Abstract
Animal experiments and limited data in humans suggest that electrical stimulation of the vestibular end organs could be used to treat loss of vestibular function. In this paper we demonstrate that canal-specific two-dimensionally (2D) measured eye velocities are elicited from intermittent brief 2 s biphasic pulse electrical stimulation in four human subjects implanted with a vestibular prosthesis. The 2D measured direction of the slow phase eye movements changed with the canal stimulated. Increasing pulse current over a 0–400 μA range typically produced a monotonic increase in slow phase eye velocity. The responses decremented or in some cases fluctuated over time in most implanted canals but could be partially restored by changing the return path of the stimulation current. Implantation of the device in Meniere's patients produced hearing and vestibular loss in the implanted ear. Electrical stimulation was well tolerated, producing no sensation of pain, nausea, or auditory percept with stimulation that elicited robust eye movements. There were changes in slow phase eye velocity with current and over time, and changes in electrically evoked compound action potentials produced by stimulation and recorded with the implanted device. Perceived rotation in subjects was consistent with the slow phase eye movements in direction and scaled with stimulation current in magnitude. These results suggest that electrical stimulation of the vestibular end organ in human subjects provided controlled vestibular inputs over time, but in Meniere's patients this apparently came at the cost of hearing and vestibular function in the implanted ear.
Keywords: vestibular, implant, human, Meniere's
animal experiments have shown that electrical stimulation of the endolymphatic or perilymphatic space in the vestibular end organ can produce robust direction specific slow phase eye movements (Chiang et al. 2011; Cohen et al. 1964; Cohen and Suzuki, 1963; Dai et al. 2011b,c; Davidovics et al. 2011, 2013; Della Santina et al. 2005, 2007; Fridman et al. 2010; Gong and Merfeld, 2000, 2002; Gong et al. 2008; Lewis et al. 2001, 2002, 2010, 2013; Merfeld et al. 2006, 2007; Nie et al. 2011, 2013; Phillips et al. 2011, 2015; Rubinstein et al. 2012; Sun et al. 2011; Suzuki and Cohen 1964). Such eye movements are presumed to reflect electrical activation of specific vestibular afferents and the resulting vestibulo-ocular reflex (VOR). Similar experiments during and after human inner ear surgery have shown that direction-specific nystagmus can be elicited by such stimulation in humans (Golub et al. 2014; Guyot et al. 2011a,b; Phillips et al. 2013; van de Berg 2012; Wall et al. 2007) and that such stimulation can partially compensate for lost vestibular function (Nguyen et al. 2014; Perez Fornos et al. 2014). Surface DC galvanic stimulation in humans similarly elicits eye movements, although in this case the activation is presumed to involve summed afferent input from many end organs of the inner ear (Cohen et al. 2012; Curthoys and Macdougall 2012; Fitzpatrick and Day 2004). To produce a viable vestibular prosthesis to replace the direction-specific velocity and acceleration information provided by the normally functioning vestibular labyrinth, site-specific stimulation of an implanted device enjoys significant theoretical advantages.
In nonhuman primates, guinea pigs, and chinchillas, biphasic pulse electrical stimulation of the semicircular canals with implanted electrodes produces eye movements largely in the plane of the implanted canal (e.g., Davidovics et al. 2013; Lewis et al. 2010). Changes in the pulse rate or current of the electrical stimulation produce changes in the slow phase velocity elicited by stimulation. The resulting eye velocities can be quite high transiently, and somewhat lower sustained velocities can also be attained (e.g., Davidovics et al. 2012). Electrical stimulation remains effective after lesion of the end organ by canal plugging, which greatly reduces movement of the endolymph and the resulting head movement modulation of canal afferent input except at frequencies above 4 Hz (Sadeghi et al. 2009), or by ototoxic lesion of the vestibular end organ with an aminoglycoside, which reduces but may not fully eliminate hair cell transduction depending on the dose of the drug (Dai et al. 2011b,c; Davidovics et al. 2011, 2013; Della Santina et al. 2005, 2007; Fridman et al. 2010; Gong and Merfeld, 2000, 2002; Gong et al. 2008; Lewis et al. 2001, 2002, 2010; Merfeld et al. 2006, 2007; Sun et al. 2011). Short-term adaptation to sustained stimulation produces a reduction in sustained slow phase eye movement velocity and also a reduction of the slow phase velocity response to transient modulated vestibular input (Davidovics et al. 2013). Animals appear to tolerate local electrical stimulation of the semicircular canal perilymphatic or endolymphatic space well (e.g., Rubinstein et al. 2012), and longitudinal experiments have demonstrated that stimulation over long periods of time with a fully implanted device can produce consistent neural responses (Phillips et al. 2012), sustained slow velocity eye movements (Phillips et al. 2015), and also consistent low-gain VOR (Lewis et al. 2010; Merfeld et al. 2007).
There are risks to implantation of a vestibular prosthesis, however. First, the device must be placed adjacent to neural elements that transduce both hearing as well as motion and orientation information. Most animal experiments have been performed with an initial intentional lesioning of the vestibular end organ, often with either canal plugging or gentamicin injection, thereby making it difficult to assess whether the implanted device itself damages vestibular function or hearing. However, in rhesus monkeys, experiments have shown that it is possible to implant multiple semicircular canals with stimulation electrode arrays and preserve hearing and vestibular function (Bierer et al. 2012; Dai et al. 2011a; Rubinstein et al. 2012). A second risk, which cannot be fully assessed in animal experiments, is whether electrical stimulation produces unpleasant sensations of pain, nausea, or loud auditory percepts. Experiments in chinchillas and rhesus monkeys show that hearing thresholds are unaffected by electrical stimulation of the vestibular end organ, but these experiments do not rule out the presence of other sensations during presumed canal-specific vestibular stimulation. Experiments in human subjects show that limited stimulation can be performed while eliciting only minimal additional sensation (Golub et al. 2014; Guyot et al. 2011a,b; Perez Fornos et al. 2014; Phillips et al. 2013; van de Berg, 2012).
Finally, it is unclear that the responses seen in animals will be fully reproduced in human experiments. The stimulation strategies and devices employed in most laboratories are unique to animal experiments. For example, most devices used in monkeys have been specially developed for vestibular stimulation in animals and require a percutaneous connection from the external device to the internal device, which is a potential source of infection and device failure. Most human studies, on the other hand, have been performed using modified cochlear implant technology. Only a few studies have been reported using such technology in nonhuman primates (e.g., Nie et al. 2011, 2013; Phillips et al. 2011, 2012; Rubinstein et al. 2012; Valentin et al. 2013). Translation of the uniquely developed animal-specific technologies and techniques to humans will involve modification of the existing paradigms. Even when devices that are developed specifically for implantation in humans are evaluated in animals, the many anatomical and physiological differences between animals and humans may produce different results in each species.
To directly test whether a vestibular prosthesis can produce canal-specific, parametrically controlled, therapeutic vestibular stimulation of the vestibular end organ over long periods of time in human subjects, we implanted subjects with intractable unilateral Meniere's disease with vestibular neurostimulators identical to those that have been implanted in rhesus monkeys in our laboratory. The devices were designed to provide therapeutic vestibular stimulation during attacks of vertigo without damaging the vestibular end organs or the cochlea. Although the implanted devices were also designed with the capacity to provide therapeutic head motion controlled stimulation to restore lost vestibular function, their use was restricted to intermittent activation in this study so that the safety of electrical stimulation could be established. We evaluated vestibular function and hearing by multiple standard clinical measures before and after implantation with the device. We assessed device activation of afferent fibers by measuring compound action potentials evoked by electrical stimulation with the device and recorded locally by the device. We assessed the efficacy of stimulation by recording the average slow phase velocity of eye movements elicited by brief trains of biphasic pulse electrical stimuli with the device. We assessed the perceptual experience of the subjects following each stimulus train by asking subjects to describe any sensation that they experienced and to report the magnitude and direction of any sensation of motion that they had. We performed longitudinal studies of each measure to determine the time course of the observed effects. A subset of these results has been presented previously (Golub et al. 2014; Phillips et al. 2013).
METHODS
All experiments were conducted in accordance with the Declarations of Helsinki and the Federal Policy for the Protection of Human Subjects: Notes and Rules. Research protocols were approved by the Human Subjects Division of the University of Washington and the Western Institutional Review Board. The implanted device was granted an investigational device exemption by the FDA for a 10-subject feasibility study for the treatment of disabling unilateral Meniere's disease.
Patients with disabling unilateral Meniere's disease who were seeking surgical treatment for their disorder were informed of the study by study personnel who discussed the potential risks and benefits of the study procedures, as well as standard treatment options with the subjects prior to their participation in any study procedures. An independent clinician obtained informed consent for the study, but one of the investigators (J. T. Rubinstein) obtained informed consent for surgery.
Clinical evaluations.
Subjects performed a battery of vestibular and auditory diagnostic tests prior to and at regular intervals following their device implantation surgery. Vestibular testing included sinusoidal and step velocity rotational chair testing, cervical vestibular evoked myogenic potential (cVEMP) testing, computerized dynamic posturography (CDP) including sensory organization test (SOT), motor control testing (MCT) and adaptation testing (ADT), subjective visual vertical assessment (SVV), sequential bithermal caloric irrigation testing, head thrust testing, and dynamic visual acuity assessment. Auditory assessment was performed with pure tone thresholds and speech discrimination testing.
Rotational chair vestibular testing was performed in the dark with a rotator (Neuro Kinetics, Pittsburgh, PA) and monocular two-dimensional (2D) recording of eye movements using video-oculography (VOG). Sinusoidal stimulation was performed about an earth vertical axis that transected the interaural axis with the subject's head restrained and oriented 20 degrees (deg) nose down. The stimuli had a peak velocity of 60 deg/s at frequencies of 0.01 to 0.64 Hz. Sinusoidal eye and chair velocities were extracted from recorded eye position records, which were desaccaded using eye velocity and acceleration criteria and digitally differentiated. The velocity data was fit using Fourier transforms, to extract relative gain, phase, and symmetry values for the angular vestibulo-ocular reflex response (aVOR). Step velocity testing was performed about the same vertical axis with sequential velocity steps of 100 deg/s to the right, left, left, and right, at 1,000 deg/s/s with 45 s of sustained velocity after each velocity step. Slow phase eye velocity data from step rotational testing was extracted from per and postrotatory nystagmus eye position records that were digitally differentiated following desaccading based on eye velocity and acceleration criteria. The peak resulting slow phase eye velocity average for a single slow phase, typically the first one, was extracted from the decaying slow phase eye velocity data, to calculate the gain of the step response. The velocity data were also fit with a single exponential to calculate the time constant of the response decay. Subject data were compared with age-matched clinical normative data collected at the University of Washington.
CDP was performed on an EquiTest platform (NeuroCom; Natus, Clackamas, OR) using standard clinical protocols and normative data supplied by the manufacturer. In brief, the testing consisted of three assessments. First, SOT testing was performed with 18 20 s trials of postural stability divided equally into six conditions: 1) support surface (SS) stable, visual surround (VS) stable, eyes open, 2) SS stable and eyes closed, 3) SS stable and VS sway referenced, 4) SS sway referenced and VS stable, 5) SS sway referenced and eyes closed, and 6) SS sway referenced and VS sway referenced. Sway angle during each trial was calculated from force data obtained from load cells in the support platform, and a calculation of the motion of the center of mass using the measured weight and height of the subject. Postural stability was assessed with an equilibrium score, which was calculated for each trial as 100 times the ratio of the maximal anterior-posterior (A-P) sway possible prior to falling/the observed sway. A composite equilibrium score was also obtained, which was a weighted average of the average equilibrium scores to each condition, i.e., [(avg cond 1 + avg cond 2)/2 + avg cond 3 + avg cond 4 + avg cond 5 + avg cond 6]/5. The average stability scores from the different conditions were also used to assess the pattern of performance on the SOT test. There were four ratios calculated which define the normal response pattern to this test: 1) somatosensory, cond 2 / cond 1, 2) visual, cond 4 / cond 1, 3) vestibular, cond 5 / cond 1, 4) visual preference, (cond 3 + cond 6) / (cond 2 + cond 5). Higher scores on these ratios suggest that the subject maintains balance with somatosensation, vision, and vestibular information and utilizes visual information in a contextually appropriate manner. Second, MCT testing was performed as a series of 18 A-P support surface translations: 3 small backward translations (BT), 3 medium BT, 3 large BT, 3 small forward translations (FT), 3 medium FT, and 3 large FT. The latency of the response was calculated by measuring the time from translation onset to the change in force exerted on the platform. In addition, weight symmetry during the response was calculated, as was the scaling of the response amplitude with increasing translation amplitude. Third, ADT was performed using a sequence of support surface rotations of equal amplitude: 5 toes up and 5 toes down. For each rotation type, postural stability was assessed from force data. Normal responses showed increasing stability with repeated exposure to each rotation type.
cVEMP testing primarily assesses saccular input to neck motorneurons and was performed with presentation of air conducted click stimuli (100 to 70 dB nHL) presented at 5 Hz to a single ear while recording unrectified bipolar sternocleidomastoid EMG with an evoked potential averager (Nicolet; Natus, San Carlos, CA). The average EMG resulting from 200 click stimuli was assessed offline for the presence of a biphasic response with a positive peak at 13 ms and a negative peak at 23 ms. Two repetitions at any sound level were required to determine the presence of a response. A normal threshold for an adult subject in our laboratory is found at 95 ± 10 dB nHL.
Subjective visual vertical testing was performed using a projected laser line with the subject sitting motionless in complete darkness (Neuro Kinetics). This test is used in some clinical laboratories to indicate central or peripheral otolith tone in the roll plane (Baier et al. 2012; Halmagyi et al. 1979) but also relies on head and trunk cues (e.g., Tarnutzer et al. 2010). The subject used two buttons to tilt a line, which was presented pseudorandomly in orientations of 5–35 deg roll tilt to the left or right, to an upright orientation. The subject indicated verbally when the line orientation was vertical. The mean angle of tilt was calculated from 10 orientation trials.
Head thrust testing (Halmagyi and Curthoys 1988) was performed with subjects seated in a head upright orientation while fixating a fixed-point target at a distance of 50 cm in the light. The head was manually rotated rapidly ≈10 deg to the right or left. The timing and the direction of the rotation were unpredictable. Eye movements were recorded by a low-intertia head-mounted VOG system (Vesticon, Portland, OR). The resulting eye position records were analyzed offline to determine the presence of a correction saccade opposite the direction of the head rotation to reestablish alignment of the eye with the fixation target. Presence of the correction saccade indicated an abnormal aVOR response.
Dynamic visual acuity assessment was performed using a wandering “E” stimulus projected on a computer monitor located 10′ from a seated subject (NeuroCom). Static acuity was obtained by asking the subject to identify the orientation of the “E” optotype of different sizes during head-stationary viewing. Perception time was assessed by having the subject repeat the orientation task with optotypes sized 0.2 logarithm of the minimum angle of resolution (logMAR) larger than the threshold static acuity. Dynamic acuity was assessed with the orientation task during repeated yaw head motion with the optotypes presented only during periods where the head was moving to the right or left at a criterion velocity. Acuity was reported as Snellen fractions, and the difference in static versus dynamic acuity was reported as ΔlogMAR, where a difference of >0.2 logMAR was considered clinically significant based on normative data collected at the University of Washington.
Bithermal caloric nystagmus testing was performed with sequential warm (50°C) or cool (24°C) air irrigation (ICS NCA-200) for 60 s in each ear at a flow rate of 8 l/min, presented as cool right (CR), cool left (CL), warm right (WR), warm left (WL) irrigations. The resulting nystagmus for each irrigation was recorded for 3 minutes in the dark using VOG (Neuro Kinetics), with a 10 s period of fixation suppression of the caloric nystagmus at roughly 2 min following the start of irrigation. Three minutes were interposed between each irrigation trial. The eye position records were desaccaded using eye velocity and acceleration criteria, and the resulting slow phase velocities were obtained by digital differentiation of the eye position recordings. Peak slow phase eye velocity averaged over a sliding window of 5 s of recording was calculated for each irrigation, and caloric weakness and directional preponderance were calculated as follows (Jongkees et al. 1962).
If the results of caloric irrigation indicated potential caloric areflexia in the irrigated ear, a second test was performed in the nonresponsive ear with ice-water irrigation (20 cc for 20 s).
Surgery.
Subjects were implanted with a specially designed nine-channel vestibular implant receiver stimulator based on a Nucleus Freedom cochlear implant (Cochlear, Sydney). The device was identical to devices previously implanted in rhesus monkeys (Rubinstein et al. 2012). The device had three leads each constructed with a fine 2.5 mm electrode array with a diameter of 150 μm for implantation in the perilymphatic space adjacent to the membranous labyrinth of each semicircular canal. Each array had three 250 μm long stimulation sites (117,810 μm2 area) separated by 200 μm. The device also had a remote ground that was implanted under the temporalis muscle. It was implanted under general anesthesia using a soft surgical technique described previously for nonhuman primate surgery (Rubinstein et al. 2012). Briefly, following a postauricular incision and mastoidectomy, the semicircular canals were blue lined (closely skeletonized) and small fenestrations were placed in the bony labyrinth adjacent to the ampullae. The tip of the leads were placed in the perilymphatic space, the fenestrations were closed with fascia while trying not to occlude the canal lumens, and the leads external to the fenestrations were tied down with suture. Subjects were monitored overnight in the hospital and were monitored and treated if necessary for nausea or vertigo. Three of four subjects required treatment for postoperative vertigo and nausea, which included administration of antiemetics and vestibular suppressant medications.
