Abstract
New routes to 2, 4, 5-trisubstituted oxazoles were established whereby the substitution pattern was established by the structure of the starting nonsymmetrical acyloins. 2-Chloromethyl-4, 5-disubstituted oxazoles were prepared by refinements of an earlier described process whereby chloroacetyl esters of symmetrical and non-symmetrical acyloins were cyclized using an ammonium acetate/acetic acid protocol. After substitution is effected, the azide moiety is then installed by substitution under mild conditions. While dibrominated and iodinated phenyloxazoles are required for further synthetic elaboration, the cyclization reaction was found to be very sensitive to the relative positions of the halogens in the starting materials.
Keywords: Oxazoles, Azides, Click Chemistry, Peptidomimetics, Biofilms
1. Introduction
The oxazole structural motif plays a central role in medicinal and natural products chemistry.1 Apart from the major classical name reactions and contemporary strategies used in preparing the oxazole nucleus, there remains unique opportunities for elaborating this central framework to provide a plethora of synthetic targets.2 Moreover, in the area of peptidomimetics, oxazole-based scaffolds, backbones and amino acid analogues continue to provide a diverse array of compounds for pharmacological evaluation in numerous therapeutic areas.3 We are presently conducting studies entailing the inhibition of dental biofilm formation using both the trisubstituted oxazole scaffold and the techniques of click chemistry. Porphyromonas gingivalis is a major pathogen associated with periodontal disease and this organism colonizes the dental biofilm by interacting with oral streptococci. Demuth and Sissons previously showed that this interaction is inhibited by a peptide comprised of two distinct structural motifs which block the interaction of minor fimbrial antigen (Mfa) of P. gingivalis with the antigen I/II (AgI/II) of oral streptococci.4 Consequently, the design and study of small-molecule, non-peptide based inhibitors of the Mfa/AgI/II interaction can involve the employment of two heterocyclic scaffolds, one being a substituted oxazole, which may be joined together via a “click” reaction. In a prior communication5 we detailed a route to the inhibitory 4, 5-diaryl-2-azidoaryl- and 4, 5-diaryl-2-azidoalkyl oxazole scaffolds, and we now describe extensions of our methodology which enables access to a great many substituted oxazoles having diverse functionality and extended heterocyclic frameworks. Our initial route entailed the cyclization of azidoalkyl- or azidoaryl esters of benzoin with ammonium acetate in acetic acid (115°C/3h) thereby affording the corresponding 2-azidoaryl- or 2-azidoalkyl-4, 5-diphenyloxazoles. We also discovered that cyclizations of benzoin esters could be accomplished by treatment with thiourea (DMF/150°C); however, these conditions were not compatible with either alkyl or aryl azides and provided complex mixtures. Essentially, the intermediate azido esters were pre-formed before the final cyclization to the target oxazole and the basic scheme relied on both the preparation of the starting haloalkyl esters and substitution of halide with azide. We now report an extension of the cyclization methodology to 2-(azidophenyl)-4-phenyl-5-alkyloxazoles 1a-i along with the corresponding 2-azido-methyl-4-phenyl-5-alkyloxazoles 2a-c. The use of the symmetrical diaryl acyloin as a starting material was detailed in our previous communication and the use of which established the basic strategy and provided the 2-alkyl- or 2-aryl-4, 5-diphenyl substitution in the target heterocycle.5 The scheme toward the 2-azidophenyl- and 2-azidomethyl-4-phenyl-5-alkyl-substituted oxazoles detailed herein required a somewhat different strategy and required the preparation and employment of non-symmetrical acyloins 5a-c as starting materials. Aromatic- aliphatic ketones were employed in the preparation of non-symmetrical acyloins through a two-step procedure involving oxidation-tosylation (Scheme 1). Hence, α-tosylation of ketones 3a-c (oxone/4-iodotoluene/p-toluenesulfonic acid/acetonitrile/ 16h, 60°C) provided the tosylates 4a-c.6,7 Base-mediated hydrolysis (LiOH/H2O/DMF/16h, 5°C to rt) of the intermediate tosylates 4ac afforded the nonsymmetrical acyloins 5a-c.7,8 The acyloins 5ac were then acylated with the appropriate acid chloride to afford the intermediate acyclic esters 6a-i.8 The acyclic esters 6a-i were then cyclized with ammonium acetate in acetic acid (3h/115°C) to provide the trisubstituted oxazoles 1a-i.8
Scheme 1.
