Abstract
The diverse structures of polyketide natural products are reflected by the equally diverse polyketide biosynthetic enzymes, namely polyketide synthases (PKSs). Three major classes of PKSs are known–noniterative type I PKSs, iterative type II PKSs and acyl carrier protein-independent type III PKSs, each of which consists of additional variants. One such variant is the noniterative type I PKS in which each PKS module lacks the cognate acyltransferase (AT) domain. The essential AT activity is instead provided by a discrete AT in trans. Termed “AT-less” type I PKSs, the loading of the malonate extender units by the discrete AT enzyme LnmG to each of the AT-less PKS modules of LnmI and LnmJ was confirmed experimentally for biosynthesis of the anticancer antibiotic leinamycin (LNM). The LNM PKS has since served as a model for the continuous discovery of numerous additional AT-less type I PKSs incorporating variable extender units. However, biochemical characterization of AT-less type I PKSs remains very limited, and the mechanism by which AT-less type I PKSs accommodate multiple extender units is unknown. This chapter provides the protocols used to establish and characterize the LNM PKS. Application of these methods to other AT-less type I PKSs should aid the biochemical characterization and hence possible exploitation of these unique PKSs for polyketide natural product structural diversity by combinatorial biosynthetic methods.
1. Introduction
Polyketides constitute one of the largest families of natural products and many are biosynthesized by noniterative type I polyketide synthases (PKSs). Type I PKSs are multifunctional enzymes organized into modules, each of which harbors a set of distinct domains responsible for the catalysis of one cycle of polyketide chain elongation. Prototypically, a type I PKS elongation module contains minimally three domains—an acyltransferase (AT), an acyl carrier protein (ACP) and a β-ketoacyl synthase (KS)—that select, activate, and catalyze a decarboxylative Claisen condensation between the extender unit and the growing polyketide chain, generating a β-ketoacyl-S-ACP intermediate. Optional domains are found between the AT and ACP domains, which carry out the variable set of reductive modifications of the β-keto group before the ensuing cycle of chain extension (Shen, 2003; Staunton and Weissman, 2001). For a prototypic type I PKS, as exemplified by the 6-deoxyerythromycin B synthase (DEBS) for biosynthesis of the aglycone of the polyketide antibacterial antibiotic erythromycin A (Donadio and Katz, 1992), an AT domain is an integral part of every PKS module, and each AT domain functions only once, for the module in which it resides. Integral AT domains in such prototypic type I PKSs were thus termed cognate ATs (Cheng et al., 2003) (Fig. 8.1A).
Figure 8.1.
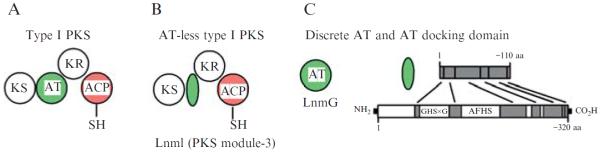
Modular organization of type I polyketide synthases (PKSs). (A) A prototypical type I PKS module contains the cognate ATdomain and other domains. (B) An AT-less type I PKS module lacks the cognate AT domain but often contains a short segment of remnant AT residues. (C) The remnant ATsegment lacks critical catalytic motifs (GHSxG and AFHS) and is therefore catalytically inactive, but may serve as a docking domain mediating the interactions between the discrete AT (LnmG) and AT-less PKS modules in LnmI and LnmJ (Tang et al., 2004).
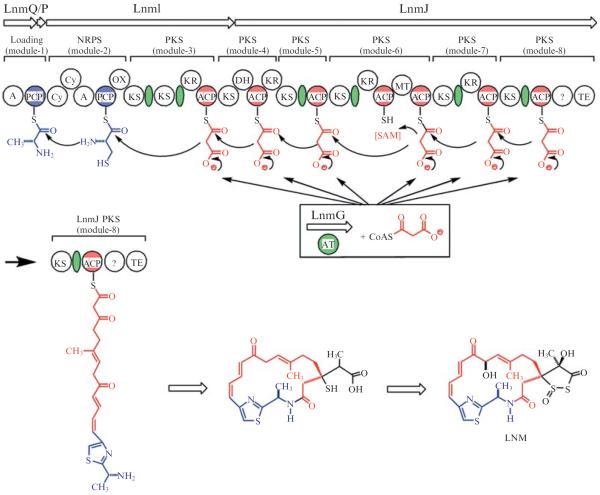
A distinct variant of the noniterative type I PKSs contains no cognate ATs but has a short segment of remnant AT sequence in some or all modules, depending on the pathway. This subclass of type I PKSs was named the AT-less type I PKSs and the remnant AT segment the AT docking domain (Fig. 8.1B and C). The essential AT activities are provided in trans by discrete AT enzymes encoded by genes that are physically separated from the PKS genes. Therefore AT-less type I PKSs require discrete ATs acting in trans to select the extender unit and load it onto the ACP domain (Cheng et al., 2003) (Figs. 8.1C and 8.2).
Figure 8.2.
A model for LNM biosynthesis featuring the LNM hybrid NRPS-PKS megasynthetase with the discrete ATenzyme LnmG that loads the malonyl CoAextender units onto all six modules on the LnmI and LnmJ PKSs (Cheng et al., 2003;Tang et al., 2004).
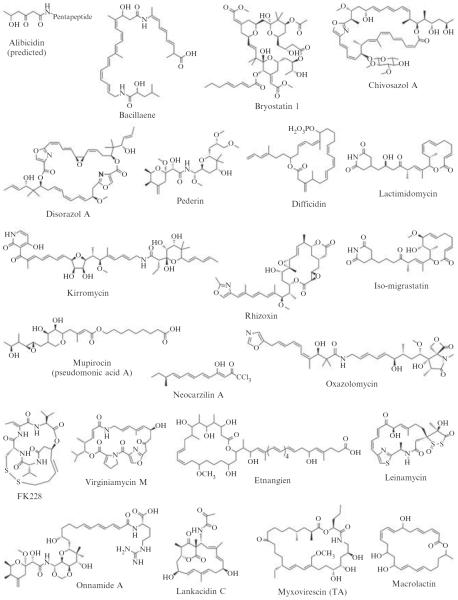
The AT-less class of type I PKSs was first discovered during genetic and biochemical studies of leinamycin (LNM) biosynthesis in Streptomyces atroolivaceus S-140 (Cheng et al., 2002; Cheng et al., 2003; Tang et al., 2004). LNM is a hybrid peptide-polyketide natural product that shows potent antitumor activity, most significantly against tumor cell lines that are resistant to clinically important anticancer drugs (Hara et al., 1989a,b). The lnm biosynthetic gene cluster consists of multiple genes encoding modular nonribosomal peptide synthetases (NRPSs), type I PKSs, and other components. The most striking feature of LNM PKSs is the lack of cognate AT domains in all six modules of the LnmI and LnmJ PKS proteins; instead, only a segment of remnant AT sequence was found in five of the six PKS modules after each KS domain (Figs. 8.1C and 8.2). Bioinformatic and genetic analyses identified lnmG as an essential AT-encoding gene, and biochemical studies subsequently confirmed LnmG as a discrete AT enzyme capable of loading the malonyl group from the extender unit substrate malonyl CoA onto the ACP domains in all six modules of the LnmI and LnmJ PKSs. On the basis of these findings, LnmI and LnmJ were hence proposed as the first AT-less type I PKSs, LnmG as the required trans-acting, discrete AT enzyme, and the remnant AT segments as AT docking domains (Figs. 8.1 and 8.2). Numerous additional AT-less type I PKSs have since been discovered (Fig. 8.3 and Table 8.1). However, unequivocal biochemical evidence validating this emerging subclass of AT-less type I PKSs remains scarce.
