interactions with actin, Rac1, and Cdc42. J Biol Chem. 2007;282:30265–30272. doi: 10.1074/jbc.M702537200. [DOI] [PubMed] [Google Scholar]
- Brown MD, Bry L, Li Z, Sacks DB. Actin pedestal formation by enteropathogenic Escherichia coli is regulated by IQGAP1, calcium, and calmodulin. J Biol Chem. 2008;283:35212–35222. doi: 10.1074/jbc.M803477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MD, Sacks DB. Protein scaffolds in MAP kinase signalling. Cell Signal. 2009;21:462–469. doi: 10.1016/j.cellsig.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Allan DS, Krzewski K, Ge B, Kopcow H, Strominger JL. CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc Natl Acad Sci U S A. 2006;103:10346–10351. doi: 10.1073/pnas.0604236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Thapa N, Hedman AC, Li Z, Sacks DB, Anderson RA. IQGAP1 is a novel phosphatidylinositol 4,5 bisphosphate effector in regulation of directional cell migration. EMBO J. 2013;32:2617–2630. doi: 10.1038/emboj.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Awasthi A, White GC, Chrzanowska-Wodnicka M, Malarkannan S. Rap1b regulates B cell development, homing, and T cell-dependent humoral immunity. J Immunol. 2008;181:3373–3383. doi: 10.4049/jimmunol.181.5.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claperon A, Therrien M. KSR and CNK: two scaffolds regulating RAS-mediated RAF activation. Oncogene. 2007;26:3143–3158. doi: 10.1038/sj.onc.1210408. [DOI] [PubMed] [Google Scholar]
- Cupit LD, Schmidt VA, Miller F, Bahou WF. Distinct PAR/IQGAP expression patterns during murine development: implications for thrombin-associated cytoskeletal reorganization. Mamm Genome. 2004;15:618–629. doi: 10.1007/s00335-004-2370-8. [DOI] [PubMed] [Google Scholar]
- Dujardin DL, Barnhart LE, Stehman SA, Gomes ER, Gundersen GG, Vallee RB. A role for cytoplasmic dynein and LIS1 in directed cell movement. J Cell Biol. 2003;163:1205–1211. doi: 10.1083/jcb.200310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JA, Chant J. An IQGAP-related protein controls actin-ring formation and cytokinesis in yeast. Curr Biol. 1997;7:921–929. doi: 10.1016/s0960-9822(06)00411-8. [DOI] [PubMed] [Google Scholar]
- Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Fu Y, Galan JE. A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 1999;401:293–297. doi: 10.1038/45829. [DOI] [PubMed] [Google Scholar]
- Fukata M, Kuroda S, Fujii K, Nakamura T, Shoji I, Matsuura Y, Okawa K, Iwamatsu A, Kikuchi A, Kaibuchi K. Regulation of cross-linking of actin filament by IQGAP1, a target for Cdc42. J Biol Chem. 1997;272:29579–29583. doi: 10.1074/jbc.272.47.29579. [DOI] [PubMed] [Google Scholar]
- Fukata M, Kuroda S, Nakagawa M, Kawajiri A, Itoh N, Shoji I, Matsuura Y, Yonehara S, Fujisawa H, Kikuchi A, Kaibuchi K. Cdc42 and Rac1 regulate the interaction of IQGAP1 with beta-catenin. J Biol Chem. 1999;274:26044–26050. doi: 10.1074/jbc.274.37.26044. [DOI] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Itoh N, Kawajiri A, Yamaga M, Kuroda S, Kaibuchi K. Involvement of IQGAP1, an effector of Rac1 and Cdc42 GTPases, in cell-cell dissociation during cell scattering. Mol Cell Biol. 2001;21:2165–2183. doi: 10.1128/MCB.21.6.2165-2183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–885. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Fusello AM, Mandik-Nayak L, Shih F, Lewis RE, Allen PM, Shaw AS. The MAPK scaffold kinase suppressor of Ras is involved in ERK activation by stress and proinflammatory cytokines and induction of arthritis. J Immunol. 2006;177:6152–6158. doi: 10.4049/jimmunol.177.9.6152. [DOI] [PubMed] [Google Scholar]
