Abstract
Background: The impact of protein intake on outcomes in pediatric critical illness is unclear.
Objective: We examined the association between protein intake and 60-d mortality in mechanically ventilated children.
Design: In a prospective, multicenter, cohort study that included 59 pediatric intensive care units (PICUs) from 15 countries, we enrolled consecutive children (age: 1 mo to 18 y) who were mechanically ventilated for ≥48 h. We recorded the daily and cumulative mean adequacies of energy and protein delivery as a percentage of the prescribed daily goal during the PICU stay ≤10 d. We examined the association of the adequacy of protein delivery with 60-d mortality and determined variables that predicted protein intake adequacy.
Results: We enrolled 1245 subjects (44% female) with a median age of 1.7 y (IQR: 0.4, 7.0 y). A total of 985 subjects received enteral nutrition, 354 (36%) of whom received enteral nutrition via the postpyloric route. Mean ± SD prescribed energy and protein goals were 69 ± 28 kcal/kg per day and 1.9 ± 0.7 g/kg per day, respectively. The mean delivery of enteral energy and protein was 36 ± 35% and 37 ± 38%, respectively, of the prescribed goal. The adequacy of enteral protein intake was significantly associated with 60-d mortality (P < 0.001) after adjustment for disease severity, site, PICU days, and energy intake. In relation to mean enteral protein intake <20%, intake ≥60% of the prescribed goal was associated with an OR of 0.14 (95% CI: 0.04, 0.52; P = 0.003) for 60-d mortality. Early initiation, postpyloric route, shorter interruptions, larger PICU size, and a dedicated dietitian in the PICU were associated with higher enteral protein delivery.
Conclusions: Delivery of >60% of the prescribed protein intake is associated with lower odds of mortality in mechanically ventilated children. Optimal prescription and modifiable practices at the bedside might enhance enteral protein delivery in the PICU with a potential for improved outcomes. This trial was registered at clinicaltrials.gov as NCT02354521.
Keywords: adequacy, children, enteral nutrition, energy, mortality, parenteral nutrition, protein
INTRODUCTION
The delivery of optimal nutrition in the pediatric intensive care unit (PICU)7 is an important objective of critical care. The achievement of nutrient intake goals during critical illness has been associated with increased 60-d mortality, acquired infections, length of stay, and nutritional morbidity (1, 2). Enteral nutrition (EN) is the preferred mode of nutrient delivery in critically ill children with a functional gastrointestinal tract and is often initiated early to facilitate the achievement of the daily goal (3, 4). However, a variety of factors impede nutrient delivery in the PICU, including multiple interruptions to EN and a lack of a uniform feeding approach at the bedside (5, 6). As a result, there is often a gap between the prescribed goal and actual delivery of nutrients in the PICU (7, 8).
In our previous study of nutrient delivery in mechanically ventilated children after admission to the PICU, we reported inadequate intakes of energy and protein (1). The adequacy of enteral energy intake was significantly associated with 60-d mortality in that cohort, and an increase in energy intake from 33% to 66% of the prescribed goal was associated with lower mortality (OR: 0.27; 95% CI: 0.11, 0.67; P = 0.002). The metabolic stress response to injury is characterized by muscle breakdown and the release of free amino acids that participate in the inflammatory response and tissue repair. Prolonged protein catabolism during illness may result in a cumulative protein deficit and consequently lead to decrements in weight and lean body mass in children (9). Hence, an adequate protein provision during critical illness is desirable. In randomized trials of protein supplementation during illness in children, protein intakes <1.5 g/kg per day were associated with negative protein balance (10). On the basis of similar data, recommendations for protein intake during critical illness have exceeded recommendations for healthy children (11, 12). However, the translation of these recommendations to the bedside practice appears to be slow. In our previous international study in mechanically ventilated patients, the average prescription of protein was 1.5 g/kg per day, but the actual delivery after 7 d in the PICU was <50% of this prescribed goal. In our current study, we aimed to explore the association of protein delivery with clinical outcome in a large cohort of mechanically ventilated children worldwide. We explored protein prescription in relation to available recommendations as well as the adequacy of actual protein delivery at the bedside. On the basis of our previous observations, we hypothesized that protein delivery in the PICU population would be inadequate, and suboptimal protein delivery would be independently associated with higher 60-d mortality. We also aimed to identify factors that are associated with optimal protein delivery in the PICU, particularly the identification of modifiable bedside practices.
METHODS
Ethical approval for the study was obtained from the Institutional Review Board of Boston Children’s Hospital and each participating site. This trial was registered at clinicaltrials.gov as NCT02354521. Centers were recruited through the membership list of the World Federation of Pediatric Intensive and Critical Care Society by emailing individual health care providers or disseminating study information through membership registries of other national and international societies, including the American Society of Parenteral and Enteral Nutrition (ASPEN) and the Society of Critical Care Medicine. Centers were eligible if they had a PICU with ≥8 beds and a dedicated dietitian or an individual with knowledge of clinical nutrition committed to data collection. Consecutive children (age: 1 mo to 18 y) admitted to the PICU with anticipated stay >48 h and who required mechanical ventilatory support were eligible for enrollment. Enrollment at each site was continued until ≥10 subjects and a maximum of 30 subjects were recruited. Patients who were not ventilated within the first 48 h of admission to the PICU, on compassionate care toward the end of life, or enrolled in any other nutritional intervention trial were excluded. Screening for eligible patients was followed by enrollment and data collection. On the basis of our previous study, we expected an average of 18 patients enrolled per site to give us a sample size of ∼1000 patients for the current study.
