SUMMARY
Objective
We studied the entire agenda of randomized clinical trials in pulmonary hyper-tension (PH) using sociological methods. We explored the geometry of the PH network to interpret the evidence on multiple competing treatments for the same indication.
Design
We searched MEDLINE, Embase and Cochrane Library Databases for published studies. We queried clinicaltrials.gov and WHO International Clinical Trials Registry platform for non-published studies.
Results
We found 75 randomized trials (41 published [n = 4136 participants] and 34 registered unpublished [planned n = 3470 participants]). Of the published randomized studies, all used placebo as the comparator arm except for two nonindustry-sponsored comparisons between phosphodiestearase-5 (PDE-5) inhibitors and endothelin receptor antagonists (ERA), and one study comparing two different regimens of treprostinil. Similarly, only five unpublished/ongoing trials used an active PH treatment as comparator (PDE-5 inhibitors versus ERA (n = 3), different doses of sildenafil (n = 1) and two formulations of epoprostenol (n = 1). Of the 75 trials, 47 were sponsored by the manufacturer of the tested active product(s), and only two trials were sponsored by two companies comparing their products.
Conclusions
The relative merits of different treatment options are not directly known, as there are very few head-to-head comparisons. A limited number of ongoing studies are using active FDA-approved PH-treatments for comparison. This lack of information can be overcome by carefully designing comparative effectiveness trials.
Keywords: Pulmonary hypertension, Treatment
Introduction
Pulmonary hypertension (PH) is a serious disease that can lead to right heart failure and death [1]. In the last decade, seven therapies for the World Health Association group I PH [2], namely pulmonary arterial hypertension (PAH), were approved by the FDA [3]. When multiple treatments are available, there is growing interest in examining the totality of the randomized evidence using trial networks [4,5]. One may use sociological methods for the analysis of the geometry of trial networks, that is, the totality of all the randomized comparisons that have been performed [6]. Identifying these geometry patterns can be very informative for detecting gaps in the existing evidence and designing the future research agenda that could improve evidence-based decisions [7]. In fact, this approach has been of great value in the evaluation of treatments for other diseases [6,8] and is certainly of importance in PH.
In the last two decades, several effective treatments for PAH have become available. The first medication that was approved for use was the prostacyclin analog epoprostenol in 1995. Since that time, a total of seven medications (nine formulations) have received FDA approval for use in PAH. These therapies are shown in Table 1. Five different companies are manufacturing these drugs, and two of them (Actelion and United Therapeutics) own six different therapies (three each), while other companies (Pfizer, Gilead and GSK) have only one PH drug each in the United States. Several other therapies are currently evaluated in clinical trials, but we will not consider them in the current analysis as they are not commercially available options as of mid-2012.
Table 1.
Food and Drug Administration-approved medications for pulmonary hypertension
| Class | Medication (Proprietary Name) |
Route of administration |
First patent [18] |
Initial U.S FDA approval [19] |
First RCT (ref) | Manufactured or marketed |
AWP (USD)a [20] |
|---|---|---|---|---|---|---|---|
| PDE-5 inhibitors | Sildenafil Revatio® | PO | 1992 | March 1998 (Viagra®) June 2005 (Revatio®) |
November 2005 [15] | Pfizeri | 20 mg Q 8 h 1856 $i |
| Tadalafil Adcirca® | PO | 1995 | November 2003 (Cialis®) | June 2009 [21] | El Lilly Lung LLCb | 40 mg Q 24 h 1601 $ |
|
| May 2009 (Adcirca®) | |||||||
| ERA | Bosentan Tracleer® | PO | 1992 | November 2001 | March 2002 [22] | Actelion | 125 mg Q 24 h 7290 $ |
| Ambrisentan Letairis® | PO | 1996 | June 2007 | June 2008 [23] | Gilead GSKh |
10 mg Q 24 h 7239 $ |
|
| Prostacyclin analogs | Epoprostenol Flolan® | IV | 1977 | September 1995 (Flolan®) | April 1990 [24] | GSK | 40 ng/kg/minc,d 4875 $ 5895 $f |
| Veletri® | IV | June 2008 (Veletri®) | Actelion | 40 ng/kg/minc,d 4095 $ |
|||
| Iloprost Ventavis® | Inh | 1980 | December 2005 | August 2002 [25] | Actelion Bayer Scheringg |
5 μg (6-9 times a day) 15,012-22,518$ |
|
| Treprostinil Remodulin® | IV, SQ | 1981 | May 2002 (Remodulin®) | March 2002 [26] | United Therapeutics | 40 ng/kg/minc,e 11,055 $ |
|
| Tyvaso® | Inh. | July 2009 (Tyvaso®) | United Therapeutics | 54 μgQ6 h 15,091$ |
ERA, endothelin receptor antagonists; FAD, Food and Drug Administration; GSK, GlaxoSmithKline; Inh, inhaled; IV, intravenous; PDE-5, phosphodiesterases; PO, oral; RCT, randomized controlled trials; SQ, subcutaneous.