Synthesis of 2-azidophenyl-4-phenyl-5-alkyloxazoles (n=2, 5, 7) from non-symmetrical acyloins:
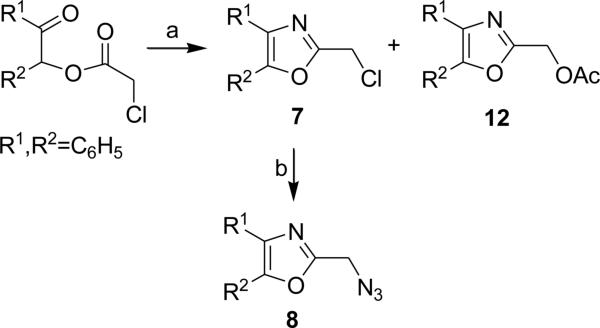
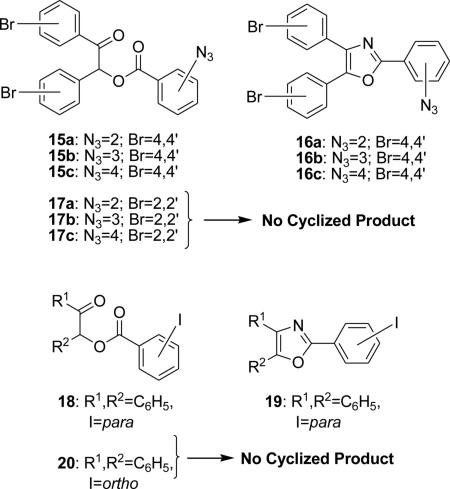
During earlier studies of the synthesis of the 2-azidomethyl-4, 5-diphenyloxazole 8 we discovered that cyclization of the chloroacetyl ester of benzoin (NH4OAc/HOAc/115°C, 3h) gave 2-chloromethyl-4, 5-diphenyl-oxazole 7, albeit in modest yield. Substitution of 7 with azide ion (NaN3/DMF/rt, 3h) gave the azidomethyloxazole 8. Thus, nucleophilic substitution of 7 with azide ion under mild conditions was a viable alternative to cyclization of the corresponding azidoacetyl ester as reported earlier (Scheme 2). The chloroacetyl esters of the nonsymmetrical acyloins 9a, 9b and 9c were cyclized in similar fashion thereby providing the corresponding 4-phenyl-5-alkyl-2-(chloromethyl) oxazoles 10a-c (Scheme 3).8 Conversion to the corresponding 2-azidomethyl compounds 2a-c was accomplished by treating 10a-c with sodium azide in DMF (rt).8 For comparison, the conversion of chloroesters 9a-c to the corresponding azidoesters 11a-c (NaN3/DMF) according to our previous route was conducted smoothly.8 The azidoesters 11a-c were then cyclized to the azidomethyloxazoles 2a-c (NH4OAc/HOAc).8 When an excess of ammonium acetate/acetic acid was used during the cyclization of the chloroacetyl ester of benzoin (Scheme 2), solvolysis to the corresponding 2-acetoxymethyl oxazole 12 was a competing reaction (7/33%, 12/16%). Decreasing the concentration of ammonium acetate in acetic acid while carefully monitoring the reaction progress increased the yield of the chloromethyloxazole 7 to 62% and decreased the formation of ester 12 to 5%.8 Interestingly, the product acetoxy ester 12 could be hydrolyzed (K2CO3/EtOH/reflux, 2h 97%) which provided the hydroxymethyl oxazole 13 in excellent yield.8 The construction of extended oxazole scaffolds might utilize the aldehyde 14 as a viable intermediate for Wittig or organometallic reaction, and to this end, 13 was oxidized to 14 with the Dess-Martin periodinane (CHCl3/reflux, 2h, 97%).8 The cyclization of the tricyclic esters to provide the triaryloxazoles using ammonium acetate in acetic acid is not without limitations and certain substitution patterns on either the benzoin portion or the benzoyl ester portion may nullify any yield of desired product. For example, the 2-azido-, 3-azido- or 4-azidobenzoyl ester of 4, 4’-dibromobenzoin 15a-c cyclized under normal conditions (NH4OAc/HOAc, 115°C) to afford the corresponding 2-azidopheny-(4,4’-dibromodiphenyl) oxazoles 16a-c, but the 2-azido, 3-azido or 4-azidobenzoyl esters of 2,2’-dibromobenzoin 17a-c do not cyclize to give the expected azidophenyl-2,2’-(dibromodiphenyl) oxazoles. Another example of steric influence resides in a comparison of the cyclization reactions of the 4-iodobenzoyl ester 18 and 2-iodobenzoyl ester 20 of benzoin. Treatment of the 4-iodobenzoyl ester 18 under normal cyclization conditions affords a clean yield (95%) of the 4-iodophenyloxazole 19, while under the same conditions, the ortho-iodobenzoyl ester 20 gave none of the expected oxazole.
