Abstract
Aim
Pulse oximetry screening of newborn infants increases early detection of critical congenital heart disease and minimises the risk of circulatory collapse before surgery. This study provides an update on the implementation of pulse oximetry screening in the Nordic countries and proposes standardised guidelines.
Methods
A questionnaire exploring pulse oximetry screening, clinical examination routines and availability of echocardiography was distributed to all 157 delivery units in the Nordic countries in June 2013.
Results
We received responses from 156 of the 157 delivery units, and 116 (74%) were using pulse oximetry screening by September 2013. Preductal and postductal screening were both used in 59 of 116 units (51%), with just postductal screening in 51 of 116 (44%) and just preductal screening alone in 6 of 116 (5%). Screening was performed before 24 h in 105 of 116 units (91%). The implementation of screening was highest in Finland (29/30, 97%), Sweden (42/46, 91%) and Norway (43/48, 90%) and lowest in Denmark (2/24, 8%) and Iceland (0/8 units).
Conclusion
In Sweden, Norway and Finland, the implementation of pulse oximetry screening is currently the highest in the world and coverage will be close to 100% in 2014. We propose uniform Nordic guidelines using preductal and postductal screening before 24 h of age.
Keywords: Congenital heart disease, Guidelines, Newborn screening, Pulse oximetry
Key notes
Pulse oximetry screening of newborn infants increases early detection of critical congenital heart disease and minimises the risk of circulatory collapse before surgery.
In Sweden, Norway and Finland, the implementation of pulse oximetry screening is currently the highest in the world and coverage will be close to 100% in 2014.
We propose uniform Nordic guidelines using preductal and postductal screening before 24 h of age.
Introduction
If newborns with critical congenital heart defects (CCHD) are left untreated, they will develop circulatory collapse and die, usually within a few days or weeks after birth. Although foetal heart screening has the potential to detect a substantial proportion of such defects, the majority of cases of CCHD are still left for postnatal diagnosis in large populations (1–3). Because symptoms and signs may be subtle or lacking, CCHD may be missed in the routine clinical examination of newborns (4,5). Subnormal arterial oxygen saturation, which is present in most cases of CCHD, is often clinically undetectable (6).
In a Swedish study, 26% of newborns with CCHD were discharged without a diagnosis (5). A UK study reported that one-third of infants with potentially life-threatening cardiac defects were discharged home with the disease undetected and 5% of them died before receiving a diagnosis (7). The trend towards earlier discharge after birth may increase the risk of this happening (5). A recent U.S. study estimated that 30% of newborns with CCHD had a late diagnosis and would have benefitted from CCHD screening (8).
Large prospective, population-based multicentre studies from Norway (9), Sweden (1), Germany (10), the United Kingdom (11) and Poland (12) have confirmed the test accuracy of universal pulse oximetry screening. In 2012, a large meta-analysis of 13 high-quality studies comprising nearly 230 000 infants came to the same conclusion (13). The most recent contribution to the evidence is also the largest. A Chinese multicentre study screened 122 738 newborns, confirming the significant improvement in the detection of CCHD by adding pulse oximetry screening and proving that this also works in a developing country (14).
In the United States, the Secretary of Health and Human Services recommended pulse oximetry screening of all newborns in 2011 (15). The strategy for implementing screening, endorsed by the American College of Cardiology Foundation, the American Heart Association and the American Academy of Paediatrics, was that screening should be:
performed using motion-tolerant oximeters cleared by the Food and Drug Administration for reporting functional oxygen saturation;
based on the Swedish screening algorithm (1) and performed by qualified personnel educated in the use of the algorithm and trained in pulse oximetry monitoring of newborns (16); and
performed between 24 and 48 h of age or shortly before discharge if <24 h of age.
To stress the importance of using proper equipment for screening, the recommendation states the use of a pulse oximeter that can read through motion and low perfusion. There are currently only four U.S. States that have not yet taken any action to implement screening. A paper addressing the challenges and opportunities of the implementation process in the United States was published in 2013 (17).
In the United Kingdom, a shift in opinion has been noted since 2010 and the vast majority of neonatologists are now in favour of screening (18).
