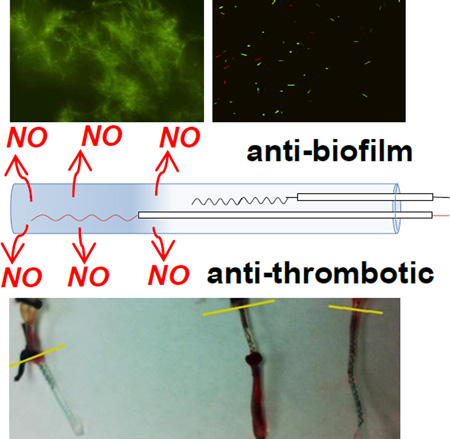
Abstract
Inexpensive nitric oxide (NO) release strategies to prevent thrombosis and bacterial infections are desirable for implantable medical devices. Herein, we demonstrate the utility of electrochemically modulated NO release from a catheter model using an inner copper wire working electrode and an inorganic nitrite salt solution reservoir. These catheters generate NO surface fluxes of >1.0×10−10 mol min−1 cm−2 for more than 60 h. Catheters with an NO flux of 1.1×10−10 mol min−1 cm−2 are shown to significantly reduce surface thrombus formation when implanted in rabbit veins for 7 h. Further, the ability of these catheters to exhibit anti-biofilm properties against bacterial species commonly causing bloodstream and urinary catheter infections is examined. Catheters releasing NO continuously during the 2 d growth of S. aureus exhibit a 6 log-unit reduction in viable surface bacteria. We also demonstrate that catheters generating NO for only 3 h at a flux of 1.0×10−10 mol min−1 cm−2 lower the live bacterial counts of both 2 d and 4 d pre-formed E. coli biofilms by >99.9%. Overall, the new electrochemical NO-release devices could provide a cost-effective strategy to greatly enhance the biocompatibility and antimicrobial properties of intravascular and urinary catheters, as well as other implantable medical devices.
Keywords: nitric oxide, copper electrode, antimicrobial catheters, thromboresistant catheters, modulated NO release
Graphical Abstract
1. Introduction
Catheters play an indispensable role in facilitating infusion, drug administration and drainage for hospitalized patients every day. However, these biomedical devices also provide a potential source of entry for microbes into the human body that can lead to severe infection, the most common being catheter-related bloodstream infections (CRBSIs) and catheter-associated urinary tract infections (CAUTIs).[1] It has been estimated that 250,000 cases of CRBSIs[2] and 500,000 incidences of CAUTIs[3] occur in hospitals annually in the U.S., thus contributing considerably to increasing healthcare costs.[4] Some 80% of CRBSIs and CAUTIs are associated with biofilm formation, thus representing a significant challenge to many clinical treatments, especially conventional antibiotic therapies.[5] Indeed, since the extracellular polymeric matrices of biofilms protect bacterial cells, most antibiotic treatments are ineffective.[6] Several strategies, such as modified surfaces[7], silver particle doped materials[8–9] and anti-quorum sensing drugs[10–11] have been suggested over the last decades to either prevent or exterminate biofilms. Yet, the problem of biofilm formation and concomitant catheter induced infections persists, suggesting that other novel and more effective strategies are needed.
In the case of intravascular catheters, another significant health risk factor is the activation of the clotting cascade and formation of surface thrombus, which can occlude a lumen of the catheter, potentially leading to complete dysfunction of the device and establishing risk for life-threatening complications via thrombus dislodgment.[12–13] This problem is currently managed by injecting intermittent heparin “lock” solutions into the catheters. Unfortunately, this approach increases the risk of systemic anticoagulation (mostly due to inadvertent overdoses) and heparin allergic response (e.g., heparin induced thrombocytopenia).
Nitric oxide (NO) releasing catheters provide a promising solution to both the biofilm-induced infection and thrombosis issues that plague current biomedical catheters. Endothelial cells produce NO to attenuate platelet activation, adhesion, and aggregation. Hence, the use of NO release agents can be useful in developing therapeutic strategies that aim to avert arterial thrombotic disorders.[14] This free radical gas molecule is also a principle component of the innate immune system and functions as a potent antimicrobial agent. Indeed, macrophages release high levels of NO as cytotoxic agent to efficiently neutralize bacteria, viruses and helminths.[15] Recent studies have also demonstrated that NO, in small doses, can act as a signaling molecule to disperse biofilms.[16] Thus, with the goal of preventing implant-associated infections, several approaches have been developed to deliver NO by either doping or modifying polymeric materials with S-nitrosothiols (RSNO) or diazeniumdiolate-based NO donors. These NO releasing materials have been shown to exhibit effective antibacterial properties.[17–19] Nevertheless, the innate drawback of these NO donor-based approaches is their instability, with RSNOs being sensitive to light and heat[20] and diazeniumdiolates being very susceptible to decomposition by moisture and potentially yielding toxic nitrosamines.[21–22] These issues have impeded their commercialization within medical devices.
Instead of S-nitrosothiols and diazeniumdiolates, inorganic nitrite is a much more attractive NO source because of its low cost and high stability. Recently, we reported the first electrochemical NO release approaches from a reservoir of inorganic nitrite by generating copper(I) species, from either a copper wire electrode or a water soluble copper complex.[23–24] In the copper wire electrode-based system, the electrode material is cost-effective but it suffered from low NO flux and large fluctuations in the NO release profile. The soluble copper complex sysytem releases a more steady and tunable NO flux, but platinum or gold wires were used as electrode materials, which is too expensive for widespread commercial use. Herein, we describe an electrochemical pulse sequence applied to a Cuo wire working electrode within a single lumen catheter model that achieves a more stable, higher flux and longer-term NO generation. The NO can be easily released on demand by turning “on” electrochemical pulse sequence, and the flux of NO can be modulated by applying different voltage pulses to the copper working electrode, which cannot be achieved by traditional chemical release. By employing this method to fabricate catheters, the NO flux from the catheter surface is increased by 100% compared with the earlier electrochemical system[23], and this enables successful in vivo demonstration of the thromboresistance of catheters with the Cu0 wire electrode-based catheter system. Further, we clearly show that by using different electrochemically modulated NO release profiles (compared to that reported in[23]), the model catheters are able to prevent biofilm formation by CRBSIs-inducing bacterial species. It is also demonstrated, for the first time, that only 3 h of electrochemically generated NO from the catheter surfaces can significantly disperse 2-d and 4-d old pre-formed biofilms by an E. coli bacterial strain that is well known to be associated with CAUTIs.
