Abstract
Background: Age-associated declines in muscle mass and function are major risk factors for an impaired ability to carry out activities of daily living, falls, prolonged recovery time after hospitalization, and mortality in older adults. New strategies that can slow the age-related loss of muscle mass and function are needed to help older adults maintain adequate performance status to reduce these risks and maintain independence.
Objective: We evaluated the efficacy of fish oil–derived n–3 (ω-3) PUFA therapy to slow the age-associated loss of muscle mass and function.
Design: Sixty healthy 60–85-y-old men and women were randomly assigned to receive n–3 PUFA (n = 40) or corn oil (n = 20) therapy for 6 mo. Thigh muscle volume, handgrip strength, one-repetition maximum (1-RM) lower- and upper-body strength, and average power during isokinetic leg exercises were evaluated before and after treatment.
Results: Forty-four subjects completed the study [29 subjects (73%) in the n–3 PUFA group; 15 subjects (75%) in the control group]. Compared with the control group, 6 mo of n–3 PUFA therapy increased thigh muscle volume (3.6%; 95% CI: 0.2%, 7.0%), handgrip strength (2.3 kg; 95% CI: 0.8, 3.7 kg), and 1-RM muscle strength (4.0%; 95% CI: 0.8%, 7.3%) (all P < 0.05) and tended to increase average isokinetic power (5.6%; 95% CI: −0.6%, 11.7%; P = 0.075).
Conclusion: Fish oil–derived n–3 PUFA therapy slows the normal decline in muscle mass and function in older adults and should be considered a therapeutic approach for preventing sarcopenia and maintaining physical independence in older adults. This study was registered at clinicaltrials.gov as NCT01308957.
Keywords: aging, muscle mass, muscle power, muscle strength, omega-3 fatty acids
INTRODUCTION
The preservation of muscle mass and function is critical for the prevention of physical frailty, mobility disability, and loss of independence in older adults. During middle age, muscle mass begins to decline at a rate of ∼0.5–1.0% per year; in addition, muscle tissue undergoes morphologic changes that are manifested by increased infiltration with fat and connective tissue, which negatively affects muscle strength (i.e., maximal force produced) and power (i.e., amount of work performed per unit of time), and results in a ∼2–3% decline of muscle function per year (1–4). Reduced muscle strength and power are key predictors of serious adverse outcomes in older adults, including the inability to carry out activities of daily living (5), increased risk of mobility disability (6–8), falls (∼20% increase in the incidence rate for each ∼15% decrease in lower-leg strength) (9–12), length of hospital stay (13), and mortality (e.g., ∼4% increase for every 1-kg decrease in grip strength) (14).
Adequate protein intake and regular physical activity, particularly resistance exercise, are the cornerstones for maintaining adequate muscle mass and physical performance throughout life (15, 16). Although most older adults eat sufficient protein (17), a plethora of health-, environment-, and self-related factors are often barriers to regular physical activity (18). Accordingly, only ∼25% of older Americans regularly engage in exercise, and fewer than 20% of older adults perform resistance exercise at least 1 time/wk (19, 20). However, even life-long exercisers and highly trained athletes suffer a progressive loss of muscle mass and physical performance with aging (21, 22). Testosterone, growth-hormone, and dehydroepiandrosterone therapy increase muscle mass and function (23–25), but their use is limited because of undesirable side effects. Therefore, new strategies that can slow the age-related loss of muscle mass and function are needed to prevent sarcopenia and help older adults maintain adequate performance status and independence.
Results from studies conducted in cancer patients, people with rheumatoid arthritis, and resistance-exercise–trained people suggested that fish oil–derived n–3 PUFAs can stimulate muscle growth and enhance strength (26–28). However, to our knowledge, a robust assessment of their effects on muscle mass and function in the older adult population has not been made. Therefore, the purpose of the current study was to test the hypothesis that fish oil–derived n–3 PUFA therapy increases (or slows the loss of) muscle mass and function in older adults. Accordingly, we conducted a 6-mo-long, double-blind, randomized controlled trial (RCT) to evaluate the effect of fish-oil–derived n–3 PUFA therapy on muscle volume, strength, and average isokinetic power in 60–85-y-old men and women.
METHODS
Participants
Sixty healthy 60–85-y-old men and women participated in the study, which was approved by the Human Research Protection Office and the Clinical Research Unit Advisory Committee at Washington University School of Medicine in St. Louis, Missouri. Written informed consent was obtained from all subjects before their participation in the study. All subjects completed a comprehensive medical evaluation, which included a history and physical examination, a 75-g oral-glucose-tolerance test, and standard blood tests, which were performed by the Clinical Laboratory Improvement Amendments–certified Core Laboratory for Clinical Studies at Washington University School of Medicine.