Dietitians (or designated health care practitioners) at each site used a remote web-based data capture tool to prospectively record site characteristics, patient demographic characteristics, illness severity score, length of PICU stay, length of hospital stay, and duration of mechanical ventilation for each subject. Nutritional variables including energy and protein goals prescribed by the local nutrition team, actual daily macronutrient delivery achieved, route of delivery, frequency and duration of feeding interruptions, and use of adjunctive drugs were also recorded. Prescribed protein goals for each subject were compared with the recommended daily protein intake in 2005 Dietary Reference Intake (DRI) and 2009 ASPEN age-based guidelines (11, 12). The endpoint for nutritional data collection was 10 d or discharge from the PICU, whichever was sooner. Energy and protein intake adequacies were calculated as the percentage of the prescribed goal that was actually delivered and an average adequacy over the PICU stay ≤10 d was derived. The primary outcome for this study was 60-d patient mortality. Outcome data were collected until 60 d after PICU admission. Ranges for individual variables, data completeness, and logic checks were incorporated into the remote data-collection tool and database. Entered data were checked for errors, inconsistencies, and omissions, and resolutions for these data were achieved with site communications.
Energy adequacy was calculated by using the average of the daily amount of calories received by EN as well as by EN plus parenteral nutrition (PN) over 10 d (or until discharge, if earlier) in the PICU. Evaluable nutrition days when no EN was received were counted as 0%. We did not determine the energy or protein adequacy of patients who received nutrition orally. Because Pediatric Risk of Mortality II, Pediatric Risk of Mortality III, and Pediatric Index of Mortality scores were used at different sites to indicate severity of illness, we classified each severity of illness score as levels 1, 2, 3, and 4, which corresponded to quartiles of the given score. Thus, a patient was considered to have severity of level 4 if their score was in the highest quartile of the group of patients in our study who used the same scoring system. In the rare case in which more than one scoring system was recorded for a patient, we used the lowest score. Patients without a recorded severity of illness score were categorized with “unknown severity of illness.”
Descriptive statistics were used for the PICU and patient characteristics as well as nutrition and clinical outcomes. Categorical variables are reported as counts and percentages, and continuous variables are summarized by their means (±SDs). Linear mixed-effects regression models were used to model the effect of PICU and patient characteristics on the percentage of nutrient prescription (energy and protein) received by the EN route (13). The relation between protein intake and mortality was examined by using 3 steps. We compared median intake adequacy between survivors and nonsurvivors by using the Mann-Whitney U test. The relation between protein intake adequacy as a continuous variable and mortality was explored. Finally, on the basis of their average protein intake adequacy during PICU stay, the cohort was arbitrarily divided on the basis of 3 levels that were clinically relevant to assess the dose-response of its relation with the outcome. Multivariable logistic regression that used a generalized estimating equation strategy and Wald’s test for the assessment of significance was applied to model the effects of PICU characteristics, patient characteristics, and nutrient intake adequacy on 60-d hospital mortality (14). Patients discharged from the hospital before 60 d were considered to be survivors. All models included the PICU site as a random effect to account for within-PICU dependence. The number of evaluable nutrition days was controlled in all models involving protein and energy adequacies. Candidate variables with P ≤ 0.15 by univariate analysis as well as other key clinical variables, such as age, sex, BMI z score, severity of illness scores, and PICU days, were selected a priori for inclusion in the multivariable model. In the event of multicollinearity between energy and protein intake adequacies, we planned to control for energy intake when examining the role of protein intake adequacy on the outcome and vice versa. All tests were 2-sided by using P < 0.05 as evidence for statistical significance. The statistical analysis was conducted with IBM/SPSS Statistics software (version 21.0; IBM). A power analysis indicated that the cohort provided 80% power (α =0.05, β = 0.20) to identify independent predictors of mortality on the basis of an estimated effect size of 0.50 that was based on the OR (version 7.0, nQuery Advisor; Statistical Solutions).
RESULTS
Data from 1245 subjects (44% female; 42% surgical) with a median age of 1.7 y (IQR: 0.4–7.0 y) were analyzed from 59 PICUs across 15 countries. Tables 1–3 describe patient and site level variables. Nutritional status on admission included a median weight-for-age z score of −0.60 (IQR: −2.01 to +0.57) and BMI z score of −0.12 (IQR: −1.47 to +1.08). On the basis of WHO criteria for weight-for-age z score, 25% of the cohort was classified as moderately malnourished on admission (i.e., weight for-age z score <−2.0). EN was the primary mode of nutrient delivery in a majority of the subjects (79%; n = 985). In 36% of enterally fed subjects (n = 354), the postpyloric route was used. PN was delivered in 363 subjects (29%), and in 29% of this subset, it was delivered as a supplement to EN.
TABLE 1.
Demographic characteristics (patient level) for the cohort (n = 1245)
| Variable | Value |
| Age, y | 1.7 (0.4–7.0)1 |
| Sex (F), n (%) | 549 (44) |
| Height, cm | 82 (60–118) |
| Weight, kg | 11.2 (5.8–23.2) |
| Weight-for-age z score | −0.60 (−2.01 to +0.57) |
| BMI, kg/m2 | 16.2 (14.3–18.7) |
| BMI z score | −0.12 (−1.47 to +1.08) |
| Admission category, n (%) | |
| Medical | 726 (58) |
| Surgical | 519 (42) |
| Severity of illness (level),2 n (%) | |
| 1 | 304 (24) |
| 2 | 290 (23) |
| 3 | 330 (27) |
| 4 | 275 (22) |
| Unknown | 46 (4) |
Median; IQR in parentheses (all such values).
Severity of illness was based on Pediatric Risk of Mortality II, Pediatric Risk of Mortality III, and Pediatric Index of Mortality scores and categorized into 4 levels on the basis of quartiles for the cohort.
TABLE 3.