Precise placement of the electrode array was facilitated by intraoperative recording of vestibular electrically evoked compound action potentials (vECAPs) during surgery to confirm that the device was activating afferent fibers. The vECAP recording was performed with neural response telemetry as described in earlier publications for nonhuman primate recordings (Nie et al. 2011; Phillips et al. 2012). Briefly, vECAPS were recorded using Nucleus Freedom Custom Sound EP 1.3 (Cochlear). In all cases, monopolar stimulation from the distal electrode of each canal array was recorded by the distal electrode of an array in an adjacent canal. Forward masking was used to minimize the stimulation artifact (Gantz et al. 1994; Miller et al. 2000). Stimulation consisted of a biphasic masker pulse, 50 μs per phase, 400 ms before a biphasic probe pulse, 50 μs per phase, followed in some cases by an artifact reduction pulse, 50 μs duration, and then, after a variable delay, a 1.6 ms recording window. Stimulations occurred 50–80 Hz and were averaged over at least 50 presentations. This same technique was used in later experiments to monitor the efficacy of the device in activating afferent fibers. The device was activated in the surgical suite, and it stimulated a site on the surgically placed electrode array and recorded the resulting action potential either from an adjacent site on the implanted array or more typically from a site on an array placed in a different canal. If vECAPs were present at low threshold, the electrode was fixed in place. If vECAPS were not present or were present only at high current thresholds, the electrode was advanced toward the ampulla following surgical expansion of the fenestration in the bony canal. An obvious limitation of this technology is that the vECAPs cannot distinguish the specific afferents that are being activated by stimulation in a specific canal.
Activation of the implanted device.
After two or more weeks of postsurgical recovery, the implanted receiver stimulator was activated in the laboratory. This laboratory testing was repeated at regular intervals thereafter. Stimulation in the laboratory was controlled with a NIC-2 research interface (Cochlear) attached via a control pod and USB interface to a desktop computer, or with a standard clinical interface (Cochlear Programming Pod, Cochlear) attached to a laptop computer. The standard clinical interface was used for recording of electrode impedances, which was performed before each recording session. Impedances were recorded in response to 67.8 μA biphasic pulses at 25 μs per phase, presented at a 1 KHz pulse rate. Following recording of the impedance data, the same interface was used to record vECAP data. The NIC-2 interface was controlled via specialized research software and graphical user interface, which allowed preprogrammed biphasic pulse stimuli to be delivered (Nie et al. 2013). Stimulation trials typically consisted of 2 s trains of constant pulse rate and current amplitude biphasic pulses, with a 100 μs phase duration and 8 μs gap. These stimulation parameters were chosen for test stimuli because they elicited consistent well-formed nystagmus with sustained slow phase eye velocity in rhesus monkeys, based on extensive recording of electrically elicited eye movements with an identical device in our laboratory. Monopolar and bipolar stimuli were presented, but the typical configuration was monopolar with a source from the most distal electrode of an implanted array and a return to both the case and remote ground. Stimuli were incremented by 10–25 μA initially while mapping the electrode array, but thereafter the currents were presented pseudorandomly, with repeated trials at each current level. All implanted electrodes were evaluated using such stimuli, with the current levels adjusted so that they did not exceed extremely conservative safe charge limits for each electrode (≤400 μA at 100 μs/phase for all stimulation sites; safety factor of 2 for safe charge limits for this electrode geometry). The brief intermittent nature of the stimulation and the low current levels were used to ensure the safety of the subjects in this early study of a new technology.
Additional stimulation was performed at home during the intervals between testing in the laboratory. At-home stimulation was performed one to three times a week at low current amplitudes (<100 μA) for a self-reported average of ∼1 min. These at-home stimulation parameters never produced an eye movement response in any of the subjects during testing in the laboratory, nor did it produce any noticeable sensation in the laboratory or at home. Such stimulation was performed specifically to maintain the impedance of the electrodes over the duration of the study.
Eye-movement recording and data analysis.
Eye movements resulting from stimulation were recorded with the subjects seated in a research rotator (Micromedical, Chatham, IL) or on a clinical bench for the initial recording in subject S1. Subjects wore a lap or chest and lap belt during testing, and the head was restrained against a headrest. The eye movements were recorded in darkness with a head-mounted monocular or binocular VOG system sampling in 2D at 100 Hz (Neuro Kinetics). VOG was chosen to monitor eye movements since the test sessions, with multiple stimulus presentations at each rate and current, took ≈4 h per session to complete, and scleral coil recordings are not tolerated for more than 30 min in our laboratory, after which the irritated eye is unsuitable for sustained infrared illumination for VOG. A limitation of this technology is that 2D eye movement recording cannot unambiguously define the precise plane of the eye movement rotations observed, only the horizontal and vertical components.
In parallel with the eye movement recordings, stimulation and data sync pulses were digitized online at 1 KHz using Spike2 software running on a CED Power 1401 computer interface (Cambridge Electronic Design, Cambridge, UK). The stimulation software also kept a record of all stimulation pulse parameters for each stimulus trial. Eye movements were also recorded on high-speed digital video by the VOG system for potential offline reanalysis. Subjects were instructed to provide a verbal report at regular intervals between stimulus trains about any sensation that they experienced during electrical stimulation and also to estimate the magnitude and direction of any sensation of motion they perceived. Subjects were not informed of the current level, pulse rate, or site of stimulation.
Data analysis was performed offline. Eye movement data and stimulus files were exported and aligned temporally using the data synch pulses. The velocities of the slow phases of evoked nystagmus were obtained by differentiation of the eye position data following removal of the fast phases using a velocity criterion. The velocity of individual slow phases was determined by a least squares fit to the data, and average slow phase velocity resulting from a single stimulus train was calculated as the time weighted average of all slow phases occurring during a 2 s interval beginning 10 ms after the onset of electrical stimulation. Statistical analyses were performed on the slow phase eye velocity data across conditions and across trials with linear regression or paired t-tests.
RESULTS
Four subjects met the inclusion criteria and agreed to participate in the study. All subjects had long-standing severe Meniere's disease in the right ear, had failed conservative management, and were reasonable candidates for endolymphatic shunts, intratympanic gentamicin, or labyrinthectomy in the affected ear. Two subjects had failed endolymphatic sac procedures previously, and one had failed intratympanic gentamicin. Two subjects were male and two were female, with an average age of 67 ± 9 yr (S1, 56-M; S2, 76-M; S3, 65-F; S4, 72-F). A total of 11 canals were implanted in the right ear with electrode arrays placed adjacent to the ampullae. Subject S2 was implanted in only two canals due to persistent bleeding that pooled over the superior canal and precluded an atraumatic fenestration. Hence, the superior canal labyrinthotomy was not performed. This subject was on anticoagulation therapy prior to surgery but had completed a presurgical protocol and had normal clotting times at the time of surgery. Subjects S1 and S3 were discharged on postoperative day 1, subjects S2 and S4 were discharged on postoperative day 2. Three of four subjects complained of postoperative vertigo and nausea. All subjects received promethazine by suppository (supp) as needed (PRN), and one subject received ondansetron IV and then ondansetron by mouth (PO) PRN on postoperative day 1. All subjects had spontaneous nystagmus immediately postoperatively that subsided before discharge. None of the subjects experienced symptoms of positional vertigo or vertigo exacerbated by pressure, sound, or straining postoperatively. Subjects S1 and S2 were discharged with oxycodone (5 mg PO, PRN), promethazine (12.5 mg PO, PRN), and ondansetron (4 mg, PO, PRN). Subject S3 was discharged with diazepam (5 mg PO, PRN), oxycodone (5 mg PO, PRN), and promethazine (25 mg supp, PRN). Subject S4 was discharged with oxycodone (5 mg PO, PRN). All subjects had completed their course of these medications by the end of the first week postoperatively, and all were required to not take medications at least 48 h prior to any test session. Subject S1 had delayed episodic postoperative vertigo beginning 1 wk after surgery and lasting for 3–4 wk, which he described as being identical to his Meniere's attacks in frequency, intensity, duration, and character. This subject did not experience auditory symptoms associated with these episodes. The subject took previously prescribed diazepam (5 mg PO, PRN) in response to some of these episodes but did not take diazepam within 48 h of the first test session on postoperative week 2.
Effect of implantation on hearing and natural vestibular function.
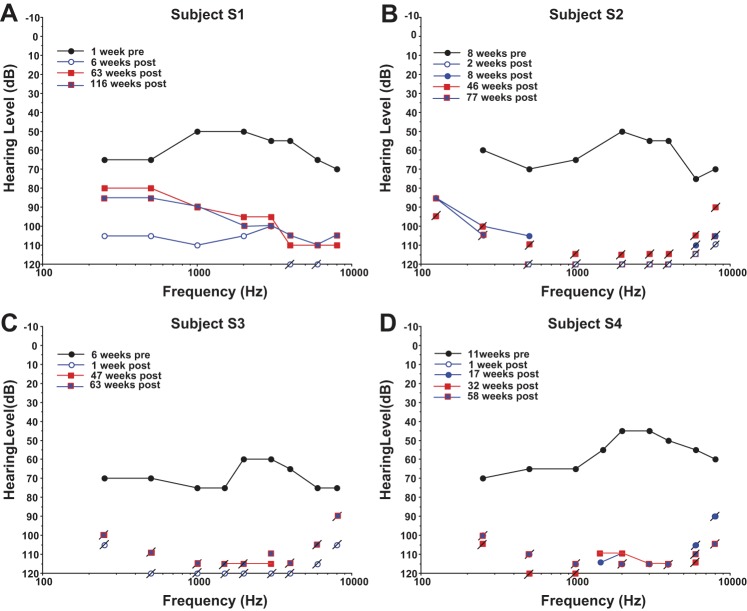
All subjects were evaluated for vestibular and hearing function pre- and postoperatively, and all subjects lost function in the implanted ear following the surgical implantation of the vestibular prosthesis. Preoperative audiograms indicated that all subjects had a moderate to moderately severe sensorineural hearing loss (SNHL) and speech discrimination scores from 16–64% at 85 dBHL in the affected ear prior to surgery (Fig. 1). Postoperative audiograms showed a further reduction in pure tone thresholds across the frequency range, resulting in a profound SNHL without measureable speech discrimination in the implanted ear. In subject S1 there was a small but significant recovery in pure tone thresholds by 63 wk postsurgery, which was sustained at 116 wk postsurgery (Fig. 1A). In the remaining subjects, there was little or no recovery of function in the implanted ear by 77 (S2), 63 (S3), or 58 wk postsurgery (S4). All subjects demonstrated stable hearing in their unimplanted ear and were quite happy to trade unilateral hearing loss for a resolution of their intractable vertigo, which resolved as a result of surgical trauma rather than electrical stimulation presented using the device.
Fig. 1.
Pure tone thresholds in the right (implanted) ear in dB HL for all subjects at different time points before (pre) and after (post) surgical implantation of the vestibular stimulator. Connected lines indicate data for sound levels at threshold. Slashed points indicate sound level limits of the audiometer for tones that were not at or above threshold.
Vestibular function was assessed with multiple measures in each subject both pre- and postoperatively. Table 1 indicates the results for cVEMP, head thrust test (HTT), SVV, dynamic visual acuity (DVA), and CDP. Three subjects (S1, S2, and S3) had absent or attenuated cVEMP bilaterally pre- and postoperatively. S4 had a weak cVEMP preoperatively and absent cVEMP bilaterally postoperatively. All subjects had abnormal head thrust toward the affected ear postoperatively, but only two subjects (S3 and S4) had normal HTT preoperatively. Two subjects (S3 and S4) had normal SVV tests preoperatively. Subject S2 had a significant leftward displacement of SVV presurgically, and subject S1 did not have preoperative testing. Postsurgically, all subjects had a clinically significant rightward displacement of SVV by the last day tested, i.e., >3 deg (S1, 10.0 deg by week 115, S2, 11.9 deg by week 46, S3, 3.3 deg by week 47, and S4, 5.4 deg by week 17). Two subjects (S1 and S2) showed abnormal DVA presurgically. For subject S1, visual acuity was decreased bidirectionally, but for subject S2 acuity was decreased only toward the affected, right, ear. Postsurgically, all subjects showed abnormal DVA bidirectionally, with the greatest decreased acuity for head movements directed toward the implanted ear. All subjects had a reduction in the composite SOT score between pre- and postoperative CDP testing. Subjects S1 and S2 had normal preoperative composite SOT scores. All other composite scores, both pre- and postoperative, were below normal for age. Postoperatively, all subjects had a vestibular deficient pattern on SOT testing, two subjects had visually deficient patterns, and two subjects had abnormal visual preference. Preoperative MCT were normal in three subjects (S2, S3, and S4). Subject S1 had abnormal latency and amplitude scaling presurgically. Postsurgically, three subjects had normal MCT (S1, S2 and S4). Subject S3 had abnormal weight symmetry postsurgically. ADT was normal pre- and postsurgically in three subject (S1, S2, and S4), and abnormal in subject S3. Overall, these tests indicate a loss of semicircular canal function in the implanted ear. The tests could not definitively identify a change in utricular or saccular function.
Table 1.
Vestibular test data for each subject by test week
| Subject | Week | ADT | MCT | SOT | SOT Type | DVA | VEMP | SVV | HTT |
|---|---|---|---|---|---|---|---|---|---|
| 1:2:3:4:5:6:C | L:S:R | R:L | |||||||
| S1 | −13 | (−) | (+)L, AS | 62:95:93:87:62:66:79 | (−) | (+)R, (+)L | (+)R | ||
| S1 | −1 | ||||||||
| S1 | 0 | 42*:22:40* | |||||||
| S1 | 6 | (−) | (−) | 94:90:85:78:50:0:59* | VE, VisPref | 55*:28:200* | (+)R, (+)L | (+)R | |
| S1 | 63 | 8.8R* | |||||||
| S1 | 115 | 10.0R* | |||||||
| S2 | −2 | (−) | (−) | 94:90:89:78:54:63:74 | (−) | 36:25:53* | 5.4L* | (+)R | |
| S2 | 9 | (−) | (−) | 87:85:72:76:0:45:54* | VE | 50*:33:166* | (+)R, (+)L | 2.7R | (+)R |
| S2 | 46 | 11.9R* | |||||||
| S3 | −3 | (+5) | (−) | 96:92:77:55:40:61:63* | VI, VE | 35:25:33 | (+)R, (+)L | 1.4L | (−) |
| S3 | 9 | (+4) | (+)R, WS | 94:93:87:44:47:19:56* | VI, VE, VisPref | 36*:17:87* | (+)R, (+)L | 3.1R* | (+)R |
| S3 | 15 | (+5) | (+)WS | 94:92:83:83:23:0:54* | VE | 32:21:53* | (+)R, (+)L | 3.5R* | (+)R |
| S3 | 47 | 3.3R* | |||||||
| S4 | −5 | (−) | (−) | 93:92:82:60:35:38:59* | VI, VE | 35:22:33 | (−) | 2.72R | (−) |
| S4 | 6 | (−) | (−) | 95:82:76:43:12:32:47* | VI, VE | 32:21:53* | (+)R, (+)L | 5.6R* | (+)R |
| S4 | 17 | (−) | (−) | 86:77:85:55:30:50:59* | Vi, VE | 50*:21:56* | (+)R, (+)L | 5.4R* | (+)R |
Negative numbers indicate weeks presurgery, and positive numbers indicate weeks postsurgery. ADT, adaptation test of the computerized dynamic posturography (CDP) battery. (−), the results were in the normal range; (+X), an abnormal score was present for X/10 rotations of the platform during toes-up and toes-down rotation. MCT, motor control testing of the CDP battery. (−), normal test results; (+), abnormal results. R or L, there were abnormal latencies to perturbation in the right or left leg, respectively. AS, abnormal amplitude scaling; WS, abnormal weight symmetry. SOT, sensory organization test of the CDP battery. The average stability scores for 3 trials of each condition are shown, as in the composite stability score (Cond1: Cond2: Cond3: Cond4: Cond5: Cond6: Composite).
Composite score was abnormal for age. SOT Type, the type of pattern displayed during SOT testing, based on ratio scores. (−), normal ratios. VE, vestibular deficient pattern; VI, visually deficient pattern; VisPref, abnormal visual preference. DVA, dynamic visual acuity testing. Scores are presented as the denominator of Snellen ratios (20/x) in the sequence (acuity during leftward head motion: static acuity: acuity during rightward head motion.