Exclusionary criteria were as follows: BMI (in kg/m2) ≤18.5 or ≥35.0; unstable body weight (i.e., >2-kg change during the past 6 mo); exercise training (i.e., ≥1.5 h of exercise per week); serious chronic disease (e.g., cardiopulmonary disease, diabetes, chronic kidney disease, or cancer); a modified Physical Performance Test score <17 of 36 (29); treatment with medications that could affect muscle function (e.g., 3-hydroxy-3methylglutaryl-CoA reductase inhibitors, corticosteroids, or androgen- or estrogen-containing compounds) ≤1 y before enrolling in the study; musculoskeletal or neuromuscular impairments that could interfere with exercise testing; metal implants that could interfere with MRI; cognitive impairments that could interfere with obtaining informed consent, treatment adherence, or testing procedures; use of tobacco products; excessive alcohol consumption (>21 and >14 units/wk for men and women, respectively); consumption of >2 servings fatty fish/wk; and use of fish-oil products.
Study design
We conducted a 6-mo, double-blind RCT to evaluate the effect of fish oil–derived n–3 PUFA therapy on muscle mass and muscle function. The primary outcome measures of this study were 1) thigh muscle volume, 2) handgrip strength, 3) one-repetition maximum (1-RM) muscle strength (composite score for leg press, chest press, knee extension, and knee flexion), and 4) average isokinetic muscle power (composite of isokinetic knee extension at 60o/s, knee flexion at 60o/s, knee extension at 180o/s, and knee flexion at 180o/s). In addition, we evaluated 1) body fat mass (to evaluate potential adverse treatment effects on body composition), 2) intermuscular fat content [which is an important determinant of muscle function (30, 31)], and 3) red blood cell n–3 PUFA content (a measure of compliance with n–3 PUFA intake). Safety measures included standard blood tests, blood pressure, oral glucose tolerance, and plasma lipid and liver enzyme concentrations.
Subjects were randomly assigned in a 2:1 fashion to either n–3 PUFA therapy [four 1-g pills/d n–3-acid ethyl esters (Lovaza, GlaxoSmithKline plc) that provided a total of 1.86 g EPA (20:5n–3) and 1.50 g DHA (22:6 n–3)/d, which is equivalent to the n–3 PUFA content of 200–400 g freshwater fatty fish (e.g., salmon, herring, and sardines) (32)] or a placebo control (4 identical looking pills/d that contained corn oil) for 6 mo. Both n–3 PUFA and corn-oil pills were kindly provided by GlaxoSmithKline plc. Subjects were instructed to consume 2 pills in the morning with breakfast and 2 pills in the evening with dinner. Compliance was assessed by a pill count at the end of the study. To help ensure the reliability of the pill count, subjects were given an excess number of pills and asked to return any remaining pills at the end of the study. Study endpoints were assessed before starting the treatment and again after ∼3 mo (12.5 ± 1.1 wk mean ± SD for all variables except body composition, thigh muscle volume, and red blood cell n–3 PUFA content) and 6 mo (25.2 ± 2.4 wk) of treatment.
Outcomes assessments
The following assessments were completed during outpatient visits to the Clinical Research Unit or the Center for Clinical Imaging Research at Washington University School of Medicine:
Body weight and body composition
Body weight was measured on a Seca 703 scale (Seca) to the nearest 0.1 kg. Total-body fat mass was evaluated by using dual-energy X-ray absorptiometry (Lunar iDXA; GE Healthcare Lunar). Thigh muscle volume and intermuscular fat content were quantified by using MRI [1.5-T superconducting magnet (Siemens) and Matlab software (Mathworks, version R2012b)]. The region of interest constituted twenty-two 8-mm-thick consecutive bilateral T1-weighted axial images, which were acquired with and without fat saturation starting 10 cm proximal to the distal edge of the femur.
Muscle strength and average isokinetic power
Subjects attended an orientation session to become familiar with the exercise equipment and testing procedures. After a median of 6 d (IQR: 3–7 d), all testing procedures were repeated, and the better of the results for each exercise on the 2 testing days was used as each subject’s baseline value. Handgrip strength was measured by using a Jamar hydraulic hand dynamometer (Patterson Medical). 1-RM muscle strength (i.e., the maximal amount of weight that each participant was able to lift just once) was evaluated by using a Hoist multistation weight machine (Hoist Fitness Systems) for the following exercises (all bilateral): leg press, chest press, knee extension, and knee flexion. The goal was to attain the 1-RM for each exercise after ∼5 incremental weight lifts; at every stage, subjects were allowed a second attempt during 1-RM testing if they were unable to lift an incremental weight the first time. Average isokinetic muscle power was evaluated by unilateral (dominant leg) testing of knee extensors and flexors at speeds of 60o/s and 180o/s by using a Biodex 3 dynamometer (Biodex Medical Systems). Each exercise was repeated 3 times, and the mean of the 2 highest average power recordings for each exercise was used for analysis.
Red blood cell fatty acid composition
Red blood cell lipids were extracted by using a modified Folch method (33). After blood samples were centrifuged 4200 × g for 10 min at 4°C, and plasma and the buffy coat were removed, lipids were extracted in a chloroform:methanol mixture (2:1) that contained 0.01% butylated hydroxytoluene. Water was added, and the lipid-containing layer was aspirated and dried under vacuum. The dried lipid fraction was reconstituted in a methanol solution that contained 10% acetyl chloride to prepare fatty acid methyl esters (34,) and the fatty acid profile was determined by using gas chromatography–mass spectrometry (MSD 5973 System; Hewlett-Packard).
