Abstract
Kilohertz electrical stimulation (KES) has been shown to induce repeatable and reversible nerve conduction block in animal models. In this study, we characterized the ability of KES stimuli to selectively block specific components of stimulated nerve activity using in vivo preparations of the rat sciatic and vagus nerves. KES stimuli in the frequency range of 5–70 kHz and amplitudes of 0.1–3.0 mA were applied. Compound action potentials were evoked using either electrical or sensory stimulation, and block of components was assessed through direct nerve recordings and muscle force measurements. Distinct observable components of the compound action potential had unique conduction block thresholds as a function of frequency of KES. The fast component, which includes motor activity, had a monotonically increasing block threshold as a function of the KES frequency. The slow component, which includes sensory activity, showed a nonmonotonic block threshold relationship with increasing KES frequency. The distinct trends with frequency of the two components enabled selective block of one component with an appropriate choice of frequency and amplitude. These trends in threshold of the two components were similar when studying electrical stimulation and responses of the sciatic nerve, electrical stimulation and responses of the vagus nerve, and sensorimotor stimulation and responses of the sciatic nerve. This differential blocking effect of KES on specific fibers can extend the applications of KES conduction block to selective block and stimulation of neural signals for neuromodulation as well as selective control of neural circuits underlying sensorimotor function.
Keywords: compound action potential, selective stimulation, nerve conduction block, sciatic nerve, vagus nerve, neuromodulation
electrical stimulation for the modulation of peripheral nerve activity is utilized for the treatment of neuropathological diseases. In some cases, it is desirable to selectively inhibit specific fibers within a peripheral nerve. Various thermal (McMullan et al. 2004; Burgess and Perl 1967), mechanical (Bentley and Schlapp 1943), and pharmacological (Martinov and Nja 2005; Güven et al. 2005; Suzuki et al. 2009) methods have been used for selective blocking but are either slow acting, or not quickly reversible, rendering them unsuitable for chronic applications. Electrical stimulation with kilohertz frequency alternating current has been shown to be effective at blocking action potential conduction in peripheral nerves (Kilgore and Bhadra 2004; Bhadra and Kilgore 2005; Bhadra et al. 2006; Tai et al. 2005; Williamson and Andrews 2005; Zhang and Tai 2006; Jensen and Durand 2009). We refer to this as kilohertz electrical stimulation (KES) throughout this article. The method is quick, reversible, and currently employed in a variety of clinical applications, including appetite control (Camilleri et al. 2008), bladder control (Boger et al. 2012), and postamputation pain relief (Narasimhan 2011).
KES conduction block has been demonstrated in a variety of animal models and nerve diameters, including sea slugs (Joseph and Butera 2011), frogs (Kilgore and Bhadra 2004; Joseph and Butera 2009), rats (Williamson and Andrews 2005; Bhadra and Kilgore 2005), cats (Bhadra et al. 2006), dogs (Lin et al. 2007), goats (Cuellar et al. 2013), pigs (Camilleri et al. 2008), and nonhuman primates (Ackermann et al. 2011b). All present implementations of KES inhibit all propagating electrical activity in the target peripheral nerve. Our in vitro study of the sciatic nerve of the leopard frog Rana pipiens demonstrated that KES stimuli can be selective towards fast and slow components of the compound action potential (CAP) (Joseph and Butera 2011), which we putatively associate with myelinated and unmyelinated nerve activity. Such selective block could enable new research protocols as well as new clinical applications.
The feasibility and evaluation of KES stimuli to selectively block fiber propagation have not been investigated in mammalian peripheral nerves, nor have they been investigated in vivo, nor functionally using sensory stimulation and motor output when appropriate. The objective of this study was to evaluate the feasibility of KES stimuli to selectively block conduction-specific components of the CAP in mammalian peripheral nerves in vivo and characterize this specificity as a function of KES frequency. We characterized the ability of KES stimuli to selectively inhibit components of the CAP in the rat sciatic and vagus nerves. We utilized both electrical and sensory stimuli and measured CAP activity as well as motor output.
MATERIALS AND METHODS
Experimental preparation.
In vivo acute experiments were performed on the left and right sciatic nerves of four Lewis rats as well as the vagus nerves of four additional Lewis rats. Rats were anesthetized with 5% isoflurane and fixed in the prone position before surgery. Anesthesia was maintained at 2–3% for the first 45 min and 1–1.5% for the remainder of the experiment using a nose cone. Ophthalmic ointment was applied to both eyes to prevent drying. The animal's toe pinch reflex was used to maintain surgical anesthetic depth throughout the experiment. Body temperature and circulation were maintained via a heating pad at 37°C.
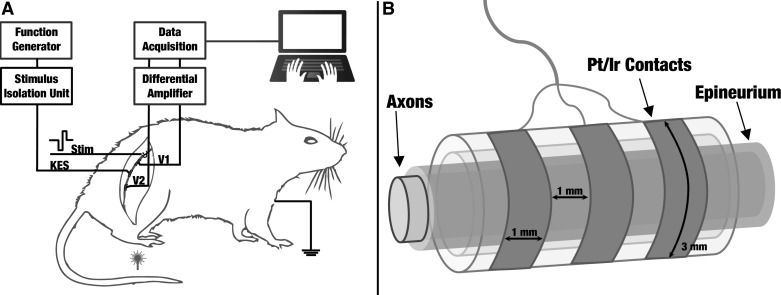
The right thigh of the animal was shaved and the biceps femoris muscle was separated for sciatic nerve preparations. The sciatic nerve was exposed from the top of the biceps femoris to the bottom of the gastrocnemius muscle in the ankle, and cuff electrodes were placed around the nerve (Fig. 1A). A total of 3–4 cm was exposed in all sciatic nerve preparations. Sterile rat ringer solution was applied throughout the experiment to prevent muscle and nerve tissue dehydration. After the experimental protocol was completed on the animal's right side, the wound site was closed with surgical clips. The same procedure was executed on the animal's left sciatic nerve. The same sterile procedures were used to expose and experiment on the left cervical vagus nerve. Preparations lasted an average of 5 h after which animals were euthanized via carbon dioxide. All experiments were conducted at room temperature. All animal experiment protocols were approved by the Georgia Tech Institutional Animal and Use Committee.