Scheme 2.
Cyclization of benzoin chloroacetate
Scheme 3.
Synthesis of 2-azidomethyl-4-phenyl-5-alkyloxazoles (n=2,5,7):

In summary, we have detailed a synthetic route to 2, 4, 5-trisubstituted oxazoles which are differentially substituted at the 4- and 5- positions with phenyl groups and alkyl groups respectively. The route utilizes non-symmetrical acyloins as starting materials and has the advantage of not involving installation of an appendage on a pre-formed oxazole. In contrast to cyclizing azidoalkyl esters to azidoalkyloxazoles, our newly-developed strategy utilizes direct cyclization of haloalkyl esters to haloalkyloxazoles so that they may be either transformed to azides or further elaborated using other nucleophiles. We found the chloromethyloxazoles to be a valuable intermediate for the preparation of many more oxazole-based scaffolds and these studies will be reported in due course.
Supplementary Material
Acknowledgments
The measurement of high resolution mass spectra by the Texas A&M University Laboratory for Biological Mass Spectrometry and Dr. Vanessa Santiago are acknowledged. Financial support from the NIH/NIDCR through grant 1RO1DE023206 (DRD and FAL) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Information
The 1H, 13C, FTIR and HRMS data for 6a-i, 1a-i, 9a-c, 10a-c, 11a-c, 2a-c, 16a-c, 7, 12, 18 and 19 and experimental procedures for compounds 4a-c, 5a-c, 6a-i, 1a-i, 9a-c, 10a-c, 2a-c, 11a-c, 7, 12, 13, 14 and 16a-c can be found in the online version at http://dxdoi.org/
References and notes
- 1.Turchi IJ, Dewar MJS. Chem. Rev. 1975;75:389–437. [Google Scholar]; Cicchi S, Cordero FM, Giomi D. In: In Progress in Heterocyclic Chemistry. Gribble GG, Joule JA, editors. Vol. 24. Elsevier; Oxford: 2012. pp. 328–329. See for example. [Google Scholar]
- 2.a Senadi GC, Hu W-P, Hsiao J-S, Vandavasi JK, Chen C-Y, Wang J-J. Org. Lett. 2012;14:4478–4481. doi: 10.1021/ol301980g. [DOI] [PubMed] [Google Scholar]; b Zheng X, Li X, Ren C, Zhang-Negrerie D, Du Y, Zhao K. J. Org. Chem. 2012;77:10353–10361. doi: 10.1021/jo302073e. [DOI] [PubMed] [Google Scholar]; c Liu X, Cheng R, Zhao F, Zhang-Negrerie D, Du Y, Zhao K. Org. Lett. 2012;14:5480–5483. doi: 10.1021/ol3025583. [DOI] [PubMed] [Google Scholar]; d Hashmi ASK, Jaimes MCB, Schuster AM, Rominger F. J. Org. Chem. 2012;77:6394–6408. doi: 10.1021/jo301288w. [DOI] [PubMed] [Google Scholar]; e Xie H, Yuan D, Ding M-W. J. Org. Chem. 2012;77:2954–2958. doi: 10.1021/jo202588j. [DOI] [PubMed] [Google Scholar]; f Acjermann L, Kornhaass C, Zhu Y. Org. Lett. 2012;14:1824–1826. doi: 10.1021/ol300514d. [DOI] [PubMed] [Google Scholar]; g He W, Li C, Zhang L. J. Am. Chem. Soc. 2011;133:8482–8485. doi: 10.1021/ja2029188. [DOI] [PubMed] [Google Scholar]; h Strotman NA, Chobainian HR, Guo Y, He J, Wilson JH. Org. Lett. 2010;12:3578–3581. doi: 10.1021/ol1011778. [DOI] [PubMed] [Google Scholar]; i Jiang H, Huang H, Cao H, Qi C. Org. Lett. 2010;12:5561–5563. doi: 10.1021/ol1023085. [DOI] [PubMed] [Google Scholar]; j Wan C, Gao L, Wang Q, Zhang J, Wang Z. Org. Lett. 2010;12:3902–3905. doi: 10.1021/ol101596s. [DOI] [PubMed] [Google Scholar]; k Wu B, Wen J, Zhang J, Li J, Xiang Y-Z, Yu X-Q. Synlett. 