AWP (average wholesale price) for a month of treatment [20]. Manufacturer/distributors offer a variety of patient assistance programs that may vary among them.
Subsidiary of United Therapeutics.
Dosage calculated for a 70 kg person and only including IV solution and no other supplies.
Calculation is based on 3 vials of 1.5 mg/day.
Calculation is based in three 20 mL vial of 2.5 mg/mL per month.
Price including diluent (2 vials per day).
Iloprost is co-marketed by Bayer Schering Pharma AG in Europe and Actelion in the USA.
Ambrisentan is co-marketed by GSK in Europe and Gilead in the USA.
Generic sildenafil is now produced by different companies and offered at lower prices than the brand name.
As the number of studies in PH continues to increase, we examined whether specific drug comparisons are disproportionately preferred or avoided in the clinical research agenda of PH. We also tested whether there is evidence for homophily, that is, whether agents in the same therapeutic class are more likely to be compared against each other than with agents of other classes [6]; and whether agents of the same company are more likely to be involved in the same trial than agents of different companies. Our main goal is to systematically study the PH treatment network on published and registered ongoing studies of FDA-approved medications for this disease, to identify potential gaps in information that would support the design of relevant studies to further advance this rapidly evolving field.
Methods
Eligibility Criteria for Randomized Controlled Trials
We considered all randomized trials involving therapies that have been approved for the treatment of PAH as of July 2012. These therapies include sildenafil, tadalafil, bosentan, ambrisentan, epoprostenol, iloprost, and treprostinil. Epoprostenol is available in two different intravenous formulations (Flolan and Veletri), meanwhile treprostinil can be administered subcutaneously (Remodulin), intravenously (Remodulin) and by inhalation (Tyvaso). Randomized trials using these medications were retained if they compared any of these treatments against each other or placebo, regardless of whether there were also common backbone interventions given to all patients. We considered backbone interventions those PAH treatments that were provided to all patients in a particular study, irrespectively of the treatment arm (e.g., patients on stable doses of epoprostenol [backbone intervention] that were randomized to receive sildenafil or placebo). We excluded trials performed only in newborns or pediatric populations as causes for PH tend to be different than those in the adult population. We also excluded trials that tested investigational drugs, lasted less than a week, used the PH-specific medications for the treatment of conditions other than PH, or were withdrawn from Clinicaltrial.gov before initiation.
Search Strategy
We searched MEDLINE (PubMed), Embase and the Cochrane library. In addition, we perused the reference lists of related articles, meta-analysis, and review articles for additional pertinent citations. We also queried the ClinicalTrials.gov registry on July 18th, 2012, to determine whether additional eligible treatment comparisons were tested but not yet reported. We retained trials regardless of whether they were completed or not. Furthermore, we examined the WHO (World Health Organization) International Clinical Trials Registry platform (www.who.int/ictrp/en/). Additional details on methodology are provided on the online supplemental file.
Data Extraction
Articles were selected, and data were scrutinized independently by two investigators (A.R.T, J.Z.). We removed redundant reports. We retrieved the full articles of all published trials that were selected by the two reviewers. If discrepancy existed regarding the inclusion of a trial, a third reviewer determined the study eligibility (J.P.A.I.). Trials sponsored by affiliates, subsidiaries or branches were merged with the mother company.
Analyses
We analyzed published and unpublished randomized trials performed in patients with PH. We tested homophily at the level of drug classes and at the level of companies. At the level of drug classes, we examined whether head-to-head comparisons are between agents in the same class or between agents in different classes. At the level of companies, we examined whether trials involve only agents (as active comparators or backbones) owned by the same company, or include treatments by different companies.
In the networks of drug comparisons, each drug is drawn by a node and randomized comparisons between drugs are shown by links between the nodes. When a drug is compared against the same agent in different dose or formulation, this is represented by an autoloop. Common backbone treatments were not considered in these networks. In the networks of companies, nodes stand for companies and autoloops around these nodes represent trials involving agents of a single company. Links between different nodes characterize trials comparing agents that belong to different companies. We examined networks of companies considering and excluding common backbone interventions, to understand whether the communication between companies was driven primarily or even exclusively by the backbone treatments.