- Gorman JA, Babich A, Dick CJ, Schoon RA, Koenig A, Gomez TS, Burkhardt JK, Billadeau DD. The cytoskeletal adaptor protein IQGAP1 regulates TCR-mediated signaling and filamentous actin dynamics. J Immunol. 2012;188:6135–6144. doi: 10.4049/jimmunol.1103487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Sato A, Adachi S, Iemura S, Natsume T, Shibuya H. IQGAP1 protein regulates nuclear localization of beta-catenin via importin-beta5 protein in Wnt signaling. J Biol Chem. 2013a;288:36351–36360. doi: 10.1074/jbc.M113.520528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Sato A, Shimizu M, Adachi S, Satoh K, Iemura S, Natsume T, Shibuya H. IQGAP1 functions as a modulator of dishevelled nuclear localization in Wnt signaling. PLoS ONE. 2013b;8:e60865. doi: 10.1371/journal.pone.0060865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmanova K, Schlaepfer D, Hess D, Gutierrez P, Beck M, Kroschewski R. Phosphorylation of IQGAP1 modulates its binding to Cdc42, revealing a new type of rho-GTPase regulator. J Biol Chem. 2004;279:48495–48504. doi: 10.1074/jbc.M408113200. [DOI] [PubMed] [Google Scholar]
- Gundersen GG. Microtubule capture: IQGAP and CLIP-170 expand the repertoire. Curr Biol. 2002;12:R645–R647. doi: 10.1016/s0960-9822(02)01156-9. [DOI] [PubMed] [Google Scholar]
- Guo W, Sutcliffe MJ, Cerione RA, Oswald RE. Identification of the binding surface on Cdc42Hs for p21-activated kinase. Biochemistry. 1998;37:14030–14037. doi: 10.1021/bi981352+. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Callow MG, Souza B, Polakis P. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 1996;15:2997–3005. [PMC free article] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Heil A, Nazmi AR, Koltzscher M, Poeter M, Austermann J, Assard N, Baudier J, Kaibuchi K, Gerke V. S100P is a novel interaction partner and regulator of IQGAP1. J Biol Chem. 2011;286:7227–7238. doi: 10.1074/jbc.M110.135095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsath L, Dvorsky R, Fiegen D, Carlier MF, Ahmadian MR. An electrostatic steering mechanism of Cdc42 recognition by Wiskott-Aldrich syndrome proteins. Mol Cell. 2005;20:313–324. doi: 10.1016/j.molcel.2005.08.036. [DOI] [PubMed] [Google Scholar]
- Heyzer-Williams MG, Davis MM. Antigen-specific development of primary and memory T cells in vivo. Science. 1995;268:106–111. doi: 10.1126/science.7535476. [DOI] [PubMed] [Google Scholar]
- Ho YD, Joyal JL, Li Z, Sacks DB. IQGAP1 integrates Ca2+/calmodulin and Cdc42 signaling. J Biol Chem. 1999;274:464–470. doi: 10.1074/jbc.274.1.464. [DOI] [PubMed] [Google Scholar]
- Huang J, Tilly D, Altman A, Sugie K, Grey HM. T-cell receptor antagonists induce Vav phosphorylation by selective activation of Fyn kinase. Proc Natl Acad Sci U S A. 2000;97:10923–10929. doi: 10.1073/pnas.97.20.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries LA, Shaffer MH, Sacirbegovic F, Tomassian T, McMahon KA, Humbert PO, Silva O, Round JL, Takamiya K, Huganir RL, Burkhardt JK, Russell SM, Miceli MC. Characterization of in vivo Dlg1 deletion on T cell development and function. PLoS ONE. 2012;7:e45276. doi: 10.1371/journal.pone.0045276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacke CM, Yarwood H. The hyaluronan receptor, CD44. Int J Biochem Cell Biol. 2002;34:718–721. doi: 10.1016/s1357-2725(01)00166-2. [DOI] [PubMed] [Google Scholar]
- Jameson KL, Mazur PK, Zehnder AM, Zhang J, Zarnegar B, Sage J, Khavari PA. IQGAP1 scaffold-kinase interaction blockade selectively targets RAS-MAP kinase-driven tumors. Nat Med. 2013;19:626–630. doi: 10.1038/nm.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HW, Li Z, Brown MD, Sacks DB. IQGAP1 binds Rap1 and modulates its activity. J Biol Chem. 2007;282:20752–20762. doi: 10.1074/jbc.M700487200. [DOI] [PubMed] [Google Scholar]