Figure 8.3.
Natural products whose biosynthetic pathways are predicted to feature AT-less type I PKSs.
Table 8.1.
A compilation of natural products biosynthesized by AT-less type I PKSs, predicted enzyme properties, and respective producing organisms
| Compound (Reference) | PKS module | Discrete AT protein | Substrate specificitya | Gene cluster status | Organism |
|---|---|---|---|---|---|
| Albicidin (Huang et al., 2001; Royer et al., 2004) | 2 PKS modules on AlbI (XabB) | 1 AT on AlbXIII | Malonyl CoA | Cluster completed and confirmed | Xanthomonas albilineans |
| Bacillaene (Butcher et al., 2007; Chen et al., 2006; Kunst et al., 1997; Straight et al., 2007) | 13 PKS modules on PksJLMNR (also known as BaeJLMNR) | 1 AT on PksC, 1 AT on PksD, and 1 AT on PksE (also known as BaeCDE) | Malonyl CoA | Cluster completed and confirmed | Bacillus subtilis (B. amyloliquefaciens FZB 42) |
| Bryostatin (Hildebrand et al., 2004; Sudek et al., 2007) | 13 PKS modules (1 nonfunctional) on BryABCD | 2 ATs on BryP | Malonyl CoA | Putative cluster complete | “Candidatus Endobugula sertula” (bacterial symbiont from bryozoan Bugula neritina) |
| Chivosazol (Perlova et al., 2006) | 15 PKS modules on ChiBCDEF | 1 AT on ChiA | Malonyl CoA | Cluster completed and confirmed | Sorangium cellulosum So ce56 |
| Difficidin (Chen et al., 2006) | 13 PKS modules on DifFGHIJKL | 1 AT on DifA | Malonyl CoA | Cluster completed and confirmed | Bacillus amyloliquefaciens FZB 42 |
| Disorazol (Carvalho et al., 2005; Kopp et al., 2005) | 10 PKS modules (3 nonfunctional) on DisABC (also known as DszABC) | 1 AT on DisD (also known as DszD) | Malonyl CoA | Cluster completed and confirmed | Sorangium cellulosum So ce12 |
| Etnangien (Menche et al., 2008) | 20 PKS modules on EtnDEFGHI | 1 AT on EtnB and 2 ATs on EtnK | Malonyl CoA Succinyl CoA | Cluster completed and confirmed | Sorangium cellulosum So ce1045 |
| FK228 (depsipeptide) (Cheng et al., 2007) | 2 PKS modules on DepBC | No discrete AT identified | Malonyl CoA | Cluster confirmed but possibly incomplete | Chromobacterium violaceum No. 968 |
| Iso-Migrastatin (Farnet et al., 2002) | 10 PKS modules on MgsEFG | 2 ATs on MgsB and 1 AT on MgsH | Malonyl CoA | Cluster completed and confirmed | Streptomyces plantensis |
| Kirromycin (Weber et al., 2008) | 14 PKS modules on KirAI-AVI with 2 AT domains on KirAVI | 2 ATs on KirCI and 1 AT on KirCII | Malonyl CoA Ethylmalonyl CoA | Cluster completed and confirmed | Streptomyces collinus Tü 365 |
| Lactimidomycin (Farnet et al., 2002) | 10 PKS modules on LtmEFG | 2 ATs on LtmB and 1 AT on LtmH | Malonyl CoA | Cluster completed and confirmed | Streptomyces amphibiosporus |
| Lankacidin (Arakawa et al., 2005; Mochizuki et al., 2003) | 5 PKS modules; on LkcACFG | 1 AT on LkcD | Malonyl CoA | Cluster completed and confirmed | Streptomyces rochei 7434AN4 |
| Leinamycin (Cheng et al., 2002, 2003; Tang et al., 2004, 2006) | 6 PKS modules on LnmIJ | 1 AT on LnmG | Malonyl CoA | Cluster completed and confirmed | Streptomyces atroolivaceus S-140 |
| Macrolactin (polyketide 2) (Chen et al., 2006; Schneider et al., 2007) | 11 PKS modules on MlnBCDEFGH | 1 AT on MlnA | Malonyl CoA | Cluster completed and confirmed | Bacillus amyloliquefaciens FZB 42 |
| Mupirocin (pseudomonic acidA) (El-Sayed et al., 2003) | 8 PKS modules on MmpABD | 2 ATs on MmpC (deposited as MmpIII) | Malonyl CoA | Cluster completed and confirmed | Pseudomonas fluorescens NCIMB 10586 |
| Myxovirescin (antibiotic TA) (Paitan et al., 1999, 2001; Simunovic et al., 2006) | 15 PKS modules (2 inactive) on TaIL, Ta-1 and TaOP | 2 ATs on TaV | Malonyl CoA | Cluster completed and confirmed | Myxococcus xanthus DK1622 |
| Neocarzilin (Otsuka et al., 2004) | 4 PKS modules on ORF4//5/6 with 2 AT domains | No discrete AT identified | Malonyl CoA | Cluster completed and confirmed | Streptomyces carzinostaticus var. F-41 |
| Onnamide (Piel et al., 2004c, 2005) | 7 PKS modules on OnnB and OnnI. Several PKS modules still missing | No discrete AT identified | Malonyl CoA | Putative cluster incomplete | Bacterial symbiont from sponge Theonella swinhoei |
| Oxazolomycin (Song et al., 2008; Zhao et al., 2006, 2009) | 10 PKS modules on OzmHIJKNQ | 2 ATs on OzmM and 1 AT on OzmC | Malonyl CoA Methoxymalonyl ACP | Cluster completed and confirmed | Streptomyces albus JA3453 |
| Pederin (Piel, 2002; Piel, et al., 2004a, 2004b, 2004c, 2004d, 2005) | 10 PKS modules on PedI and PedF; 4 optional PKS modules on PedH | 2 ATs on PedC and 1 AT on PedD | Malonyl CoA | Putative cluster complete | Bacterial symbiont of Paederus ssp. beetles |
| Rhizoxin (Partida-Martinez and Hertweck, 2007) | 12 PKS modules on RhiABCDEF (also known as RzxABCDEF) | 2 ATs on RhiG (also known as RzxG) | Malonyl CoA | Cluster completed and confirmed | Burkholderia rhizoxina |
| Rhizoxin S2 and analogues (Brendel et al., 2007) | 12 PKS modules on RzxABCDEF | 2 ATs on RzxG | Malonyl CoA | Cluster completed and confirmed | Pseudomonas fluorescens Pf-5 |
| Unknown (Fritzler and Zhu, 2007; Zhu et al., 2002) | 7 PKS modules with 2 AT domains on CpPKS1 | No discrete AT identified | Malonyl CoA | Putative cluster incomplete | Cryptosporidium parvum |
| Virginiamycin M (Pulsawat et al., 2007) | 7 PKS modules on VirAFGH | 1 AT on VirI | Malonyl CoA | Cluster completed and confirmed | Streptomyces virginiae |
Only the substrate specificity of LnmG has been experimentally confirmed.