Results
Published Randomized Trials in PH
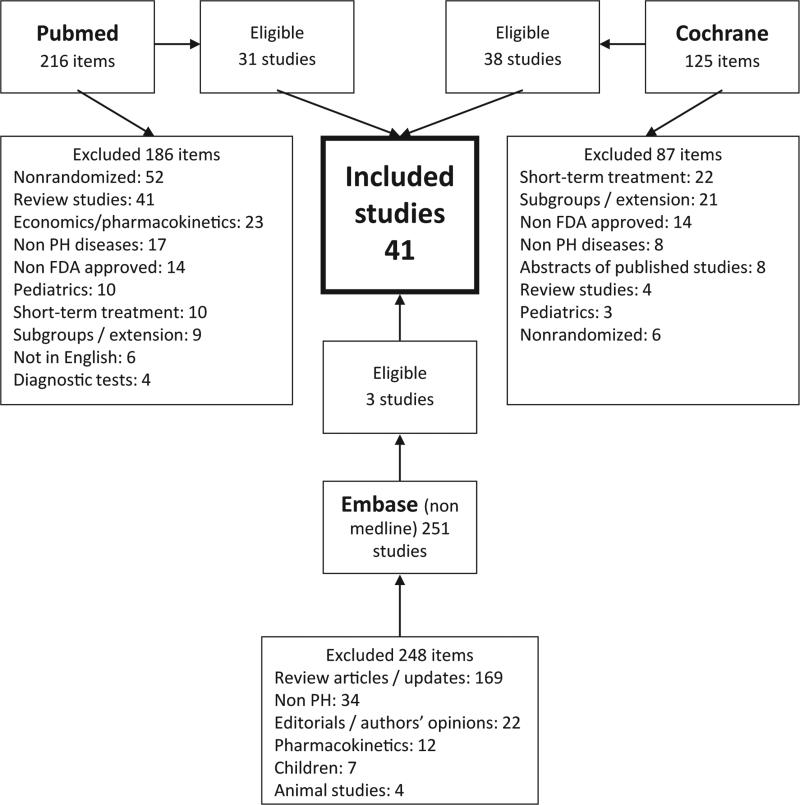
Using Pubmed, Cochrane and Embase databases, we identified 41 eligible published trials (Figure 1 and Table S1) with a total number of 4136 participants. The median (interquartile range) of participants in the trials was 44 (26–171). Trials design was parallel in 38 and crossover in three studies. Studies were published between 1990 and 2012 (83% of them between 2002 and 2010). Of these published randomized studies, 30 only involved patients with PAH (4th World Symposium PH group I [2]). The rest included patients with PH due to heart disease (group II, n = 3), lung disease (group III, n = 1), chronic thromboembolic disease (group IV, n = 3), sarcoidosis (group V, n = 1), or combinations of groups (n = 3).
Figure 1.
Flowchart of eligible published randomized trials. Some of the eligible studies from different sources overlapped, and thus, the included studies are less than the sum of the eligible ones by source.
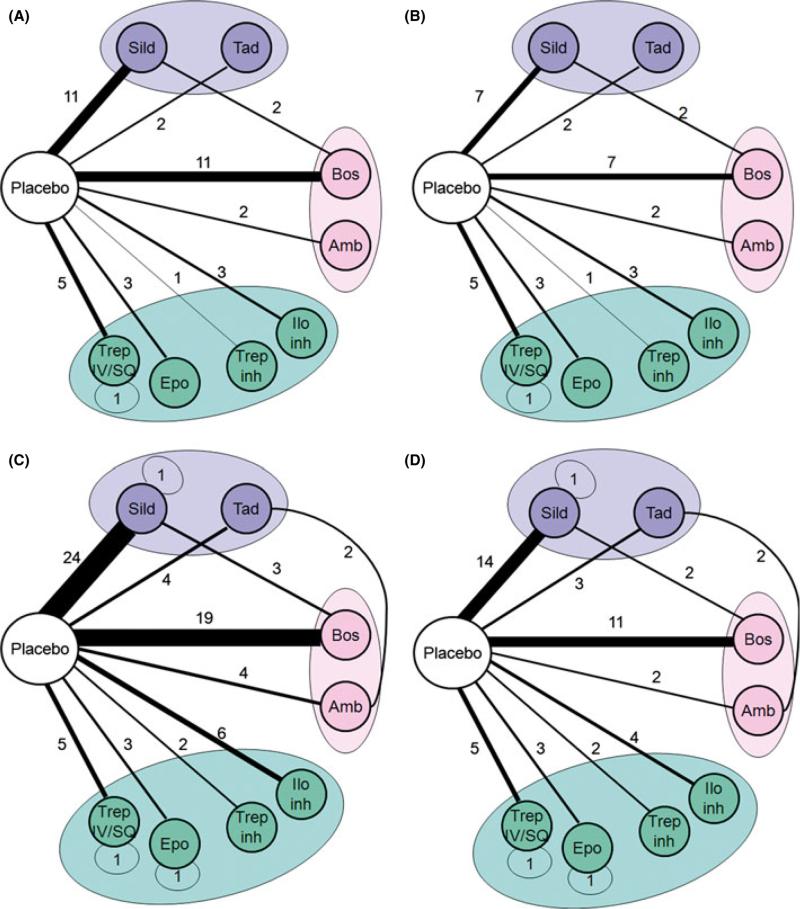
A total of 2545 PH patients received active PH medication. The studied agents were more commonly bosentan (n = 13 trials; patients receiving treatment = 633) and sildenafil (n = 13 trials; patients receiving treatment = 593) (Table 2). Placebo was used as the comparator arm in 38 studies (patients receiving placebo = 1643). Of the patients that received placebo, 52 participants were part of crossover studies with sildenafil. The most frequently used comparisons were bosentan versus placebo (n = 11) and sildenafil versus placebo (n = 11). Seven trials, all in patients with PAH, selected patients who were already receiving a PH-specific therapy as a backbone intervention (epoprostenol = 2, bosentan = 4, bosentan or sildenafil = 1) and underwent randomization to active PH medication or placebo. Figure 2, panel A shows the overall network of trial comparisons.