- Johnson M, Sharma M, Brocardo MG, Henderson BR. IQGAP1 translocates to the nucleus in early S-phase and contributes to cell cycle progression after DNA replication arrest. Int J Biochem Cell Biol. 2011;43:65–73. doi: 10.1016/j.biocel.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Johnson M, Sharma M, Henderson BR. IQGAP1 regulation and roles in cancer. Cell Signal. 2009;21:1471–1478. doi: 10.1016/j.cellsig.2009.02.023. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Henderson BR. The scaffolding protein IQGAP1 co-localizes with actin at the cytoplasmic face of the nuclear envelope: implications for cytoskeletal regulation. Bioarchitecture. 2012;2 doi: 10.4161/bioa.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Sharma M, Mok MT, Henderson BR. Stimulation of in vivo nuclear transport dynamics of actin and its co-factors IQGAP1 and Rac1 in response to DNA replication stress. Biochim Biophys Acta. 2013;1833:2334–2347. doi: 10.1016/j.bbamcr.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Johnson P, Ruffell B. CD44 and its role in inflammation and inflammatory diseases. Inflamm Allergy Drug Targets. 2009;8:208–220. doi: 10.2174/187152809788680994. [DOI] [PubMed] [Google Scholar]
- Joyal JL, Annan RS, Ho YD, Huddleston ME, Carr SA, Hart MJ, Sacks DB. Calmodulin modulates the interaction between IQGAP1 and Cdc42. Identification of IQGAP1 by nanoelectrospray tandem mass spectrometry. J Biol Chem. 1997;272:15419–15425. doi: 10.1074/jbc.272.24.15419. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr Opin Cell Biol. 1999;11:591–596. doi: 10.1016/s0955-0674(99)00014-9. [DOI] [PubMed] [Google Scholar]
- Kanwar N, Wilkins JA. IQGAP1 involvement in MTOC and granule polarization in NK-cell cytotoxicity. Eur J Immunol. 2011;41:2763–2773. doi: 10.1002/eji.201040444. [DOI] [PubMed] [Google Scholar]
- Kholmanskikh SS, Koeller HB, Wynshaw-Boris A, Gomez T, Letourneau PC, Ross ME. Calcium-dependent interaction of Lis1 with IQGAP1 and Cdc42 promotes neuronal motility. Nat Neurosci. 2006;9:50–57. doi: 10.1038/nn1619. [DOI] [PubMed] [Google Scholar]
- King AJ, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall MS. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature. 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- Kurella VB, Richard JM, Parke CL, Lecour LF, Jr, Bellamy HD, Worthylake DK. Crystal structure of the GTPase-activating protein-related domain from IQGAP1. J Biol Chem. 2009;284:14857–14865. doi: 10.1074/jbc.M808974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, Shoji I, Matsuura Y, Yonehara S, Kaibuchi K. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Kaibuchi K. Cdc42, Rac1, and their effector IQGAP1 as molecular switches for cadherin-mediated cell-cell adhesion. Biochem Biophys Res Commun. 1999;262:1–6. doi: 10.1006/bbrc.1999.1122. [DOI] [PubMed] [Google Scholar]
- Le CC, Schlaepfer D, Ferrari A, Klingauf M, Grohmanova K, Veligodskiy A, Didry D, Le D, Egile C, Carlier MF, Kroschewski R. IQGAP1 stimulates actin assembly through the N-WASP-Arp2/3 pathway. J Biol Chem. 2007;282:426–435. doi: 10.1074/jbc.M607711200. [DOI] [PubMed] [Google Scholar]
- Li R, Debreceni B, Jia B, Gao Y, Tigyi G, Zheng Y. Localization of the PAK1-, WASP-, and IQGAP1-specifying regions of Cdc42. J Biol Chem. 1999;274:29648–29654. doi: 10.1074/jbc.274.42.29648. [DOI] [PubMed] [Google Scholar]
- Li S, Wang Q, Chakladar A, Bronson RT, Bernards A. Gastric hyperplasia in mice lacking the putative Cdc42 effector IQGAP1. Mol Cell Biol. 2000;20:697–701. doi: 10.1128/mcb.20.2.697-701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, McNulty DE, Marler KJ, Lim L, Hall C, Annan RS, Sacks DB. IQGAP1 promotes neurite outgrowth in a phosphorylation-dependent manner. J Biol Chem. 2005;280:13871–13878. doi: 10.1074/jbc.M413482200. [DOI] [PubMed] [Google Scholar]
