Abstract
The interaction of amyloid β-proteins (Aβs) with membrane lipids has been postulated as an early event in Aβ fibril formation in Alzheimer’s disease. We evaluated the effects of several putative bioactive Aβs and gangliosides on neural stem cells (NSCs) isolated from embryonic mouse brains or the subventricular zone of adult mouse brains. Incubation of the isolated NSCs with soluble Aβ1–40 alone did not cause any change in the number of NSCs, but soluble Aβ1–42 increased their number. Aggregated Aβ1–40 and Aβ1–42 increased the number of NSCs but soluble and aggregated Aβ25–35 decreased the number. Soluble Aβ1–40 and Aβ1–42 did not affect the number of apoptotic cells but aggregated Aβ1–40 and Aβ1–42 did. When NSCs were treated with a combination of GM1 or GD3 and soluble Aβ1–42, cell proliferation was enhanced, indicating that both GM1 and GD3 as well as Aβs are involved in promoting cell proliferation and survival of NSCs. These observations suggest the potential of beneficial effects of using gangliosides and Aβs for promoting NSC proliferation.
Keywords: Alzheimer’s disease, Amyloid β-peptide, Ganglioside, Neural stem cell Cell, proliferation
Introduction
Alzheimer’s disease (AD) is the most common form of dementia with clinical symptoms that include deficits in memory, judgment, thinking, and behavior. Those symptoms usually develop slowly and become worse over time, interfere with daily tasks, and ultimately lead to death. It is well accepted that deposition of aggregated amyloid β-protein (Aβ) to form amyloid plaques, also known as senile plaques (SPs), together with associated reactive astrocytosis and dystrophic neuritis, represent major pathological hallmarks of AD [1]. During neurodegeneration, the physicochemical properties of membranes are altered, which results in imbalances in the proportion of membranous lipids. Among the various lipid changes, the most notable are observed with gangliosides during the progression of AD [2].
Gangliosides are sialic acid-containing glycosphingolipids expressed primarily in the outer leaflet of the plasma membrane of all vertebrate cells and are particularly abundant in the nervous system [3, 4]. The expression of neuronal gangliosides changes dramatically during cellular differentiation and brain development. For instance, in rodent brains a shift from the synthesis of simple gangliosides, such as GM3 and GD3, to the synthesis of the more complex gangliosides in the a- and b-series during brain development has been well documented [5, 6]. Remarkably, GD3 is predominant in embryonic brain, particularly in NSCs, and accounts for over 80 % of the total ganglioside species in those cells. On the other hand, the level of GM1 increases in mature brain and differentiated neural cells. Several reports have indicated that gangliosides can interact with Aβs with high affinities [7, 8], and that this interaction may result in conformational changes of Aβs [8–10]. Histochemical analysis of amyloid plaques in the SPs of AD brains revealed the presence of the GM1-bound Aβs, which may act as an endogenous seed for Aβ fibrillogenesis [11].
Unfortunately, at present there has not been any effective therapy to halt the progression of AD. Cognitive enhancers such as donepezil and memantine have been used [12], but these drugs can only help to ameliorate the symptoms. With the advantage of cell replacement therapy, it is expected that the use of neural stem cells (NSCs) may contribute to the treatment of AD as well as several other neurodegenerative disorders. NSCs are undifferentiated neural cells characterized by their high proliferative potential and the capacity for self-renewal with retention of multipotency, i.e., generating brain-forming cells such as neurons, astrocytes, and oligodendrocytes. For the therapeutic use of NSCs in AD, detailed clarification of the effects of Aβs and gangliosides on NSCs seems to be warranted. In this regard, it is worth noting that Aβs have been reported to possess neurogenic effects on NSCs [13– 17]. Nevertheless, the effects of Aβs on NSCs are still controversial because Aβs have also been reported to be cytotoxic in some other studies [18–21]. This discrepancy may result from the difference of the patterns and levels of gangliosides expressed in the NSCs. In fact, we have recently reported that a combination of Aβ1–40 and GM1 at relatively high concentrations (submillimolar) co-operatively exerted a cytotoxic effect on mouse embryonic NSCs [22]. Furthermore, the effects of other forms of Aβs (e.g., Aβ25–35 and Aβ1–42) and gangliosides on NSCs have never been evaluated. In addition, the effects of the aggregated forms of Aβs, which are known to be present in physiological conditions, with gangliosides on NSCs also have not been investigated. In this research, we carried out a systematic study to investigate the effects of various forms of Aβs (Aβ25–35, Aβ1–40, and Aβ1–42) and several representative gangliosides (GM1, GD3, and GM3) on mouse NSCs isolated from embryonic brains and the subventricular zone (SVZ) of adult brains.