2. Experimental Details
2.1 Materials
Sodium nitrite, sodium chloride, ethylenediaminetetraacetic acid (EDTA) disodium salt, sodium phosphate dibasic heptahydrate, and sodium phosphate monobasic monohydrate were purchased from Sigma-Aldrich (St. Louis, MO) and used as received. Luria Bertani (LB) broth and LB agar were obtained from Fisher Scientific Inc. (Pittsburgh, PA). All aqueous solutions were prepared with deionized water from a Milli-Q system (18MΩ cm−1; Millipore Corp., Billerica, MA).
Polytetrafluoroethylene (PTFE)-coated silver wire (bare wire 0.125 mm O.D.; PTFE coated wire 0.176 mm O.D.) was purchased from Sigmund Cohn Corp. (Mount Vernon, NY). PTFE-coated Cuo wire (bare wire 0.127 mm O.D.; PTFE coated wire 0.152 mm O.D.) was a product of Phoenix Wire Inc. (South Hero, VT). Single-lumen standard silicone tubing (0.51 mm I.D.; 0.94 mm O.D.) was purchased from Helix Medical (Carpinteria, CA). Silicone rubber sealant was obtained from Dow Corning (Midland, MI).
E. coli K-12 MG16653 and S. aureus ATCC 45330 were from the American Type Culture Collection.
2.2 Catheters Fabrication
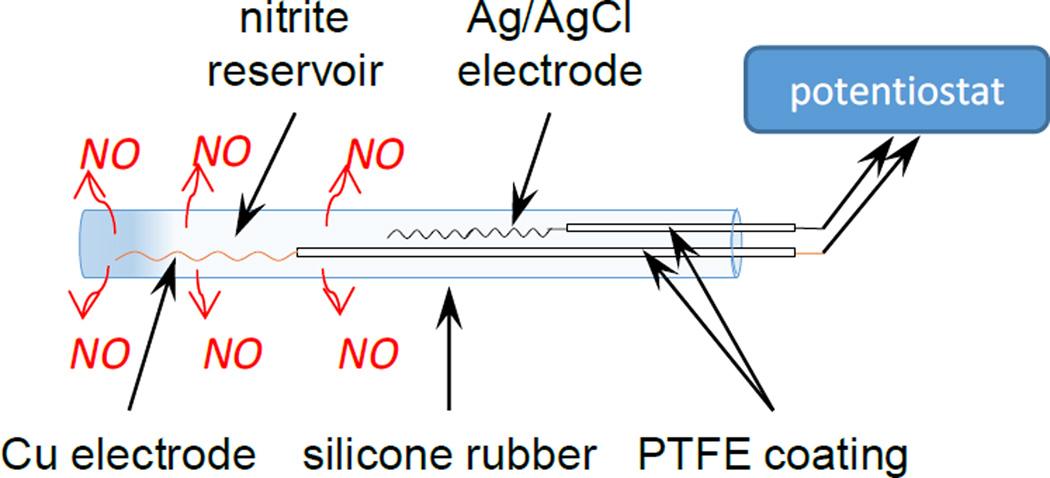
The model catheter configuration consisted of a single-lumen, silicone rubber tubing (length: 3 cm), that was sealed at one end with silicone rubber sealant. A PTFE coated silver/silver chloride wire was utilized as the reference electrode (with 0.235 cm2 surface area exposed) and a PTFE-coated Cuo wire (with ~0.012 cm2 surface area exposed) served as the working electrode within the lumen. The reservoir solution within the tubing was 1 M NaNO2, 0.30 M NaCl, 0.02 M EDTA, 1 M phosphate buffer (pH 7.05). The PTFE was removed from the ends of both Ago and Cuo wires to expose a length of 60 mm and 3 mm, respectively. Both wires were coiled and inserted in the lumen in a manner that prevents direct metallic contact with each other. Finally, the other end of the silicone rubber tubing was sealed with silicone rubber sealant, and left to cure in water for approximately 12 h.
2.3 Electrochemical Generation of Nitric Oxide
The NO releasing profile from each catheter was measured via a chemiluminescence NO analyzer (NOA) (Seivers 280, Boulder CO). The catheter was placed inside a glass cell, containing DI water; the solution was constantly purged with a stream of N2 gas, and the electrochemically generated NO emitted from the outer surface of the catheter tubing was carried to the NOA. The Cuo and Ago wires coming out of the catheters were connected to a portable potentiostat (CH Instruments 1206B, Austin TX). To generate NO, a pulse sequence was applied to the Cuo wire working electrode. One cycle of the pulse sequence included a period of a cathodic pulse (−1.1, −1.2, −1.3 or −1.4 V vs. Ag/AgCl wire), and another period involved an anodic pulse (+0.2 V vs. Ag/AgCl wire). Unless otherwise noted (for the studies aimed at undertanding how oxygen levels influence the observed NO release levels), the NO flux values indicated througout this manuscript are those obtained using the conditions outlineed above, in the presence of N2 as the purge gas.
2.4 In Vivo Anti-thrombotic Experiment in Rabbits
Briefly, one group of NO releasing catheters (low flux group, n=3) was tested using pulse sequence of 15 s at −1.25 V and 15 s at +0.20 V and the other group (high flux group, n=3) was tested using pulse sequence of 15 s at −1.3 V and 15 s at +0.20 V. Two or three catheters were implanted into the jugular or femoral veins of each rabbit (~ 3 kg). One of the catheters was turned “on” by applying a pulse sequence described above and the other was turned “off” with open circuit. After 7 h, the catheters were explanted, a digital picture was taken, and the red pixels were counted using Image J software to represent the clot area. Animal handling and surgical procedures were approved by the University Committee on the Use and Care of Animals in accordance with university and federal regulations and a detailed protocol was reported elsewhere.[24]
2.5 Biofilm Growth Conditions and Plate Counting
S. aureus biofilms
Staphylococcus aureus ATCC 25923 was used for the test. Catheters were mounted aseptically in the middle of a drip flow biofilm reactor (DFR 1110-4, BioSurface Technologies, Bozeman, MO) chambers. Each reactor chamber including the catheter was initially inoculated with 10 mL of a 100 fold-diluted bacterial culture that had been grown overnight with shaking at 37 °C. After one hour incubation, fresh 10% strength of LB broth was dripped into the flow chamber at a flow rate of 100 mL/h. Biofilms were grown for 48 h at 37 °C, by utilizing the drip flow biofilm reactor. The channels of the reactor were constantly flushed with 10% LB broth for the entire time of the biofilm growth. Nitric oxide release was turned “on” during the 48 h experiment by applying cycles of 15 s at −1.3 V and 15 s at +0.2 V. At the end of the 48 h, each catheter was removed from the drip flow biofilm reactor and placed separately into a centrifuge tube containing 2 mL of 10 mM phosphate buffer saline, pH 7.4 (PBS). Each catheter was then homogenized in order to disintegrate the biofilm clumps and form a homogenous cell suspension. Finally, each sample was 10-fold serially diluted and plated using the spread plating technique to assess cell viability.
