Abstract
Background: Dietary lipids are one of the most effective stimulators of carotenoid absorption, but very limited data exist on the impact of endogenous food sources of lipids to enhance carotenoid absorption. The co-consumption of whole egg with carotenoid-rich foods may increase overall carotenoid absorption via lipid-rich egg yolk.
Objective: We designed this study to assess the effects of egg consumption on carotenoid absorption from a carotenoid-rich, raw mixed-vegetable salad.
Design: Healthy young men (n = 16) consumed the same salad (all served with 3 g canola oil) with no egg (control), 75 g scrambled whole eggs (1.5 eggs) [low egg (LE)], and 150 g scrambled whole eggs (3 eggs) [high egg (HE)] (a randomized crossover design). Control, LE, and HE meals contained 23 mg, 23.4 mg (0.4 mg from eggs), and 23.8 mg (0.8 mg from eggs) total carotenoids and 3 g, 10.5 g (7.5 g from eggs), and 18 g (15 g from eggs) total lipids, respectively. Blood was collected hourly for 10 h, and the triacylglycerol-rich lipoprotein (TRL) fraction was isolated. Total and individual carotenoid contents, including lutein, zeaxanthin , α-carotene, β-carotene, and lycopene in TRL were analyzed, and composite areas under the curve (AUCs) were calculated.
Results: The total mean (±SE) carotenoid AUC0–10h in TRL was higher for the HE meal than for LE and control meals [125.7 ± 19.4a compared with 44.8 ± 9.2b compared with 14.9 ± 5.2b nmol/L · 10 h, respectively (values without a common superscript letter differ); P < 0.0001]. The TRL AUC0–10h of lutein and zeaxanthin increased 4–5-fold (P < 0.001), and the TRL AUC0–10h of carotenoid not present in eggs, including α-carotene, β-carotene, and lycopene, increased 3–8-fold (P < 0.01) for the HE meal compared with the control meal.
Conclusion: These findings support the claim that co-consuming cooked whole eggs is an effective way to enhance carotenoid absorption from other carotenoid-rich foods such as a raw mixed-vegetable salad. This trial was registered at clinicaltrials.gov as NCT01951313.
Keywords: carotenoid absorption, triacylglycerol absorption, eggs, triacylglycerol rich lipoprotein fraction, vegetable salad
INTRODUCTION
The 2010 Dietary Guidelines for Americans recommends the consumption of 4.5 cups fruit and vegetables/d, but the average fruit and vegetable intake of US adults is only 2.6 cups/d (1). This low consumption of fruit and vegetables may result in low intakes of fat-soluble, health-promoting, bioactive compounds such as carotenoids from these foods (2). Dietary carotenoids have beneficial biological properties including antioxidant and anti-inflammatory effects, and research has supported the potential for protective effects of carotenoids against many degenerative diseases, including cardiovascular diseases, age-related macular degeneration, and some types of cancer (3–7).
The absorption of carotenoids from a meal can be affected by several factors, including the food matrix, type of food processing or preparation, interactions with other dietary compounds during digestion and absorption, gut status, which may affect digestion and absorption processes, and nutritional status (2). Of these factors that influence the bio-accessibility and bio-availability of carotenoids, the co-consumption of carotenoid-rich foods with dietary lipids may be one of the most effective stimulators of carotenoid absorption (8). For example, healthy young adults had ∼12.5-, ∼40-, and ∼3-fold increases in postprandial α-carotene, β-carotene, and lycopene absorption, respectively, when they consumed a salad with full-fat salad dressing (28 g) compared with a fat-free salad dressing (9). Also, an acute feeding study designed to examine the impact of both the amount and source of dietary lipids on postprandial absorption of carotenoids from a mixed-vegetable salad revealed that the amount of co-consumed lipids was a more critical effector than source (with a varying degree of saturation) on carotenoid absorption (10). However, over the past 10 y, low-fat versions of food products typically co-consumed with fruit and vegetables (e.g., salad dressings) are readily available and limit or omit fat from salads (11). Collectively, either low intake of carotenoids from fruit and vegetables or inefficient bio-availability because of a decreased co-consumption of dietary lipids may reduce the potential for carotenoids to effectively promote health and prevent disease in humans.
Eggs are already established as a highly bioavailable source of specific carotenoids, lutein, and zeaxanthin (12) that is due, in part, to the presence of known promoters of carotenoid intestinal absorption. Both the lipid content and high amount of phospholipids in egg yolk (13, 14) are known to enhance carotenoid intestinal micellarization and absorption (12, 15, 16). The highly bioavailable nature of carotenoids from eggs suggests that egg-derived factors may be further leveraged to improve the bio-availability of carotenoids found in co-consumed fruit and vegetables. Although promising, very limited data exist on the impact of a co-consumed food source of lipid, such as eggs, to enhance carotenoid absorption. Therefore, the aim of this study was to assess the effects of co-consuming cooked whole eggs with a carotenoid-rich, raw mixed-vegetable salad on postprandial carotenoid absorption. We hypothesized that a salad with 75 g (∼1.5 eggs) or 150 g (∼3 eggs) of scrambled whole egg would progressively increase postprandial carotenoid absorption compared with a salad without egg.
METHODS
Subjects
A total of 17 subjects were recruited from the greater Lafayette, Indiana, region and 16 of 17 subjects completed the study (Figure 1). One subject withdrew because of a personal medical condition unrelated to the study. Sixteen healthy young men (8 Asian, 7 Caucasian, and one African American) participated, and their average (mean ± SE) age and BMI (in kg/m2) were 24 ± 1 y and 23.9 ± 0.6, respectively. Exclusion criteria for this study included weight change >3 kg ≤3 mo prestudy, exercising vigorously during the past 3 mo, intestinal disorders including lipid malabsorption or lactose intolerance, abnormal liver- or kidney-function tests, fasting blood glucose concentration >110 mg/dL, smoking, drinking >2 alcoholic drinks/d, and taking lipid-lowering medications or dietary supplements that affect the plasma cholesterol concentration. The study protocol was approved by the Purdue University Biomedical Institutional Review Board, and all subjects provided written informed consent and received monetary compensation for their participation. This trial was registered at clinicaltrials.gov as NCT01951313.
FIGURE 1.
Consort flow diagram.
