Abstract
Aim
This study examined the relationship between hypothalamic-associated hormones and behavioural and eating disorders in children with low birthweight.
Methods
We included 100 children (mean age 9.7 years): 39 were born preterm at <32 gestational weeks, 28 were full-term, but small for gestational age, and 33 were full-term controls. Behavioural histories were analysed, together with fasting blood samples of leptin, insulin, insulin-like growth factor-1 (IGF-I), prolactin, glucagon and cortisol.
Results
Preterm children had lower prolactin (p = 0.01) and higher IGF-I than controls (p < 0.05, adjusted for confounders), despite being significantly shorter than the predicted target height (p < 0.001). More preterm children displayed behavioural disorders (38% versus 10%, p < 0.001) and eating disorders (26% versus 8%, p < 0.05) than full-term children. These disorders were associated with lower leptin (p < 0.01), insulin (p < 0.05) and IGF-I (p < 0.05), but correlations between these hormones and leptin were similar among the groups. Combined behavioural and eating disorders were only observed in preterm children, who were also the shortest in height.
Conclusion
Behavioural and eating disorders among preterm children were associated with low leptin, insulin and IGF-1. Low prolactin in all preterm children indicated an increased dopaminergic tonus, which might inhibit body weight incrementation. This raises speculation about IGF-I receptor insensitivity.
Keywords: Dopaminergic tonus, Insulin-like growth factor-1, Leptin, Prolactin, Target height deviation
Introduction
Children who are born prematurely are at an increased risk of developing long-term behavioural disorders, particularly attention deficit and hyperactivity disorders (1–4), and this risk is escalated in individuals born extremely preterm (4). Previous reports have also shown that preterm birth was associated with eating disorders during infancy and childhood (5,6). Health and disease states during childhood are often reflected in growth patterns.
Several factors, including epigenetic factors, can act early in life to imprint and modify physiological systems later in life (7). Perinatal life, infancy and childhood are periods of increased vulnerability for systems involved in stress response. The presence of adverse stressors during these critical periods may affect behaviour and physiological functions, such as growth, metabolism, reproduction and the inflammatory/immune response (8), by altering the activity of the hypothalamic–pituitary–adrenal (HPA) axis.
Animal models of early stress have shown that stress-related events that occur during foetal and early postnatal periods may have lifelong programming effects on the HPA axis and various body functions, particularly disease susceptibility (9). Exposure of the developing brain to severe and, or, prolonged stress may result in negative effects after birth. These effects may include the following: hyperactivity in the stress response and the HPA axis, defective glucocorticoid-negative feedback, altered cognition, novelty seeking behaviour, increased vulnerability to addictive behaviour and mood-related disorders involving the monoamine system in the brain. Moreover, young adult humans who were born preterm with very low birthweights showed a reduced cortisol response during a stress test (10).
Key notes
This study evaluated relationships between various hormones and behavioural and eating disorders in 9-year-olds, who had been born preterm, small for gestational age and full-term.
It showed that among preterm children, those with behavioural or eating disorders had lower leptin, insulin and insulin-like growth factor-1 IGF-1.
Preterm children also exhibited higher IGF-1 and lower prolactin levels, and this might reflect an increased dopaminergic tonus, which may inhibit body weight incrementation.
Leptin, a 16 kDa protein hormone, is a product of the obese gene and is secreted by adipocytes. Serum leptin levels are thought to reflect fat mass, and in children, they correlate very well with the body mass index (BMI) standard deviation score (SDS) (11). Through its action on the leptin receptor, leptin is centrally involved in a negative feedback loop that regulates feeding signals, including appetite and hunger. Leptin also decreases depression-like activity and anxiety-related behaviour in animal models (12). Furthermore, leptin dampens the HPA axis response to stress, both centrally and peripherally, partly by affecting adrenocorticotropic hormone levels (13).
The insulin-like growth factor-binding hormone-1 (IGFBP-1) is thought to reflect diurnal insulin levels. Insulin and glucagon, both produced by the pancreas, have opposing actions that regulate blood glucose levels; insulin rapidly reduces glucose, and glucagon is secreted to counteract hypoglycaemia. Insulin-like growth factor-1 (IGF-I) is the main hormone regulator of growth during infancy and puberty. IGF-I is produced by the liver and regulated by growth hormone in children and adults. Growth hormone and prolactin have closely similar activities and they are both partly produced by the same cells in the pituitary. Prolactin is a hormone involved in lactation, but it has also been suggested that it promotes body weight gain, both in experimental animals (14) and in humans. Hyperprolactinaemia is associated with insulin resistance (15).
This study aimed to investigate whether low birthweight, due to preterm birth or intrauterine growth restriction, might be associated with behavioural, eating or sleeping disorders in childhood. We also examined the circulating levels of hormones associated with growth, glucose metabolism and adipose tissue in children at the start of puberty. We hypothesised that there may be relationships between birthweight-associated disorders, alterations in HPA hormone levels, growth and the accumulation of fat mass.