E. coli biofilms
Escherichia coli K-12 was used or this test. The bacterial strain was grown or either 48 or 96 h at 22 °C with catheters in the channel of the reactor but not turned “on” with the applied voltage pulse sequence. This specific temperature was chosen as it allowed E. coli biofilms to develop that had greater thickness and biomass. At the end of the 48 or 96 h, NO release was turned “on” for 3 h by applying cycles of 15 s at −1.3 V and 15 s at +0.2 V. The other growth conditions were the same as those used for S. aureus biofilms. Procedures to assess cell viability were also performed exactly as described above.
2.6 Biofilm Imaging
After either 2 or 4 d of biofilm growth, the NO releasing and control catheters (same assembly as NO release but electrodes in the catheters were not connected to potentiostat) used for fluorescent imaging were removed from the drip flow biofilm reactor and gently rinsed with 10 mM PBS. Unlike catheters utilized for plate-counting, these catheters were not homogenized; instead, they were stained with fluorescent dyes by using Live/Dead BacLight Bacterial Vaiability kit (Invitrogen, Carlsbad, CA) for 20 min in the dark, exactly per the kit’s instructions. Finally, each catheter was singly placed onto a cover-slip and its outer surface was visualized using an inverted fluorescent microscope (Olympus 1×71, Center Valley, PA), equipped with the appropriate filter sets.
3. Results and Discussions
3.1 Electrochemical Generation of Nitric Oxide
The model catheter configuration consists of a single-lumen, silicone rubber tubing that contains a nitrite resvior and Cu0 and Ag/AgCl wire electrodes (Scheme 1). A pulse sequence of applied voltages to the Cuo wire electrode is used to generate NO continuously from the nitrite ion reservoir within the catheter. The anodic pulse provides a transient level of Cu(I) ions that are capable of reducing nitrite to NO in accordance with the following reaction:.
Cu(I) + NO2− + 2 H+ → Cu(II) + NO + H2O
Scheme 1.
Schematic of a single-lumen electrochemically modulated NO releasing catheter.
However, oxidation of the copper electrode surface forms oxides of copper that passivate the surface, requiring applied cathodic voltages to regenerate a clean Cuo surface that can create more Cu(I) ions upon re-oxidation.[23] Therefore, the pulse sequence of applied voltages must include repeated cycles of a cathodic voltage pulse followed by an anodic pulse (Fig. 1a). In earlier work,[23] a pulse sequence containing cycles of 3 min at −0.92 V and 3 min at 0 V (vs. Ag/AgCl) was used to generate an average NO flux of 0.5×10−10 mol min−1 cm−2, which flucates periodically from 0.25 – 0.75×10−10 mol min−1 cm−2 (see Fig. 1b). This NO flux is not sufficient to prevent clotting in vivo and the fluctuation complicates interpreting the relationship between NO flux and in vivo results. In order to obtain higher and more stable NO fluxes, in this work we compared the effect of different pulse sequences on the NO release profile. By using the pulse sequence of +0.2 V and −1.3 V, the average measured NO flux is now consistently >1.0×10−10 mol min−1 cm−2 (in absence of significant levels of oxygen, see below). As shown in Fig. 1c, it is evident that fluctuations on the NO release profile are much larger if the pulse cycle time is every 6 min as compared with every 30 s. Note that for pulse cycles of every 6 min for 2 h, 20 spikes can be identified from the NO release profile detected by the NOA. This is because NO is only generated on the electrode during the anodic pulse—which is half of the cycle.
Fig. 1.