Fig. 1.
A: experimental setup for selective kilohertz electrical stimulation (KES) conduction block in the rat sciatic nerve. All experiments were conducted in a faraday cage to minimize interference from external noise sources. A hook electrode is used to elicit compound action potentials (CAPs) at either the distal or proximal end of the nerve. Cuff electrodes are used to record nerve activity as well as deliver the KES stimulus. Sensory stimulation was delivered to the animal's hindleg using a convective heat source. B: schematic of nerve cuff electrodes used for electrophysiology trials with exact dimensions (schematic not to scale).
Electrophysiological configuration and measurement.
Recordings of CAP propagation along the nerve were used as an output measure to detect and monitor the status of selective conduction block in both sciatic and vagus nerves. A combination of hook and tripolar cuff electrodes was used to conduct these studies. A bipolar stainless steel hook electrode was used to electrically elicit CAPs. Tripolar cuff electrodes (Fig. 1B) were used to record CAPs and deliver the KES block stimulus. The sampling rate of the data acquisition system (Digidata 1322; Molecular Devices, Foster City, CA; full scale range: 10.096 V) was 50 kHz per channel. Nerve recordings were differentially amplified (model 1700; A-M Systems, Sequim, WA) with a gain of 1,000× and filtered using a second-order bandpass (100 Hz-5 kHz) filter. In some cases, an additional digital first-order bandpass (300 Hz-3 kHz) filter was enabled in the acquisition system to enhance visualization of the CAP during application of the KES stimuli. This experimental setup provided direct monitoring of the neural activity along the nerve and the status of selective KES conduction block. Figure 1 displays the experimental setup used for sciatic nerve studies. No significant modifications were made to this setup for the vagus nerve studies. Vagus nerve studies positioned the electrode on the cervical section of the vagus nerve.
Electrode design and fabrication.
Nerve recordings and application of the KES block stimulus were performed using custom-made, tripolar, longitudinally slit cuff electrodes as shown in Fig. 1B. Cuff electrodes were made using silicone tubing, stainless steel wire, and platinum-iridium (Pt-Ir, 90/10) contacts (3 × 1 mm). Average cuff diameter and length for sciatic nerve studies were 1.25–1.5 and 5 mm, respectively, with 1 mm between Pt-Ir contacts. Average cuff diameter and length for vagus nerve studies were 1.0–1.2 mm and 3 mm, respectively, with 0.75 mm between Pt-Ir contacts. Impedance was characterized for all cuffs using an impedance conditioning module (FHC, Bowdoin, ME). The impedance ranges for the recording and KES stimuli cuffs were 1.6–2.0 and 1–1.2 kΩ, respectively at 1 kHz.
Stimulation.
Electrical stimulation was used to elicit CAPs in both sciatic and vagus nerves. Supramaximal cathode-first biphasic electrical stimulus pulses (5 V, 0.2 ms) were generated by the data acquisition system to trigger CAPs in the nerve. Sensory stimulation in the form of a hot air gun was used to deliver a low flow, high heat noxious stimulus to the forepaw of the animal. The KES block stimulus (continuous sinusoidal waveform) was generated using a function generator (DS345; Stanford Research Systems, Sunnyvale, CA). Both the stimulation pulses and KES block stimulus were converted to current sources (1 mA/V) by optically isolated stimulus isolation units (model 2200; A-M Systems). All stimulation equipment was calibrated and offsets were zeroed before experimentation to ensure no leakage of current from equipment.
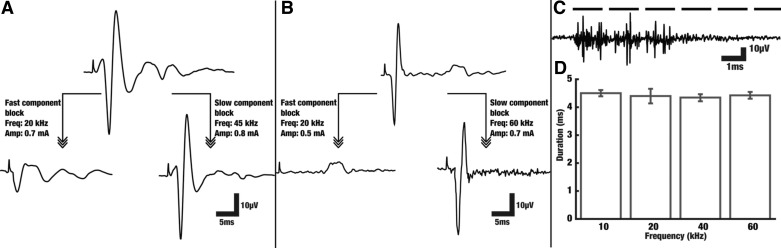
Evoked CAPs have two visually distinguishable components that we refer to as the fast and slow responses, in reference to their time of appearance relative to the stimulus artifact that occurs when a nerve is electrically stimulated. The fast response is attributed to large diameter, predominantly (but not exclusively) myelinated fibers with fast conduction velocities (e.g., Aα, Aβ, and Aγ), and the slow response is attributed to small diameter, predominantly (but not exclusively) unmyelinated fibers with slow conduction velocities (e.g., C) (Gasser 1941). Components with conduction velocities <2 m/s were classified as slow and components with conduction velocities >2 m/s were classified as fast. In addition, the waveforms associated with these two components were visually recognizable (Fig. 2, A and B). During experimentation, fast and slow components were identified by latencies between stimulus onset and the recording electrodes. Fast and slow components were associated with latencies in the range of 0.5–1.5 and 9–13 ms for electrode V1, respectively. In addition, fast and slow components were associated with latencies in the range of 3–5 and 15–20 ms for electrode V2, respectively.