Study design
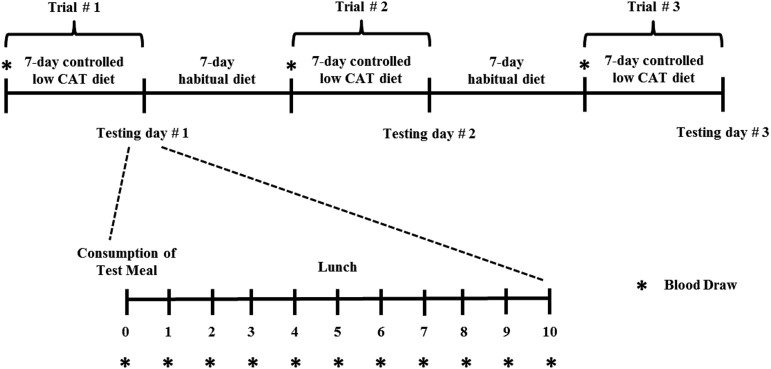
Each subject completed 3 trials (a randomized, single-blind, crossover design); the treatment-order random assignment was done with SAS 9.2 software (SAS Institute Inc.) (Figure 2). Although subjects and dietitians were not blinded, investigators were blinded until all subjects finished the protocol and all sample analyses were completed. Seven days before each testing day, subjects arrived at the Purdue clinical research center in the morning after a 12-h fast, and blood was collected to analyze plasma carotenoid concentrations. For the next 7 d of each trial, all subjects consumed a low-carotenoid diet designed to reduce plasma carotenoid concentrations (10). On each of the 3 testing days, subjects returned to the clinical research center after a 12-h overnight period of fasting, and a catheter was placed into an antecubital vein. After a baseline blood sample was collected, subjects consumed a carefully portioned raw mixed-vegetable salad without eggs (control), with 75 g scrambled whole eggs (∼1.5 eggs) [low egg (LE)5], and with 150 g scrambled whole eggs (∼3 eggs) [high egg (HE)]. After the test meal was consumed, blood samples were collected hourly for 10 h. A low-carotenoid, low-fat lunch was consumed after blood was collected at hour 5. On completion of the first and second trials, 1-wk periods were scheduled during which subjects consumed their habitual unrestricted diets.
FIGURE 2.
Study design. On the 3 testing days, each subject consumed the test salad with different amounts of scrambled whole eggs (0, 75, and 150 g) as designated by the randomized crossover design. CAT, carotenoid.
Test meal
All subjects consumed a controlled low-carotenoid diet for 7 d before each testing day to reduce plasma carotenoid concentrations and minimize the contribution of carotenoid in the triacylglycerol-rich lipoprotein (TRL) fraction because the carotenoid content in the TRL fraction is known to be elevated from previously consumed meals (17). During the first 5 d of these controlled diet periods, subjects were counseled to consume a very-low carotenoid diet; meals on these days were designed to contain ≤750 μg carotenoids/d. On days 6 and 7 of the controlled diet periods (the 2 d before each testing day), subjects were provided completely controlled, low-carotenoid meals with <500 μg carotenoids/d. On each of the 3 testing days, subjects consumed a carefully portioned test salad including tomatoes, shredded carrots, baby spinach, lettuce, and Chinese wolfberry as sources of carotenoids (Table 1). All salads were served with 3 g canola oil. Control, LE, and HE salads included 3 g, 10.5 g (7.5 g from eggs), and 18 g (15 g from eggs) total dietary lipids, respectively. Scrambled whole eggs were prepared uniformly from large eggs on the basis of the American Egg Board’s recommendation (18) and portioned appropriately to dose. The low-carotenoid, low-fat lunch consumed at hour 5 provided 638 kcal, 25 g protein, 130 g carbohydrate, 1.95 g fat, and 172 μg carotenoids. Consumption of water throughout the day was allowed ad libitum. All menus were developed by a registered dietitian with Pronutra software (version 3.3; Viocare Inc.), and all foods were prepared, portioned, and provided to subjects by research staff in the Department of Nutrition Science Metabolic Kitchen at Purdue University. All dietary counseling and meals provided before and during trials were the same as in our previous study (10).
TABLE 1.
Nutrient contents of vegetables, salad oil, and test salad (vegetables plus oil)1
| Energy, kcal | Protein,2 g | Carbohydrate,2 g | Lipids,2 g | Fiber, g | Carotenoids, μg | |
| Vegetables | 65 | 3 (18) | 12 (74) | 0.6 (8) | 4 | 25,210 |
| Oil | 27 | 0 | 0 | 3.0 (100) | 0 | 0 |
| Test salad (vegetables + oil) | 92 | 3 (13) | 12 (52) | 3.6 (35) | 4 | 25,210 |
Vegetables included 100 g beefsteak tomatoes, 62 g shredded carrots, 70 g baby spinach, 25 g romaine lettuce, and 5 g Chinese wolfberry.
Values in parentheses are total energy content, %.
Sample collection and analysis
Blood sample collection and plasma and TRL-fraction isolation
All blood samples were collected into heparinized tubes and centrifuged (3000 × g for 15 min at 4°C) to obtain plasma. Aliquots of fasting-state plasma samples were flushed with nitrogen gas and stored at −80°C until thawed for carotenoid analysis. On each of the 3 testing days, aliquots of fresh plasma from each blood sample were processed to isolate the TRL fraction as previously reported (10). Briefly, 10 mL plasma was transferred to Ultra Clear centrifuge tubes (Denville Scientific Inc.) and preserved with stabilizers including aprotinin, phenylmethylsulfonyl fluoride, sodium azide, and EDTA (Sigma Chemical Co.). Preserved samples were overlaid with a 1.006-g/mL density solution and centrifuged (L8-M 70; Beckman Instruments Inc.) in a swinging bucket rotor (SW 41 TI; Beckman Instruments Inc.) at 152,000 × g for 35 min at 20°C. The isolated TRL fraction was pipetted into cryostorage tubes, which were flushed with nitrogen gas and stored at −80°C until thawed for analysis. All isolation processes were conducted in a dark room with red light to minimize photo-oxidative reactions.
Lipid analysis
Triacylglycerol and total cholesterol concentrations in TRL fractions were measured in duplicate by using a Cobas MIRAS Plus chemistry analyzer (Roche Analytic Instruments).
