Abstract
Introduction
In the United States, Federally Qualified Health Centers (FQHCs) are safety-net clinics that provide cervical cancer screening and human papillomavirus (HPV) vaccination to medically underserved women, some of whom may be at risk for developing cervical cancer. National guidelines recommend against using screening test results or sexual history to determine vaccine eligibility. Documenting HPV vaccine recommendations and beliefs of primary care providers in FQHCs may aid in promoting evidence-based practices and prioritizing health interventions for vulnerable populations.
Methods
Between 2009 and 2010, we collected data from 98 primary care providers in 15 FQHC clinics in IL, USA using a cross-sectional survey. Questions assessed provider and practice characteristics, HPV vaccine recommendations, and provider’s belief about whether their screening and management procedures would change for women who were vaccinated.
Results
93% of providers recommended the HPV vaccine, most frequently for females aged 13–26 years (98%). Some providers reported sometimes to always using HPV test results (12%), Pap test results (7%), and number of sexual partners (33%) to determine vaccine eligibility. More than half of providers (55%) reported they will not change their screening and management practices for vaccinated females, yet believe vaccination will yield fewer abnormal Pap tests (71%) and referrals for colposcopy (74%).
Conclusion
Study providers routinely recommended the HPV vaccine for their patients. However, providers made fewer recommendations to vaccinate females ages 9–12 years (which includes the target age for vaccination) compared to older females, and used pre-vaccination assessments not recommended by U.S. guidelines, such as screening test results and number of sexual partners. In order to maximize the public health benefit of the HPV vaccine to prevent cervical cancer, adherence to guidelines is necessary, especially in settings that provide care to medically underserved women.
Keywords: HPV vaccine, Cervical cancer screening, Federally Qualified Health Center (FQHC), Underserved, Low-income
1. Introduction
Opportunities for cervical cancer prevention and control have evolved dramatically over the last 10 years, in part due to the development of human papillomavirus (HPV)-targeted prevention and detection technologies. In the United States, quadrivalent and bivalent vaccines that protect against the high-risk HPV types associated with most cervical cancers and precancers [1,2] are recommended for cancer prevention, yet largely underutilized [3,4]. Routine vaccination is recommended for males and females ages 11–12 years; vaccination can begin at 9 years of age, and is recommended as a catch-up for females through age 26 years and through age 21 years for males [5,6]. HPV vaccines are most effective when administered prior to HPV exposure, however U.S. guidelines specify that age-eligible females with a history of abnormal Papanicolaou (Pap) tests or positive HPV tests can also be vaccinated [5]. Cervical cancer screening and management procedures should not change for females who have been vaccinated [5]. Likewise, use of screening test results or sexual history is not recommended when determining vaccine eligibility [5,7], and could result in unnecessary clinical intervention and costs as well as missed opportunities for vaccination.
HPV vaccination rates are low among socioeconomic disadvantaged groups, and in states and regions with low cervical cancer screening participation and greater cervical cancer morbidity and mortality [8]. Uninsured and low-income women suffer disproportionate cervical cancer morbidity, mortality and late-stage diagnosis [9,10]. Provider recommendation is a key facilitator to vaccination among low-income, medically underserved populations [11,12]. However, little is known about vaccine recommendations, or beliefs regarding the anticipated impact of the vaccine on cervical cancer outcomes among providers who serve medically underserved women [13]. Focused surveys and interventions are necessary to develop appropriate and effective messages and outreach methods for uptake of HPV vaccination [14]. To better facilitate the adoption of HPV technologies in a medically under-served population the Centers for Disease Control and Prevention (CDC) launched the Cervical Cancer (Cx3) Study [15], a pilot study that assessed patient and provider knowledge, attitudes and practices related to cervical cancer screening and HPV vaccination. The objective of this manuscript is to present the HPV vaccine recommendations and beliefs of Cx3 Study providers.
