Abstract
Low levels of reactive oxygen species (ROS) modulate signaling pathways required for human sperm activation, but high levels impair sperm function, leading to infertility. Peroxiredoxins (PRDXs) are enzymes with a dual role as ROS scavengers and modulators of ROS-dependent signaling. The present study aimed to characterize PRDXs in human spermatozoa and possible modifications resulting from hydrogen peroxide (H2O2). We found PRDX1, PRDX4, PRDX5, and PRDX6 in both seminal plasma and spermatozoa. Using immunocytochemistry, we demonstrated that these PRDXs are differentially localized in the head, acrosome, mitochondrial sheath, and flagellum. These observations were confirmed by immunoblotting using cytosolic, Triton-soluble and -insoluble, and head and flagella sperm fractions. PRDXs are dose-dependently modified by H2O2, as seen by the formation of disulfide bridges and high-molecular-mass complexes. This first study, to our knowledge, on PRDXs in human spermatozoa indicates that PRDX1, PRDX4, PRDX5, and PRDX6 are modified when spermatozoa are challenged with H2O2. This suggests that PRDXs may protect these cells at high levels of H2O2 but could also control H2O2 levels within different cell compartments so that normal sperm activation can occur.
Keywords: antioxidant enzymes, antioxidants, male reproductive tract, oxidative stress, reactive oxygen species, sperm, sperm activation, spermatozoa, stress
The differential localization and hydrogen peroxide reactivity of various peroxiredoxins in human sperm suggest that these enzymes play different roles in controlling ROS levels within sperm compartments.
INTRODUCTION
To fertilize a mature oocyte, the human spermatozoon must accomplish a series of metabolic and morphological changes called capacitation [1, 2]. Among these changes, the most significant are the phosphorylations of proteins by specific kinases [3–6] in a time-dependent manner [7–9]. The capacitated spermatozoon binds the zona pellucida and undergoes the acrosome reaction to penetrate the zona pellucida, fuse with the oolemma, and fertilize the oocyte [1, 7].
Low concentrations of reactive oxygen species (ROS), such as superoxide anion, nitric oxide, and hydrogen peroxide (H2O2), are produced by human spermatozoa during capacitation and acrosome reaction [2, 10–12] and mediate most of the known transduction pathways involved in these complex phenomena [8, 9]. Compounds aimed at reacting with sulfhydryl groups, such as phenylarsine oxide, diamide, dithiopyridine, N-ethylmaleimide, maleimidylpropionyl biocytin, p-chloromercuribenzoic acid, and bromobimane analogs, when used at low levels, trigger capacitation in human spermatozoa, suggesting that the protein sulfhydryl-disulfide status is important to regulate sperm capacitation [13, 14].
Paradoxically, high levels of ROS, produced by spermatozoa themselves or by other cells present in the semen (e.g., leukocytes), are harmful for normal sperm function [15, 16] and are associated with male infertility [17, 18]. The antioxidant system present in semen [19, 20] of patients is often not sufficient to protect spermatozoa from ROS-dependent damages, such as peroxidation of membrane lipids [21], DNA fragmentation and oxidation of bases [22], low mitochondrial membrane potential [23, 24], and loss of motility and viability [25].
Members of the glutathione peroxidase (GPX) family are found in spermatozoa but with species specificities. Phospholipid hydroperoxide GPX is important for sperm chromatin remodeling during the epididymal maturation but is an enzymatically inactive structural protein only in ejaculated mature spermatozoa (associated with mitochondrial sheath) [26, 27]. GPX5 is found in monkey and rat spermatozoa but not in human testis, epididymis, spermatozoa, or seminal plasma [28]. In view of these reports on GPXs, we were interested in the peroxiredoxin (PRDX) family as potential antioxidants protecting human spermatozoa against H2O2. Along with thioredoxins (TRXs), PRDXs are the major enzymatic regulators of the redox homeostasis and signaling within somatic cells [29]. A testis- and sperm-specific TRX (TXNDC) has been found in humans [30], but nothing is presently known about PRDX in human spermatozoa.
The PRDXs are ancestral sulfhydryl-dependent, selenium-free, and heme-free peroxidases and are highly expressed in virtually all living species, from prokaryotes to eukaryotes [29, 31]. They are acidic proteins of approximately 20–31 kDa, with one or two Cys residues at the active site and required for their activity [32]. Six isoforms are classified in three groups: 1) the 2-Cys PRDX proteins (isoforms 1–4) contain both the N- and C-terminal-conserved Cys residues that are essential for function; 2) the atypical 2-Cys PRDX protein (isoform 5) contains only the N-terminal Cys but requires one additional, nonconserved Cys residue for activity; and 3) the 1-Cys PRDX protein (isoform 6) contains and requires only the N-terminal Cys for function [29, 33, 34]. All PRDXs can reduce both organic and inorganic hydroperoxides [35] and PRDX5 and PRDX6 can reduce peroxynitrite [36, 37] by coupling with the TRX/TRX reductase system [38, 39]. The major role of PRDXs as H2O2 scavengers and sensors [34] is emphasized by their wide cellular distribution (cytosol, nucleus, mitochondria, endoplasmic reticulum, and plasma membrane [39–41]).
Increasing evidence supports the involvement of PRDX in the regulation of H2O2-dependent signaling [29, 34, 42]. Kinetic modeling identifies PRDXs as direct targets for H2O2, being readily oxidized in cells exposed to low levels of this ROS [43, 44].
Limited information about PRDXs in the male reproductive tract is currently available, especially for humans. The studies so far have presented partial characterization of one member of the family and have usually been performed on rodents. An extensive literature search indicates that only one review paper [33] referred to the presence of expressed sequence tag for the PRDX1 gene in human and mice spermatogonia, spermatocytes, Sertoli cells, and prostate using the UniGene database. Unfortunately, no further analysis to determine the expression of the protein was performed. The other five PRDXs are found in testis from the rat, boar, mouse, and bull [45–49] and in spermatozoa from the mouse and boar [50]. PRDX4 is associated with acrosome formation during rat spermatogenesis [46] and has a protective role in the male reproductive tract, because the phenotype of mice lacking this isoform includes testicular atrophy and increased sperm DNA damage [51]. PRDX5 is associated with boar sperm plasma membrane and could be a protein candidate for binding with the zona pellucida in porcine species [52].
Considering the major effects of ROS on human sperm function, both of activation and of inhibition depending on the concentration, and the parallel that can be drawn with the peculiar roles of PRDXs in finely modulating the effects of low or high levels of ROS, we looked for the presence and localization of representative members of the PRDX family (PRDX1, PRDX4, PRDX5, and PRDX6) in human spermatozoa and aimed to determine whether these enzymes are modified by the treatment of cells with exogenous H2O2.
MATERIALS AND METHODS
Materials
Percoll was obtained from GE Healthcare. Rabbit polyclonal anti-PRDX1 (ab41906), rabbit polyclonal anti-PRDX4 (ab59542; used for immunocytochemistry), monoclonal anti-PRDX4 (ab16943; used for immunoblotting), monoclonal anti-PRDX5 (ab16944), monoclonal anti-PRDX6 (ab16947), polyclonal anti-PRDX6 sulfonated form (ab28444), and the antigenic peptide (ab41919; used to rise the anti-PRDX1) were purchased from Abcam, Inc. Nitrocellulose (pore size, 0.22 μm; Osmonics, Inc.), donkey anti-rabbit immunoglobulin (Ig) G and goat anti-mouse IgG (both conjugated to horseradish peroxidase; Cedarlane Laboratories Ltd.), an enhanced chemiluminescence kit (Lumi-Light; Roche Molecular Biochemicals), and radiographic films (Fuji) were also used for immunodetection of blotted proteins. Both biotinylated goat anti-rabbit IgG (H+L) and biotinylated horse anti-mouse IgG (H+L) were purchased from Vector Laboratories, Inc. Alexa Fluor 555 conjugate of streptavidin and Prolong Antifade were purchased from Invitrogen, Inc. SDS, phosphotungstic acid, butylated hydroxytoluene, 2-thiobarbituric acid, and malonaldehyde bis(dimethyl acetal) were purchased from Sigma-Aldrich Chemical Co. Catalase was purchased from EMD Biosciences. Other chemicals were of at least reagent grade.