Table 2.
Food and Drug Administration-approved PH-specific medications used in PH randomized published studies
| Medication | Route of administration | Trials in all PH groups (n = 41) | Number of patients treated with the medication | Trials in PAH (n = 33) | Number of patients treated with the medication |
|---|---|---|---|---|---|
| Sildenafil | PO | 13c | 593a | 9c | 531a |
| Tadalafil | PO | 2 | 334 | 2 | 334 |
| Bosentan | PO | 13c | 633 | 9c | 458 |
| Ambrisentan | PO | 2d | 262 | 2d | 262 |
| Epoprostenol | IV | 3 | 107 | 3 | 107 |
| Iloprost | Inhaled | 3 | 154 | 3 | 154 |
| Treprostini | Inhaled/IV/SQ | 7 | 462b | 7 | 462b |
IV, intravenous; SQ, subcutaneous.
52 patients were part of crossover studies that used placebo as the comparator arm.
264 SQ, 83 IV and 115 Inhaled treprostinil.
Two studies compared sildenafil with bosentan.
ARIES 1 and 2 were published in one manuscript.
Figure 2.
Trials network of randomized studies on FDA-approved PH medications. Each regimen is shown by a circle and the same color is used for treatments in the same class. An autoloop is generated when different doses of the same medication are compared. The thickness of the lines either in the autoloops or connecting the nodes is proportional to the number of studies that have compared the regimens. Trep, treprostinil; Epo, epoprostenol; Trep Inh, inhaled treprostinil; Ilo Inh, inhaled iloprost; Amb, ambrisentan; Bos, Bosentan; Tad, Tadalafil; Sild, sildenafil. Panel A: published studies in all PH groups, Panel B: published studies in PH group I, Panel C: both published and unpublished studies in all PH groups, Panel D: both published and unpublished studies in PH group I.
