Summary
Granulocytes and monocytes/macrophages of the myeloid lineage are the chief cellular agents of innate immunity. Here, we have examined the inflammatory response in mice with conditional knockouts of the hypoxia responsive transcription factor HIF-1α, its negative regulator VHL, and a known downstream target, VEGF. We find that activation of HIF-1α is essential for myeloid cell infiltration and activation in vivo through a mechanism independent of VEGF. Loss of VHL leads to a large increase in acute inflammatory responses. Our results show that HIF-1α is essential for the regulation of glycolytic capacity in myeloid cells: when HIF-1α is absent, the cellular ATP pool is drastically reduced. The metabolic defect results in profound impairment of myeloid cell aggregation, motility, invasiveness, and bacterial killing. This role for HIF-1α demonstrates its direct regulation of survival and function in the inflammatory microenvironment.
Introduction
Microenvironmental conditions found in injured tissues are characterized by low levels of oxygen and glucose, as well as high concentrations of lactate and reductive metabolites (Saadi et al., 2002; Schor et al., 2000). Thus, effector cells of the innate immune system have an acute need to respond to these demanding conditions to maintain viability and activity. This is especially the case in regions where the vascular network has itself been wounded or incapacitated.
At sites of inflammation, approximately 95% of the myeloid cells are recruited to, rather than resident at, those sites (Lewis et al., 1999); thus they need to move against oxygen gradients in order to migrate toward relevant areas of inflammation (Turner et al., 1999). The hypoxic arenas in which myeloid cells are found include sites of cutaneous inflammation, e.g., skin infections and wounds (Arnold et al., 1987), arthritis (Mapp et al., 1995), and in particular, central necrotic areas of solid tumors (Denko and Giaccia, 2001; Hockel and Vaupel, 2001). Low oxygen levels have been described in all of these areas of myeloid cell activity and in virtually every other site of extensive inflammation (Korhonen, 2000; Najafipour and Ferrell, 1995; Ott, 1987; Sawyer et al., 1991; Silver, 1975; Simmen et al., 1994). Hypoxic conditions have also been shown to profoundly affect a broad range of myeloid cell properties in vitro, e.g., phagocytosis, cell surface marker expression, secretion of cytokines, chemokine receptor levels, adhesion, migration, and cell survival (Lewis et al., 1999).
Studies extending back almost a century have demonstrated that neutrophils and macrophages are highly dependent on the process of anaerobic glycolysis for the production of ATP (Bakker, 1927; Fleischmann and Kubowitz, 1927; Kempner, 1939; Levene and Meyer, 1912a, 1912b). Glycolytic inhibitors have been shown to greatly reduce both cellular ATP concentrations and functional activity of myeloid cells; inhibitors of mitochondrial respiration, on the other hand, typically have no effect on the inflammatory reponse (Borregaard and Herlin, 1982; Kellett, 1966). Since glycolysis represents the chief means of generating ATP in the absence of oxygen, the reliance of neutrophils and other myeloid cells on this metabolic pathway strongly suggests that they are highly adapted to a hypoxic mode of existence.
These observations in turn argue for a pronounced dependence of neutrophils and macrophages on the known functions of the hypoxia inducible transcription factor-1 (HIF-1), one of the principal mediators of adaptation to critically low oxygen levels (Semenza, 2001c). A number of laboratories have demonstrated that HIF-1 is implicated in most aspects of hypoxia-induced gene expression and is essential for hypoxia-induced increases in glycolysis and angiogenesis in tumor cells as well as normal tissues (Semenza, 2001b). The HIF-1 heterodimer consists of two helix-loop-helix proteins; these are termed HIF-1α, which is the oxygen-responsive component, and HIF-1β. The latter, also known as the aryl hydrocarbon receptor nuclear translocator (ARNT), is constitutively expressed. In contrast, HIF-1α is typically only detected under low oxygen concentrations and is rapidly degraded by the ubiquitin-proteasome pathway under ambient conditions (Semenza, 2001a).
A central component of the complex regulating HIF-1α turnover is the product of the tumor suppressor gene vhl, encoding the von Hippel-Lindau protein (Maxwell et al., 1999). Mutations of the vhl gene are found in patients suffering from the von Hippel-Lindau disease, as well as in many spontaneous renal cell carcinomas. Patients with the familial disease are prone to development of malignant tumors at a young age. These tumors show high levels of HIF-1α expression and have pronounced vascular beds with enhanced permeability; these are particularly indicative of high levels of expression of one HIF-1 target, the angiogenic/vascular permeability factor VEGF (Maxwell et al., 1999).
HIF-1α and VEGF are expressed in activated macrophages (Burke et al., 2002; Hollander et al., 2001; Talks et al., 2000). However, there has been no previous demonstration of what their roles are during inflammation. Presented here is an extensive study of the role of the hypoxic response during inflammation, employing conditional gene targeting in the myeloid cell lineage. In this study, we have deleted HIF-1α, its target gene VEGF, and its upstream regulator, the von Hippel-Lindau factor (VHL) in separate mouse strains expressing cre recombinase in granulocytes and monocytes/macrophages. As will be described, these deletions clearly show the essentiality of this pathway for inflammatory activation and, in particular, demonstrate the critical role of HIF-1α in infiltration at the very earliest stages of inflammatory cell recruitment. We present evidence that HIF-1α controls inflammatory response through its regulation of the metabolic switch to glycolysis, a switch that is intrinsic to myeloid cell survival and function. This finding ties HIF-1α-controlled transcriptional regulation to almost a century of investigations of glycolytic metabolism, hypoxia, and inflammatory responses in neutrophils and macrophages and demonstrates its potential as a target for modulation of inflammation.
Results
Deletion of HIF-1α, VEGF, and VHL in Macrophages and Neutrophils
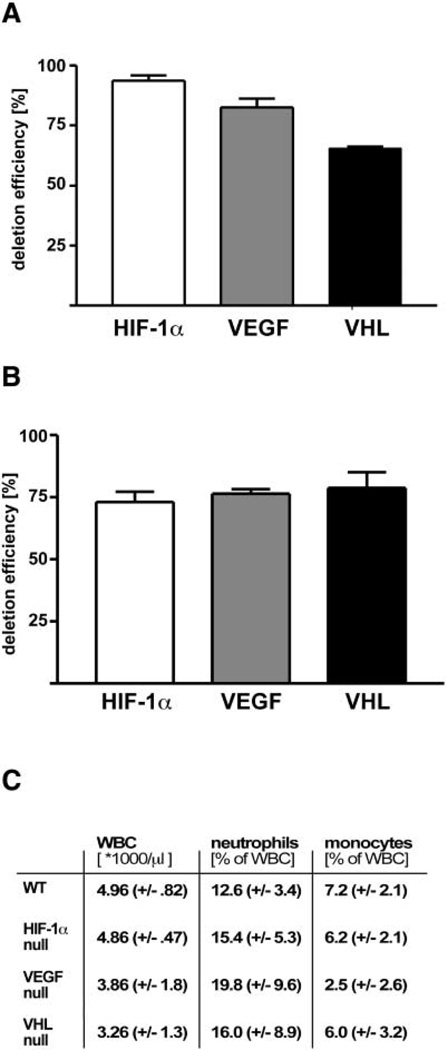
We created targeted deletions of the HIF-1α transcription factor via crosses into a background of cre expression driven by the lysozyme M promoter (lysMcre) (Clausen et al., 1999), which allows specific deletion of the factor in the myeloid lineage. This deletion results in an extensive loss of the HIF-1α gene in isolated peritoneal macrophages, with real time PCR of genomic macrophage DNA demonstrating excision in 91% of isolated cells (Figure 1A). As can be seen in Figure 1, there is a substantial but somewhat lower level of deletion in the VEGF and VHL conditional allele crosses, at 82% and 60% deletion, respectively. We also determined the almost tent of deletion in elicited neutrophils (Figure 1B); these also show extensive deletion, all averaging an approximate 75% deletion rate, as determined in a 95% pure neutrophil population. The mice harboring the described mutations have normal viability postnatally and do not display any obvious phenotypes when housed under standard sterile barrier conditions. In addition, none of these deletions results in large changes in the numbers of circulating monocytes or neutrophils, with overall levels similar in wild-type littermates and HIF-1α, VEGF, and VHL conditionally null animals (Figure 1C).