The difference in acuity between the static and dynamic scores exceeded 0.2 logMAR and was clinically significant. VEMP, cervical vestibular evoked myogenic potential testing. (−), the results were in the normal range; (+), the VEMP was absent or attenuated at 100 dB nHL. R and L, stimulation of the right or left ear. SVV, static subjective visual vertical testing. The average deviation of the line from true vertical is indicated in degrees. R or L, rightward or leftward deviation of the top of the line from vertical.
The deviation exceeded 3° and was considered clinically significant based on in-house normative data. HTT, head thrust testing in the horizontal plane. (−), the results were in the normal range; (+), the results were positive on inspection of the traces for a correction saccade opposite to the direction of the preceding head thrust.
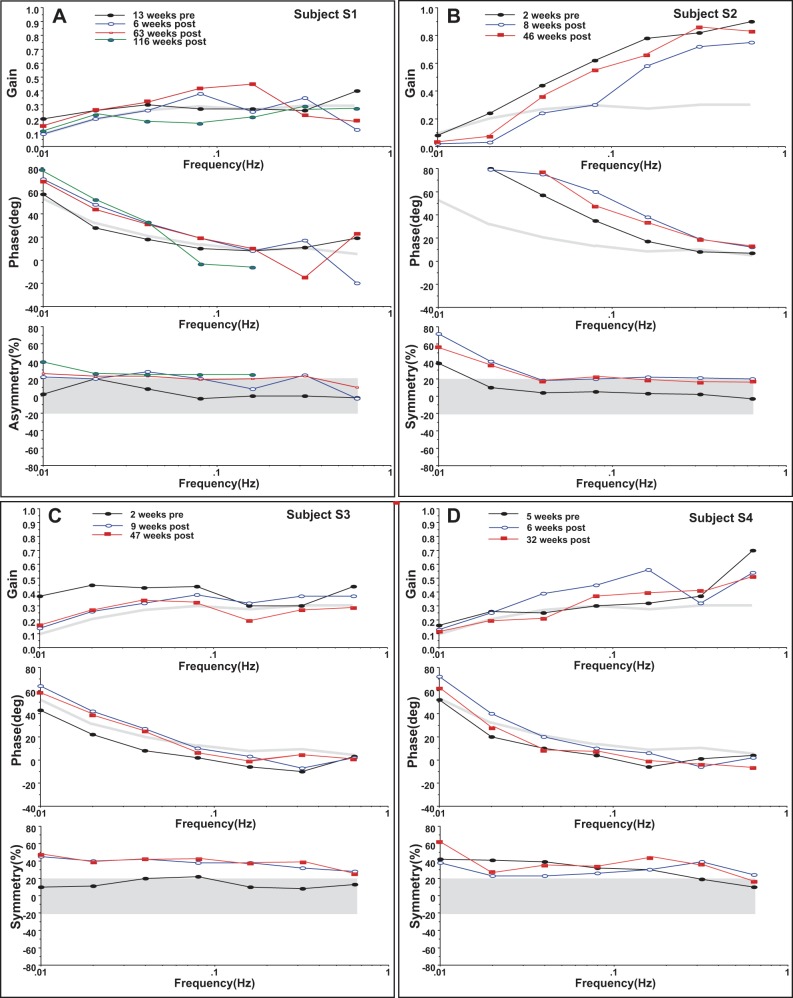
Rotational testing was also performed in all subjects. En bloc vertical axis sinusoidal rotation testing revealed below-normal gain in three of four subjects presurgically and showed a reduction in gain from preoperative levels at low frequencies in all subjects postoperatively (Fig. 2). Subject S4 had abnormally low gain at low- to-intermediate frequency preoperatively, and this was unchanged or improved by 6 wk postoperatively but subsequently decreased to below preoperative levels by 32 wk postoperatively (Fig. 2D). All subjects showed an advance in phase from preoperative values, particularly at lower rotational frequencies, following implantation of the vestibular prosthesis. Two of four subjects had an abnormal phase advance at several rotational frequencies presurgically. All subjects also showed a rightward gain asymmetry following surgery; i.e., reduced VOR gain for rightward vs. leftward head rotation. Three of four subjects had a rightward asymmetry presurgically consistent with a presurgical loss of vestibular function in the implanted ear. Again, subject S4 (Fig. 2D) had a very large rightward asymmetry presurgically, which actually improved by 6 wk postoperatively and then worsened to close to preoperative levels by postoperative week 32.
Fig. 2.
Sinusoidal angular vestibulo-ocular reflex behavior pre- and postsurgically for all subjects. Filled black circles are presurgical values. Thick gray lines and shading indicate 2 SD limits for control values for the respective measures. Top panel for each subject is gain with the 2 SD lower gain limit displayed for comparison. Middle panel for each subject is phase with the 2 SD phase advance limit displayed for comparison. Perfectly compensatory phase is defined to be 0.0 degrees (deg). Bottom panel for each subject is gain symmetry with the ±2 SD normal limit shaded for comparison. Positive values indicate a rightward asymmetry; i.e., rotation rightward produces lower eye velocity. Symmetric responses have 0.0% asymmetry.
Step rotational testing, performed pre- and postoperatively revealed a similar loss of vestibular function relative to preoperative measurements in the implanted ear following surgery in three of four subjects (Fig. 3). Preoperative gains were normal for steps in both directions in subjects S1, S2, and S3 but borderline below normal bidirectionally for subject S4 (Fig. 3A). There was a significant asymmetry in preoperative gain in subject S3, with the affected ear having lower gain. Preoperative time constants were shorter than normal bidirectionally in subjects S1 and S2, and there was a significant asymmetry in time constants in subject S4, with the time constant being shorter for rotations toward the right (affected) ear (Fig. 3B). Postsurgically, gains decreased from preoperative levels in the right (implanted) ear of all subjects by the last postoperative measurement day. Gain increased slightly in the implanted ear of subject S4 from preoperative week 5 to postoperative week 6 but then fell back to below preoperative levels by postoperative week 32. Average time constants for steps toward the implanted right ear decreased slightly in all subjects from preoperative testing to the last postoperative day. In subject S1, time constants increased from preoperative testing to postoperative week 6 but then decreased again by postoperative day 116.
Fig. 3.
Velocity step rotational test results pre- and postsurgically for all subjects. A: step gains calculated as (−1) times the ratio of the velocity of the first slow phase to the step velocity. Leftward steps are indicated by lighter gray bars and rightward steps are indicated by darker (gray and black) bars. The subject and week of testing are indicated. Negative weeks indicate presurgical data. B: step time constants in seconds calculated from a single exponential fit to the slow phase velocity data with the same color scheme as in A. Post Right, postrotatory nystagmus after rightward rotation; Post Left, postrotatory nystagmus after leftward rotation; Per Right, per-rotatory nystagmus during rightward rotation; Per Left, per-rotatory nystagmus during leftward rotation. Note: all subjects were implanted in the right ear.
Bithermal caloric testing showed a clear loss of vestibular function from preoperative levels in the implanted ear of all subjects postoperatively (Fig. 4). Two of four subjects (S2 and S4) had bilateral caloric hypofunction presurgically, and two of four subjects had clinically significant caloric weakness (CW) in the affected ear presurgically (CW = −25, S1; −16, S2; −55, S3; −13, S4). Three of four subjects had abnormal directional preponderance (DP = 32, S1; −24, S2; 64, S3; −52, S4), and subject S4 had borderline normal DP. All subjects had clinically significant CW postsurgically, indicating severe caloric hypofunction or areflexia in the right (implanted) ear (CW = −100, S1; −97, S2; −100, S3; −99, S4). In all subjects, ice-water calorics performed postoperatively failed to enhance the responses obtained during bithermal irrigation, indicating a complete loss of low-frequency vestibular function in the implanted (right) ear. All subjects had left beating DP consistent with an uncompensated loss of function in the implanted ear postsurgically (DP = −39, S1; −90, S2; −84, S3; −78, S4). In subject S1, there was a small improvement in symmetry of the caloric response over time, with CW decreasing from −100% to −71% between postsurgical days 42 and 608, and DP decreasing from −39% to −25%. However, these changes were primarily due to a reduction in the response to irrigation of the unimplanted, left, ear.
Fig. 4.
Caloric responses for all subjects. Left ear irrigation is indicated by lighter gray bars, and right ear irrigation is indicated by darker (gray and black) bars. Peak slow phase velocities in deg/s are plotted. Negative velocities indicate leftward slow phase eye movement. LC, 24°C irrigation of the left ear; LW, 50°C irrigation of the left ear; RC, 24°C irrigation of the right ear; RW, 50°C irrigation of the right ear. The subject and week of testing are indicated. Negative weeks indicate presurgical data.
Taken together, the above results indicate that although the subjects had limited vestibular function and relatively poor hearing presurgically, following implantation of the vestibular prosthesis, the subjects lost hearing and vestibular function in the implanted ear relative to their preoperative levels. Long-term changes in function were evaluated in all subjects, and these data suggest that there was limited recovery of auditory function in one subject after many weeks, but there was no recovery of vestibular function.
Postoperative changes in the implanted electrodes.
To assess the viability of the implanted canal electrode sites, we obtained electrode impedance measurements intraoperatively and at multiple time points postsurgically. The results are displayed in Table 2, which shows the electrode impedance measurements from all of the implanted canals across time. The changes in impedance were relatively small across time. In subjects S1 and S2, all electrode sites in all canals showed an increase in impedance between intraoperative and the first post-operative measurement. In subject S4, six of nine sites showed such an increase. In subject S3, three electrodes showed an increase and six showed a decrease in impedance. Longitudinal measurement of the impedances over the next 48–63 wk, showed mixed changes, with impedances decreasing at 7/9 sites in subject S1 at 63 wk, in 5 of 6 sites in subject S2 at 46 wk, in 5/9 sites in subject S3 at 63 wk, and in 3/9 sites in subject S4 at 58 wk. The average postoperative change in impedance from the first postoperative measure to the last at 48–63 wk was 1.89 kOhm for S1, −1.65 kOhm for S2, 0.9 kOhm for S3, and −6.8 kOhm for S4. Additional data were also available for subject S1, from 63 to 131 wk postoperatively. During this time, the impedance of all sites in all canals dropped by an average of 11 K Ohm. Since our safe-charge limits were set very conservatively, the voltage compliance of the stimulator was sufficient so that impedance changes did not limit our ability to deliver electrical stimuli within those limits.
Table 2.
Impedance data by test week
| Superior Canal Array |
Lateral Canal Array |
Posterior Canal Array |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject # | prox | mid | dis | prox | mid | dis | prox | mid | dis | |
| S1 | week 0 | 19.51 | 21.31 | 21.23 | 16.23 | 16.48 | 12.95 | 25.37 | 20.36 | 19.06 |
| S1 | week 2 | 25.36 | 30.69 | 30.13 | 29.79 | 27.55 | 27.32 | 37.54 | 32.73 | 33.07 |
| S1 | week 6 | 31 | 33.59 | 26.96 | 30.65 | 29.85 | 30.09 | 37.37 | 41.44 | 37.48 |
| S1 | week 63 | 29.89 | 30.57 | 28.38 | 23.73 | 21.61 | 23.51 | 32.99 | 34.58 | 31.95 |
| S1 | week 87 | 31.09 | 27.73 | 28.38 | 24.09 | 21.62 | 23.79 | 29.5 | 32.82 | 31.21 |
| S1 | week 116 | 30.35 | 28.97 | 27.69 | 22.61 | 22.09 | 22.82 | 29.42 | 33.57 | 30.6 |
| S1 | week 131 | 24.61 | 24.35 | 21.65 | 21.48 | 19.97 | 17.93 | 30.21 | 31.81 | 26.99 |
| S2 | week 0 | x | x | x | 20.93 | 21.83 | 21.95 | 22.79 | 19.95 | 23.35 |
| S2 | week 2 | x | x | x | 25.5 | 27.77 | 31.7 | 30.49 | 28.2 | 36.12 |
| S2 | week 4 | x | x | x | ||||||
| S2 | week 9 | x | x | x | 25.46 | 31.43 | 18 | 27.09 | 25.39 | 19.44 |
| S2 | week 25 | x | x | x | 21.78 | 29.62 | 26.41 | 25.43 | 23.22 | 25.07 |
| S2 | week 31 | x | x | x | 24.75 | 25.72 | 19.84 | 22.55 | 18.36 | 23.48 |
| S2 | week 46 | x | x | x | 14.06 | 39.54 | 22.41 | 21.96 | 20.6 | 22.09 |
| S3 | week 0 | 20.55 | 21.33 | 24.84 | 17.89 | 18.56 | 19.59 | 17.38 | 17.7 | 20.03 |
| S3 | week 2 | 21.67 | 28.98 | 32.11 | 12.97 | 14.38 | 16.5 | 16.84 | 17 | 18.35 |
| S3 | week 15 | 24.85 | 20.96 | 31.43 | 16.76 | 25.26 | 21.63 | 29.76 | 28.95 | 26.76 |
| S3 | week 63 | 21.64 | 19.92 | 28.28 | 30.65 | 27.35 | 17.17 | 10.6 | 28.77 | 16.35 |
| S4 | week 0 | 18.71 | 18.31 | 20.48 | 16.66 | 16.83 | 19.81 | 19.24 | 20.93 | 23.69 |
| S4 | week 1 | 16.85 | 18.66 | 25.3 | 18.76 | 18.82 | 20.48 | 13.24 | 13.2 | 17.44 |
| S4 | week 6 | 25.83 | 22.79 | 23.82 | 25.89 | 27.54 | 25.22 | 28.79 | 28.6 | 31.38 |
| S4 | week 32 | 26.7 | 19.86 | 19.77 | 19.16 | 17.48 | 19.16 | 30.35 | 32.33 | 38.30 |
| S4 | week 58 | 33.48 | 22.51 | 21.52 | 19.87 | 18.39 | 20.42 | 33.64 | 28.3 | 38.07 |
Week 0 indicates intraoperative testing, and positive numbers indicate weeks postsurgery. Values in kOhms are listed for the 3 sites on each stimulation array in the superior, lateral, and posterior canal. prox, Proximal; mid, middle; dis, distal; x, the array was not implanted.
Ability to drive vestibular afferents electrically.
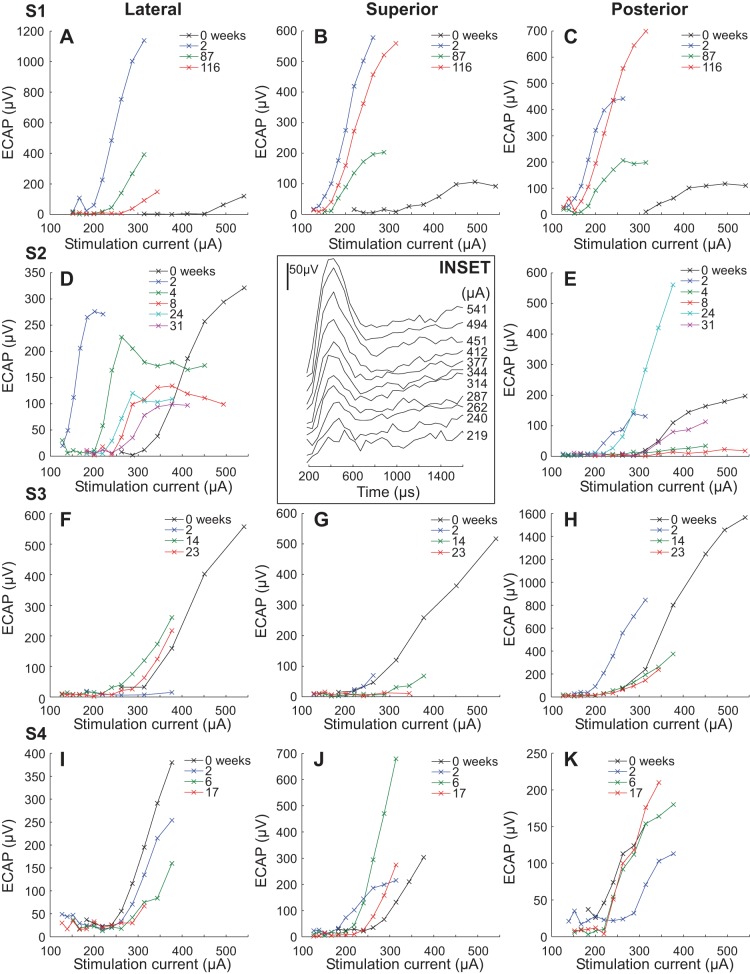
To evaluate the ability of the vestibular prosthesis to drive vestibular afferents, we recorded compound action potentials in the vestibular end organ during electrical stimulation with the vestibular prosthesis. The compound action potentials (vECAP) were recorded intraoperatively and postoperatively at two or more time points for all implanted canals. A representative intraoperative vECAP recording from subject S1 is presented in Fig. 5, inset. The vECAP shows an increasing amplitude with increasing current of stimulation, suggesting that the electrical stimulation is recruiting increasing numbers of afferent fibers as the stimulation current level increases. This suggests that recruitment may be a useful mechanism for modulation of vestibular input with electrical stimulation of the end organ. Electrical stimulation of the superior canal with recording in the lateral canal in Fig. 5, inset, produces a robust intraoperative vECAP with a threshold of 262 μA.