Statistical analysis
Statistical analyses were carried out with SPSS version 21 for Windows software (IBM). All variables were tested for normality by using the Kolmogorov-Smirnov test. Student’s t test (for normally distributed variables) and the Mann-Whitney U test (for skewed variables) were used to compare subject characteristics in the n–3 PUFA and control groups at baseline. An ANCOVA with the baseline value as a covariate was used to evaluate the effect of n–3 PUFA therapy on thigh muscle volume, body weight, fat mass, intermuscular fat content, blood pressure, plasma lipid and liver enzyme concentrations, and glucose tolerance, which were evaluated at baseline and 6 mo of treatment only. A linear mixed-model ANOVA was used to evaluate differences in handgrip strength, 1-RM muscle strength, and average isokinetic muscle power between the control and n–3 PUFA groups; when significant, group × time interactions were detected, and post hoc analyses were used to locate differences. A composite 1-RM muscle-strength score (i.e., sum of 1-RM leg press, chest press, knee extension, and knee flexion) and a composite average isokinetic muscle-power score (sum of average isokinetic muscle power during knee-extension exercises at 60o/s and 180o/s and knee-flexion exercises at 60o/s and 180o/s) were used for statistical analyses.
P ≤ 0.05 was considered statistically significant. Baseline data are presented as means (±SDs) for normally distributed data sets or median (quartiles) for skewed data sets. Changes over time and treatment effects are presented as mean changes and between-group differences and their 95% confidence bounds, respectively.
Our power calculation was based on previously published changes in thigh muscle volume (evaluated by using MRI in our research facility) (35) and handgrip strength (36) in response to nutrition interventions in older adults (i.e., a −6.9 ± 3.4% change in thigh muscle volume and a 0.6 ± 1.9-kg change in handgrip strength). With the use of the reported SDs, a sample size of 48 subjects (i.e., n = 16 in the control group and n = 32 in the n–3 PUFA group, with the assumption of a 20% dropout rate in both groups), and 2-tailed tests, we estimated that we had enough power (0.80) to detect a ≥3.0% difference in thigh muscle volume and a ≥1.7-kg difference in handgrip strength between control and n–3 PUFA groups at the ≤0.05 significance level.
RESULTS
Subject flow, characteristics, and compliance
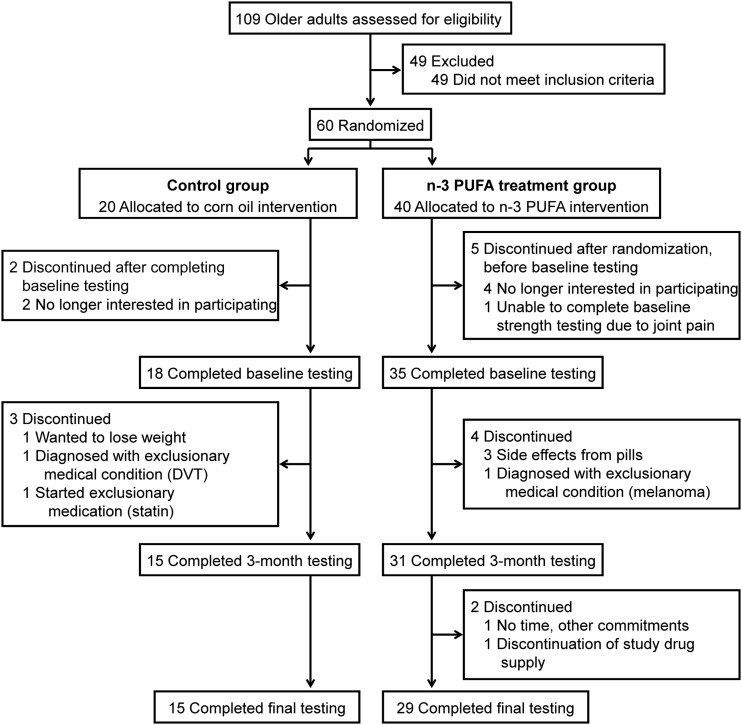
A total of 44 subjects [29 subjects (73%) in the n–3 PUFA group and 15 subjects (75%) in the control group] completed the study and were included in the analysis. The flow of study subjects is shown in Figure 1. Baseline characteristics (sex, age, body weight, body composition, physical function, blood pressure, and metabolic profile) of subjects in treatment and control groups who completed the study were not statistically or clinically significantly different (Table 1). Baseline characteristics of subjects who dropped out of the study were also not different from those who completed the study (data not shown). The average compliance of subjects who completed the study, as judged by the leftover pill count, was 93.6 ± 7.4% in the n–3 PUFA group and 91.8 ± 8.1% in the control group. The red blood cell n–3 PUFA content increased by 135% (95% CI: 115%, 154%) in the n–3 PUFA group (from 5.8 ± 1.0% to 13.2 ± 1.9% of the total fatty acid content) and did not change (2%; 95% CI: −6%, 9%) in the control group (5.9 ± 1.0% of the total fatty acid content before and 6.0 ± 1.2% after).
FIGURE 1.
Flow of study participants. DVT, deep vein thrombosis.
TABLE 1.