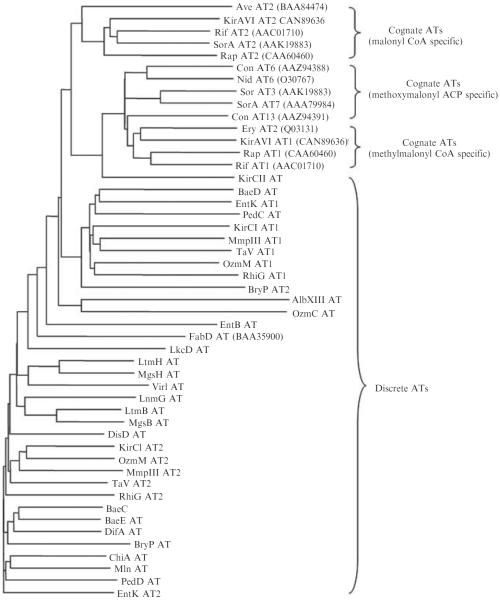
Noniterative type I PKSs use malonyl CoA, methylmalonyl CoA, ethylmalonyl CoA, methoxymalonyl ACP, and in rare cases, hydroxymalonyl ACP or aminomalonyl ACP, as extender units in the biosynthesis of diverse polyketide natural products (Chan et al., 2006; Khosla et al., 1999). (See also Chapter 7 of this volume.) Biochemical studies (for LnmG) and substrate specificity predictions (for all others) suggest that most, if not all, discrete ATs known for AT-less type I PKSs use malonyl CoA as a substrate (Table 8.1 and Fig. 8.4). Possible exceptions to this finding include EtnB from the etnangien biosynthetic pathway (Menche et al., 2008) and KirCII from the kirromycin biosynthetic pathway (Weber et al., 2008), which appear to use succinyl CoA and ethylmalonyl CoA, respectively. An AT-less type I PKS that incorporates methoxymalonyl ACP as an extender unit has also been proposed, for the biosynthesis of oxazolomycin, the predicted discrete AT (OzmC) for which, however, shows little sequence homology to typical ATs known for PKSs. With the exception of LnmG, the actual substrate specificities for all discrete ATs identified for AT-less type I PKSs to date remain to be experimentally proven.
Figure 8.4.
Phylogenetic analysis of cognate and discrete acyltransferases (ATs), highlighting substrate specificities. Phylogenetic analysis was performed by the neighbor-joining method (Bruno et al., 2000). DiscreteATs form a loose but distinctive clade distant from the cognate ATs of prototypical PKSs. For discrete ATs, see Table 8.1 for references. For cognate ATs, NCBI accession numbers are given in parentheses.
This chapter describes protocols for biochemical characterization of AT-less type I PKSs, as previously applied to establish and characterize the LNM PKS. They include (1) heterologous expression and overproduction of ACPs from AT-less type I PKS modules; (2) in vitro preparation of holo-ACPs; (3) heterologous expression and overproduction of discrete ATs; (4) in vitro assay of AT substrate specificity; and (5) in vitro assay of AT-catalyzed loading of extender unit substrate onto holo-ACPs. The biochemical reactions examined are depicted in Fig. 8.5.
Figure 8.5.
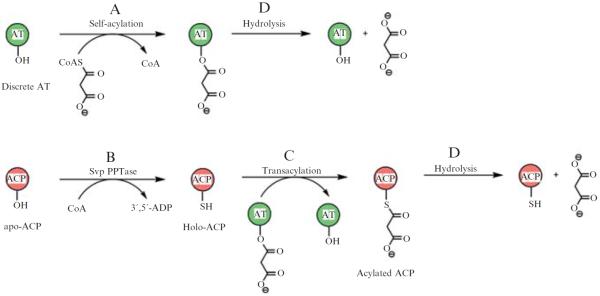
Scheme depicting the reactions assayed by the protocols provided. (A) Self-acylation of a discrete AT at the active site Ser residue with the extender substrate malonyl CoA. (B) Conversion of apo-ACP to holo-ACP by attaching the 4′-phosphopantetheine group from CoA to the active site Ser residue by PPTases such as Svp. (C) Transfer of activated substrate from acylated AT to the SH-group of holo-ACP. (D) Spontaneous hydrolysis of acylated ATor acylated ACP.
2. Methods
2.1. Heterologous expression and overproduction of apo-ACPs from AT-less PKS modules
Conduct sequence analysis (e.g., by ClustalW program [Thompson et al., 2002]) to determine the boundaries of individual ACP domains from AT-less type I PKS modules (Donadio and Katz, 1992).
Choose a host/vector system for heterologous gene expression [e.g., Escherichia coli BL21(DE3)/pET28a or pET37b from Novagen for overproduction of the gene product as His6-tagged fusion protein] and a subsequent protein purification system (e.g., by affinity chromatography on Ni-NTA resin from Qiagen).
Design and synthesize PCR primers for the amplification of DNA encoding the target ACP domains. Restriction sites (e.g., CATATG for NdeI and AAGCTT for HindIII) are routinely added to the primers to facilitate directional cloning.
Amplify the DNA by PCR with a high-fidelity DNA polymerase (e.g., Pfu Turbo DNA polymerase from Strategene), clone the amplicon into pET28a or pET37b, and sequence the construct to verify DNA amplification fidelity.
Introduce individual expression constructs into host strain E. coli BL21 (DE3) cells by transformation or electroporation. Select for transformants on LB agar supplemented with 25 mg/ml of kanamycin.
Optimize culture conditions on a small scale for overproduction of soluble ACPs by following the manufacturer's recommendations (e.g., the QIAexpressionist from Qiagen).
Scale up the culture volume for protein overproduction and proceed to purify sufficient amounts of individual ACPs for assays. We routinely grow 1 to 4 l of culture at 18 to 25° for 0.5 to 2 days and add 1 to 100 μM (final concentration) isopropyl β-D-1-thiogalactopyranoside (IPTG) to induce gene expression. We also routinely employ lysozyme and sonication to lyse cells, and use gravity Ni-NTA affinity chromatography and subsequently anion exchange FPLC (e.g., the ÄKTA system from Amersham Pharmacia) to obtain pure ACPs.
Examine protein fractions by SDS-PAGE and pool together the fractions with at least 95% purity.
Dialyze pooled protein samples against 25 mM Tris-HCl, pH 8.0, 25 mM NaCl, 2 mM DTT and 10% glycerol.