Site-level characteristics of PICUs participating in the study (n = 59)1
| Variable | Value |
| Hospital size (beds), n | 360 ± 2522 |
| PICU size (beds), n | 21 ± 12 |
| 8–15, n (%) | 20 (34) |
| 16–20, n (%) | 14 (24) |
| 21–29, n (%) | 16 (27) |
| ≥30, n (%) | 9 (15) |
| PICU type, n (%) | |
| Open | 14 (24) |
| Closed3 | 45 (76) |
| Multiple PICUs in hospital | 22 (37) |
| Nutrition protocol/guideline used | |
| Yes | 26 (44) |
| No | 33 (56) |
| Presence of dedicated PICU dietitian(s) | |
| Yes | 53 (90) |
| No | 6 (10) |
| FTE dietitian per 10 PICU beds | 0.36 ± 0.22 |
FTE, full time equivalent; PICU, pediatric intensive care unit.
Mean ± SD (all such values).
Intensive care unit specialists were responsible for all medical decision making. Other disciplines may have consulted on the patient during the intensive care unit stay.
TABLE 2.
Patient-level nutrient intake variables for the study cohort of mechanically ventilated children (n = 1245 unless otherwise specified)1
| Variable | Value |
| Route of nutrient delivery, n (%) | |
| Patients who received any EN | 985 (79) |
| Gastric | 631 (64) |
| Postpyloric | 354 (36) |
| Patients who received any PN | 363 (29) |
| Nutrient goals (prescription) | |
| Prescribed energy goal, kcal/kg per day | 69 ± 282 |
| Prescribed protein goal, g/kg per day | 1.9 ± 0.7 |
| Nutrient delivery | |
| Energy delivery adequacy (from EN)3 | 36 ± 35 |
| Protein delivery adequacy (from EN)3 | 37 ± 38 |
| Energy delivery adequacy (from EN+PN)3 | 74 ± 74 |
| Protein delivery adequacy (from EN+PN)3 | 41 ± 43 |
| Actual protein delivery, g/kg per day | 0.67 ± 0.65 |
| Actual protein adequacy based on ASPEN recommendations, % | 38 ± 36 |
| Enteral nutrient delivery (n = 985) | |
| Time to initiation of en after admission to PICU, n (%) | |
| Patients receiving EN by day 1 | 268 (27) |
| Patients receiving EN by day 2 | 324 (33) |
| Patients receiving EN by day 3 | 193 (20) |
| Patients receiving EN by day 4 or later | 200 (20) |
| Interruptions to EN | |
| Patients with at least one interruption, n (%) | 724 (74) |
| Frequency of interruptions (n = 724), d | 2 (1–3)4 |
| Duration of interruptions (n = 724), h/d | 8 (5–12) |
| Antacid used, n (%) | 763 (61) |
| Motility agents used, n (%) | 988 (79) |
ASPEN, American Society of Parenteral and Enteral Nutrition; EN, enteral nutrition; EN+PN, total intake via enteral nutrition and parenteral nutrition; PICU, pediatric intensive care unit; PN, parenteral nutrition.
Mean ± SD (all such values).
Adequacy equaled the percentage of the goal (energy or protein) that was actually delivered on average over the course of the pediatric intensive care unit stay ≤10 d.
Median; IQR in parentheses (all such values).
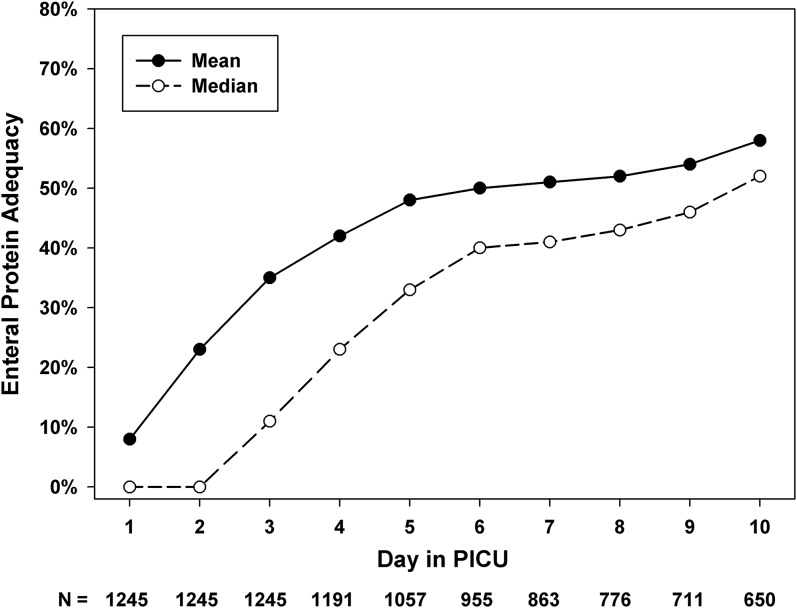
Prescribed goals for energy and protein were 69 ± 28 kcal/kg per day and 1.9 ± 0.7 g/kg per day, respectively. On the basis of ASPEN recommendations for the age-based daily protein intake goal, protein was underprescribed in 466 subjects (37%) in our study. Protein intake adequacy in comparison to ASPEN recommended values was 38 ± 36%. On the basis of 2005 DRI guidelines for daily protein intake in healthy children by the Institute of Medicine, protein was underprescribed in 91 subjects (7%) in the cohort. Actual protein intake (g/kg per day) was significantly lower than age-based protein goals recommended by ASPEN guidelines (0.66 compared with 1.7; P < 0.001). On average, the difference between the recommended and delivered protein for the cohort was ∼1g/kg per day. Figure 1 shows daily cumulative enteral protein intake adequacy in the cohort.
FIGURE 1.
Daily cumulative protein intake adequacy in relation to the day since admission to the PICU in mechanically ventilated children (n = 1245). Adequacy = amount delivered ÷ goal prescribed × 100. Mean protein prescribed in this cohort was 1.9 g/kg per day. PICU, pediatric intensive care unit.