2. Methods
In the United States, Federally Qualified Health Centers (FQHCs) are clinics funded by Section 330 of the U.S. Public Health Service Act. FQHCs are safety-net providers, and are mandated to serve an underserved area or population, offer a sliding fee scale, and provide preventive primary care services. Services provided in FQHCs include, but are not limited to, well child care, immunizations, family planning, chronic disease screenings, vision and hearing screening, and risk assessment and counseling (https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/fqhcfactsheet.pdf) There are approximately 1228 FQHCs in the United States, of which 37 are located in Illinois (http://kff.org/other/state-indicator/total-fqhcs/#map). The Cx3 Study was conducted in 15 clinics associated with six FQHCs across the state of Illinois. The Cx3 Study selected FQHCs as the study site because the client base is predominately low income and under- or uninsured, and assessing practices in these settings will help CDC provide technical assistance to its national cancer programs. Illinois was chosen as the study location because of the state’s elevated cervical cancer incidence rates, and the Illinois Breast and Cervical Cancer Early Detection Program’s (IBCCEDP) high Pap test volume and follow-up rate. Clinics that partnered with the IBCCEDP were selected to participate in the study by convenience sampling and are not meant to be representative of all FQHCs in Illinois.
All providers within the participating clinics who routinely performed cervical cancer screening were eligible for the study, which included physicians, nurse practitioners, certified nurse midwives, and physician assistants. Between 2009 and 2010, self-administered surveys and a $50 cash incentive were sent to providers prior to study initiation with a stamped, self-addressed envelope for return. Clinic coordinators would follow-up weekly with non-responding providers, and many were encouraged multiple times to complete the survey.
The provider survey was developed specifically for this study, and is based upon national primary care provider surveys [16,17]. The survey was pilot tested with seven primary care providers in the Atlanta, GA area to estimate respondent burden, format, appropriateness and relevance of survey questions. Provider demographic and practice characteristics were collected along with information on HPV vaccine recommendations and beliefs regarding the impact of the vaccine on future screening test outcomes. HPV vaccine recommendations were assessed by asking providers: (1) To what age groups do you recommend patients get the HPV vaccine (response options based on patient age and gender); (2) How often do you: (a) recommend the HPV vaccine to females with a history of an abnormal Pap test result (atypical squamous cells-undetermined significance or higher)? (b) recommend the HPV vaccine to females with a positive HPV test? (c) use the HPV test to determine who should get the HPV vaccine? (d) perform a Pap test to determine who should get the HPV vaccine? and (e) use the number of sexual partners to determine who should get the HPV vaccine? (response options: rarely or never; sometimes; usually; always or almost always; unknown); and (3) Will your cervical cancer screening and management procedures change for females who have been fully vaccinated with the HPV vaccine? (response options: yes; no; do not know). For this analysis, responses to “sometimes”, “usually”, and “always or almost always” were collapsed.
Beliefs about the impact of the vaccine on screening outcomes were assessed by asking providers their level of agreement with the following: vaccinating female patients will result in (a) fewer numbers of abnormal Pap tests, (b) fewer referrals for colposcopy, and (c) fewer cervical intraepithelial neoplasia (CIN) results (response options: agree; disagree; unsure). CDC’s Institutional Review Board approved the study. Results are descriptive and presented as percent and mean distributions. Additional details on study design and procedures have been published [15].
3. Results
Surveys were completed by 98 of 109 eligible providers (89.9% response rate). Most providers were female (77%), physicians (66%), specialized in obstetrics/gynecology (55%) or family medicine (35%), and reported an average of 8.8 years providing clinical care. Non-physician providers included Nurse Practitioners (20%), Physician Assistants (7%), and Certified Nurse Midwives (6%). Providers mainly served female (mean 85%) patients, and provided care to all ages: <18 years (18%), 18–29 years (35%), 30–65 years (33%), and >65 years (14%). Almost all providers (93%) currently recommended or planned to recommend the HPV vaccine to their patients, most frequently for females aged 13–26 years (98%) followed by females aged 9–12 years (68%), males aged 13–26 years (16%) and males aged 9–12 years (13%) (data not reported in a table or figure).
When asked how they determine vaccine administration, 12% of providers reported sometimes to always using results from an HPV test, 7% reported sometimes to always using results from a Pap test, and one-third of providers (33%) reported sometimes to always using the number of sexual partners to determine vaccine administration. About three-quarters of providers reported sometimes to always recommending the vaccine to patients with a history of an abnormal Pap test (79%) or a positive HPV test (73%) (Table 1).
Table 1.