Figure 1. Efficiency of CRE Recombinase-Mediated Deletion in Myeloid Cells.
Genomic DNA was isolated from peritoneal macrophages (A) and neutrophils (B) from HIF-1α-, VEGF-, and VHL-LysM-CRE mice and subjected to real-time PCR with primers spanning the targeted region as well as primers for an undeleted control gene for normalization. Efficiency of deletion was calculated by quantitative PCR. (C) Differential leukocyte counts were performed on samples harvested by retinal bleeding.
Hypoxia-Induced Gene Expression in Macrophages Requires HIF-1α
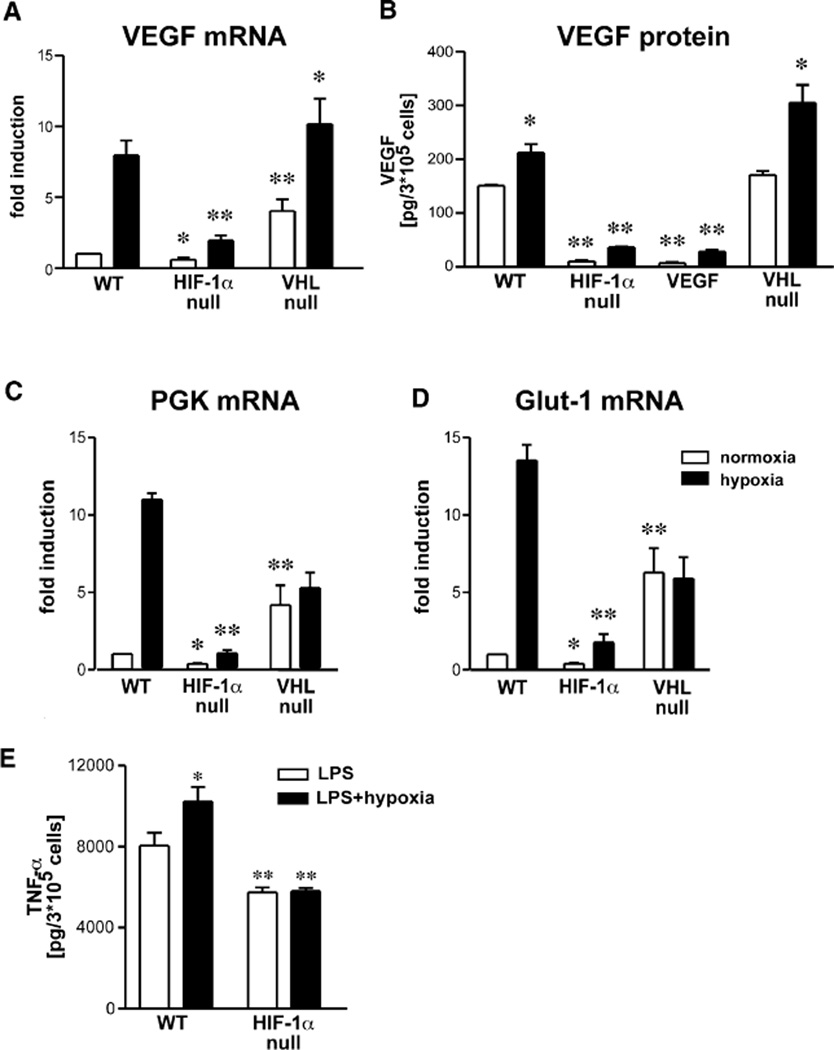
In a first attempt to characterize the loss of HIF-1α, VEGF, and VHL in myeloid cells, we studied the expression patterns of three known HIF-1α target genes in peritoneal macrophages under normoxic and hypoxic conditions. Most strikingly, the functional inactivation of HIF-1α significantly reduced normoxic as well as hypoxic expression of all the analyzed hypoxia-responsive genes (Figure 2). As expected, hypoxic induction of VEGF mRNA levels is reduced by approximately 75% in HIF-1α null macrophages, whereas the loss of VHL results in high normoxic expression levels; these increase approximately 2-fold under hypoxic conditions (Figure 2A). VEGF mRNA expression was not analyzed in VEGF mutant cells due to the continuing presence of mutant, albeit nontranslatable, transcript in VEGF conditionally null cells. An ELISA for secreted VEGF demonstrated highly reduced amounts of VEGF protein in conditioned supernatant of lysMcre/VEGF macrophages (Figure 2B). In addition, this assay confirmed the data obtained by RT-PCR and showed that loss of HIF-1α led to almost complete loss of VEGF expression in isolated hypmacrophages, regardless of oxygen levels (Figure 2B). These unexpected findings suggest that HIF-1α is an obligate regulator of VEGF expression in macrophages even under normoxic conditions.
Figure 2. Functional Inactivation of Hypoxic Response-Related Genes Significantly Impairs Gene Expression Patterns of Macrophages.
(A) Peritoneal macrophages were cultured under normoxic (open bars) or hypoxic (closed bars) conditions for 8 hr, and total RNA was isolated and VEGF mRNA expression determined by real-time PCR. Values were normalized to RT-PCR results for ribosomal RNA. Normoxic WT levels were arbitrarily set as one. VEGF RNA was not analyzed in VEGF mutants due to the expression of a nontranslatable transcript in these cells. (B) Peritoneal macrophages were cultured under normoxic (open bars) or hypoxic (closed bars) conditions for 24 hr, and conditioned supernatant was harvested and VEGF protein analyzed by ELISA. Values were normalized to cell number. (C) Hypoxic induction of gene expression of the glycolytic enzyme phosphoglyceratekinase (PGK) and (D) glucose transporter 1 (Glut-1) was characterized by means of real-time PCR. Normoxic WT levels were arbitrarily set as one and normalization performed as outlined under (A). (E) Macrophages were stimulated with LPS and cultured under normoxic or hypoxic conditions for 8 hr, and conditioned supernatant was assayed for TNF-α protein by ELISA and values normalized to cell number. Statistical analysis was performed using the unpaired Student’s t test, *p < 0.05, **p < 0.01.
The glycolytic enzyme phosphoglycerate kinase (PGK) and the glucose transporter GLUT-1 both have typically HIF-1α-dependent hypoxic induction of mRNA expression (Figures 2C and 2D). The loss of VHL results in a loss of hypoxic induction and a high basal rate of expression, as expected for this mutant (Figures 2C and 2D). These results, taken together, demonstrate that the role of HIF-1α in myeloid cells is similar to its role in other cell types during hypoxia-induced transcription and that loss of VHL has the effect of inducing normoxic expression of these same genes, presumably via increased accumulation of active HIF-1 complexes. However, they also indicate that normoxic expression of these genes is reduced in macrophages, indicating an unsuspected role for the transcription factor in normoxic gene regulation.
In order to establish whether other critical pathways of inflammatory response are affected during normoxia, we examined the response of macrophages to LPS treatment in vitro. As can be seen in Figure 2E, loss of HIF-1α does alter the acute secretion of TNF-α in response to LPS treatment, reducing it approximately 25%; more strikingly, it completely eliminates the hypoxia-induced augmentation of the TNF-α response (Figure 2E) (Hempel et al., 1996; VanOtteren et al., 1995).
HIF-1α Regulates Glycolysis and Energy Metabolism in Myeloid Cells
The sufficient activation of myeloid cells during the course of inflammation is dependent on a number of energy-requiring pathways (Buttgereit et al., 2000; Krauss et al., 2001). Macrophages and neutrophils typically produce most of their ATP through glycolysis (Kawaguchi et al., 2001; Lewis et al., 1999); this is likely tied to their need to function in microenvironments low in oxygen and glucose. It has been shown through a large number of studies that inhibition of glycolysis can directly inhibit ATP production and subsequently myeloid cell properties as diverse as adhesion, extravasation, motility, and invasion (Simchowitz et al., 1979; Weisdorf et al., 1982a; Weisdorf et al., 1982b). HIF-1α is a key regulator of glycolysis (Ryan et al., 1998; Seagroves et al., 2001), and this led us to conduct a number of experiments to determine the role of HIF-1α in glucose metabolism and energy generation of myeloid cells.