Subject characteristics at baseline1
| Control (n = 15) | n–3 PUFA (n = 29) | P2 | |
| Age, y | 69 ± 73 | 68 ± 5 | 0.82 |
| Sex, n (%) | |||
| M | 5 (33) | 10 (34) | — |
| F | 10 (67) | 19 (66) | — |
| Blood pressure, mm Hg | |||
| Systolic | 125 ± 12 | 124 ± 12 | 0.91 |
| Diastolic | 73 ± 6 | 73 ± 5 | 0.86 |
| Plasma concentrations4 | |||
| Triglycerides, mmol/L | 0.82 (0.73, 1.08)5 | 0.94 (0.64, 1.31) | 0.75 |
| HDL cholesterol, mmol/L | 1.56 ± 0.34 | 1.81 ± 0.47 | 0.08 |
| LDL cholesterol, mmol/L | 2.87 ± 0.69 | 3.17 ± 0.61 | 0.14 |
| AST, IU/L | 20 ± 5 | 19 ± 4 | 0.39 |
| ALT, IU/L | 16 (13, 21) | 15 (13, 21) | 0.59 |
| Glucose, mmol/L | 5.21 ± 0.35 | 5.05 ± 0.38 | 0.18 |
| Glucose, 2 h post-OGTT, mmol/L | 7.13 ± 1.37 | 6.65 ± 1.64 | 0.33 |
| Body mass and composition | |||
| BMI, kg/m2 | 25.3 ± 4.2 | 26.1 ± 4.1 | 0.57 |
| Body mass, kg | 72.4 ± 15.6 | 73.6 ± 12.4 | 0.79 |
| Body fat, kg | 23.0 ± 10.3 | 23.4 ± 8.1 | 0.89 |
| Body fat, % | 31.1 ± 11.0 | 31.7 ± 8.7 | 0.83 |
| Thigh muscle volume, cm3 | 3,185 ± 554 | 3,427 ± 731 | 0.27 |
| Thigh intermuscular fat volume, cm3 | 32 (23, 49) | 37 (32, 58) | 0.34 |
| Physical function | |||
| PPT6 | 34 (33, 35) | 34 (33, 35) | 0.79 |
| Handgrip strength, kg | 35 ± 10 | 35 ± 9 | 0.83 |
| Leg press, 1-RM strength, kg | 48 ± 13 | 56 ± 16 | 0.12 |
| Chest press, 1-RM strength, kg | 39 ± 16 | 40 ± 17 | 0.90 |
| Knee extension, 1-RM strength, kg | 52 ± 24 | 52 ± 22 | 0.95 |
| Knee flexion, 1-RM strength, kg | 48 ± 18 | 50 ± 18 | 0.73 |
| Sum of 1-RM strength, kg | 187 ± 65 | 197 ± 67 | 0.65 |
| Knee extension, power at 60o/s, W | 70 (67, 86) | 79 (63, 92) | 0.61 |
| Knee flexion, power at 60o/s, W | 44 ± 12 | 47 ± 15 | 0.50 |
| Knee extension, power at 180°/s, W | 174 ± 66 | 184 ± 63 | 0.63 |
| Knee flexion, power at 180o/s, W | 108 ± 33 | 105 ± 36 | 0.75 |
| Sum average isokinetic power, W | 377 (338, 431) | 391 (308, 484) | 0.74 |
Data represent baseline characteristics for subjects who completed the study in treatment and control groups. ALT, alanine aminotransferase; AST, aspartate aminotransferase; OGTT, oral-glucose-tolerance test; PPT, Physical Performance Test; 1-RM, one-repetition maximum.
Comparisons between groups were performed by using Student’s t test for independent samples (for normally distributed data sets) or the Mann Whitney U test (for skewed data sets).
Mean ± SD (all such values for normally distributed data sets).
Values (except for 2 h post-OGTT) were obtained after an overnight fast.
Median; quartiles in parentheses (all such values for skewed data sets).
Values ranged from 0 to 36. Scores 32–36 indicate no clinically significant deficit (29).