Only four European countries (Switzerland, Ireland, Poland and Norway) have a national recommendation to screen despite the fact that all but one of the largest published multicentre studies were conducted in Europe (1,9–12).
Recently, at a meeting in Turin, steps were taken by an international group of neonatologists, paediatric cardiologists and screening experts to promote CCHD screening across Europe (19).
The purpose of this study was to examine the extent of pulse oximetry screening in the Nordic countries, as well as details of the screening methods used, and to discuss whether the results could provide a basis for uniform Nordic guidelines.
Patients and Methods
A form containing 28 questions exploring pulse oximetry screening, clinical examination routines and availability of echocardiography was distributed to all 157 delivery units in the Nordic countries in June 2013. The results were presented at the Nordic Paediatric Cardiology meeting in Copenhagen in September 2013 and describe the situation at that point in time. Following this, a proposal for uniform Nordic guidelines was agreed by the authors.
Descriptive statistics are presented as numbers and percentages. No statistical comparisons were made.
Results
We received responses to the questionnaire from 156 of the 157 delivery units (Table 1). The number of delivery units and live births, routines for clinical examination of newborns and access to echocardiography in each country are shown in Table 1. It can be seen that Sweden, Finland and Denmark have a centralised birth pattern, while in Norway, births are more decentralised. In Iceland, 73% of the 4500 deliveries per year are centralised to Reykjavik, but the remaining deliveries are distributed between seven units.
Table 1.
Number of delivery hospitals and live births, newborn examination routines, access to echocardiography and pulse oximetry screening routines in the Nordic countries in September 2013. Percentages were calculated based on the number of responding units unless otherwise stated
| Sweden | Norway | Finland | Denmark | Iceland | Total | |
|---|---|---|---|---|---|---|
| Number of delivery units | 46 | 48 | 30 | 25 | 8 | 157 |
| Number of responding units | 46 | 48 | 30 | 24 | 8 | 156 |
| Live births 2012 | 113 137 | 61 149 | 59 038 | 59 527a | 4 450 | 297 301 |
| Number of units with | ||||||
| <500 deliveries | 2 (4.3%) | 24 (50.0%) | 5 (16.7%) | 2 (8.3%) | 7 (87.5%) | 40 (25.6%) |
| 500–1500 | 12 (26.1%) | 11 (22.9%) | 11 (36.7%) | 6 (25.0%) | 0 | 40 (25.6%) |
| >1500 | 32 (69.6%) | 13 (27.1%) | 14 (46.7%) | 16 (66.7%) | 1 (12.5%) | 76 (48.7%) |
| Number of examinations by physicianb | ||||||
| 0 | 0 | 0 | 0 | 24 (100.0%) | 0 | 24 (15.4%) |
| 1 | 37 (80.4%) | 43 (89.6%) | 26 (86.7%) | 0 | 0 | 106 (67.9%) |
| 2 | 9 (19.6%) | 5 (10.4%) | 4 (13.3%) | 0 | 8 (100.0%) | 26 (16.7%) |
| Transport always needed for echo | 4 (8.7%) | 25 (52.1%) | 5 (16.7%) | 16 (66.7%) | 6 (75.0%) | 56 (35.9%) |
| Pulse oximetry screening status | ||||||
| Screening by September 2013 | 42 (91.3%) | 43 (89.6%) | 29 (96.7%) | 2 (8.3%) | 0 | 116 (74.4%) |
| Will start during 2013 | 4 (8.7%) | 3 (6.3%) | 0 | 0 | 0 | 7 (4.5%) |
| Will start during 2014 | 0 | 2 (4.2%) | 1 (3.3%) | 2 (8.3%) | 0 | 5 (3.2%) |
| Proportion of neonates screenedc | 95% | 89% | 99% | ≅10% | 0 | ≅ 76% |
| Units screening at <24 hd | 32 (76.2%) | 42 (97.7%) | 29 (100.0%) | 2 (100.0%) | – | 105 (90.5%) |
| Screening methodd | ||||||
| Measuring in right hand and one foot | 42 (100.0%) | 7 (16.3%) | 10 (34.5%) | 0 | – | 59 (50.9%) |
| Measuring in right hand only | 0 | 5 (11.6%) | 1 (3.4%) | 0 | – | 6 (5.2%) |
| Measuring in one foot only | 0 | 31 (72.1%) | 18 (62.1%) | 2 (100.0%) | – | 51 (44.0%) |
2011.