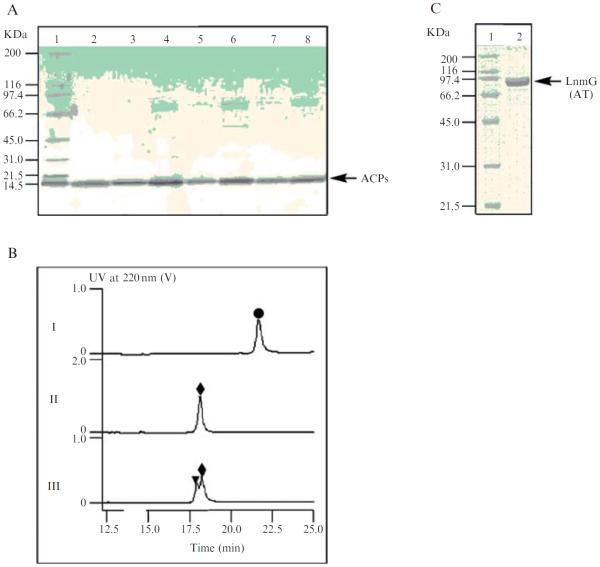
Re-examine the quality of the purified ACPs by SDS-PAGE (Fig. 8.6A), and determine their concentrations by Bradford assay (Bradford, 1976).
Aliquot (e.g., 500 μl per 1.5-ml tube) and store the ACP samples at −80° until use.
Figure 8.6.
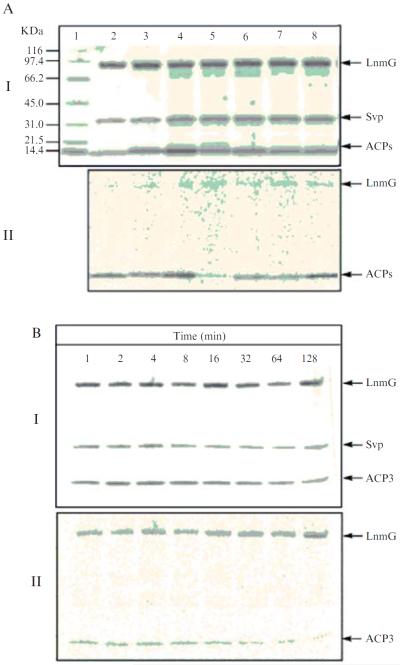
(A) Purified LnmI and LnmJACPs on a 4 to 15% SDS-PAGE: lane1, molecular weight standards; lane 2, ACP3; lane 3, ACP4; lane 4, ACP5; lane 5, ACP6-1; lane 6, ACP6-2; lane 7, ACP7; lane 8, ACP8. The numbers after the ACPs refer to the PKS modules from which they are derived, with 6–1 and 6–2 to indicate the first and second ACPs, respectively, for PKS module 6. (B) HPLC analysis of carrier protein modification and LnmG-catalyzed loading of the extender substrate onto holo-ACP, as exemplified by ACP3: (I) apo-ACP3 (●); (II) Svp-catalyzed conversion of apo-ACP to holo-ACPs (◆); and (III) LnmG-catalyzed loading of malonyl CoA onto holo-ACP, showing holo-ACP3 (◆) converted to malonyl-S-ACP3 (▾). (C) Purified discrete AT (LnmG) on a 4 to15% SDS-PAGE: lane1, molecular weight standards; lane 2, LnmG.
2.2. In vitro preparation of holo-ACPs
PKS ACPs overproduced in E. coli under the conditions described above are generally in their nonphosphopantatheinylated apo-form. Apo-ACPs need to be converted into their functional holo-forms by attachment of the 4′-phosphopantetheine group from CoA to the active-site Ser residue. This post-translational modification of apo-ACPs to holo-ACPs can be carried out in vitro with promiscuous 4-phosphopantetheinyl transferases (PPTases) such as Svp (Fig. 8.5) and followed by HPLC, MS, or autoradiography analysis (Lambalot et al., 1996; Sanchez et al., 2001).
2.2.1. In vitro preparation of holo-ACPs from apo-ACPs
Set up an analytical in vitro phosphopantetheinylation reaction in 100-μl volume as follows: (1) 25 μl of 4 X reaction buffer (400 mM Tris · HCl, pH 7.5, 50 mM MgCl2, 10 mM DTT)l; (2) 10 μl of 2.5 mM CoA stock (final concentration 250 μM) (use 3H-labeled CoA for autoradiography analysis); (3) 10 μl of apo-ACP (final concentration 10 μM ]; (4) 2 μl of Svp (final concentration 2 μM); and (5) add H2O to a total reaction volume of 100 μl.
Incubate the reaction at 25° for 30 to 60 min.
Add 900 μl of acetone to quench the reaction, and mix briefly by vortexing.
Freeze sample tube at −80° for at least 1 h to precipitate proteins, and pellet the proteins by centrifugation at 14,000 RPM for 20 min at 4°.
Decant supernatant, and dry pellet briefly in the air with the cap open.
Redissolve pellet in 30 μl of 25 μM Tris-HCl, pH 7.5, 25 μM NaCl, 2 mM DTT and 10% glycerol.
Separate apo- and holo-ACPs from the reactions by HPLC with a Jupiter C-18 column (5 μM, 300Å, 250 × 4.6 mM, Phenomenex), and a gradient elution from 85% buffer A (H2O + 0.1% formic acid) to 90% buffer B (acetonitrile + 0.1% formic acid) in 25 min at a flow rate of 1 ml/min, with UV detection at 220 nm (Fig. 8.6B). Individual protein peaks are collected, lyophilized, and redissolved in 50 μl of H2O.
Determine the apo- and holo-ACPs by ESI-MS analysis in positive ionization mode on an Agilent 1000 HPLC-MSD SL instrument under standard operational conditions (Table 8.2).
Scale up the reaction volume three- to five-fold for preparative reactions, and aliquot and store the holo-ACP samples from Step 6 at −80° until use.
Table 8.2.
ESI-MS analysis of apo-, holo-, and malonyl-S-ACPs of the LnmI and LnmJ PKSs
| apo-ACP [M + H]+ |
holo-ACP [M + H]+ |
Malonyl-S-ACP [M + H]+ |
||||
|---|---|---|---|---|---|---|
| ACPsa | Calculated | Found | Calculated | Found | Calculated | Found |
| LnmI-ACP3 | 11,702 | 11,700 | 12,042 | 12,040 | 12,128 | 12,126 |
| LnmJ-ACP4 | 12,245 | 12,241 | 12,585 | 12,582 | 12,671 | 12,669 |
| LnmJ-ACP5 | 12,520 | 12,517 | 12,860 | 12,857 | 12,946 | 12,943 |
| LnmJ-ACP6-1 | 12,209 | 12,206 | 12,549 | 12,546 | 12,635 | 12,632 |
| LnmJ-ACP6-2 | 12,151 | 12,147 | 12,491 | 12,486 | 12,577 | 12,572 |
| LnmJ-ACP7 | 12,322 | 12,318 | 12,662 | 12,665 | 12,748 | 12,751 |
| LnmJ-ACP8 | 12,090 | 12,087 | 12,430 | 12,427 | 12,516 | 12,512 |
The numbers after ACPs refer to the LNM PKS modules from which they are derived, with 6-1 and 6-2 to indicate the first and second ACPs, respectively, for LNM PKS module 6.