Provider use of screening tests and sexual history to determine HPV vaccine recommendations (row percent).
| Rarely or never
|
Sometimes to alwaysa
|
Otherb
|
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Practices discouraged by guidelinesc | ||||||
| Use an HPV test to determine who should receive HPV vaccine? (n = 96) | 80 | 83 | 12 | 12 | 4 | 4 |
| Perform Pap to determine who should receive the HPV vaccine? (n = 96) | 85 | 89 | 7 | 7 | 4 | 4 |
| Use number of sexual partners to determine who should get the HPV vaccine? (n = 95) | 59 | 62 | 31 | 33 | 5 | 5 |
| Guideline-consistent practicesd | ||||||
| Recommend HPV vaccine to females with history of abnormal Pap test result? (n = 93) | 12 | 13 | 74 | 79 | 7 | 8 |
| Recommend HPV vaccine to females with positive HPV test? (n = 94) | 20 | 21 | 68 | 73 | 6 | 6 |
“Sometimes”, “Usually”, and “Always or almost always” responses collapsed.
“Unknown”, “Not applicable”, and “Do not ask” responses collapsed.
According to Advisory Committee on Immunization Practices and American College of Obstetricians and Gynecologists [5,7].
Unable to determine age range for these female patients; practices acceptable according to Advisory Committee on Immunization Practices [5].
Providers were asked how routine HPV vaccination may affect their cervical cancer screening and management practices. For fully vaccinated female patients, more than half of providers (55%) reported they would not change their cervical cancer screening and management practices, consistent with current guidelines. Just more than one-quarter (27%) would change their practices, and 18% were unsure whether their practices would change (not reported in a table or figure). However, most providers believed that vaccinating female patients would result in fewer abnormal Pap tests (71%), fewer referrals for colposcopy (74%), and fewer CIN results (79%) (Fig. 1).
Fig. 1.
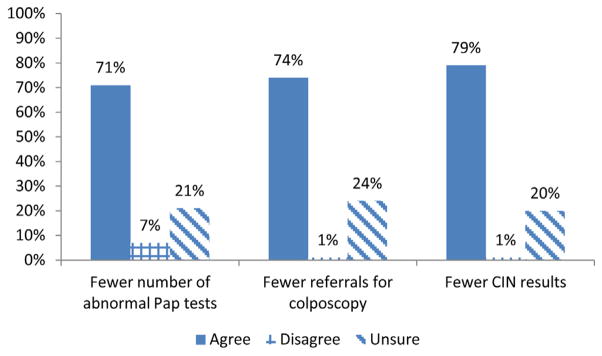
Provider beliefs regarding the impact of HPV vaccination on future screening test outcomes (n = 98). IN = cervical intraepithelial neoplasia.
4. Discussion
Understanding the cervical cancer prevention recommendations and beliefs of primary care providers working with medically underserved women is essential to the integration of the HPV technologies, such as the HPV vaccine, that can reduce the cancer burden in this population. Our study found that primary care providers working in 6 FQHCs across Illinois were generally supportive of, and routinely recommended the HPV vaccine for their patients. However they made fewer recommendations to vaccinate females ages 9–12 years (which includes the target age for vaccination), compared to older adolescents and adults (13–26 years of age). In addition, they reported using pre-vaccination assessments to determine vaccine eligibility that are not supported by U.S. guidelines, such as screening test results, and number of sexual partners [5,7].
While it is preferable to administer the vaccine before exposure to HPV through sexual activity, those already exposed to HPV and have HPV-associated outcomes (e.g., females with Pap test abnormalities and persons with a history of genital warts) should also be vaccinated, making Pap test results, HPV test results, and number of sexual partners irrelevant to determining vaccination. It is not surprising that one-third of providers sometimes to always use sexual history to determine vaccination because these same providers also reported the number of their patient’s current (71%) and lifetime (67%) sexual partners as important factors to consider when determining her cervical cancer screening interval [23]. We do not know if pre-vaccination assessment using the number of sexual partners means providers are more or less likely to vaccinate against HPV. Regardless, this is a practice we hope has diminished over time.
More than half of providers reported they would not change their screening and management practices for females who have been vaccinated; encouraging since current screening guidelines do not differentiate between vaccinated and non-vaccinated women, however this may change with new surveillance data and more women being vaccinated. Of concern are the more than one-quarter of providers who would change their patient care after vaccination. Many reported believing that vaccination will reduce the number of abnormal test results and need for colposcopy, as documented in previous studies [24,25]. Beliefs about the impact of vaccination on screening practices and outcomes is relevant, as the age group of females for which almost all providers recommend the vaccine (aged 13–26 years) includes women also eligible for cervical cancer screening (aged 21–26 years). Pap test providers must take into consideration that vaccination will have no therapeutic effect on an existing HPV infection or abnormal Pap test [5].