Methods
This study included 100 school-aged children, with no known somatic diseases, who were entering puberty, with a mean age of 9.73 and a range of 9.68–9.79 years. The children were divided into three groups: 39 children were born prematurely at <32 gestational weeks (preterm group), 28 children were born full-term at 37–42 gestational weeks, but small for gestational age (term-SGA group), and 33 children were born full-term with normal birthweights (control group) (Table S1). These subjects were described previously (16,17). In this study, seven subjects were born prematurely and were also SGA, and they were included in the preterm children group. Gestational age was determined by ultrasound during early pregnancy (18). SGA was defined as a birthweight of <2 standard deviations (SD) below the mean, which was defined as below the sex-specific 2.5th percentile for gestational age, according to Swedish reference data for normal foetal growth (19). Target height (Table 1) was calculated according to parental height: we added the father's height and the mother's height, divided that number by two and then added 6.5 cm for the boys and subtracted 6.5 cm for the girls. The target height standard deviation score (SDS) was based on the Swedish reference material (20).
Table 1.
Follow-up characteristics of children born prematurely (preterm), full-term, but small for gestational age (term-SGA), or full-term with normal birthweight (control)
| Characteristic | 1. Preterm (n = 39) | 2. Term-SGA (n = 28) | 3. Control (n = 33) | p | 1 vs. 2† | 1 vs. 3† | 2 vs. 3† |
|---|---|---|---|---|---|---|---|
| Age, years | 9.6 (9.5; 9.7) | 9.8 (9.7; 9.9) | 9.8 (9.8; 9.9) | 0.002 | <0.05 | <0.001 | 0.18 |
| Weight, kg | 32.0 (29.7; 34.3) | 31.4 (28.6; 34.2) | 36.3 (33.8; 38.9) | 0.018 | 0.73 | 0.02 | 0.01 |
| Weight SDS | −0.14 (−0.65; 0.37) | −0.32 (−0.92; 0.37) | 0.81 (0.25; 1.36) | 0.012 | 0.64 | 0.01 | <0.01 |
| Height, cm | 134 (132; 136) | 137 (134; 139) | 141 (139; 144) | <0.001 | 0.13 | <0.001 | 0.01 |
| Height SDS | −0.81 (−1.17; −0.46) | −0.49 (−0.91; −0.07) | 0.30 (−0.08; 0.69) | <0.001 | 0.24 | <0.001 | <0.01 |
| HC, cm | 53.0 (52.5; 53.5) | 53.3 (52.6; 53.9) | 54.7 (54.2; 55.3) | <0.001 | 0.52 | <0.001 | <0.001 |
| BMI, kg/m2 | 17.7 (16.8; 18.7) | 16.7 (15.6; 17.8) | 18.1 (17.1; 19.2) | 0.15 | 0.15 | 0.58 | 0.06 |
| Catch-upa weight SDS | 0.89 (0.30; 1.49) | 2.87 (2.17; 3.57) | 1.07 (0.42; 1.72) | <0.001 | <0.001 | <0.001 | 0.68 |
| Catch-upa height SDS | 0.43 (−0.12; 0.99) | 2.18 (1.53; 2.83) | 0.94 (0.35; 1.54) | <0.001 | <0.001 | <0.01 | 0.22 |
| Target height SDS | −0.13 (−0.37; 0.10) | −0.49 (−0.76; −0.21) | −0.09 (−0.34; 0.16) | 0.08 | 0.06 | 0.80 | 0.04 |
| Height SDS deviation (from target height SDS) | −0.65 (−0.98; −0.31) | 0.02 (−0.37; 0.41) | 0.33 (−0.02; 0.70) | <0.001 | 0.01 | <0.001 | 0.23 |
Values represent the number (%) or mean (95% CI).
SDS = Standard deviation score; HC = Head circumference; BMI = Body mass index; target height SDS = For description see Methods; aCatch-up = Refers to the compensatory weight or height gained from birth to follow-up.
Significant p-values, p < 0.05, are in italics, according to analysis of variance or Pearson's chi-square, when comparing all groups; and †according to Fisher's post hoc test for comparisons between two groups.
All children were singletons born in northern Stockholm at Karolinska University Hospital, Solna. No children had chromosomal anomalies, congenital infections or life-threatening congenital anomalies. The inclusion criteria were described previously (17). Of the 257 children invited to participate in this study, 57 were preterm children. Of those contacted, 105 children accepted the invitation to participate in the study. The children who did not participate were not different in gestational ages and birthweights from those who participated in the three study groups. Children included in this study had reached a Tanner stage of two or lower, according to breast/pubic development. Two term-SGA children and three control children had reached a Tanner stage of more than two and were therefore excluded from analysis. This meant that 100 children were included in this study. Of these, five children had reached a Tanner stage of two: one preterm and three control girls and one preterm boy. The three control girls did not differ in serum blood values or anthropometrical data to the other control girls (data not shown). This study was approved by the Ethics Committee at Karolinska University Hospital, and written informed signed consent was obtained from the parents and children.