Sperm Preparation and H2O2 of Spermatozoa
Healthy volunteers (n = 14) participating in the present study signed an informed consent document and provided semen samples that were normal according to World Health Organization criteria [53]. Informed consent was also obtained from the volunteers, and the ethics board of the Royal Victoria Hospital approved the present study.
Liquefied semen samples were washed on four-layer (95%-65%-40%-20%) Percoll gradient buffered in Hepes-balanced saline (115 mM NaCl, 4 mM KCl, 0.5 mM MgCl2, 14 mM fructose, and 25 mM Hepes [pH 8.0]). Samples were centrifuged for 30 min at 2300 × g, and spermatozoa at the 65%-95% Percoll interface and in the 95% Percoll layer were pooled and diluted to 400 × 106 cells/ml with the 95% Percoll solution. Special care was taken to prevent contamination of the sperm layers with the seminal plasma found at the top of the Percoll gradient. Only samples in which progressive sperm motility was greater than 70% after the Percoll gradient were used. Spermatozoa were further diluted to 100 × 106 cells/ml in Biggers, Whitten, and Whittingham (BWW) medium [54] devoid of bicarbonate and bovine serum albumin (BSA) and containing 1 mM CaCl2 and 25 mM Hepes (pH 8.0) and then treated at 37°C with H2O2 at different concentrations for 30 min. Sperm suspensions were mixed with electrophoresis sample buffer with or without (reducing or nonreducing condition, respectively) 100 mM dithiothreitol (DTT), incubated at 95°C for 5 min, and then centrifuged at 21 000 × g for 5 min. After centrifugation, supernatants of samples in nonreducing buffer were separated, and the respective pellet was resuspended with reducing sample buffer and incubated again at 95°C for 5 min before loading on electrophoresis gels.
Seminal Plasma Preparation
Seminal plasma was collected from the top of the four-layer (95%-65%-40%-20%) Percoll gradient (see above), diluted 1:25 in PBS, and centrifuged at 21 000 × g for 5 min. Then, the diluted seminal plasma was prepared for SDS-PAGE under reducing conditions as described above.
Lipid Peroxidation Determination
Spermatozoa were treated with H2O2 at different concentrations with or without 0.5 mg/ml of catalase for 30 min at 37°C to test whether H2O2 induced oxidative stress under the experimental conditions. We determined the levels of 2-thiobarbituric acid reactive substances (TBARS), as a marker for lipid peroxidation, as previously described [55] by spectrofluorometry using a multimode microplate reader (Fluostar Optima; BMG Labtech). Malondialdehyde, generated by the acid hydrolysis of malonaldehyde bis(dimethyl acetal), was used as standard [56], and the values presented as nmol TBARS/106 spermatozoa.
SDS-PAGE and Immunoblotting
Sperm proteins were electrophoresed on 12% (or 10%) (see Figs. 6 and 7) polyacrylamide gels and electrotransferred to nitrocellulose membranes using transfer buffer (192 mM glycine and 25 mM Tris [pH 8.3]) containing 20% methanol. The membranes were incubated with a solution of skim milk (5% w/v) in 20 mM Tris (pH 7.8)-buffered saline containing Tween 20 (0.1% v/v; TBS-T) for 30 min. The anti-PRDX1, anti-PRDX4, anti-PRDX5, anti-PRDX6, and anti-PRDX6 sulfonated form antibodies were used at 1:1000, 1:2000, 1:500, 1:10 000, and 1:2000 (v/v), respectively, according to manufacturers' instructions. After overnight incubation, membranes were washed, incubated with donkey anti-rabbit IgG or goat anti-mouse IgG conjugated with horseradish peroxidase (diluted 1:5000 v/v in TBS-T) for 45 min at 20°C and then washed again with TBS-T. Positive immunoreactive bands were detected using the LumiLight chemiluminescence kit. At the end of each experiment, blots were rinsed in distilled water and silver stained to ascertain that the amount of protein loaded in each well was the same.
For the specificity studies, 0.4 μg/ml of anti-PRDX1 antibody were incubated with 2 μg/ml of its antigenic peptide in TBS-T supplemented with 3% BSA for 2 h at room temperature. We also ascertained that our secondary antibodies, the donkey anti-rabbit IgG or goat anti-mouse IgG conjugated with horseradish peroxidase, did not recognize by themselves any bands when incubated on a set of membranes blotted with sperm samples (see Figs. 1 and 5E). The mentioned controls were performed for the immunocytochemistry studies (Fig. 2).
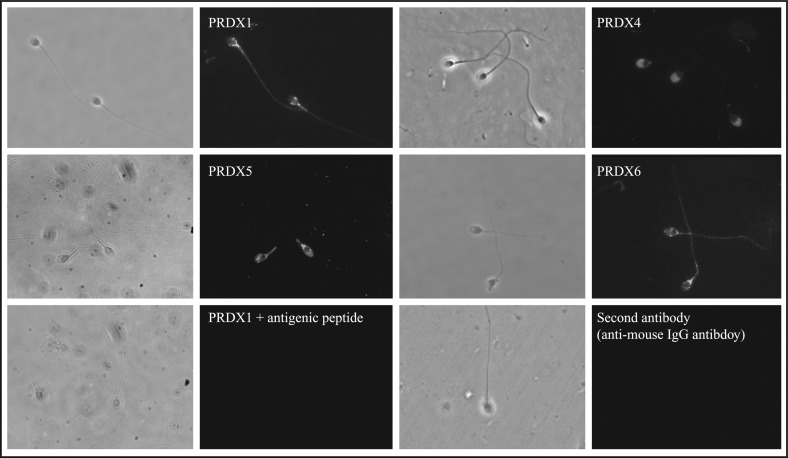
FIG. 2.
Peroxiredoxins are differentially localized in human spermatozoa. Fluorescence and phase contrast of the same microscopic field (observed at a magnification of ×1000) are presented. Percoll-washed spermatozoa were speared, fixed with methanol in the presence of Triton X-100, and then incubated with anti-PRDX antibodies overnight at 4°C. As mentioned in Figure 1, PRDX1 specificity and absence of labeling with the second antibody were also confirmed. Results of one experiment representative of two others (n = 3) performed on spermatozoa from different donors are shown.
Cellular Fractionation and Immunolocalization of PRDXs in Spermatozoa
Percoll-washed spermatozoa were diluted to 250 × 106 cells/ml, frozen at −80°C for 15 min, and then thawed at 37°C to disrupt sperm membranes and allow cytosolic contents to be released. After thawing and centrifugation at 12 000 × g at 4°C, the supernatant (cytosol-enriched fraction) was collected, and the pellet was incubated with Triton X-100 (0.2% v/v) for 10 min on ice. The Triton-soluble and -insoluble fractions were collected after centrifugation of these samples (12 000 × g). Then, the Triton-insoluble fraction was sonicated (three times for 15 sec each time at 30% output) with a Sonic Vibracell (Sonics and Materials, Inc.), and the dissociation of the heads and tails was monitored by phase-contrast microscopy. Heads and flagella fragments were then separated by a 5-min centrifugation (2000 × g at 4°C) over a discontinuous, 45%-90% Percoll gradient in Hepes-balanced saline. Flagellar fragment (45%-90% Percoll interface)- and head (pellet)-enriched fractions were solubilized in electrophoresis sample buffer under reducing conditions. Preliminary experiments performed with the inclusion of protease inhibitor cocktail (Bioshop Canada, Inc.) in all the steps for sperm preparation gave results (data not shown) similar to those presented here.
For the immunolocalization of PRDX, human sperm smears were prepared on SuperFrost Plus slides (Fisher Scientific). After permeabilization by methanol and rehydration, smears were treated with 5% normal goat serum in PBS containing 0.1% Triton X-100 (PBS-T; for PRDX1 and PRDX4) or 5% normal horse serum in PBS-T (for PRDX5 and PRDX6) for 30 min. Then, smears were washed with PBS-T and incubated with the anti-PRDX1 (1:25), anti-PRDX4 (1:50), anti-PRDX5 (1:25), or anti-PRDX6 (1:10) antibody overnight at 4°C. Finally, smears were washed with PBS-T and incubated with biotinylated goat anti-rabbit (1:200) or biotinylated horse anti-mouse (1:150) antibody for 1 h at room temperature and then with an Alexa Fluor 555 conjugate of streptavidin (1:500 v/v) in PBS-T. Smears were mounted with Prolong Antifade and observed under a Carl Zeiss Axiophot microscope (exciter filter BP450–490) at 1000× magnification. As controls, smears were incubated with the anti-PRDX1 antibody preadsorbed with the antigenic peptide (see above), with the biotinylated goat anti-rabbit antibody alone, or with the biotinylated horse anti-mouse antibody alone.