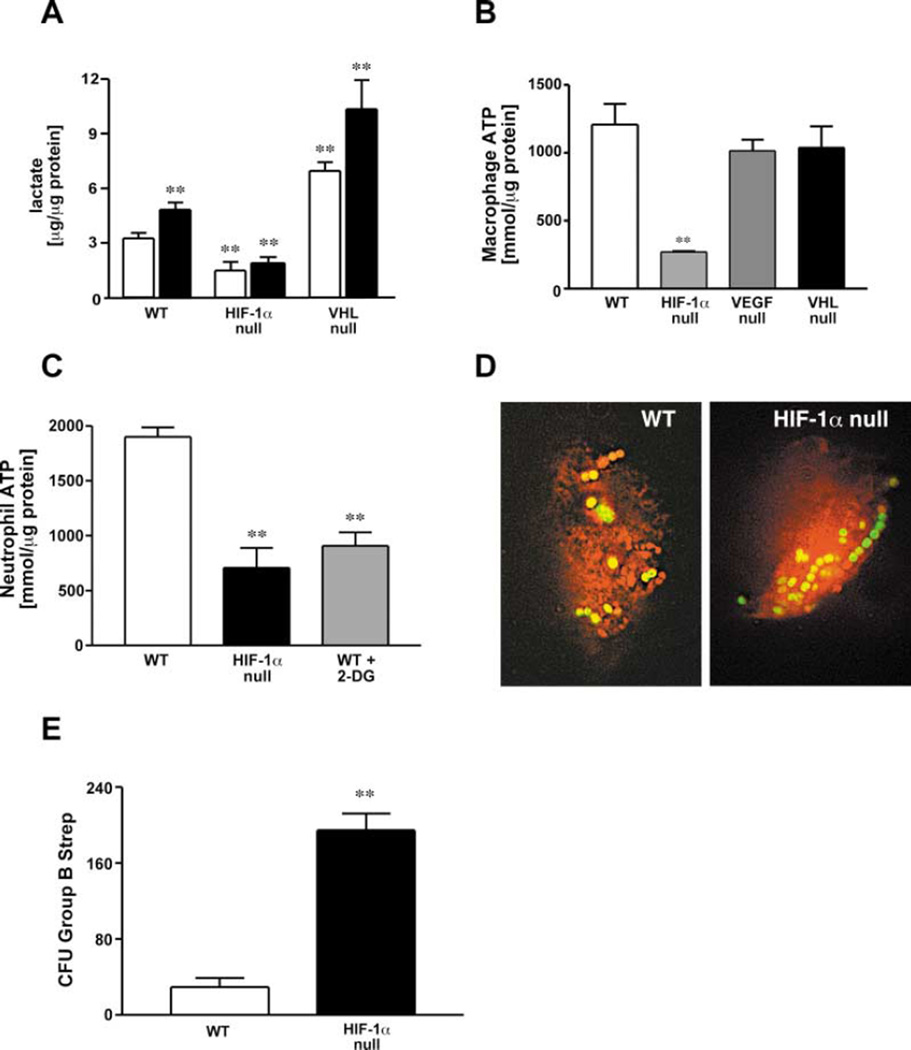
One marker of enhanced glycolytic function and inflammatory activation of macrophages is a large increase in lactate levels (Haji-Michael et al., 1999). As shown in Figure 3A, lactate release is significantly lower in HIF-1α null macrophages, relative to wild-type cells, and fails to be induced by LPS stimulation. In contrast, lactate production is significantly increased in VHL null cells, indicating that the loss of VHL results in increased glycolytic activity of macrophages under both basal and LPS-stimulated conditions. As expected, levels of lactate production are not changed in VEGF null myeloid cells (data not shown).
Figure 3. Glycolysis and Energy Generation in Myeloid Cells Are Severely Affected by the Loss of HIF-1α.
(A) Lactate concentrations in macrophage supernatant were quantified under either control conditions (open bars) or after the addition of LPS (closed bars). Values were normalized to total protein content.
(B) Peritoneal macrophages were isolated from WT, HIF-1α-, VEGF-, and VHL-LysM-CRE mice and cultured under ambient conditions for 24 hr. Cell lysates were harvested and intracellular ATP concentrations measured by means of a luciferase-based chemiluminescent assay. Values were normalized to total protein content.
(C) Peritoneal neutrophils were harvested and assayed for ATP synthesis. 2-deoxyglucose (500 mg/kg b.w.) was injected i.p. 60 min prior to harvest to block glycolysis in peritoneal exudate cells.
(D) Engulfment of viable bacteria was characterized by inoculating macrophages with GFP-expressing GBS for 2 hr. Deconvolution fluorescence microscopy was used for documentation.
(E) Bone marrow derived macrophages were inoculated with Group B streptococci (GBS) at a MOI of 2.5 and intracellular killing analyzed by determination of viable colony forming units in the macrophage lysates after washing and antibiotic treatment to remove nonengulfed bacteria.
Statistical analysis was performed using the unpaired Student’s t test, **p < 0.01.
We then assayed ATP levels in isolated macrophages (Figure 3B) and neutrophils (Figure 3C). They were found to be dramatically reduced in HIF-1α null macrophages, to approximately 15%–20% of normal levels. This very high reduction in overall ATP levels in macrophages is much greater than that seen in other HIF-1α null cell types (Seagroves et al., 2001). Further, this reduction in ATP occurs in normoxic culture conditions, indicating that HIF-1α impacts ATP levels even in highly oxygenated tissue culture media. Decreases in neutrophil ATP were on the order of 40%; this is functionally significant and similar to the level of reduction in ATP caused by the glycolytic inhibitor 2-deoxyglucose, at a dose known to cause decreased neutrophilic activity (Figure 3C) Boxer et al., 1977). The reduction in cellular ATP in these cells clearly indicates that HIF-1α activity is required for the maintenance of intracellular energy homeostasis in myeloid cells. Since a large number of studies link ATP levels in myeloid cells to capacity for inflammation (Kalbhen et al., 1967; Kellett, 1966; Kittlick, 1986; Manns, 1967; Weisdorf et al., 1982b), we postulated that the large decrease in ATP levels caused by decreased glycolysis in myeloid cells could inhibit or eliminate inflammatory responses in lysMcre/HIF-1α animals.
Intracellular Killing of Bacterial Pathogens Is Inhibited by Loss of HIF-1α
Response to pathogenic organisms is a well-studied aspect of myeloid cell function; although it has been shown that opsonin-dependent engulfment is not ATP dependent (Michl et al., 1976), we wished to determine if loss of HIF-1α affected this process or altered the rate of subsequent intracellular destruction of pathogens. We performed assays to determine the rate of uptake of Group B Streptococcus (GBS) by normal and HIF-1α-deficient macrophages. The rate of phagocytic uptake of GFP-tagged GBS was not affected by HIF-1α deletion, as determined by deconvolution microscopy (Figure 3D) and FACS analysis (data not shown) of washed cultures of peritoneal macrophages following incubation with GFP-labeled bacteria. In contrast, quantification of bacterial colony forming units following macrophage lysis revealed approximately 7-fold more viable bacteria present within the HIF-1α-deficient macrophages than in wild-type cells (Figure 3E). Intracellular killing of pathogens is critically dependent on energetically intensive processes, such as peroxide generation (Babior, 2000),which occur independently of respiration, but require ATP (Sbarra and Karnovsky, 1959). This finding indicates a marked defect in the ability of HIF-1α macrophages to kill bacteria through those respiration-independent processes.