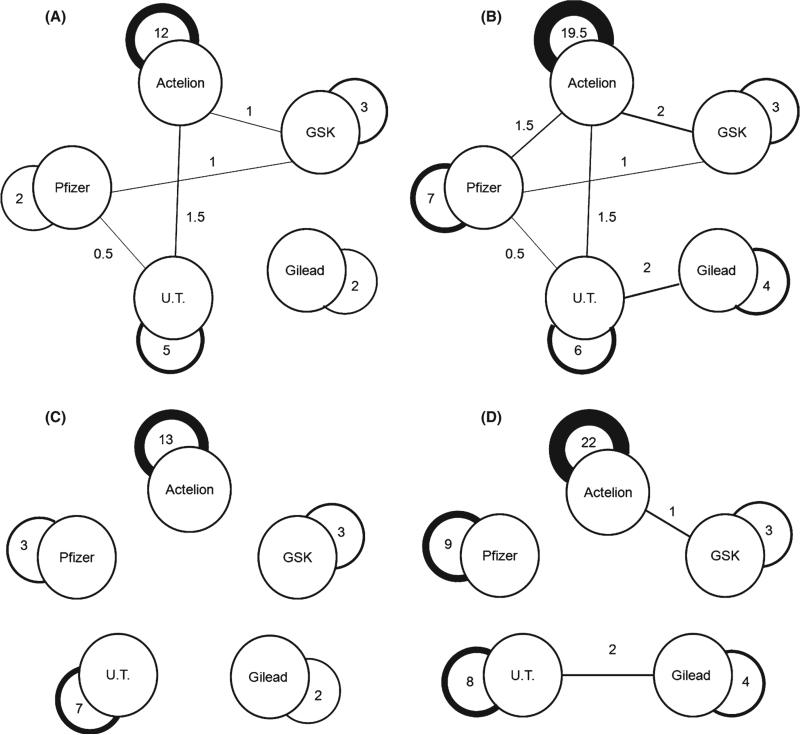
Studies that used placebo as the comparator arm (n = 38) were for the most part sponsored by the pharmaceutical company that owned the product (n = 28 studies [74%]) (Figure 3, panel A). The only two published head-to-head comparisons of different medications (sildenafil against bosentan) were not sponsored by pharmaceutical companies, but by the British Heart Foundation [13] and the Italian Health Authority [14]. Of these two studies, one include 26 patients (14 randomized to sildenafil and 12 to bosentan) [13] and the other is only available in abstract form [14]. Furthermore, one study, sponsored by the European Commission, compared two dosing regimens of treprostinil.
Figure 3.
Network of companies manufacturing/commercializing PH-specific medications. Panel A: published trials only, considering the common backbone interventions, panel B: published and unpublished studies, considering also the common backbone interventions, panel C: published trials only, without considering the common backbone interventions, and panel D: published and unpublished studies, without considering the common backbone interventions. Only industry-sponsored trials are shown in all panels. The active medication versus placebo contributes to an autoloop around the company name. If backbone medication is used, an autoloop is generated if both the active and backbone medication belong to the same company; however, if products are from different companies, these two nodes were linked. If background therapy belongs to two different companies then half-a-point is given to each link. The thickness of the lines either in the autoloops or connecting the nodes is proportional to the number of sponsored studies. Iloprost is co-marketed by Bayer Schering Pharma AG in Europe and Actelion in the USA. Ambrisentan is co-marketed by GSK in Europe and Gilead in the USA. Lung LLC is a subsidiary of United Therapeutics. Cotherix was acquired by Actelion. UT, united therapeutics.
Of the seven studies that used a backbone PH-specific therapy, one was sponsored by an unrestrictive grant from the Swedish Orphan Biovitrum, and two used medications from the same pharmaceutical company (bosentan and iloprost) for background and active arm treatments; in the other 4, the company sponsoring the active experimental treatment was not manufacturing any other PH medication of the same class as the type of backbone therapy used (Table S1).
Published Randomized Studies in PAH (Only PH Group I)
Thirty-three studies included patients with PAH with a total number of 3702 participants. A total of 2308 PH patients received active PH medication. The studied PH agents were more commonly bosentan (n = 9 trials; patients receiving treatment = 458) and sildenafil (n = 9 trials; patients receiving treatment = 531) (Table 2). Placebo was used as the comparator arm in 30 studies (patients that received placebo = 1446). Of the patients that received placebo, 52 participants were part of crossover studies with sildenafil. The most frequent comparisons were sildenafil versus placebo (seven trials) and bosentan versus placebo (seven trials) (Figure 2, panel B).
Registered Unpublished Randomized Studies in PH (all PH Groups)
We identified 217 studies in ClinicalTrials.gov and of those the reasons for exclusion were: 63 were nonrandomized, 34 tested non-FDA-approved medications (at the time of this writing), 27 studied diseases other than PH, 24 only included newborns or children, 12 were a 1 day study, 17 were already published, five were withdrawn before initiation and one study tested a device for IV medication administration. We found 224 trials in the WHO International Clinical Trials Registry Platform; however, the selected ones were also included in ClinicalTrials.gov. No new study was identified with this latter approach.
