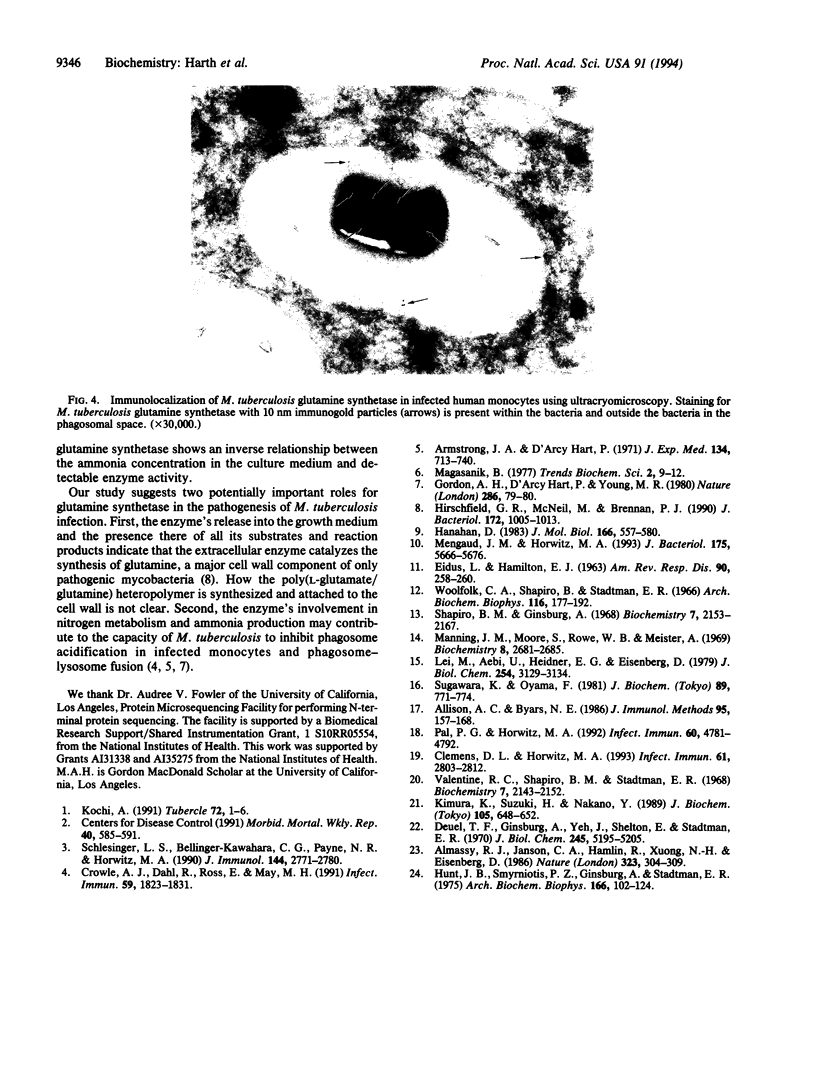
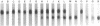Abstract
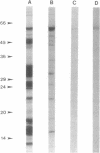
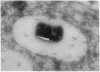
We have investigated the activity and extracellular release of glutamine synthetase [L-glutamate:ammonia ligase (ADP-forming), EC 6.3.1.2] of Mycobacterium tuberculosis. The purified, homogeneous M. tuberculosis glutamine synthetase appears to consist of 12 most likely identical subunits of M(r) 58,000, arranged in two superimpose hexagons. In the catalysis of L-glutamine, the enzyme has an apparent Km for L-glutamate of approximately 3 mM at the pH optimum of 7.5. M. tuberculosis releases a large proportion (approximately 30%) of its total measurable enzyme activity into the culture medium, a feature that is highly specific for pathogenic mycobacteria. Immunogold electron microscopy revealed that M. tuberculosis also releases the enzyme into its phagosome in infected human monocytes. Two potentially important roles for glutamine synthase in the pathogenesis of M. tuberculosis infection are (i) the synthesis of L-glutamine, a major component of the cell wall of pathogenic but not nonpathogenic mycobacteria, and (ii) the modulation of the ammonia level in the M. tuberculosis phagosome, which may in turn influence phagosomal pH and phagosomelysosome fusion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Byars N. E. An adjuvant formulation that selectively elicits the formation of antibodies of protective isotypes and of cell-mediated immunity. J Immunol Methods. 1986 Dec 24;95(2):157–168. doi: 10.1016/0022-1759(86)90402-3. [DOI] [PubMed] [Google Scholar]
- Almassy R. J., Janson C. A., Hamlin R., Xuong N. H., Eisenberg D. Novel subunit-subunit interactions in the structure of glutamine synthetase. 1986 Sep 25-Oct 1Nature. 323(6086):304–309. doi: 10.1038/323304a0. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens D. L., Horwitz M. A. Hypoexpression of major histocompatibility complex molecules on Legionella pneumophila phagosomes and phagolysosomes. Infect Immun. 1993 Jul;61(7):2803–2812. doi: 10.1128/iai.61.7.2803-2812.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Dahl R., Ross E., May M. H. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect Immun. 1991 May;59(5):1823–1831. doi: 10.1128/iai.59.5.1823-1831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Ginsburg A., Yeh J., Shelton E., Stadtman E. R. Bacillus subtilis glutamine synthetase. Purification and physical characterization. J Biol Chem. 1970 Oct 25;245(20):5195–5205. [PubMed] [Google Scholar]
- EIDUS L., HAMILTON E. J. IN VITRO TESTS WITH 4,4'-DIISOAMYLOXYTHIOCARBANILIDE. Am Rev Respir Dis. 1964 Aug;90:258–260. doi: 10.1164/arrd.1964.90.2.258. [DOI] [PubMed] [Google Scholar]