RESULTS
PRDXs Are Present in Human Semen
Peroxiredoxins are classified in three groups according to the number of N- and C-terminal-conserved Cys residues that are essential for function. In the present study, we characterized at least one representative member of each group of the PRDX family: isoforms 1 and 4 (2-Cys PRDX), isoform 5 (atypical 2-Cys PRDX), and isoform 6 (1-Cys PRDX). We included PRDX4 in the present study because this isoform is associated with acrosome formation during spermatogenesis [46] and Prdx4−/− male mice have testicular atrophy and increased sperm DNA damage [51].
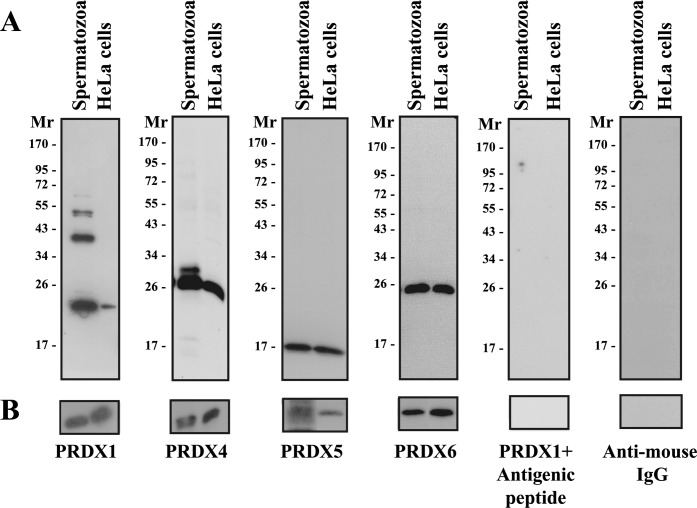
Using SDS-PAGE under reducing conditions, PRDX1, PRDX4, PRDX5, and PRDX6 were detected in ejaculated human spermatozoa (Fig. 1A) and seminal plasma (Fig. 1B). The anti-PRDX1 antibody recognized four distinctive bands of 23, 42/54 (doublet), and 65 kDa, and its specificity has been confirmed. The recognition of other bands with anti-PRDX antibodies has been described previously: Multiple bands of PRDX1 (20, ∼28, 75, and ∼250 kDa) and PRDX3 (25, 50, and 100 kDa) are found in rat heart [57]. PRDX2 is present as multiple bands in boar testis (20, 41–53, and 80 kDa), spermatozoa (20 and 80 kDa), and seminal plasma (41 and 80 kDa) [50]. PRDX1 is Triton-insoluble in human spermatozoa (Fig. 3), and these extra bands may correspond to dimers or trimers formed by bonds other than disulfide bridges.
FIG. 1.
Peroxiredoxins are present in human spermatozoa and seminal plasma. A) Percoll-washed spermatozoa were solubilized in SDS-PAGE sample buffer (reducing conditions) and solubilized proteins from 0.1–0.5 × 106 spermatozoa were loaded in each well (depending on the PRDX). B) Seminal plasma collected from the top of the Percoll gradient was diluted 1:25 in PBS (pH 7.4), centrifuged at 21 000 × g for 5 min, and finally supplemented with electrophoresis sample buffer (with reducer). Immunoblotting using the anti-PRDX1, anti-PRDX4, anti-PRDX5, or anti-PRDX6 antibody was performed as described in Materials and Methods. Specificity of the PRDX1 antibody was ascertained by preincubation with the antigenic peptide. The anti-mouse IgG antibody alone did not recognize any protein bands. Results of one experiment representative of five others (n = 6) using semen from different donors are shown. HeLa cells solubilized in electrophoresis sample buffer were used as positive control. The position of molecular mass standards (× 10−3) is given on the left of each immunoblot.
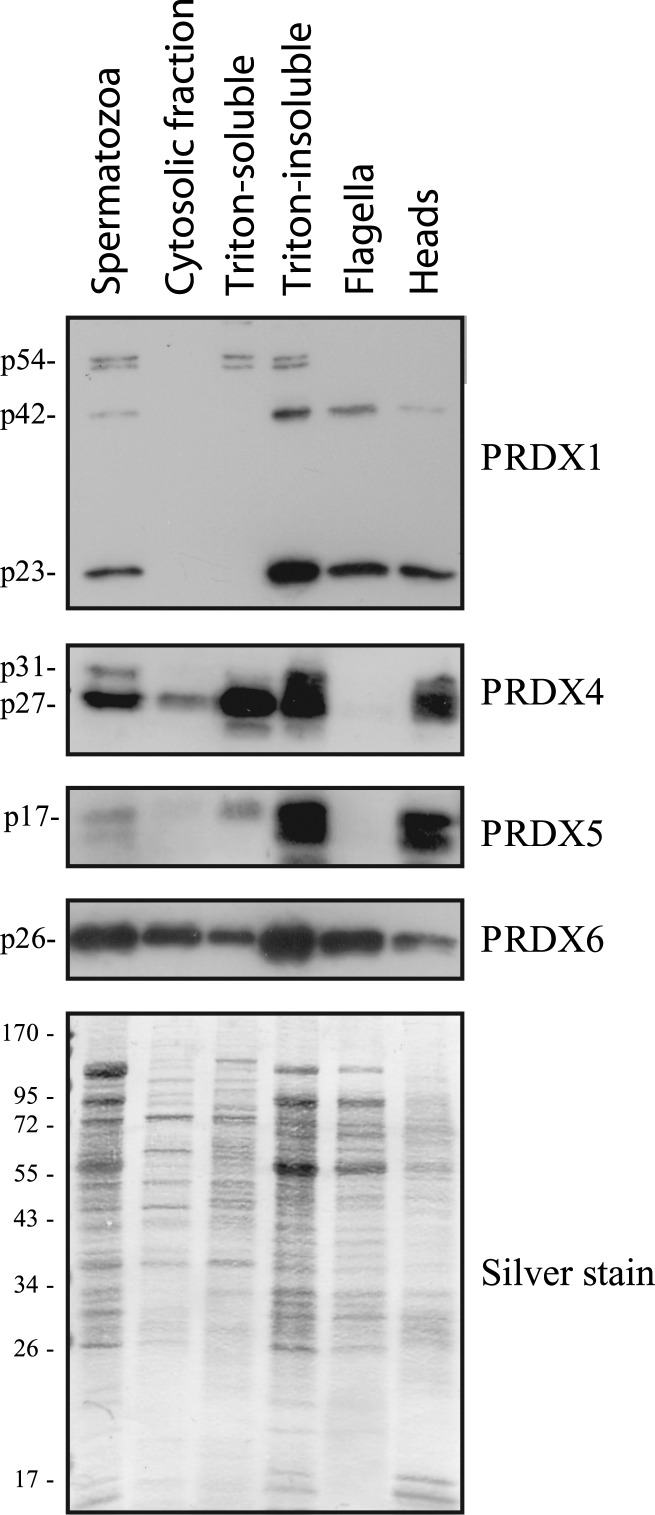
FIG. 3.
Presence of PRDXs in sperm extracts. Proteins from whole spermatozoa (1 × 106 cells/well), cytosol-enriched fraction (equivalent to 2.5 × 106 cells/well), Triton-soluble (equivalent to 2.5 × 106 cells/well) and Triton-insoluble (equivalent to 2.5 × 106 cells/well) extracts, as well as from flagella-enriched and heads-enriched (both equivalent to 5 × 106 cells/well) fractions, were immunoblotted as described previously. Results representative of two others (n = 3) performed with spermatozoa from different donors are shown.
The anti-PRDX4 antibody recognized two bands of 27 and 31 kDa in sperm cells, but only the 27-kDa band was found in HeLa cells (positive control). PRDX5 and PRDX6 were present in human spermatozoa and HeLa cells, with an apparent molecular mass of 17 and 26 kDa, respectively. The antigenic peptides for the anti-PRDX4, anti-PRDX5, and anti-PRDX6 antibodies are not commercially available. However, the protein bands that were recognized had similar molecular masses in both spermatozoa and HeLa cells, and the anti-mouse IgG antibody alone did not react with any proteins bands.