Effects of n–3 PUFA therapy on body weight, body fat, and muscle mass and function
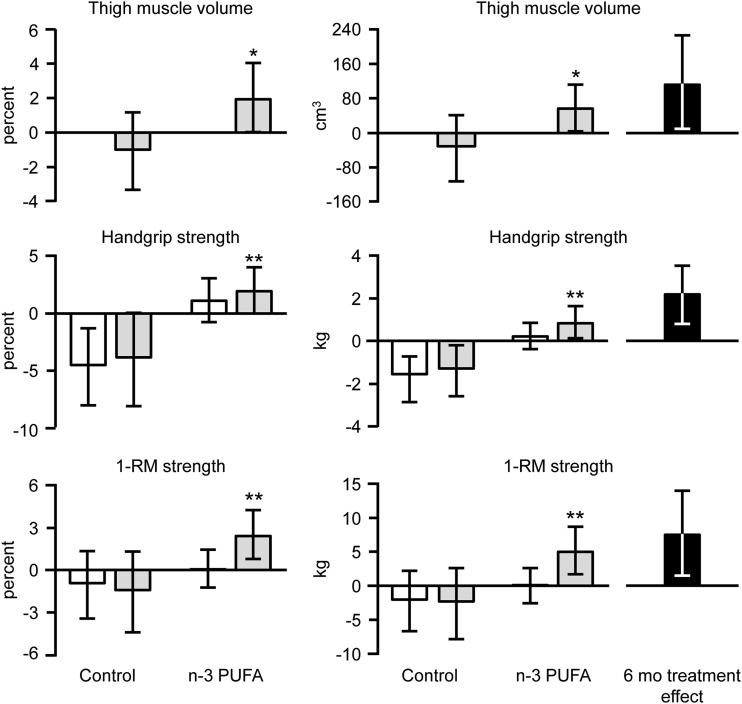
Six months of n–3 PUFA therapy did not significantly affect body weight, total-body fat mass, or the intermuscular fat content (Table 2). However, compared with the control group, 6 mo of n–3 PUFA therapy increased thigh muscle volume (treatment effect at 6 mo: 3.6%; 95% CI: 0.2%, 7.0%; P < 0.05), handgrip strength (2.3 kg; 95% CI: 0.8, 3.7 kg; P < 0.01), and 1-RM muscle strength (4.0%; 95% CI: 0.8%, 7.3%; P < 0.05) (Figure 2). Average isokinetic muscle power tended to be increased by n–3 PUFA therapy at 6 mo (treatment effect: 5.6%; 95% CI: −0.6%, 11.7%; P = 0.075) but not at 3 mo (3.3%; 95% CI: −0.9%, 7.5%; P = 0.12).
TABLE 2.
Changes in body weight, fat mass, intermuscular fat content, blood pressure, plasma lipid and liver enzyme concentrations, and glucose tolerance after 6 mo1
| ANCOVA2 |
||||
| Control (n = 15) | n–3 PUFA (n = 29) | Treatment effect | P | |
| Body weight, kg | 0.0 (−0.9, 0.9) | 0.6 (−0.3, 1.4) | 0.6 (−0.8, 2.0) | 0.39 |
| Body fat, kg | −0.2 (−0.9, 0.6) | 0.3 (−0.2, 0.8) | 0.5 (−0.5, 1.4) | 0.31 |
| Intermuscular fat content, cm3 | −0.8 (−5.2, 3.7) | −6.0 (−10.3, −1.7) | −4.0 (−10.8, 2.8) | 0.24 |
| Blood pressure, mm Hg | ||||
| Systolic | −5 (−12, 2) | −2 (−7, 4) | 3 (−5, 12) | 0.46 |
| Diastolic | −4 (−8, 0) | −1 (−3, 2) | 3 (−2, 8) | 0.22 |
| Plasma concentrations3 | ||||
| Triglycerides, mmol/L | −0.10 (−0.26, 0.05) | −0.17 (−0.27, −0.07) | −0.03 (−0.17, 0.12) | 0.69 |
| HDL cholesterol, mmol/L | −0.06 (−0.21, 0.10) | 0.02 (−0.06, 0.09) | 0.14 (−0.01, 0.29) | 0.06 |
| LDL cholesterol, mmol/L | −0.13 (−0.29, 0.04) | 0.03 (−0.11, 0.17) | 0.15 (−0.09, 0.38) | 0.23 |
| AST, IU/L | −0.4 (2.2, 1.4) | 0.3 (−0.7, 1.3) | 0.3 (−1.5, 2.2) | 0.73 |
| ALT, IU/L | −0.4 (2.8, 2.0) | 1.3 (−0.4, 3.0) | 1.7 (−1.2, 4.6) | 0.25 |
| Glucose, mmol/L | 0.06 (−0.17, 0.28) | 0.26 (0.11, 0.42) | 0.13 (−0.13, 0.39) | 0.33 |
| Glucose, 2 h post-OGTT, mmol/L | 0.16 (−0.54, 0.86) | −0.43 (−0.90, 0.05) | −0.72 (−1.56, 0.13) | 0.09 |
All values are means with upper and lower 95% CI bounds in parentheses. Values represent changes from baseline at 6 mo for subjects who completed the study in treatment and control groups. ALT, alanine aminotransferase; AST, aspartate aminotransferase; OGTT, oral-glucose-tolerance test.
Effect of n–3 PUFA treatment (compared with the control) was evaluated by using an ANCOVA with the baseline value as the covariate.
Values (except for 2 h post-OGTT) were obtained after an overnight fast.
FIGURE 2.
Changes (95% CIs) in thigh muscle volume, handgrip strength, and 1-RM muscle strength in the n–3 PUFA and control groups. Data represent average absolute and relative changes from baseline after 3 mo (white bars) and 6 mo (gray bars) of treatment with corn oil (control) and fish oil–derived n–3 PUFA and treatment effects at 6 mo (black bars). n = 15 in the control group (all figures); n = 29 in the n–3 PUFA group for thigh muscle volume and n = 28 for handgrip strength and 1-RM muscle strength because one subject could not complete the tests because of shoulder and arm pain at 3 and 6 mo. 1-RM strength represents the composite score (i.e., sum of all 1-RMs) for the following exercises: leg press, chest press, knee extension, and knee flexion. An ANCOVA with the baseline value as a covariate was used to evaluate the effect of n–3 PUFA treatment on thigh muscle volume, which was measured only at baseline and 6 mo of treatment; *significantly different from the control group, P < 0.05. A linear mixed model ANOVA (with raw data collected at baseline and 3 and 6 mo) was used to evaluate differences in handgrip strength and 1-RM muscle strength between control and n–3 PUFA groups. Significant group × time interactions were identified, and post hoc analyses revealed the following: **significantly different from the corresponding value before treatment, P < 0.05. 1-RM, one-repetition maximum.