Fig. 5.
Longitudinal recording of vestibular evoked compound action potential (vECAP) in all subjects. The amplitude of the recorded waveform is plotted against the stimulation current for different times (in weeks) following the surgery. The symbols indicate the week of the recording. Each row indicates data from a single subject, and each column indicates data from stimulation with a specific canal array. Insert: vECAP recording intraoperatively in subject S1. The stimulation currents are listed to the right of each trace. The amplitude calibration is 50 μV. Time is the latency from commanded stimulation onset in μs.
To obtain a quantitative indicator of recruitment of vestibular afferents over time with electrical stimulation we measured the amplitude difference between the P1 and the N1 wave of the vECAP at different currents longitudinally in each subject (Fig. 5). To demonstrate statistically significant differences at different time points, we performed a linear regression analysis looking for significant differences in the slopes of the regression of the relationship between vECAP amplitude and stimulation current (P ≤ 0.05). The vECAP amplitude vs. current slope increased significantly in four canals between intraoperative recording in the open surgical field and the first postoperative recording (S1 superior, S1 posterior, S2 lateral, S3 superior) and decreased significantly in three canals (S3 lateral, S4 lateral, and S4 posterior). In the superior canal of subject S3, the increase in vECAP amplitude vs. current slope, though statistically significant, was very small. In the lateral canal of subject S1, it was not possible to perform a comparison between intraoperative vECAP recordings and later recordings, because different stimulation parameters were used (i.e., different masking settings).
Later postoperative vECAP recordings were obtained in 11 canals. Seven of 11 canals showed a significant decrement in amplitude vs. current slope between the last recorded test day and the first postoperative time point (S1 lateral, S1 superior, S2 lateral, S2 posterior, S3 superior, S3 posterior, S4 lateral). Two of 11 canals showed a significant increase in vECAP amplitude versus current slope over the same period (S3 lateral, S4 posterior). vECAP amplitudes in the superior and posterior canals of subject S1 showed a late rebound between 87 and 116 wk, suggesting that there were still dynamic changes occurring in this subject at these later time points. Such changes paralleled the changes in the audiogram and vestibular function in this subject. Similar changes in vECAP amplitude were observed in the posterior canal of subject S2 between 8 and 24 wk, in the lateral canal of subject S3 between 2 and 14 wk, and in the superior and posterior canals of subject S4 between 2 and 6 wk.
Overall, the results of vECAP recording suggest that the vestibular prosthesis was activating vestibular afferents postsurgically and that there was recruitment of afferent fibers with increasing current. The variability in vECAP between intraoperative and postoperative recording may be due to the different recording conditions encountered in the open surgical field and in the closed postsurgical recording condition. Most canals showed a decrease in vECAP amplitude vs. current slope with time postsurgically. This change may reflect a progressive loss of vestibular afferent input, a change in the efficacy of the electrical stimulation, or a change in the electrical environment surrounding the electrodes. However, in two of 11 canals, the vECAP amplitude vs. current slopes actually increased over time, suggesting an improvement in the efficacy of stimulation over time, and many canals showed at least transient increases.
Ability to drive vestibulo-ocular slow phase eye movements with electrical stimulation.
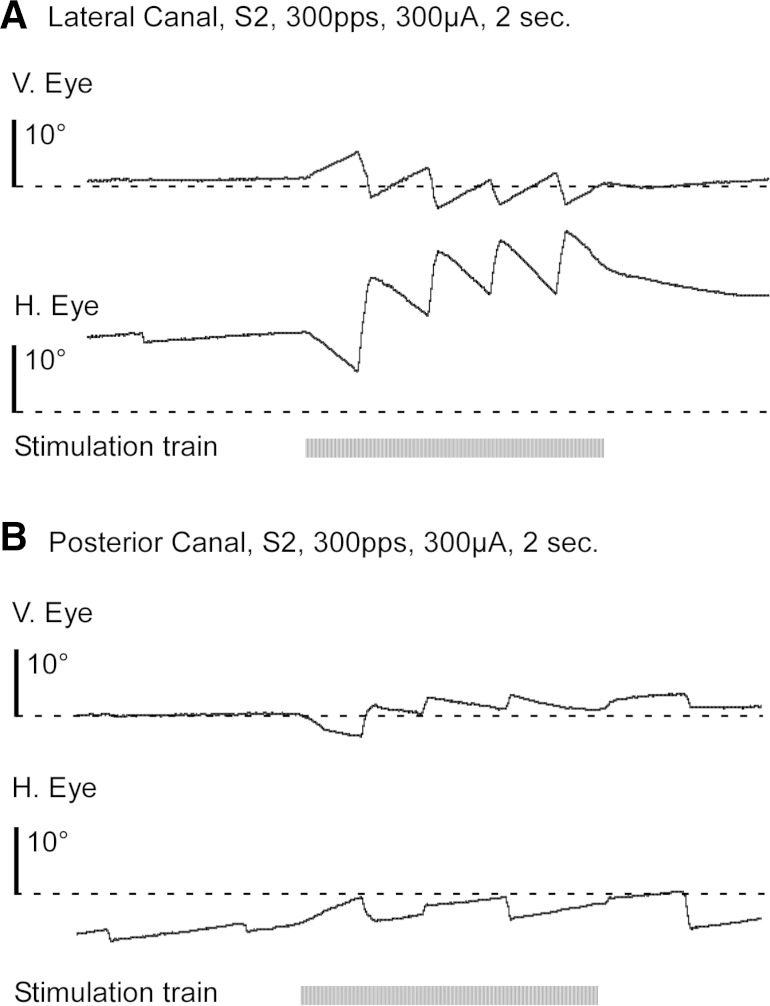
To assess the ability of a vestibular prosthesis to drive slow phase eye movements in our human subjects, we stimulated each canal with 2 s train of constant current and constant rate biphasic pulses at 100 μs per phase and with an 8 μs interphase gap. Subsequent experiments indicated that changing the gap sequentially from 8 to 16 μs in 2 μs steps had no effect on the observed slow phase eye velocities. During a single day of recording, we performed many short stimulations in each subject. The mean total duration per day of the short 2 s stimulation trains was 401 ± 117 s (mean ± SD) across all subjects. The 2 s stimulation trains typically elicited 2 s periods of constant velocity jerk nystagmus as shown in Fig. 6. Figure 6A shows the eye position changes resulting from 2 s train of biphasic pulses delivered to the lateral canal of subject S2. Stimulation of the lateral canal ampullar nerve alone should elicit a constant velocity right beating nystagmus. However, in subject S2 there is also a constant velocity upward vertical component to the response, suggesting current spread to ampulla of the adjacent superior semicircular canal. The response is primarily in the plane of the canal, but the presence of the vertical component suggests that stimulation involves multiple afferent nerves. Similarly, Fig. 6B shows stimulation of the posterior canal in subject S2, which produces both a constant velocity downward nystagmus, and a weak left beating nystagmus, suggesting stimulation primarily in the plane of the posterior canal. Also seen in this trace is a transient period of increased velocity at the outset of electrical stimulation. This response characteristic was noted during stimulation in two canals but was not typically present in the recordings.
Fig. 6.
Representative traces of slow phase eye movement resulting from a 2 s train of 300 μA biphasic pulses (100 μs per phase and 8 μs gap) monopolar electrical stimulation at 300 pps in subject S2. Horizontal (H) and vertical (V) eye position are displayed as is the stimulation train (black horizontal bar). A: stimulation of the right lateral canal. B: stimulation of the right posterior canal.
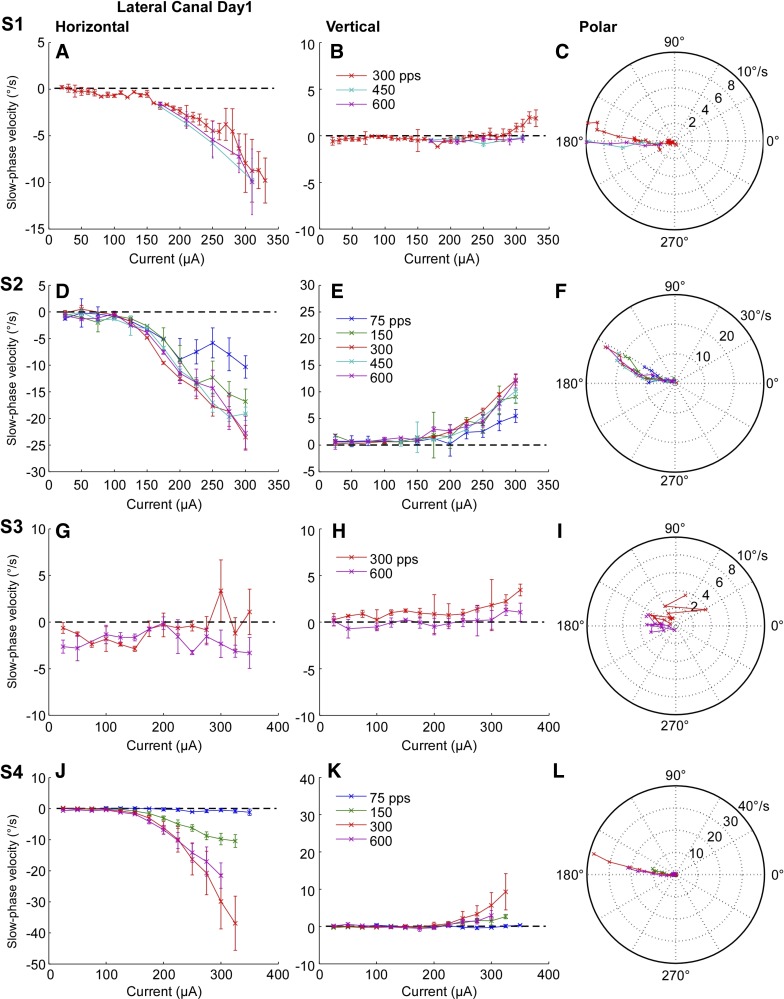
To evaluate the effect of rate and amplitude of electrical stimulation on electrically evoked VOR eye movement slow phase velocity, we plotted time-weighted average slow phase eye velocity vs. pulse current amplitude and rate for all canals. Data for electrical stimulation of the right lateral canal are shown in Fig. 7, which displays the horizontal and vertical component average slow phase velocity and a polar plot indicating vector velocity for each subject on the first postoperative day. For three of four subjects, average horizontal slow phase velocity increases clearly with stimulation current (Fig. 7, A, D, J). In subject S3 there is only a low horizontal slow phase velocity (maximally 3 deg/s) associated with stimulation (Fig. 7G), while the other three subjects displayed much higher maximal slow phase velocities (11 deg/s, S1; 24 deg/s, S2; 37 deg/s, S4). In addition, in all subjects there is an increase in horizontal slow phase velocity with increasing stimulus rate, although the dynamic range of the rates that produce this effect is somewhat different in each subject. Subjects S2 and S4 show a clear relationship between either stimulation current or rate and horizontal slow phase velocity, but the dynamic range of rates is limited to between 75 and 300 pps (Fig. 7, D and J). Stimulation at higher rates of 450 or 600 pps produced either the same or lower horizontal slow phase velocities as 300 pps. In subject S1, the range of pulse rates that was evaluated was limited, but stimulation at 600 pps produced slightly higher horizontal slow phase velocities than stimulation at either 300 or 450 pps (Fig. 7A). In subject S3, horizontal slow phase velocities in response to stimulation at 600 pps were higher than those at 300 pps, but the response velocities were very low (Fig. 7G).
Fig. 7.
Average slow phase eye velocity vs. stimulation current at different stimulus rates for 2 s trains of biphasic pulses applied to the right lateral canal on the 1st postsurgical test day. Rows show data from different test subjects. Columns from left to right display horizontal slow phase velocity vs. current, vertical slow phase velocity vs. current, and a polar plot of 2D eye velocity vs. current. Negative values indicate leftward or downward slow phase velocity. Error bars display ± 1 SD of the slow phase velocity of all slow phases collected over all trials.
The vertical slow phase eye velocities that were evoked by right lateral canal stimulation were lower than the horizontal slow phase eye velocities at all amplitudes and rates of stimulation except in subject S3, and they had a higher current threshold (Fig. 7, B, E, K). The maximal vertical slow phase eye velocities elicited were 2 deg/s in S1, 12 deg/s in S2, 3 deg/s in S3, and 9 deg/s in S4. Since the thresholds were different between the horizontal and vertical velocity components for subjects S1, S2, and S4, the vector eye velocities changed direction somewhat with stimulation current, such that low current stimulation produced largely horizontal eye movements in the canal plane, whereas higher current stimulation produced oblique trajectories of slow phase eye movement, leftward and upward (Fig. 7, C, F, L). These later trajectories may have been due to current spread to the superior canal ampullar nerve afferents at higher currents. The trajectories for subject S3 (Fig. 7I) are highly variable given the relatively low velocities of the elicited slow phase eye movements.
To assess whether the observed changes in slow phase velocity were statistically significant across currents we performed a linear regression analysis at each pulse rate for each lateral canal. The slope of the linear regression of the horizontal slow phase velocity vs. current relationship was significantly different from zero (P ≤ 0.05) for all lateral canals at all pulse rates except for the lateral canal of subject S3 at 600 pps. The slope of the vertical slow phase velocity vs. current relationship was significantly different from zero (P ≤ 0.05) for all lateral canals at all pulse rates except for the lateral canal of subject S1 at 450 and 600 pps and the lateral canal of subject S4 at 75 pps.
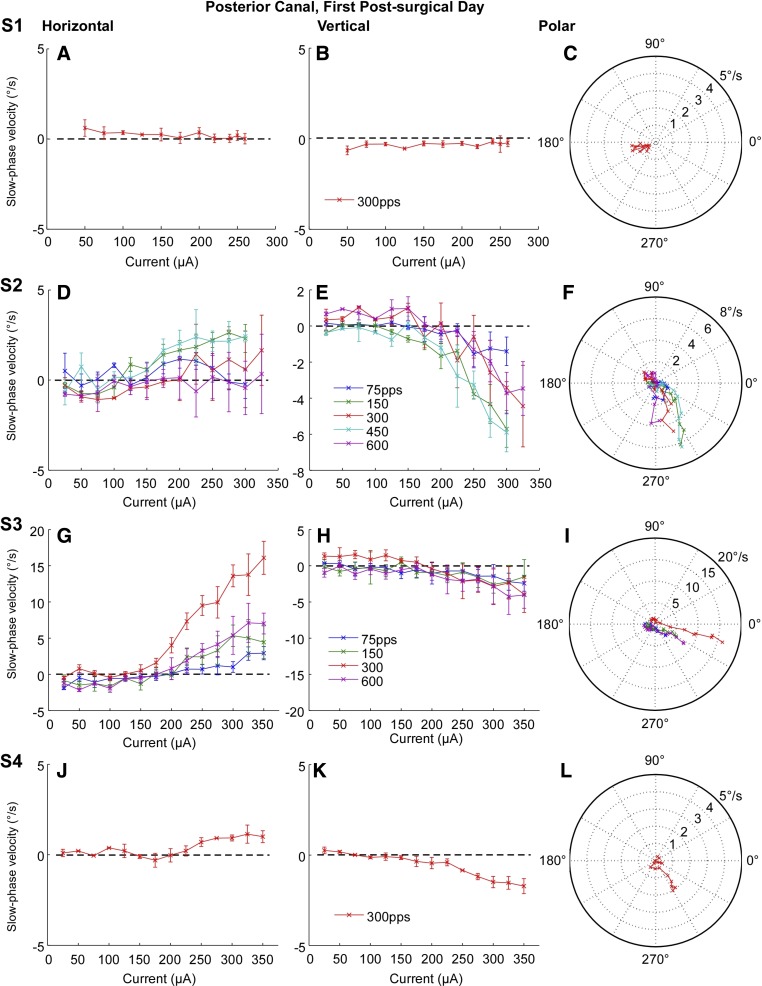
Comparable average slow phase eye velocity data was also obtained for posterior canal electrical stimulation in all subjects on the first postsurgical test day (Fig. 8). Posterior canal stimulation was ineffective in eliciting any slow phase eye velocity in subject S1 (Fig. 8, A and B). In the remaining subjects, posterior canal stimulation did elicit downward and rightward slow phase velocity in varying proportions (Fig. 8, D, E, G, H, J, K). The elicited slow phase eye velocities were largely downward in subjects S2 and S4 (Fig. 8, F and L), but the slow phase eye velocities elicited in subject S4 were very low. In subject S3, there was considerable slow phase eye velocity, but it was largely rightward with a smaller downward component (Fig. 8, G and H). Maximal average slow phase eye velocities were lower for posterior canal stimulation than for lateral canal stimulation in three of four subjects. Maximal downward average slow phase eye velocities were 6 deg/s in S2, 5 deg/s in S3, and 2 deg/s in S4 and maximal rightward average slow phase velocities were 3 deg/s in S2, 15 deg/s in S3, and 1.5 deg/s in S4. The thresholds for activation of horizontal and vertical slow phase eye velocity were roughly equivalent in subjects S3 and S4, resulting in consistent direction of motion across a range of currents (Fig. 8, I and L).