We have found PRDX1, PRDX4, PRDX5, and PRDX6 in seminal plasma (Fig. 1B). The anti-PRDX1 and anti-PRDX4 antibodies recognized only single bands of 23 and 27 kDa, respectively. PRDX5 and PRDX6 were at the same apparent molecular mass as observed in spermatozoa. The amount of these PRDXs in seminal plasma was high (samples must be diluted 25-fold for immunoblotting studies). Therefore, these enzymes are an important component of antioxidant protection after ejaculation.
Localization of PRDXs in Human Spermatozoa
The localization of PRDXs in spermatozoa appeared to be isoform-specific (Fig. 2). PRDX1 and PRDX6 were located on the sperm tail and in the equatorial segment and postacrosomal region of the head. PRDX4 was mainly present on the acrosome and less so in the postacrosomal region, and PRDX5 was found on the head (mostly the postacrosomal region) and mitochondrial sheath region.
Subcellular fractions have been prepared. PRDX4 (p27 band) and PRDX6 were present in the cytosol-enriched and Triton-soluble and -insoluble fractions, whereas PRDX1 (p23, p42, and p54 bands) and PRDX5 were found mostly in the Triton-insoluble fraction (Fig. 3). Both forms of PRDX4 (p27 and p31) were present in the Triton-insoluble fractions.
We then used the Triton-insoluble sperm material to prepare flagella- or heads-enriched fractions (Fig. 3). As expected from Figure 2, PRDX1 and PRDX6 were found in both fractions, and PRDX4 and PRDX5 were found only in the heads fraction. Interestingly, the p54 band recognized by the anti-PRDX1 antibody was present in the Triton-soluble and -insoluble fractions, whereas p42 was found in the Triton-insoluble and flagella fractions and less so in the heads fraction.
Effect of H2O2 Treatment on Sperm PRDXs
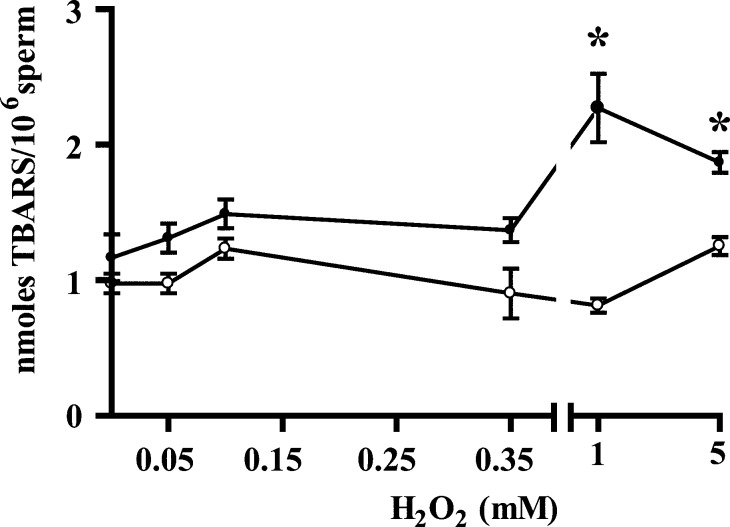
We challenged spermatozoa with H2O2, both at low concentrations known to activate these cells and at higher levels known to affect not only motility and viability but also mitochondrial and nuclear sperm DNA [15, 58]. When used at high concentrations (1 and 5 mM), H2O2 produces an oxidative stress, as confirmed by higher levels of TBARS, a marker of lipid peroxidation (Fig. 4). As expected, catalase, a scavenger for H2O2, prevented this effect.
FIG. 4.
Lipid peroxidation (as measured by TBARS) in human spermatozoa treated with H2O2. Percoll-washed spermatozoa incubated in BWW medium at 37°C for 30 min with H2O2. After treatment, samples were divided into two aliquots with or without 0.5 mg/ml of catalase. Levels TBARS were determined (see Materials and Methods). Results are presented as the mean ± SEM of three measurements performed with sperm samples from different donors. An asterisk indicates a value significantly (P < 0.05) higher than the control (0 mM H2O2) as analyzed by one-way ANOVA plus Bonferroni post hoc test.
Spermatozoa were then prepared for SDS-PAGE under nonreducing conditions to evaluate the possible formation of disulfide bridges resulting from the treatment with H2O2. The insoluble portion of the samples (pellet of samples boiled in the absence of reducer) was then treated with sample buffer containing DTT to determine whether PRDX were associated with other proteins via disulfide bridges.
Increasing concentrations of H2O2 caused a decrease in the signal of all PRDX bands in sperm samples evaluated under nonreducing conditions (Fig. 5). PRDX4 and PRDX5 have the same molecular mass in sperm samples under nonreducing or reducing conditions. Interestingly, doublets of PRDX1 and PRDX6 were observed: two doublets for PRDX1 (51/54 and 37/42 kDa) and one for PRDX6 (25.5/26 kDa), apparently formed by disulfide (-S-S-) interaction, because the addition of DTT reversed the effect (Fig. 5A, third panel). The p51 and p42 bands recognized by the anti-PRDX1 antibody likely are dimers of the p23 (23 ± 2 kDa, mean ± SD), a band that disappears when samples are in nonreducing conditions (Fig. 1A, first panel). The H2O2 treatment (as low as 50 μM) caused the PRDX6 doublet to merge into a strong single band, the intensity of which decreased at high H2O2 concentrations (1 and 5 mM) (Fig. 5). The possibility of unequal loading of the wells was excluded when the same sperm samples were also electrophoresed under reducing conditions and the levels of PRDXs were found to be identical (Fig. 5, third panel for each PRDX).
FIG. 5.
Peroxiredoxins are differentially modified by exogenous H2O2. A–D) Percoll-washed spermatozoa incubated in BWW medium at 37°C for 30 min with H2O2 were prepared for SDS-PAGE under nonreducing conditions and centrifuged, and the supernatant was loaded (Supernatant). The remaining pellets were supplemented with sample buffer containing 100 mM DTT [reducing conditions; Pellet (+DTT)]. An aliquot of spermatozoa was also supplemented with sample buffer with DTT (Supernatant + DTT) to ascertain that the total PRDX was constant in all samples. Note the absence of the bands at high molecular mass seen in the first panel for PRDX1 and PRDX6, confirming that molecular mass complexes are formed by disulfide bridges. Immunoblotting was performed as described in Materials and Methods. Results of one experiment representative of three others (n = 4) made with semen samples from different donors are shown. E) Samples from spermatozoa incubated in BWW medium at 37°C for 30 min with 0, 1, or 5 mM H2O2 were prepared for SDS-PAGE under nonreducing conditions and immunoblotted with the anti-PRDX1 antibody preincubated with the antigenic peptide (left) or the anti-mouse IgG antibody used to recognize the anti-PRDX6 antibody. HeLa cells were used as positive control. Results of one experiment representative of two others (n = 3) made with semen samples from different donors are shown.