Effect of pulse cycle on NO release profile from surface of the silicone catheter from a copper wire electrode: (a) Schematic of applied voltage pulse sequence; (b) NO release profile from 6 min cyles using old pulse sequence (as in[23]); with an anodic pulse of 3 min at 0 V and a cathodic pulse of 3 min at −0.92 V (vs. Ag/AgCl wire); (c) NO release profile of 6 min cycles and 30 s cycles, with an anodic pulse of +0.2 V and a cathodic pulse of −1.3 V (vs. Ag/AgCl wire).
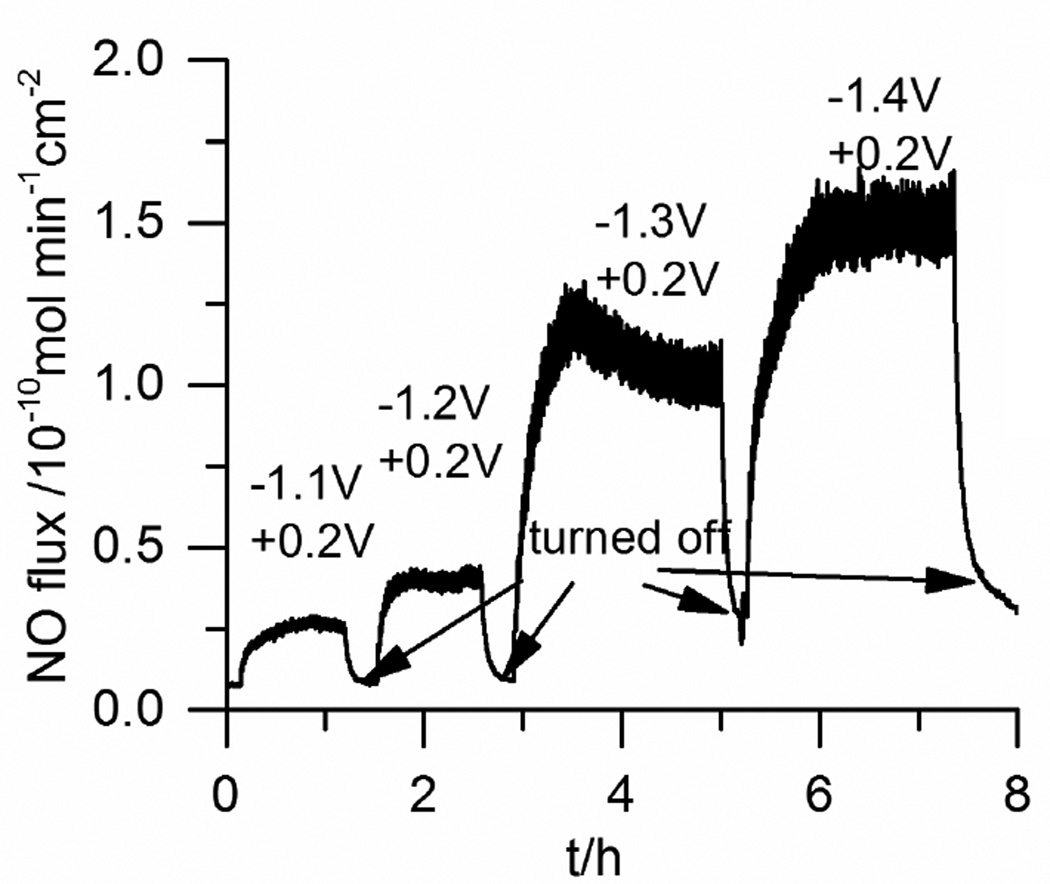
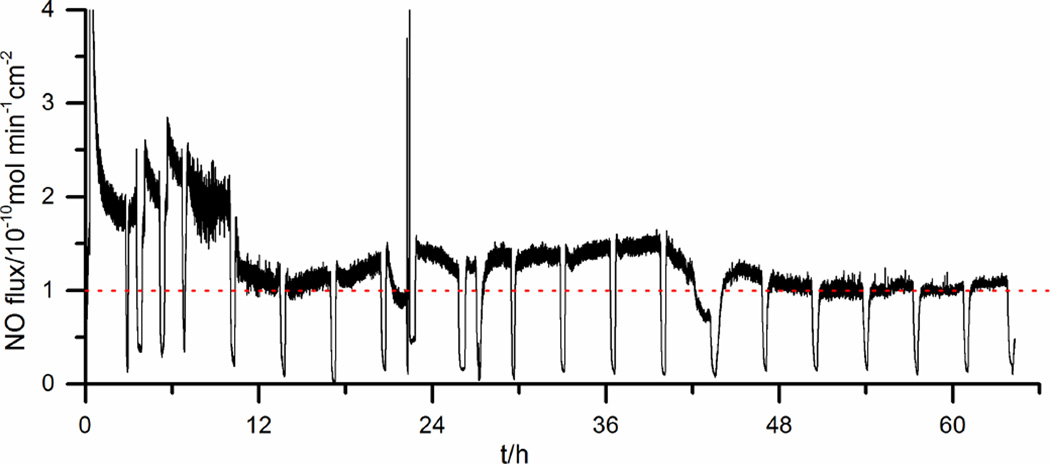
In another experiment, it was found that by further increasing the cathodic voltages from −1.0 V to −1.4 V (vs. Ag/AgCl wire), increased NO fluxes from the surface of the catheter tubing could be obtained (Fig. 2). This is likely due to the fact that the more negative voltage can better regenerate a clean Cuo surface. However, we noticed that when cathodic pulses above −1.35 V are used, the baseline NO level (hen the sequence is turned “o ”) also increases, probably due to the fact that the surface regenerated at more negative voltage is too active. When cathodic pulses < −1.35 V are used, the NO baseline level is consitently low (<0.2×10−10 mol min−1 cm−2) when the system is turned “off”. Therefore, for the remainder of the studies reported here, a pulse sequence with 15 s at +0.2 V and 15 s at −1.3 V is used and with this sequence the catheters can release NO continuously for > 60 h (Fig. 3).
Fig. 2.
Effect of cathodic pulse voltage on NO release from a single-lumen catheter at 25 °C. Each pulse cycle includes a 15 s cathodic voltage pulse and a 15 s anodic voltage pulse. The potentials are vs. Ag/AgCl wire
Fig. 3.
NO release from a single-lumen silicone catheter using repeated pulse cycles of 15 s at −1.3 V and 15 s at +0.2 V at 37 °C. The dotted line indicates a surface NO flux of 1.0×10−10 mol min−1 cm−2. Note that the catheter is intentionally turned “off” periodically to demonstrate the NO release can be modulated. The potentials are vs. Ag/AgCl wire
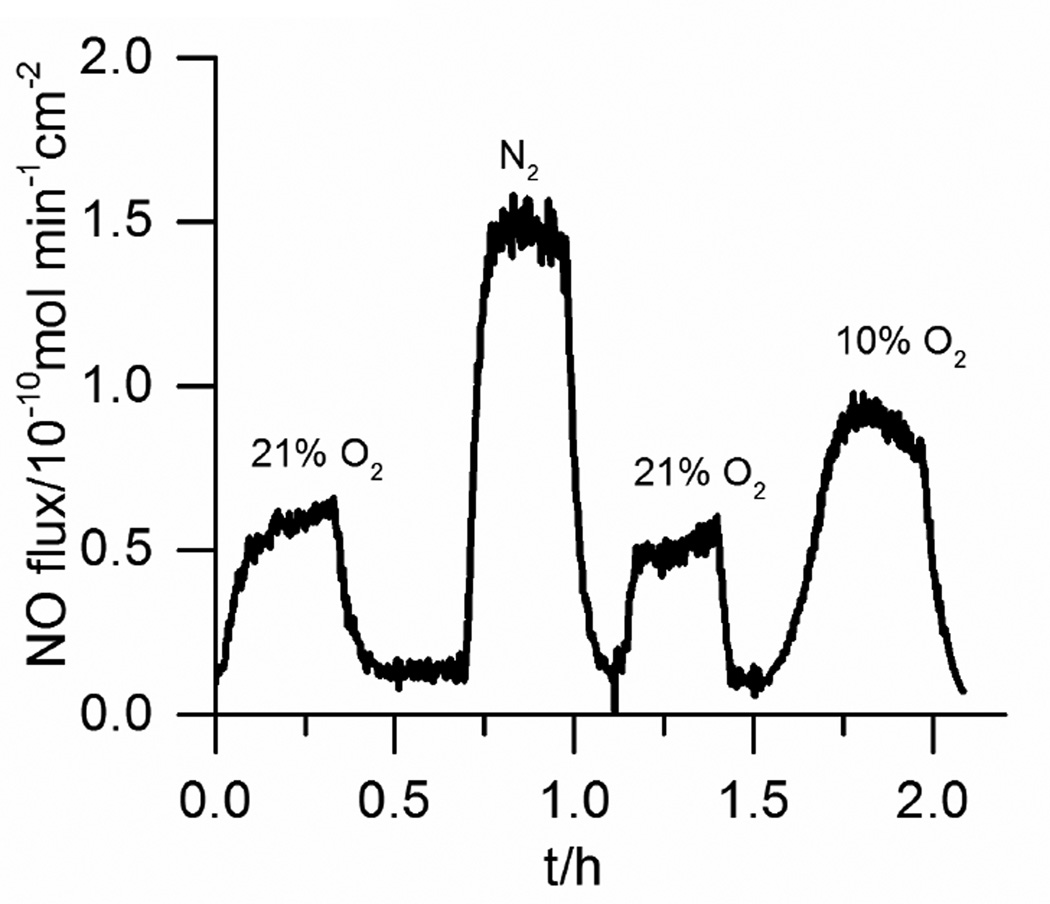
The effect of O2 level in the test solution in which the catheters were placed was also studied. In the presence of 10% and 21% O2, the detected NO flux is reduced by 39% and 53%, respectively (Fig. 4). This decrease in measured NO can result from two processes. First, the generated NO can react with O2 directly, within the catheter and within the gas phase that is purging the NO into the chemiluminescence instrument. Second, it is known that some Cu(I) species can reduce O2 to the superoxide radical, which can scanvage NO to form peroxynitrite.[25] To clarify the contribution of these two processes, we also tested chemical donor based NO releasing devices under similar conditions and found 18% and 21% decrease in measured NO under 10% and 21% O2, respectively compared to when only N2 is the purge gas (data not shown). The NO release for these materials is based on decomposition of dizeniumdiolate NO donors which involes no copper. Therefore, this result means that ~40% of the observed total reduction in NO flux observed from the electrochemical NO releasing catheter in the presence of O2 results from the effect of O2 on the NO detection method, and the other ~60% is likely from the reaction of oxygen with Cu(I) as well as the faster rate of oxygen reaction with the higher NO level near the surface of the Cu0 electrode as this reaction is second order in NO. Of note, the venous O2 content is typically is much less than 10%, and arterial O2 is fairly close to this level (e.g., corresponding to 80–120 mm Hg). Hence, for the proposed catheters described here, it is likely that in vivo, the NO fluxes at the surface of the catheters should typically be > 0.8×10−10 mol min−1 cm−2, whether the catheters are implanted in veins or arteries.