4.1. Strengths and limitations
This study contributes to the literature because it is believed to be the first to examine HPV vaccine practices and beliefs of providers in FQHCs. Additionally, some recommendations made by providers in this study were similar to recommendations made by primary care providers across the United States, validating our findings [20]. At the time of survey, the vaccine was not routinely recommended for males, and these providers primarily served female patients, possibly explaining low recommendation for male vaccination. However, a one-year follow-up survey of this same cohort of providers identified a significant increase in recommendations for males. The survey included response options for patient age at time of vaccination (9–12 years and 13–26 years) that may be inconsistent with other surveys examining preferences for patient age at vaccination. Due to small sample size, data are not stratified according to provider demographics. These data were collected five years ago and may not reflect current provider beliefs. Additionally, the results were from a pilot study of 15 FQHC clinics in Illinois and results may not be generalizable.
4.2. Conclusion
Community-based clinical settings that serve the medically underserved, such as FQHCs, often contend with competing health priorities [26] and are under pressure to provide health services efficiently with limited resources [27]. As FQHCs receive funding to expand clinical services (http://bphc.hrsa.gov/about/healthcenterfactsheet.pdf), providing evidence-based care is especially critical. However, as this study and previous research highlights, provider HPV vaccine recommendations and cervical cancer screening practices [23,28] in some FQHCs may not be consistent with U.S. guidelines. Effective integration of screening and immunization services to provide the maximum health benefit for women is a critical challenge [29]. In order for providers to regularly adhere to recommendations, they must be aware of the guidelines, intellectually agree, and actively adopt [30]. Reimbursement considerations and office-based reminder systems are also necessary to realize the public health benefits of the HPV vaccine [31]. This study highlights many opportunities for provider education regarding the relationship between screening and vaccination.
Monitoring cervical cancer prevention practices and beliefs, including those regarding HPV vaccination, may aid in promoting evidence-based practices and prioritizing health interventions for vulnerable populations. Continued surveillance on the influence of HPV vaccine on screening attitudes and practices and [29] how FQHCs and other community-based clinical settings are implementing and monitoring recommendations for the HPV vaccine and other preventive services is necessary. These data illustrate that the primary care providers sampled, who also screen women for cervical cancer, are positioned to deliver the HPV vaccine to their age-eligible patients, many of whom who may be at greater risk for developing cervical cancer. Closer adherence to vaccine guidelines is necessary to ensure maximum benefit of the HPV vaccine to achieve public health goals.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This manuscript was written in the course of employment by the United States Government and it is not subject to copyright in the United States. The authors gratefully acknowledge the medical directors, administrators and providers from the FQHCs who participated in the study, and the Illinois Breast and Cervical Cancer Early Detection Program.
Funding: Centers for Disease Control and Prevention, Contract number 200-2002-00573, Task Order No. 0006 supported field work and data collection.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report, or financial disclosures.
Data analysis: The Task Order also supported data analysis, in addition to field work and data collection.
References
- 1.Human papillomavirus-associated cancers – United States, 2004–2008. MMWR Morb Mortal Wkly Rep. 2012;61:258–61. [PubMed] [Google Scholar]
- 2.Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 3.National and state vaccination coverage among adolescents aged 13–17 years – United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(34):671–7. [PubMed] [Google Scholar]
- 4.Human papillomavirus vaccination coverage among adolescent girls, 2007–2012, and postlicensure vaccine safety monitoring, 2006–2. MMWR Morb Mortal Wkly Rep. 2013;62(29):591–5. [PMC free article] [PubMed] [Google Scholar]
- 5.FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59(20):626–9. [PubMed] [Google Scholar]
- 6.FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59(20):630–2. [PubMed] [Google Scholar]
- 7.ACOG Committee Opinion No. 344: Human papillomavirus vaccination. Obstet Gynecol. 2006;108(3 Pt 1):699–705. doi: 10.1097/00006250-200609000-00047. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benard VB, Johnson CJ, Thompson TD, et al. Examining the association between socioeconomic status and potential human papillomavirus-associated cancers. Cancer. 2008;113(Suppl 10):2910–8. doi: 10.1002/cncr.23742. [DOI] [PubMed] [Google Scholar]