- Liu J, Guidry JJ, Worthylake DK. Conserved sequence repeats of IQGAP1 mediate binding to Ezrin. J Proteome Res. 2014;13:1156–1166. doi: 10.1021/pr400787p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue JS, Whiting JL, Tunquist B, Sacks DB, Langeberg LK, Wordeman L, Scott JD. AKAP220 organizes signaling elements that impact cell migration. J Biol Chem. 2011 doi: 10.1074/jbc.M111.277756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias MJ, Wiesner S, Sudol M. WW and SH3 domains, two different scaffolds to recognize proline-rich ligands. FEBS Lett. 2002;513:30–37. doi: 10.1016/s0014-5793(01)03290-2. [DOI] [PubMed] [Google Scholar]
- Malarkannan S, Awasthi A, Rajasekaran K, Kumar P, Schuldt KM, Bartoszek A, Manoharan N, Goldner NK, Umhoefer CM, Thakar MS. IQGAP1: a regulator of intracellular spacetime relativity. J Immunol. 2012;188:2057–2063. doi: 10.4049/jimmunol.1102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataraza JM, Briggs MW, Li Z, Frank R, Sacks DB. Identification and characterization of the Cdc42-binding site of IQGAP1. Biochem Biophys Res Commun. 2003;305:315–321. doi: 10.1016/s0006-291x(03)00759-9. [DOI] [PubMed] [Google Scholar]
- Mateer SC, McDaniel AE, Nicolas V, Habermacher GM, Lin MJ, Cromer DA, King ME, Bloom GS. The mechanism for regulation of the F-actin binding activity of IQGAP1 by calcium/calmodulin. J Biol Chem. 2002;277:12324–12333. doi: 10.1074/jbc.M109535200. [DOI] [PubMed] [Google Scholar]
- Mateer SC, Morris LE, Cromer DA, Bensenor LB, Bloom GS. Actin filament binding by a monomeric IQGAP1 fragment with a single calponin homology domain. Cell Motil Cytoskeleton. 2004;58:231–241. doi: 10.1002/cm.20013. [DOI] [PubMed] [Google Scholar]
- Mbele GO, Deloulme JC, Gentil BJ, Delphin C, Ferro M, Garin J, Takahashi M, Baudier J. The zinc- and calcium-binding S100B interacts and co-localizes with IQGAP1 during dynamic rearrangement of cell membranes. J Biol Chem. 2002;277:49998–50007. doi: 10.1074/jbc.M205363200. [DOI] [PubMed] [Google Scholar]
- McCallum SJ, Wu WJ, Cerione RA. Identification of a putative effector for Cdc42Hs with high sequence similarity to the RasGAP-related protein IQGAP1 and a Cdc42Hs binding partner with similarity to IQGAP2. J Biol Chem. 1996;271:21732–21737. doi: 10.1074/jbc.271.36.21732. [DOI] [PubMed] [Google Scholar]
- McNulty DE, Li Z, White CD, Sacks DB, Annan RS. MAPK scaffold IQGAP1 binds the EGF receptor and modulates its activation. J Biol Chem. 2011;286:15010–15021. doi: 10.1074/jbc.M111.227694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T. The biochemistry, ultrastructure, and subunit assembly mechanism of AMPA receptors. Mol Neurobiol. 2010;42:161–184. doi: 10.1007/s12035-010-8149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhaei-Nejad M, Zhang QX, Murray AG. Endothelial IQGAP1 Regulates Efficient Lymphocyte Transendothelial Migration. Eur J Immunol. 2009 doi: 10.1002/eji.200839214. [DOI] [PubMed] [Google Scholar]
- Nakhaei-Nejad M, Zhang QX, Murray AG. Endothelial IQGAP1 regulates efficient lymphocyte transendothelial migration. Eur J Immunol. 2010;40:204–213. doi: 10.1002/eji.200839214. [DOI] [PubMed] [Google Scholar]
- Nauert JB, Rigas JD, Lester LB. Identification of an IQGAP1/AKAP79 complex in beta-cells. J Cell Biochem. 2003;90:97–108. doi: 10.1002/jcb.10604. [DOI] [PubMed] [Google Scholar]
- Neel NF, Sai J, Ham AJ, Sobolik-Delmaire T, Mernaugh RL, Richmond A. IQGAP1 Is a Novel CXCR2-Interacting Protein and Essential Component of the “Chemosynapse”. PLoS ONE. 2011;6:e23813. doi: 10.1371/journal.pone.0023813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudauer CL, Joberty G, Tatsis N, Macara IG. Distinct cellular effects and interactions of the Rho-family GTPase TC10. Curr Biol. 1998;8:1151–1160. doi: 10.1016/s0960-9822(07)00486-1. [DOI] [PubMed] [Google Scholar]