Fig. 2.
A: selective KES block of electrically evoked fast and slow CAP components in rat sciatic nerve. The top trace is the raw evoked CAP (recorded with V1). The fast component block threshold (bottom left) was found to be 0.7 mA at 20 kHz and the slow component block threshold (bottom right) was found to be 0.8 mA at 45 kHz for this nerve. The bottom 2 traces correspond to recordings obtained with electrode V2. B: selective KES block of electrically evoked fast and slow CAP components in the rat vagus nerve. The top trace is the raw evoked CAP (recorded with V1). The fast component block threshold (bottom left) was found to be 0.5 mA at 20 kHz and the slow component block threshold (bottom right) was found to be 0.7 mA at 60 kHz for this nerve. The bottom 2 traces correspond to recordings obtained with electrode V2. C: example recording of the onset response as recorded by electrode V2 when KES (20 kHz at 1 mA) is delivered to the nerve with a bandpass (100 Hz-10 kHz) filter. Dashed black bar above the recording depicts when KES was on. D: onset response duration (mean and SD, n = 3) for select KES frequencies at 1 mA.
Force transduction.
Force transduction experiments were carried out to validate the functionality of selective block of the fast component. The posterior segment of the leg was shaved and the biceps femoris was exposed to allow dissection of the gastrocnemius-soleus muscle complex. The tibia was fixed to the experimental rig and the Achilles tendon was attached to a force transducer (model 724490; Harvard Apparatus) using a hemostat.
KES conduction block trials.
Block (or no block) of the slow component was verified via visual classification. First, block was visually verified in-line during experimentation. Block did not occur when there was a repeatedly triggered and identifiable waveform in the latency window of the slow component. Block occurred when there was no identifiable and repeatable waveform in this latency window. This visual analysis was repeated during post hoc data analysis.
Furthermore, we quantified the change in rectified and integrated area of both the fast and slow components of the CAP. Fast and slow components were identified using measured latencies and conduction velocities. The identified components were numerically integrated using Romberg's method for numerical integration (Burden and Faires 2001). Romberg's method was chosen because of its simplicity and ability to eliminate error without the need for oversampling. Because of these qualities, Romberg's method is more suitable for integrating experimental data, which tend to be noisy.
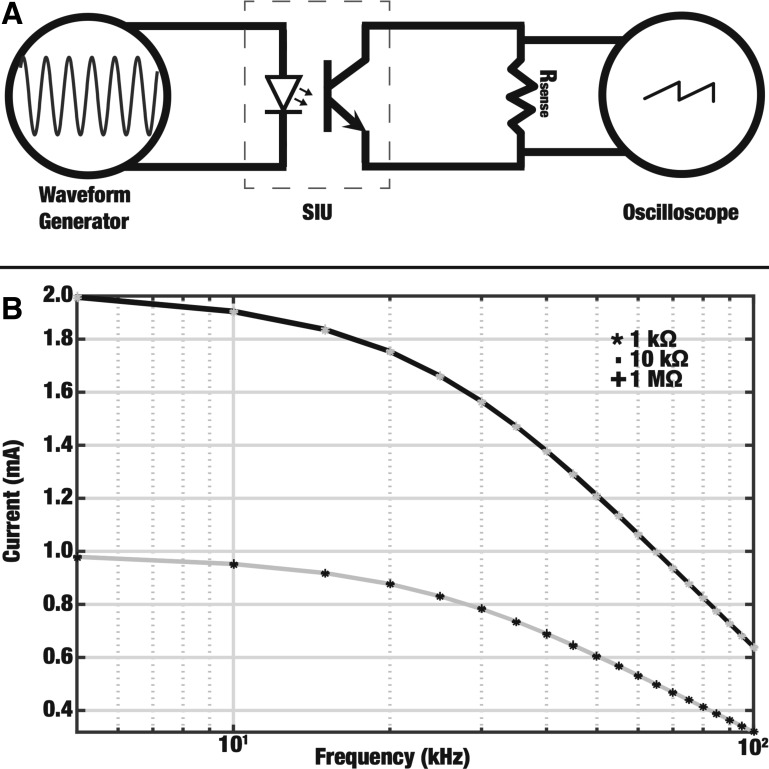
Attenuation of stimulus isolation units at high frequencies.
Frequencies up to 50 and 70 kHz were tested for the sciatic and vagus nerve experiments, respectively. These frequency ranges are beyond the rating (up to 40 kHz) of the stimulus isolation units (SIUs) driven by the waveform generator. The SIUs can provide outputs at frequencies higher than the ratings; however, the output is of a lower amplitude than specified. We measured this attenuation and used this information to calculate the true current output at a given frequency. Attenuation of the KES waveform was characterized by measuring the current across varying resistances (1 kΩ, 10 kΩ, and 1 MΩ) in parallel with the output terminal of the SIU, as shown in Fig. 3A. The resulting current across the resistor was calculated using voltage measurements made using an oscilloscope (HP 54602B; Hewlett Packard, Palo Alto, CA). The resulting calibration curves (Fig. 3B) were used to calculate the true current provided by the KES. These adjusted values are reported in this article. The same trends qualitatively reported are also evident in the nonadjusted data.