In patients who received EN, it was initiated by day 2 in 60% of this group and by day 3 in 80% of this group. EN was interrupted at least once in 724 subjects (58%). The median number of EN interruptions for the cohort was 2 (IQR: 1–3); and the median duration of interruption was 8 h (IQR: 5–12 h). Motility agents were used in 79% (n = 988) of the cohort as an EN adjunct. Antacid use was recorded in 61% of subjects (n = 763). The percentage of adequacy, i.e.,
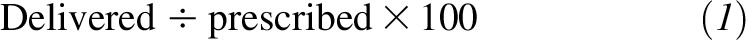 |
for nutrient intake for this cohort over the PICU course for ≤10 d was 36 ± 35% for energy and 37 ± 38% for protein. Because energy and protein intake adequacies were highly correlated, we proceeded by adjusting for each other while examining their individual effect on outcome.
Table 4 describes the results of the univariable analysis for predictors of 60-d mortality in our cohort. In the multivariable analysis (Table 5) after adjustment for PICU site, evaluable EN days, PICU length of stay, and severity of illness, there was a significant association between the adequacy of enteral protein intake and 60-d mortality (P < 0.001). This effect was independent of the enteral energy adequacy. The enteral energy adequacy was not associated with 60-d mortality (P = 0.16).
TABLE 4.
Independent predictors (patient and site levels) of 60-d mortality in mechanically ventilated children (n = 1245): results of univariable analysis1
| Univariable analysis |
|||
| Mortality (n = 82; 6.6%) |
|||
| Variable | Survivors | Nonsurvivors | P |
| Patient characteristics | |||
| Age, y | 1.7 (0.4–7.0)2 | 1.6 (0.3–8.7) | 0.97 |
| Sex, n (%) | 0.21 | ||
| M | 656 (56) | 40 (49) | |
| F | 507 (44) | 42 (51) | |
| BMI, kg/m2 | 17.3 ± 13.53 | 17.2 ± 4.7 | 0.92 |
| BMI z score | −0.20 ± 2.08 | −0.27 ± 2.19 | 0.75 |
| Weight-for-age z score | −0.7 ± 2.13 | −0.8 ± 3.2 | 0.91 |
| Severity of illness level, n (%) | <0.001 | ||
| 1 | 293 (96) | 11 (4) | |
| 2 | 277 (95) | 13 (5) | |
| 3 | 309 (94) | 21 (6) | |
| 4 | 242 (88) | 33 (12) | |
| Unknown | 42 (91) | 4 (9) | |
| Diagnostic category, n (%) | 0.36 | ||
| Medical | 674 (93) | 52 (7) | |
| Surgical | 489 (94) | 30 (6) | |
| Acquired infection | 0.16 | ||
| Yes | 138 (91) | 14 (9) | |
| No | 1025 (94) | 68 (6) | |
| Nutrient intake | |||
| Evaluable nutrition, d | 10 (6–10) | 10 (7–10) | 0.12 |
| EN energy adequacy | 30 (3–60) | 12 (0–53) | 0.01 |
| EN protein adequacy | 32 (2–61) | 12 (0–48) | 0.002 |
| EN+PN energy adequacy | 66 (5–120) | 25 (0–100) | 0.005 |
| EN+PN protein adequacy | 35 (4–65) | 16 (0–52) | 0.004 |
| Site characteristics | |||
| PICU type, n (%) | — | — | 0.60 |
| Open | 305 (94) | 19 (6) | |
| Closed4 | 858 (93) | 63 (7) | |
| PICU beds, n | 20 (14–28) | 16 (14–24) | 0.04 |
Adequacy equals the percentage of the goal (energy or protein) that was actually delivered on average over the course of the PICU stay ≤10 d. EN, enteral nutrition; EN+PN, total intake via enteral nutrition and parenteral nutrition; ICU, intensive care unit; PICU, pediatric intensive care unit.
Median; IQR in parentheses (all such values).
Mean ± SD (all such values).
ICU specialists were responsible for all medical decision making. Other disciplines may have consulted on the patient during the ICU stay.
TABLE 5.
Adjusted odds for 60-d mortality in mechanically ventilated children (n = 1245) by using a multivariable logistic regression analysis1
| Variable | β coefficient ± SE | Wald’s test | P | OR (95% CI) |
| Age (y) | 0.02 ± 0.02 | 0.74 | 0.39 | 1.02 (0.98, 1.06) |
| Sex | −0.31 ± 0.23 | 1.80 | 0.18 | 0.73 (0.46, 1.16) |
| BMI z score | −0.10 ± 0.06 | 0.03 | 0.86 | 0.99 (0.89, 1.11) |
| Severity of illness, level 4 | 0.85 ± 0.25 | 11.94 | 0.001 | 2.34 (1.44, 3.79) |
| Enteral protein adequacy | — | 9.52 | 0.009 | — |
| 20–60 compared with <20 | −1.01 ± 0.38 | 7.16 | 0.007 | 0.37 (0.17, 0.76) |
| >60 compared with <20 | −1.96 ± 0.66 | 8.69 | 0.003 | 0.14 (0.04, 0.52) |
| Enteral energy adequacy | 0.01 ± 0.01 | 1.97 | 0.16 | 1.01 (0.99, 1.03) |
| PICU length of stay | 0.09 ± 0.05 | 3.67 | 0.06 | 1.09 (0.99, 1.20) |
After adjustment for pediatric intensive care unit site, evaluable enteral nutrition days, and severity of illness level. Adequacy equals the percentage of the goal (energy or protein) that was actually delivered on average over the course of the PICU stay ≤10 d. PICU, pediatric intensive care unit.