- Nguyen A, Burack WR, Stock JL, Kortum R, Chaika OV, Afkarian M, Muller WJ, Murphy KM, Morrison DK, Lewis RE, McNeish J, Shaw AS. Kinase suppressor of Ras (KSR) is a scaffold which facilitates mitogen-activated protein kinase activation in vivo. Mol Cell Biol. 2002a;22:3035–3045. doi: 10.1128/MCB.22.9.3035-3045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AN, Ikner AD, Shiozaki M, Warren SM, Shiozaki K. Cytoplasmic localization of Wis1 MAPKK by nuclear export signal is important for nuclear targeting of Spc1/Sty1 MAPK in fission yeast. Mol Biol Cell. 2002b;13:2651–2663. doi: 10.1091/mbc.02-03-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H, Adachi M, Matsui T, Okawa K, Tsukita S, Tsukita S. IQGAP3 regulates cell proliferation through the Ras/ERK signalling cascade. Nat Cell Biol. 2008;10:971–978. doi: 10.1038/ncb1757. [DOI] [PubMed] [Google Scholar]
- Nuriya M, Oh S, Huganir RL. Phosphorylation-dependent interactions of alpha-Actinin-1/IQGAP1 with the AMPA receptor subunit GluR4. J Neurochem. 2005;95:544–552. doi: 10.1111/j.1471-4159.2005.03410.x. [DOI] [PubMed] [Google Scholar]
- Osman MA, Cerione RA. Iqg1p, a yeast homologue of the mammalian IQGAPs, mediates cdc42p effects on the actin cytoskeleton. J Cell Biol. 1998;142:443–455. doi: 10.1083/jcb.142.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman MA, Konopka JB, Cerione RA. Iqg1p links spatial and secretion landmarks to polarity and cytokinesis. J Cell Biol. 2002;159:601–611. doi: 10.1083/jcb.200205084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman MA, Sarkar FH, Rodriguez-Boulan E. A molecular rheostat at the interface of cancer and diabetes. Biochim Biophys Acta. 2013;1836:166–176. doi: 10.1016/j.bbcan.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D, Campbell LJ, Littlefield K, Evetts KA, Li Z, Sacks DB, Lowe PN, Mott HR. The IQGAP1-Rac1 and IQGAP1-Cdc42 interactions: interfaces differ between the complexes. J Biol Chem. 2008;283:1692–1704. doi: 10.1074/jbc.M707257200. [DOI] [PubMed] [Google Scholar]
- Palazzo AF, Joseph HL, Chen YJ, Dujardin DL, Alberts AS, Pfister KK, Vallee RB, Gundersen GG. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr Biol. 2001;11:1536–1541. doi: 10.1016/s0960-9822(01)00475-4. [DOI] [PubMed] [Google Scholar]
- Pathmanathan S, Elliott SF, McSwiggen S, Greer B, Harriott P, Irvine GB, Timson DJ. IQ motif selectivity in human IQGAP1: binding of myosin essential light chain and S100B. Mol Cell Biochem. 2008;318:43–51. doi: 10.1007/s11010-008-9855-9. [DOI] [PubMed] [Google Scholar]
- Ren JG, Li Z, Crimmins DL, Sacks DB. Self-association of IQGAP1: characterization and functional sequelae. J Biol Chem. 2005;280:34548–34557. doi: 10.1074/jbc.M507321200. [DOI] [PubMed] [Google Scholar]
- Ren JG, Li Z, Sacks DB. IQGAP1 modulates activation of B-Raf. Proc Natl Acad Sci U S A. 2007;104:10465–10469. doi: 10.1073/pnas.0611308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren JG, Li Z, Sacks DB. IQGAP1 integrates Ca2+/calmodulin and B-Raf signaling. J Biol Chem. 2008;283:22972–22982. doi: 10.1074/jbc.M804626200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Tomassian T, Zhang M, Patel V, Schoenberger SP, Miceli MC. Dlgh1 coordinates actin polymerization, synaptic T cell receptor and lipid raft aggregation, and effector function in T cells. J Exp Med. 2005;201:419–430. doi: 10.1084/jem.20041428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Li Z, Sacks DB. IQGAP1 binds ERK2 and modulates its activity. J Biol Chem. 2004;279:17329–17337. doi: 10.1074/jbc.M308405200. [DOI] [PubMed] [Google Scholar]
- Roy M, Li Z, Sacks DB. IQGAP1 is a scaffold for mitogen-activated protein kinase signaling. Mol Cell Biol. 2005;25:7940–7952. doi: 10.1128/MCB.25.18.7940-7952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks DB. The role of scaffold proteins in MEK/ERK signalling. Biochem Soc Trans. 2006;34:833–836. doi: 10.1042/BST0340833. [DOI] [PubMed] [Google Scholar]