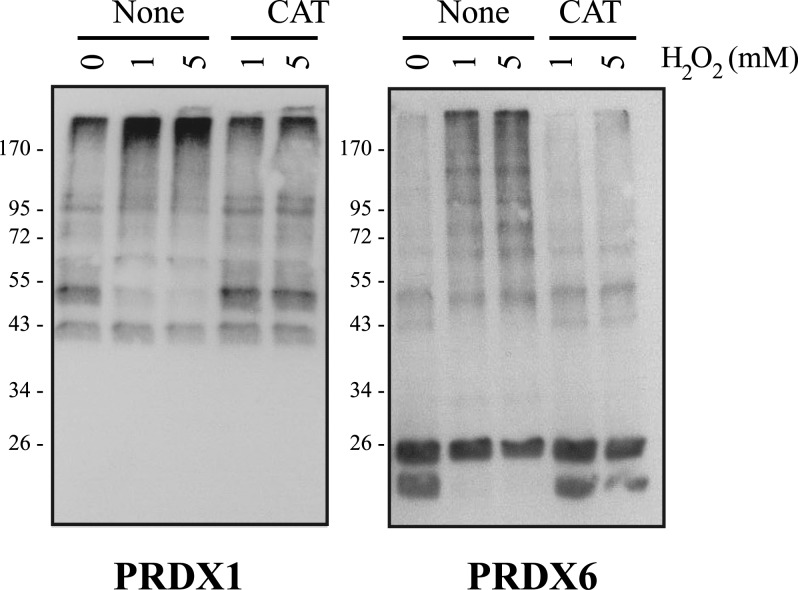
Because of this, the portion of the sperm samples that was not solubilized in the nonreducing conditions was then treated with complete electrophoresis buffer (containing 100 mM DTT). Interestingly, an increase in the levels of all the PRDXs was then found, suggesting that H2O2 treatment causes the formation of disulfide bridges between PRDXs and, potentially, other proteins and of macromolecular complexes that are too big to enter into gel and/or are insoluble under nonreducing conditions. These findings are also in agreement with the observation that higher concentrations of H2O2 caused the appearance of bands with high molecular mass recognized by the anti-PRDX1 and anti-PRDX6 antibodies under nonreducing conditions (Fig. 5). These high-molecular-mass bands were not observed when DTT was added to the sperm suspension for PRDX1 (Fig. 5A, third panel) and for PRDX6 (Fig. 5D, third panel), suggesting the presence of disulfide bridges. Moreover, these bands were not detected with the anti-PRDX1 antibody previously incubated with its antigenic peptide or with the anti-mouse IgG used to recognize the anti-PRDX6 antibody (Fig. 5E). The decrease of intensity and the production of high-molecular-mass complexes were prevented by catalase (Fig. 6), confirming that these changes on PRDXs are the result of H2O2. Superoxide dismutase did not prevent oxidation or the formation of high-molecular-mass complexes, thus suggesting that superoxide anion is not involved in these events (data not shown). The decrease of PRDX signal in the sperm proteins solubilized under nonreducing conditions (supernatant) was accompanied by an increase of the signal in the insoluble fraction of the sperm sample (pellet from the sample in nonreducing conditions, treated with DTT).
FIG. 6.
Catalase prevented the formation of high-molecular-mass complexes containing PRDX1 or PRDX6. Percoll-washed spermatozoa incubated in BWW medium at 37°C for 30 min with H2O2 in the presence or absence of 0.1 mg/ml of catalase were prepared for SDS-PAGE (10% acrylamide gels) under nonreducing conditions and immunoblotted with the anti-PRDX1 or anti-PRDX6 antibody as described in Materials and Methods. Results of one experiment representative of another (n = 2) made with semen samples from different donors are shown.
We further investigated the presence of hyperoxidated PRDX6 in spermatozoa treated with H2O2 and prepared under nonreducing and reducing conditions for immunoblotting (Fig. 7). In samples supplemented with DTT (reducing conditions), H2O2 promoted hyperoxidation of PRDX6, which was maximum at a concentration of 5 mM (Fig. 7A). Interestingly, a strong band of high molecular mass was observed in samples treated with 5 mM H2O2 and supplemented with sample buffer without DTT (nonreducing conditions) (Fig. 7B).
FIG. 7.
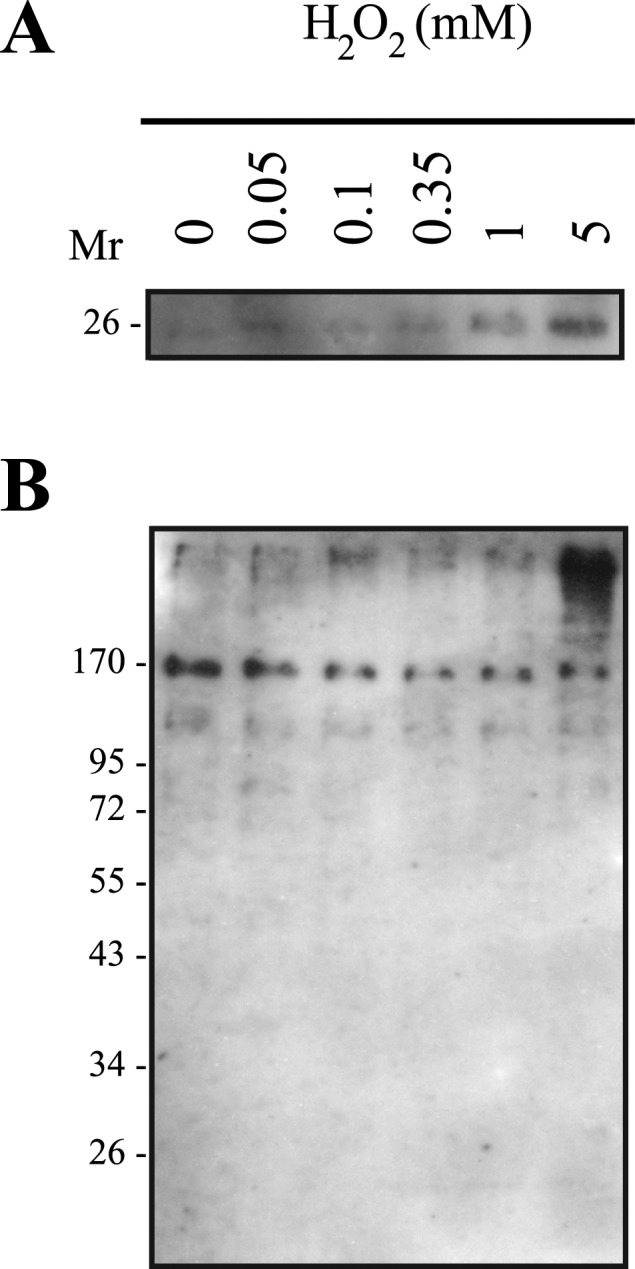
PRDX6 is hyperoxidized by high concentrations of H2O2 and is present with a high molecular mass in human spermatozoa. Percoll-washed spermatozoa were incubated in BWW medium at 37°C for 30 min with H2O2, prepared for SDS-PAGE (10% acrylamide gels) under reducing (A) and nonreducing (B) conditions, and immunoblotted with the antibody that recognizes the sulfonated form of PRDX6. Results of one experiment representative of two others (n = 3) made with semen samples from different donors are shown.
DISCUSSION
In the present study, we characterized, to our knowledge for the first time, the presence and location of PRDX1, PRDX4, PRDX5, and PRDX6 in human spermatozoa. The dose-dependent modification resulting from exogenous H2O2 suggests the formation of macromolecular complexes involving, in turn, the formation of disulfide bridges and the hyperoxidation of PRDXs in those complexes.
Spermatozoa are highly specialized and terminal cells with a unique goal: to deliver the paternal DNA into the oocyte. Thus, sperm motility and DNA integrity are two major requirements for achieving this purpose. High levels of ROS have a negative impact on both sperm motility machinery and chromatin [22, 25].
We found that PRDX1, PRDX4, PRDX5, and PRDX6 are present in human seminal plasma (Fig. 1B). These findings are in accordance with those of a previous proteomics study on this biological fluid [59], and they suggest that these enzymes are part of the antioxidant defense required to protect ejaculated spermatozoa from high levels of ROS [20, 60, 61]. Immunocytochemistry studies revealed that PRDX1, PRDX4, PRDX5, and PRDX6 have different localization in human spermatozoa (Fig. 2). PRDX1 and PRDX6 are present in the equatorial segment and postacrosomal region of the head and in the tail, whereas PRDX4 is strongly expressed in the acrosomal region, and PRDX5 in the postacrosomal region of the head and the midpiece. These differences could be associated with what is presently known of ROS in sperm function and suggest a role for PRDXs as modulators of ROS action in different compartment of human spermatozoa.
High amounts of ROS cause the depletion on ATP in human spermatozoa and, thus, decrease motility [25]. The glycolytic enzyme glyceraldehyde-3-phosphodehydrogenase, an essential component of the sperm tail necessary for motility and male fertility [62], can be inactivated by ROS [63]. The presence of PRDX1 and PRDX6 in the tail (Fig. 2) and in the flagella-enriched and Triton-insoluble fractions (Fig. 3) suggests that these enzymes might be part of the mitochondrial sheath (PRDX1) and principal piece (PRDX1 and PRDX6) of human spermatozoa and may play a role in protecting vital components of this complex structures against oxidative stress. Interestingly, a similar situation occurs with PRDX2 that immunoprecipitates along with GPX4 and sperm mitochondria-associated Cys-rich protein in boar seminiferous tubule extracts, which would possibly make PRDX2 a functional component of the sperm mitochondrial sheath [50]. It is tempting to extrapolate and suggest that a similar association occurs in spermatozoa, but this is only a hypothesis mainly because these data were obtained from extracts that contained other cell types (e.g., Sertoli cells). In agreement with this and with the use of immunohistochemistry, we found that PRDX2 (and also PRDX1, PRDX4, and PRDX6) are expressed in rat Sertoli cells (data not shown).