Fig. 3.
A: setup for measuring attenuated output from optically isolated stimulus isolation units (SIUs). The function generator output was connected to the input of the SIU. The SIU was set to provide an output of 1 mA/V and the output was connected to varying loads (Rsense). B: attenuation of current output by SIUs. The current across 3 different loads (Rsense) was measured at frequencies up to 100 kHz for input values of 1 V (grey) and 2 V (black). Curves for the 3 different loads at both input values overlap, showing that the attenuation is independent of the tested load and input value.
RESULTS
Each experimental preparation was tested for normal conduction properties before beginning selective KES trials. CAPs were triggered using suprathreshold electrical or sensory stimulation. Evoked nerve activity was recorded using cuff electrodes along the length of the nerve. The distance between stimulating and recording electrodes was used to determine conduction velocities for the CAP components.
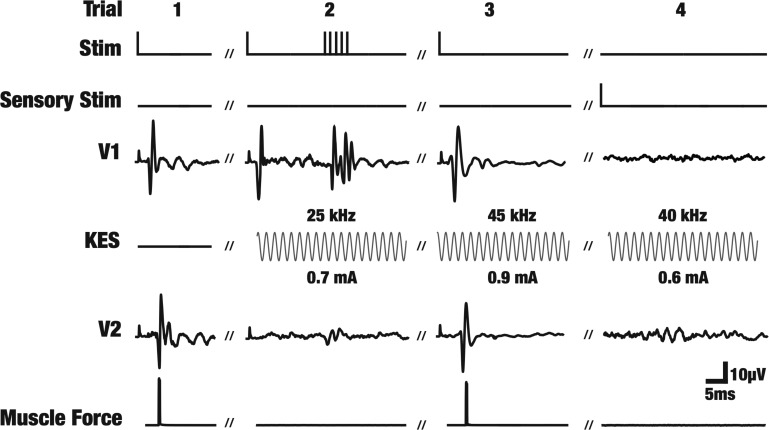
Figure 4 shows single trial data from one experiment demonstrating selective KES block. The labels on the left correspond to the electrodes shown in Fig. 1. Stimulus artifacts precede the fast component. Trial 1 shows the standard baseline trial conducted throughout every experiment to verify CAP initiation and propagation. A suprathreshold stimulation pulse is delivered to the nerve at the proximal end and the propagating CAP and associated muscle twitch are recorded. Trial 2 depicts the case where a CAP is triggered and the fast component is selectively blocked via KES. This block of the fast component led to an absence of the muscle twitch but maintained propagation of the slow component. Additional stimulation pulses were delivered to the nerve during selective block of the fast component to ensure true block had occurred. The additional stimulation pulses evoked the fast component response in the proximal recording electrode but not in the distal recording electrode, demonstrating the continued effects of the KES-selective block. This resulted in continued absence of muscle force generation while maintaining propagation of the slow component. Trial 3 demonstrates a scenario where the slow component is selectively blocked, leaving the fast component and muscle force intact. Trial 4 shows the use of sensory stimulation (heat) to evoke the slow component only. The stimulus is applied to the hindleg of the animal, resulting in the CAP appearing on electrode V2 first and then being blocked (absent on V1). Individual trials, as shown in Fig. 4, are aggregated to produce block threshold characterization curves (Figs. 5–7).
Fig. 4.
Raw data demonstrating selective KES conduction block in the rat sciatic nerve. All data shown is from individual trials from one single experiment. The labels correspond to the electrodes in Fig. 1. The Stim electrode was used to deliver electrical stimulation pulses to evoke CAPs. CAPs first show up on recording electrode V1. The KES trace shows the delivered KES block stimulus. The modulated CAP is then recorded by electrode V2. Simultaneous muscle force measurements were made using a force transducer. Sensory stimuli were also delivered to the hindleg of the animal to evoke sensory CAPs. In such cases the CAP appears on V2 before V1. The trial numbers (1-4) are arbitrarily labeled for referencing and do not represent the actual trial numbers from the experiment.
Fig. 5.
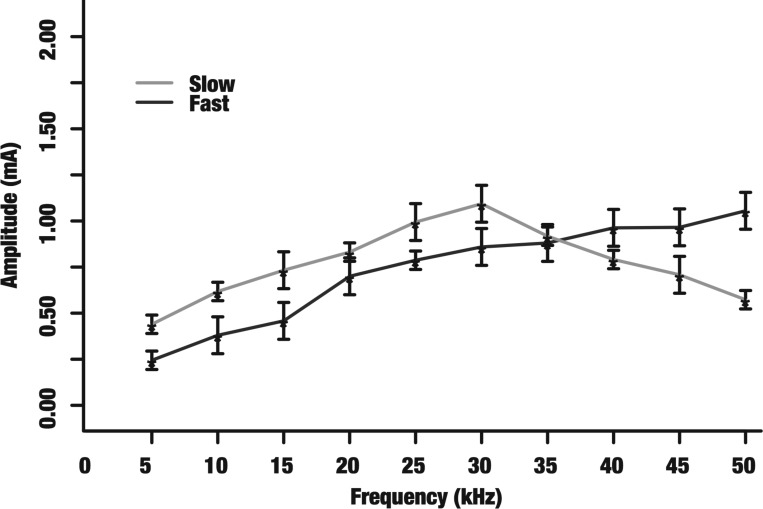
Block thresholds of sensory evoked CAPs and electrically evoked motor output in the rat sciatic nerve. The mean block thresholds (denoted by asterisks) and their corresponding SDs are shown for 5–50 kHz. Only CAP measurements were used to determine block thresholds for the slow component (sensory-evoked CAPs) and only motor output was used to determine block thresholds for the fast component (electrically evoked motor output).