- Gordon A. H., Hart P. D., Young M. R. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature. 1980 Jul 3;286(5768):79–80. doi: 10.1038/286079a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hirschfield G. R., McNeil M., Brennan P. J. Peptidoglycan-associated polypeptides of Mycobacterium tuberculosis. J Bacteriol. 1990 Feb;172(2):1005–1013. doi: 10.1128/jb.172.2.1005-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. B., Smyrniotis P. Z., Ginsburg A., Stadtman E. R. Metal ion requirement by glutamine synthetase of Escherichia coli in catalysis of gamma-glutamyl transfer. Arch Biochem Biophys. 1975 Jan;166(1):102–124. doi: 10.1016/0003-9861(75)90370-7. [DOI] [PubMed] [Google Scholar]
- Kimura K., Suzuki H., Nakano Y. Physical and chemical characterization of glutamine synthetase purified from Mycobacterium phlei. J Biochem. 1989 Apr;105(4):648–652. doi: 10.1093/oxfordjournals.jbchem.a122719. [DOI] [PubMed] [Google Scholar]
- Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991 Mar;72(1):1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- Lei M., Aebi U., Heidner E. G., Eisenberg D. Limited proteolysis of glutamine synthetase is inhibited by glutamate and by feedback inhibitors. J Biol Chem. 1979 Apr 25;254(8):3129–3134. [PubMed] [Google Scholar]
- Manning J. M., Moore S., Rowe W. B., Meister A. Identification of L-methionine S-sulfoximine as the diastereoisomer of L-methionine SR-sulfoximine that inhibits glutamine synthetase. Biochemistry. 1969 Jun;8(6):2681–2685. doi: 10.1021/bi00834a066. [DOI] [PubMed] [Google Scholar]
- Mengaud J. M., Horwitz M. A. The major iron-containing protein of Legionella pneumophila is an aconitase homologous with the human iron-responsive element-binding protein. J Bacteriol. 1993 Sep;175(17):5666–5676. doi: 10.1128/jb.175.17.5666-5676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal P. G., Horwitz M. A. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992 Nov;60(11):4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger L. S., Bellinger-Kawahara C. G., Payne N. R., Horwitz M. A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990 Apr 1;144(7):2771–2780. [PubMed] [Google Scholar]
- Shapiro B. M., Ginsburg A. Effects of specific divalent cations on some physical and chemical properties of glutamine synthetase from Escherichia coli. Taut and relaxed enzyme forms. Biochemistry. 1968 Jun;7(6):2153–2167. doi: 10.1021/bi00846a018. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Oyama F. Fluorogenic reaction and specific microdetermination of ammonia. J Biochem. 1981 Mar;89(3):771–774. doi: 10.1093/oxfordjournals.jbchem.a133257. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Woolfolk C. A., Shapiro B., Stadtman E. R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]