2009:500–504. [Google Scholar]; l Besselievre F, Lebrequier S, Mahuteau-Betzer F, Riguel S. Synthesis. 2009:3511–3512. [Google Scholar]; m Yazmin N, Ray JK. Synlett. 2009:2825–2827. [Google Scholar]; n Pan Y-M, Zheng F-J, Lin H-X, Zhan Z-P. J. Org. Chem. 2009;74:3148–3151. doi: 10.1021/jo8027533. [DOI] [PubMed] [Google Scholar]; o Sanz-Cervera JF, Blasco R, Piera J, Cynamon M, Ibanez I, Murguia M, Fustero S. J. Org. Chem. 2009;74:8988–8996. doi: 10.1021/jo9016265. [DOI] [PubMed] [Google Scholar]; p Merkul E, Grotkop O, Muller T. J. Synthesis. 2009:502–507. [Google Scholar]; q Verrier C, Martin T, Hoarau C, Marsais F. J. Org. Chem. 2008;73:7383–7386. doi: 10.1021/jo801093n. [DOI] [PubMed] [Google Scholar]; r Martin R, Cuenca A, Buchwald S. Org. Lett. 2007;9:5521–5524. doi: 10.1021/ol7024718. [DOI] [PubMed] [Google Scholar]; s Kumar MP, Liu R-S. J. Org. Chem. 2006;71:4951–4955. doi: 10.1021/jo0606711. [DOI] [PubMed] [Google Scholar]; t Herrera A, Martinez-Alvarez R, Ramiro P, Molero D, Almy J. J. Org. Chem. 2006;71:3026–3032. doi: 10.1021/jo052619v. [DOI] [PubMed] [Google Scholar]; u Cuny G, Gamez-Montano R, Zhu J. Tetrahedron. 2004;60:4879–4885. [Google Scholar]
- 3.a Falorni M, Dettori G, Giacomelli G. Tetrahedron Asymmetry. 1998;9:1419–1426. [Google Scholar]; b Mann E, Kessler H. Org. Lett. 2003;5:4567–4570. doi: 10.1021/ol035673b. [DOI] [PubMed] [Google Scholar]; c Bailey JL, Sudini RR. Tetrahedron Lett. 2014;55:3674–3677. [Google Scholar]; d Neildé K, Crozet MD, Terme T, Vanell P. Tetrahedron Lett. 2014;55:3652–3657. [Google Scholar]; e Rynearson KD, Dutta S, Tran K, Dibrov SM, Hermann T. Eur. J. Org. Chem. 2013:7337–7342. [Google Scholar]
- 4.a Filoche S, Wong L, Sissons CH. J. Dent. Res. 2010;89:8–18. doi: 10.1177/0022034509351812. [DOI] [PubMed] [Google Scholar]; b Daep C, James D, Lamont R, Demuth D. Infection and Immunity. 2006;74:5756–5762. doi: 10.1128/IAI.00813-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Daep C, Lamont R, Demuth D. Infection and Immunity. 2008;76:3273–3280. doi: 10.1128/IAI.00366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Daep C, Novak E, Lamont R, Demuth D. Infection and Immunity. 2011;79:67–74. doi: 10.1128/IAI.00361-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loner CM, Luzzio FA, Demuth DR. Tetrahedron Lett. 2012;53:5641–5644. doi: 10.1016/j.tetlet.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka A, Togo H. Synlett. 2009:3360–3364. [Google Scholar]
- 7.Konosu T, Miyaoka T, Tajima Y, Oida S. Chem. Pharm. Bull. 1991;39:2241–2246. doi: 10.1248/cpb.39.2581. [DOI] [PubMed] [Google Scholar]
- 8.See Supplementary Information for general procedures.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.