We included 34 unpublished randomized studies that met inclusion criteria (Table S2). The planned overall enrollment was 3055 subjects. The planned median (interquartile range) enrollment, per study, was 50 (30–76) participants. Of all these trials, 17 were still recruiting, nine were terminated, four were completed, and four were not yet opened to recruitment. The studies were included in these registries between 2005 and 2012. These trials planned to include PH groups I (n = 18 studies), II (n = 4 studies), III (n = 11 studies), and V (n = 1 study). PH-specific agents to be studied were more commonly sildenafil (n = 15 trials; planned total enrollment = 1502) and bosentan (n = 9 trials; planned total enrollment = 639) (Table 3). Placebo was used as the comparator arm in 29 studies. The most frequent comparisons were sildenafil versus placebo (13 trials) and bosentan versus placebo (eight trials). Of all studies in this group, five trials (all including group I PH patients) used other FDA PH-specific medications as required backbone therapy for inclusion (sildenafil, sildenafil or bosentan, bosentan, treprostinil or tadalafil). Of these studies, three used medications from the same pharmaceutical company (bosentan and iloprost [n = 1] or tadalafil and treprostinil [n = 2]) for back ground and study arm (Table 3); in the other two, the company sponsoring the active experimental treatment did not have a PH medication of the same class as the one used as backbone therapy.
Table 3.
Food and Drug Administration-approved PH-specific medications used in registered and unpublished randomized studies
| Medication | Route of administration | Trials in all PH groups (n = 34)a,b | Planned total enrollment | Trials in PAH (n = 18)a | Planned total enrollment |
|---|---|---|---|---|---|
| Sildenafil | PO | 15 | 1502b | 8 | 689 |
| Tadalafil | PO | 4 | 601a | 3 | 481a |
| Bosentan | PO | 9 | 639b | 4 | 389 |
| Ambrisentan | PO | 4 | 475a | 2 | 415a |
| Epoprostenol | IV | 1 | 30 | 1 | 30 |
| Iloprost | Inhaled | 3 | 193 | 1 | 67 |
| Treprostini | Inhaled/IV/SQ | 1 | 30 | 1 | 30 |
Two studies are comparing tadalafil with ambrisentan with a total planned enrollment of 415 participants.
Includes a study comparing sildenafil with bosentan in PH group III (planned enrollment of 60 subjects).
Of the studies that used placebo as the comparator arm, 13 were nonindustry sponsored, 16 were directly sponsored by a single company. The five studies (four in PH group I and one in PH group III patients) that did not use placebo as comparator (tadalafil vs. ambrisentan vs. the combination of both [n = 2], comparison of two formulations of epoprostenol [n = 1], evaluation of different doses of sildenafil [n = 1], and sildenafil against bosentan [n = 1]) were sponsored by pharmaceutical companies that owned one of the two study drugs (n = 4) or non-industry organizations (All India Institute of Medical sciences, n = 1).
Registered Unpublished Randomized Studies in PH (Only PH Group I)
Eighteen studies included patient with PAH with a total number of 1686 expected participants. Of these trials eight were still recruiting, seven were terminated, two were completed, and one was not yet opened to recruitment. The studied PH agents were more commonly sildenafil (n = 8 trials; planned total enrollment = 689) and bosentan (n = 4 trials; planned total enrollment = 389) (Table S2). Placebo was used as the comparator arm in 14 studies. Similarly, the most frequent comparisons were sildenafil versus placebo (seven trials) and bosentan versus placebo (four trials). One study tested different doses of sildenafil.
Overall Network Geometry
Figure 2 shows the network of comparison for all published trials on PH (2A) or only PH group I (2B), specifically, as well as the respective networks including both published and unpublished trials (2C and 2D, respectively). As shown in all of these networks, there is a dearth of head-to-head comparisons, even when all trials are considered, including those that are ongoing and those that have not started recruiting yet. Of five head-to-head comparisons of different agents (not just different doses, routes of administration, or formulations) pertained to agents from different drug classes, so there was no evidence of homophily based on medication class.
Figure 3 shows the network of the pharmaceutical companies who produce FDA-approved PH medications, with panel A mapping the published trials and panel B mapping both published and registered unpublished trials, considering also the backbone interventions; panels C and D are the respective networks of published (panel C) and published plus registered unpublished trials (panel D), without consideration of the common backbone interventions. We only incorporated in the figures industry-sponsored trials. As shown, there are strong autoloop patterns (trials involving a single active agent), but when backbone interventions are considered, there are also some interaction links among the different manufacturers. Almost all interaction links disappear when backbone interventions are not considered.
Discussion
We have studied the geometry of the randomized evidence for PH treatment. As anticipated, we observed that in published studies, mostly carried out in PAH patients, head-to-head comparisons between FDA-approved PH-specific medications are rare. Interestingly, this is also noted in registered ongoing trials as placebo continues to be the comparator of choice. However, there are three promising registered ongoing studies that are comparing endothelin receptor antagonists against phospodiestearase-5 inhibitors.