Adverse events
No serious adverse events related to n–3 PUFA therapy occurred (Table 3), and there were no n–3 PUFA therapy–related changes in blood pressure, plasma lipid and liver enzyme concentrations, and glucose tolerance (Table 2). The most-commonly reported side effect was a fishy aftertaste, which was reported by one-third of subjects in the n–3 PUFA group. Gastrointestinal symptoms were reported by 6 subjects (33%) in the control group and 11 subjects (31.4%) in the n–3 PUFA group.
TABLE 3.
Summary of adverse events in all subjects who initiated the n–3 PUFA and corn-oil (control) treatments1
| Control (n = 18) | n–3 PUFA (n = 35) | |
| Definitely or possibly related to treatment, n (%) | ||
| Fishy aftertaste | — | 12 (34.3) |
| Diarrhea | 2 (11.1) | 4 (11.4) |
| Constipation | 1 (5.5) | 2 (5.7) |
| Gastrointestinal discomfort | 2 (11.1) | — |
| Indigestion | — | 3 (8.6) |
| Intestinal gas | — | 1 (2.9) |
| More-frequent bowel movements | — | 1 (2.9) |
| Unlikely related to treatment, n (%) | ||
| Back pain | — | 4 (11.4) |
| Chest pain | — | 2 (5.7) |
| Common cold | 1 (5.5) | — |
| Deep vein thrombosis | 1 (5.5) | — |
| Gastroenteritis | 1 (5.5) | — |
| Memory problems | 1 (5.5) | — |
| Shoulder pain | — | 2 (5.7) |
| Dislocated shoulder | — | 1 (2.9) |
| Neck pain | — | 1 (2.9) |
| Sore elbow | — | 1 (2.9) |
| Sore knee | — | 1 (2.9) |
| Dry skin | — | 1 (2.9) |
| Ear infection | — | 1 (2.9) |
| Facial flushing | — | 1 (2.9) |
| Fatigue | — | 1 (2.9) |
| Melanoma | — | 1 (2.9) |
Adverse events are listed for all subjects who initiated n–3 PUFA and corn-oil (control) treatments, including those who dropped out of the study. No adverse events are reported for the 7 subjects who were randomly assigned but did not complete the baseline testing.
DISCUSSION
Age-associated declines in muscle mass, strength, and power can have adverse effects on the ability to carry out activities of daily living and live independently in older adults (1, 2, 4–6, 8, 9, 12, 37). Exercise, particularly resistance-exercise training, and treatment with testosterone, growth hormone, or dehydroepiandrosterone (23–25, 28) have beneficial effects on muscle mass and function. However, in clinical practice, compliance with an effective exercise program is difficult to achieve, and the safety of long-term use of anabolic agents, such as testosterone and growth hormone, is unclear because they can cause serious side effects (including myocardial infarction). Therefore, new, safe, and effective therapies to prevent the aging-associated decline in muscle mass and function are much needed and could have considerable clinical and public health implications. In this RCT, we showed that 6 mo of n–3 PUFA therapy had both statistically and clinically significant beneficial effects on thigh muscle volume, handgrip strength, and upper- and lower-body 1-RM muscle strength and tended to increase the average isokinetic leg muscle power. In addition, treatment was well tolerated with only minor adverse effects. These data show that fish oil–derived n–3 PUFA supplementation deserves consideration as a potential therapy to slow, and possibly prevent, the aging-associated decline in physical function.
Changes in muscle mass and function induced by n–3 PUFA therapy in our subjects were less than those reported with exercise training (28, 38) but the same or greater than those achieved with testosterone (24, 25), growth-hormone (25), or dehydroepiandrosterone (23) therapy in older adults and clearly clinically relevant. The difference in muscle volume between n–3 PUFA and control groups at 6 mo was ∼3.5%, and the difference in muscle strength was ∼6%, suggesting that 6 mo of n–3 PUFA therapy can prevent 2–3 y of normal age-associated losses in muscle mass (∼0.5–1.0%/y) and function (∼2–3%/y) (1–4).
The exact mechanisms by which fish oil–derived n–3 PUFA therapy increases muscle mass are not known but likely involve alterations in both anabolic and catabolic pathways. We previously reported that fish oil–derived n–3 PUFA therapy increased the rate of muscle protein synthesis (39), and studies conducted in isolated mouse muscles showed that n–3 PUFAs attenuated muscle protein breakdown (40). The beneficial effects of n–3 PUFA therapy on muscle strength and average isokinetic muscle power could also be related to their beneficial effect on the muscle lipid content and mitochondrial function, which are important determinants of muscle function (31, 41, 42). Several studies (43–45) conducted in cell cultures and animal models indicated that fish oil–derived n–3 PUFAs stimulate muscle mitochondrial biogenesis and mitochondrial content and function and reduce both total muscle and intramyocellular triglyceride contents. In addition, it is possible that the beneficial effects of n–3 PUFA therapy on muscle function were mediated through their known neuroprotective and motor-neuron excitability properties (28, 46).