Fig. 8.
Average slow phase eye velocity vs. stimulation current at different stimulus rates for 2 s trains of biphasic pulses applied to the right posterior canal on the 1st postsurgical test day. Rows show data from different test subjects. Columns from left to right display horizontal slow phase velocity vs. current, vertical slow phase velocity vs. current, and a polar plot of 2D eye velocity vs. current. Negative values indicate leftward or downward slow phase velocity. Error bars display ± 1 SD of the slow phase velocity of all slow phases collected over all trials.
To assess whether the observed changes in slow phase velocity were statistically significant across currents we performed a linear regression analysis at each pulse rate for each posterior canal. The slope of the linear regression of the vertical slow phase velocity vs. current relationship was significantly different from zero (P ≤ 0.05) for all posterior canals at all pulse rates. The slope of the linear regression of the horizontal slow phase velocity vs. current relationship was significantly different from zero (P ≤ 0.05) for all posterior canals at all pulse rates except for the posterior canal of subject S2 at 75 pps.
The superior semicircular canal was implanted in three subjects, and electrical stimulation of that canal produced upward average slow phase eye velocity in each subject (Fig. 9, B, E, H). The maximal upward slow phase eye velocity was 8 deg/s in subject S1, 6 deg/s in S3, and 3 deg/s in S4. Subjects S3 and S4 had horizontal eye velocity that was rightward in subject S3 (Fig. 9D) and leftward at the highest currents in subject S4 (Fig. 9H). The directions of the vector velocities were fairly consistent across different stimulation currents, but inconsistent between subjects (Fig. 9, C, F, I). Subjects S1 and S4 had largely upward directed slow phases, while subject S3 had primarily rightward-directed eye movement. The current thresholds for activation of slow phase eye movement were ∼225 to 250 μA in subjects S1 and S4. Subject S3 displayed some slow phase velocity at all current levels, suggesting a suppression of underlying spontaneous nystagmus during all stimulation trials (Fig. 9E).
Fig. 9.
Average slow phase eye velocity vs. stimulation current at different stimulus rates for 2 s trains of biphasic pulses applied to the right superior canal on the 1st postsurgical test day. Rows show data from different test subjects. Note that the superior canal was not implanted in subject S2. Columns from left to right display horizontal slow phase velocity vs. current, vertical slow phase velocity vs. current, and a polar plot of 2D eye velocity vs. current. Negative values indicate leftward or downward slow phase velocity. Error bars display ± 1 SD of the slow phase velocity of all slow phases collected over all trials.
To assess whether the observed changes in slow phase velocity were statistically significant across currents we performed a linear regression analysis at each pulse rate for each superior canal. The slope of the linear regression of the vertical slow phase velocity vs. current relationship was significantly different from zero (P ≤ 0.05) for all superior canals at all pulse rates. The slope of the linear regression of the horizontal slow phase velocity vs. current relationship was significantly different from zero (P ≤ 0.05) for all superior canals at all pulse rates except for the superior canal of subject S1 at 450 and 600 pps.
Longitudinal vestibulo-ocular slow phase eye movements with electrical stimulation.
Electrical stimulation produced relatively robust slow phase eye movements initially, but it was unclear that such eye movements would be sustained for long periods in our human subjects. To assess the longitudinal efficacy of electrical stimulation, we performed repeated stimulations on multiple days in each subject. We noted the average slow phase velocity elicited by stimulation at a fixed rate of 300 pps across a range of currents on each test day. There is an important caveat here, which is that these results describe only intermittent stimulation with the implanted stimulator. Although the subjects were instructed to regularly activate all channels of the stimulator at very low current levels several times a week at home for periods of several minutes, they were not required to log such activations. The stimulation current and frequency of the at-home stimulation map and settings were fixed such that those settings during stimulation in the laboratory failed to produce any sensation, or any slow phase eye movements in the dark or light, at any time. Earlier experiments in rhesus monkeys suggest that higher current intermittent activation such as we performed in the laboratory during test sessions was sufficient to drive robust responses for a year or more, with little decrement in velocity (Phillips et al. 2015, see also Golub et al. 2014, supplemental figures for an example).
Based on intermittent activation in human subjects, we observed that lateral canal average slow phase velocity generally decreased over time in all subjects. Data were collected on weeks 2, 63, 87, 116 and 130 postsurgery in subject S1, on weeks 2, 4, 9, 25, 32, and 52 postsurgery in subject S2, on weeks 2, 15, 24, and 42 postsurgery in subject S3 and on weeks 1, 6, 17, and 31 postsurgery in subject S4 (Fig. 10). In subject S1, there was a decrease in slow phase velocity between weeks 2 and 63, but a significant recovery in slow phase eye velocity at higher currents between weeks 63 and 130. In subject S2, there was a progressive decrease in electrically elicited average slow phase velocity at each current level with time over the first 9 wk postsurgery, and then there was a very small recovery in slow phase velocity between weeks 9 and 52. In subject S3, there was minimal response to stimulation at 2 wk postsurgery, and this response further reduced at later time points up to 42 wk postsurgery. In subject S3, there was a reduction in slow phase velocity between 1 wk and 17 wk postsurgery, and then there was a recovery in slow phase velocity by week 31. Responses were still elicited in three of four subjects at the highest current at the end of longitudinal recording, with moderate to low slow phase velocities (8 deg/s, S1, week 130; 3 deg/s, S2, week 52; and 21 deg/s, S4, week 31; Fig. 10, A, C, G). The response in subject S3 was very weak and highly variable across currents and was in the wrong direction, slow phase right, at the highest current, on the last week of recording (week 42). To assess the significance of these results, we performed paired t-tests of slow phase eye velocities elicited at 300 pps in the lateral canal of each subject at the highest current available on the first and last recording weeks. Horizontal velocity differences were significant (P ≤ 0.05) in three of four subjects. Vertical velocity differences were significant (P ≤ 0.05) in 3/4 subjects. Subject S1 showed no statistically significant difference in either horizontal or vertical slow phase velocities between the first and last week of recording, although there were changes during the intervening weeks.
Fig. 10.
Superimposed traces of average slow phase eye velocity vs. stimulation current for 2 s trains of biphasic pulses applied to the right lateral canal at different time points postsurgery. Rows show data from different test subjects. Columns from left to right display horizontal slow phase velocity vs. current and vertical slow phase velocity vs. current. Negative values indicate leftward or downward slow phase velocity. Error bars display ± 1 SD of the slow phase velocity of all slow phases collected over all trials.
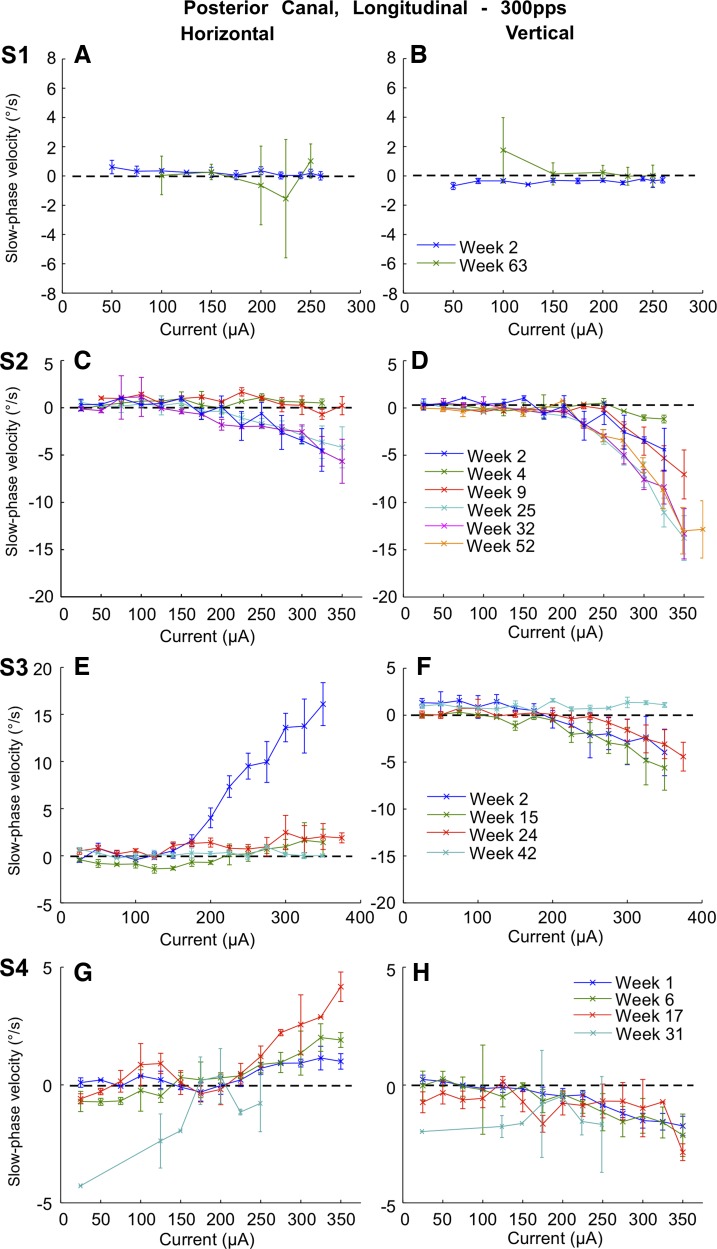
Posterior canal average slow phase velocities showed highly variable changes with time across the four subjects. Data were collected on days in weeks 2 and 63 postsurgery in subject S1, on weeks 2, 4, 9, 25, 32, and 52 postsurgery in subject S2, on weeks 2, 15, 24, and 42 postsurgery in subject S3, and on weeks 1, 6, 17, and 31 postsurgery in subject S4 (Fig. 11). There was little change overall in the slow phase velocity in the vertical dimension in subject S3 through week 24, but there was a complete loss of vertical slow phase velocity by week 42 in that subject (Fig. 11H). In contrast, there was an immediate (by week 6) and dramatic decrease in the rightward slow phase velocity in the same subject (Fig. 11E). In subject S4, there was an increase in rightward slow phase velocity through week 17, followed by a decrease and change in direction of the horizontal slow phase velocity by week 31 (Fig. 11G). The slow phase velocities in both dimensions were dramatically increased in subject S2 by 25 wk postsurgery and were largely sustained through 52 wk postsurgery. In this subject, the maximum downward slow phase velocities increased from 1.5 deg/s at 4 wk postsurgery, to 7 deg/s at 9 wk postsurgery, and to 13–14 deg/s at 25, 32, and 52 wk postsurgery. This was the only canal that showed such an increase with time. Subject S1 had little or no response to stimulation on either week 2 or week 63. To assess the significance of the longitudinal results, we performed paired t-tests of slow phase eye velocities elicited at 300 pps in the posterior canal of each subject at the highest current available on the first and last recording weeks. Vertical velocity differences were significant (P ≤ 0.05) in 3/4 subjects. Horizontal velocity differences were significant (P ≤ 0.05) in 3/4 subjects. Subject S1 showed no statistically significant difference in either horizontal or vertical slow phase velocities between the first and last week of recording.
Fig. 11.
Superimposed traces of average slow phase eye velocity vs. stimulation current for 2 s trains of biphasic pulses applied to the right posterior canal at different time points postsurgery. Rows show data from different test subjects. Columns from left to right display horizontal slow phase velocity vs. current and vertical slow phase velocity vs. current. Negative values indicate leftward or downward slow phase velocity. Error bars display ± 1 SD of the slow phase velocity of all slow phases collected over all trials.
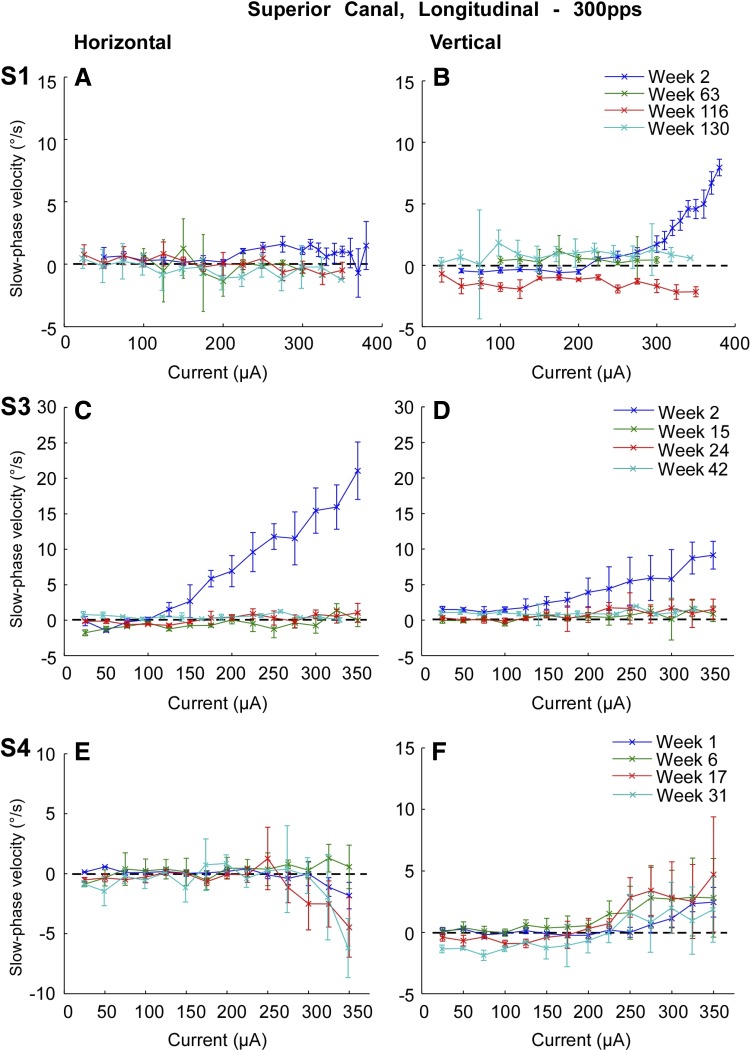
Superior canal average slow phase velocities decreased in two subjects, S1 and S3, and increased slightly in a third subject, S4 (Fig. 12). Data were collected on weeks 2, 63, 116, and 130 postsurgery in subject S1, on weeks 2, 15, 24, and 42 postsurgery in subject S3, and on weeks 1, 6, 17, and 31 postsurgery in subject S4. In subject S4, maximal slow phase velocities at the highest current reached 4 deg/s at 350 μA at 17 weeks postsurgery and fell to 2 deg/s by 31 wk postsurgery (Fig. 12F). At the same time, the leftward slow phase velocities increased from 2 to 6 deg/s (Fig. 12E). To assess the significance of the longitudinal results, we performed paired t-tests of slow phase eye velocities elicited at 300 pps in the superior canal of each subject at the highest current available on the first and last recording days. Vertical velocity differences were significant (P ≤ 0.05) in 3/4 subjects. Horizontal velocity differences were significant (P ≤ 0.05) in all subjects. Subject S1 showed no statistically significant difference in either vertical slow phase velocities between the first and last week of recording.
Fig. 12.
Superimposed traces of average slow phase eye velocity vs. stimulation current for 2 s trains of biphasic pulses applied to the right superior canal at different time points postsurgery. Rows show data from different test subjects. Note that the superior canal was not implanted in subject S2. Columns from left to right display horizontal slow phase velocity vs. current and vertical slow phase velocity vs. current. Negative values indicate leftward or downward slow phase velocity. Error bars display ± 1 SD of the slow phase velocity of all slow phases collected over all trials.
To assess whether repeated 2 s trials of electrical stimulation produced a decrease in the observed slow phase velocity of the elicited eye movements on each trial day in each subject, we compared the responses to repeats of the same stimulus (first and last 2 s trials) during the course of each session. The results showed that there was no significant difference in slow phase velocities between the first and last trials with the same stimulus parameters in any of our subjects by a paired t-test (P > 0.05).
Taken together, these data show that for these four subjects with Meniere's disease, there was a general decrease over time in the slow phase velocity elicited by electrical stimulation in most canals. Such changes were not observed with repeated trials on each day of stimulation. Some canals showed fluctuations in slow phase velocity over several test days. The notable exception to this was the posterior canal in subject S2, which showed a remarkable increase in average slow phase velocity with increasing current over the first 32 wk postsurgery. Since the electrode impedances remained sufficiently low for the voltage compliance of our device in all subjects (Table 2), it is unlikely that the device was failing to deliver appropriate current. Rather, we hypothesized that the reason for the reduction in slow phase velocity, and a similar reduction in the amplitude of the vECAPs (Fig. 5), was that the current was now being shunted away from the ampulla of the implanted canal. To test this hypothesis, we performed a cross canal stimulation experiment.