Fig. 4.
Effect of solution phase O2 concentration on NO release from a single-lumen silicone catheter (37 °C). Repeated pulse cycles of 15 s at −1.3 V and 15 s at +0.2 V are applied. Note that NO release is turned “o ” when changing the purging gas.
3.2 Anti-thrombotic Properties of NO Releasing Catheters In Vivo
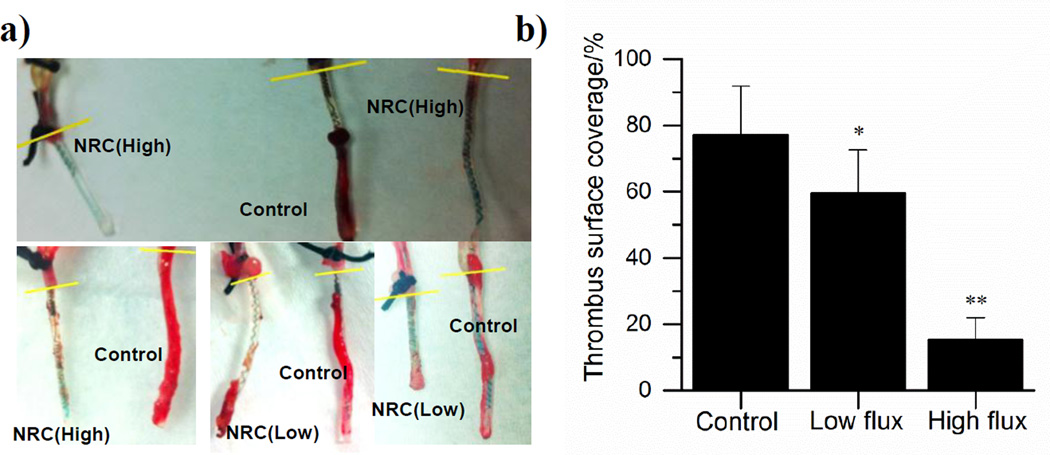
To assess the antithrombotic activity of the electrochemical NO releasing catheters, the devices (both active and control catheters) were implanted into rabbit veins for 7 h. Catheters in the low flux group exhibit an NO flux of ~0.7×10−10 mol min−1 cm−2 and those in the high flux group exhibit fluxes of ~1.1×10−10 mol min−1 cm−2. As shown in Fig. 5a, less thrombus formation is observed on the surface of the NO releasing catheters as compared with control catheters. However, the NO flux plays a critical role in this experiment, with a higher flux (~1.1×10−10 mol min−1 cm−2) reducing the overall thrombus coverage by 62% (n=3, p<0.001), while a lower flux (~0.7×10−10 mol min−1 cm−2) reduces thrombus area by only 17% (Fig. 5b; n=3, p<0.05) (reductions are relative to average of control catheters without NO release). This agrees well with the fact that the endothelium layer of cells on the inner walls of blood vessels produces NO at the flux of 0.5–4×10−10 mol min−1 cm−2 to prevent clotting, and dysfunction of NO producing the enzyme, eNOS, increases susceptibility to thrombophilia.[26] Note that NO released from the surface of the catheter has a short lifetime in blood (<1 s) due to reaction with oxyhemoglobin and, thus, will only induce a very localized antithrombotic effect, as compared with heparin, which usually induces a systemic effect.[27]
Fig. 5.
Antithrombotic effect of NO releasing catheters (NRC) in 7 h rabbit experiment: a) representative photos of high flux (NRC (High) ~1.1×10−10 mol min−1 cm−2), low flux (NRC (Low) ~0.7×10−10 mol min−1 cm−2) and control (Control, <0.1×10−10 mol min−1 cm−2) catheters after explantation; b) thrombus surface coverage quantified by counting the red pixels on the catheters from the photos (Low flux catheters: n=3 rabbits, * p<0.05; High flux catheters: n=3 rabbits, **p<0.01).
3.3. Biofilm Prevention Study
Since bacterial infections pose major clinical problems[28], in our prior work with the Cu-wire based NO release catheters, we studied the effect of continous e-chem NO release on the adhesion of E. coli and A. baumannii bacteria to catheter surfaces within a CDC bioreactor. In this study, a drip flow bioreactor is used to allow growth of high biomass-biofilms close to the air liquid interface, which provides a standardized model that better represents in vivo conditions.[29] We first investigated the effect of NO on bacterial biofilm formation from a microbial species that is more prelevant with CRBSI, S. aureus. We also examined whether effective dispersal of pre-formed, mature biofilms can be achieved with only 3 h of NO release turned “on” after the catheters were placed in the drip flow bioreactor to allow E. coli biofilm formation of over 2 and 4 d periods.