The strengths of our study included the RCT design, long duration of the intervention, and comprehensive assessment of muscle mass and function. However, our study had some limitations. The fishy aftertaste of the n–3 PUFA pills might have prevented the true blinding of some of our study subjects, and we did not control or monitor dietary intake of our subjects, although they were asked to maintain their habitual dietary intakes throughout the entire study period. In addition, we a priori decided to evaluate 4 key outcome measures related to muscle size and function (i.e., muscle volume, handgrip strength, 1-RM muscle strength, and average isokinetic muscle power), which increased the chance of a false-positive effect. All analyses were performed on a completers-only basis. However, characteristics of subjects who dropped out were not different from those who completed the study; reasons for dropout in n–3 PUFA and control groups were well balanced and similar in the 2 groups, and all completers were included irrespective of how well they adhered to the intervention. Last, our study only involved healthy older adults who did not have clinically significant physical impairments or diminished physical function. Therefore, our study could not determine whether n–3 PUFAs have therapeutic effects in the older adult population who has the greatest immediate need for maintaining mobility and physical function. Alternatively, it is possible that the magnitude of gains in muscle size and strength would have been even greater in a population with significant deficits. Additional studies are needed to determine whether n–3 PUFA therapy can reduce the rate of loss or even increase muscle mass and function in sarcopenic older adults.
In summary, results from our study show that fish oil–derived n–3 PUFA therapy has clinically important muscle anabolic and physical performance–enhancing effects in older adults. Additional studies are needed to determine whether long-term n–3 PUFA therapy can sufficiently slow the declines in muscle mass and function that normally occur in older adults to significantly delay or even prevent sarcopenia and a loss of physical independence or cure it in already sarcopenic persons.
Acknowledgments
We thank Rachel Burrows and Lynda Bowers for help with subject recruitment, scheduling, and testing, and Kathryn Gratza, Jennifer Shew, Freida Custodio, and Adewole Okunade for their technical assistance.
The authors’ responsibilities were as follows—BM: designed the study, was responsible for the overall study supervision, and had primary responsibility for the final content of the manuscript; GIS, SJ, DRS, and BM: conducted the study; DNR and SK: provided medical supervision for the study; GIS and BM: performed the statistical analysis; GIS and BM: drafted the manuscript; SJ, DNR, DRS, and SK: critically revised the manuscript for important intellectual content; and all authors: read and approved the final manuscript. No funding entity had any role in the design, implementation, analysis, or interpretation of the data. None of the authors reported a conflict of interest related to the study.
REFERENCES
- 1.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–64. [DOI] [PubMed] [Google Scholar]
- 4.Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65-89 years. Age Ageing 1994;23:371–7. [DOI] [PubMed] [Google Scholar]
- 5.Ishizaki T, Watanabe S, Suzuki T, Shibata H, Haga H. Predictors for functional decline among nondisabled older Japanese living in a community during a 3-year follow-up. J Am Geriatr Soc 2000;48:1424–9. [DOI] [PubMed] [Google Scholar]
- 6.Hicks GE, Shardell M, Alley DE, Miller RR, Bandinelli S, Guralnik J, Lauretani F, Simonsick EM, Ferrucci L. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2012;67:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Impaired voluntary neuromuscular activation limits muscle power in mobility-limited older adults. J Gerontol A Biol Sci Med Sci 2010;65:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbat-Artigas S, Rolland Y, Cesari M, Abellan van Kan G, Vellas B, Aubertin-Leheudre M. Clinical relevance of different muscle strength indexes and functional impairment in women aged 75 years and older. J Gerontol A Biol Sci Med Sci 2013;68:811–9. [DOI] [PubMed] [Google Scholar]
- 9.Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci 1995;50:64–7. [DOI] [PubMed] [Google Scholar]
- 10.Whipple RH, Wolfson LI, Amerman PM. The relationship of knee and ankle weakness to falls in nursing home residents: an isokinetic study. J Am Geriatr Soc 1987;35:13–20. [DOI] [PubMed] [Google Scholar]
- 11.Sieri T, Beretta G. Fall risk assessment in very old males and females living in nursing homes. Disabil Rehabil 2004;26:718–23. [DOI] [PubMed] [Google Scholar]
- 12.Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc 2004;52:1121–9. [DOI] [PubMed] [Google Scholar]
- 13.Roberts HC, Syddall HE, Cooper C, Aihie Sayer A. Is grip strength associated with length of stay in hospitalised older patients admitted for rehabilitation? Findings from the Southampton grip strength study. Age Ageing 2012;41:641–6. [DOI] [PubMed] [Google Scholar]
- 14.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 2006;61:72–7. [DOI] [PubMed] [Google Scholar]