2.3. Heterologous expression and overproduction of discrete ATs
The majority of genes encoding discrete ATs for AT-less type I PKSs encode a single AT domain, but a few are predicted to encode tandem AT domains (see Table 8.1). For those that encode a single AT domain, use the entire open reading frame for heterologous expression. For those that encode tandem AT domains, conduct sequence analysis [e.g., by ClustalW program (Thompson et al., 2002)] to determine the boundaries of individual AT domains for heterologous expression, taking as much advantage as possible of their natural N- or C-terminus (Donadio and Katz, 1992).
Follow Steps 2 through 8 as described in Section 2.1, but replace the target genes with those encoding discrete ATs.
Dialyze pooled protein samples against 25 μM Tris-HCl, pH 7.0, 25 μM NaCl, 2 μM DTT and 10% glycerol.
Re-examine the quality of the purified ATs by SDS-PAGE (Fig. 8.6C), and determine their concentrations by Bradford assay (Bradford, 1976).
Aliquot (e.g., 500 μl per 1.5-ml tube) and store the AT samples at −80°until use.
2.4. In vitro assay for AT substrate specificity
The protocol for a quasi-kinetic assay of AT substrate specificity was modified from previously published procedures (Liou et al., 2003; Szafranska et al., 2002). Acyl CoAs (use 14C-labeled acyl CoAs only autoradiography analysis) used include malonyl CoA, methylmalonyl CoA, butyryl CoA, propionyl CoA, and acetyl CoA.
Set up a typical in vitro AT self-acylation reaction in a 200-μl volume for each acyl CoA as follows: (1) 100 μl of 2 X reaction buffer (100 μM phosphate buffer, pH 7.0, 0.4 μM DTT, 0.2 μM EDTA); (2) 10 μl of acyl CoA and (final acyl CoA concentration 20 μM and ~8 × 108 DPM if 14C-labeled acyl CoA is included); (3) 5 μl of LnmG (final concentration 2 μM and add in Step 3 to initiate reaction); and (4) add H2O to a total reaction volume of 200 μl
Equilibrate all reagents on ice (0°) for 15 min or longer, and perform assay on ice to slow down the reaction.
Initiate reaction by adding LnmG and quickly mix by pipetting up and down several times.
Transfer 25 μl of reaction mixture to a tube containing 50 μl of ice-cold 10% TCA at each of the seven time points: 0.5, 1, 2, 4, 8, 16 and 32 min.
Add 25 μl of 10-mg/ml BSA to each tube as a carrier protein and allow proteins to precipitate on ice for 15 min.
Pellet proteins by centrifugation at 14,000 RPM for 20 min at 4°.
Wash protein pellet twice with 100 μl of 10% ice-cold TCA.
Solubilize the pellet in 100 μl of 10 μM NaCl, 2% SDS solution at ambient temperature.
Mix with 5 μl of scintillation cocktail and count radioactivity in a scintillation counter (Beckman Coulter).
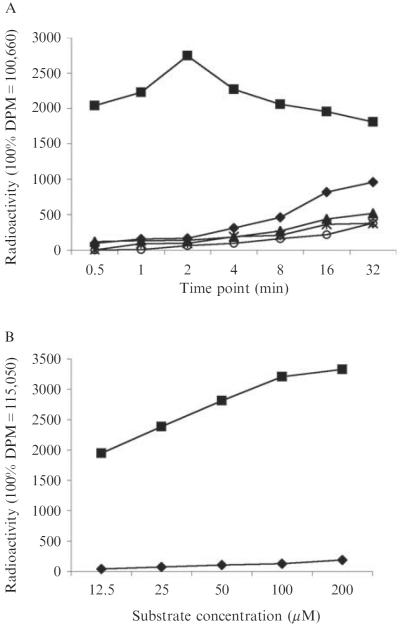
Plot the radioactivity counts as a function of time for each acyl CoA. Use the count of a 25-μl fraction directly from the original reaction (Step 4) as 100% DPM value. Normalize the 100% DPM values of individual acyl CoAs to that of malonyl CoA (Fig. 8.7A).
Similarly, assays can be performed with a fixed amount of AT enzyme (2 μM of LnmG) and variable concentrations of acyl CoA in 25-μl reaction for 2 min on ice (Fig. 8.7B).
Figure 8.7.
Determination of malonyl CoA as the preferred substrate of the discrete AT (LnmG): (A) time-course assays of ATself-acylation with malonyl CoA (∎), methylmalonyl CoA (◆), butyryl CoA (▴), propionyl CoA (★), or acetyl CoA (◯); and (B) assays with a fixed amount of AT enzyme (2 μM of LnmG) and variable concentrations of malonyl CoA (∎) or methylmalonyl CoA (◆).
2.5. In vitro assay of AT-catalyzed loading of acyl CoA extender substrate to holo-ACPs
This assay can be carried out in two completely separate steps (continuing from the holo-ACPs prepared from Step 9, Section 2.2) or in two consecutive steps in a single tube (continuing from the holo-ACPs prepared from Step 2, Section 2.2). The protocol described below was modified from Lambalot et al. (1996) and Sanchez et al. (2001) and was developed for a typical analytical reaction. The loading of the acyl group from the acyl CoA substrate (malonyl CoA in the current example) onto holo-ACP can be followed by HPLC, MS or autoradiography analysis. Only the protocol for assay in a single tube is provided, which could be readily adapted for assay in two separate steps. For preparative reactions, scale up reaction volumes three- to five-fold.
-
1
Add to the reaction tube from the Step 2 of Section 2.2 a 25-μl solution containing 2 μM LnmG and 200 μM [2-14C] malonyl CoA (~5 × 108 DPM).
-
2a
For assaying one ACP in a time-course (Fig. 8.8A), incubate the reactions at 25° and quench the reactions by adding 900 μl of acetone at various time points (1, 2, 4, 8, 16, 32, 64, and 128 min). Mix briefly by vortexing.
-
2b
For assaying multiple ACPs at a fixed time point (Fig. 8.8B), incubate the reactions at 25° and quench by adding 900 μl of acetone at a fixed time point (2 min). Mix briefly by vortexing.
-
3
Freeze samples at −80° for at least 1 h to precipitate proteins and pellet the proteins by centrifugation at 14,000 RPM for 30 min at 4°.
-
4
Decant supernatant, and dry pellet briefly in the air with the cap open.
-
5
Follow Steps 6 to 8 of Section 2.2 to analyze the resultant acyl-S-ACPs by HPLC (Fig. 8.6B) and ESI-MS (Table 8.2).
-
6
Alternatively, redissolve protein pellets in 25 μl of 1 × sampler buffer.
-
7
Analyze protein samples on a 4 to 15% SDS-PAGE gel (Bio-Rad), visualize proteins by Coomassie blue staining and record the gel image (Fig. 8.8).
-
8
Air dry the gel and expose it to a low-energy (LE) phosphor screen (Amersham Pharmacia) at ambient temperature for 3 to 7 days to acquire autoradiography with a phosphorimager (Molecular Dynamics) (Fig. 8.8).