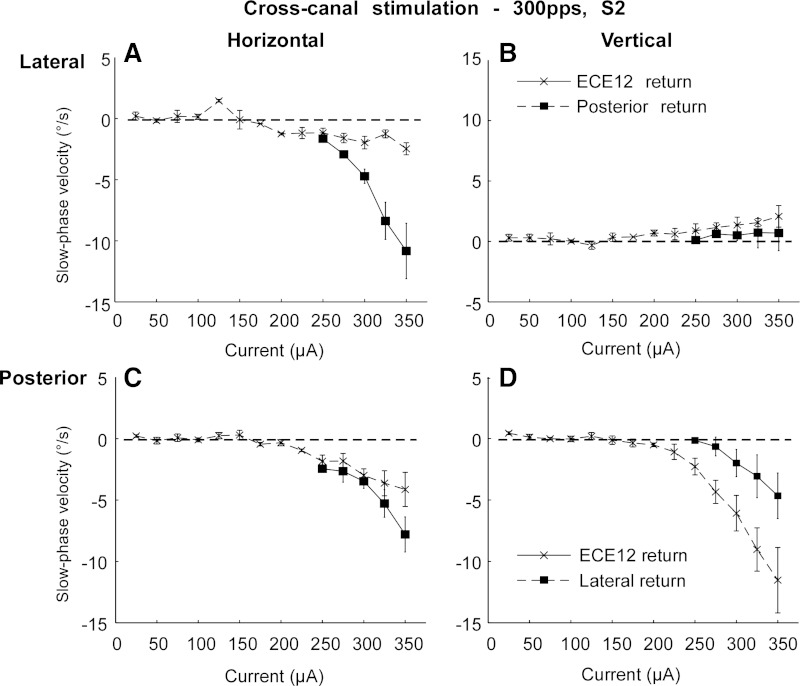
Effect of cross canal electrical stimulation.
All of the electrical stimulation data presented above were collected during monopolar stimulation from the most distal tip electrode of each implanted array, with a combination of a remote ground and the case ground of the receiver/stimulator as returns. In addition to this stimulation paradigm, in subject S2 on week 25, we also performed stimulation between the distal electrode in one canal with the return in an adjacent canal. We hypothesized that this stimulation could steer the current toward the ampulla, thereby increasing recruitment of ampullar afferent fibers of the implanted canal. The result of this stimulation experiment is displayed in Fig. 13. In this subject, lateral canal stimulation with a remote ground produced a very weak response even at high currents at this late time point (Fig. 13, A and B). However, stimulation with a return path within the implanted labyrinth, using the distal tip electrode of the posterior canal array as the return, restored the electrically elicited horizontal slow phase velocity. The maximal horizontal slow phase velocity of the response went from 3 deg/s to 12 deg/s. Furthermore, the off-direction vertical velocity remained very low, increasing from a maximal average slow phase velocity of <1 deg/s to 3 deg/s at the same current. This result was consistent with our hypothesis that the current was available but was not appropriately directed with monopolar stimulation at the later time points.
Fig. 13.
Average slow phase velocity vs. current for monopolar electrical stimulation and for cross canal stimulation in subject S2. A: horizontal average slow phase velocities vs. stimulation current for stimulation of the distal site of the right lateral canal array with a return to either a remote ground and case ground (ECE12 return) or to the distal site of the right posterior canal array (Posterior return). B: vertical average slow phase velocities for stimulation as in A. C: horizontal average slow phase velocities vs. stimulation current for stimulation of the distal site of the right posterior canal array with a return to either a remote ground and case ground or to the distal site of the right lateral canal array (Lateral return). D: vertical average slow phase velocities for stimulation as in C. Negative values indicate leftward or downward slow phase velocity. Error bars display ± 1 SD of the slow phase velocity of all slow phases collected over all trials.
To test the generality of this observation, we also performed the reverse experiment, where we stimulated the posterior canal tip electrode using a return path through the lateral canal tip electrode, and compared that result with monopolar stimulation. Monopolar electrical stimulation in the posterior canal produced high slow phase velocities at week 17 in subject S4 (Fig. 13, C and D). Stimulation with the lateral canal electrode as the return produced a reduced response in both dimensions. Therefore, in the electrode that was performing optimally with monopolar stimulation, the new current path reduced the observed slow phase velocity response, whereas, in the electrode that was performing poorly, the new current path greatly enhanced, and largely restored, the response.
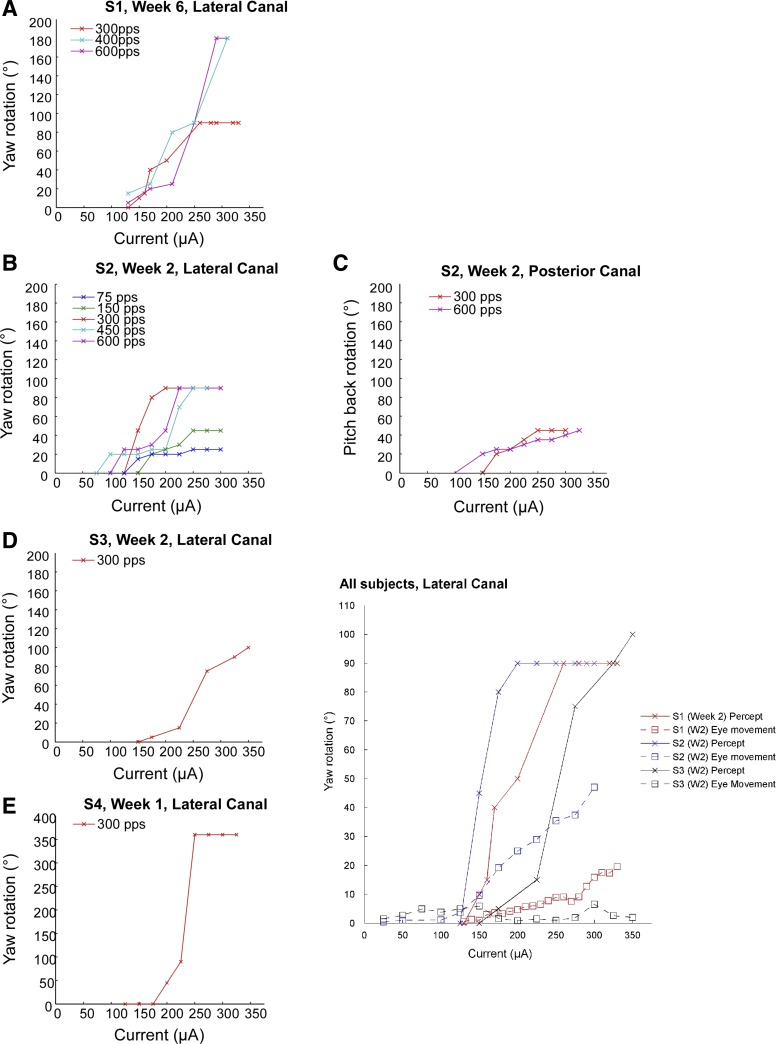
Perceptual responses to electrical stimulation of the semicircular canals.
All subjects provided extensive descriptions of their subjective experience during electrical stimulation of their semicircular canals. Three of the four subjects, subjects S1, S2, and S3, also provided verbal estimates of any perceived motion during electrical stimulation. None of the subjects experienced nausea or electrically evoked auditory percepts in association with the electrical stimulation. Two subjects experienced a sensation of high frequency vibration, which was primarily present at low current amplitude or low pulse rate. This could have been due to activation of irregular otolith afferents, which can follow vibration frequencies up to very high rates (Curthoys, 2006). Such afferents would be expected to have low galvanic thresholds, consistent with this initial sensation (Goldberg et al. 1982, 1984). As the current was increased, these sensations generally gave way to sensations of whole body motion. Two subjects additionally experienced a sensation of pain when stimulated at higher currents during the last recording sessions. In both subjects, the canals that were stimulated no longer elicited slow phase eye movements.
Subject S1 described different sensations on week 2 depending on the stimulated canal. Right lateral canal stimulation produced a sensation of roll right with low levels of electrical stimulation. This may have been due to activation of the utricle in the right side, but this was not possible to determine unambiguously with our monocular 2D recording of eye movement. The threshold for this movement sensation was 140 μA, which corresponded to a threshold of 160 μA to elicit slow phase eye movements during the same stimulation. The sensation of roll right increased in amplitude to roughly 15 deg with increasing current to 220 μA and thereafter remained static during stimulation at higher currents. The subject experienced a reverse roll left to upright at the end of the 2s stimulus. At a threshold of 130 μA, subject S1 began to experience a sensation of rightward yaw rotation combined with a fixed 15 deg roll. The rightward yaw sensation increased in velocity and amplitude over the 2s stimulation. This sensation was preserved over time and was present on week 6 as shown in Fig. 14, which plots perceived rotational amplitude vs. stimulation current for three subjects. Figure 14A shows that perceived yaw rotational amplitude increased with increasing current in subject S1, with a threshold of 130 μA. There was not a clear relationship of stimulus rate and perceived rotation except at the higher currents, where 300 pps stimulation for 2s produced a smaller perceived rotation than 450 or 600 pps stimulation. The perceived rotational amplitude greatly exceeded the amplitude calculated from integration of eye velocity over the same 2 s period. For example, at 300 μA and 600 pps, the subject estimate 180 deg of perceived rotation, corresponding to an average rotational velocity of 90 deg/s (Fig. 14A). This compares to an average horizontal slow phase eye velocity of 10 deg/s during the same stimulation. In this subject, the end of stimulation was accompanied with a perceived rotation back to the original prestimulation body position.
Fig. 14.
Verbally reported perceived amplitude of rotation vs. current for 2 s trains of single canal electrical stimulation at different stimulus rates on the 1st test day in all subjects. A, B, D, E: perceived yaw rotation amplitude leftward vs. current for right lateral canal stimulation in subjects S1, S2, S3, and S4. C: perceived pitch rotation amplitude backward vs. current for posterior canal stimulation in subject S2. Inset: perceived yaw rotation and cumulative eye movement amplitude vs. current for 2 s trains of electrical stimulation at 300 pps for 3 subjects.
Subject S1 also experienced a sensation of rotation with electrical stimulation of the right superior canal on week 2. The sensations were not described quantitatively, but the subject described being pushed forward and down to the left. The sensation, which had a threshold of 120 μA, increased in intensity with increasing current and was limited to the duration of the stimulation. Following the end of the 2 s stimulation train, subject S1 described a complementary rotation in the same plane back to upright. Stimulation of the posterior canal in subject S1 produced no sensation of movement but, rather, produced a sensation of pressure at the site of the surgical incision.
Subject S2 experienced primarily right yaw rotation in response to right lateral canal stimulation on week 2 postsurgery. The perceived rotation had a current threshold that ranged from 100 to 175 μA depending on stimulation pulse rate. As with subject S1, the yaw rotation amplitude in subject S2 increased with increasing stimulation current (Fig. 14B). Changes in stimulus rate produced different perceived rotational velocities, but the relationships were not predictable. Unlike subject S1, subject S2 did not perceive a return to initial position after each stimulation. Rather, this subject perceived sequential rotations with serial stimulation trials, resulting in a perception of complete rotations about a vertical axis after multiple stimulation trials.
Subject S2 also experienced a sense of rotation in response to 2 s stimulation of the posterior canal. In this case, the subject reported a sensation of backward pitch rotation. The amplitude of the rotation scaled with stimulation current (Fig. 14C) and had a threshold of between 140 to 160 μA, depending on stimulus rate. Subject S2 reported the perception of a complementary rotation to upright following each stimulation trial. The amplitude of the perceived pitch and yaw rotation exceeded the amplitude calculated from the integrated velocity of the elicited slow phase eye movements.
Despite displaying only very minimal slow phase eye movement velocity with electrical stimulation of the lateral canal on postsurgery week 2, subject S3 reported a large yaw rotation to the right with electrical stimulation at several currents. The rotations reached a maximal value of 100 deg for 2 s stimulation at a current of 350 μA; e.g., 50 deg/s average velocity. The threshold for perceived rotation was at 155 μA. These data suggest that the electrical stimulation can affect either the VOR or vestibular perception, typically both, in a predictable manner. Increasing current produces increased sensation of movement and increased slow phase eye velocity on average, although in some instances only one effect is produced. In both cases, the directions of either eye or perceived movement are consistent with the directionality of the implanted and stimulated semicircular canal. This can be seen in Fig. 14, inset, which displays the relative cumulative slow phase eye amplitudes and the associated perceived whole body rotations for lateral canal stimulation at 300 pps in subjects S1, S2, and S3.
DISCUSSION
The results of this study replicate, in humans, many of the features of electrical stimulation in the perilymphatic space in individual semicircular canals of animals. However, there are differences that may have important implications for the further development of this technology.
In this study, we observed that electrical stimulation of individual canals using implanted electrodes appears well tolerated by human subjects. This is similar to observations with identical devices in animals (Rubinstein et al. 2012) and is also similar to the results of experiments using surface galvanic stimulation and prosthetic stimulation in human subjects (Golub et al. 2014; Guyot et al. 2011a,b; Nguyen et al. 2014; Perez Fornos et al. 2014; Phillips et al. 2013; St George and Fitzpatrick 2011; Van De Berg et al. 2012; Wall et al. 2007). The subjects in this experiment did not report significant pain, nausea, or auditory sensations as a result of stimulation that produced clear slow phase eye movements. At later test dates, two subjects did report pain with stimulation at two electrode sites. On these dates, stimulation did not elicit eye movements, although it had previously.
We also observed that constant pulse rate and current amplitude electrical stimulation over short periods appears to produce relatively constant velocity slow phase eye movement. This is similar to the results of electrical stimulation in rhesus monkeys implanted with identical (Phillips et al. 2015; Rubinstein et al. 2012; see also Golub et al. 2014, supplementary figures) or similar (Davidovics et al. 2013) devices. This is different than results obtained in other species where sustained slow phase velocities are not obtained, but rather brief transient slow phase eye movements are recorded (Davidovics et al. 2013).
In these experiments, slow phase eye velocity typically scaled with stimulus current amplitude. This is a consistent finding in animal models (Davidovics et al. 2011, 2013). The slow phase velocities that were obtained were far lower than those produced by electrical stimulation in nonhuman primates (e.g., Davidovics et al. 2013; Phillips et al. 2015) and varied greatly between canals. However, the slow phase velocities that we observed for brief lateral canal stimulation are comparable to the velocities obtained by Perez Fornos et al. (2014) for sinusoidal current amplitude modulated stimulation in human subjects. It is well known that precise placement of the electrodes is critical to the efficacy of such stimulation (Lewis et al. 2001, 2002, 2010; Nie et al. 2011). Similar to our previous monkey experiments, we used vECAPs to guide placement of our electrodes. However, the vECAP response amplitudes were also far smaller than those recorded in nonhuman primates at comparable currents (Nie et al. 2011; Phillips et al. 2012). This suggests that in the larger ear, higher currents or different placement strategies may be required to drive responses comparable to those in nonhuman primates. Since our device was constructed with very small stimulation sites, and we used very conservative charge-density limits in our studies, our ability to apply the higher currents required to explore this question was limited.
Another observation of this study was that the slow phase eye movement components produced during electrical stimulation in human subjects, which were directionally inconsistent with those expected with eye rotations in the canal plane, were quite large. This result is different than the responses elicited in rhesus monkeys (Dai et al. 2013; Golub et al. 2014; Phillips et al. 2015), and may have been due to the higher currents typically used to elicit the responses. Such higher currents would be expected to produce more current spread to adjacent canals, which would then activate afferents with different directional sensitivities. However, several groups have shown in animals that adaptation of the VOR to sustained electrical stimulation (Fridman et al. 2010; Gong et al. 2008; Lewis et al. 2010; Merfeld et al. 2007) can produce a reduction in the misalignment of the eye (Dai et al. 2013; Davidovics et al. 2013; Fridman et al. 2010). Of course, our inferences about the specific direction of the elicited eye movements are only approximate, since we used 2D VOR to record eye movement and therefore could not completely define the plane of the observed movements as has been done with 3D coil recording in animal models (e.g., Dai et al. 2011; Davidovics et al. 2013).
A surprising finding was that pulse rate was far less effective than current amplitude in modulating slow phase velocity in the human subjects, whereas it is highly effective in animals (Davidovics et al. 2013). Previous experiments have shown that both current modulation and pulse rate modulation work well in modulating slow phase velocity. In a human subject, Guyot et al. (2011) stated that pulse rate modulation was effective in modulating slow phase eye velocity over small amplitudes (≤0.5°) from an electrode implanted close to the left posterior ampullar nerve. Indeed, some authors have proposed a combined amplitude and rate modulation strategy for modulation of slow phase velocity (Davidovics et al. 2012). In the initial experiments in subject S1, we only evaluated this at the higher pulse rates, well above the natural sustained discharge rates of the afferents but less than their expected peak discharge rates, where one would be less likely to observe an effect due to velocity saturation. However, in later experiments in the other subjects, where we did observe an effect of pulse rate on slow phase velocity by varying rate across a larger range, there was often not a monotonic relationship between rate and slow phase velocity. This was a surprising finding, considering the fact that rate modulation of action potentials in vestibular afferents is the primary code for relaying head velocity information to the brain. Based on the vECAP data presented here and in nonhuman primates, it would appear that recruitment of increasing numbers of afferent fibers with increasing current amplitude occurs in both monkeys and humans; i.e., the vECAP increases with increasing current in both species (Nie et al. 2011; Phillips et al. 2012; Rubinstein et al. 2012). Since this increase in the compound action potential occurs in parallel with an increase in slow phase velocity, it is reasonable to postulate that recruitment of afferent fibers underlies the increasing velocity of slow phase eye movement seen with comparable current increases. Recruitment is not often a critical feature of the natural discharge of vestibular afferents due the their tonic resting rate, so this mode of modulating slow phase velocity is rather aphysiological: driving the system with constant rate input but modulating the number of afferents contributing to the response.