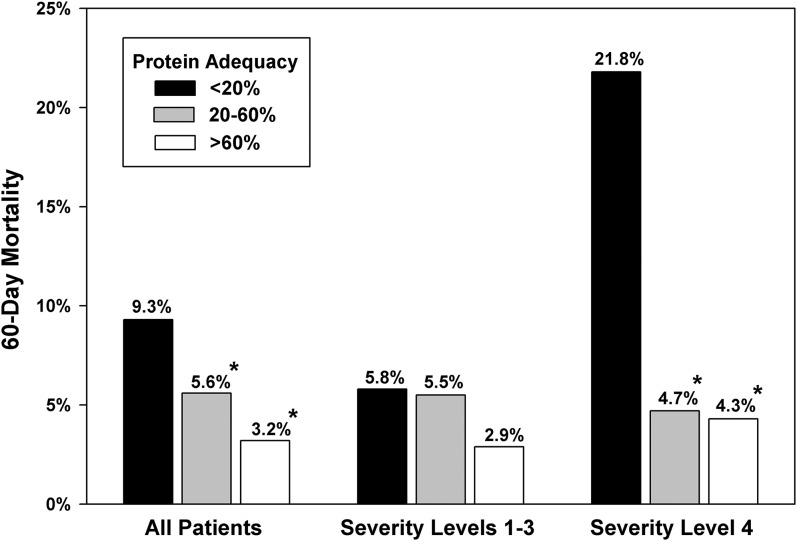
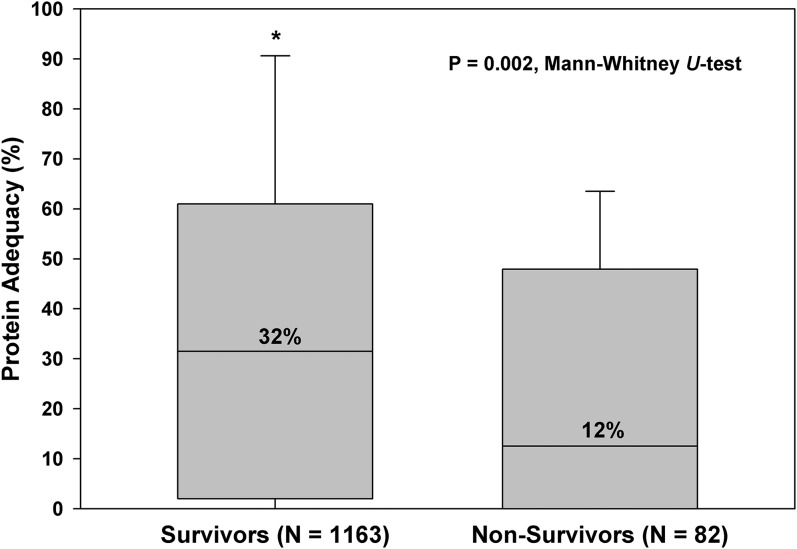
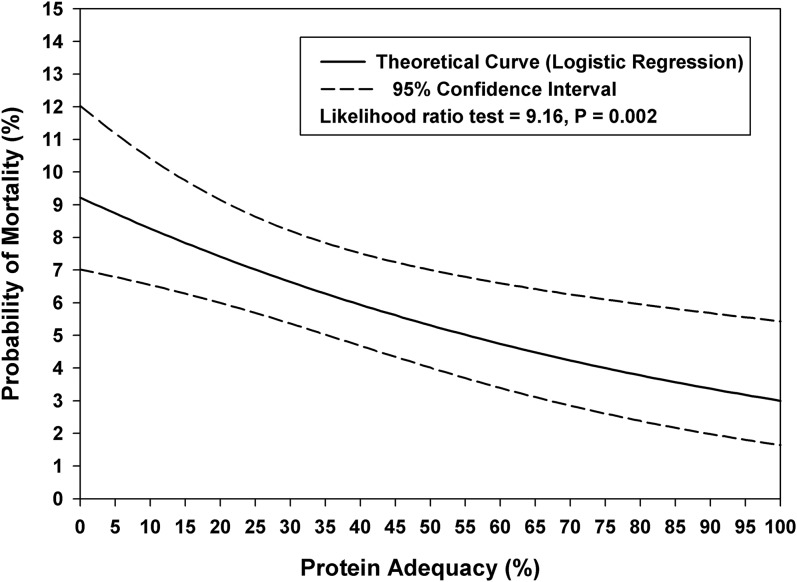
We observed a significant incremental relation between different protein intake adequacies and the 60-d outcome. Compared with patients with protein intake adequacy<20% of the prescribed protein goal, the OR for 60-d mortality was 0.37 (95% CI: 0.17, 0.76; P = 0.007) for patients with protein intake adequacy of 20–60% and 0.14 (95% CI: 0.04, 0.52; P = 0.003) for patients with a protein intake adequacy >60%. Figure 2 describes the relation between protein intake and mortality in relation to the severity of illness. For the entire cohort and for a subset with higher illness severity (level 4), 60-d mortality was significantly lower in patients who received 20–60% and >60% of their prescribed goals. The effect of protein adequacy on mortality was amplified in subjects with higher severity of illness. Figures 3 shows median values of protein intake in survivors and nonsurvivors. Figure 4 shows the linear relation between enteral protein intake adequacy (as a continuous variable) and 60-d mortality.
FIGURE 2.
Relation between enteral protein adequacy and 60-d mortality in relation to the severity of illness at admission in mechanically ventilated children (n = 1245). Adequacy equals the delivered amount as a percentage of prescribed goal. *Significantly lower mortality than in reference category of <20%, P < 0.01 (Fisher’s exact test). The interaction between the severity of illness and protein intake adequacy was significant, P = 0.014 (Wald’s test = 8.62 on 2 df).
FIGURE 3.
Median and interquartile values of protein intake adequacy (as a percentage of the prescribed goal) in survivors and nonsurvivors: a cohort of mechanically ventilated children (n = 1245).
FIGURE 4.