- Sackstein R. The biology of CD44 and HCELL in hematopoiesis: the ‘step 2-bypass pathway’ and other emerging perspectives. Curr Opin Hematol. 2011;18:239–248. doi: 10.1097/MOH.0b013e3283476140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbroggio M, Carnevale D, Bertero A, Cifelli G, De BE, Mascio G, Hirsch E, Bahou WF, Turco E, Silengo L, Brancaccio M, Lembo G, Tarone G. IQGAP1 regulates ERK1/2 and AKT signalling in the heart and sustains functional remodelling upon pressure overload. Cardiovasc Res. 2011;91:456–464. doi: 10.1093/cvr/cvr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt VA, Scudder L, Devoe CE, Bernards A, Cupit LD, Bahou WF. IQGAP2 functions as a GTP-dependent effector protein in thrombin-induced platelet cytoskeletal reorganization. Blood. 2003;101:3021–3028. doi: 10.1182/blood-2002-09-2807. [DOI] [PubMed] [Google Scholar]
- Schrick C, Fischer A, Srivastava DP, Tronson NC, Penzes P, Radulovic J. N-cadherin regulates cytoskeletally associated IQGAP1/ERK signaling and memory formation. Neuron. 2007;55:786–798. doi: 10.1016/j.neuron.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon KB, Li R. The multiple roles of Cyk1p in the assembly and function of the actomyosin ring in budding yeast. Mol Biol Cell. 1999;10:283–296. doi: 10.1091/mbc.10.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Findlay GM, Bandukwala HS, Oberdoerffer S, Baust B, Li Z, Schmidt V, Hogan PG, Sacks DB, Rao A. Dephosphorylation of the nuclear factor of activated T cells (NFAT) transcription factor is regulated by an RNA-protein scaffold complex. Proc Natl Acad Sci U S A. 2011;108:11381–11386. doi: 10.1073/pnas.1019711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AS, Filbert EL. Scaffold proteins and immune-cell signalling. Nat Rev Immunol. 2009;9:47–56. doi: 10.1038/nri2473. [DOI] [PubMed] [Google Scholar]
- Skandalis SS, Kozlova I, Engstrom U, Hellman U, Heldin P. Proteomic identification of CD44 interacting proteins. IUBMB Life. 2010;62:833–840. doi: 10.1002/iub.392. [DOI] [PubMed] [Google Scholar]
- Smith JM, Hedman AC, Sacks DB. IQGAPs choreograph cellular signaling from the membrane to the nucleus. Trends Cell Biol. 2015 doi: 10.1016/j.tcb.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal FJ, Clevers H. Tcf/Lef transcription factors during T-cell development: unique and overlapping functions. Hematol J. 2000;1:3–6. doi: 10.1038/sj.thj.6200001. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Luis TC. Wnt signaling in hematopoiesis: crucial factors for self-renewal, proliferation, and cell fate decisions. J Cell Biochem. 2010;109:844–849. doi: 10.1002/jcb.22467. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- Stephenson LM, Sammut B, Graham DB, Chan-Wang J, Brim KL, Huett AS, Miletic AV, Kloeppel T, Landry A, Xavier R, Swat W. DLGH1 is a negative regulator of T-lymphocyte proliferation. Mol Cell Biol. 2007;27:7574–7581. doi: 10.1128/MCB.00439-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Fu C, Ishikawa S, Stella A, Kojima M, Shitoh K, Schreiber EM, Day BW, Liu B. APC is essential for targeting phosphorylated beta-catenin to the SCFbeta-TrCP ubiquitin ligase. Mol Cell. 2008;32:652–661. doi: 10.1016/j.molcel.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Subramani J, Ghosh M, Rahman MM, Caromile LA, Gerber C, Rezaul K, Han DK, Shapiro LH. Tyrosine phosphorylation of CD13 regulates inflammatory cell-cell adhesion and monocyte trafficking. J Immunol. 2013;191:3905–3912. doi: 10.4049/jimmunol.1301348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Chikamatsu Y, Takahashi K. Requirement of protein phosphatase 2A for recruitment of IQGAP1 to Rac-bound beta1 integrin. J Cell Physiol. 2005;203:487–492. doi: 10.1002/jcp.20249. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Takahashi K. Regulation of lamellipodia formation and cell invasion by CLIP-170 in invasive human breast cancer cells. Biochem Biophys Res Commun. 2008;368:199–204. doi: 10.1016/j.bbrc.2008.01.069. [DOI] [PubMed] [Google Scholar]