Second examination before discharge or at follow-up.
Estimated based on birth rates 2012 (2011 for Denmark) and screening policy in September 2013.
Percentages were calculated based on number of units screening by September 2013.
In all Nordic countries, with the notable exception of Denmark, newborns are routinely examined, at least once, by a physician before discharge. In Sweden and Finland, the newborn examination is always performed by a paediatric consultant or resident, whereas in some units in Norway and Iceland, nonpaediatric physicians undertake this clinical screening. In Denmark, the physical examination of newborns by a physician was cancelled in most units in 2007, because it was considered that there was not enough scientific evidence of its benefits (20). Instead, newborns are examined by a midwife in 75% of units. Auscultation of the heart and palpation of femoral pulses, however, are not part of this examination.
Urgent echocardiography 24 h a day is available for all newborns in the Nordic countries, but sometimes only after transport. This is especially so in Norway, where 25 of the 48 delivery units have to transport a baby to another hospital for echocardiography (Table 1).
Local guidelines regarding investigation of cardiac malformations in newborns are in use in 85% of all delivery units in Sweden, 77% in Norway, 87% in Finland and 76% in Denmark. In Iceland, there are no written guidelines.
Pulse oximetry screening
In total, 116 of the 156 (74%) responding delivery units have implemented pulse oximetry screening for CCHD (Table 1). The implementation of screening is highest in Finland, Sweden and Norway and lowest in Denmark and Iceland (Table 1).
There were no national guidelines for pulse oximetry screening in any of the Nordic countries when the questionnaire was distributed (June 2013). However, during that month, the Norwegian Directorate of Health endorsed universal pulse oximetry screening (21). Regardless of the lack of guidelines, screening was gradually implemented in Sweden, Norway and Finland from 2004. A high prenatal detection rate was cited as the main reason for not screening in Denmark and a high prenatal detection rate combined with a very low neonatal mortality rate in Iceland. This was the lowest in Europe for 3 years in a row at 0.9–1.1 per 1000 in 2010–2012. Other reasons mentioned by units for not screening were lack of national guidelines and concerns about false positives.
Table 1 shows data for pulse oximetry screening routines. In Sweden, all units use the Granelli protocol, which calls for preductal and postductal screening (1), whereas in Norway, most units use only postductal screening (9). In Finland, there is a mixture of both protocols. The majority of units in all three countries screen before 24 h of age and most use motion-tolerant oximeters. In both Sweden and Norway, 95% of the screening units use reusable sensors in contrast to Finland, where 55% use disposable sensors. A mix of midwives and nurses perform the screening, with midwives making up the majority.
The result of screening is stored electronically in all Swedish units (17% also keep paper records), 88% in Norway (17% paper records) and 80% in Finland (12% paper records). However, none of the units in the Nordic countries store the results in a digital database enabling retrieval of data for research purposes, without having to check each individual patient file.
Uniform guidelines
We propose uniform Nordic guidelines (Table 2) based on the Swedish algorithm with the modification to always screen within 24 h of birth. This algorithm is presented in Figure1.
Table 2.
The proposed uniform Nordic pulse oximetry screening guidelines
| Screening should be done with a motion-tolerant pulse oximeter reading through low perfusion and reporting functional oxygen saturation |
| Screening should be based on the endorsed screening algorithm and performed by qualified personnel who have been educated in the use of the algorithm and trained in pulse oximetry monitoring of newborns |
| A positive screen occurs when |
| One oxygen saturation value is <90% |
| Oxygen saturation is <95% in both right hand and one foot or there is a >3% absolute difference in oxygen saturation between the right hand and one foot on three repeated measurements. Any measurement that is >95% in either extremity with <3% absolute difference in oxygen saturation between the upper and lower extremity would be considered a pass and screening would end |
| The screening should be conducted within the first 24 h of life |
| Standards should be developed for digital reporting of the screening result that can be retrieved for follow-up |
Figure 1.