In the experiments reported here, we hypothesize that the eye movement responses of subjects are due primarily to direct activation of the afferent fibers and not mediated through activation of hair cells. The reason for this is that the clinical vestibular testing suggests that the natural hair cell transduction mechanism is nonfunctional in the implanted ear of all of our subjects. We believe that this was due to changes in hair cell function and not due to occlusion of the membranous labyrinth, because in the smaller canals of monkeys this did not occur with implantation (Rubinstein et al. 2012). While this does not rule out a contribution from electrical stimulation of nonfunctional but electrically responsive hair cells, this seems unlikely. The rotary chair and caloric tests, as well as the HTT and DVA testing, all indicate a loss of lateral canal function. Indeed, the subjective visual vertical tests imply, but cannot conclusively demonstrate, that the subjects experienced a loss of balanced utricular input to central nervous system as well. We feel that this is significant because although the devices were designed to preserve vestibular function and hearing similar to the results of studies in nonhuman primates (Bierer et al. 2012; Rubinstein et al. 2012), and the study was designed to evaluate the function of the device in subjects with preserved but intermittently dysfunctional vestibular input, the implantation of the device appears to have produced a clear loss of function unilaterally, with preservation of electrically elicited behavioral and perceptual responses.
We do not know the reason for the loss of natural hearing and balance function in our subjects, but one possibility is that violation of the fluid space of the membranous labyrinth produced a slow loss of fluid and damage to the end organ (Parnes and McClure 1990, 1991). Indeed, the presence of Meniere's disease in these subjects may have made the inner ear more susceptible to surgical injury (Luetje 1992). We should emphasize here that there are multiple approaches to allow implantation of stimulating electrodes for stimulation of the vestibular nerve, including placement of electrodes in the canal adjacent to the ampulla (Nie et al. 2011, 2013, Perez Fornos et al. 2014; Phillips et al. 2012, Rubinstein et al. 2012), in the ampulla itself (Davidovics et al. 2011, 2012, 2013; Fridman et al. 2010; Gong et al. 2008; Gong and Merfeld 2000, 2002; Lewis et al. 2002, 2010; Merfeld et al. 2006, 2007; Tang et al. 2009), adjacent to the individual ampullar nerves (Feigl et al. 2009; Guyot et al. 2011a,b; van de Berg et al. 2012; Wall et al. 2007), and even implantation in Scarpa's ganglia. Each approach has theoretical advantages and disadvantages. Other surgical techniques, not used here, may place the end organ at less surgical risk and may minimize the loss of natural function that we observed.
One of the more interesting aspects of the results reported here is that the efficacy of electrical vestibular stimulation in these four human subjects with Meniere's disease changed significantly over time as assessed with either vECAP or with slow phase eye velocity. The vECAP amplitudes resulting from a given current in most, but not all, canals decreased significantly over time. Such decreases could reflect changes either in the activation of targeted vestibular afferents or in the activation of other nearby neurons. Similarly, the slow phase velocities elicited by electrical stimulation also tended to decrease or fluctuate over time. This is an important observation, because it suggests that there are changes in galvanic sensitivity of individual afferents as reflected by the evoked potential, and concurrent changes in the velocity signal delivered to the brain. There are many potential explanations for these changes, which were not seen consistently following implantation of identical devices in either intact or lesioned nonhuman primates in our laboratory (Golub et al. 2014).
The first possibility is that the electrodes became covered or damaged over time in our human subjects. This seems unlikely, however, since longitudinal recording of electrode impedances suggested that these remained relatively constant over time post surgically, at least in three of our subjects. A second possibility is that the electrically elicited responses remained unchanged in the nerve but adapted centrally over time. Such a phenomenon is clearly present in primates and other species (Dai et al. 2011c, 2013; Davidovics et al. 2013; Fridman et al. 2010; Gong et al. 2008; Lewis et al. 2010, 2013; Merfeld et al. 2007). This seems somewhat unlikely since the subjects were not exposed to continuous stimulation with the device in these trials, and the test stimuli were of short duration. However, the subjects were receiving regular intermittent stimulation at home. Another possibility is that the device failed to deliver consistent stimulus current over time. While this is possible, it is unlikely because the changes in velocity occur idiosyncratically from canal to canal in individual subjects. Furthermore, we demonstrated that the same electrodes, when used with a different return path (e.g., cross canal stimulation), produces a robust response after the monopolar response is dramatically reduced. Another possibility is that the pathophysiology of Meniere's disease produces changes in the afferents or in the immediate electrical environment around the afferents. Since our surgery may have produced an exaggerated biological response in the Meniere's ear, it is certainly possible that this produced changes to the afferent fibers that we stimulate electrically. Of course, it is also possible that the electrodes migrated in some canals or became dislodged or covered in bone. A small movement of the implanted electrode array could dramatically reduce the effect of electrical stimulation in that canal.
Another possibility is that the device became less effective because, when the afferents were deprived of input from the hair cells postsurgically, they retracted toward the cell bodies in Scarpa's ganglion; i.e., the device became ineffective because the afferents were no longer present to be activated by a local electrical field. It is well known that disease and surgical damage to the end organ can result in a loss of afferent fibers in the ampullae, and a die-back of afferents toward Scarpa's ganglia (Cass et al. 1989; Gacek 1974; McCall et al. 2009; Schuknecht 1982; Tsuji et al. 2000a,b; Velázquez-Villaseñor et al. 2000). No device that seeks to differentially activate individual ampullar nerves will remain functional if the afferent fibers die back to the ganglion, so this is a potentially serious concern.
In the cochlea, the cell bodies of the afferent fibers are localized in the spiral ganglion, which is anatomically close to the hair cells of the end organ and has a frequency map that is at least monotonically related to that of the nearby receptors. Also, it is close to the electrode array of the implanted receiver stimulator of a cochlear implant. In animals, Scarpa's ganglia are located farther away from the ampullae of the implanted canals and they contains neurons that have a variety of directional specificities, with separate afferents from a single ganglion innervating one of two to four otolith or semicircular canal end organs. In humans, who are far larger than the animals tested to date, the ganglia are also quite distant from the canals. Electrical stimulation of the end organ or ampullar nerves may fail to drive afferent fibers if they retract from the ampullae. While the nonampullar approaches to electrode placement would tolerate more retraction of afferent fibers, they do not overcome this limitation entirely. If the afferents retract fully to the ganglia, then the directional specificity of electrical stimulation theoretically becomes quite problematic.
One piece of evidence that argues against the overall loss of afferent fibers in the ampullae was the fluctuation in slow phase eye velocity that was seen in all subjects postsurgically. This seems to suggest that the afferent fibers were present, but intermittently functional. Also, in several canals we observed similar fluctuation of the vECAP. Also, we have no direct evidence of changes in afferent innervation, or whether if it is occurring, it is primarily due to the pathophysiology of Meniere's disease (Tsuji et al. 2000a). Furthermore, since we limited our study to intermittent stimulation with the implanted device, we may have eliminated the potential benefits of continuous electrical stimulation to maintaining afferent sensory projections that have been demonstrated in the auditory system (e.g., Leake et al. 1999). Also, adaptation experiments in monkeys (Merfeld et al. 2006, 2007) and in a human subject (Guyot et al. 2011a) have shown that long duration constant frequency electrical stimulation that elicits a behavioral response initially will produce a reduced response over time. In humans, this time period seems to be limited to 18 h, which is far less than the period between our stimulation experiments. Although our intermittent 2 s trains of stimulation are of much shorter duration, it is possible that the electrical stimulation performed in the laboratory or by our subjects at home provided such an adapting stimulus or produced habituation of the response. However, the stimulation performed in the laboratory did not show any change in efficacy between the first and last trials of randomly delivered 2 s stimulation trains. The slow phase velocities were unchanged for comparable stimulation performed at any time during a given day. This means that the stimulation on a given day would have to produce long-term effects not seen during that day. While possible, this seems rather unlikely. Similarly, the current and pulse rate used in the map and instructions for home stimulation were selected to be well below the threshold for producing a behavioral response or subjective sensation. Therefore, we do not believe that this was a significant confound in the studies reported here. However, we cannot rule out habituation or other response reductions mediated by repeated stimulation.
Finally, we observed that subjects reported perceived motion that was roughly consistent with the direction of their eye movements and scaled with current and eye velocity. This is an important preliminary finding because it suggests that the vestibular inputs that we are generating with electrical stimulation in human subjects are coherent in terms of their contribution both to eye movement and to cortical motion processing and perception. However, the consistency is not perfect. Subjects tend to overestimate their rotation in these experiments by up to an order of magnitude. One subject reported a substantial rotational percept in the absence of slow phase eye movement. Most subjects reported a sensation of motion at current thresholds below the threshold for slow phase eye movement. The reason for this may simply be that human subjects, and perhaps especially subjects with Meniere's disease, are not good at performing such estimations, produce estimations that are inconsistent with eye movement or actual rotational measures, or are interpreting other peculiar sensations as movement because they have an expectation of motion from the stimulation (Guedry 1974, 1992; Holly 2011; Parker 2003; Wood 2007). Indeed, a significant literature suggests that perceptual motion thresholds are highly dependent on the method of report, the requirement for detection vs. direction recognition, and the overall context. VOR eye movement thresholds and scaling have been reported to differ significantly from these rather variable perceptual thresholds (Agrawal et al. 2013; Barnett-Cowan and Harris 2009; Benson et al. 1986, 1989; Chaudhuri et al. 2013; Clark 1967; Clark and Stewart 1968; Dong et al. 2011; Doty 1969; Grabherr et al. 2008; Groen and Jongkees 1948; Janssen et al. 2011; MacNeilage et al. 2010; Mallery et al. 2010; Naseri and Grant 2012; Nesti et al. 2014; Ormsby 1974; Roditi and Crane 2012; Soyka et al. 2011; Zupan and Merfeld 2008).
Another intriguing possibility is that the afferent input that is being provided by prosthetic electrical stimulation may preferentially activate thalamocortical pathways over direct VOR pathways. At any current level, electrical stimulation is activating only a subset of afferents, and some afferent types are far more galvanically sensitive than others (Baird et al. 1988; Goldberg et al. 1984; Lysakowski et al. 1995). As we have demonstrated, there are differences between the threshold and scaling of postural responses and eye movements resulting from vestibular prosthesis stimulation in human subjects (Phillips et al. 2013), implying perhaps similar differences in the galvanic sensitivity of descending and VOR pathways. Also, electrical stimulation synchronously activates all recruited afferents, which differs from the stochastic process that naturally defines the activity of the vestibular nerve afferents. Therefore, in terms of the precise subset of afferent types and the synchrony of the afferent input, electrical stimulation provides an effective but aphysiological vestibular stimulus. It is possible that such a stimulus is more effective cortically or descending systems than at the level of the oculomotor system. This suggests that it may not be adequate to use the VOR response to “map” the receiver stimulator and determine the necessary transfer functions for optimal operation of the prosthesis. It is possible that if we used another dependent measure, such as the perceptual responses that result from electrical stimulation, our resulting map of the device and optimal stimulation parameters would be significantly different than those that we currently utilize.
These results in total suggest new directions for vestibular research in human subjects. We have demonstrated that selective electrical stimulation of the vestibular end organ can result in parametrically controllable behavioral and neural responses, which are very similar in many regard to those in animals. Therefore, this technology creates not only a possible new treatment modality, but also opportunity for controlled experiments evaluating the hypotheses that we have previously evaluated primarily in animal models.
GRANTS
The prosthesis used in this study was developed with support from the National Institute on Deafness and Other Communication Disorders (NIDCD) Contract N01-DC-6-005, the National Center for Research Resources ITHS Ignition Award RR-00166, and The Wallace H. Coulter Foundation. This work was directly supported by The Wallace H. Coulter Foundation, Cochlear Ltd., and a gift from Sara Kranwinkle. Publication was supported by NIDCD Grant R01-DC-014002.
DISCLOSURES
The University of Washington and authors J. O. Phillips, L. Ling, K. Nie, and J. T. Rubinstein have intellectual property associated with the device used in these experiments. J. T. Rubinstein is a paid consultant for Cochlear Ltd.
AUTHOR CONTRIBUTIONS
Author contributions: J.O.P., L.L., K.N., C.M.P., and J.T.R. conception and design of research; J.O.P., L.L., K.N., E.J., C.M.P., A.L.N., and J.T.R. performed experiments; J.O.P., L.L., K.N., E.J., C.M.P., A.L.N., and J.S.G. analyzed data; J.O.P., L.L., and C.M.P. interpreted results of experiments; J.O.P., L.L., C.M.P., and A.L.N. prepared figures; J.O.P. drafted manuscript; J.O.P., L.L., K.N., E.J., C.M.P., A.L.N., J.S.G., and J.T.R. edited and revised manuscript; J.O.P., L.L., K.N., E.J., C.M.P., A.L.N., J.S.G., and J.T.R. approved final version of manuscript.
ACKNOWLEDGMENTS
New address for J. S. Golub: Dept. of Otolaryngology-HNS, Columbia University, New York, NY.