Information about maternal and neonatal characteristics was collected from the Swedish birth records. Medical and behavioural histories were obtained from parents and study participants using a questionnaire that was sent to them prior to a home visit. The questionnaire contained questions and a place for free comments. The parents could either check the ‘normal’ box or describe how their child showed discrepancies from normal behaviour. The questionnaire was followed up by a visit, where a medical doctor (MV) went through the questionnaire with the parent(s) that were present and carefully discussed and investigated any abnormal behaviours pointed out by the parent(s). The questionnaire included descriptions of different behavioural problems, which are categorised as follows: (i) behavioural disorder, defined according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, text revision (DSM-IV-TR) as hyperactivity, concentration problems or a combination of both (21); (ii) eating disorder, defined as eating small portions, having a poor appetite and/or having no time/interest in eating (22); (iii) sleeping disorder, defined as difficulty falling asleep, nightmares, sleeping lightly and waking up during the night (20); and (iv) affective disorder, defined as impulsive aggression, affective seizures and mood outbreaks. When a child met the criteria for both behavioural and eating disorders, the child was categorised as having a combined behavioural and eating disorder. The physician also performed a physical examination. The same physician was present when 90 of the children were assessed, and two other physicians assessed the other 10 children with the same strict protocol methods. A single research nurse obtained anthropometric measurements for all children.
Six of the eight children with combined and eating disorders were born extremely preterm (≤27 gestational weeks). These six children exhibited a higher prevalence of respiratory distress syndrome (6/6, 100% versus 8/17, 47%, respectively, p = 0.02), were more likely to have been delivered vaginally (5/6, 83% versus 6/17, 35%, respectively, p = 0.04; Pearson's χ2 test) and had a lower gestational age at birth [mean (±SD) 24.5 ± 0.5 weeks versus 25.3 ± 0.6 weeks, p = 0.009, analysis of variances (ANOVA), t-test], when compared with 17 other children born extremely preterm.
One boy who had been born preterm was taking a psychostimulant medication for a behavioural disorder and attention deficit. Two of the preterm children with combined behavioural and eating disorders had received growth hormone treatment for 1 year prior to the study. These individuals were excluded from the comparison of target height SDS deviation between the groups and from the comparison of prolactin levels between preterm children with and without disorders.
Prolactin, growth hormone, glucagon, IGF-1, leptin, insulin and IGFBP-1 were analysed in 80 children, comprising 28 preterm, 23 term-SGA and 29 controls. No blood samples were available from the remaining 20 children, either due to refusal by the child or parent or due to an unsuccessful draw. The same nurse took blood samples from all 80 subjects. Blood samples were drawn after an overnight fast at 9.30 am to 10 am for all subjects. Blood sampling and blood pressure measurements were taken after at least 15 (15–30) min of rest in a horizontal position. The blood pressure measurements did not differ between groups.
Prolactin was analysed by immunofluorescence with an AutoDELFIA® prolactin kit (Perkin Elmer, Inc., Waltham, MA, USA). The analysing interval was 0.04–250 μg/L, the reference interval was 3–19 μg/L, the interassay coefficient of variation (CV%) was 2.8%, and the intra-assay CV was <10%. Growth hormone was analysed by immunofluorescence with the AutoDELFIA® hGH kit (Perkin Elmer, Inc.). The analysing interval was approximately 0.01–38.5 μg/L, and the reference interval was <15 μg/L. Adrenocorticotropic hormone was analysed by chemiluminescence with a regent kit (Nichols Advantage; Nichols Institute diagnostics, Valencia, USA). The analysing interval was 0–330 pmol/L and the reference interval was 2.0–10 pmol/L. Serum cortisol level was analysed with the AutoDELFIA® cortisol kit (Perkin Elmer, Inc.). The analysing interval was 5–1600 nmol/L and the reference interval was 230–700 nmol/L. Glucagon was analysed with an EURIA-glucagon radioimmunoassay kit (Euro Diagnostica, Malmo, Sweden). The gamma counter analysing interval was 4.7–300 pmol/L and the reference interval was <60 pmol/L. The laboratory methods for IGF-1, leptin, insulin and IGFBP-1 were described previously, and the unadjusted levels of IGF-1, leptin, insulin and IGFBP-1 from this cohort were published previously (11,16).
Statistics
Measurements are expressed as the number of individuals (percentage), the mean (95% CI) for anthropometrical data or the geometrical mean (95% CI) for all hormone values, because they were not normally distributed. All hormone measurements were initially log-transformed. For comparisons among all groups, either an analysis of variance (ANOVA), for comparisons of means, or Pearson's χ2, for comparisons of frequencies, was performed. Analyses of covariances (ANCOVAs) were used to compare mean hormone measurements, with age, gender, weight and height (i.e. adjusting for body composition) as covariance variables. Post hoc tests were performed with Fisher's test, or a planned comparison between two groups followed by the Bonferroni correction; post hoc p-values were multiplied by three to account for the number of comparisons being performed. For comparisons between two groups, we also used ANOVAs and the t-test. Linear regression analysis was performed to compare IGF-1 with catch-up height, catch-up weight and with prolactin and to compare leptin (as a dependent variable) with the following hormones: IGF-1, IGFBP-1, insulin, glucagon, prolactin and growth hormone. Linear regression analyses between the body length SDS and the head circumference SDS at birth and at follow-up were also performed in the group of preterm children. p < 0.05 was considered statistically significant; p < 0.10 was considered a tendency. The results were analysed with Stat Soft, version 10 (Tulsa, OK, USA).