- 15.Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznaric Z, Nair KS, et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin Nutr 2014;33:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, Doehner W, Fearon KC, Ferrucci L, Hellerstein MK, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc 2010;11:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulgoni VL., 3rd Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003-2004. Am J Clin Nutr 2008;87:1554S–7S. [DOI] [PubMed] [Google Scholar]
- 18.Schutzer KA, Graves BS. Barriers and motivations to exercise in older adults. Prev Med 2004;39:1056–61. [DOI] [PubMed] [Google Scholar]
- 19.Schoenborn CA, Adams PF, Peregoy JA. Health behaviors of adults: United States, 2008-2010. Vital Health Stat 10 2013;257:1–184. [PubMed] [Google Scholar]
- 20.Adams PF, Benson V. Current estimates from the National Health interview survey, 1989. Vital Health Stat 10 1990;176:1–221. [PubMed] [Google Scholar]
- 21.Pearson SJ, Young A, Macaluso A, Devito G, Nimmo MA, Cobbold M, Harridge SD. Muscle function in elite master weightlifters. Med Sci Sports Exerc 2002;34:1199–206. [DOI] [PubMed] [Google Scholar]
- 22.Korhonen MT, Cristea A, Alen M, Hakkinen K, Sipila S, Mero A, Viitasalo JT, Larsson L, Suominen H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol (1985) 2006:101:906–17. [DOI] [PubMed] [Google Scholar]
- 23.Baker WL, Karan S, Kenny AM. Effect of dehydroepiandrosterone on muscle strength and physical function in older adults: a systematic review. J Am Geriatr Soc 2011;59:997–1002. [DOI] [PubMed] [Google Scholar]
- 24.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, van der Schouw YT. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA 2008;299:39–52. [DOI] [PubMed] [Google Scholar]
- 25.Giannoulis MG, Sonksen PH, Umpleby M, Breen L, Pentecost C, Whyte M, McMillan CV, Bradley C, Martin FC. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab 2006;91:477–84. [DOI] [PubMed] [Google Scholar]
- 26.Murphy RA, Mourtzakis M, Chu QS, Baracos VE, Reiman T, Mazurak VC. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer 2011;117:1775–82. [DOI] [PubMed] [Google Scholar]
- 27.Berbert AA, Kondo CR, Almendra CL, Matsuo T, Dichi I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition 2005;21:131–6. [DOI] [PubMed] [Google Scholar]
- 28.Rodacki CL, Rodacki AL, Pereira G, Naliwaiko K, Coelho I, Pequito D, Fernandes LC. Fish-oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr 2012;95:428–36. [DOI] [PubMed] [Google Scholar]
- 29.Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci 2000;55:M350–5. [DOI] [PubMed] [Google Scholar]
- 30.Fukumoto Y, Ikezoe T, Yamada Y, Tsukagoshi R, Nakamura M, Mori N, Kimura M, Ichihashi N. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur J Appl Physiol 2012;112:1519–25. [DOI] [PubMed] [Google Scholar]
- 31.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 2005;60:324–33. [DOI] [PubMed] [Google Scholar]
- 32.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106:2747–57. [DOI] [PubMed] [Google Scholar]
- 33.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 34.Patterson BW, Mittendorfer B, Elias N, Satyanarayana R, Klein S. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J Lipid Res 2002;43:223–33. [PubMed] [Google Scholar]
- 35.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Ehsani AA, Holloszy JO. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol 2007;102:634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grady D, Halloran B, Cummings S, Leveille S, Wells L, Black D, Byl N. 1,25-Dihydroxyvitamin D3 and muscle strength in the elderly: a randomized controlled trial. J Clin Endocrinol Metab 1991;73:1111–7. [DOI] [PubMed] [Google Scholar]
- 37.Puthoff ML, Nielsen DH. Relationships among impairments in lower-extremity strength and power, functional limitations, and disability in older adults. Phys Ther 2007;87:1334–47. [DOI] [PubMed] [Google Scholar]
- 38.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr 2011;93:402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitehouse AS, Smith HJ, Drake JL, Tisdale MJ. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res 2001;61:3604–9. [PubMed] [Google Scholar]
- 41.Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci USA 2007;104:1057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW, Manini TM, Wohlgemuth SE, Leeuwenburgh C, Cummings SR, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci 2013;68:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jucker BM, Cline GW, Barucci N, Shulman GI. Differential effects of safflower oil versus fish oil feeding on insulin-stimulated glycogen synthesis, glycolysis, and pyruvate dehydrogenase flux in skeletal muscle: a 13C nuclear magnetic resonance study. Diabetes 1999;48:134–40. [DOI] [PubMed] [Google Scholar]
- 44.Vaughan RA, Garcia-Smith R, Bisoffi M, Conn CA, Trujillo KA. Conjugated linoleic acid or omega 3 fatty acids increase mitochondrial biosynthesis and metabolism in skeletal muscle cells. Lipids Health Dis 2012;11:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neschen S, Moore I, Regittnig W, Yu CL, Wang Y, Pypaert M, Petersen KF, Shulman GI. Contrasting effects of fish oil and safflower oil on hepatic peroxisomal and tissue lipid content. Am J Physiol Endocrinol Metab 2002;282:E395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim SN, Huang W, Hall JC, Ward RE, Priestley JV, Michael-Titus AT. The acute administration of eicosapentaenoic acid is neuroprotective after spinal cord compression injury in rats. Prostaglandins Leukot Essent Fatty Acids 2010;83:193–201. [DOI] [PubMed] [Google Scholar]