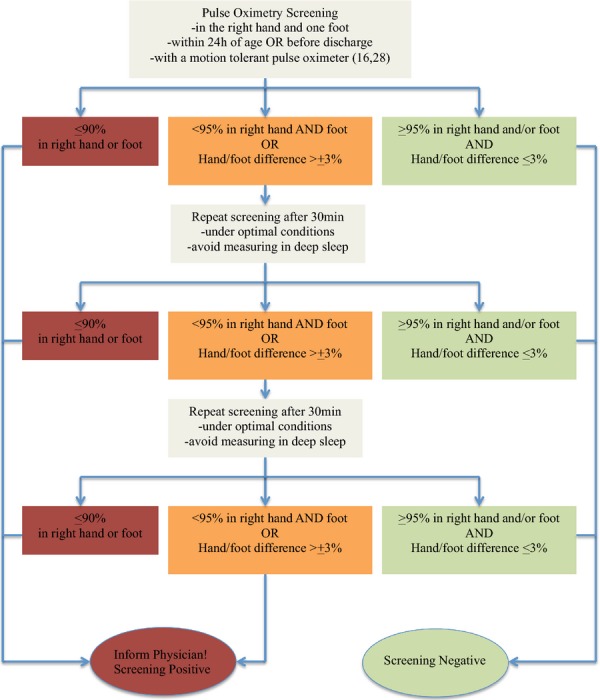
Proposed screening algorithm for the Nordic countries.
Discussion
In this update on pulse oximetry screening routines in the five Nordic countries, we found that screening had been successfully implemented in three of them (Sweden, Norway and Finland). This has occurred as a bottom-up approach, in the absence of national recommendations, special funding or training. Screening has reached over 90% coverage across these three countries, currently the greatest proportion in the world. This shows the feasibility of the method and clearly dismisses concerns that this method only works in large research settings with dedicated staff and funding (22).
In Denmark, the general belief has been that prenatal detection rates are so high that screening would not be cost-effective, although actual detection rates have not been widely acknowledged. In Iceland, an active decision was made not to screen on the same grounds. We did not examine prenatal detection rates. However, all five countries have programmes for foetal heart screening in the second trimester. These programmes always include the four-chamber view and in a majority of units also outflow views and the three-vessel view. The effectiveness of prenatal screening programmes is, however, highly variable within countries as well as between countries (2,23,24). Some areas in Sweden, for example, report more than 75% prenatal detection of CCHD, whereas other areas detect <25% (personal communications, unpublished data). The situation in the United Kingdom is similar, with a wide variation in detection rates (3). In Denmark, the general assumption has been that the prenatal detection rate is high, but at the annual Danish neonatology meeting in 2011, it was reported that the prenatal detection of CCHD was 34% in 2008–2009 (25). Partly as a result of the present study, at least two Danish units are now planning to start pulse oximetry screening, with a bottom-up approach, while waiting for a national recommendation.
When employing pulse oximetry screening on national levels, the diagnostic yield may be lower in areas served by large university hospitals with effective prenatal screening programmes compared to other areas, but screening should be promoted on all levels to optimise the diagnostic yield on a national level. We encourage a combined approach with effective prenatal screening according to International Society of Ultrasound in Obstetrics and Gynaecology guidelines (26) and postnatal pulse oximetry screening using the algorithm presented here to optimise perinatal care of foetuses and newborns with CCHD.
Differences between recommended screening algorithms
One of the main obstacles preventing or delaying implementation of pulse oximetry screening, reported by physicians in the United Kingdom and the Nordic countries, has been a lack of national recommendations and/or guidelines. When the Norwegian Directorate of Health endorsed universal pulse oximetry screening in June 2013 (21), it recommended postductal screening after 24 h of age, thus diverging both from the United States (preductal and postductal, 24–48 h of age) and our proposal for common Nordic recommendations (preductal and postductal before 24 h). We believe screening within the first 24 h after birth is justified despite a higher proportion of false positives (11), because the majority of the false positives resulting from pulse oximetry screening have true hypoxia requiring special or intensive care (early infections, pulmonary hypertension and other pulmonary disorders), and thus can be considered as secondary targets and an additional advantage with screening (1,9,11,12,14). In a recent study, Singh et al. (27) evaluated the impact of CCHD screening before 24 h of age and found that of 208 (0.8%) who screened positive, 17 had CHD and 148 had other clinical conditions requiring further intervention, confirming the additional benefit of screening confirming this additional benefit of screening. In a hypothetical future situation, with close to 100% prenatal detection rate of CCHD across all delivery units in a country, including small delivery units in decentralised organisations, such as in Norway, there may still therefore be a place for postnatal pulse oximetry screening. Another justification for this recommendation is that the screening is already performed within 24 h in nearly all units in Sweden, Norway and Finland. Screening may take place either in the delivery unit before discharge if very early discharge is planned or before transfer to the nursery, or in the nursery before 24 h of age.