- Swiech L, Blazejczyk M, Urbanska M, Pietruszka P, Dortland BR, Malik AR, Wulf PS, Hoogenraad CC, Jaworski J. CLIP-170 and IQGAP1 cooperatively regulate dendrite morphology. J Neurosci. 2011;31:4555–4568. doi: 10.1523/JNEUROSCI.6582-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai CY, Dujardin DL, Faulkner NE, Vallee RB. Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J Cell Biol. 2002;156:959–968. doi: 10.1083/jcb.200109046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Suzuki K. Regulation of protein phosphatase 2A-mediated recruitment of IQGAP1 to beta1 integrin by EGF through activation of Ca2+/calmodulin-dependent protein kinase II. J Cell Physiol. 2006;208:213–219. doi: 10.1002/jcp.20657. [DOI] [PubMed] [Google Scholar]
- Takeda S, Fujimoto A, Yamauchi E, Hiyoshi M, Kido H, Watanabe T, Kaibuchi K, Ohta T, Konishi H. Role of a tyrosine phosphorylation of SMG-9 in binding of SMG-9 to IQGAP and the NMD complex. Biochem Biophys Res Commun. 2011;410:29–33. doi: 10.1016/j.bbrc.2011.05.099. [DOI] [PubMed] [Google Scholar]
- Tekletsadik YK, Sonn R, Osman MA. A conserved role of IQGAP1 in regulating TOR complex 1. J Cell Sci. 2012;125:2041–2052. doi: 10.1242/jcs.098947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Tirnauer JS. A new cytoskeletal connection for APC: linked to actin through IQGAP. Dev Cell. 2004;7:778–780. doi: 10.1016/j.devcel.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Tran NH, Frost JA. Phosphorylation of Raf-1 by p21-activated kinase 1 and Src regulates Raf-1 autoinhibition. J Biol Chem. 2003;278:11221–11226. doi: 10.1074/jbc.M210318200. [DOI] [PubMed] [Google Scholar]
- Tran NH, Wu X, Frost JA. B-Raf and Raf-1 are regulated by distinct autoregulatory mechanisms. J Biol Chem. 2005;280:16244–16253. doi: 10.1074/jbc.M501185200. [DOI] [PubMed] [Google Scholar]
- Umemoto R, Nishida N, Ogino S, Shimada I. NMR structure of the calponin homology domain of human IQGAP1 and its implications for the actin recognition mode. J Biomol NMR. 2010;48:59–64. doi: 10.1007/s10858-010-9434-8. [DOI] [PubMed] [Google Scholar]
- Vallee RB, Tai C, Faulkner NE. LIS1: cellular function of a disease-causing gene. Trends Cell Biol. 2001;11:155–160. doi: 10.1016/s0962-8924(01)01956-0. [DOI] [PubMed] [Google Scholar]
- Wang JB, Sonn R, Tekletsadik YK, Samorodnitsky D, Osman MA. IQGAP1 regulates cell proliferation through a novel CDC42-mTOR pathway. J Cell Sci. 2009;122:2024–2033. doi: 10.1242/jcs.044644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Watanabe T, Noritake J, Fukata M, Yoshimura T, Itoh N, Harada T, Nakagawa M, Matsuura Y, Arimura N, Kaibuchi K. IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite outgrowth. J Cell Sci. 2007;120:567–577. doi: 10.1242/jcs.03356. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang A, Wang F, Wang M, Zhu M, Ma Y, Wu R. IQGAP1 activates Tcf signal independent of Rac1 and Cdc42 in injury and repair of bronchial epithelial cells. Exp Mol Pathol. 2008;85:122–128. doi: 10.1016/j.yexmp.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Noritake J, Kaibuchi K. Roles of IQGAP1 in cell polarization and migration. Novartis Found Symp. 2005;269:92–101. [PubMed] [Google Scholar]
- Watanabe T, Noritake J, Kakeno M, Matsui T, Harada T, Wang S, Itoh N, Sato K, Matsuzawa K, Iwamatsu A, Galjart N, Kaibuchi K. Phosphorylation of CLASP2 by GSK-3beta regulates its interaction with IQGAP1, EB1 and microtubules. J Cell Sci. 2009;122:2969–2979. doi: 10.1242/jcs.046649. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7:871–883. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Weissbach L, Bernards A, Herion DW. Binding of myosin essential light chain to the cytoskeleton-associated protein IQGAP1. Biochem Biophys Res Commun. 1998;251:269–276. doi: 10.1006/bbrc.1998.9371. [DOI] [PubMed] [Google Scholar]