Linear relation between enteral protein intake adequacy (as a percentage of the prescribed goal) as a continuous variable and 60-d mortality in a cohort of mechanically ventilated children (n = 1245).
The multivariable analysis for predictors of enteral protein adequacy in our study cohort is described in Table 6. Enteral protein adequacy was significantly and directly associated with the early initiation of EN, use of the postpyloric route, decreased duration of EN interruption, PICU size (≥20 beds), and the full-time equivalent for a dedicated dietitian per 10 patients in the PICU. The use of antacids or prokinetic agents, PICU length of stay, diagnostic category, age, or sex was not associated with the adequacy of enteral protein delivery.
TABLE 6.
Significant independent predictors of enteral protein adequacy (percentage of prescribed goal actually delivered) in critically ill children (n = 1245): multivariable regression analysis1
| Multivariable model2 |
||
| Variables | β coefficient ± SE | P |
| Time to initiating EN after admission (d) | −8.06 ± 0.89 | <0.001 |
| Route of EN delivery (postpyloric compared with gastric) | 1.94 ± 0.10 | 0.05 |
| Total duration of EN interruption (h) | −1.14 ± 0.24 | <0.001 |
| Presence of a dedicated PICU dietitian | 6.53 ± 2.80 | 0.02 |
| PICU beds (≥20) | 7.15 ± 2.80 | 0.01 |
Other variables included in the multivariable linear regression analysis included age, sex, diagnostic category (medical compared with surgical), weight-for-age z score, parenteral nutrition use, use of feeding protocol in the unit, and PICU length of stay. EN, enteral nutrition; PICU, pediatric intensive care unit.
After adjusting for severity of illness, evaluable EN days, and PICU length of stay.
DISCUSSION
We have reported the bedside delivery of energy and protein in >1200 critically ill, mechanically ventilated children admitted to 59 PICUs at academic institutions from 15 countries. Our results demonstrate a significant association between higher enteral protein adequacy and lower 60-d mortality in this cohort, independent of the disease severity. We observed a significant dose-response for this association, and this effect was independent of energy intake. Our study highlights opportunities for improving protein prescription and delivery and the potential for improving clinical outcomes in this vulnerable cohort.
Critical illness is associated with an adaptive metabolic stress response that is characterized by muscle catabolism (15). A reduced supply of amino acids from the diet or increased demand for amino acids from catabolic diseases will contribute to increased higher protein degradation from muscle, which is the largest reservoir of protein, to ensure bodily functions. Cumulative protein deficits have been associated with decline in mid-arm circumference (9). Regardless of its cause, muscle wasting in patients with acute lung injury has been associated with weakness, disability, and an impaired quality of life (16). Hence, the preservation of muscle mass and maintenance of protein balance in the face of protein catabolism should be one of the most-important goals of critical care nutrition.
A protein-enriched diet was shown to increase protein synthesis and, thereby, improve the protein balance in the setting of increased protein catabolism during illness (17). The most recent US DRI recommendations, which were published in 2005, include the recommended protein intake for healthy children (Table 7). We previously reported that a minimum of 1.5 g/kg per day of protein intake may be necessary to achieve a positive protein balance in critically ill children (10). Over the years, recommendations for protein requirements in critically ill children have evolved, with higher daily protein intakes (compared with DRIs) recommended in the 2009 ASPEN guidelines. In more than one-third of our cohort, the protein prescription was lower than the age-based recommended range of the ASPEN guidelines. The average protein prescription in the study cohort was 1.9 g/kg per day. On the basis of previous randomized controlled trials in critically ill children, this value may not be enough to prevent a negative protein balance in some patients (10). The translation of evidence into recommendations and, then, into practice at the bedside appears to be delayed in the pediatric critically ill population.
TABLE 7.
Recommended daily protein intake for healthy and critically ill children1
| Age range | Recommended protein, g/kg per day | |
| DRI 2005 | 0–6 mo | 1.52 |
| 7–12 mo | 1.2 | |
| 1–3 y | 1.05 | |
| 4–13 y | 0.95 | |
| 14–18 y | 0.85 | |
| ASPEN 2009 | 0–2 | 2–3 |
| 2–3 | 1.5–2 | |
| 3–18 | 1.5 |
ASPEN, American Society of Parenteral and Enteral Nutrition; DRI, Dietary Reference Intake recommendations by the Institute of Medicine (2005).
The delivery of this prescribed protein goal at the bedside remains challenging. In our current study, larger units with dedicated dietitians appeared to achieve higher adequacy of protein delivery in mechanically ventilated children. The presence of a dedicated dietitian or a nutrition support team in the PICU setting was previously shown to help improve nutrient delivery at the bedside (18, 19). In single centers, the implementation of a nutrition education program and nutrition support team has been associated with a decrease in PN use and incremental increase in EN use with decreased mortality (19). The dose-response relation between increasing enteral protein intake and lower mortality in our current study was most striking in patients with a higher severity of illness, in whom optimal protein intake must be prioritized.