The recommendation to screen both preductally and postductally is based on the fact that this increases the chances of detecting some types of CCHD. In transposition of the great arteries, with or without aortic arch obstruction, only postductal screening may miss the hypoxia as these babies can have a higher, and sometimes normal, postductal saturation with concomitant critically low preductal saturation (28). Newborns with coarctation of the aorta sometimes have a lower postductal than preductal saturation and will then be detected by screening if it is performed in both the right hand and a foot (28). However, most newborns with coarctation of the aorta have normal saturations both pre- and postductally and will therefore be missed by pulse oximetry screening (1). The reason for this is presumably a large left-to-right shunt across the foramen ovale of fully saturated blood and a large pulmonary blood flow resulting in a normal or near-normal saturation in the blood shunted right to left across the ductus arteriosus. Another mechanism could be that in some neonates, the coarctation may be mild initially, when pulse oximetry screening is performed, with a pure left-to-right shunt through the ductus arteriosus. This is an important limitation of the method as coarctation of the aorta is the CCHD most commonly overlooked in the newborn examination (5). Careful examination of the newborn, including palpation of the femoral pulses and echocardiography before discharge in all neonates with weak or absent femoral pulses, should therefore be mandatory also in units employing pulse oximetry screening.
To improve quality in screening for CCHD, the use of a computer-based tool to interpret the algorithm was recently proposed (29). The authors found the manual algorithm to be susceptible to human error. Their findings underline the importance of proper training of the personnel to understand the algorithm for CCHD screening and to make correct interpretations of their measurements.
Limitations
One limitation of this study was that we lacked up-to-date data on prenatal detection rates for duct-dependent cardiac defects in all areas of all Nordic countries. Such data would have been of value to analyse the role of postnatal pulse oximetry screening routines and recommendations in the complete perspective of pre- and postnatal detection of CCHD.
Another limitation was that none of the Nordic countries employing pulse oximetry screening store the screening results in a way that data can be retrieved for whole populations. When such data are available for all newborns, together with data on whether there was a prenatal diagnosis or not, a detailed analysis of the relative contributions of the two screening methods on a national level will be possible.
Challenges for the future
Coarctation of the aorta is the duct-dependent cardiac defect most often missed on clinical examination of the newborn, as well as by pulse oximetry screening. Coarctation is also often overlooked in prenatal ultrasound screening programmes (3). It is therefore an important research objective to try to improve pre- and postnatal detection of coarctation. There is some promise that the additional use of perfusion index may improve the detection of aortic arch obstructions after birth (30).
It is hoped that a consensus can be reached by the respective organisations in the Nordic countries to endorse uniform guidelines for pulse oximetry screening based on our proposal. An important next step will be to stimulate also other countries to consider national recommendations for pulse oximetry screening of all newborns.
Acknowledgments
The authors thank Nils-Gunnar Pehrsson for statistical support. We also thank the Nordic colleagues who answered the questionnaire. Special thanks to Karl Viktor Perminow, Oslo University Hospital, Oslo, Norway; Lars Björklund, Skane University Hospital, Lund, Sweden; Gunnar Bergman, Karolinska Institutet, Stockholm, Sweden; and Olli Pitkänen, Hospital for Children and Adolescents, University of Helsinki, Helsinki, Finland, for valuable advice and support.
Financial Disclosure
This study was made possible by funding from the Swedish Heart-Lung Foundation (Mats Mellander).
Conflict of Interest
The authors declare no conflict of interest in relation to the manuscript.