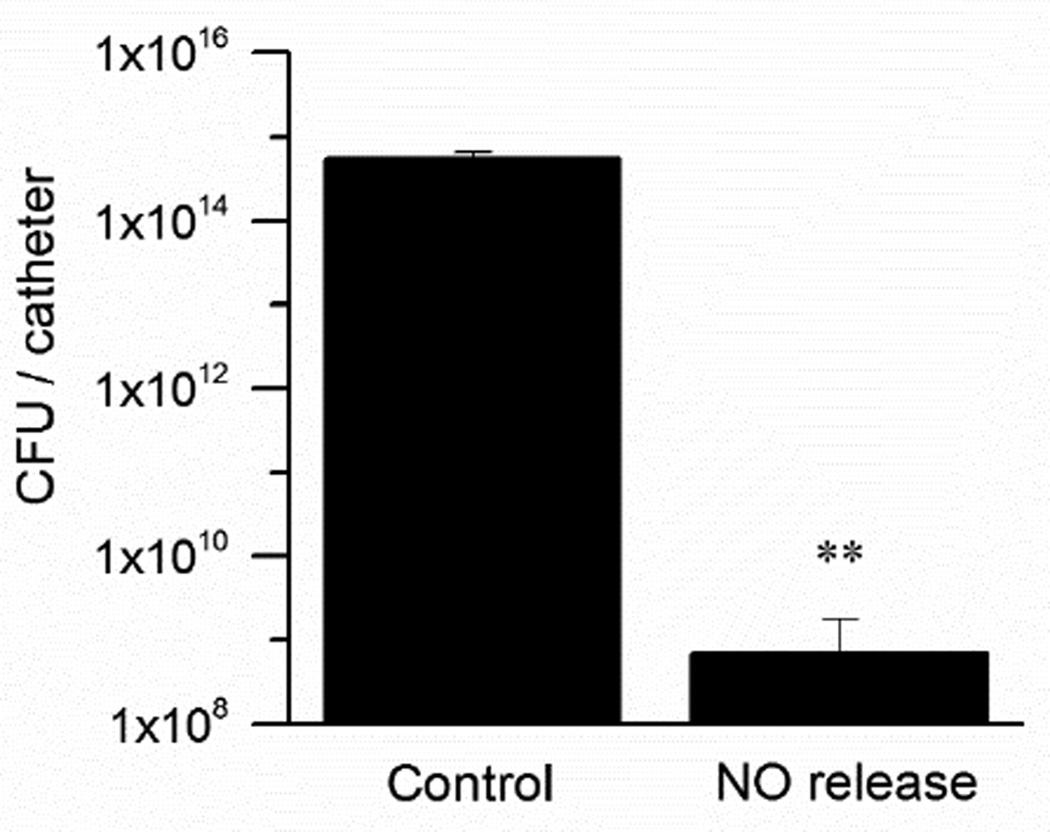
Since intravascular catheters necessitate continuous NO release to prevent clotting formation on their surfaces, biofilm dispersal was first studied by employing catheters that release NO continuously during the time exposed to S. aureus and media within the drip flow bioreactor. The S. aureus biofilms were grown for 48 h at 37 °C, and NO was constantly generated electrochemically for the active catheters using pulse sequence of 15 s at −1.3 V and 15 s at +0.2 V. Corresponding controls containing the same nitrite reservoir as well as the Cuo and Ag/AgCl wires but not connected to the potentiostat were also examined. Plate counts of viable bacteria present on the surface of the catheters demonstrate a 6 log-unit difference between control and NO releasing catheters (Fig. 6; n=3, p<0.01).
Fig. 6.
S. aureus biofilms developed on single-lumen catheters in a drip flow biofilm reactor for 48 h with the electrochemical NO release turned “on” continuously (only for NO release catheters) throughout the entire period of the biofilm growth. Plate counts of viable bacteria attached to the catheter surface after this period. (n=3, **p<0.01)
It should be noted that S. aureus is an opportunistic pathogen capable of forming biofilms and causing chronic infections with high morbidity and mortality.[30–31] Furthermore, because S. aureus biofilms are purported to promote horizontal transfer of antibiotic determinants, they present an even greater challenge to successfully eradicate.[32] Thus, since the NO releasing catheter devices significantly lower the viable cell count of S. aureus biofilms, this approach may represent an efficacious and relatively simple strategy to reduce this current serious complication in hospitalized patients.
It is also interesting to note that some bacterial infections can cause concomitant thrombosis. Indeed, invasive staphylococcal and streptococcal bacterial infections can promote hemostatic system malfunctions, which also lead to thrombosis.[33–34] Colonization by S. aureus, in addition to causing the majority of intravascular catheters-related infections[35], can also induce platelet aggregation in vitro and induce the development of deep vein thrombosis in vivo.[36–37]
Because the plate count data for S. aureus biofilms showed a dramatic difference between continuous NO releasing and the control catheters, this result prompted us to investigate whether releasing NO only at the end of the biofilm growth period may still be sufficient to reduce bacterial viability on the surface of the catheter. Moreover, this strategy would conserve the nitrite reservoir thus significantly increasing the operational NO release lifetime of any medical catheters developed based on this chemistry. This approach could be more viable for urinary catheters rather than intravascular devices, since thrombosis would not be an issue with such catheters and hence continuous NO release would not likely be required.
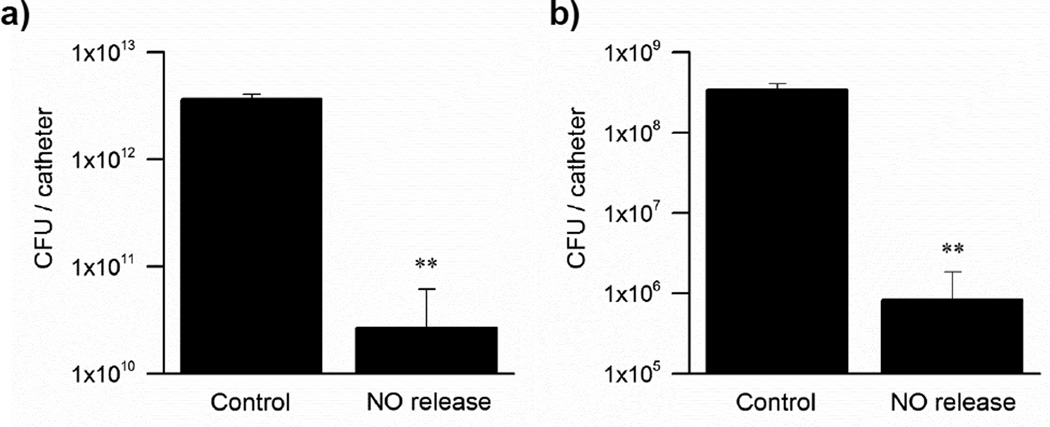
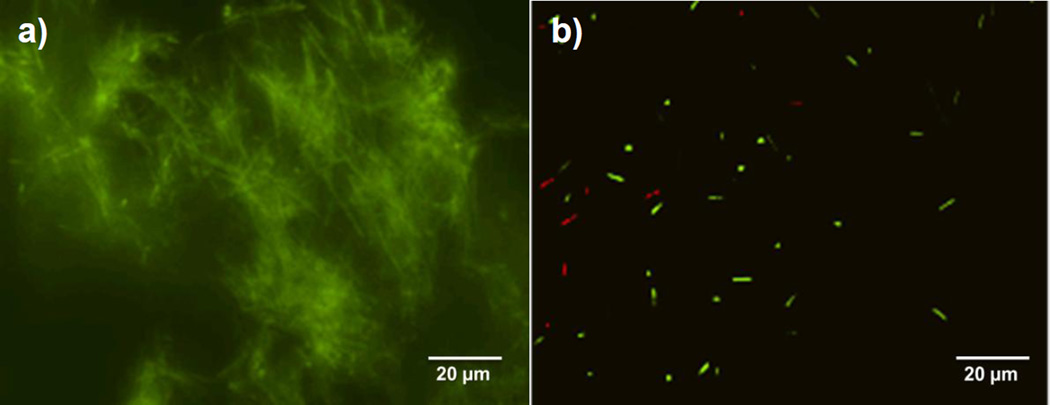
Since E. coli is the cause of 80% of urinary tract infections (UTIs) in humans[38] and can easily adhere onto abiotic surfaces and mediate strong biofilm growth[39–40], this bacterium was chosen to assess whether the catheters that are only turned “on” to generate NO at the end of the biofilm maturation process, can still abate cell viability significantly. Remarkably, as shown in Fig. 7a, the electrochemical NO-release catheters can efficiently disperse 48 h E. coli biofilms by generating NO for only 3 h at relevant biological fluxes (1.0×10−10 mol min−1 cm−2). Indeed, the bacterial count on the surface of the NO producing catheters is 3 log units lower than that found on the control catheters (n=4, p<0.01). This finding is further validated by the fluorescent imaging data that bacterial surface coverage of the NO releasing catheters is noticeably less than the control catheters (Fig. 8).