Figure 8.8.
In vitro assays of LnmG-catalyzed loading of malonyl CoA onto holo-ACPs of the LnmI and LnmJ PKSs: (A) Complete assay of individual ACPs in reactions with Svp, CoA, LnmG and [2-14C]malonyl CoA, as visualized on a 4 to 15% SDS-PAGE (I) and by phosphorimaging (II) (lane1, molecular mass standards and lanes 2 to 8, ACP3 to ACP8); and (B) time course of LnmG-catalyzed loading of [2-14C]malonyl CoA onto holo-ACP3, as visualized on a 4 to 15% SDS-PAGE (I) and by phosphorimaging (II).
3. Conclusion
Bioinformatics analysis of gene clusters continues to unveil candidates encoding AT-less type I PKSs for natural product biosynthesis (Fig. 8.3 and Table 8.1). The LNM PKS, however, remains the only AT-less type I PKS that has been confirmed experimentally, with LnmG acting in trans as a discrete AT responsible for loading the extender substrate malonyl CoA to all six modules of the LnmI and LnmJ PKS proteins (Cheng et al., 2003). A significant knowledge gap exists between the predicted and experimentally validated involvement of AT-less type I PKSs in natural product biosynthesis, yet such information is absolutely required to realize the full potential of this subclass of novel PKSs in combinatorial biosynthesis for natural product structural diversity. It is hoped that methods devised for studies of the LNM PKS will be applicable to other AT-less type I PKSs, thereby aiding their biochemical and mechanistic characterization. Although gene expression in heterologous hosts such as E. coli is intrinsically unpredictable, overproduction of functional ACPs and discrete ATs generally does not constitute an insurmountable problem. ACPs overproduced in E. coli are often in the apo-form, but conversion to the functional holo-form is readily achievable thanks to the availability of promiscuous PPTases such as Svp (Sanchez et al., 2001) and Sfp (Lambalot et al., 1996). While conceptually straightforward, care must be taken in executing assays for the substrate specificity of discrete ATs, since AT self-acylation reaction is often rapid and followed by a gradual spontaneous hydrolysis, and assays for discrete AT-catalyzed loading of extender substrates to holo-ACPs, because the general protocol often requires specific optimization, and acylated ACPs also undergo slow hydrolysis under the assay conditions (Fig. 8.5).
AT-less type I PKSs provide tremendous opportunities for PKS engineering. For instance, given that LnmG is responsible for the loading of the malonyl CoA extender unit to all six modules of the LnmI and LnmJ PKS, it was reasoned that LnmG could be a rate-limiting factor for LNM biosynthesis. Subsequent overexpression of lnmG under a strong promoter in the LNM-producing-strain S, atroolivaceus S-140 indeed resulted in improved LNM titers by three- to five-fold (Cheng et al., 2003; Tang et al., 2004). It is also very tempting to envision the application of lnmG and other discrete AT-encoding genes to either prototypical type I PKSs or AT-less type I PKSs to alter the extender-unit specificity by combinatorial methods, thereby further expanding the size and diversity of polyketide natural products.
Finally, it is exciting to consider how these established methods designed with LNM in mind will also be applicable to decipher the mechanism by which AT-less type I PKSs accommodate multiple extender units. Take for instance the recently discovered discrete ATs from the etnangien biosynthetic pathway (EtnB) (Menche et al., 2008), the kirromycin biosynthetic pathway (KirCII) (Weber et al., 2008), and the oxazolomycin biosynthetic pathway (OzmC) (Zhao et al., 2009), whose predicted substrate specificities are for succinyl CoA, ethylmalonyl CoA, and methoxymalonyl ACP, respectively, in addition to other dedicated discrete ATs for malonyl CoA in the same pathway. If experimentally proven, EtnB, KirCII, and OzmC, together with their corresponding AT-less PKS machinery, would provide additional opportunities to introduce uncommon extender units for engineering novel natural products by combinatorial biosynthesis methods.
ACKNOWLEDGMENT
This work was supported in part by National Institutes of Health grants CA106150 and CA113297.
REFERENCES
- Arakawa K, Sugino F, Kodama K, Ishii T, Kinashi H. Cyclization mechanism for the synthesis of macrocyclic antibiotic lankacidin. Streptomyces rochei. Chem. Biol. 2005;12:249–256. doi: 10.1016/j.chembiol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brendel N, Laila P, Partida-Martinez LP, Scherlach K, Hertweck C. A cryptic PKS-NRPS gene locus in the plant commensal Pseudomonas fluorescens Pf–5 codes for the biosynthesis of an antimitotic rhizoxin complex. Org. Biomol. Chem. 2007;5:2211–2213. doi: 10.1039/b707762a. [DOI] [PubMed] [Google Scholar]
- Bruno WJ, Socci ND, Halpern AL. Weighted neighbor joining: A likelihood-based approach to distance-based phylogeny reconstruction. Mol. Biol. Evol. 2000;17:189–197. doi: 10.1093/oxfordjournals.molbev.a026231. [DOI] [PubMed] [Google Scholar]
- Butcher RA, Schroeder FC, Fischbach MA, Straight PD, Kolter R, Walsh CT, Clardy J. The identification of bacillaene, the product of the PksX megacomplex in Bacillus subtilis. Proc. Natl. Acad. Sci. USA. 2007;104:1506–1509. doi: 10.1073/pnas.0610503104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho R, Reid R, Viswanathan N, Gramajo H, Julien B. The biosynthetic genes for disorazoles, potent cytotoxic compounds that disrupt microtubule formation. Gene. 2005;359:91–98. doi: 10.1016/j.gene.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Chan YA, Boyne MT, Podevels AM, Klimowicz AK, Handelsman J, Kelleher NL, Thomas MG. Hydroxymalonyl-acyl carrier protein (ACP), and aminomalonyl-ACP are two additional type I polyketide synthase extender units. Proc. Natl. Acad. Sci. USA. 2006;103:14349–14354. doi: 10.1073/pnas.0603748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XH, Vater J, Piel J, Franke P, Scholz R, Schneider K, Koumoutsi A, Hitzeroth G, Grammel N, Strittmatter AW, Gottschalk G, Sussmuth RD, et al. Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB 42. J. Bacteriol. 2006;188:4024–4036. doi: 10.1128/JB.00052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YQ, Tang GL, Shen B. Identification and localization of the gene cluster encoding biosynthesis of the antitumor macrolactam leinamycin in Streptomyces atroolivaceus S-140. J. Bacteriol. 2002;184:7013–7024. doi: 10.1128/JB.184.24.7013-7024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YQ, Tang GL, Shen B. Type I polyketide synthase requiring a discrete acyltransferase for polyketide biosynthesis. Proc. Natl. Acad. Sci. USA. 2003;100:3149–3154. doi: 10.1073/pnas.0537286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YQ, Yang M, Matter AM. Characterization of a gene cluster responsible for the biosynthesis of anticancer agent FK228 in Chromobacterium violaceum No. 968. Appl. Environ. Microbiol. 2007;73:3460–3469. doi: 10.1128/AEM.01751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadio S, Katz L. Organization of the enzymatic domains in the multifunctional polyketide synthase involved in erythromycin formation in Saccharopolyspora erythraea. Gene. 1992;111:51–60. doi: 10.1016/0378-1119(92)90602-l. [DOI] [PubMed] [Google Scholar]