Results
General description
At follow-up, the 100 children had a mean age (95% CI) of 9.7 years (9.6–9.8) (Table 1). The preterm children were younger than the term children at follow-up (Table 1). At follow-up, there were significant differences between the groups regarding weight, height and head circumference (Table 1). Term-SGA children had a significantly lower target height SDS than the controls (p < 0.05; Table 1), but the deviation from target was not significantly different from that of the controls (height SDS deviation). Preterm children deviated significantly more from their target height than the other groups at the start of puberty (height SDS deviation, Table 1).
Hormone levels in the different groups
Prolactin was inversely correlated with weight (r = −0.33, p < 0.01) and height (r = −0.23, p < 0.05) in all subjects (n = 80). The preterm group had lower prolactin levels than the term-SGA group (p = 0.029, adjusted for age, gender, weight and height) and the control group (p = 0.015, adjusted for age, gender, weight and height) (Table 2). Adding catch-up group as a covariable, the difference in prolactin levels between the preterm group and controls became more significant (p = 0.007, adjusted for age, gender, weight, height and catch-up height). Prolactin did not differ between genders in the preterm group (p = 0.43). IGF-I levels did not correlated significantly with catch-up height (preterm group r = 0.15, p = 0.44, term-SGA r = 0.08, p = 0.72 and controls r = 0.27, p = 0.15) or with catch-up weight (preterm r = 0.37, p = 0.06, term-SGA r = 0.23, p = 0.28 and controls r = 0.02, p = 0.91). Adjusted IGF-I levels were higher in the preterm group than in the control group (p = 0.021), and adding catch-up height as a covariable had no effect on this calculation. The adjusted serum cortisol levels tended to be lower in the preterm group compared with controls (p = 0.05). No differences were observed between the groups regarding the other hormones (Table 2). IGF-I levels were inversely correlated with prolactin in the preterm group (r = −0.39, p < 0.05), but this correlation was not observed in the combined group of term-SGA and controls (Fig.1).
Table 2.
Serum prolactin, growth hormone, adrenocorticotropic hormone (ACTH), cortisol, glucagon and IGF-1 in children born prematurely (preterm), full-term, but small for gestational age (term-SGA), or full-term with normal birthweight (control)
| Hormone (μg/L, or pmol/L for ACTH) | 1. Preterm (n = 28) | 2. Term-SGA (n = 23) | 3. Control (n = 29) | p | *p | 1 vs. 2† | 1 vs. 3 |
|---|---|---|---|---|---|---|---|
| Prolactin | 4.72 (3.91; 5.70) | 6.84 (5.63; 8.32) | 7.09 (5.91; 8.50) | 0.008 | 0.013 | 0.029 | 0.015 |
| Growth hormone | 0.87 (0.45; 1.70) | 0.94 (0.47; 1.88) | 0.78 (0.41; 1.51) | 0.94 | 0.51 | 1.0 | 1.0 |
| ACTH | 54 (47; 61) | 48 (43; 54) | 50 (44; 56) | 0.49 | 0.38 | 0.69 | 1.0 |
| Cortisol | 254 (216; 298) | 294 (249; 347) | 339 (291; 394) | 0.06 | 0.16 | 0.66 | 0.05 |
| Glucagon | 8.9 (6.7; 11.7) | 10.2 (8.1; 13.0) | 13.0 (10.1; 16.7) | 0.17 | 0.89 | 1.0 | 0.20 |
| IGF-I | 242 (213; 275) | 200 (176; 227) | 186 (164; 210) | 0.021 | 0.05 | 0.12 | 0.021 |
IGF-I = Insulin-like growth factor-1.
Because none of the hormones were normally distributed, values represent geometrical means (95% CI).
Significant p-values, p < 0.05, are in italics, according to an age, gender, weight and height-adjusted analysis of covariance (ANCOVA) between multiple groups and †post hoc test was performed by planned comparison and the Bonferroni correction; post hoc p-values were multiplied by three to account for the number of comparisons being performed.
p: p -values calculated according to an ANCOVA, but adjusted for age and gender only.
Figure 1.
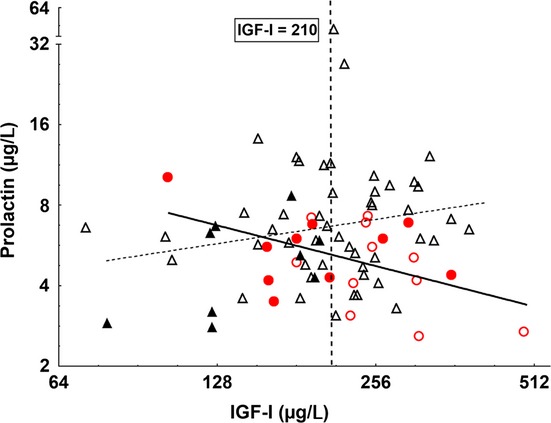
Correlation between insulin-like growth factor-1 (IGF-I) and prolactin levels in the different groups. Symbols represent preterm children with (•) or without (o) behavioural/eating disorders and term- small for gestational age (SGA) and controls (full-term group) with ( ) or without (
) or without ( ) behavioural or eating disorders. IGF-1 was inversely correlated with prolactin in the preterm group (solid line; r = −0.38, p = 0.046); in contrast, the combined term-SGA and controls showed a positive correlation (dashed line; r = 0.23, p < 0.1). The mean IGF-I in the entire cohort was 207 μg/L. When the rounded-off mean value (210 μg/L) was used as a threshold value, it was clear that preterm children with IGF-1 levels below 210 μg/L had a significantly higher incidence (p < 0.05) of behavioural/and or eating disorders than those with IGF-1 levels above the threshold (Pearson's χ2 test).