Fig. 7.
E. coli biofilm developed on single-lumen catheters in a drip flow biofilm reactor for a) 48 h and b) 96 h and NO release was then turned “on” only at the end of these periods for 3 h. Plate counts of viable bacteria attached to the catheter surface.. (n=4 for both experiments, **p<0.01).
Fig. 8.
Representative fluorescent micrographs of surfaces of a) control and b) NO releasing catheters with live/dead staining. E. coli biofilms was grown for 48 h and NO is turned “on” for the NO releasing catheter for 3 h at the end of the growth before the imaging.
The matrix of extracellular polymeric substances, by increasing over colonization time, contributes to the adhesion properties of bacteria and enhances biofilm stability.[41–43] Because of this, both dispersal and eradication of biofilm structures are typically more problematic at later stages of biofilm development. Nonetheless, as demonstrated here, the electrochemical method of NO production from the surface of a catheter is efficient at inducing dispersal of 96 h old E. coli biofilms (Fig. 7b). In fact, it is shown that the bacterial counts on the surfaces of catheters releasing NO for 3 h after a 4 d growth of biofilms is almost 3 log units lower than those on the control catheters (n=4, p<0.01). Consequently, these results demonstrate how the electromodulated NO releasing approach may also provide a novel strategy to reduce biofilm-induced UTIs over an extended time period of catheter placement, via a periodic generation of NO.
Because the proposed electrochemical NO generating system has considerable anti-biofilm properties, in future studies, it would be interesting to investigate whether local NO delivery at these low, non-toxic fluxes can also be efficient against antibiotic resistant, biofilm-forming microbial isolates. It has already been shown that NO can have an antimicrobial effect against multidrug-resistant uropathogenic E. coli.[44] In addition, a manganese nitrosyl, entrapped in the mesoporous material MCM-41 has been discovered to strongly inhibit the growth of drug-resistant A. baumannii.[45] Nevertheless, most of these studies to date have focused on the bactericidal properties of NO on planktonic cells. Because cells within biofilms are much more resistant than planktonic cells to antimicrobial agents[6][46], it will be necessary to investigate whether NO released from the catheter configuration described in this work can also eradicate antibiotic-resistant isolates that have been grown to a mature biofilm stage on the surface of such devices.
4. Conclusions
In summary, we have demonstrated that electrochemical release of NO from model catheters, which employ a copper wire electrode to generate Cu(I) ions to reduce nitrite to NO, can produce a relatively steady flux of NO from their surfaces for an extended time period by using the proper applied voltage pulse sequence. Such catheters were further shown to exhibit significant thromboresistance in vivo as well as anti-biofilm properties against bacteria that are known to be associated with high rates of hospital infections for both intravascular and urinary catheters. It should be noted that the single-lumen configuration used in this work is not practical for preparing operational catheters that could be employed clinically to infuse fluids or withdraw blood. However, we have already demonstrated that electrochemical NO generation from multi-lumen catheters is possible, using the alternate Cu(II)-ligand mediated electrochemical reduction of nitrite within one lumen of the multi-lumen device.[24] Hence, efforts will now focus on using either of the two methods (Cuo wire-based or Cu(II)-complex based) within full sized multi-lumen catheter configurations to demonstrate the anti-clotting and antimicrobial capability of this new approach with both intravascular and urinary cathters that could ultimately be employed in human studies.
Highlights.
Electrochemical NO release from a nitrite reservoir is demonstrated in a catheter.
NO release from the catheter surface can be turned “on” and “off”.
These catheters reduce surface thrombus in vivo by up to 67%.
The NO release catheters reduce S. aureus and E. coli biofilm formation by >99.9%.