- El-Sayed AK, Hothersall J, Cooper SM, Stephens E, Simpson TJ, Thomas CM. Characterization of the mupirocin biosynthesis gene cluster from Pseudomonas fluorescens NCIMB 10586. Chem. Biol. 2003;10:419–430. doi: 10.1016/s1074-5521(03)00091-7. [DOI] [PubMed] [Google Scholar]
- Farnet CM, Zazopoulos E, Staffa A, Yang X. International Patent WO 02/088176. 2002. [Google Scholar]
- Fritzler JM, Zhu G. Functional characterization of the acyl-[acyl carrier protein] ligase in the Cryptosporidium parvum giant polyketide synthase. Int. J. Parasitol. 2007;37:307–316. doi: 10.1016/j.ijpara.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Asano K, Kawamoto I, Takiguchi T, Katsumata S, Takahashi K, Nakano H. Leinamycin, a new antitumor antibiotic from Streptomyces: Producing organism, fermentation and isolation. J. Antibiot. (Tokyo) 1989a;42:1768–1774. doi: 10.7164/antibiotics.42.1768. [DOI] [PubMed] [Google Scholar]
- Hara M, Takahashi I, Yoshida M, Asano K, Kawamoto I, Morimoto M, Nakano H. DC 107, a novel antitumor antibiotic produced by a Streptomyces sp. J. Antibiot. (Tokyo) 1989b;42:333–335. doi: 10.7164/antibiotics.42.333. [DOI] [PubMed] [Google Scholar]
- Hildebrand M, Waggoner LE, Liu H, Sudek S, Allen S, Anderson C, Sherman DH, Haygood M. BryA: An unusual modular polyketide synthase gene from the uncultivated bacterial symbiont of the marine bryozoan Bugula neritina. Chem. Biol. 2004;11:1543–1552. doi: 10.1016/j.chembiol.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Huang G, Zhang L, Birch RG. A multifunctional polyketide-peptide synthetase essential for albicidin biosynthesis in Xanthomonas albilineans. Microbiology. 2001;147:631–642. doi: 10.1099/00221287-147-3-631. [DOI] [PubMed] [Google Scholar]
- Khosla C, Gokhale RS, Jacobsen JR, Cane DE. Tolerance and specificity of polyketide synthases. Annu. Rev. Biochem. 1999;68:219–253. doi: 10.1146/annurev.biochem.68.1.219. [DOI] [PubMed] [Google Scholar]
- Kopp M, Irschik H, Pradella S, Muller R. Production of the tubulin destabilizer disorazol in Sorangium cellulosum: Biosynthetic machinery and regulatory genes. ChemBioChem. 2005;6:1277–1286. doi: 10.1002/cbic.200400459. [DOI] [PubMed] [Google Scholar]
- Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- Lambalot RH, Gehring AM, Flugel RS, Zuber P, LaCelle M, Marahiel MA, Reid R, Khosla C, Walsh CT. A new enzyme superfamily—the phosphopantetheinyl transferases. Chem. Biol. 1996;3:923–936. doi: 10.1016/s1074-5521(96)90181-7. [DOI] [PubMed] [Google Scholar]
- Liou GF, Lau J, Cane DE, Khosla C. Quantitative analysis of loading and extender acyltransferases of modular polyketide synthases. Biochemistry. 2003;42:200–207. doi: 10.1021/bi0268100. [DOI] [PubMed] [Google Scholar]
- Menche D, Arikan F, Perlova O, Horstmann N, Ahlbrecht W, Wenzel SC, Jansen R, Irschik H, Muller R. Stereochemical determination and complex biosynthetic assembly of etnangien, a highly potent RNA polymerase inhibitor from the myxobacterium Sorangium cellulosum. J. Am. Chem. Soc. 2008;130:14234–14243. doi: 10.1021/ja804194c. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Hiratsu K, Suwa M, Ishii T, Sugino F, Yamada K, Kinashi H. The large linear plasmid pSLA2-L of Streptomyces rochei has an unusually condensed gene organization for secondary metabolism. Mol. Microbiol. 2003;48:1501–1510. doi: 10.1046/j.1365-2958.2003.03523.x. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Ichinose K, Fujii I, Ebizuka Y. Cloning, sequencing, and functional analysis of an iterative type I polyketide synthase gene cluster for biosynthesis of the antitumor chlorinated polyenone neocarzilin in “Streptomyces carzinostaticus”. Antimicrob. Agents Chemother. 2004;48:3468–3476. doi: 10.1128/AAC.48.9.3468-3476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paitan Y, Alon G, Orr E, Ron EZ, Rosenberg E. The first gene in the biosynthesis of the polyketide antibiotic TA of Myxococcus xanthus codes for a unique PKS module coupled to a peptide synthetase. J. Mol. Biol. 1999;286:465–474. doi: 10.1006/jmbi.1998.2478. [DOI] [PubMed] [Google Scholar]
- Paitan Y, Orr E, Ron EZ, Rosenberg E. An unusual beta-ketoacyl:acyl carrier protein synthase and acyltransferase motifs in TaK, a putative protein required for biosynthesis of the antibiotic TA in Myxococcus xanthus. FEMS Microbiol. Lett. 2001;203:191–197. doi: 10.1111/j.1574-6968.2001.tb10840.x. [DOI] [PubMed] [Google Scholar]
- Partida-Martinez LP, Hertweck C. A gene cluster encoding rhizoxin biosynthesis in “Burkholderia rhizoxina,” the bacterial endosymbiont of the fungus Rhizopus microsporus. ChemBioChem. 2007;8:41–45. doi: 10.1002/cbic.200600393. [DOI] [PubMed] [Google Scholar]
- Perlova OK, Kaiser O, Hans A, Muller R. Identification and analysis of the chivosazol biosynthetic gene cluster from the myxobacterial model strain Sorangium cellulosum So ce56. J. Biotechnol. 2006;121:174–191. doi: 10.1016/j.jbiotec.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Piel J. A polyketide synthase-peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles. Proc. Natl. Acad. Sci. USA. 2002;99:14002–14007. doi: 10.1073/pnas.222481399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel J, Butzke D, Fusetani N, Hui D, Platzer M, Wen G, Matsunaga S. Exploring the chemistry of uncultivated bacterial symbionts: Antitumor polyketides of the pederin family. J. Nat. Prod. 2005;68:472–479. doi: 10.1021/np049612d. [DOI] [PubMed] [Google Scholar]