Carotenoid extraction
The extraction of carotenoids from plasma and TRL fractions was done as described by Goltz et al. (10). A total of 200 μL plasma and 2000 μL TRL fractions were deproteinated with methanol and acetone:petroleum ether [1:2 with 0.1% butylated hydroxytoluene (BHT)], and petroleum ether layers were transferred into 11-mL glass vials. This step was repeated twice more by using petroleum ether (0.1% BHT), and the combined petroleum ether layers were dried under nitrogen gas. These extracts were immediately resolubilized and analyzed as described in Carotenoid Analysis.
On each testing day, duplicate test salads and scrambled whole eggs were each homogenized by using a blender (KitchenAid), and aliquots were put into storage tubes, flushed with nitrogen gas, and stored at −80°C until thawed for analysis. The extraction of carotenoids from salad homogenates was conducted as described by Goltz et al. (10) with minor modifications. A total of 0.5 g homogenate was combined with ∼0.25 g sodium bicarbonate and ∼0.5 g celite, and carotenoids were extracted with a 1:1 solution of acetone:petroleum ether (0.1% BHT). The salad solvent suspension was vacuum filtered through 2 no. 1 Whatman filter papers (VWR International) into a 125-mL Erlenmeyer flask. Two additional extractions were performed with petroleum ether (0.1% BHT) until no residual color remained in the plant tissue filtrate. Combined acetone and petroleum ether layers were saponified by mixing with 40% KOH in methanol on a magnetic stir plate for 45 min at 30°C. The saponified extract was quantitatively transferred to a separator funnel and washed with distilled water and saturated sodium chloride. The aqueous layer was drained, and the petroleum ether layer was poured through a funnel that contained glass wool and a layer of anhydrous sodium sulfate to remove residual water and collected into a 50-mL volumetric flask. Subsequently, the petroleum ether layer was diluted to a total volume of 50 mL with petroleum ether, and 50-mL aliquots were dried under nitrogen gas and stored at −80°C until analysis.
Extractions of carotenoids in homogenized samples of scrambled whole eggs were done as described by Handelman et al. (12) with modification. Briefly, 0.3 g scrambled whole eggs were mixed with ultrapure water and ethanol (0.1% BHT) and placed in a 60°C water bath for 5 min. Subsequently, KOH (30% weight:volume in water) was added, and the mixture was placed in a 60°C water bath for 30 min. The mixture was immediately placed on ice, and cold ultrapure water, isopropanol, and hexane:ethyl acetate (9:1) were added. After centrifugation, the top organic layer was transferred into an 11-mL glass vial, and this step was repeated 2 more times with hexane:ethyl acetate (9:1). The combined organic layers were dried under nitrogen gas and stored at −80°C until analysis. All extractions were done in a dark room with red light to minimize photo-oxidative reactions.
Carotenoid analysis
Dried samples from plasma, the TRL fraction, test salad, and scrambled whole eggs were resolubilized in ethyl acetate:methanol (1:1) and injected and analyzed by using HPLC. As previously described (10), injected samples were analyzed by using a Hewlett-Packard model 1090A HPLC pump, model 79880A diode array detector, and a YMC Carotenoid C30 column (2.0 × 150 mm; 3-μm particle size). All carotenoids (lutein, zeaxanthin, α-carotene, β-carotene, α-cryptoxanthin, and β-cryptoxanthin) were detected at 450 nm except for lycopene (470 nm). ChemStation software and data-management system [Rev. A.10.02 (1757); Agilent Technologies] were used to collect, integrate, and store chromatographic data. The most abundant individual carotenoids in test meals, including lutein, zeaxanthin, α-carotene, β-carotene, and lycopenem were analyzed. Less-abundant carotenoids in test meals (i.e., α-cryptoxanthin and β-cryptoxanthin), were also analyzed, but data for these compounds were only included in the calculation of the total carotenoid content in each TRL fraction.
Power calculation and statistical analysis
The primary outcome of this research was the difference in the postprandial carotenoid-absorption response from the raw mixed-vegetable salad that contained 3 scrambled whole eggs compared with from the same salad without eggs, which contained 18 and 3 g dietary lipids, respectively. A power calculation was conducted by using findings from our research that determined the total carotenoid concentration AUC0–10h between the same salad used for the current study with 20 g dietary lipids and the salads with both 3 and 8 g dietary lipids (10). To detect a similar difference in the salad when 3 eggs were consumed, a group size ≥15 participants provided ≥90% power at α = 0.05.
Postprandial triacylglycerol, total cholesterol, and total and individual carotenoid contents in TRL fractions, including lutein, zeaxanthin, α-carotene, β-carotene, and lycopene, were baseline corrected by subtracting fasting values from each time point. Also, baseline corrected total and individual carotenoid contents in TRL fractions at each time point were normalized by the carotenoid contents in the test salad and scrambled whole eggs consumed each testing day and then the 0–10-h positive incremental AUCs of total and individual carotenoid contents in TRL fractions were calculated. A repeated-measures ANOVA was used to determine the main effects of time and trial and the time-by-trial interaction. A 1-factor ANOVA with a post hoc Tukey test was applied to examine differences in the baseline corrected carotenoid content at each time point and baseline-corrected 0–10-h positive incremental AUCs of total cholesterol, triacylglycerol, and total and individual carotenoid contents. Paired t tests were used to compare plasma carotenoid concentrations before and after the 7-d low-carotenoid–diet periods that proceeded each testing day. All analyses were performed with SAS 9.2 software (SAS Institute Inc.). Data are presented as means (±SEs), and statistical significance was accepted at P < 0.05 (2 tailed).
RESULTS
Compliance with 7-d pretesting controlled diets
Average reductions in individual and total plasma carotenoid concentrations after the 7-d controlled low-carotenoid–diet periods ranged from 22% to 33% (P < 0.0001), consistent with the dietary compliance leading into the testing days (Table 2).
TABLE 2.
Change and percentage of change in individual and total carotenoids in plasma before and after the 7-d controlled low-carotenoid–diet periods1
| Lutein | Zeaxanthin | α-Carotene | β-Carotene | Lycopene | Total carotenoids | |
| Pre, nmol/L | 123.9 ± 6.82 | 53.0 ± 3.0 | 137.0 ± 10.9 | 427.1 ± 42.8 | 832.6 ± 48.7 | 1982.0 ± 111.0 |
| Post,3 nmol/L | 83.3 ± 3.6 | 39.0 ± 1.8 | 108.0 ± 7.6 | 322.9 ± 30.9 | 614.7 ± 38.9 | 1475.5 ± 77.2 |
| Change,4 nmol/L | −40.4 ± 4.9 | −13.7 ± 2.4 | −29.8 ± 5.8 | −108.3 ± 17.9 | −225.1 ± 29.8 | −517.2 ± 62.0 |
| Change,5 % | −33 | −26 | −22 | −25 | −27 | −26 |
n = 16 subjects. Pre, pre–7-d controlled low-carotenoid diet, Post; post–7-d controlled low-carotenoid diet.