- Weissbach L, Settleman J, Kalady MF, Snijders AJ, Murthy AE, Yan YX, Bernards A. Identification of a human rasGAP-related protein containing calmodulin-binding motifs. J Biol Chem. 1994a;269:20517–20521. [PubMed] [Google Scholar]
- Weissbach L, Settleman J, Kalady MF, Snijders AJ, Murthy AE, Yan YX, Bernards A. Identification of a human rasGAP-related protein containing calmodulin-binding motifs. J Biol Chem. 1994b;269:20517–20521. [PubMed] [Google Scholar]
- White CD, Brown MD, Sacks DB. IQGAPs in cancer: a family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009;583:1817–1824. doi: 10.1016/j.febslet.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CD, Erdemir HH, Sacks DB. IQGAP1 and its binding proteins control diverse biological functions. Cell Signal. 2012;24:826–834. doi: 10.1016/j.cellsig.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CD, Li Z, Dillon DA, Sacks DB. IQGAP1 protein binds human epidermal growth factor receptor 2 (HER2) and modulates trastuzumab resistance. J Biol Chem. 2011;286:29734–29747. doi: 10.1074/jbc.M111.220939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom SA, Lange A, Hess MW, Polleux J, Spatz JP, Kruger M, Pfaller K, Lambacher A, Bloch W, Mann M, Huber LA, Fassler R. Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev Cell. 2010;19:574–588. doi: 10.1016/j.devcel.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier R, Rabizadeh S, Ishiguro K, Andre N, Ortiz JB, Wachtel H, Morris DG, Lopez-Ilasaca M, Shaw AC, Swat W, Seed B. Discs large (Dlg1) complexes in lymphocyte activation. J Cell Biol. 2004;166:173–178. doi: 10.1083/jcb.200309044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka-Tojo M, Ushio-Fukai M, Hilenski L, Dikalov SI, Chen YE, Tojo T, Fukai T, Fujimoto M, Patrushev NA, Wang N, Kontos CD, Bloom GS, Alexander RW. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species--dependent endothelial migration and proliferation. Circ Res. 2004;95:276–283. doi: 10.1161/01.RES.0000136522.58649.60. [DOI] [PubMed] [Google Scholar]
- Yan J, Yang Y, Zhang H, King C, Kan HM, Cai Y, Yuan CX, Bloom GS, Hua X. Menin interacts with IQGAP1 to enhance intercellular adhesion of beta-cells. Oncogene. 2009;28:973–982. doi: 10.1038/onc.2008.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang M, Hayne C, Luo Z. Interaction between active Pak1 and Raf-1 is necessary for phosphorylation and activation of Raf-1. J Biol Chem. 2002;277:4395–4405. doi: 10.1074/jbc.M110000200. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wang ZX, Zheng Y. Characterization of the interactions between the small GTPase Cdc42 and its GTPase-activating proteins and putative effectors. Comparison of kinetic properties of Cdc42 binding to the Cdc42-interactive domains. J Biol Chem. 1997;272:21999–22007. doi: 10.1074/jbc.272.35.21999. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zaal KJ, Sheridan J, Mehta A, Gundersen GG, Ralston E. Microtubule plus-end binding protein EB1 is necessary for muscle cell differentiation, elongation and fusion. J Cell Sci. 2009a;122:1401–1409. doi: 10.1242/jcs.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yang J, Liu Y, Chen X, Yu T, Jia J, Liu C. PR55 alpha, a regulatory subunit of PP2A, specifically regulates PP2A-mediated beta-catenin dephosphorylation. J Biol Chem. 2009b;284:22649–22656. doi: 10.1074/jbc.M109.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Kaibuchi K, Quilliam LA. The insert region of RhoA is essential for Rho kinase activation and cellular transformation. Mol Cell Biol. 2001;21:5287–5298. doi: 10.1128/MCB.21.16.5287-5298.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]