Early initiation of EN was a predictor of optimal enteral protein intake in our study. Although total energy and protein intake (EN plus PN) were significant predictors of mortality in the univariable analysis, these variables did not achieve significance in the multivariable modeling. The timing and impact of supplementary PN in critically ill children are currently being investigated. Over the past decade, EN has been the preferred mode of nutrient delivery (3, 6, 8, 20). EN was initiated by the third day in the PICU in 80% of our cohort. Early EN delivery has been recommended in patients who are hemodynamically stable and have a functioning gastrointestinal tract (11). Early EN may be associated with favorable metabolic and endocrine profiles and even with lower mortality in critically ill children (3, 21). In children with a burn injury, early EN has been associated with reductions in mortality, hospital length of stay, infectious complications, caloric deficits, weight loss, protein breakdown, and altered small-bowel mucosal permeability (21, 22). After initiation, EN was interrupted in a majority of subjects, and the duration of interruptions was a significant predictor of the inadequacy of EN delivery in our study cohort. EN interruptions have been described in both pediatric and adult critically ill patients (6). A significant proportion of these interruptions may be avoidable (6, 23). A variety of strategies have been used to avoid unintended EN interruptions in the intensive care unit, including the use of the postpyloric route, stepwise algorithms, and volume-based feeding (24–26). Compared with gastric feeding, postpyloric feeding did not improve outcomes in a randomized controlled trial in the PICU environment (27). Postpyloric feeding has been associated with EN tolerance and an earlier achievement of nutrient delivery goals in children with sepsis and cardiac disease (28, 29). Our results suggest that postpyloric feeding deserves additional examination in well-designed trials conducted in centers with local expertise and resources for the placement of specialized enteral access. The use of stepwise EN-delivery algorithms was shown to improve EN delivery in single centers (20, 26). A uniform approach to EN delivery at the bedside may help identify candidates for EN adjuncts such as postpyloric feeding, decrease unintended interruptions, improve the management of EN intolerance, and facilitate the early identification of cumulative nutrient imbalances.
In our previous study, we described the association between enteral energy adequacy and outcome (1). In this current study, the effect of energy on the outcome was not independent of protein delivery. Indirect calorimetry provides accurate measurements of resting energy expenditure and was recommended as a guide to energy prescription for patients in adult and pediatric intensive care units (11, 30). Most centers do not have access to indirect calorimetry and, instead, rely on inaccurate equations to predict resting energy expenditure, thereby risking unintended underfeeding or overfeeding (31). In our current study, energy prescriptions were most-often based on equations and individualized by a dedicated dietitian. Hence, the true adequacy of energy intake in the absence of measured resting energy expenditure could not be determined.
To our knowledge, our current study represents the largest multicenter effort to describe protein delivery and its association with mortality during pediatric critical illness. Dedicated dietitians at each participating site recorded nutritional and clinical outcome data prospectively. Unlike retrospective studies, the database was specifically developed for this study, and individual site coordinators were trained in data entry. A systematic multistep process allowed real-time data checks and verification. However, our study was limited to units with ≥8 beds, and the observations may not be applicable to patients in smaller PICUs or to patients who are not mechanically ventilated. The adequacies of energy and protein intakes were based on daily goals determined by the local team, and accurate energy requirements for this cohort could not be determined. Enrolling sites represented diverse regions with variations in expertise, resources, and regional nutrient-prescription practices. Finally, the results of this study represent merely an association between nutritional delivery and mortality. The inference made from these observations should not be taken as proof of causation. The biological plausibility of these observations should prompt well-designed studies aimed at exploring the role of optimal protein in this cohort.
In conclusion, energy and protein intake adequacies during the first week of illness remain alarmingly low in mechanically ventilated children in PICUs worldwide. Adequacy of enteral protein intake was significantly associated with mortality in this prospective cohort study of mechanically ventilated children. Increments in protein adequacy were associated with significant reductions in odds of mortality in this cohort. Protein prescriptions at the bedside remain lower than age-based recommendations in the literature. Protein intake in the PICU can be optimized with early EN initiation, a decreased duration of EN interruption, the use of the postpyloric route, and the presence of a dedicated PICU dietitian. Well-designed trials aimed at optimizing protein intake and describing its effect on protein balance, lean body mass preservation, and muscle function in critically ill children are urgently needed.
Acknowledgments
The authors’ responsibilities were as follows—NMM, LJB, DZ, CPD, and DKH: designed the research (project conception, development of overall research plan, and study oversight); NMM and LJB: conducted the research and data collection; NMM, LJB, and DZ: analyzed data and performed the statistical analysis; NMM: had primary responsibility for the final content of the manuscript; and all authors: wrote the manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: ASPEN, American Society of Parenteral and Enteral Nutrition; DRI, Dietary Reference Intake; EN, enteral nutrition; PICU, pediatric intensive care unit; PN, parenteral nutrition.
REFERENCES
- 1.Mehta NM, Bechard LJ, Cahill N, Wang M, Day A, Duggan CP, Heyland DK. Nutritional practices and their relationship to clinical outcomes in critically ill children–an international multicenter cohort study. Crit Care Med 2012;40:2204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elke G, Wang M, Weiler N, Day AG, Heyland DK. Close to recommended caloric and protein intake by enteral nutrition is associated with better clinical outcome of critically ill septic patients: secondary analysis of a large international nutrition database. Crit Care 2014;18:R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mikhailov TA, Kuhn EM, Manzi J, Christensen M, Collins M, Brown AM, Dechert R, Scanlon MC, Wakeham MK, Goday PS. Early enteral nutrition is associated with lower mortality in critically ill children. JPEN J Parenter Enteral Nutr 2014;38:459–66. [DOI] [PubMed] [Google Scholar]
- 4.Mehta NM. Feeding the gut during critical illness–it is about time. JPEN J Parenter Enteral Nutr 2014;38:410–4. [DOI] [PubMed] [Google Scholar]
- 5.Rogers EJ, Gilbertson HR, Heine RG, Henning R. Barriers to adequate nutrition in critically ill children. Nutrition 2003;19:865–8. [DOI] [PubMed] [Google Scholar]
- 6.Mehta NM, McAleer D, Hamilton S, Naples E, Leavitt K, Mitchell P, Duggan C. Challenges to optimal enteral nutrition in a multidisciplinary pediatric intensive care unit. JPEN J Parenter Enteral Nutr 2010;34:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor RM, Preedy VR, Baker AJ, Grimble G. Nutritional support in critically ill children. Clin Nutr 2003;22:365–9. [DOI] [PubMed]
- 8.Kyle UG, Jaimon N, Coss-Bu JA. Nutrition support in critically ill children: underdelivery of energy and protein compared with current recommendations. J Acad Nutr Diet 2012;112:1987–92. [DOI] [PubMed] [Google Scholar]
- 9.Hulst JM, van Goudoever JB, Zimmermann LJ, Hop WC, Albers MJ, Tibboel D, Joosten KF. The effect of cumulative energy and protein deficiency on anthropometric parameters in a pediatric ICU population. Clin Nutr 2004;23:1381–9. [DOI] [PubMed] [Google Scholar]
- 10.Bechard LJ, Parrott JS, Mehta NM. Systematic review of the influence of energy and protein intake on protein balance in critically ill children. J Pediatr 2012;161:333-9–e1. [DOI] [PubMed]
- 11.Mehta NM, Compher C; A.S.P.E.N. Board of Directors . A.S.P.E.N. Clinical Guidelines: nutrition support of the critically ill child. JPEN J Parenter Enteral Nutr 2009;33:260–76. [DOI] [PubMed] [Google Scholar]
- 12.“Protein and amino acids.” Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). The National Academies Press, Washington (DC): 2005. p. 589–786.