Loss of HIF-1α Significantly Impairs Myeloid Cell Aggregation, Invasion, and Motility
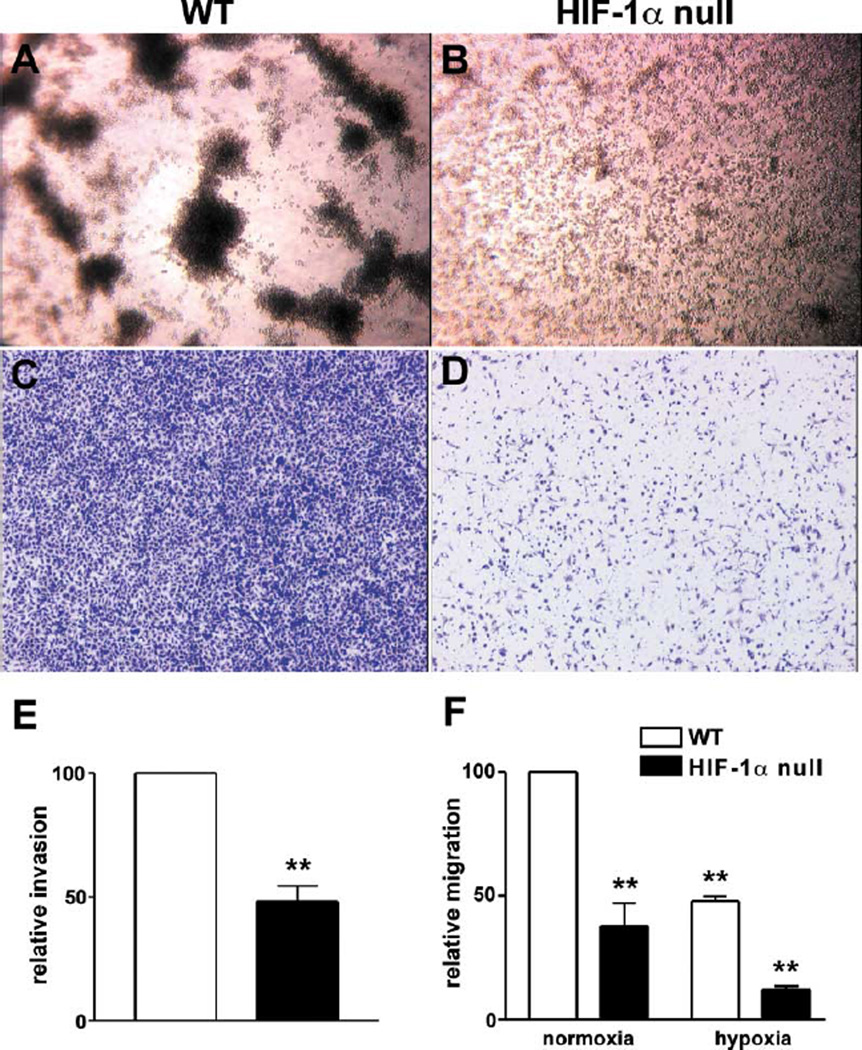
One of the clearest ties in the literature between glycolysis, ATP generation, and inflammatory response has been established for the energy-requiring processes of aggregation, motility, and tissue infiltration of granulocytes and macrophages (Weisdorf et al., 1982a, 1982b). To determine how the loss of HIF-1α and glycolytic response affected these processes, we conducted a number of in vitro studies using wild-type and HIF-1α null peritoneal macrophages. The first series of experiments determined the role of HIF-1α in homotypic adhesion (HA). HA is a process whereby tissue recruitment of leukocytes is amplified by adhesion of monocytes and neutrophils to each other. HA has also been widely used as an in vitro assay to mirror leukocyte-endothelial cell interactions (Manjunath et al., 1993). As can be seen in Figure 4A, wild-type macrophages respond to plating on growth factor-reduced matrigel by rapid and pronounced homotypic adhesion; however, HIF-1α null macrophages show no evidence of homotypic adhesion, although they maintain viability in culture even during extended incubation (Figure 4B). This result indicates that the loss of metabolic response in these cells completely inhibits self-aggregation; further, these results replicate those obtained with chemical inhibition of myeloid cell glycolysis (Weisdorf et al., 1982b).
Figure 4. HIF-1α Is an Essential Prerequisite for Aggregation, Motility, and Invasion of Macrophages.
Macrophages were isolated from the peritoneum and plated at a concentration of 106/ml on growth factor-reduced matrigel. Cells were allowed to aggregate for 24 hr: (A) WT, (B) HIF-1α null macrophages. Invasive capacity of peritoneal macrophages was studied using modified Boyden chambers. Cell culture inserts were coated with matrigel, (C) WT, or (D) HIF-1α null cells added to the upper wells. Macrophages were allowed to invade toward 5% FCS in the lower wells for 24 hr, stained with 0.1% Alcian blue, and photographed. Bound stain was extracted with acetic acid and quantified spectophotometrically (E) WT levels were set arbitrarily as 100. (F) Migration of macrophages was analyzed in modified Boyden chambers without added matrigel. Cells were allowed to migrate for 24 hr and further processed as outlined above. Statistical analysis was performed using the unpaired Student’s t test, **p < 0.01.
Next, we sought to analyze the ability of HIF-1α null macrophages to move and invade through extracellular matrix in vitro; both of these are energy-requiring processes and have also been clearly linked to glycolytic function and myeloid cell ATP levels (Weisdorf et al., 1982b). HIF-1α null cells were severely deficient in their capacity to invade matrigel toward a chemotactic agent, in this case 5% serum (Figures 4C and 4D). Quantification of invading cells by staining and subsequent dye extraction demonstrated that there was an approximately 60% reduction in the penetration of HIF-1α null macrophages through the artificial extracellular matrix (Figure 4E). Loss of HIF-1α also reduced directed motility in the absence of matrigel by 50% at normoxia and by 75% under hypoxic conditions (Figure 4F), indicating that this energy-dependent process is also greatly inhibited by the loss of HIF-1α-regulated metabolic responses.
HIF-1α and VEGF Have Nonoverlapping Effects on Inflammatory Responses
We next analyzed the effects of the conditional loss of HIF-1α, VEGF, and VHL during inflammatory response in vivo. We applied the phorbol ester TPA cutaneously to one side of the ears of control and mutant mouse strains; this is a well-established and widely used model of acute skin inflammation, which induces rapid edema and massive infiltration (Nakadate et al., 1985; Raick, 1973). As can be seen in Figure 5A, in wild-type ears there is clear evidence of infiltration and edema on the painted (upper) portion of the ear (ears are shown stained with anti-CD45 antibody [leukocyte common antigen]). In contrast, the lysM-cre/HIF-1α mouse ear displays little evidence of infiltration or edema (Figure 5B). The central importance of HIF-1α in regulating inflammatory cell VEGF expression (as outlined in Figure 2) raised the question whether reduced VEGF levels might account for the observed phenotype of lysMcre/HIF-1α animals. However, application of TPA on ears of lysMcre/VEGF mice lead to a quite different phenotype: extensive leukocyte infiltration, although with greatly reduced levels of edema (Figure 5C).
Figure 5. Phorbol Ester-Induced Ear Inflammation Is Differentially Regulated by Members of the Hypoxic Response Pathway in Myeloid Cells.
Ears of (A) WT, (B) HIF-1α-, (C) VEGF-, and (D) VHL-LysM-CRE mice were painted once with 2.5 µg TPA in acetone carrier solution. 24 hr later, ears were harvested and fixed in 4% PFA. The leukocyte common antigen (CD45) was immunolocalized on paraffin sections and detected with DAB. (E) Ears were treated with TPA as outlined above, and 24 hr later a defined area of 6mm was punched out. Weight was determined as a measurement of edema formation. (F) Tissue was homogenized and myeloperoxidase activity assayed by spectophotometrically monitored conversion of o-dianisidine. (G and H). Total RNA was isolated from ear samples and subjected to RPA analysis using murine chemokine (G) and cytokine (H) probe sets. Statistical analysis was performed using the unpaired Student’s t-test, **p < 0.01.
This demonstrates that loss of HIF-1α and VEGF in the myeloid lineages do not result in overlapping phenotypes and also shows that loss of HIF-1α results in a blockage of inflammatory response functionally upstream of VEGF expression. It has been hypothesized that VEGF controls or facilitates inflammatory cell extravasation due to its role as an inducer of increased vessel permeability. However, our results show that VEGF secreted by myeloid cells acts primarily to induce edema and is not a critical factor in infiltration in this model.
As further proof that the HIF-1 pathway is critical to inflammatory progression, animals carrying the lysMcre allele in a VHL floxed background, which should have elevated levels of HIF-1α expression, show evidence of greatly increased inflammation (Figure 5D). This hyperinflammatory response is especially intriguing given the data presented in Figure 3A, showing an exaggerated lactate production and thus higher levels of glycolytic activity in VHL mutant animals. This implies that exaggerated metabolic function may act to stimulate the inflammatory response.