References
- 1.de-Wahl Granelli A, Wennergren M, Sandberg K, Mellander M, Bejlum C, Inganäs L, et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39 821 newborns. BMJ. 2009;8:338. doi: 10.1136/bmj.a3037. a3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meberg A, Andreassen A, Brunvand L, Markestad T, Moster D, Nietsch L, et al. Pulse oximetry screening as a complementary strategy to detect critical congenital heart defects. Acta Paediatr. 2009;98:682–6. doi: 10.1111/j.1651-2227.2008.01199.x. [DOI] [PubMed] [Google Scholar]
- 3.Gardiner H, Kovacevic A, van der Heijden L, Pfeiffer P, Franklin R, Gibbs J, et al. Prenatal screening for major congenital heart disease: assessing performance by combining national cardiac audit with maternity data. Heart. 2014;100:375–382. doi: 10.1136/heartjnl-2013-304640. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Harb M, Hey E, Wren C. Death in infancy from unrecognised congenital heart disease. Arch Dis Child. 1994;71:3–7. doi: 10.1136/adc.71.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mellander M, Sunnegårdh J. Failure to diagnose critical heart malformations in newborns before discharge – an increasing problem? Acta Paediatr. 2006;95:407–13. doi: 10.1080/08035250500541910. [DOI] [PubMed] [Google Scholar]
- 6.O'Donnell CPF, Kamlin COF, Davis PG, Carlin JB, Morley CJ. Clinical assessment of infant colour at delivery. Arch Dis Child Fetal Neonatal Ed. 2007;92:F465–7. doi: 10.1136/adc.2007.120634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wren C, Reinhardt Z, Khawaja K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. Arch Dis Child Fetal Neonatal Ed. 2008;93:F33–5. doi: 10.1136/adc.2007.119032. [DOI] [PubMed] [Google Scholar]
- 8.Peterson C, Ailes E, Riehle-Colarusso T, Oster ME, Olney RS, Cassell CH, et al. Late detection of critical congenital heart disease among US infants. Estimation of the potential impact of proposed universal screening using pulse oximetry. JAMA Pediatr. 2014;168((4)):361–370. doi: 10.1001/jamapediatrics.2013.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meberg A, Brügmann-Pieper S, Due R, Jr, Eskedal L, Fagerli I, Farstad T, et al. First day of life pulse oximetry screening to detect congenital heart defects. J Pediatr. 2008;152:761–5. doi: 10.1016/j.jpeds.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 10.Riede FT, Wörner C, Dähnert I, Möckel A, Kostelka M, Schneider P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine –results from a prospective multicentre study. Eur J Pediatr. 2010;169:975–81. doi: 10.1007/s00431-010-1160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewer AK, Middleton LJ, Furmston AT, Bhoyar A, Daniels JP, Thangaratinam S, et al. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study. Lancet. 2011;378:785–94. doi: 10.1016/S0140-6736(11)60753-8. [DOI] [PubMed] [Google Scholar]
- 12.Turska-Kmieć A, Borszewska-Kornacka MK, Błaż W, Kawalec W, Żuk M. Early screening for critical congenital heart defects in asymptomatic newborns in Mazovia province: experience of the POLKARD pulse oximetry programme 2006–2008 in Poland. Kardiol Pol. 2012;70:4. 370–6. [PubMed] [Google Scholar]
- 13.Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet. 2012;379:2459–64. doi: 10.1016/S0140-6736(12)60107-X. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Q, Ma X, Ge X, Liu F, Yan W, Wu L, et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014 doi: 10.1016/S0140-6736(14)60198-7. [Epub ahead of print], published online April 23: doi: 10.1016/S0140-6736(14)60198-7. [DOI] [PubMed] [Google Scholar]
- 15.Sebelius K. Secretary of Health and Human Services recommendation for pulse oximetry screening. Washington, DC: Department of Health and Human Services; 2011. http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendations/correspondence/cyanoticheartsecre09212011.pdf. [Google Scholar]