) behavioural or eating disorders. IGF-1 was inversely correlated with prolactin in the preterm group (solid line; r = −0.38, p = 0.046); in contrast, the combined term-SGA and controls showed a positive correlation (dashed line; r = 0.23, p < 0.1). The mean IGF-I in the entire cohort was 207 μg/L. When the rounded-off mean value (210 μg/L) was used as a threshold value, it was clear that preterm children with IGF-1 levels below 210 μg/L had a significantly higher incidence (p < 0.05) of behavioural/and or eating disorders than those with IGF-1 levels above the threshold (Pearson's χ2 test).
Behavioural, eating and sleeping disorders at follow-up
Compared with the term-SGA/control group, preterm children presented significantly higher incidences of behavioural (38% versus 10%), eating (26% versus 8%) and sleeping disorders (23% versus 5%), but the groups were not different in the incidence of affective disorders (Table 3). No significant gender differences were found among the participants, with behavioural disorders observed in 15% of the girls and 29% of the boys (p = 0.091, Pearson's χ2 test). Among children born preterm, behavioural disorders were observed in 32% of the girls and 47% of the boys (p = 0.33, Pearson's χ2 test). Combined behavioural and eating disorders were found in eight preterm children, but in none of the children in the combined term-SGA/control group (Table 3).
Table 3.
Behavioural, eating, sleeping and affective disorders at follow-up for children born prematurely (preterm), full-term, but small for gestational age (term-SGA), or full-term with normal birthweight (controls)
| Disorder | 1. Preterm (n = 39) | 2. Term-SGA (n = 28) | 3. Controls (n = 33) | p | 1 vs. 2+3 |
|---|---|---|---|---|---|
| Behaviour | 15 (38) | 3 (11) | 3 (9) | 0.003 | <0.001 |
| Eating | 10 (26) | 4 (15) | 2 (6) | 0.07 | 0.02 |
| Sleeping | 9 (23) | 2 (7) | 1 (3) | 0.022 | 0.006 |
| Combined behaviour and eating | 8 (21) | 0 (0) | 0 (0) | 0.001 | <0.001 |
| Affective | 6 (15) | 3 (11) | 2 (6) | 0.45 | 0.26 |
Values represent the, number of individuals (%); Significant p-values, p < 0.05 are in italics, according to Pearson's chi-square test.
Relationships between disorders and growth parameters
Among preterm children, those with and those without combined behavioural and eating disorders had similar head circumference SDS values at birth (p = 0.27). However, at follow-up, the eight children with combined behavioural and eating disorders had significantly smaller head circumferences (mean 51.3, 95% CI: 49.7–52.9 cm versus mean 53.4, 95% CI: 53.0–53.8 cm, p < 0.001) and greater deviations from the target height SDS (mean −2.03, 95% CI: −3.13 to −0.92 versus mean −0.35, 95% CI: −0.71 to 0.00), p < 0.001) than the 31 preterm children without the combined disorders. There appeared to be a larger difference between groups in height than in head circumference, which suggested a head-sparing effect (Figure S1).
Relationships between disorders and hormone levels in preterm children
According to the t-test and ANOVAs, the 12 children in the preterm group with behavioural and/or eating disorders had lower leptin (mean 2.1 μg/L, 95% CI: 1.0–4.3 versus mean 8.2 μg/L, 95% CI: 4.8–13.8; p < 0.01), insulin (mean 3.1 pmol/L, 95% CI: 2.0–4.7 versus mean 5.3 pmol/L, 95% CI: 4.2–6.8; p < 0.05) and IGF-I levels (mean 195 μg/L, 95% CI: 155–245 versus mean 263 μg/L, 95% CI: 232–299; p < 0.05) than the 16 children born preterm without the disorders. However, there were no differences between the groups in prolactin (p = 0.11), glucagon (p = 0.38) or cortisol levels (p = 0.65). Nevertheless, the correlations between leptin and IGF-I, IGFBP-1, insulin, growth hormone, prolactin and glucagon were similar in preterm children with and without behavioural and/or eating disorders (Figure S2 and Table 4). Among the two full-term groups, only the correlation between leptin and IGF-1 differed between term-SGA and controls. It was positive in the term-SGA children, but not significant in the controls (Table 4). Glucagon correlated inversely with leptin in both full-term groups, but not in the preterm children (Table 4).
Table 4.
Correlations between serum levels of leptin and IGF-I, IGFBP-1, insulin, growth hormone, prolactin and glucagon in all children born prematurely (preterm), preterm with disorders, full-term, but small for gestational age (term-SGA), or full-term with normal birthweight (control)
| Hormone | 1. Preterm (all, n = 28) | 2. Preterm with disorders (n = 12) | 3. Term-SGA (n = 23) | 4. Control (n = 29) |
|---|---|---|---|---|
| Insulin | 0.78*** | 0.66* | 0.45* | 0.45* |
| IGFBP-1 | −0.70*** | −0.54 | −0.45* | −0.69*** |
| Glucagon | 0.10 | −0.11 | −0.42* | −0.55** |
| IGF-I | 0.48* | 0.44 | 0.45* | 0.29 |
| Prolactin | −0.23 | −0.28 | 0.08 | −0.03 |
| Growth hormone | −0.54* | −0.35 | 0.23 | −0.26 |
IGFBP-1 = insulin-like growth factor-binding protein-1, IGF-I = insulin-like growth factor-1.