REFERENCES
- Agrawal Y, Bremova T, Kremmyda O, Strupp M, Macneilage PR. Clinical testing of otolith function: perceptual thresholds and myogenic potentials. J Assoc Res Otolaryngol 14: 905–915, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier B, Thömke F, Wilting J, Heinze C, Geber C, Dieterich M. A pathway in the brainstem for roll-tilt of the subjective visual vertical: evidence from a lesion-behavior mapping study. J Neurosci 32: 14854–14858, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird RA, Desmadryl G, Fernández C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol 60: 182–203, 1988. [DOI] [PubMed] [Google Scholar]
- Barnett-Cowan M, Harris LR. Perceived timing of vestibular stimulation relative to touch, light, and sound. Exp Brain Res 198: 221–231, 2009. [DOI] [PubMed] [Google Scholar]
- Benson AJ, Spencer MB, Stott JR. Thresholds for the detection of the direction of whole- body, linear movement in the horizontal plane. Aviat Space Environ Med 57: 1088–1096, 1986. [PubMed] [Google Scholar]
- Benson AJ, Hutt E, Brown S. Thresholds for the perception of whole body angular movement about a vertical axis. Aviat Space Environ Med 60: 205–213, 1989. [PubMed] [Google Scholar]
- Bierer SM, Ling L, Nie K, Fuchs AF, Kaneko CR, Oxford T, Nowack AL, Shepherd SJ, Rubinstein JT, Phillips JO. Auditory outcomes following implantation and electrical stimulation of the semicircular canals. Hear Res 287: 51–56, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass SP, Davidson P, Goshgarian H. Survival of the vestibular nerve after labyrinthectomy in the cat. Otolaryngol Head Neck Surg 101: 459–465, 1989. [DOI] [PubMed] [Google Scholar]
- Chaudhuri SE, Karmali F, Merfeld DM. Whole-body motion-detection tasks can yield much lower thresholds than direction-recognition tasks: implications for the role of vibration. J Neurophysiol 110: 2764–2772, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang B, Fridman GY, Chenkai D, Rahman MA, Della Santina CC. Design and performance of a multichannel vestibular prosthesis that restores semicircular canal sensation in rhesus monkey. IEEE Trans Neural Syst Rehabil Eng 19: 588–598, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. Thresholds for the perception of angular acceleration in man. Aerospace Med 38: 443–450, 1967. [PubMed] [Google Scholar]
- Clark B, Stewart JD. Comparison of three methods to determine thresholds for perception of angular acceleration. Am J Psychol 81: 207–216, 1968. [PubMed] [Google Scholar]
- Cohen B, Suzuki J, Bender MB. Eye movements from semicircular canal nerve stimulation in cat. Ann Otol Rhinol Laryngol 73: 153–169, 1964. [DOI] [PubMed] [Google Scholar]
- Cohen B, Suzuki JI. Eye movements induced by ampullary nerve stimulation. Am J Physiol 204: 347–351, 1963. [DOI] [PubMed] [Google Scholar]
- Cohen B, Yakushin SB, Holstein GR. What does galvanic vestibular stimulation actually activate: response. Front Neurol 3: 148, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys IS, Macdougall HG. What galvanic vestibular stimulation actually activates. Front Neurol 3: 117, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys IS, Halmagyi GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vestib Res 5: 67–107, 1995. [PubMed] [Google Scholar]
- Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res 175: 256–267, 2006. [DOI] [PubMed] [Google Scholar]
- Dai C, Fridman GY, Della Santina CC. Effects of vestibular prosthesis electrode implantation, and stimulation on hearing in rhesus monkeys. Hear Res 277: 204–210, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Fridman GY, Davidovics NS, Chiang B, Ahn JH, Della Santina CC. Restoration of 3D vestibular sensation in rhesus monkeys using a multichannel vestibular prosthesis. Hear Res 281: 74–83, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Fridman GY, Chiang B, Davidovics NS, Melvin T, Cullen KE, Della Santina CC. Crossaxis adaptation improves 3D vestibulo-ocular reflex alignment during chronic stimulation via a head-mounted multichannel vestibular prosthesis. Exp Brain Res 210: 595–606, 2011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Fridman GY, Chiang B, Rahman MA, Ahn JH, Davidovics NS, Della Santina CC. Directional plasticity rapidly improves 3D vestibulo-ocular reflex alignment in monkeys using a multichannel vestibular prosthesis. J Assoc Res Otolaryngol 14: 863–877, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovics NS, Fridman GY, Chiang B, Della Santina CC. Effects of biphasic current pulse frequency, amplitude, duration, and interphase gap on eye movement responses to prosthetic electrical stimulation of the vestibular nerve. IEEE Trans Neural Syst Rehabil Eng 19: 84–94, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovics NS, Fridman GY, Della Santina CC. Co-modulation of stimulus rate and current from elevated baselines expands head motion encoding range of the vestibular prosthesis. Exp Brain Res 218: 389–400, 2012. [DOI] [PubMed] [Google Scholar]
- Davidovics NS, Rahman MA, Dai C, Ahn J, Fridman GY, Della Santina CC. Multichannel vestibular prosthesis employing modulation of pulse rate and current with alignment precompensation elicits improved VOR performance in monkeys. J Assoc Res Otolaryngol 14: 233–248, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Santina C, Migliaccio A, Patel A. Electrical stimulation to restore vestibular function development of a 3-d vestibular prosthesis. Conf Proc IEEE Eng Med Biol Soc 7: 7380–7385, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Santina CC, Migliaccio AA, Patel AH. A multichannel semicircular canal neural prosthesis using electrical stimulation to restore 3-D vestibular sensation. IEEE Trans Biomed Eng 54: 1016–1030, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Robinson CJ, Fulk G. Psychophysical detection thresholds in anterior horizontal translations of seated, and standing blindfolded subjects. Conf Proc IEEE Eng Med Biol Soc 2011: 4112–4115, 2011. [DOI] [PubMed] [Google Scholar]
- Doty RL. Effect of duration of stimulus presentation on the angular acceleration threshold. J Exp Psychol 80: 317–321, 1969. [DOI] [PubMed] [Google Scholar]
- Feigl GC, Fasel JH, Anderhuber F, Ulz H, Rienmul̈ler R, Guyot JP, Kos IM. Superior vestibular neurectomy: a novel transmeatal approach for a denervation of the superior and lateral semicircular canals. Otol Neurotol 30: 586–591, 2009. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol 96: 2301–2316, 2004. [DOI] [PubMed] [Google Scholar]
- Fridman G, Davidovics N, Dai C, Migliaccio A, Della Santina C. Vestibulo-ocular reflex responses to a multichannel vestibular prosthesis incorporating a 3D coordinate transformation for correction of misalignment. J Assoc Res Otolaryngol 11: 367–381, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacek RR. Transection of the posterior ampullary nerve for the relief of benign paroxysmal positional vertigo. Ann Otol Rhinol Laryngol 83: 596–605, 1974. [DOI] [PubMed] [Google Scholar]
- Gacek RR. A perspective on recurrent vertigo. ORL J Otorhinolaryngol Relat Spec 75: 91–107, 2013. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Brown CJ, Abbas PJ. Intraoperative measures of electrically evoked auditory nerve compound action potential. Am J Otol 15: 137–144, 1994. [PubMed] [Google Scholar]
- Goldberg JM, Fernández C, Smith CE. Responses of vestibular-nerve afferents in the squirrel monkey to externally applied galvanic currents. Brain Res Dec 252: 156–160, 1982. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernández C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol 51: 1236–1256, 1984. [DOI] [PubMed] [Google Scholar]
- Golub JS, Ling L, Nie K, Nowack A, Shepherd SJ, Bierer SM, Jameyson E, Kaneko CR, Phillips JO, Rubinstein JT. Prosthetic implantation of the human vestibular system. Otol Neurotol 35: 136–147, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong WS, Merfeld DM. Prototype neural semicircular canal prosthesis using patterned electrical stimulation. Ann Biomed Eng 28: 572–581, 2000. [DOI] [PubMed] [Google Scholar]
- Gong WS, Merfeld DM. System design, and performance of a unilateral horizontal semicircular canal prosthesis. IEEE Trans Biomed Eng 49: 175–181, 2002. [DOI] [PubMed] [Google Scholar]
- Gong WS, Haburcakova C, Merfeld DM. Vestibulo-ocular responses evoked via bilateral electrical stimulation of the lateral semicircular canals. IEEE Trans Biomed Eng 55: 2608–2619, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr L, Nicoucar K, Mast FW, Merfeld DM. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res 186: 677–681, 2008. [DOI] [PubMed] [Google Scholar]
- Groen JJ, Jongkees LBW. The threshold of angular acceleration perception. J Physiol 107: 1–7, 1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedry FE. Psychophysics of vestibular sensation, in: Vestibular System, Part 2: Psychophysics and Applied Aspects and General Interpretations VI/2 edited by Kornhuber HH. Berlin: Springer, 1974, p. 3–154. [Google Scholar]
- Guedry FE, Rupert AH, McGrath BJ, Oman CM. The dynamics of spatial orientation during complex and changing linear and angular acceleration. J Vestib Res 2: 259–283, 1992. [PubMed] [Google Scholar]
- Guyot JP, Gay A, Izabel Kos M, Pelizzone M. Ethical, anatomical, and physiological issues in developing vestibular implants for human use. J Vestib Res 22: 3–9, 2012. [DOI] [PubMed] [Google Scholar]
- Guyot JP, Sigrist A, Pelizzone M, Kos MI. Adaptation to steady-state electrical stimulation of the vestibular system in humans. Ann Otol Rhinol Laryngol 120: 143–149, 2011a. [DOI] [PubMed] [Google Scholar]
- Guyot JP, Sigrist A, Pelizzone M, Feigl GC, Kos MI. Eye movements in response to electrical stimulation of the lateral, and superior ampullary nerves. Ann Otol Rhinol Laryngol 120: 81–87, 2011b. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Gresty MA, Gibson WP. Ocular tilt reaction with peripheral vestibular lesion. Ann Neurol 6: 80–83, 1979. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol 45: 737–739, 1988. [DOI] [PubMed] [Google Scholar]
- Holly JE, Davis SM, Sullivan KE. Differences between perception and eye movements during complex motions. J Vestib Res 21: 193–208, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen M, Lauvenberg M, van der Ven W, Bloebaum T, Kingma H. Perception threshold for tilt. Otol Neurotol 32: 818–825, 2011. [DOI] [PubMed] [Google Scholar]
- Jonkees LB, Maas JP, Philipszoon AJ. Clinical nystagmography. A detailed study of electro-nystagmography in 341 patients with vertigo. Pract Otorhinolaryngol (Basel) 24: 65–93, 1962. [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol 412: 543–562, 1999. [DOI] [PubMed] [Google Scholar]
- Lewis RF, Nicoucar K, Gong W, Haburcakova C, Merfeld DM. Adaptation of vestibular tone studied with electrical stimulation of semicircular canal afferents. J Assoc Res Otolaryngol 14: 331–340, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RF, Merfeld DM, Gong WS. Cross-axis vestibular adaptation produced by patterned electrical stimulation. Neurology 56: A18, 2001. [Google Scholar]
- Lewis RF, Gong WS, Ramsey M, Minor L, Boyle R, Merfeld DM. Vestibular adaptation studied with a prosthetic semicircular canal. J Vestib Res 12: 87–94, 2002. [PubMed] [Google Scholar]
- Lewis RF, Haburcakova C, Gong W, Makary C, Merfeld DM. Vestibuloocular reflex adaptation investigated with chronic motion-modulated electrical stimulation of semicircular canal afferents. J Neurophysiol 103: 1066–1079, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetje CM. Hearing results following posterior semicircular canal injury during antesigmoid retrolabyrinthine selective vestibular nerve section. Am J Otol 13: 600–602, 1992. [PubMed] [Google Scholar]
- Lysakowski A, Minor LB, Fernández C, Goldberg JM. Physiological identification of morphologically distinct afferent classes innervating the cristae ampullares of the squirrel monkey. J Neurophysiol 73: 1270–1281, 1995. [DOI] [PubMed] [Google Scholar]
- MacNeilage PR, Turner AH, Angelaki DE. Canal-otolith interactions and detection thresholds of linear and angular components during curved-path self-motion. J Neurophysiol 104: 765–773, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallery RM, Olomu OU, Uchanski RM, Militchin VA, Hullar TE. Human discrimination of rotational velocities. Exp Brain Res 204: 11–20, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall AA, Ishiyama GP, Lopez IA, Bhuta S, Vetter S, Ishiyama A. Histopathological and ultrastructural analysis of vestibular endorgans in Meniere's disease reveals basement membrane pathology. BMC Ear Nose Throat Disord 9: 4, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merfeld DM, Gong WS, Morrissey J, Saginaw M, Haburcakova C, Lewis RF. Acclimation to chronic constant-rate peripheral stimulation provided by a vestibular prosthesis. IEEE Trans Biomed Eng 53: 2362–2372, 2006. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Haburcakova C, Gong W, Lewis RF. Chronic vestibulo-ocular reflexes evoked by a vestibular prosthesis. IEEE Trans Biomed Eng 54: 1005–1015, 2007. [DOI] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Brown CJ. An improved method of reducing stimulus artifact in the electrically evoked whole-nerve potential. Ear Hear 21: 280–290, 2000. [DOI] [PubMed] [Google Scholar]
- Naseri AR, Grant PR. Human discrimination of translational accelerations. Exp Brain Res 218: 455–464, 2012. [DOI] [PubMed] [Google Scholar]
- Nesti A, Barnett-Cowan M, Macneilage PR, Bülthoff HH. Human sensitivity to vertical self-motion. Exp Brain Res 232: 303–314, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TA, Ranieri M, DiGiovanna J, Peter O, Genovese V, Perez Fornos A, Micera S. A real-time research platform to study vestibular implants with gyroscopic inputs in vestibular deficient subjects. IEEE Trans Biomed Circuits Syst 8: 474–484, 2014. [DOI] [PubMed] [Google Scholar]
- Nie K, Bierer SM, Ling L, Oxford T, Rubinstein JT, Phillips JO. Characterization of the electrically evoked compound action potential of the vestibular nerve. Otol Neurotol 32: 88–97, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie K, Ling L, Bierer SM, Kaneko CR, Fuchs AF, Oxford T, Rubinstein JT, Phillips JO. An experimental vestibular neural prosthesis: design and preliminary results with rhesus monkeys stimulated with modulated pulses. IEEE Trans Biomed Eng 60: 1685–1692, 2013. [DOI] [PubMed] [Google Scholar]
- Ormsby C. Model of Human Dynamic Orientation (PhD thesis) Cambridge, MA: MIT, 1974. [Google Scholar]
- Parker DE. Spatial perception changes associated with space flight: implications for adaptation to altered inertial environments. J Vestib Res 13: 331–343, 2003. [PubMed] [Google Scholar]
- Parnes LS, McClure JA. Posterior semicircular canal occlusion in the normal hearing ear. Otolaryngol Head Neck Surg 104: 52–57, 1991. [DOI] [PubMed] [Google Scholar]
- Parnes LS, McClure JA. Posterior semicircular canal occlusion for intractable benign paroxysmal positional vertigo. Ann Otol Rhinol Laryngol 99: 330–334, 1990. [DOI] [PubMed] [Google Scholar]
- Perez Fornos A, Guinand N, van de Berg R, Stokroos R, Micera S, Kingma H, Pelizzone M, Guyot JP. Artificial balance: restoration of the vestibulo-ocular reflex in humans with a prototype vestibular neuroprosthesis. Front Neurol 5: 66, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C, Defrancisci C, Ling L, Nie K, Nowack A, Phillips JO, Rubinstein JT. Postural responses to electrical stimulation of the vestibular end organs in human subjects. Exp Brain Res 229: 181–195, 2013. [DOI] [PubMed] [Google Scholar]
- Phillips C, Ling L, Oxford T, Nowack A, Nie K, Rubinstein JT, Phillips JO. Longitudinal performance of an implantable vestibular prosthesis. Hear Res 322: 200–211, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JO, Bierer SM, Ling L, Nie K, Rubinstein JT. Real-time communication of head velocity and acceleration for an externally mounted vestibular prosthesis. Conf Proc IEEE Eng Med Biol Soc 2011: 3537–3541, 2011. [DOI] [PubMed] [Google Scholar]
- Phillips JO, Shepherd SJ, Nowack AL, Ling L, Bierer SM, Kaneko CRS, Phillips CMT, Nie K, Rubinstein JT. Longitudinal performance of a vestibular prosthesis as assessed by electrically evoked compound action potential recording. Conf Proc IEEE Eng Med Biol Soc 2012: 6128–6131, 2012. [DOI] [PubMed] [Google Scholar]
- Roditi RE, Crane BT. Directional asymmetries and age effects in human self-motion perception. J Assoc Res Otolaryngol 13: 381–401, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein JT, Bierer S, Kaneko C, Ling L, Nie K, Oxford T, Newlands S, Santos F, Risi F, Abbas PJ, Phillips JO. Implantation of the semicircular canals with preservation of hearing and rotational sensitivity: a vestibular neurostimulator suitable for clinical research. Otol Neurotol 33: 789–796, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Goldberg JM, Minor LB, Cullen KE. Effects of canal plugging on the vestibuloocular reflex and vestibular nerve discharge during passive and active head rotations. J Neurophysiol 102: 2693–2703, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuknecht HF. Behavior of the vestibular nerve following labyrinthectomy. Ann Otol Rhinol Laryngol Suppl 97: 16–32, 1982. [PubMed] [Google Scholar]
- Soyka F, Robuffo GP, Beykirch K, Bul̈thoff HH. Predicting direction detection thresholds for arbitrary translational acceleration profiles in the horizontal plane. Exp Brain Res 209: 95–107, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George RJ, Fitzpatrick RC. The sense of self-motion, orientation and balance explored by vestibular stimulation. J Physiol 589: 807–813, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun DQ, Rahman MA, Fridman G, Chenkai D, Chiang B, Della Santina CC. Chronic stimulation of the semicircular canals using a multichannel vestibular prosthesis: effects on locomotion, and angular vestibulo-ocular reflex in chinchillas. Conf Proc IEEE Eng Med Biol Soc: 3519–3523, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki JI, Cohen B. Head, eye, body and limb movements from semicircular canal nerves. Exp Neurol 10: 393–405, 1964. [DOI] [PubMed] [Google Scholar]
- Tang S, Melvin TA, Della Santina CC. Effects of semicircular canal electrode implantation on hearing in chinchillas. Acta Otolaryngol 129: 481–486, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnutzer AA, Bockisch CJ, Straumann D. Roll-dependent modulation of the subjective visual vertical: contributions of head-, and trunk-based signals. J Neurophysiol 103: 934–994, 2010. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Velázquez-Villaseñor, Rauch SD, Glynn RJ, Wall C 3rd, Merchant SN. Temporal bone studies of the human peripheral vestibular system. Meniere's disease. Ann Otol Rhinol Laryngol Suppl 181: 26–31, 2000a. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Velázquez-Villaseñor L, Rauch SD, Glynn RJ, Wall C 3rd, Merchant SN. Temporal bone studies of the human peripheral vestibular system. Aminoglycoside ototoxicity. Ann Otol Rhinol Laryngol Suppl 181: 20–25, 2000b. [DOI] [PubMed] [Google Scholar]
- Valentin NS, Hageman KN, Dai C, Della Santina CC, Fridman GY. Development of a multichannel vestibular prosthesis prototype by modification of a commercially available cochlear implant. IEEE Trans Neural Syst Rehabil Eng 21: 830–839, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Berg R, Guinand N, Guyot JP, Kingma H, Stokroos RJ. The modified ampullar approach for vestibular implant surgery: feasibility and its first application in a human with a long-term vestibular loss. Front Neurol 3: 18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez-Villaseñor L, Merchant SN, Tsuji K, Glynn RJ, Wall C 3rd, Rauch SD. Temporal bone studies of the human peripheral vestibular system. Normative Scarpa's ganglion cell data. Ann Otol Rhinol Laryngol Suppl 181: 14–19, 2000. [DOI] [PubMed] [Google Scholar]
- Wall C, Kos I, Guyot JP. Eye movements in response to electrical stimulation of the human posterior ampullary nerve. Ann Otol Rhinol Laryngol 116: 369–74, 2007. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Reschke MF, Sarmiento LA, Clément G. Tilt and translation motion perception during off-vertical axis rotation. Exp Brain Res 182: 365–377, 2007. [DOI] [PubMed] [Google Scholar]
- Zupan LH, Merfeld DM. Interaural self-motion linear velocity thresholds are shifted by roll vection. Exp Brain Res 191: 505–511, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]