Fig. 7.
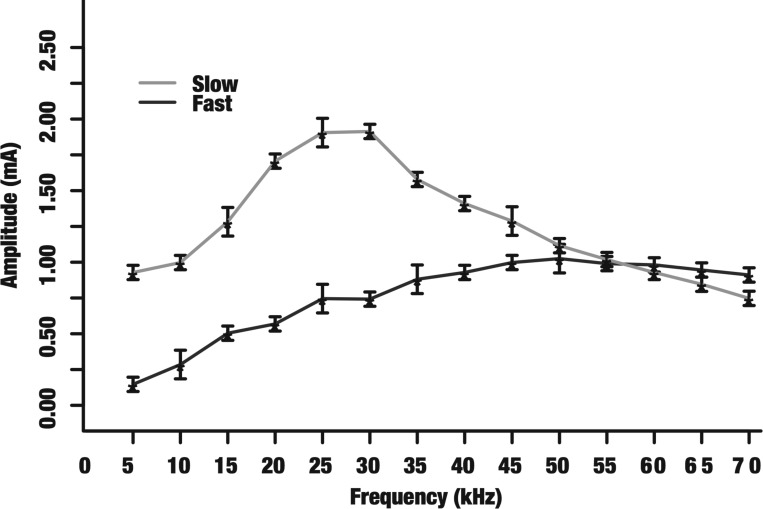
Block thresholds of electrically evoked fast and slow CAP components in the vagus nerve. The mean block thresholds (denoted by asterisks) and their corresponding SDs are shown for 5–70 kHz. The fast component block threshold monotonically increases while the slow component displays a nonmonotonic trend.
The primary results from these studies are the block threshold characterization curves (Figs. 5–7). These curves provide a visual representation of the frequency and amplitude pairs that allow for selective KES block of CAP components. Figure 5 depicts that KES amplitudes greater than or equal to the dark grey line but less than the light grey line provide for selective block of the fast component for frequencies up to 35 kHz. Similarly, KES amplitudes greater than or equal to the light grey line but less than the dark grey line provide for selective block of the slow component for frequencies between 35–50 kHz. This interpretation applies to all the block threshold characterization curves presented here.
CAP components corresponding to sensory stimuli and motor output are selectively blocked.
We utilized selective KES block to demonstrate loss of either motor function or sensory evoked CAPs when either the fast or slow component was selectively blocked. Sensory stimulation was applied to the hindleg of the animal to evoke sensory CAPs and evaluate block of the slow component. Electrical stimulation was used to evoke motor output and evaluate block of the fast component. Motor block was verified by force measurements (Fig. 4, trial 2) and sensory CAP generation and block was verified by direct nerve recordings (Fig. 4, trial 4). The block threshold was characterized for both sensory evoked CAPs and motor output (Fig. 5). The fast component block threshold increased with frequency while the slow component block threshold displayed a nonmonotonic trend peaking around 30 kHz.
Fast and slow components of the triggered CAP are selectively blocked by KES.
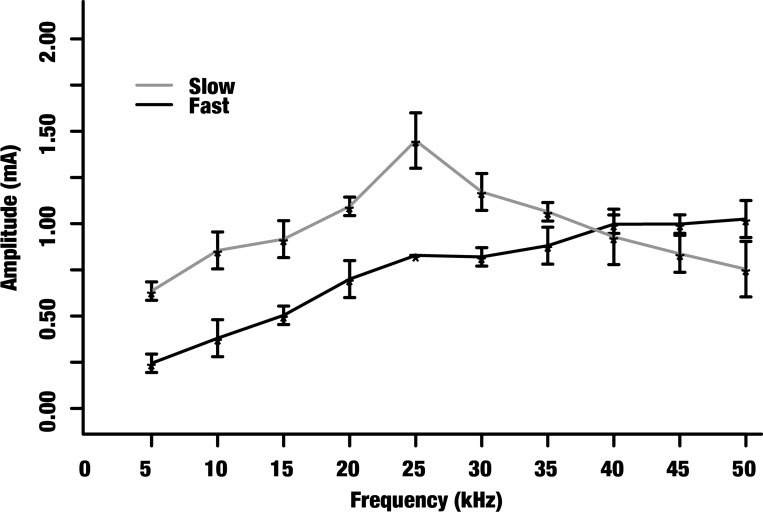
We achieved selective KES block of the fast (Fig. 4, trial 2) and slow (Fig. 4, trial 3) components of electrically triggered CAPs in eight rat sciatic nerves. Figure 6 shows the block threshold characterization for KES stimuli up to 50 kHz. The nonmonotonic block threshold trend peaked ∼25 kHz.
Fig. 6.
Block thresholds of electrically evoked fast and slow CAP components in the rat sciatic nerve. The mean block thresholds (denoted by asterisks) and their corresponding SDs are shown for 5–50 kHz. The light grey and dark grey data points correspond to the slow and fast components, respectively. The fast component block threshold monotonically increases while the slow component block threshold displays a nonmonotonic trend.
Selective block of CAP components can be achieved in multiple nerve types.