Fortunately, for patients with PAH, well done trials have led to the FDA approval of seven different therapies for the treatment of this condition, a major breakthrough for a rare disease (Table 1). More importantly, these treatments have reduced both morbidity and mortality of this serious condition [9,10]. As seen in other diseases, medications are not frequently compared against licensed regimens belonging to other companies [11,12]. The vast majority of published randomized studies compared a single PH-specific therapy against placebo. This holds true even when we considered studies performed in PAH subjects or when analyzing registered ongoing trials. Explanations for this biased network could be challenges in performing trials in a rare disease in which investigators might have to choose between testing a novel medication or compare FDA-approved ones, FDA requirements for approving a particular medication and systematic avoidance of head-to-head comparisons that may potentially show that one medication is better than other and jeopardize the marketability of a product.
Pulmonary hypertension-specific agents are expensive (Table 1) and companies undertake major marketing efforts to educate physicians and promote their products. Newer PH-specific medications are generally sold under the assumption that are equally effective as older options but offer a more favorable administration profile (e.g., once daily dosing, no need for refrigeration, subcutaneous route of delivery), fewer adverse effects, or a better price [3]. Comparative evidence is largely not available, a condition that leaves a gap in the evidence on PH treatment and leads to no particular drug or strategy of choice for treating the disease. The ongoing industry-sponsored AMBITION study (NCT01178073) is the first to compare PH medications owned by different companies. Additional antagonistic head-to-head trials like this are needed to remedy a research agenda based primarily on commercial criteria instead of scientific merit. A coordinated approach involving the industry, academia and government may help achieve this goal.
It is a matter of continuous debate whether placebo should be used as a comparator in controlled trials. It may be appropriate to use placebo when there is no established therapy such as in PH groups other than PAH or in certain subgroups of PAH in which there is no known effective treatment like PH associated with sickle cell disease. There is insufficient data to determine whether significant short- and/or long-term declines occur with the randomization to placebo, and if any potential deterioration is reversible after the subsequent introduction of therapy. In fact, investigators have expressed concern regarding the lack of “catch-up” in placebo-treated PH patients [15] and some have suggested to conduct research to determine the long-term risks associated with withholding PH therapies, before further placebo-controlled trials without background therapies are conducted in PAH [16]. The use of active comparators will require more patients to achieve adequate statistical power, which may be challenging in a relatively uncommon disease like PH. However, this can in part be overcome by a more efficient recruitment in head-to-head trials, as physicians strongly prefer active-controlled trials as they believe the enrolled patients will not be exposed to unnecessary risks and the information obtained may be more valuable [17].
Our analysis has some potential limitations. First, we cannot totally exclude that we may have missed some unpublished trials on some particular comparisons. Second, we did not take into consideration the quality of the trials, length of follow-up or outcomes; however, this would not affect our results because treatment comparisons other than against placebo were very rare anyhow. Third, it is possible that we may have missed the industry sponsorship of some trials that was not reported in the manuscript. Fourth, most of the trials have been conducted by the industry with the specific purpose of obtaining FDA approval; hence, their design was subjected to FDA standards. In spite these limitations, the present evaluation of the PH network geometry sensitizes us on the fact that there is no evidence on important comparisons, a factor that limits our capacity to select the best treatment for PH patients and strengthens the argument for making nonindustry funding available to perform comparative effectiveness trials that can identify the most efficacious PH-specific drugs for each particular setting.
Conclusions
The PH trial network geometry shows that FDA-approved PH-specific therapies continue to be compared almost exclusively against placebo, leading to a biased research agenda that includes mostly trials run by single companies on their own agents. This lack of comparative information can be overcome by designing trials that contrast main treatment strategies for this disease.
Supplementary Material
Acknowledgement
The authors thank Abdalla Ammar, Pharm.D and Marcia Wyman, Pharm. D., R.Ph. for their help in investigating the costs of different pulmonary hypertension medications.
Funding sources
This publication was made possible by CTSA KL2 [Grant # RR024990] (A.R.T.) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
Authors’ Contributions
Adriano R. Tonelli, MD: Participated in the conception and design of the study, data collection, study selection, analysis and interpretation of the results, writing and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Joe Zein, MD: Participated in the design of the study, data collection, study selection, analysis and interpretation of the results, writing of the manuscript and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
John P.A. Ioannidis, MD, DSc: Participated in the conception and design of the study, study selection, application of sociological methods, analysis and interpretation of the results, writing and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Adriano Tonelli is the guarantor of the article, taking responsibility for the integrity of the work as a whole, from inception to published article.