The similar strong labeling on the base of the head (Fig. 2) and the Triton insolubility (Fig. 3) could suggest that PRDX1 and PRDX6 are associated with the perinuclear theca. This complex sperm structure was elegantly characterized by Oko and Maravei [64] and by Sutovsky et al. [65] and can be regionalized in the subacrosomal layer, involved in acrosomal assembly during spermiogenesis [66], and in the postacrosomal sheath, involved in sperm-egg interaction during fertilization [65, 67].
Sperm cytosol-enriched fractions did not contain PRDX1 (Fig. 3), in contrast to what has been observed in the cytosol of somatic cells [40, 68]. This finding, along with the localization in the tail and in the Triton-insoluble sperm fraction, reinforces a role of PRDX1 as antioxidant and regulator of ROS action in the sperm flagellum. PRDX5 was found in the midpiece and the head of spermatozoa (Fig. 2); similar to what occurs in somatic cells, this isoform could control ROS effects within the sperm mitochondria and nucleus.
During rat and mouse spermatogenesis, PRDX4 is involved in acrosome formation [46, 51]. Moreover, mice lacking the Prdx gene have testicular atrophy, low epididymal sperm count, increased spermatogenic cell death, and increased susceptibility to oxidative stress [51]. Interestingly, these mice are fertile, which suggests that PRDX4 is not essential for male fertility [51]. However, rodents have normal numbers of offspring even with extremely low sperm counts, such as those resulting from suppression of spermatogenesis by androgen administration [69]. Thus, a relevant role for PRDX4 in male reproduction can be still considered, especially in men with idiopathic infertility.
We observed that human ejaculated spermatozoa have both forms of PRDX4 (Fig. 1), mainly associated with the acrosome region (Fig. 2) and the heads-enriched fraction (Fig. 3). Contrary to the 27-kDa secreted form, the 31-kDa isoform, which is also present in rat and mouse testis [46, 51], was found only in the Triton-insoluble and heads-enriched fractions (Fig. 3), suggesting differential actions depending on the subcellular localization between these two forms. Two successive extractions with Triton X-100 did not abolish the signal from the heads-enriched fraction (data not shown), suggesting that both PRDX4 isoforms are associated with the sperm nucleus and may be part of the perinuclear theca.
In human spermatozoa, PRDX5 was found in the acrosome and postacrosomal region, midpiece (Fig. 2), and the Triton-soluble and -insoluble and heads-enriched fractions (Fig. 3). This enzyme is present in the cytosol and mitochondria of somatic cells [70] and in the acrosomal membranes involved in zona pellucida binding in porcine species [52]. Therefore, PRDX5 may play a role in regulating ROS actions in the mitochondria. The localization in the postacrosomal region and the presence in the sperm Triton-insoluble fraction suggest that PRDX5 may be part of the perinuclear theca and may be involved in sperm-egg fusion events [67]. Although we used two different techniques to ascertain PRDX localization in human spermatozoa, we are aware that immunocytochemistry at the electron-microscope level could provide final confirmation of the biochemical and immunofluorescence results presented here.
Beside the confirmation that human spermatozoa possess PRDXs (at least PRDX1, PRDX4, PRDX5, and PRDX6), we evaluated the effects of H2O2 on these enzymes. We selected various concentrations of H2O2 from 0.05 mM (induction of capacitation and associated phosphorylation events) [9] and 0.35 to 5 mM (damage to mitochondrial and nuclear sperm DNA, respectively) [58]. A major dose-dependent decrease was observed in the intensity of all the PRDX bands (nonreducing conditions) (Fig. 5). Interestingly, this was accompanied by increases in both the level of high-molecular-mass proteins (PRDX1 and PRDX6) and the intensity of the respective bands in the pellet of these sperm samples subsequently incubated with 100 mM DTT (reducing conditions).
We found that both PRDX1 and PRDX6 appeared as high-molecular-mass bands (>170 kDa) in spermatozoa submitted to H2O2 at 1 and 5 mM (Fig. 5, A and D). These results suggest the formation of macromolecular complexes (e.g., homodecamers) as reported before when purified PRDX were submitted to strong oxidative stress [31, 71] or, alternatively, between PRDX molecules and other sperm proteins. We confirm that these complexes contained PRDX1 or PRDX6, because they were not recognized by the anti-PRDX1 antibody previously treated with its antigenic peptide or by the anti-mouse IgG antibody (Fig. 5E), thus discarding nonspecific binding. Eukaryotic as well as prokaryotic typical 2-Cys PRDXs (e.g., PRDX1) adopt different conformation states, which are linked to switches in function (e.g., peroxidase, chaperone, binding partner, enzyme activator, and/or redox sensor) [72]. The peroxidase activity of 2-Cys PRDXs is inhibited by the oxidation of their active Cys to sulfonic acid, thus enhancing their chaperone activity [73]. The reduced and hyperoxidized dimers strongly tend to form decamers or dodecamers [31], whereas the oxidized form is preferentially present as dimer [71]. Taken together, these results suggest that under a strong oxidative stress, both sperm PRDX1 and PRDX6 become hyperoxidized and form high-molecular-mass complexes. PRDX1 and PRDX3 formed high-molecular-mass complexes when isolated rat hearts were perfused with high concentrations of H2O2 [57]. It has been demonstrated that PRDX2 (a 2-Cys PRDX) forms high-molecular-mass complexes in HeLa cells treated with H2O2, thus protecting them from H2O2-induced cell death [74]. It is possible that PRDX1 and PRDX6 are converted in high-molecular-mass complexes to prevent misfolding or unfolding of proteins or that they become molecular chaperones [75] to protect unfolded proteins in human spermatozoa challenged during a strong oxidative stress. Interestingly, the high-molecular-mass complexes detected in H2O2-treated spermatozoa (nonreducing conditions) are not solubilized when cells are extracted with RIPA buffer (150 mM NaCl, 0.1% SDS, 1% Triton X-100, and 0.5% sodium deoxycholate) or BWW medium supplemented with 0.2% Triton X-100. PRDX1 or PRDX6 were then found only in the RIPA- or Triton-insoluble fractions (data not shown). The high-molecular-mass band in H2O2-treated spermatozoa can be partially extracted with SDS (as seen on SDS-PAGE) but only at concentrations that prevent immunoprecipitation. Therefore, the identification of proteins (PRDXs as well as potential partners linked by disulfide bridges) in these complexes will require special extraction techniques. Although the DTT treatment abolished the presence of these high-molecular-mass complexes, suggesting the presence of disulfide bridges among PRDX1 or PRDX6 molecules forming decamers or dodecamers [31, 71], we cannot rule out the possibility that other proteins may be present in the complexes.
Hyperoxidation of the active Cys is necessary for PRDX to switch the peroxidative activity to chaperones [76, 77]. We found that PRDX6 is hyperoxidized by high levels of H2O2 (Fig. 7). This result suggests that PRDX6 present in the high-molecular-mass complexes is hyperoxidized and may act as a chaperone. The 2-Cys PRDXs (including PRDX1) can also be hyperoxidized and switch from peroxidative to chaperone activity [77, 78], but confirmation of this hypothesis must wait for the development of a suitable and efficient antibody. However, sufficient data in the literature encourage us to suggest that PRDX1 can act as chaperone in human spermatozoa. The prominence of several PRDXs in all sperm compartments as well as their behavior in H2O2-treated cells suggest that a major role for these enzymes in the regulation of ROS signaling and, because of their ability to switch to chaperone activity, in protecting against strong oxidative stress of ejaculated human spermatozoa.
The addition of 50 μM of H2O2 triggers capacitation and the associated protein tyrosine phosphorylation without decreasing motility of human spermatozoa [9]. The doublet of PRDX6 bands is present in untreated spermatozoa (nonreducing conditions) (Fig. 5D) and becomes a singlet, but with double intensity, when cells are challenged with 50 μM H2O2. The finding that PRDX6 is susceptible even to these very low levels of ROS suggests that PRDX6 may be involved in the redox control of capacitation.
In conclusion, we present here the first study, to our knowledge, of the PRDX family in human spermatozoa. PRDXs are specifically present in all subcellular compartments; they appear to actively react with H2O2, which could provide protection to spermatozoa against this ROS. The fact that low levels of H2O2 also modified PRDXs suggests their role in controlling ROS levels within different compartments necessary for normal sperm function in humans.