Mean ± SE (all such values).
There was a significant difference between prediet and postdiet periods for each carotenoid (P < 0.0001).
Post − Pre.
(Post − Pre) ÷ Pre × 100.
Total carotenoid contents in the test salad and scrambled whole eggs
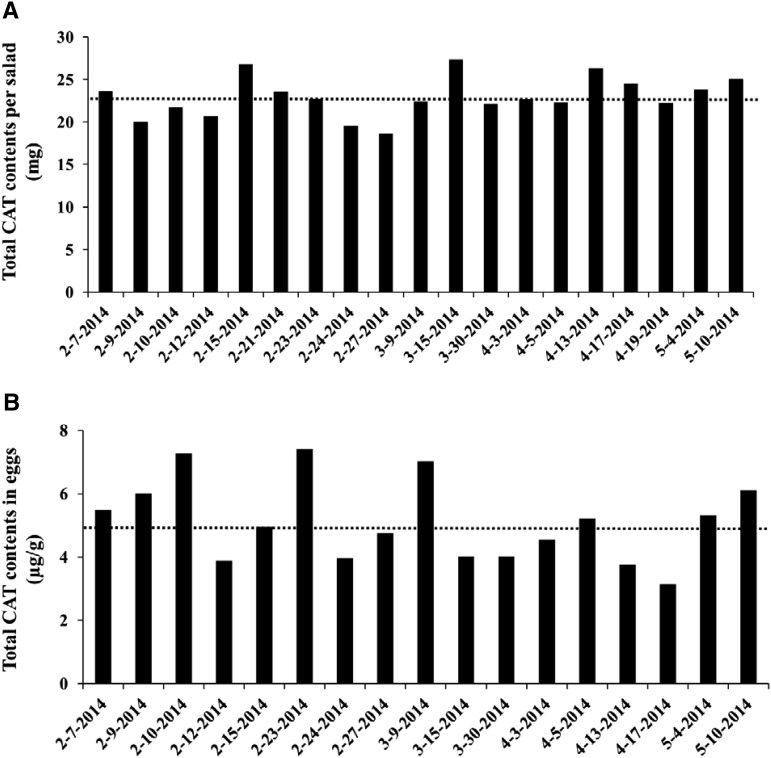
During the study period, total carotenoid content in test salads and in scrambled whole eggs was 23.0 ± 0.5 mg/serving (range: 18.7–27.3; CV: 0.11) (Figure 3A) and was 5.0 ± 0.3 μg/g of scrambled whole eggs (range: 3.1-7.4; CV: 0.26) (Figure 3B), respectively. Therefore, 75 and 150 g scrambled whole eggs provided 0.4 and 0.8 mg total carotenoids, respectively, to the test meal. Thus, 100%, 98.3%, and 96.6% of total carotenoids in control, LE, and HE test meals, respectively, were from the salad, and 0%, 1.7%, and 3.4% of total carotenoids, respectively, were from scrambled whole eggs. Individual carotenoid contents from salad and scrambled whole eggs were also measured (Supplementa1 Table 1).
FIGURE 3.
Reproducibility of total CAT contents in the test salad (A) and scrambled whole eggs (B) during the testing phase of the study. CAT, carotenoid.
Triacylglycerol and total cholesterol contents in TRL fraction
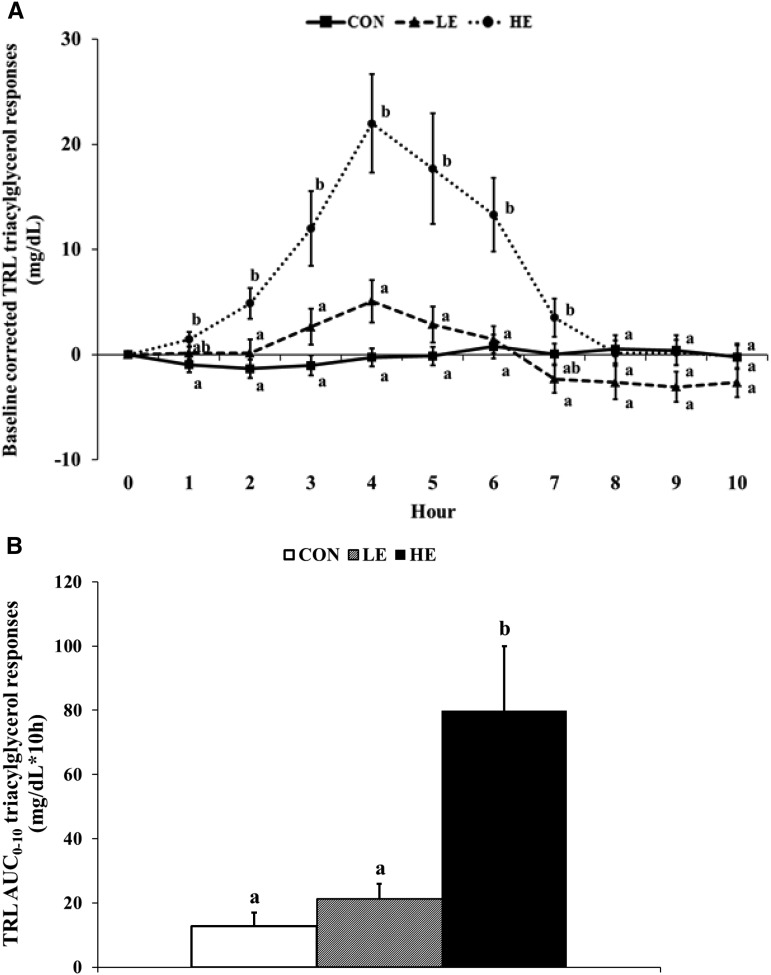
From hours 2 to 6, the HE consumption presented a higher baseline corrected triacylglycerol content in the TRL fraction than did LE and control consumption (Figure 4A), and the composite triacylglycerol AUC0–10h in the TRL fraction was higher for the HE consumption than for LE and control consumption (79.8 ± 20.1 compared with 21.1 ± 4.9 compared with 12.7 ± 4.2 mg/dL · 10 h; P < 0.001) (Figure 4B). The TRL fraction total cholesterol content was not different in trials during the 10 h of testing (except for hour 4) and the total cholesterol AUC0–10h in TRL was not different in trials (Supplementa1 Figure 1).