The robustness of selective KES conduction block was also examined in the rat vagus nerve preparation. The experimental setup (Fig. 1) was modified by using smaller cuff electrodes for interfacing with the rat vagus nerve. Electrical stimulation was used to evoke CAPs and nerve recordings were used to assess status of block (Fig. 2B). Figure 7 shows the mean and SD for selective KES block thresholds in the rat vagus nerve. The trends are similar to the sciatic nerve results in Fig. 6. The fast component block threshold increases monotonically while the slow component block threshold displays a nonmonotonic trend peaking around 30 kHz.
Changes in rectified and integrated area of CAP components.
We quantified the resolution of selective KES conduction block in the rat sciatic nerve by rectifying and integrating the fast and slow components. Table 1 depicts example data from two arbitrarily chosen frequencies and increasing amplitudes. The fast and slow components were rectified and integrated to quantify the percent reduction in the rectified and integrated area of each component compared with baseline (0% reduction) as a function of the block frequency and amplitude. It can be seen that the rectified and integrated area of the fast and slow components of the CAP decrease in a frequency and amplitude-dependent manner. For example, the fast component area decreases significantly as the KES amplitude is increased at low frequencies (20 kHz). Simultaneously, the slow component demonstrates a decrease, until both are no longer identifiable by our classification method. The same trend is observed at high frequencies (40 kHz), in which the slow component rectified and integrated area significantly decreases with increasing amplitude.
Table 1.
Average percent reduction in integrated electrically evoked CAP area
| Frequency, kHz | Amplitude, mA | Fast | Slow |
|---|---|---|---|
| 0 | 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 20 | 0.5 | 13.6 ± 1.5 | 2.0 ± 0.3 |
| 20 | 1.0 | 99.0 ± 0.5 | 12.3 ± 0.8 |
| 20 | 1.5 | 99.0 ± 0.5 | 99.0 ± 0.5 |
| 40 | 0.5 | 8.7 ± 2.4 | 24.1 ± 3.6 |
| 40 | 0.9 | 18.2 ± 5.3 | 99.0 ± 0.5 |
| 40 | 1.5 | 99.0 ± 0.5 | 99.0 ± 0.5 |
Percent changes were computed by normalizing each components area to baseline (0 kHz, 0 mA). In specific cases, such as 1.5 mA at both 20 and 40 kHz, both components were no longer distinguishable. A reduction of 99.0% ± 0.5 means that our classification methods were not able to detect those components.
CAP, compound action potential.
DISCUSSION
This investigation is the first to experimentally explore the frequency-amplitude relationship of the different components of the CAP of a mixed composition mammalian nerve to KES stimulation. The results show that KES stimulation can induce selective conduction block in whole nerves. This is also the first study to demonstrate the differential response of fast and slow mammalian fibers to KES stimulation. In addition, it is also the first to demonstrate that the fast and slow components of CAPs can consistently and selectively be blocked in multiple different mammalian nerves. This was validated via electrical measurements in the sciatic and vagus nerves as well as in response to sensory stimuli and motor output in the sciatic nerve.
Our combined experimental setup, using direct measures of nerve activity through CAP recordings and muscle force output, offers a powerful technique to investigate the effect of KES on different peripheral nerves and identify frequency-amplitude regions where specific fiber types may be selectively blocked. This setup enables direct observation and interpretation of the effects of KES stimuli on evoked CAPs and their distinct components.
Published work on KES conduction block shows that the block threshold increases with frequency for motor nerves (Ackermann et al. 2011b). Computational studies have shown similar relationships, that block threshold is inversely proportional to fiber diameter. They also suggest that small diameter unmyelinated fibers have a higher block threshold compared with large diameter myelinated fibers at all frequencies. While block thresholds of larger diameter myelinated fibers continually increased with frequency, the block thresholds of the small diameter unmyelinated fibers decreased with frequencies above 25 kHz (sciatic) and 30 kHz (vagus). The fast component block thresholds showed a monotonic relationship with frequency, as suggested by previously published literature. The slow component block thresholds displayed a nonmonotonic relationship with frequency, consistent with previous experimental results conducted in purely unmyelinated nerves of Aplysia californica (Joseph and Butera 2009) and our previous in vitro studies, which blocked the slow component in the sciatic nerve of the frog (Joseph and Butera 2011).
While qualitatively similar, the block threshold curves between the sciatic and vagus nerve differed in their quantitative details. The slow component block thresholds were higher while fast component block thresholds were lower for the vagus nerve (Fig. 7) compared with the sciatic nerve (Fig. 6). We hypothesize that this occurs due to the differences in composition of the sciatic (Schmalbruch 1986) and vagus (Yoo et al. 2013) nerves in terms of the number, types, and organization of fibers within the nerve. In addition, block threshold results from the sensory stimuli and motor output study (Fig. 5) were lower compared with slow component block thresholds from the selective block study results (Fig. 6). This is believed to be a result of the differences in fiber recruitment between electrical and sensory stimulation. Supramaximal electrical pulses activate all the fibers within the nerve while sensory stimulation only activates a small subset of the fibers.
In addition to the trends shown in Figs. 5, 6, and 7, changes in rectified and integrated area of each component of the CAP suggest that higher resolutions of selectivity may be feasible with KES. Table 1 depicts changes in rectified and integrated area of both the fast and slow components of the CAP as a function of frequency and amplitude. Specific frequency and amplitude pairs demonstrate a preference for blocking specific components of the CAP. As the amplitude increased, the selectivity increased (as observed by increasing reduction in individual component areas). All fibers are blocked once the amplitude is above the threshold for both slow and fast fibers. While this investigation focused on the use of KES conduction block for all-or-none inhibition, there may be applications in which reduction of specific components of the CAP may provide varying levels of neuromodulation. Continued investigations in our laboratory are aimed at understanding the resolution and sensitivity of KES conduction block.