Acknowledgments
We thank Dr. Eve de Lamirande for her valuable comments and Mr. Stefan Patrascu and Ms. Shasha Gong for their technical assistance. We also thank the volunteers who participated in the present study.
Footnotes
Supported by a grant from the Institute for Human Development, Child and Youth Health of the Canadian Institutes of Health Research and by a grant from the Research Institute-McGill University Health Centre and the Urology Division, McGill University, to C.O.
REFERENCES
- Yanagimachi R. Mammalian fertilization. Knobil E, Neill D. (Eds.), The Physiology of Reproduction. New York: Raven Press; 1994: 189 318. [Google Scholar]
- de Lamirande E, Leclerc P, Gagnon C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol Hum Reprod 1997; 3: 175 194. [DOI] [PubMed] [Google Scholar]
- Leclerc P, de Lamirande E, Gagnon C. Cyclic adenosine 3′,5′-monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod 1996; 55: 684 692. [DOI] [PubMed] [Google Scholar]
- Luconi M, Krausz C, Barni T, Vannelli GB, Forti G, Baldi E. Progesterone stimulates p42 extracellular signal-regulated kinase (p42erk) in human spermatozoa. Mol Hum Reprod 1998; 4: 251 258. [DOI] [PubMed] [Google Scholar]
- O'Flaherty C, de Lamirande E, Gagnon C. Phosphorylation of the arginine-X-X-(serine/threonine) motif in human sperm proteins during capacitation: modulation and protein kinase A dependency. Mol Hum Reprod 2004; 10: 355 363. [DOI] [PubMed] [Google Scholar]
- O'Flaherty C, de Lamirande E, Gagnon C. Reactive oxygen species and protein kinases modulate the level of phospho-MEK-like proteins during human sperm capacitation. Biol Reprod 2005; 73: 94 105. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, O'Flaherty C. Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta 2008; 1784: 106 115. [DOI] [PubMed] [Google Scholar]
- O'Flaherty C, de Lamirande E, Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radic Biol Med 2006; 41: 528 540. [DOI] [PubMed] [Google Scholar]
- O'Flaherty C, de Lamirande E, Gagnon C. Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic Biol Med 2006; 40: 1045 1055. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C. Capacitation-associated production of superoxide anion by human spermatozoa. Free Radic Biol Med 1995; 18: 487 495. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Harkiss D, Knox W, Paterson M, Irvine DS. A novel signal transduction cascade in capacitating human spermatozoa characterized by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J Cell Sci 1998; 111: 645 656. [DOI] [PubMed] [Google Scholar]
- Herrero B, Chatterjee S, Lefievre L, de Lamirande E, Gagnon C. Nitric oxide interacts with the cAMP pathway to modulate capacitation of human spermatozoa. Free Radic Biol Med 2000; 29: 522 536. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C. Paradoxical effect of reagents for sulfhydryl and disulfide groups on human sperm capacitation and superoxide production. Free Radic Biol Med 1998; 25: 803 817. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C. Redox control of changes in protein sulfhydryl levels during human sperm capacitation. Free Radic Biol Med 2003; 35: 1271 1285. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C. Impact of reactive oxygen species on spermatozoa: a balancing act between beneficial and detrimental effects. Hum Reprod 1995; 10 (suppl 1): 15 21. [DOI] [PubMed] [Google Scholar]
- Sikka SC, Rajasekaran M, Hellstrom WJ. Role of oxidative stress and antioxidants in male infertility. J Androl 1995; 16: 464 468. [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol 2006; 18: 325 332. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Baker MA. Oxidative stress, sperm survival and fertility control. Mol Cell Endocrinol 2006; 250: 66 69. [DOI] [PubMed] [Google Scholar]
- Lewis SEM, Sterling ES, Young IS, Thompson W. Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. Fertil Steril 1997; 67: 142 147. [DOI] [PubMed] [Google Scholar]
- Zini A, de Lamirande E, Gagnon C. Reactive oxygen species in semen of infertile patients: levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. Int J Androl 1993; 16: 183 188. [DOI] [PubMed] [Google Scholar]
- Storey BT. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol Hum Reprod 1997; 3: 203 213. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, Irvine DS. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod 1998; 59: 1037 1046. [DOI] [PubMed] [Google Scholar]
- Gallon F, Marchetti C, Jouy N, Marchetti P. The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil Steril 2006; 86: 1526 1530. [DOI] [PubMed] [Google Scholar]
- Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, Aitken RJ. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab 2008; 93: 3199 3207. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C. Reactive oxygen species and human spermatozoa. II. Depletion of adenosine triphosphate plays an important role in the inhibition of sperm motility. J Androl 1992; 13: 379 386. [PubMed] [Google Scholar]
- Conrad M, Moreno SG, Sinowatz F, Ursini F, Kolle S, Roveri A, Brielmeier M, Wurst W, Maiorino M, Bornkamm GW. The nuclear form of phospholipid hydroperoxide glutathione peroxidase is a protein thiol peroxidase contributing to sperm chromatin stability. Mol Cell Biol 2005; 25: 7637 7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini F, Heim S, Kiess M, Maiorino M, Roveri A, Wissing J, Flohe L. Dual function of the selenoprotein PHGPx during sperm maturation. Science 1999; 285: 1393 1396. [DOI] [PubMed] [Google Scholar]
- Williams K, Frayne J, Hall L. Expression of extracellular glutathione peroxidase type 5 (GPX5) in the rat male reproductive tract. Mol Hum Reprod 1998; 4: 841 848. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 2005; 38: 1543 1552. [DOI] [PubMed] [Google Scholar]
- Miranda-Vizuete A, Sadek CM, Jimenez A, Krause WJ, Sutovsky P, Oko R. The mammalian testis-specific thioredoxin system. Antioxid Redox Signal 2004; 6: 25 40. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Schroder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci 2003; 28: 32 40. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life 2001; 52: 35 41. [DOI] [PubMed] [Google Scholar]
- Immenschuh S, Baumgart-Vogt E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid Redox Signal 2005; 7: 768 777. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol 2005; 17: 183 189. [DOI] [PubMed] [Google Scholar]
- Zhang P, Liu B, Kang SW, Seo MS, Rhee SG, Obeid LM. Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of Bcl-2. J Biol Chem 1997; 272: 30615 30618. [DOI] [PubMed] [Google Scholar]
- Dubuisson M, Vander Stricht D, Clippe A, Etienne F, Nauser T, Kissner R, Koppenol WH, Rees JF, Knoops B. Human peroxiredoxin 5 is a peroxynitrite reductase. FEBS Lett 2004; 571: 161 165. [DOI] [PubMed] [Google Scholar]
- Peshenko IV, Shichi H. Oxidation of active center cysteine of bovine 1-Cys peroxiredoxin to the cysteine sulfenic acid form by peroxide and peroxynitrite. Free Radic Biol Med 2001; 31: 292 303. [DOI] [PubMed] [Google Scholar]
- Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J Biol Chem 1998; 273: 6297 6302. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 2003; 300: 650 653. [DOI] [PubMed] [Google Scholar]
- Immenschuh S, Baumgart-Vogt E, Tan M, Iwahara Si, Ramadori G, Fahimi HD. Differential cellular and subcellular localization of heme-binding protein 23/peroxiredoxin I and heme oxygenase-1 in rat liver. J Histochem Cytochem 2003; 51: 1621 1631. [DOI] [PubMed] [Google Scholar]
- Seo MS, Kang SW, Kim K, Baines IC, Lee TH, Rhee SG. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J Biol Chem 2000; 275: 20346 20354. [DOI] [PubMed] [Google Scholar]