FIGURE 4.
Mean (±SE) baseline-corrected triacylglycerol content in the TRL fraction during the 10-h testing day (A) and the composite triacylglycerol AUC0–10h in the TRL fraction (B). Different superscript letters indicate statistical differences in CON, LE, and HE conditions (P < 0.001). CON, control; HE, high egg; LE, low egg; TRL, triacylglycerol-rich lipoprotein.
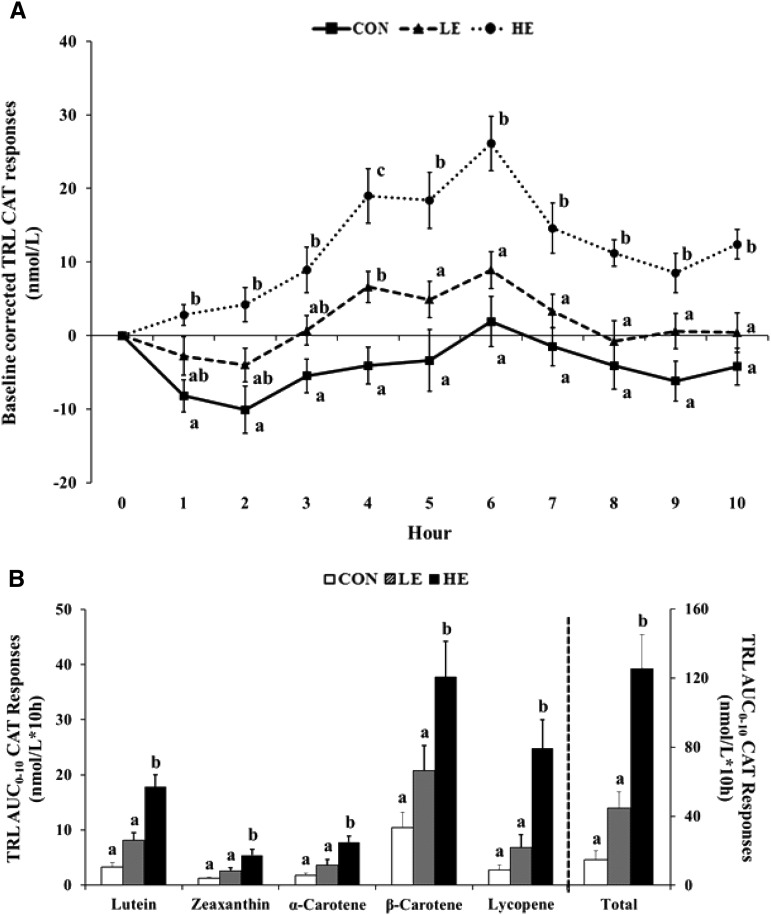
Changes in total and individual carotenoid contents in TRL fraction
TRL total and individual carotenoid responses supported the impact of egg consumption on carotenoid absorption. Until hour 3, the HE meal presented a significantly higher total carotenoid content in TRL than did the control meal, and at hour 4, dose-dependent progressive responses were observed. From hours 5 to 10, the HE meal still presented a higher total carotenoid content in TRL than did LE and control meals (Figure 5A). Lutein and zeaxanthin TRL response were also influenced by co-consumed scrambled whole eggs. As with total carotenoid, dose-dependent progressive responses were observed at hour 4, and the HE meal still presented a higher lutein content in TRL than did LE and control meals from hours 5 to 10. The HE meal also presented a higher zeaxanthin response than did the control meal from hours 1 to 10. Similar responses were observed for carotenoids beyond those shown in eggs such as α-carotene, β-carotene, and lycopene. The HE meal presented higher α-carotene, β-carotene, and lycopene contents in TRL than did the control meal during 10 h (Supplemental Figure 2).
FIGURE 5.
Mean (±SE) normalized baseline-corrected total CAT content in the TRL fraction during the 10-h testing day (A) and the composite total and individual CAT AUC0–10h in the TRL fraction (B). Different superscript letters indicate statistical differences in CON, LE, and HE conditions (P < 0.0001). CAT, carotenoid; CON, control; HE, high egg; LE, low egg; TRL, triacylglycerol-rich lipoprotein.
The total carotenoid AUC0–10h in TRL was also higher for the HE meal than for LE and control meals (125.7 ± 19.4 compared with 44.8 ± 9.2 compared with 14.9 ± 5.2 nmol/L · 10 h; P < 0.0001). Moreover, lutein, zeaxanthin, α-carotene, β-carotene, and lycopene AUCs0–10h in TRL were higher for the HE meal than for LE and control meals (Figure 5B).
DISCUSSION
It is well known that dark-green leafy vegetables and eggs are good sources of carotenoids, in particular, lutein and zeaxanthin (19). Although dark-green leafy vegetables contain more lutein and zeaxanthin than do eggs (19), eggs are highly bioavailable presumably because of the presence of the lipid matrix, which is composed of cholesterol, triacylglycerol, and phospholipids in egg yolk (20). One large whole egg (∼50 g) generally contains 4.8 g total lipids, which are mostly present in egg yolk, with 1.8 g MUFAs, 1.0 g PUFAs, and 1.6 g SFAs (21). Research that assessed the bio-availability of lutein from lutein supplements, spinach, and eggs documented a higher lutein bio-availability with egg consumption than with other treatments (22). The high bio-availability of carotenoid from eggs suggests that egg-derived factors may be leveraged to improve the bio-availability of other carotenoids in co-consumed fruit and vegetables. The current study used a carotenoid-rich raw mixed-vegetable salad to assess the impact of an endogenous food source of lipids to enhance carotenoid absorption. The eggs consumed with the LE and HE salads provided ∼0.4 and ∼0.8 mg total carotenoids (1.7% and 3.4% of total carotenoids consumed in these trials). Thus, 100.0%, 98.3% and 96.6% of total carotenoids consumed from control, LE, and HE test meals were from the salad. The findings from the current study support that co-consuming 150 g (3 eggs) cooked whole eggs is an effective way to enhance the absorption of carotenoids from the salad that are not found in eggs.