In order to quantify levels of edema, ears from 16 mice were painted with TPA, defined regions weighed, and results calculated in comparison to weights of acetone-treated control ears. As can be seen in Figure 5E, loss of either HIF-1α or VEGF resulted in an almost complete inhibition of edema formation, as measured by increased tissue weight. However, VHL deletion in the myeloid lineage resulted in greatly increased levels of edema relative to that seen in wild-type treated tissue (Figure 5E). In order to quantify the degree of infiltration, treated ear tissue was characterized for levels of myeloperoxidase (MPO), a marker of infiltration (Bradley et al., 1982). This assay confirmed the findings obtained by immunolocalization of CD45 (Figures 5A–5D) and showed that loss of HIF-1α results in decreased inflammatory cell infiltration (Figure 5F) but that loss of VEGF does not. It also demonstrates that loss of VHL in myeloid cells actually promotes infiltration at sites of inflammation. These findings clearly separate the roles of HIF-1α and VEGF in myeloid cell function and demonstrate that the loss of VEGF does not affect myeloid cell infiltration during inflammation.
Loss of HIF-1α Does Not Alter Cytokine Expression in Resident Myeloid Cells
Histological examination demonstrated that the numbers of resident myeloid cells were not different in HIF-1α null tissues examined (data not shown). To determine whether cell signaling and cytokine response was deficient in mutant resident cells, we performed an acute treatment with TPA, followed by isolation of tissue RNA at 4 hr posttreatment and thus prior to infiltration. In Figure 5G, RNase protection assay of cytokine profiles show that the acute resident cell responses to TPA are normal in both HIF-1α null and VHL null tissues. This data indicates that resident cell numbers as well as responses to inflammatory stimuli are intact in these mutants.
Cutaneous Inflammation Demonstrates Requirement of HIF-1α for Infiltration
Due to the dramatic effect of the loss of HIF-1α on the TPA-induced cutaneous inflammatory response, we focused on the lysMcre/HIF-1α model in two further in vivo assays of inflammation. In the first of these, we employed a relatively nonspecific, macrophage-driven model of skin inflammation and irritation. This model promotes disruption of barrier function via daily epicutaneous painting with a 5% SDS solution in PBS (Thepen et al., 2000). This treatment leads to an inflammatory response in less than 3 days in wild-type mice, marked by leukocyte invasion, vasodilation, epidermal hyperproliferation, and edema. As can be seen in Figure 6, loss of HIF-1α in the myeloid lineage results in an almost complete ablation of the inflammatory response in the skin. Grossly, this is seen as an absence of visible irritation of the skin posttreatment, whereas the wild-type mouse demonstrates superficial cutaneous inflammation and keratosis (Figures 6A and 6B). The corresponding histological phenotypes are depicted in Figures 6C and 6D: extensive edema, epidermal hyperproliferation, and inflammatory cell infiltration are evident in wild-type skin; all of these are absent in HIF-1α null mutants. In order to determine the level of inflammatory infiltration, we assayed tissue sections with an antibody against CD45 (Figures 6E and 6F); this demonstrated that there little or no accumulation of leukocytes at the dermal-epidermal border following SDS treatment in HIF-1α mutant animals.
Figure 6. Loss of HIF-1α in Myeloid Cells Impairs Chronic Cutaneous Inflammation.
The back skin of mice was freed of hair and 5% SDS solution applied epicutaneously once daily for a total of 10 days. Macroscopic appearance of skin after (A) 5 and (B) 10 days of treatment. (C and D) Histological analysis of skin after 5 days of SDS application, H&E, magnification 100×. (E and F) Immunolocalization of CD45 in mouse skin after 5 days of 5% SDS treatment. Magnification 200×.
Passively Induced Arthritis Requires HIF-1α Expression in Myeloid Cells
To determine whether the defect seen in this nonspecific inflammatory response was also found in a more specific and noncutaneous model of inflammation, we injected animals with a passive inducer of joint inflammation. This involves use of isolated serum from a strain-specific rheumatoid arthritis model (Matsumoto et al., 1999); serum is injected at day 1 and day 3 of treatment, and then joint inflammation is monitored over a period of 3 weeks. As can be seen in Figures 7A and 7B, loss of myeloid cell HIF-1α eliminates swelling of the ankle joints and the gross edema that is seen in wild-type mice. Histological examination of involved joints, seen in Figure 7C and 7D, reveals that loss of the factor reduces the synovialis infiltration, pannus formation, and subsequent cartilage destruction that are typical of this model. Figure 7E shows the significance of blind scoring of the joints for involvement. This assay shows that activation of HIF-1α in the myeloid lineage is also an important aspect of this model of inflammation and that it acts within the joint during the progression of this destructive inflammatory response.
Figure 7. Passively Induced Arthritis Is Dependent on Functional HIF-1α in Myeloid Cells.
Animals were injected twice with heterologous serum from KbxN-TCR transgenic mice and monitored over a period of 3 weeks. Macroscopic appearance of hindpaws from (A)WT and (B) HIF-1α null mice 21 days after the initiation of serum injection. Limbs were fixed in 4% PFA and paraffin embedded. Histological analysis of (C) WT and (D) HIF-1α null ankle joints 23 days after arthritis induction. H&E, magnification 100×. (E) Throughout the course of the experiment, mice were blind scored every second day for clinical signs of arthritis as outlined in Experimental Procedures. Statistical analysis was performed using the unpaired Student’s t test, **p < 0.01.
Discussion
Neutrophils and monocytes/macrophages are the key cellular components of the innate immune system, the body’s first line of defense against invading microorganisms (Medzhitov and Janeway, 2000). In order to maintain tissue integrity, it is of pivotal importance that these cells are able to exert their highly specialized functions in hostile areas like wounds or abscesses, where oxygen and nutrient levels are often extremely low. It is therefore reasonable to assume that cellular pathways controlling microenvironmental responses are especially important for myeloid cell biology.
The finding that leukocytes may be adapted to hypoxia and exhibit high degrees of lactate accumulation, even under aerobic conditions, was first made by Levene and Meyer in 1912 (1912b). One of the most important next steps in determining the role of energetics in inflammation was the demonstration that neutrophils produce their energy by glycolysis, under both aerobic and anaerobic conditions (Sbarra and Karnovsky, 1959). This established that, unlike almost all other cells and tissues, myeloid cells do not typically shift to mitochondrial respiration even in highly oxygenated environments. In fact, neutrophils have few mitochondria, and remain reliant on glycolysis for ATP production under virtually all conditions, utilizing stored glycogen for glycogenolysis when limited for glucose (Reiss and Roos, 1978, 1979). In human neutrophils, approximately 85% of glucose uptake is ultimately incorporated into lactate, even under resting aerobic conditions (Borregaard and Herlin, 1982). Neutrophils have evolved to utilize different glucose sources for different cellular functions, with motility, chemotaxis, and aggregation being fueled by extracellular glucose uptake (Weisdorf et al., 1982b). This reliance on the glycolytic pathway is thus a hallmark of the unique and extravascular mode of existence of these cells.
Classical studies with glycolytic inhibitors have also shown that modulating glycolysis inhibits chemotaxis, aggregation, and invasion by macrophages and neutrophils, whereas inhibitors of mitochondrial respiration have little or no effect on these processes (Kay et al., 1980; O’Flaherty et al., 1977). These studies clearly delineate the functional role of glycolytic ATP production in myeloid cell types, since they show how inhibition of glycolysis and dimunition of ATP in these cells does not result in a loss of viability but does prevent normal responses to chemotactic agents and inflammatory stimuli.
We show here that the transcription factor HIF-1α is essential for myeloid cell function in vitro and inflammatory responses in vivo. Most strikingly, the loss of inflammatory capacity is correlated with defects in metabolic activation and occurs without a change in the normal development and differentiation of the myeloid lineage. Thus, HIF-1α is functioning as a basal regulator of energy metabolism in these cells; given the role of HIF-1 in regulating glycolytic enzyme expression, our data also demonstrates a central role of glycolytic energy production for myeloid cell function and, ultimately, inflammation.