- 10.Fedewa SA, Cokkinides V, Virgo KS, Bandi P, Saslow D, Ward EM. Association of insurance status and age with cervical cancer stage at diagnosis: National Cancer Database, 2000–2007. Am J Public Health. 2012;102(9):1782–90. doi: 10.2105/AJPH.2011.300532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerry SL, De Rosa CJ, Markowitz LE, et al. Human papillomavirus vaccine initiation among adolescent girls in high-risk communities. Vaccine. 2011;29(12):2235–41. doi: 10.1016/j.vaccine.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 12.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76–82. doi: 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saslow D, Castle PE, Cox JT, et al. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57(1):7–28. doi: 10.3322/canjclin.57.1.7. [DOI] [PubMed] [Google Scholar]
- 14.Ricciotti HA, Girouard S, Pokorney G, Dodge LE, Hacker MR. Lessons learned from a Boston community health center promoting the human papilloma virus vaccine in a minority adult population. J Prim Care Community Health. 2010;1(1):50–4. doi: 10.1177/2150131909356109. [DOI] [PubMed] [Google Scholar]
- 15.Benard VB, Saraiya M, Greek A, et al. Overview of the CDC Cervical Cancer (Cx3) Study: an educational intervention of HPV testing for cervical cancer screening. J Womens Health (Larchmt) 2014;23(3):197–203. doi: 10.1089/jwh.2013.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benard VB, Saraiya MS, Soman A, Roland KB, Yabroff KR, Miller J. Cancer screening practices among physicians in the national breast and cervical cancer early detection program. J Womens Health (Larchmt) 2011;20(10):1479–84. doi: 10.1089/jwh.2010.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roland KB, Soman A, Benard VB, Saraiya M. Human papillomavirus and Papanicolaou tests screening interval recommendations in the United States. Am J Obstet Gynecol. 2011;205(5):447–8. doi: 10.1016/j.ajog.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Kepka D, Berkowitz Z, Yabroff KR, Roland K, Saraiya M. Human papillomavirus vaccine practices in the USA: do primary care providers use sexual history and cervical cancer screening results to make HPV vaccine recommendations? Sex Transm Infect. 2012;88(6):433–5. doi: 10.1136/sextrans-2011-050437. [DOI] [PubMed] [Google Scholar]
- 23.Roland KB, Benard VB, Greek A, Hawkins NA, Manninen D, Saraiya M. Primary care provider practices and beliefs related to cervical cancer screening with the HPV test in Federally Qualified Health Centers. Prev Med. 2013;57(5):419–25. doi: 10.1016/j.ypmed.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young JL, Bernheim RG, Korte JE, Stoler MH, Guterbock TM, Rice LW. Human papillomavirus vaccination recommendation may be linked to reimbursement: a survey of Virginia family practitioners and gynecologists. J Pediatr Adolesc Gynecol. 2011;24(6):380–5. doi: 10.1016/j.jpag.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Duval B, Gilca V, McNeil S, et al. Vaccination against human papillomavirus: a baseline survey of Canadian clinicians’ knowledge, attitudes and beliefs. Vaccine. 2007;25(45):7841–7. doi: 10.1016/j.vaccine.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 26.Allen CL, Harris JR, Hannon PA, et al. Opportunities for improving cancer prevention at federally qualified health centers. J Cancer Educ. 2014;29(1):30–7. doi: 10.1007/s13187-013-0535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Gutierrez J, Jhingan E, Angulo A, Jimenez R, Thompson B, Coronado GD. Cancer screening at a federally qualified health center: a qualitative study on organizational challenges in the era of the patient-centered medical home. J Immigr Minor Health. 2013;15(5):993–1000. doi: 10.1007/s10903-012-9701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coronado GD, Petrik A, Spofford M, et al. Perceptions of under and overutilization of cervical cancer screening services at Latino-serving community health centers. J Community Health. 2013;38(5):915–8. doi: 10.1007/s10900-013-9701-1. [DOI] [PubMed] [Google Scholar]
- 29.Wong C, Berkowitz Z, Saraiya M, Wideroff L, Benard VB. US physicians’ intentions regarding impact of human papillomavirus vaccine on cervical cancer screening. Sex Health. 2010;7(3):338–45. doi: 10.1071/SH09115. [DOI] [PubMed] [Google Scholar]
- 30.Pathman DE, Konrad TR, Freed GL, Freeman VA, Koch GG. The awareness-to-adherence model of the steps to clinical guideline compliance. The case of pediatric vaccine recommendations. Med Care. 1996;34(9):873–89. doi: 10.1097/00005650-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Vadaparampil ST, Malo TL, Kahn JA, et al. Physicians’ human papillomavirus vaccine recommendations, 2009 and 2011. Am J Prev Med. 2014;46(1):80–4. doi: 10.1016/j.amepre.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