Dietary lipids are an important determinant of carotenoid absorption (8, 23) by enhancing carotenoid intestinal micellarization and the incorporation of carotenoid into a chylomicron. The extent of micellarization of carotenoid increases when the amount of soybean oil added to a salad puree and the amount of oil are increased (24). Human clinical trials also supported that the amount (9, 10) and type (10, 25) of dietary lipids influence carotenoid absorption. The triacylglycerol content in TRL fractions generally represents the response of the ingestion of dietary lipids (26, 27). We showed that the change in the triacylglycerol content in TRL fractions was greater after ingestion of the HE meal than after ingestion of LE and control meals, which indicated that the endogenous lipids in eggs were digested and absorbed. It was reported that increasing amounts of dietary cholesterol also impact postprandial lipoprotein responses in humans (28), and HE consumption provided ≤560 mg dietary cholesterol (21). The total cholesterol content in TRL fractions presented similar responses as the total carotenoid content in TRL fractions during 10 h of testing, but we did not observe a statistical difference of the total cholesterol AUC0–10h in TRL fractions in trials.
Consistent with the triacylglycerol response, HE consumption increased total and individual carotenoid absorption compared with LE and control consumption, including 2- and 5–6-fold increases in the TRL AUC0–10h of lutein and zeaxanthin, respectively, and 2–3 and 4–9-fold increases in the TRL AUC0–10h of carotenoids beyond those in eggs, including α-carotene, β-carotene, and lycopene. These results are consistent with previous clinical studies that showed greater increases in individual carotenoid absorption when 20 g dietary lipids (10) and 28 g salad dressing (9) were consumed. The consumption of a high-fat mixed meal (29) and a meal containing beef tallow (25) increased β-carotene absorption, and another lipid-rich food source including avocado also increased carotenoid absorption from a vegetable, whereby 150 g avocado (24 g lipids) increased lutein, α-carotene, and β-carotene absorption (30). In addition, differences have been observed in the micellarization of carotenoids (in vitro) between meals coformulated with meat (ham, chicken, or beef) and vegetable oils (31), but in all cases, the inclusion of dietary lipids in any form appeared to consistently potentiate absorption. Collectively, this group of studies, including the current study, consistently shows that the consumption of lipid-rich foods is an effective strategy to enhance carotenoid absorption. Little information is available regarding the optimal amount of dietary lipids to maximally enhance carotenoid absorption, but our study suggests that 18 g dietary lipids from eggs were sufficient to increase the absorption of all individual carotenoids from raw mixed-vegetable salads.
Except for lutein, no differences of total and individual carotenoid absorption were observed between control and LE consumption in the current study. This observation contrasts with research that reported that the co-consumption of 75 g avocado presented higher α-carotene and β-carotene absorption (30). Seventy-five grams of avocado contains a slightly higher amount of dietary lipids (12 g lipids) than that in our LE meal (10.5 g lipids), and presumably the difference in the fatty acid composition may explain this differential response. LE consumption, compared with the consumption of 75 g avocado, provides more SFAs (2.4 compared with 2.0 g, respectively) and PUFAs (1.5 compared with 1.0 g, respectively) but less MUFAs (5.7 compared with 7.0 g, respectively). Although limited data exist regarding the impact of the dietary lipid source on carotenoid absorption, some observations suggested that the absorption of carotenoids is enhanced by dietary lipids that are rich in MUFAs than rich in PUFAs (10, 32, 33). PUFAs may make carotenoids more susceptible to oxidation in the intestinal chyme, resulting in less carotenoid available for absorption (34). Also, the diffusion of micelles through the unstirred water layer near to the enterocyte is slow because micelles that contain PUFAs are larger in size, thereby decreasing the absorption rate of carotenoids (34). In addition, PUFAs compete with carotenoids (β-carotene in particular) for binding to fatty acid binding proteins (33), which may decrease the incorporation of carotenoids into chylomicrons. Although inconsistency exists (25), SFAs are considered relatively poor carotenoid-absorption enhancers from in vitro (24, 35) and in vivo (10) studies.
In contrast to other individual carotenoids, lutein absorption was greater with LE consumption than with control consumption (P = 0.037). This result could have stemmed from the LE meal containing ∼6% more lutein than that of the control meal (3.8 compared with 3.6 mg) because of the 0.2 mg highly bioavailable lutein contained in the eggs (22). Also, lutein may be more readily incorporated into mixed micelles than are other hydrocarbon carotenoids such as α-carotene, β-carotene, and lycopene because of its more polar characteristics (36–38). Zeaxanthin absorption was not greater for LE consumption that for control consumption, even though the eggs provided an additional 0.4 mg zeaxanthin to the test salad (which contained 4.9 mg) or ∼7% more zeaxanthin than with control consumption. This finding is consistent with previous studies (10, 24). Research on the absorption of zeaxanthin from eggs compared with nonegg sources is inconsistent (39), and the eggs used in this study contained very little zeaxanthin compared with that in the test salad. Also, our use of Chinese wolfberry as the source of zeaxanthin in the salad may have affected the total zeaxanthin absorption. Wolfberry zeaxanthin is primarily a di-ester form (40, 41), which was reported to have a lower efficiency of micellarization than does zeaxanthin in free form (40).
The novelty of the current study is supported by the limited amount of human studies focused on the inclusion of food source of lipids, rather than bulk oils or fats, to enhance carotenoid absorption from a co-consumed carotenoid-rich mixed meal. Strengths of this research included the successful completion of a randomized crossover study with well-controlled 7-d low-carotenoid–diet periods and investigators remaining fully blinded until after all testing and sample analyses were completed. In addition, we measured the carotenoid content within TRL fractions because it primarily represents newly absorbed carotenoids appearing in plasma (17). Although minor, the TRL fractions (density <1.006 g/mL) may also include VLDL, which can presumably originate from both the liver and intestine. Previous studies showed parallel postprandial carotenoid increases in both chylomicrons and VLDL fractions (25, 42), which may have resulted in the overestimation of newly absorbed carotenoid from our isolated TRL fractions. Not only a high lipid content but also the high amount of phospholipids in egg yolk may enhance carotenoid absorption (15, 43, 44), but this study was not designed to assess the specific impact of phospholipids in eggs on carotenoid absorption. Future research is needed to differentiate the impact of components of the lipid matrix in egg yolk on carotenoid absorption. We recognize that our power calculations to estimate the sample size may be viewed as strength or limitation. Although we drew on results from our previous carotenoid-absorption study which used the exact-same salad components and quantities (10), the sources, quantities, and matrices of lipids differed.