Interestingly, phenotypes resulting from the loss of HIF-1α and its target VEGF are not overlapping, indicating that the loss of inflammatory capacity in HIF-1α null cells is clearly separable from the function of VEGF. Although the role of granulocyte and macrophage-derived VEGF in inflammation needs to be more thoroughly explored, it is clear from the work described here that the loss of HIF-1α does not alter inflammatory progression solely because of alterations in VEGF expression. In fact, the chief difference between the phenotypes of HIF-1α and VEGF deletion in the myeloid lineage is in the differential degree of infiltration and invasion. Infiltration accounts for the majority of cells entering inflamed tissue, and the loss of VEGF does not alter this process or in any discernable way inhibit it in our acute model of cutaneous inflammation. The loss of VEGF most clearly affects tissue edema; this is likely due to the well-characterized function of VEGF as a permeability factor during inflammation (Dvorak, 2000).
Epidermal overexpression of HIF-1α results in increased expression of VEGF and increased angiogenesis in a transgenic model (Elson et al., 2001). We have approached the overexpression of HIF-1α in an alternative fashion here and show that loss of vhl in myeloid cells results in a hyperinflammatory response in one model of acute inflammation and is also coupled to increased lactate production and tissue edema. Although the targets of VHL-mediated ubiquitination and degradation include HIF-1α, they also include a number of other substrates (Kondo and Kaelin, 2001), and further analysis will be needed to determine the factor(s) contributing to the hyperinflammatory phenotype in these mutants.
In summary, we have found that HIF-1α critically regulates pathways essential for the maintenance of energy homeostasis in myeloid cell types. Functional inactivation of HIF-1α causes greatly inhibited motility, invasiveness, and homotypic adhesion in isolated peritoneal macrophages. Furthermore, various in vivo assays of acute and chronic inflammation demonstrate a profound reliance on HIF-1α function for infiltration, edema formation, and tissue destruction caused by granulocytes and macrophages. Deletion of the HIF-1α regulator VHL causes a concomitant hyperinflammatory response; but loss of the HIF-1α target VEGF eliminates tissue edema and, thereby, shows that the phenotype resulting from the loss of HIF-1α is not solely due to decreased VEGF expression. These data demonstrate the importance of HIF-1α for myeloid cell function and the orchestration of inflammation and, further, show the opportunities for intercession in metabolic and angiogenic pathways regulated by this transcription factor.
Experimental Procedures
Harvesting of Cells
Mice were killed by cervical dislocation, and the abdominal skin was treated twice with 70% ethanol. Resident peritoneal macrophages were isolated by injecting 10 ml sterile PBS (Ca2+ and Mg2+ free) into the peritoneal cavity followed by gently massaging the abdomen. Abdominal skin was cut and the resulting peritoneal fluid collected. Cells were centrifuged at 400 × g for 10 min in the cold, washed once with PBS, dissolved in sterile water for 15 s to lyse any contaminating erythrocytes, and finally, the cell pellet was taken up in RPMI (10% fetal calf serum, 1% penicillin-streptomycin, and 1% glutamine). Cells were plated and allowed to adhere for 2 to 4 hr. Nonadherent cells were washed off with PBS, and new culture medium was added. Thioglycollate-elicited macrophages were harvested in an identical manner 3 days after the peritoneal injection of 2.5 ml 3% thioglycollate broth (Difco). PMN were isolated from the peritoneal cavity 3 hr after instillation of thioglycollate as previously described (Clausen et al., 1999). Isolated cells displayed a viability of >98% as demonstrated by trypan blue exclusion.
Determination of Deletion Frequency by Quantitative PCR
Thioglycollate-elicited macrophages were used for isolation of genomic DNA. 24 hr after harvesting, cells were scraped into 1 ml of PBS, centrifuged at 400 × g for 5 min, and the resulting pellet was digested in 10 mM Tris-Hcl (pH 7.5), 100 mM NaCl, 10 mM EDTA, and 0.5% SDS with 0.4 mg/ml proteinase K (Roche) overnight at 65°C. DNA was extracted with phenol:chloroform:isoamylaclohol (25:24:1 [pH 8.2]) and precipitated with three volumes of ethanol and one/two volumes of 6M ammonium acetate. The resulting pellet was resuspended in 10 mM Tris, 1mM EDTA (pH 8.0). Primer Express software (Applied Biosystems) was used to design forward, reverse, and fluorescein dye-tagged oligonucleotides (Operon) for use in real-time PCR. The following primers and probes were used: HIF-permeability 1α forward 5′-CTATGGAGGCCAGAAGAGGGTAT-3′, HIF-1α reverse 5′-CCCACATCAGGTGGCTCATAA-3′, HIF-1α probe 5′-(6FAM)AGATCCCTTGAAGCTAG(BHQ~6FAM)-3′. The degree of excision was calculated by comparison of HIF-1α intact DNA relative to an unexcised gene.
Real-Time PCR
For real-time PCR analyses, cDNAs were diluted to a final concentration of 10 ng/µl. For PCR reactions, TaqMan-Universal Mastermix (Applied Biosytems) was used. 50 ng cDNA was used as template to determine the relative amount of mRNA by real-time PCR (ABI Prism 7700 sequence detection system), using specific primers and probes with the following sequences: VEGF reverse 5′-ATCCGCATGATCTGCATGG-3′, VEGF forward 5′-AGTCCCATGAAGTGATCAAGTTCA-3′, VEGF probe (6FAM)TGCCCACGTCAGAGAGCAACATCAC(BHQ~6FAM)-3′, PGK reverse 5′-CAGGACCATTCCAAACAATCTG-3′, PGK forward 5′-CTGTGGTACTGAGAGCAGCAAGA-3′, PGK probe 5′-(6FAM)TAGCTCGACCCACAGCCTCGGCATAT(BHQ-~6FAM)-3′, Glut-1 reverse 5′-ACGAGGAGCACCGTGAAGAT-3′, Glut-1 forward 5′-GGGCATGTGCTTCCAGTATGT-3′, Glut-1 probe (6FAM)CAACTGTGCGGCCCCTACGTCTTC(BHQ~6FAM).
In Vivo Model of Chronic Cutaneous Irritation
Mice were anaesthetized with 1.5% avertin, the backs were shaved with surgical clippers, and the remaining hair was removed by topical application of Nair for 3 min. After this procedure, mice were allowed to rest for 3 days. Then, 5% SDS/PBS (v/v) was administered epicutaneously once per day for the indicated amount of total days, maximally 10.
Bacterial Uptake and Killing Assays
For flourescence visualization, Group B Streptococcus (GBS) was transformed with the GFP-expressing plasmid pSB027 (gift of S. Beres). Bacteria were grown to logarithmic phase in Todd-Hewitt broth media (O.D. 600 = 0.4 or ~10e8 cfu/ml), pelleted and washed in PBS, then resuspended and diluted in RPMI + 0.1% BSA to the desired concentration. Bacteria were added to monolayers of normal and HIF-1α null macrophages at an inoculum of 2.5 bacteria per cell, the culture plates were centrifuged (500 × g for 10 min) to place bacteria on the monolayer surface, then incubated for 2 hr at 37°C. To assess uptake of GFP-tagged GBS, the monolayers were next washed three times with PBS and incubated with 0.04% Trypan blue × 10 min at 37°C to quench fluorescence of surface-associated bacteria and verify macrophage viability. Standard FACS analysis for GFP and deconvolution microscopy was used to quantify uptake. Intracellular killing of bacteria was assessed using an antibiotic protection assay wherein monolayers were washed as above, extracellular and surface-associated organisms killed by treatment with penicillin and gentamicin, and intracellular GBS colony-forming units enumerated following liberation and lysis of macrophage monolayers using trypsin-EDTA and 0.025% Triton X-100 (Nizet et al., 1997).
TPA-Induced Ear Inflammation
TPA (2.5 µg in acetone, 20 µl total volume/site) was topically applied to the left outside ear of anaesthetized mice. The right ear was painted the same way with acetone alone as a carrier control. After the indicated time points, tissue was harvested for further analysis.
Immunohistochemistry
Routine sections (5 µm) were cut, stained with H&E, and analyzed. Immunohistochemistry was performed with an antibody specific for murine CD45 (anti-leukocyte common antigen [LCA], Pharmingen, San Diego, CA) as described in detail elsewhere (Grunstein et al., 1999).