Prior simulation studies have also indicated that numerous factors, including KES frequency, computational model of choice, and possible interactions between nodes of Ranvier, are key issues in achieving localized electrical nerve block and these factors may also affect the block threshold at each frequency (Bhadra et al. 2007; Joseph et al. 2007; Schnabel and Struijk 2001; Bédard and Destexhe 2008; Rattay and Aberham 1993; Rattay 1989). However, all of these computational studies suggest a monotonic relationship with frequency for all fiber types, including small diameter unmyelinated fibers. Our laboratory is currently investigating the biophysical mechanisms that may underlie the distinct block threshold plots through computational modeling and experimental approaches.
Block induction using KES waveforms is preceded with an onset response and a period of asynchronous firing (Fig. 2, C and D). This onset response is known to initially increase and then decrease as the KES amplitude or frequency is increased and has been investigated by others (Ackermann et al. 2011a; Miles et al. 2007; Gerges et al. 2010; Vrabec and Bhadra 2013; Pyragas et al. 2013). In our current study, this onset response presented itself as rapid transients in the nerve response and as rapid muscle contractions.
The technique presented here may enable greater control of peripheral nerve stimulation for clinical and scientific purposes. While the work presented here demonstrates fiber specificity in the form of specific CAP components, we acknowledge that both the fast and slow components consist of activity from multiple different fiber types (e.g., Aδ, Aβ, and C). For example, Aδ sensory fibers likely propagate with a velocity closer to what we associate with the fast component. The ability of KES to selectively block conduction in these specific fiber types is not clear and requires further investigation using single fiber recordings. Present and ongoing studies are aimed at understanding this phenomenon in greater detail, as well as how KES stimuli affect physiological states. Present clinical implementations of KES conduction block are nonspecific, blocking all activity in target nerves. In cases such as neuromodulation for diet control (Camilleri et al. 2008), systemic inflammation (Borovikova et al. 2000), pain (Narasimhan 2011), or motor inhibition in the central nervous system (Fisher et al. 2014), it is undesirable to modulate activity in all fibers of a target nerve, as this may lead to unknown long-term side-effects. The use of KES to selectively block conduction of specific fiber types may enhance the efficacy of treatments while removing unnecessary side-effects. In addition, the use of KES to selectively block conduction may enable more controlled investigation of neural circuits underlying a variety of neural pathologies. For example, a KES-enabled reversible vagotomy would offer many advantages over irreversible vagotomy procedures presently used in both scientific and clinical applications.
GRANTS
This work was supported by internal funding from Georgia Tech and National Institutes of Health Grant 2R01-EB-016407-09A1 (Principal Investigator: D. Christini, Weill Cornell Medical College) subcontracted to R. J. Butera.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.A.P. and R.J.B. conception and design of research; Y.A.P. performed experiments; Y.A.P. and R.J.B. analyzed data; Y.A.P. and R.J.B. interpreted results of experiments; Y.A.P. prepared figures; Y.A.P. drafted manuscript; Y.A.P. and R.J.B. edited and revised manuscript; Y.A.P. and R.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank L. O'Farrell for providing training related to animal experiments. We also thank members of the Butera laboratory for comments on the manuscript.
REFERENCES
- Ackermann MD, Bhadra N, Foldes EL, Kilgore KL. Conduction block of whole nerve without onset firing using combined high frequency and direct current. Med Biol Eng Comput 49: 241–251, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann MD, Ethier C, Foldes EL, Oby ER, Tyler D, Bauman M, Bhadra N, Miller L, Kilgore KL. Electrical conduction block in large nerves: high frequency current delivery in the nonhuman primate. Muscle Nerve 43: 897–899, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard C, Destexhe A. A modified cable formalism for modeling neuronal membranes at high frequencies. Biophys J 94: 1133–1143, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley F, Schlapp W. The effects of pressure on conduction in peripheral nerve. J Physiol 102: 72–82, 1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra N, Bhadra N, Kilgore K, Gustafson KJ. High frequency electrical conduction block of the pudendal nerve. J Neural Eng 3: 180–187, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra N, Kilgore KL. High-frequency electrical conduction block of mammalian peripheral motor nerve. Muscle Nerve 32: 782–790, 2005. [DOI] [PubMed] [Google Scholar]
- Bhadra N, Lahowetz EA, Foldes ST, Kilgore KL. Simulation of high-frequency sinusoidal electrical block of mammalian myelinated axons. J Comput Neurosci 22: 313–326, 2007. [DOI] [PubMed] [Google Scholar]
- Boger AS, Bhadra N, Gustafson KJ. High frequency sacral root nerve block allows bladder voiding. Neurourol Urodynamics 31: 677–682, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000. [DOI] [PubMed] [Google Scholar]