In conclusion, the absorption of carotenoids contained in carotenoid-rich foods such as raw-vegetable salads can be effectively enhanced by co-consuming cooked whole eggs. These results support that egg, which is a nutrient-rich food that contains essential amino acids, unsaturated fatty acids, and B vitamins (21), may be used to increase the nutritive value of vegetables, which are underconsumed by the majority of people living in the United States (1, 45).
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—JEK, MGF, and WWC: designed the research and analyzed data; JEK and SLG: conducted the research; JEK and WWC: wrote the manuscript and had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: BHT, butylated hydroxytoluene; HE, high egg; LE, low egg; TRL, triacylglycerol-rich lipoprotein.
REFERENCES
- 1.United States Department of Health and Human Services, USDA, United States Dietary Guidelines Committee. Report of the Dietary Guidelines Advisory Committee on the dietary guidelines for Americans, 2010. 2010.
- 2.Yonekura L, Nagao A. Intestinal absorption of dietary carotenoids. Mol Nutr Food Res 2007;51:107–15. [DOI] [PubMed] [Google Scholar]
- 3.Hozawa A, Jacobs DR Jr, Steffes MW, Gross MD, Steffen LM, Lee DH. Circulating carotenoid concentrations and incident hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Hypertens 2009;27:237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Odorico A, Martines D, Kiechl S, Egger G, Oberhollenzer F, Bonvicini P, Sturniolo GC, Naccarato R, Willeit J. High plasma levels of alpha- and beta-carotene are associated with a lower risk of atherosclerosis: results from the Bruneck study. Atherosclerosis 2000;153:231–9. [DOI] [PubMed] [Google Scholar]
- 5.Bendich A, Olson JA. Biological actions of carotenoids. FASEB J 1989;3:1927–32. [PubMed] [Google Scholar]
- 6.Piermarocchi S, Saviano S, Parisi V, Tedeschi M, Panozzo G, Scarpa G, Boschi G, Lo Giudice G; Carmis Study Group. Carotenoids in Age-related Maculopathy Italian Study (CARMIS): two-year results of a randomized study. Eur J Ophthalmol 2012;22:216–25. [DOI] [PubMed] [Google Scholar]
- 7.Ito Y, Suzuki K, Ishii J, Hishida H, Tamakoshi A, Hamajima N, Aoki K. A population-based follow-up study on mortality from cancer or cardiovascular disease and serum carotenoids, retinol and tocopherols in Japanese inhabitants. Asian Pac J Cancer Prev 2006;7:533–46. [PubMed] [Google Scholar]
- 8.Goltz SR, Ferruzzi MG. Carotenoid bioavailability: influence of dietary lipid and fiber. In: Tanumihardjo SA, editor. Carotenoids and human health. New York: Springer Science & Business Media; [Internet]. 2013 [cited 2013 Mar 4]. Available from: http://download.springer.com/static/pdf/600/chp%253A10.1007%252F978-1-62703-203-2_6.pdf?auth66=13637981504ee3ec14d79630924ec3a08a7aa944da&ext=.pdf.
- 9.Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr 2004;80:396–403. [DOI] [PubMed] [Google Scholar]
- 10.Goltz SR, Campbell WW, Chitchumroonchokchai C, Failla ML, Ferruzzi MG. Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. Mol Nutr Food Res 2012;56:866–77. [DOI] [PubMed] [Google Scholar]
- 11.Sandrou DK, Arvanitoyannis IS. Low-fat/calorie foods: current state and perspectives. Crit Rev Food Sci Nutr 2000;40:427–47. [DOI] [PubMed] [Google Scholar]
- 12.Handelman GJ, Nightingale ZD, Lichtenstein AH, Schaefer EJ, Blumberg JB. Lutein and zeaxanthin concentrations in plasma after dietary supplementation with egg yolk. Am J Clin Nutr 1999;70:247–51. [DOI] [PubMed] [Google Scholar]
- 13.American Egg Board [Internet]. Nutrient composition. [cited 2012 Nov 29]. Available from: http://www.aeb.org/food-manufacturers/egg-nutrition-and-trends/nutrient-composition.
- 14.Human Nutrition Information Service, US Department of Agriculture [Internet]. Composition of foods. Agriculture handbook no. 8, supplement no. 8. Washington (DC): US Government Printing Office; 1989. [Google Scholar]
- 15.Baskaran V, Sugawara T, Nagao A. Phospholipids affect the intestinal absorption of carotenoids in mice. Lipids 2003;38:705–11. [DOI] [PubMed] [Google Scholar]
- 16.Lakshminarayana R, Raju M, Keshava Prakash MN, Baskaran V. Phospholipid, oleic acid micelles and dietary olive oil influence the lutein absorption and activity of antioxidant enzymes in rats. Lipids 2009;44:799–806. [DOI] [PubMed] [Google Scholar]
- 17.van Vliet T, Schreurs WH, van den Berg H. Intestinal β-carotene absorption and cleavage in men: response of β-carotene and retinyl esters in the triglyceride-rich lipoprotein fraction after a single oral dose of β-carotene. Am J Clin Nutr 1995;62:110–6. [DOI] [PubMed] [Google Scholar]
- 18.American Egg Board [Internet]. General egg preparation. [cited 2013 Feb 25]. Available from: http://www.aeb.org/foodservice-professionals/egg-safety.
- 19.Ribaya-Mercado JD, Blumberg JB. Lutein and zeaxanthin and their potential roles in disease prevention. J Am Coll Nutr 2004;23(6 Suppl):567S–87S. [DOI] [PubMed] [Google Scholar]
- 20.Cotterill OJ, Marion WW, Naber EC. A nutrient re-evaluation of shell eggs. Poult Sci 1977;56:1927–34. [DOI] [PubMed] [Google Scholar]
- 21.US Department of Agriculture Agricultural Research Service [Internet]. USDA National Nutrient Database for Standard Reference, Release 23, Nutrient Data Laboratory Home Page. 2010. [cited 2014 Dec 9]. Available from: http://www.ars.usda.gov/nutrientdata.