Quantification of VEGF in Conditioned Medium
Secreted VEGF was determined by using the DuoSet ELISA development kit for mouse VEGF (R&D Systems, Minneapolis, MN). ELISA analysis was performed according to the manufacturer’s instructions.
Measurement of Lactate
Conditioned medium from triplicate macrophage cell cultures was harvested and assayed for lactate content by colorimetric detection using the Lactate kit (Sigma, St. Louis, MO) according to the manufacturer’s instructions. Values were calculated with the help of a lactate standard curve and normalized to cell number.
Measurement of Intracellular ATP
Cells were harvested and cellular ATP content was determined using the CLSII Assay Kit (Boehringer-Mannheim, Mannheim, Germany) as described in detail previously (Seagroves et al., 2001).
In Vitro Migration and Invasion Assays
Migration was tested in a modified Boyden chamber assay using cell culture inserts with a polycarbonate-filter (PVP-free, pore size 8 µM, Corning Incorporated, Corning, NY). Analysis of invasive properties was achieved by using cell culture inserts covered with growth factor-reduced matrigel (Becton Dickinson, Bedford, MA). For both assays, 250 µl of cell suspension (2.5 × 105 cells) was added to the upper wells. The lower compartment was filled with RPMI supplemented with either 5% FCS or 0.1% BSA. Chambers were incubated for 24 hr at 37°C in a 5% CO2 atmosphere. Cells on the lower side of the filter were quantitated by dissolving the cell bound crystal violet in 10% acetic acid for 5 min and subsequent spectrophotometric analysis at 450 nm. Data was expressed as the fold increase compared to random migration.
Induction of Arthritis
The K/BxN T cell receptor (TCR) transgenic mice were generated by crossing the KRN-TCR transgenic strain on the C57Bl/6 background with the NOD strain (Kouskoff et al., 1996). Sera from K/BxN TCR transgenic mice (40 to 60 days of age) were pooled for induction of arthritis.
Passive arthritis was induced in mice by transfer of the serum from K/BxN TCR transgenic mice as previously described (Korganow et al., 1999). Thirteen-week-old mice (wild-type mice< n = 8 and conditional HIF-1α null mice, n = 8) were administered 100 µl serum from K/BxN TCR transgenic mice intraperitoneally on day 0. On day 3, serum injection was repeated. Arthritis scores were assessed using a semiquantitative clinical scoring system. Five-micron sections were cut, mounted on glass slide, and stained with hematoxylin and eosin (H&E).
RNase Protection Assay (RPA)
Mouse ears treated with TPA or acetone as the carrier control were ground to a fine powder in liquid nitrogen and subsequently homogenized in TRIzol reagent for 30 s at full speed. Total RNA was isolated and hybridized with mouse cytokine (mCK-2b) and chemokine (mCK-5b) RNA probes using a Riboquant Multiprobe RPA System (Pharmingen, San Diego, CA), following the manufacturer’s instructions.
Acknowledgments
This work was sponsored by grants from the Deutsche Forschungs-gemeinschaft to T.C. (Cr 133/1-1) and from the National Institutes of Health (CA82515) to R.S.J. We would like to thank Gabriele Bergers, Zena Werb, Michael Karin, Wayne McNulty, Tiffany Seagroves, Nan Tang, Sang-Ki Park, Dominique Sawka, Mercedes Ricote, Annabel Valledor, and members of the Johnson laboratory for helpful suggestions and assistance, and Kit Pogliano and Joseph Pogliano for their timely aid with deconvolution microscopy.
References
- Arnold F, West D, Kumar S. Wound healing: the effect of macrophage and tumour derived angiogenesis factors on skin graft vascularization. Br. J. Exp. Pathol. 1987;68:569–574. [PMC free article] [PubMed] [Google Scholar]
- Babior BM. Phagocytes and oxidative stress. Am. J. Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- Bakker A. Einige Übereinstimmungen im Stoffwechsel der Carcinomzellen und Exsudatleukocyten. Klin. Wochenschr. 1927;6:252. [Google Scholar]
- Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J. Clin. Invest. 1982;70:550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer LA, Baehner RL, Davis J. The effect of 2-deoxyglucose on guinea pig polymorphonuclear leukocyte phagocytosis. J. Cell. Physiol. 1977;91:89–102. doi: 10.1002/jcp.1040910110. [DOI] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neuastrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Burke B, Tang N, Corke KP, Tazzyman D, Ameri K, Wells M, Lewis CE. Expression of HIF-1α by human macrophages: implications for the use of macrophages in hypoxia-regulated cancer gene therapy. J. Pathol. 2002;196:204–212. doi: 10.1002/path.1029. [DOI] [PubMed] [Google Scholar]
- Buttgereit F, Burmester GR, Brand MD. Bioenergetics of immune functions: fundamental and therapeutic aspects. Immunol. Today. 2000;21:192–199. doi: 10.1016/s0167-5699(00)01593-0. [DOI] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Denko NC, Giaccia AJ. Tumor hypoxia, the physiological link between Trousseau’s syndrome (carcinoma-induced coagulopathy) and metastasis. Cancer Res. 2001;61:795–798. [PubMed] [Google Scholar]
- Dvorak HF. VPF/VEGF and the angiogenic response. Semin. Perinatol. 2000;24:75–78. doi: 10.1016/s0146-0005(00)80061-0. [DOI] [PubMed] [Google Scholar]
- Elson DA, Thurston G, Huang LE, Ginzinger DG, McDonald DM, Johnson RS, Arbeit JM. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1α. Genes Dev. 2001;15:2520–2532. doi: 10.1101/gad.914801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann W, Kubowitz F. Über den Stoffwechsel der Leukocyten. Biochem. Z. 1927;181:395. [Google Scholar]
- Grunstein J, Roberts WG, Mathieu-Costello O, Hanahan D, Johnson RS. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res. 1999;59:1592–1598. [PubMed] [Google Scholar]
- Haji-Michael PG, Ladriere L, Sener A, Vincent JL, Malaisse WJ. Leukocyte glycolysis and lactate output in animal sepsis and ex vivo human blood. Metabolism. 1999;48:779–785. doi: 10.1016/s0026-0495(99)90179-8. [DOI] [PubMed] [Google Scholar]
- Hempel SL, Monick MM, Hunninghake GW. Effect of hypoxia on release of IL-1 and TNF by human alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 1996;14:170–176. doi: 10.1165/ajrcmb.14.2.8630267. [DOI] [PubMed] [Google Scholar]
- Hockel M, Vaupel P. Biological consequences of tumor hypoxia. Semin. Oncol. 2001;28:36–41. [PubMed] [Google Scholar]
- Hollander AP, Corke KP, Freemont AJ, Lewis CE. Expression of hypoxia-inducible factor 1α by macrophages in the rheumatoid synovium: implications for targeting of therapeutic genes to the inflamed joint. Arthritis Rheum. 2001;44:1540–1544. doi: 10.1002/1529-0131(200107)44:7<1540::AID-ART277>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Kalbhen DA, Koch HJ, Domenjoz R. The energy situation in the course of acute experimental inflammation. Med. Pharmacol. Exp. Int. J. Exp. Med. 1967;16:425–431. [PubMed] [Google Scholar]
- Kawaguchi T, Veech RL, Uyeda K. Regulation of energy metabolism in macrophages during hypoxia. Roles of fructose 2,6-bisphosphate and ribose 1,5-bisphosphate. J. Biol. Chem. 2001;276:28554–28561. doi: 10.1074/jbc.M101396200. [DOI] [PubMed] [Google Scholar]
- Kay NE, Bumol TF, Douglas SD. Effects of 2-deoxy-D-glucose on human monocyte metabolism and function. J. Reticu-Canloendothel. Soc. 1980;28:367–379. [PubMed] [Google Scholar]
- Kellett DN. 2-Deoxyglucose and inflammation. J. Pharm. Pharmacol. 1966;18:199–200. doi: 10.1111/j.2042-7158.1966.tb07853.x. [DOI] [PubMed] [Google Scholar]