- Kang SW, Rhee SG, Chang TS, Jeong W, Choi MH. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol Med 2005; 11: 571 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baty JW, Hampton MB, Winterbourn CC. Proteomic detection of hydrogen peroxide-sensitive thiol proteins in Jurkat cells. Biochem J 2005; 389: 785 795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin AV, Low FM, Paton LN, Maghzal GJ, Hampton MB, Winterbourn CC. The high reactivity of peroxiredoxin 2 with H2O2 is not reflected in its reaction with other oxidants and thiol reagents. J Biol Chem 2007; 282: 11885 11892. [DOI] [PubMed] [Google Scholar]
- Matsuki S, Sasagawa I, Iuchi Y, Fujii J. Impaired expression of peroxiredoxin 4 in damaged testes by artificial cryptorchidism. Redox Rep 2002; 7: 276 278. [DOI] [PubMed] [Google Scholar]
- Sasagawa I, Matsuki S, Suzuki Y, Iuchi Y, Tohya K, Kimura M, Nakada T, Fujii J. Possible involvement of the membrane-bound form of peroxiredoxin 4 in acrosome formation during spermiogenesis of rats. Eur J Biochem 2001; 268: 3053 3061. [DOI] [PubMed] [Google Scholar]
- Lee K, Park JS, Kim YJ, Soo Lee Y, Sook Hwang T, Kim DJ, Park EM, Park YM. Differential expression of Prx I and II in mouse testis and their up-regulation by radiation. Biochem Biophys Res Commun 2002; 296: 337 342. [DOI] [PubMed] [Google Scholar]
- Onorato TM, Brown PW, Morris PL. Mono-(2-ethylhexyl) phthalate increases spermatocyte mitochondrial peroxiredoxin 3 and cyclooxygenase 2. J Androl 2008; 29: 293 303. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shen H, Ma L, Shen B, Xu Z, Wang X. Differential expression of peroxiredoxin 6 in fetal rat testis following in utero exposure to di(n-butyl) phthalate. Toxicology 2007; 240: 86 95. [DOI] [PubMed] [Google Scholar]
- Manandhar G, Miranda-Vizuete A, Pedrajas JR, Krause WJ, Zimmerman S, Sutovsky M, Sutovsky P. Peroxiredoxin 2 and peroxidase enzymatic activity of mammalian spermatozoa. Biol Reprod 2009; 80: 1168 1177. [DOI] [PubMed] [Google Scholar]
- Iuchi Y, Okada F, Tsunoda S, Kibe N, Shirasawa N, Ikawa M, Okabe M, Ikeda Y, Fujii J. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochem J 2009; 419: 149 158. [DOI] [PubMed] [Google Scholar]
- van Gestel RA, Brewis IA, Ashton PR, Brouwers JF, Gadella BM. Multiple proteins present in purified porcine sperm apical plasma membranes interact with the zona pellucida of the oocyte. Mol Hum Reprod 2007; 13: 445 454. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for Examination of Human Semen and Semen-Cervical Mucus Interaction. Cambridge, UK: Cambridge University Press; 1999: 1 20. [Google Scholar]
- Bigers JD, Whitten WK, Whittngham DG. The culture of mouse embryos in vitro. Daniel JC. (Ed.), Methods in Mammalian Embryology. San Francisco: Freeman; 1971: 86 116. [Google Scholar]
- Aitken RJ, Harkiss D, Buckingham D. Relationship between iron-catalyzed lipid peroxidation potential and human sperm function. J Reprod Fertil 1993; 98: 257 265. [DOI] [PubMed] [Google Scholar]
- Saba M, Morales CR, de Lamirande E, Gagnon C. Morphological and biochemical changes following acute unilateral testicular torsion in prepubertal rats. J Urol 1997; 157: 1149 1154. [PubMed] [Google Scholar]
- Schroder E, Brennan JP, Eaton P. Cardiac peroxiredoxins undergo complex modifications during cardiac oxidant stress. Am J Physiol Heart Circ Physiol 2008; 295: H425 H433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetts LE, Aitken RJ. A comparative study of oxidative DNA damage in mammalian spermatozoa. Mol Reprod Dev 2005; 71: 77 87. [DOI] [PubMed] [Google Scholar]
- Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biology 2006; 7: R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ. A free radical theory of male infertility. Reprod Fertil Dev 1994; 6: 19 23. [DOI] [PubMed] [Google Scholar]
- Shamsi MB, Venkatesh S, Tanwar M, Talwar P, Sharma RK, Dhawan A, Kumar R, Gupta NP, Malhotra N, Singh N, Mittal S, Dada R. DNA integrity and semen quality in men with low seminal antioxidant levels. Mutat Res 2009; 665: 29 36. [DOI] [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O'Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A 2004; 101: 16501 16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colussi C, Albertini MC, Coppola S, Rovidati S, Galli F, Ghibelli L. H2O2-induced block of glycolysis as an active ADP-ribosylation reaction protecting cells from apoptosis. FASEB J 2000; 14: 2266 2276. [DOI] [PubMed] [Google Scholar]
- Oko R, Maravei D. Distribution and possible role of perinuclear theca proteins during bovine spermiogenesis. Microsc Res Tech 1995; 32: 520 532. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Manandhar G, Wu A, Oko R. Interactions of sperm perinuclear theca with the oocyte: implications for oocyte activation, antipolyspermy defense, and assisted reproduction. Microsc Res Tech 2003; 61: 362 378. [DOI] [PubMed] [Google Scholar]
- Mountjoy JR, Xu W, McLeod D, Hyndman D, Oko R. RAB2A: a major subacrosomal protein of bovine spermatozoa implicated in acrosomal biogenesis. Biol Reprod 2008; 79: 223 232. [DOI] [PubMed] [Google Scholar]
- Oko R, Sutovsky P. Biogenesis of sperm perinuclear theca and its role in sperm functional competence and fertilization. J Reprod Immunol 2009; 83: 2 7. [DOI] [PubMed] [Google Scholar]
- Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med 2005; 38: 1422 1432. [DOI] [PubMed] [Google Scholar]
- Robaire B, Smith S, Hales BF. Suppression of spermatogenesis by testosterone in adult male rats: effect on fertility, pregnancy outcome and progeny. Biol Reprod 1984; 31: 221 230. [DOI] [PubMed] [Google Scholar]
- Banmeyer I, Marchand C, Verhaeghe C, Vucic B, Rees JF, Knoops B. Overexpression of human peroxiredoxin 5 in subcellular compartments of Chinese hamster ovary cells: effects on cytotoxicity and DNA damage caused by peroxides. Free Radic Biol Med 2004; 36: 65 77. [DOI] [PubMed] [Google Scholar]
- Barranco-Medina S, Lazaro JJ, Dietz KJ. The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Lett 2009; 583: 1809 1816. [DOI] [PubMed] [Google Scholar]
- Chang TS, Jeong W, Choi SY, Yu S, Kang SW, Rhee SG. Regulation of peroxiredoxin I activity by Cdc2-mediated phosphorylation. J Biol Chem 2002; 277: 25370 25376. [DOI] [PubMed] [Google Scholar]
- Lim JC, Choi HI, Park YS, Nam HW, Woo HA, Kwon KS, Kim YS, Rhee SG, Kim K, Chae HZ. Irreversible oxidation of the active site cysteine of peroxiredoxin to cysteine sulfonic acid for enhanced molecular chaperone activity. J Biol Chem 2008; 283: 28873 28880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JC, Hah YS, Kim WY, Jung BG, Jang HH, Lee JR, Kim SY, Lee YM, Jeon MG, Kim CW, Cho MJ, Lee SY. Oxidative stress-dependent structural and functional switching of a human 2-Cys peroxiredoxin isotype II that enhances HeLa cell resistance to H2O2-induced cell death. J Biol Chem 2005; 280: 28775 28784. [DOI] [PubMed] [Google Scholar]
- Kumsta C, Jakob U. Redox-regulated chaperones. Biochemistry 2009; 48: 4666 4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HH, Kim SY, Park SK, Jeon HS, Lee YM, Jung JH, Lee SY, Chae HB, Jung YJ, Lee KO, Lim CO, Chung WS, et al. Phosphorylation and concomitant structural changes in human 2-Cys peroxiredoxin isotype I differentially regulate its peroxidase and molecular chaperone functions. FEBS Lett 2006; 580: 351 355. [DOI] [PubMed] [Google Scholar]
- Matsumura T, Okamoto K, Iwahara Si, Hori H, Takahashi Y, Nishino T, Abe Y. Dimer-oligomer interconversion of wild-type and mutant rat 2-Cys peroxiredoxin: disulfide formation at dimer-dimer interfaces is not essential for decamerization. J Biol Chem 2008; 283: 284 293. [DOI] [PubMed] [Google Scholar]
- Aran M, Ferrero DS, Pagano E, Wolosiuk RA. Typical 2-Cys peroxiredoxins—modulation by covalent transformations and noncovalent interactions. FEBS J 2009; 276: 2478 2493. [DOI] [PubMed] [Google Scholar]