Acknowledgements
The research described in this paper was supported by grants from the National Institutes of Health (NIH-EB-000783; NIH-R56-HL-119403-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Infect. Control Hosp. Epidemiol. 2011;32:101. doi: 10.1086/657912. [DOI] [PubMed] [Google Scholar]
- 2.Miller DL, O’Grady NP. J. Vasc. Interv. Radiol. 2012;23:997. doi: 10.1016/j.jvir.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oman KS, Makic MBF, Fink R, Schraeder N, Hulett T, Keech T, Wald H. Am. J. Infect. Control. 2012;40:548. doi: 10.1016/j.ajic.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. JAMA Intern. Med. 2013;173:2039. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 5.Davies D. Nat. Rev. Drug Discov. 2003;2:114. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 6.Mah TF, O’Toole GA. Trends Microbiol. 2001;9:34. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 7.Chung KK, Schumacher JF, Sampson EM, Burne RA, Antonelli PJ, Brennan AB. Biointerphases. 2007;2:89. doi: 10.1116/1.2751405. [DOI] [PubMed] [Google Scholar]
- 8.Iconaru SL, Chapon P, Le Coustumer P, Predoi D. Sci. World J. 2014;2014:165351. doi: 10.1155/2014/165351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stobie N, Duffy B, McCormack DE, Colreavy J, Hidalgo M, McHale P, Hinder SJ. Biomaterials. 2008;29:963. doi: 10.1016/j.biomaterials.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 10.Ding X, Yin B, Qian L, Zeng Z, Yang Z, Li H, Lu Y, Zhou S. J. Med. Microbiol. 2011;60:1827. doi: 10.1099/jmm.0.024166-0. [DOI] [PubMed] [Google Scholar]
- 11.Jones SM, Dang TT, Martinuzzi R. Int. J. Antimicrob. Agents. 2009;34:360. doi: 10.1016/j.ijantimicag.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Kuter DJ. Oncologist. 2004;9:207. doi: 10.1634/theoncologist.9-2-207. [DOI] [PubMed] [Google Scholar]
- 13.van Rooden CJ, Schippers EF, Barge RM, Rosendaal FR, Guiot HF, van der Meer FJ, Meinders AE, Huisman MV. J. Clin Oncol. 2005;23:2655. doi: 10.1200/JCO.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Loscalzo J. Circ. Res. 2001;88:756. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 15.MacMicking J, Xie QW, Nathan C. Annu. Rev. Immunol. 1997;15:323. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 16.Barraud N, Schleheck D, Klebensberger J, Webb JS, Hassett DJ, Rice SA, Kjelleberg S. J. Bacteriol. 2009;191:7333. doi: 10.1128/JB.00975-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai W, Wu J, Xi C, Meyerhoff ME. Biomaterials. 2012;33:7933. doi: 10.1016/j.biomaterials.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols SP, Schoenfisch MH. Biomater. Sci. 2013;1:1151. doi: 10.1039/C3BM60130G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riccio DA, Schoenfisch MH. Chem. Soc. Rev. 2012;41:3731. doi: 10.1039/c2cs15272j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams DLH. Acc. Chem. Res. 1999;32:869. [Google Scholar]
- 21.Keefer LK. ACS Chem. Biol. 2011;6:1147. doi: 10.1021/cb200274r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mowery KA, Schoenfisch MH, Saavedra JE, Keefer LK, Meyerhoff ME. Biomaterials. 2000;21:9. doi: 10.1016/s0142-9612(99)00127-1. [DOI] [PubMed] [Google Scholar]
- 23.Höfler L, Koley D, Wu J, Xi C, Meyerhoff ME. RSC Adv. 2012;2:6765. doi: 10.1039/C2RA20853A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren H, Wu J, Xi C, Lehnert N, Major TC, Bartlett RH, Meyerhoff ME. ACS Appl. Mater. Interfaces. 2014;6:3779. doi: 10.1021/am406066a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jourd'heuil D, Jourd'heuil FL, Kutchukian PS, Musah RA, Wink DA, Grisham MB. J. Biol. Chem. 2001;276:28799. doi: 10.1074/jbc.M102341200. [DOI] [PubMed] [Google Scholar]
- 26.Chen AF, Ren J, Miao CY. Jpn. J. Pharmacol. 2002;89:327. doi: 10.1254/jjp.89.327. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR. J. Biol. Chem. 1998;273:18709. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 28.Khanna V, Mukhopadhayay C, Eshwara VK, Verma M, Dabke P. J. Pathog. 2013;2013:936864. doi: 10.1155/2013/936864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goeres DM, Hamilton MA, Beck NA, Buckingham-Meyer K, Hilyard JD, Loetterle LR, Lorenz LA, Walker DK, Stewart PS. Nat. Protoc. 2009;4:783. doi: 10.1038/nprot.2009.59. [DOI] [PubMed] [Google Scholar]
- 30.Götz F. Mol. Microbiol. 2002;43:1367. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 31.Parsek MR, Singh PK. Annu. Rev. Microbiol. 2003;57:677. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 32.Savage VJ, Chopra I, O’Neill AJ. Antimicrob. Agents Chemother. 2013;57:1968. doi: 10.1128/AAC.02008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boersma RS, Jie KSG, Verbon A, van Pampus ECM, Schouten HC. Ann. Oncol. 2008;19:433. doi: 10.1093/annonc/mdm350. [DOI] [PubMed] [Google Scholar]
- 34.Donabedian H, Boyle MD. Infect. Immun. 1998;66:2362. doi: 10.1128/iai.66.5.2362-2364.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiedrowski MR, Horswill AR. Ann. N. Y. Acad. Sci. 2011;1241:104. doi: 10.1111/j.1749-6632.2011.06281.x. [DOI] [PubMed] [Google Scholar]
- 36.Kerrigan SW, Clarke N, Loughman A, Meade G, Foster TJ, Cox D. Arterioscler. Thromb. Vasc. Biol. 2008;28:335. doi: 10.1161/ATVBAHA.107.152058. [DOI] [PubMed] [Google Scholar]
- 37.Martin E, Cevik C, Nugent K. Thromb. Res. 2012;130:302. doi: 10.1016/j.thromres.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Campanhã MT, Hoshino-Shimizu S, Baquerizo Martinez M. Rev. Panam. Salud Publica. 1999;6:89. doi: 10.1590/s1020-49891999000700002. [DOI] [PubMed] [Google Scholar]
- 39.Gander RM, Thomas VL. Infect. Immun. 1987;55:293. doi: 10.1128/iai.55.2.293-297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulett GC, Mabbett AN, Fung KC, Webb RI, Schembri MA. Microbiology. 2007;153:2321. doi: 10.1099/mic.0.2006/004648-0. [DOI] [PubMed] [Google Scholar]
- 41.Danese PN, Pratt LA, Kolter R. J. Bacteriol. 2000;182:3593. doi: 10.1128/jb.182.12.3593-3596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lüdecke C, Jandt KD, Siegismund D, Kujau MJ, Zang E, Rettenmayr M, Bossert J, Roth M. PLoS One. 2014;9:e84837. doi: 10.1371/journal.pone.0084837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reisner A, Haagensen JA, Schembri MA, Zechner EL, Molin S. Mol. Microbiol. 2003;48:933. doi: 10.1046/j.1365-2958.2003.03490.x. [DOI] [PubMed] [Google Scholar]
- 44.Bang CS, Kinnunen A, Karlsson M, Önnberg A, Söderquist B, Persson K. BMC Microbiol. 2014;14:1. doi: 10.1186/1471-2180-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heilman BJ, John JS, Oliver SR, Mascharak PK. J. Am. Chem. Soc. 2012;134:11573. doi: 10.1021/ja3022736. [DOI] [PubMed] [Google Scholar]
- 46.Nickel JC, Ruseska I, Wright JB, Costerton JW. Antimicrob. Agents Chemother. 1985;27:619. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]; [a] Jourd'heuil D, Jourd'heuil FL, Kutchukian PS, Musah RA, Wink DA, Grisham MB. J. Biol. Chem. 2001;276:28799. doi: 10.1074/jbc.M102341200. [DOI] [PubMed] [Google Scholar]; [b] Lewis RS, Deen WM. Chem. Res. Toxicol. 1994;7:568. doi: 10.1021/tx00040a013. [DOI] [PubMed] [Google Scholar]