*p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
We found that children born preterm had lower prolactin levels than those born full-term. To our knowledge, this finding has not been previously reported in children. Prolactin has been proposed to promote body weight in humans, and prolactin levels were reduced with chronic energy intake restriction (23).
The reduced prolactin in preterm children might be the result of a higher dopaminergic tonus in the brains of these children. Dopaminergic neurons exert negative feedback on prolactin release (24) and positive feedback on growth hormone release (25). We did not detect significantly elevated growth hormone in the preterm group, but growth hormone release is pulsatile, with peaks during the night. We also observed a difference in IGF-1 among preterm children compared with control subjects. This difference might relate to an increased brain dopamine tonus, because serum IGF-1 concentrations reflect growth hormone secretion rates (26). In another study, we also observed increased IGF-1 levels among preterm participants who had birthweights appropriate for gestational age compared with control subjects (11). The inverse correlation between prolactin and IGF-1 indicated that higher IGF-1 levels were associated with lower prolactin levels in children born preterm. Despite relatively elevated IGF-1, preterm children grew to a relatively short height. These findings implied that preterm subjects may have reduced sensitivity in the IGF-1 receptors, which then required higher IGF-1 levels for sufficient signalling. Furthermore, in contrast to our expectations, we found a tendency to lower cortisol levels in the preterm group compared with the controls. This finding was consistent with findings from a previous study with older children born preterm, who were submitted to a stress test (10).
In contrast to the full-term groups (SGA/controls), no inverse correlation was found between glucagon and leptin in the preterm children. The robust correlation between insulin and leptin observed among the preterm children indicated that increases in insulin elicited a prompt promotion of fat mass incrementation, and thus increased leptin, in this group. This strong positive correlation between insulin and leptin might explain the lack of inverse correlation between glucagon and leptin among these subjects, as reductions in glucagon might be inhibited to prevent hypoglycaemia in the presence of elevated leptin and insulin (27).
We also found a higher prevalence of behavioural, eating and sleeping disorders among children born preterm compared with children born at term. More than 50% of the children born preterm with behavioural problems also reported eating problems and 59% of the children born at <27 gestational weeks reported at least one disorder. Subjects with both disorders showed the largest deviation from predicted target height at puberty onset, and they had smaller head circumferences than preterm children without disorders. Among the children born preterm, those with behavioural and/or eating disorders had lower leptin, insulin and IGF-I levels than those without disorders.
Our results confirm previous findings that children born preterm show an increased prevalence of behavioural and eating disorders (1–6). Hack et al. (28) studied 9-year-old children born with very low birthweight and found that a small head circumference was associated with poor academic achievement, low IQ and behavioural problems. In our study, among the children born extremely preterm, those with combined disorders had the smallest head circumferences.
We found lower levels of leptin, insulin and IGF-I in preterm children with behavioural and/or eating disorders compared with preterm children without those disorders. However, the associations between hormones and leptin were similar between these groups. This indicated that several hormones were associated with leptin (i.e. the percentage of fat tissue) in a similar way in the two groups. Therefore, the regulation of these hormones in relation to leptin appeared to be functioning properly in subjects with behavioural and/or eating disorders. As a consequence, the cause of lower hormone levels in these subjects is unknown. One may speculate that the preterm birth alters a neuronal set point, which induces lower levels of IGF-I, insulin and leptin in this group compared with children born preterm without disorders. Conversely, behavioural/eating disorders may also result in these findings (29). Unfortunately, we lack information about the thyroid function/status in this cohort. Based on our findings, we propose a hypothesis that integrates our findings (Fig.2).
Figure 2.
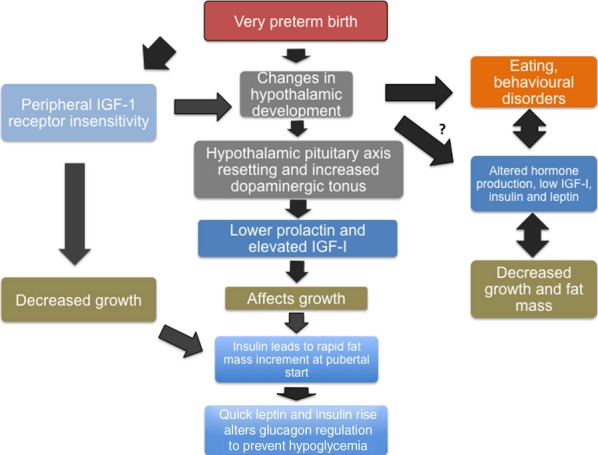
A schematic of our hypothesis based on our findings.