- 22.Chung HY, Rasmussen HM, Johnson EJ. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J Nutr 2004;134:1887–93. [DOI] [PubMed] [Google Scholar]
- 23.van Het Hof KH, West CE, Weststrate JA, Hautvast JG. Dietary factors that affect the bioavailability of carotenoids. J Nutr 2000;130:503–6. [DOI] [PubMed] [Google Scholar]
- 24.Failla ML, Chitchumronchokchai C, Ferruzzi MG, Goltz SR, Campbell WW. Unsaturated fatty acids promote bioaccessibility and basolateral secretion of carotenoids and alpha-tocopherol by Caco-2 cells. Food Funct 2014;5:1101–12. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, Jandacek RJ, White WS. Intestinal absorption of β-carotene ingested with a meal rich in sunflower oil or beef tallow: postprandial appearance in triacylglycerol-rich lipoproteins in women. Am J Clin Nutr 2000;71:1170–80. [DOI] [PubMed] [Google Scholar]
- 26.Havel RJ. Postprandial lipid metabolism: an overview. Proc Nutr Soc 1997;56:659–66. [DOI] [PubMed] [Google Scholar]
- 27.Smilowitz JT, German JB, Zivkovic AM. Food intake and obesity: the case of fat. In Montmayeur JP, le Coutre J, editors. Fat detection: taste, texture, and post ingestive effects. Boca Raton (FL): CRC Press; 2010. [Internet]. [cited 2015 Jan 26]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK53555/.
- 28.Dubois C, Armand M, Mekki N, Portugal H, Pauli AM, Bernard PM, Lafont H, Lairon D. Effects of increasing amounts of dietary cholesterol on postprandial lipemia and lipoproteins in human subjects. J Lipid Res 1994;35:1993–2007. [PubMed] [Google Scholar]
- 29.Dimitrov NV, Meyer C, Ullrey DE, Chenoweth W, Michelakis A, Malone W, Boone C, Fink G. Bioavailability of beta-carotene in humans. Am J Clin Nutr 1988;48:298–304. [DOI] [PubMed] [Google Scholar]
- 30.Unlu NZ, Bohn T, Clinton SK, Schwartz SJ. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr 2005;135:431–6. [DOI] [PubMed] [Google Scholar]
- 31.Garrett DA, Failla ML, Sarama RJ. Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J Agric Food Chem 1999;47:4301–9. [DOI] [PubMed] [Google Scholar]
- 32.Lakshminarayana R, Raju M, Krishnakantha TP, Baskaran V. Lutein and zeaxanthin in leafy greens and their bioavailability: olive oil influences the absorption of dietary lutein and its accumulation in adult rats. J Agric Food Chem 2007;55:6395–400. [DOI] [PubMed] [Google Scholar]
- 33.Hollander D, Ruble PE Jr. beta-Carotene intestinal absorption: bile, fatty acid, pH, and flow rate effects on transport. Am J Physiol 1978;235:E686–91. [DOI] [PubMed] [Google Scholar]
- 34.Clark RM, Yao L, She L, Furr HC. A comparison of lycopene and astaxanthin absorption from corn oil and olive oil emulsions. Lipids 2000;35:803–6. [DOI] [PubMed] [Google Scholar]
- 35.van Greevenbroek MM, van Meer G, Erkelens DW, de Bruin TW. Effects of saturated, mono-, and polyunsaturated fatty acids on the secretion of apo B containing lipoproteins by Caco-2 cells. Atherosclerosis 1996;121:139–50. [DOI] [PubMed] [Google Scholar]
- 36.Deming DM, Erdman JW Jr. Mammalian carotenoid absorption and metabolism. Pure Appl Chem 1997;71:2213–23. [cited 2015 Jan 29]. Available from: http://old.iupac.org/publications/pac/1999/71_12_pdf/7112deming_2213.pdf. [Google Scholar]
- 37.Gartner C, Stahl W, Sies H. Preferential increase in chylomicron levels of the xanthophylls lutein and zeaxanthin compared to beta-carotene in the human. Int J Vitam Nutr Res 1996;66:119–25. [PubMed] [Google Scholar]
- 38.Borel P, Grolier P, Armand M, Partier A, Lafont H, Lairon D, Azais-Braesco V. Carotenoids in biological emulsions: solubility, surface-to-core distribution, and release from lipid droplets. J Lipid Res 1996;37:250–61. [PubMed] [Google Scholar]
- 39.Thurnham DI. Macular zeaxanthins and lutein–a review of dietary sources and bioavailability and some relationships with macular pigment optical density and age-related macular disease. Nutr Res Rev 2007;20:163–79. [DOI] [PubMed] [Google Scholar]
- 40.Chitchumroonchokchai C, Failla ML. Hydrolysis of zeaxanthin esters by carboxyl ester lipase during digestion facilitates micellarization and uptake of the xanthophyll by Caco-2 human intestinal cells. J Nutr 2006;136:588–94. [DOI] [PubMed] [Google Scholar]
- 41.Breithaupt DE, Bamedi A, Wirt U. Carotenol fatty acid esters: easy substrates for digestive enzymes? Comp Biochem Physiol B Biochem Mol Biol 2002;132:721–8. [DOI] [PubMed] [Google Scholar]
- 42.Paetau I, Chen H, Goh NM, White WS. Interactions in the postprandial appearance of beta-carotene and canthaxanthin in plasma triacylglycerol-rich lipoproteins in humans. Am J Clin Nutr 1997;66:1133–43. [DOI] [PubMed] [Google Scholar]
- 43.Marisiddaiah R, Baskaran V. Bioefficacy of beta-carotene is improved in rats after solubilized as equimolar dose of beta-carotene and lutein in phospholipid-mixed micelles. Nutr Res 2009;29:588–95. [DOI] [PubMed] [Google Scholar]
- 44.Sugawara T, Kushiro M, Zhang H, Nara E, Ono H, Nagao A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J Nutr 2001;131:2921–7. [DOI] [PubMed] [Google Scholar]
- 45.US Department of Agriculture Agricultural Research Service [Internet]. What We Eat in America. NHANES 2009-2010 [cited 2015 Mar 2]. Available from: http://www.ars.usda.gov/SP2UserFiles/Place/80400530/pdf/0910/tables_1-40_2009-2010.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.