Conflict of Interest
The authors declare no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Published randomized studies on FDA approved PH treatments.
Table S2. Registered and unpublished randomized studies on FDA approved PH treatments (ClinicalTrials.gov).
References
- 1.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009 Jun 30;54(1 Suppl.):S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Barst RJ, Gibbs JS, Ghofrani HA, et al. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 2009 Jun 30;54(1 Suppl.):S78–S84. doi: 10.1016/j.jacc.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. Oct 15. 2005;331:897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. Jun. 2008;17:279–301. doi: 10.1177/0962280207080643. [DOI] [PubMed] [Google Scholar]
- 6.Rizos EC, Salanti G, Kontoyiannis DP, Ioannidis JP. Homophily and co-occurrence patterns shape randomized trials agendas: illustration in antifungal agents. J Clin Epidemiol. 2011;64:830–842. doi: 10.1016/j.jclinepi.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Ioannidis JP, Karassa FB. The need to consider the wider agenda in systematic reviews and meta-analyses: breadth, timing, and depth of the evidence. BMJ. 2010;341:c4875. doi: 10.1136/bmj.c4875. [DOI] [PubMed] [Google Scholar]
- 8.Kappagoda S, Ioannidis JP. Neglected tropical diseases: survey and geometry of randomised evidence. BMJ. 2012;345:e6512. doi: 10.1136/bmj.e6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macchia A, Marchioli R, Tognoni G, Scarano M, Marfisi R, Tavazzi L, Rich S. Systematic review of trials using vasodilators in pulmonary arterial hypertension: why a new approach is needed. Am Heart J. 2010;159:245–257. doi: 10.1016/j.ahj.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 10.Galie N, Manes A, Negro L, Palazzini M, Bacchi-Reggiani ML, Branzi A. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J. 2009;30:394–403. doi: 10.1093/eurheartj/ehp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lathyris DN, Patsopoulos NA, Salanti G, Ioannidis JP. Industry sponsorship and selection of comparators in randomized clinical trials. Eur J Clin Invest. 2010;40:172–182. doi: 10.1111/j.1365-2362.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- 12.Heres S, Davis J, Maino K, Jetzinger E, Kissling W, Leucht S. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: an exploratory analysis of head-to-head comparison studies of second-generation antipsychotics. Am J Psychiatry. 2006;163:185–194. doi: 10.1176/appi.ajp.163.2.185. [DOI] [PubMed] [Google Scholar]
- 13.Wilkins MR, Paul GA, Strange JW, et al. Sildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) study. Am J Respir Crit Care Med. 2005;171:1292–1297. doi: 10.1164/rccm.200410-1411OC. [DOI] [PubMed] [Google Scholar]
- 14.Palazzini M, Mazzanti G, Gotti E, et al. A randomized open labe study comparing bosentan to sildenafil first-line treatment in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2010;181:A3357. [Google Scholar]
- 15.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 16.Halpern SD, Doyle R, Kawut SM. The ethics of randomized clinical trials in pulmonary arterial hypertension. Proc Am Thorac Soc. 2008;5:631–635. doi: 10.1513/pats.200802-019SK. [DOI] [PubMed] [Google Scholar]
- 17.Halpern SD, Ubel PA, Berlin JA, Townsend RR, Asch DA. Physicians’ preferences for active-controlled versus placebo-controlled trials of new antihypertensive drugs. J Gen Intern Med. 2002;17:689–695. doi: 10.1046/j.1525-1497.2002.11024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Merk index: an encyclopedia of chemicals, drugs and biologicals. 14th edn. John Wiley & Sons, Inc.; NJ, USA: 2006. [Google Scholar]
- 19.Stolz D, Rasch H, Linka A, Di Valentino M, Meyer A, Brutsche M, Tamm M. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J. 2008;32:619–628. doi: 10.1183/09031936.00011308. [DOI] [PubMed] [Google Scholar]
- 20.Stolz D, Breidthardt T, Christ-Crain M, et al. Use of B-type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. 2008;133:1088–1094. doi: 10.1378/chest.07-1959. [DOI] [PubMed] [Google Scholar]
- 21.Galie N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 22.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 23.Galie N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 24.Rubin LJ, Mendoza J, Hood M, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med. 1990;112:485–491. doi: 10.7326/0003-4819-112-7-485. [DOI] [PubMed] [Google Scholar]
- 25.Olschewski H, Simonneau G, Galie N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 26.Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.