- Piel J, Hofer I, Hui D. Evidence for a symbiosis island involved in horizontal acquisition of pederin biosynthetic capabilities by the bacterial symbiont of Paederus fuscipes beetles. J. Bacteriol. 2004a;186:1280–1286. doi: 10.1128/JB.186.5.1280-1286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel J, Hui D, Fusetani N, Matsunaga S. Targeting modular polyketide synthases with iteratively acting acyltransferases from metagenomes of uncultured bacterial consortia. Environ. Microbiol. 2004b;6:921–927. doi: 10.1111/j.1462-2920.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- Piel J, Hui D, Wen G, Butzke D, Platzer M, Fusetani N, Matsunaga S. Antitumor polyketide biosynthesis by an uncultivated bacterial symbiont of the marine sponge Theonella swinhoei. Proc. Natl. Acad. Sci. USA. 2004c;101:16222–16227. doi: 10.1073/pnas.0405976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel J, Wen G, Platzer M, Hui D. Unprecedented diversity of catalytic domains in the first four modules of the putative pederin polyketide synthase. ChemBio-Chem. 2004d;5:93–98. doi: 10.1002/cbic.200300782. [DOI] [PubMed] [Google Scholar]
- Pulsawat N, Kitani S, Nihira T. Characterization of biosynthetic gene cluster for the production of virginiamycin M, a streptogramin type A antibiotic, in Streptomyces virginiae. Gene. 2007;393:31–42. doi: 10.1016/j.gene.2006.12.035. [DOI] [PubMed] [Google Scholar]
- Royer M, Costet L, Vivien E, Bes M, Cousin A, Damais A, Pieretti I, Savin A, Megessier S, Viard M, Frutos R, Gabriel DW, et al. Albicidin pathotoxin produced by Xanthomonas albilineans is encoded by three large PKS and NRPS genes present in a gene cluster also containing several putative modifying, regulatory, and resistance genes. Mol. Plant Microbe Interact. 2004;17:414–427. doi: 10.1094/MPMI.2004.17.4.414. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Du L, Edwards DJ, Toney MD, Shen B. Cloning and characterization of a phosphopantetheinyl transferase from Streptomyces verticillus ATCC15003, the producer of the hybrid peptide-polyketide antitumor drug bleomycin. Chem. Biol. 2001;8:725–738. doi: 10.1016/s1074-5521(01)00047-3. [DOI] [PubMed] [Google Scholar]
- Schneider K, Chen XH, Vater J, Franke P, Nicholson G, Borriss R, Süssmuth RD. Macrolactin is the polyketide biosynthesis product of the pks2 cluster of Bacillus amyloliquefaciens FZB42. J. Nat. Prod. 2007;70:1417–1423. doi: 10.1021/np070070k. [DOI] [PubMed] [Google Scholar]
- Shen B. Polyketide biosynthesis beyond the type I, II and III polyketide synthase paradigms. Curr. Opin. Chem. Biol. 2003;7:285–295. doi: 10.1016/s1367-5931(03)00020-6. [DOI] [PubMed] [Google Scholar]
- Simunovic V, Zapp J, Rachid S, Krug D, Meiser P, Muller R. Myxovirescin A biosynthesis is directed by hybrid polyketide synthases/nonribosomal peptide synthetase, 3-hydroxy-3-methylglutaryl-CoA synthases, and trans-acting acyltransferases. ChemBioChem. 2006;7:1206–1220. doi: 10.1002/cbic.200600075. [DOI] [PubMed] [Google Scholar]
- Song D, Coughlin J, Ju J, Zhou X, Shen B, Zhao C, Deng Z. Alternative method for site-directed mutagenesis of complex polyketide synthase in Streptomyces albus JA3453. Acta Biochim. Biophys. Sin. (Shanghai) 2008;40:319–326. doi: 10.1111/j.1745-7270.2008.00408.x. [DOI] [PubMed] [Google Scholar]
- Staunton J, Weissman KJ. Polyketide biosynthesis: A millennium review. Nat. Prod. Rep. 2001;18:380–416. doi: 10.1039/a909079g. [DOI] [PubMed] [Google Scholar]
- Straight PD, Fischbach MA, Walsh CT, Rudner DZ, Kolter R. A singular enzymatic megacomplex from Bacillus subtilis. Proc. Natl. Acad. Sci. USA. 2007;104:305–310. doi: 10.1073/pnas.0609073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudek S, Lopanik NB, Waggoner LE, Hildebrand M, Anderson C, Liu H, Patel A, Sherman DH, Haygood MG. Identification of the putative bryostatin polyketide synthase gene cluster from “Candidatus Endobugula sertula” the uncultivated microbial symbiont of the marine bryozoan Bugula neritina. J. Nat. Prod. 2007;70:67–74. doi: 10.1021/np060361d. [DOI] [PubMed] [Google Scholar]
- Szafranska AE, Hitchman TS, Cox RJ, Crosby J, Simpson TJ. Kinetic and mechanistic analysis of the malonyl CoA ACP transacylase from Streptomyces coelicolor indicates a single catalytically competent serine nucleophile at the active site. Biochemistry. 2002;41:1421–1427. doi: 10.1021/bi012001p. [DOI] [PubMed] [Google Scholar]
- Tang GL, Cheng YQ, Shen B. Leinamycin biosynthesis revealing unprecedented architectural complexity for a hybrid polyketide synthase and nonribosomal peptide synthetase. Chem. Biol. 2004;11:33–45. doi: 10.1016/j.chembiol.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Tang GL, Cheng YQ, Shen B. Polyketide chain skipping mechanism in the biosynthesis of the hybrid nonribosomal peptide-polyketide antitumor antibiotic leinamycin in Streptomyces atroolivaceus S-140. J. Nat. Prod. 2006;69:387–393. doi: 10.1021/np050467t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using. In: Clustal W, Clustal X, editors. Current Protocols in Bioinformatics. unit 2.3. chapter 2. John Wiley & Son, Inc.; Hoboken, NJ: 2002. [DOI] [PubMed] [Google Scholar]
- Weber T, Laiple KJ, Pross EK, Textor A, Grond S, Welzel K, Pelzer S, Vente A, Wohlleben W. Molecular analysis of the kirromycin biosynthetic gene cluster revealed beta-alanine as precursor of the pyridone moiety. Chem. Biol. 2008;15:175–188. doi: 10.1016/j.chembiol.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Zhao C, Ju J, Christenson SD, Smith WC, Song D, Zhou X, Shen B, Deng Z. Utilization of the methoxymalonyl-acyl carrier protein biosynthesis locus for cloning the oxazolomycin biosynthetic gene cluster from Streptomyces albus JA3453. J. Bacteriol. 2006;188:4142–4147. doi: 10.1128/JB.00173-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Coughlin JM, Song D, Ju J, Zhu D, Wendt-Pienkowski E, Zhou X, Wang Z, Shen B, Deng Z. The oxazolomycin biosynthetic gene cluster from Streptomyces albus JA3453 unveils a hybrid peptide-polyketide pathway featuring an scyltransferase-less polyketide synthase that incorporates two distinct extender units. 2009. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, LaGier MJ, Stejskal F, Millership JJ, Cai X, Keithly JS. Cryptosporidium parvum: The first protist known to encode a putative polyketide synthase. Gene. 2002;298:79–89. doi: 10.1016/s0378-1119(02)00931-9. [DOI] [PubMed] [Google Scholar]