There were some limitations in this study. First, we were unable to obtain blood samples from the entire cohort. In the preterm group, 30% of the serum samples were missing, which could skew the results. Furthermore, the morning fasting values of growth hormone and prolactin might not reflect their diurnal production rates. Finally, the assessment of disorders was based on a written form, followed up with a visit and discussion of the general health history and a history of behavioural patterns, but it was not based on a formal behavioural questionnaire.
In conclusion, we found that preterm children had lower prolactin and higher IGF-I levels than controls. These differences might relate to early life imprinting mechanisms. We also noted the presence of a combined behavioural and eating disorder in the preterm subjects alone, which was not emphasised in earlier studies. This subgroup of preterm children exhibited the largest deviation from the target height at puberty onset. When examining prematurely born children, special attention and care may be required to take notice of this combined disorder. Eating and behavioural disorders in children born preterm were associated with lower leptin, insulin and IGF-1 levels, but the coordinated regulation of these hormones appeared to function properly. Lower prolactin and higher IGF-I levels suggested that dopaminergic tonus may be increased in children born preterm; this observation raises speculation about IGF-I receptor insensitivity.
Acknowledgments
The authors wish to thank Professor Kerstin Hall for fruitful discussions and Agneta Hilding at the Karolinska Institutet for valuable statistical advice. We also thank Giovanna Marchini at Astrid Lindgren Children's Hospital for her help and assistance. We would also like to express our sincere gratitude to the children that took part in this study and their families.
Glossary
- ANCOVA
Analysis of covariance
- ANOVA
Analysis of variance
- BMI
Body mass index
- HPA
Hypothalamic–pituitary–adrenal
- IGFBP-1
IGF-binding protein-1
- IGF-I
Insulin-like growth factor-1
- SD
Standard deviation
- SGA
Small for gestational age
Funding
This study was supported by the von Kantzow Foundation, the Magnus Bergvall Foundation, the Samaritan Foundation, the Mjolkdroppen Foundation, the Barnavard Society, Children Research Foundation at Astrid Lindgren Children's Hospital, a Freemason Scholarship and the Mayflower Annual Campaign for Children's Health.
Conflict of Interest
The authors declare no conflict of interests with the study sponsors. The study sponsors played no role in the study design or the collection, analysis and interpretation of the data.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Figure S1 Body length SDS and head circumference (HC) SDS at birth and at follow-up among preterm children.
Figure S2 Correlations between leptin and the various hormones.
Table S1 Birth characteristics for children born prematurely (preterm), full term, but small for gestational age (term-SGA), or at term with normal birthweight (control).
References
- 1.Delobel-Ayoub M, Arnaud C, White-Koning M, Casper C, Pierrat V, Garel M, et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009;123:1485–92. doi: 10.1542/peds.2008-1216. [DOI] [PubMed] [Google Scholar]
- 2.Schaap AH, Wolf H, Bruinse HW, Smolders-de Haas H, van Ertbruggen I, Treffers PE. School performance and behaviour in extremely preterm growth-retarded infants. Eur J Obstet Gynecol Reprod Biol. 1999;86:43–9. doi: 10.1016/s0301-2115(99)00041-x. [DOI] [PubMed] [Google Scholar]
- 3.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–28. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 4.Samara M, Marlow N, Wolke D. Pervasive behavior problems at 6 years of age in a total-population sample of children born at </= 25 weeks of gestation. Pediatrics. 2008;122:562–73. doi: 10.1542/peds.2007-3231. [DOI] [PubMed] [Google Scholar]
- 5.Douglas JE, Bryon M. Interview data on severe behavioural eating difficulties in young children. Arch Dis Child. 1996;75:304–8. doi: 10.1136/adc.75.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samara M, Johnson S, Lamberts K, Marlow N, Wolke D. Eating problems at age 6 years in a whole population sample of extremely preterm children. Dev Med Child Neurol. 2010;52:e16–22. doi: 10.1111/j.1469-8749.2009.03512.x. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–7. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seckl JR. Glucocorticoid programming of the fetus; adult phenotypes and molecular mechanisms. Mol Cell Endocrinol. 2001;185:61–71. doi: 10.1016/s0303-7207(01)00633-5. [DOI] [PubMed] [Google Scholar]
- 9.Maccari S, Morley-Fletcher S. Effects of prenatal restraint stress on the hypothalamus-pituitary-adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendocrinology. 2007;32(Suppl 1):S10–5. doi: 10.1016/j.psyneuen.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Kaseva N, Wehkalampi K, Pyhala R, Moltchanova E, Feldt K, Pesonen AK, et al. Blunted hypothalamic-pituitary-adrenal axis and insulin response to psychosocial stress in young adults born preterm at very low birth weight. Clin Endocrinol. 2014;80:101–6. doi: 10.1111/cen.12251. [DOI] [PubMed] [Google Scholar]