- Kempner W. The nature of leukemic blood cells as deter- mined by their metabolism. J. Clin. Invest. 1939;18:291–300. doi: 10.1172/JCI101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittlick PD. Inflammation, glycolyticmetabolism, and glyco-besaminoglycans. Exp. Pathol. 1986;30:1–19. doi: 10.1016/s0232-1513(86)80051-2. [DOI] [PubMed] [Google Scholar]
- Kondo K, Kaelin WG., Jr The von Hippel-Lindau tumor suppressor gene. Exp. Cell Res. 2001;264:117–125. doi: 10.1006/excr.2000.5139. [DOI] [PubMed] [Google Scholar]
- Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- Korhonen K. Hyperbaric oxygen therapy in acute necrotizing infections. With a special reference to the effects on tissue gas tensions. Ann. Chir. Gynaecol. 2000;89(Suppl 214):7–36. [PubMed] [Google Scholar]
- Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- Krauss S, Brand MD, Buttgereit F. Signaling takes a breath–new quantitative perspectives on bioenergetics and signal transduction. Immunity. 2001;15:497–502. doi: 10.1016/s1074-7613(01)00205-9. [DOI] [PubMed] [Google Scholar]
- Levene PA, Meyer GM. The action of leucocytes on glucose. J. Biol. Chem. 1912a;11:361–370. [Google Scholar]
- Levene PA, Meyer GM. On the action of leucocytes on glucose, second communication. J. Biol. Chem. 1912b;12:265–273. [Google Scholar]
- Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: relevance to disease mechanisms. J. Leukoc. Biol. 1999;66:889–900. doi: 10.1002/jlb.66.6.889. [DOI] [PubMed] [Google Scholar]
- Manjunath N, Johnson RS, Staunton DE, Pasqualini R, Ardman B. Targeted disruption of CD43 gene enhances T lymphocyte adhesion. J. Immunol. 1993;151:1528–1534. [PubMed] [Google Scholar]
- Manns G. Determination of glycolysis metabolites in inflamed and non-inflamed paw tissue of rats. Med. Pharmacol. Exp. Int. J. Exp. Med. 1967;17:557–575. [PubMed] [Google Scholar]
- Mapp PI, Grootveld MC, Blake DR. Hypoxia, oxidative stress and rheumatoid arthritis. Br. Med. Bull. 1995;51:419–436. doi: 10.1093/oxfordjournals.bmb.a072970. [DOI] [PubMed] [Google Scholar]
- Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C., Jr Innate immunity. N. Engl. J. Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- Michl J, Ohlbaum DJ, Silverstein SC. J. Exp. Med. 1976;144:1484–1493. doi: 10.1084/jem.144.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafipour H, Ferrell WR. Comparison of synovial PO2 and sympathetic vasoconstrictor responses in normal and acutely inflamed rabbit knee joints. Exp. Physiol. 1995;80:209–220. doi: 10.1113/expphysiol.1995.sp003841. [DOI] [PubMed] [Google Scholar]
- Nakadate T, Yamamoto S, Aizu E, Kato R. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced increase in vascular permeability in mouse skin by lipoxygenase inhibitors. Jpn. J. Pharmacol. 1985;38:161–168. doi: 10.1254/jjp.38.161. [DOI] [PubMed] [Google Scholar]
- Nizet V, Kim KS, Stins M, Jonas M, Chi EY, Nguyen D, Rubens CE. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 1997;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Flaherty JT, Kreutzer DL, Showell HJ, Ward PA. Influence of inhibitors of cellular function on chemotactic factor induced neutrophil aggregation. J. Immunol. 1977;119:1751–1756. [PubMed] [Google Scholar]
- Ott A. Inflammation and transcutaneous measurement of oxygen pressure in dermatology. Adv. Exp. Med. Biol. 1987;220:79–82. doi: 10.1007/978-1-4613-1927-6_14. [DOI] [PubMed] [Google Scholar]
- Raick AN. Ultrastructural, histological, and biochemical alterations produced by 12-O-tetradecanoyl-phorbol-13-acetate on mouse epidermis and their relevance to skin tumor promotion. Cancer Res. 1973;33:269–286. [PubMed] [Google Scholar]
- Reiss M, Roos D. Differences in oxygen metabolism of phagocytosing monocytes and neutrophils. J. Clin. Invest. 1978;61:480–488. doi: 10.1172/JCI108959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D, Reiss M, Balm AJ, Palache AM, Cambier PH, Van Der Stijl-Neijenhuis JS. A metabolic comparison between human blood monocytes and neutrophils. Adv. Exp. Med. Biol. 1979;121:29–36. doi: 10.1007/978-1-4684-3593-1_3. [DOI] [PubMed] [Google Scholar]
- Ryan HE, Lo J, Johnson RS. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadi S, Wrenshall LE, Platt JL. Regional manifestations and control of the immune system. FASEB J. 2002;16:849–856. doi: 10.1096/fj.01-0690hyp. [DOI] [PubMed] [Google Scholar]
- Sawyer RG, Spengler MD, Adams RB, Pruett TL. The peritoneal environment during infection. The effect of monomicrobial and polymicrobial bacteria on pO2 and pH. Ann. Surg. 1991;213:253–260. doi: 10.1097/00000658-199103000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbarra A, Karnovsky M. The biochemical basis of phagocytosis. I. Metabolic changes during ingestion of particles by polymorphonuclear leukocytes. J. Biol. Chem. 1959;234:1355–1362. [PubMed] [Google Scholar]
- Schor H, Vaday GG, Lider O. Modulation of leukocyte behavior by an inflamed extracellular matrix. Dev. Immunol. 2000;7:227–238. doi: 10.1155/2000/51902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves TN, Ryan HE, Lu H, Wouters BG, Knapp M, Thibault P, Laderoute K, Johnson RS. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol. Cell. Biol. 2001;21:3436–3444. doi: 10.1128/MCB.21.10.3436-3444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001a;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: control of oxygen homeostasis in health and disease. Pediatr. Res. 2001b;49:614–617. doi: 10.1203/00006450-200105000-00002. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol. Med. 2001c;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- Silver IA. Measurement of pH and ionic composition of pericellular sites. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1975;271:261–272. doi: 10.1098/rstb.1975.0050. [DOI] [PubMed] [Google Scholar]
- Simchowitz L, Mehta J, Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: effect of metabolic inhibitors and antiinflammatory drugs. Arthritis Rheum. 1979;22:755–763. doi: 10.1002/art.1780220711. [DOI] [PubMed] [Google Scholar]
- Simmen HP, Battaglia H, Giovanoli P, Blaser J. Analysis of pH, pO2 and pCO2 in drainage fluid allows for rapid detection of infectious complications during the follow-up period after abdominal surgery. Infection. 1994;22:386–389. doi: 10.1007/BF01715494. [DOI] [PubMed] [Google Scholar]
- Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol. 2000;157:411–421. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thepen T, van Vuuren AJ, Kiekens RC, Damen CA, Vooijs WC, van De Winkel JG. Resolution of cutaneous inflammation after local elimination of macrophages. Nat. Biotech. 2000;18:48–51. doi: 10.1038/71908. [DOI] [PubMed] [Google Scholar]
- Turner L, Scotton C, Negus R, Balkwill F. Hypoxia inhibits macrophage migration. Eur. J. Immunol. 1999;29:2280–2287. doi: 10.1002/(SICI)1521-4141(199907)29:07<2280::AID-IMMU2280>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- VanOtteren GM, Standiford TJ, Kunkel SL, Danforth JM, Strieter RM. Alterations of ambient oxygen tension modulate the expression of tumor necrosis factor and macrophage inflammatory protein-1 α from murine alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 1995;13:399–409. doi: 10.1165/ajrcmb.13.4.7546769. [DOI] [PubMed] [Google Scholar]
- Weisdorf DJ, Craddock PR, Jacob HS. Glycogenolysis versus glucose transport in human granulocytes: differential activation in phagocytosis and chemotaxis. Blood. 1982a;60:888–893. [PubMed] [Google Scholar]
- Weisdorf DJ, Craddock PR, Jacob HS. Granulocytes utilize different energy sources for movement and phagocytosis. Inflammation. 1982b;6:245–256. doi: 10.1007/BF00916406. [DOI] [PubMed] [Google Scholar]