- Burden RL, Faires JD. Numerical Analysis. Wadsworth, OH: Wadsworth Group, 2001. [Google Scholar]
- Burgess P, Perl E. Myelinated afferent fibres responding specificaly to noxious stimulation of the skin. J Physiol 190: 541–562, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, Toouli J, Herrera MF F, Kulseng B, Kow L, Pantoja JP, Marvik R, Johnsen G, Billington CJ, Moody FG, Knudson MB, Tweden KS, Vollmer M, Wilson RR, Anvari M. Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery 143: 723–731, 2008. [DOI] [PubMed] [Google Scholar]
- Cuellar JM, Alataris K, Walker A, Yeomans DC, Antognini JF. Effect of high-frequency alternating current on spinal afferent nociceptive transmission. Neuromodulation 16: 318–327, 2013. [DOI] [PubMed] [Google Scholar]
- Fisher KM, Jillani NE, Oluoch GO, Baker SN. Blocking central pathways in the primate motor system using high frequency sinusoidal current. J Neurophysiol 113: 1670–1680, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser H. The classification of nerve fibers. Ohio J Sci 41: 145–159, 1941. [Google Scholar]
- Gerges M, Foldes EL, Ackermann MD, Bhadra N, Bhadra N, Kilgore KL. Frequency- and amplitude transitioned waveforms mitigate the onset response in high-frequency nerve block. J Neural Eng 7: 066003, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güven M, Özgünen K, Günay I. Conduction blocks of lidocaine on crushed rat sciatic nerve: an in-vitro study. Int J Neurosci 115: 725–734, 2005. [DOI] [PubMed] [Google Scholar]
- Jensen AL, Durand DM. High frequency stimulation can block axonal conduction. Exp Neurol 220: 57–70, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph L, Butera RJ. Unmyelinated aplysia nerves exhibit a nonmonotonic blocking response to high-frequency stimulation. IEEE Trans Neural Syst Rehabil Eng 17: 537–544, 2009. [DOI] [PubMed] [Google Scholar]
- Joseph L, Butera RJ. High-frequency stimulation selectively blocks different types of fibers in frog sciatic nerve. IEEE Trans Neural Syst Rehabil Eng 19: 550–557, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph L, Haeffele BD, Butera RJ. Conduction block induced by high frequency AC stimulation in unmyelinated nerves. Annu Int Conf IEEE Eng Medicine Biol Soc 2007: 1719–1722, 2007. [DOI] [PubMed] [Google Scholar]
- Kilgore KL, Bhadra N. Nerve conduction block utilising high-frequency alternating current. Med Biol Eng Comput 42: 394–406, 2004. [DOI] [PubMed] [Google Scholar]
- Lin E, Kilgore KL, Bhadra N, Lahowetz EA. Chronic high-frequency nerve block with an implanted waveform generator. Int Funct Electr Stimul Soc Conf 1: 2007. [Google Scholar]
- Martinov VN, Nja A. A microcapsule technique for long-term conduction block of the sciatic nerve by tetrodotoxin. J Neurosci Methods 141: 199–205, 2005. [DOI] [PubMed] [Google Scholar]
- McMullan S, Simpson DA, Lumb BM. A reliable method for the preferential activation of C- or A-fibre heat nociceptors. J Neurosci Methods 138: 133–139, 2004. [DOI] [PubMed] [Google Scholar]
- Miles JD, Kilgore KL, Bhadra N, Lahowetz E. Effects of ramped amplitude waveforms on the onset response of high-frequency mammalian nerve block. J Neural Eng 4: 390–398, 2007. [DOI] [PubMed] [Google Scholar]
- Narasimhan A. Commercialization of HFAC Electronic Nerve Block Technology to Treat Chronic Post Surgical Pain (PhD Dissertation) Cleveland, OH: Case Western University, 2011. [Google Scholar]
- Pyragas K, Novičenko V, Tass PA. Mechanism of suppression of sustained neuronal spiking under high-frequency stimulation. Biol Cybern 107: 669–684, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattay F. Analysis of models for extracellular fiber stimulation. IEEE Trans Biomed Eng 36: 676–682, 1989. [DOI] [PubMed] [Google Scholar]
- Rattay F, Aberham M. Modeling axon membranes for functional electrical stimulation. IEEE Trans Biomed Eng 40: 1201–1209, 1993. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H. Fiber composition of the rat sciatic nerve. Anat Rec 215: 71–81, 1986. [DOI] [PubMed] [Google Scholar]
- Schnabel V, Struijk JJ. Evaluation of the cable model for electrical stimulation of unmyelinated nerve fibers. IEEE Trans Biomed Eng 48: 1027–1033, 2001. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Gerner P, Colvin AC, Binshtok AM. C-fiber-selective peripheral nerve blockade. Open Pain J 2: 24–29, 2009. [Google Scholar]
- Tai C, Groat WC, Roppolo JR. Simulation analysis of conduction block in unmyelinated axons induced by high-frequency biphasic electrical currents. IEEE Trans Biomed Eng 52: 1323–1332, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabec TL, Bhadra N. A novel waveform for no-onset nerve block combining direct current and kilohertz frequency alternating current. In: 2013 6th Int IEEE EMBS Conf on Neural Eng Neural Eng (NER). San Diego, CA: IEEE, 2013, p. 283–286. [Google Scholar]
- Williamson RP, Andrews BJ. Localized electrical nerve blocking. IEEE Trans Biomed Eng 52: 362–370, 2005. [DOI] [PubMed] [Google Scholar]
- Yoo PB, Lubock NB, Hincapie JG, Ruble SB, Hamann JJ, Grill WM. High-resolution measurement of electrically-evoked vagus nerve activity in the anesthetized dog. J Neural Eng 10: 1–9, 2013. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tai C. Mechanism of nerve conduction block induced by high-frequency biphasic electrical currents. IEEE Trans Biomed Eng 53: 1–19, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]