- 11.Kistner A, Vanpee M, Hall K. Leptin may enhance hepatic insulin sensitivity in children and women born small for gestational age. Endocr Connect. 2013;2:38–49. doi: 10.1530/EC-12-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ates M, Dayi A, Kiray M, Sisman A, Agilkaya S, Aksu I, et al. Anxiety- and depression-like behavior are correlated with leptin and leptin receptor expression in prefrontal cortex of streptozotocin-induced diabetic rats. Biotech Histochem. 2014;89:161–71. doi: 10.3109/10520295.2013.825319. [DOI] [PubMed] [Google Scholar]
- 13.Roubos EW, Dahmen M, Kozicz T, Xu L. Leptin and the hypothalamo-pituitary-adrenal stress axis. Gen Comp Endocrinol. 2012;177:28–36. doi: 10.1016/j.ygcen.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Murphy LJ, Tachibana K, Friesen HG. Stimulation of hepatic insulin-like growth factor-I gene expression by ovine prolactin: evidence for intrinsic somatogenic activity in the rat. Endocrinology. 1988;122:2027–33. doi: 10.1210/endo-122-5-2027. [DOI] [PubMed] [Google Scholar]
- 15.Jiang XB, He DS, Mao ZG, Fan X, Lei N, Hu B, et al. BMI, apolipoprotein B/apolipoprotein A-I ratio, and insulin resistance in patients with prolactinomas: a pilot study in a Chinese cohort. Tumour Biol. 2013;34:1171–6. doi: 10.1007/s13277-013-0660-z. [DOI] [PubMed] [Google Scholar]
- 16.Kistner A, Rakow A, Legnevall L, Marchini G, Brismar K, Hall K, et al. Differences in insulin resistance markers between children born small for gestational age or born preterm appropriate for gestational age. Acta Paediatr. 2012;101:1217–24. doi: 10.1111/apa.12005. [DOI] [PubMed] [Google Scholar]
- 17.Johansson S, Norman M, Legnevall L, Dalmaz Y, Lagercrantz H, Vanpee M. Increased catecholamines and heart rate in children with low birth weight: perinatal contributions to sympathoadrenal overactivity. J Intern Med. 2007;261:480–7. doi: 10.1111/j.1365-2796.2007.01776.x. [DOI] [PubMed] [Google Scholar]
- 18.Hogberg U, Larsson N. Early dating by ultrasound and perinatal outcome. A cohort study. Acta Obstet Gynecol Scand. 1997;76:907–12. doi: 10.3109/00016349709034900. [DOI] [PubMed] [Google Scholar]
- 19.Niklasson A, Albertsson-Wikland K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr. 2008;8:8. doi: 10.1186/1471-2431-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wikland KA, Luo ZC, Niklasson A, Karlberg J. Swedish population-based longitudinal reference values from birth to 18 years of age for height, weight and head circumference. Acta Paediatr. 2002;91:739–54. doi: 10.1080/08035250213216. [DOI] [PubMed] [Google Scholar]
- 21.Rydelius PA. Children and youth psychiatry and certain neuropsychiatric issues. In: Lindberg T, Lagercrantz H, editors. Barnmedicin. 3rd ed. Studentlitteratur Denmark: Narayana Press; 2007. pp. 498–529. [Google Scholar]
- 22.Isager T. Psychiatric and psychosomatic diseases. In: Krasilnikoff PA, Holmberg L, Lie SO, Schiotz PO, Visakorpi JK, editors. Nordisk laerebog i Paediatri. Kopenhavn: Munksgaard; 1993. pp. 542–7. [Google Scholar]
- 23.Hamada N, Engelman RW, Tomita Y, Chen RF, Iwai H, Good RA, et al. Prolactin effects on the dietary regulation of mouse mammary tumor virus proviral DNA expression. Proc Natl Acad Sci USA. 1990;87:6733–7. doi: 10.1073/pnas.87.17.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinberg DL, Noel GL, Frantz AG. Chlorpromazine stimulation and L-dopa suppression of plasma prolactin in man. J Clin Endocrinol Metab. 1971;33:873–6. [Google Scholar]
- 25.Brown GM, Reichlin S. Psychologic and neural regulation of growth hormone secretion. Psychosom Med. 1972;34:45–61. doi: 10.1097/00006842-197201000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Blum WF, Albertsson-Wikland K, Rosberg S, Ranke MB. Serum levels of insulin-like growth factor I (IGF-I) and IGF binding protein 3 reflect spontaneous growth hormone secretion. J Clin Endocrinol Metab. 1993;76:1610–6. doi: 10.1210/jcem.76.6.7684744. [DOI] [PubMed] [Google Scholar]
- 27.Jarhult J, Farnebo LO, Hamberger B, Holst J, Schwartz TW. The relation between catecholamines, glucagon and pancreatic polypeptide during hypoglycaemia in man. Acta Endocrinol. 1981;98:402–6. doi: 10.1530/acta.0.0980402. [DOI] [PubMed] [Google Scholar]
- 28.Hack M, Breslau N, Weissman B, Aram D, Klein N, Borawski E. Effect of very low birth weight and subnormal head size on cognitive abilities at school age. N Engl J Med. 1991;325:231–7. doi: 10.1056/NEJM199107253250403. [DOI] [PubMed] [Google Scholar]
- 29.Nakai Y, Hamagaki S, Kato S, Seino Y, Takagi R, Kurimoto F. Leptin in women with eating disorders. Metabolism. 1999;48:217–20. doi: 10.1016/s0026-0495(99)90037-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Body length SDS and head circumference (HC) SDS at birth and at follow-up among preterm children.
Figure S2 Correlations between leptin and the various hormones.
Table S1 Birth characteristics for children born prematurely (preterm), full term, but small for gestational age (term-SGA), or at term with normal birthweight (control).