- 16.Kemper AR, Mahle WT, Martin GR, Cooley WC, Kumar P, Morrow WR, et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011;128:e1259–67. doi: 10.1542/peds.2011-1317. [DOI] [PubMed] [Google Scholar]
- 17.Martin RG, Beekman RH, 3rd, Bradshaw Mikula E, Fasules J, Garg LF, Kemper AR, et al. Implementing recommended screening for critical congenital heart disease. Pediatrics. 2013;132:e185–92. doi: 10.1542/peds.2012-3926. [DOI] [PubMed] [Google Scholar]
- 18.Singh A, Ewer AK. Pulse oximetry screening for critical congenital heart defects: a UK national survey. Lancet. 2013;381:535. doi: 10.1016/S0140-6736(13)60278-0. [DOI] [PubMed] [Google Scholar]
- 19.Ewer AK, de-Wahl Granelli A, Manzoni P, Sanchez-Luna M, Martin GR. Pulse oximetry screening for congenital heart defects. Lancet. 2013;382:856–7. doi: 10.1016/S0140-6736(13)61859-0. [DOI] [PubMed] [Google Scholar]
- 20. Afrapportering fra udvalg vedrörende rutineundersögelse af tilsyneladende raske börn. Dansk Paediatrisk Selskab og Dansk Selskab for obstetrik og gynekologi (Report from the committee concerning prophylactic health examinations of apparently healthy children in Denmark. Danish Paediatric Society and the Danish Society of Obstetrics and Gynecology) (In Danish) 2007.
- 21. Nytt liv og trygg barseletid for familien. Kortversjon av retningslinje for barseleomsorgen. Oslo: Helsedirektoratet. Nasjonale faglige retningslinjer;IS-2086 (New life and safe child birth time for the family. Summary of the Guidelines for maternity care. Oslo: Norwegian Health Directorate. National Guidelines IS-2086) (In Norwegian) June 2013.
- 22.Walsh W. Evaluation of pulse oximetry screening in Middle Tennessee: cases for consideration before universal screening. J Perinatol. 2011;31:125–9. doi: 10.1038/jp.2010.70. [DOI] [PubMed] [Google Scholar]
- 23.Tegnander E, Williams W, Johansen OJ, Blass H-GK, Eik-Nes S. Prenatal detection of heart defects in a non-selected population of 30 149 fetuses – detection rates and outcome. Ultrasound Obstet Gynecol. 2006;27:252–65. doi: 10.1002/uog.2710. [DOI] [PubMed] [Google Scholar]
- 24.Ojala T, Ritvanen A, Pitkänen O. Prenatal screening and diagnosis of severe congenital heart defects in Finland. Duodecim. 2013;129:2367–74. [PubMed] [Google Scholar]
- 25.Wehner B, Steensberg J, Reimers J, Andersen H. Saturation screening in Denmark. Oral presentation Annual Danish Neonatology meeting. G1 Avernaes 2011.
- 26.Carvalho JS, Allan LD, Chaoui R, Copel JA, DeVore GR, Hecher K, et al. ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol. 2013;41:348–59. doi: 10.1002/uog.12403. [DOI] [PubMed] [Google Scholar]
- 27.Singh A, Vishna Rasiah S, Ewer AK. The impact of routine predischarge pulse oximetry screening in a regional neonatal unit. Arch Dis Child Fetal Neonatal Ed. 2014:F1–6. doi: 10.1136/archdischild-2013-305657. [Epub ahead of print]. doi: 10.1136/archdischild-2013-305657. [DOI] [PubMed] [Google Scholar]
- 28.de-Wahl Granelli A, Mellander M, Sunnegårdh J, Sandberg K, Östman-Smith I. Screening for duct-dependent congenital heart disease with pulse oximetry: a critical evaluation of strategies to maximize sensitivity. Acta Paediatr. 2005;94:1590–6. doi: 10.1111/j.1651-2227.2005.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 29.Oster ME, Kuo KW, Mahle WT. Quality improvement in screening for critical congenital heart disease. J Pediatr. 2014;164:67–71. doi: 10.1016/j.jpeds.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 30.Granelli AW, Östman-Smith I. Noninvasive peripheral perfusion index as a possible tool for screening for critical left heart obstruction. Acta Paediatr. 2007;96:1455–9. doi: 10.1111/j.1651-2227.2007.00439.x. [DOI] [PubMed] [Google Scholar]