- 13.Fahrmeir L, Tutz G. Multivariate statistical modelling based on generalized linear models. Second ed. Springer, New York (NY). 2001. p. 283–329.
- 14.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression methods in biostatistics: linear, logistic, survival, and repeated-measures models. Springer, New York (NY) 2005. p. 157–209.
- 15.Shew SB, Jaksic T. The metabolic needs of critically ill children and neonates. Semin Pediatr Surg 1999;8:131–9. [DOI] [PubMed] [Google Scholar]
- 16.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, Himmelfarb CR, Desai SV, Ciesla N, Herridge MS, et al. . Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med 2014;42:849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Betue CT, van Waardenburg DA, Deutz NE, van Eijk HM, van Goudoever JB, Luiking YC, Zimmermann LJ, Joosten KF. Increased protein-energy intake promotes anabolism in critically ill infants with viral bronchiolitis: a double-blind randomised controlled trial. Arch Dis Child 2011;96:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakeham M, Christensen M, Manzi J, Kuhn EM, Scanlon M, Goday PS, Mikhailov TA. Registered dietitians making a difference: early medical record documentation of estimated energy requirement in critically ill children is associated with higher daily energy intake and with use of the enteral route. J Acad Nutr Diet 2013;113:1311–6. [DOI] [PubMed] [Google Scholar]
- 19.Gurgueira GL, Leite HP, Taddei JA, de Carvalho WB. Outcomes in a pediatric intensive care unit before and after the implementation of a nutrition support team. JPEN J Parenter Enteral Nutr 2005;29:176–85. [DOI] [PubMed] [Google Scholar]
- 20.Petrillo-Albarano T, Pettignano R, Asfaw M, Easley K. Use of a feeding protocol to improve nutritional support through early, aggressive, enteral nutrition in the pediatric intensive care unit. Pediatr Crit Care Med 2006;7:340–4. [DOI] [PubMed] [Google Scholar]
- 21.Gottschlich MM, Jenkins ME, Mayes T, Khoury J, Kagan RJ, Warden GD. The 2002 Clinical Research Award. An evaluation of the safety of early vs delayed enteral support and effects on clinical, nutritional, and endocrine outcomes after severe burns. J Burn Care Rehabil 2002;23:401–15. [DOI] [PubMed] [Google Scholar]
- 22.Khorasani EN, Mansouri F. Effect of early enteral nutrition on morbidity and mortality in children with burns. Burns 2010;36:1067–71. [DOI] [PubMed] [Google Scholar]
- 23.Keehn A, O'Brien C, Mazurak V, Brunet-Wood K, Joffe A, de Caen A, Larsen B. Epidemiology of Interruptions to nutrition support in critically ill children in the pediatric intensive care unit. JPEN J Parenter Enteral Nutr 2013;2015;39:211–7. [DOI] [PubMed] [Google Scholar]
- 24.McClave SA, Saad MA, Esterle M, Anderson M, Jotautas AE, Franklin GA, Heyland DK, Hurt RT. Volume-based feeding in the critically ill patient. JPEN J Parenter Enteral Nutr 2014 Jun 18 (Epub ahead of print; DOI: 10.1177/0148607114540004). [DOI] [PubMed] [Google Scholar]
- 25.Khlevner J, Antino J, Panesar R, Chawla A. Establishing early enteral nutrition with the use of self-advancing postpyloric feeding tube in critically ill children. JPEN J Parenter Enteral Nutr 2012;36:750–2. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton S, McAleer DM, Ariagno K, Barrett M, Stenquist N, Duggan CP, Mehta NM. A stepwise enteral nutrition algorithm for critically ill children helps achieve nutrient delivery goals. Pediatr Crit Care Med 2014;15:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meert KL, Daphtary KM, Metheny NA. Gastric vs small-bowel feeding in critically ill children receiving mechanical ventilation: a randomized controlled trial. Chest 2004;126:872–8. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez C, Lopez-Herce J, Carrillo A, Mencia S, Vigil D. Early transpyloric enteral nutrition in critically ill children. Nutrition 2007;23:16–22. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez C, Lopez-Herce J, Carrillo A, Bustinza A, Sancho L, Vigil D. Transpyloric enteral feeding in the postoperative of cardiac surgery in children. J Pediatr Surg 2006;41:1096–102. [DOI] [PubMed] [Google Scholar]
- 30.Martindale RG, McClave SA, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med 2009;37:1757–61. [DOI] [PubMed] [Google Scholar]
- 31.Mehta NM, Bechard LJ, Dolan M, Ariagno K, Jiang H, Duggan C. Energy imbalance and the risk of overfeeding in critically ill children. Pediatr Crit Care Med 2011;12:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]