Materials and Methods
Materials
GM1, GD3 and GM3 used in this study were isolated from either human brains or bovine buttermilk in our laboratory by established procedures [23–25]. Aβ1–40, Aβ1–42 and Aβ25–35 were purchased from Bachem Americas (Torrance, CA). To prepare the aggregated forms of Aβs, the peptide solutions were incubated at 37 °C for 3 days before use [13, 14].
Neural Stem Cell Culture
Mouse NSCs were prepared from embryonic brains or SVZ of adult brains in the form of neurospheres, which are floating clonal aggregates formed by NSCs in vitro [26]. In brief, single-cell suspensions prepared from the striata of embryonic mouse brains at E14.5 or the SVZ of adult mouse brains by trypsinization and mechanical trituration were cultured in Neurobasal A medium (Invitrogen, Carlsbad, CA) supplemented with B27 (Invitrogen), 20 ng/ml of basic fibroblast growth factor (bFGF) (Peprotech, Rocky Hill, NJ), and 20 ng/ml of epidermal growth factor (EGF) (Peprotech). Neurospheres formed after 1 week were collected for passage or further analyses. The use of animals for this study was approved by the Institutional Animal Care and Use Committees at Georgia Regents University and Charlie Norwood Veterans Affairs Medical Center, Augusta, GA.
WST-8 Assay
The number of NSCs cultured in the presence or absence of gangliosides and/or Aβs was estimated by the WST-8 assay using a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan). For neurosphere experiments (Fig. 1), NSCs dissociated from neurospheres were plated onto non-coated 96-well plates at a density of 1 × 104 cells per well. At the time the culture was started, gangliosides and Aβ were dissolved in media. For monolayer culture of NSCs (Figs. 2, 3, 4, and 5), the dissociated NSCs from neurospheres were plated onto 96-well plates that had been coated with poly-l-ornithine (Sigma-Aldrich, St. Louis, MO) and fibronectin (Sigma-Aldrich). On the next day, gangliosides and/or Aβ dissolved in media were added to each well. The total volume of culture media was 100 μl/well. After 3 days of culture, a 10-μl WST-8 solution was added to each well. After incubating 3 h in a CO2 incubator, the absorbance of WST-8-formazan produced by the dehydrogenase activity in living neural cells was measured at a wavelength of 450 nm using a Benchmark Plus Microplate Spectrophotometer (Bio-Rad Laboratories, Hercules, CA). The absorbance measured by this assay was highly correlative with the number of living NSCs.
Fig. 1.

Effects of gangliosides or Aβs on cellular proliferation of neurospheres. The number of cells in the neurospheres treated with gangliosides and Aβs was estimated by the WST-8 assay. The absorbance measured at a wavelength of 450 nm (reference: 650 nm) in this assay is highly correlated to the number of NSCs. The y-axis represents relative absorbance (Abs.). The relative absorbance of the WST-8 assay revealed the percentage of absorbance against vehicle treatment. a Primary structures of Aβ peptides. b Dissociated embryonic neurospheres were cultured for 3 days in the presence of different concentrations (10, 50, or 200 μM) of GM1 or GD3. c Dissociated neurospheres were cultured for 3 days in the presence of different concentrations (1, 10, or 50 μM) of aggregated Aβ1–40, Aβ1–42, or Aβ25–35. Each bar represents mean ± SEM of 3 independent experiments (n = 3). Comparison was made between vehicle versus each treatment. *p < 0.05
Fig. 2.

Effects of low concentrations of gangliosides on embryonic NSCs. The number of NSCs in monolayer cultures in the presence of GM1 or GM3 ganglioside at 0.001, 0.01, 0.1, 1 or 10 μM was estimated by the WST-8 assay. The relative absorbance of the WST-8 assay revealed the percentage of absorbance against vehicle treatment. The bar denotes mean ± SEM of 8 independent experiments (n = 8). Comparison was made between vehicle versus each treatment. *p < 0.05
Fig. 3.
Effects of low concentrations of gangliosides and soluble Aβs (Aβ1–40 or Aβ1–42) on embryonic NSCs in culture. The number of NSCs on monolayer cultures in the presence of gangliosides (0, 0.001, 0.01, 0.1, 1 or 10 μM of GM1 or GD3) and soluble Aβs (10 μM) was estimated by the WST-8 assay. a Aβ1–40; b Aβ1–42. The relative absorbance of the WST-8 assay revealed the percentage of absorbance against vehicle treatment. Each bar denotes mean ± SEM of 8 independent experiments (n = 8). *p < 0.05: Comparison was made between vehicle versus each treatment. **p < 0.05: Comparison was made between Aβ only (0 μM of ganglioside, white column) versus each treatment
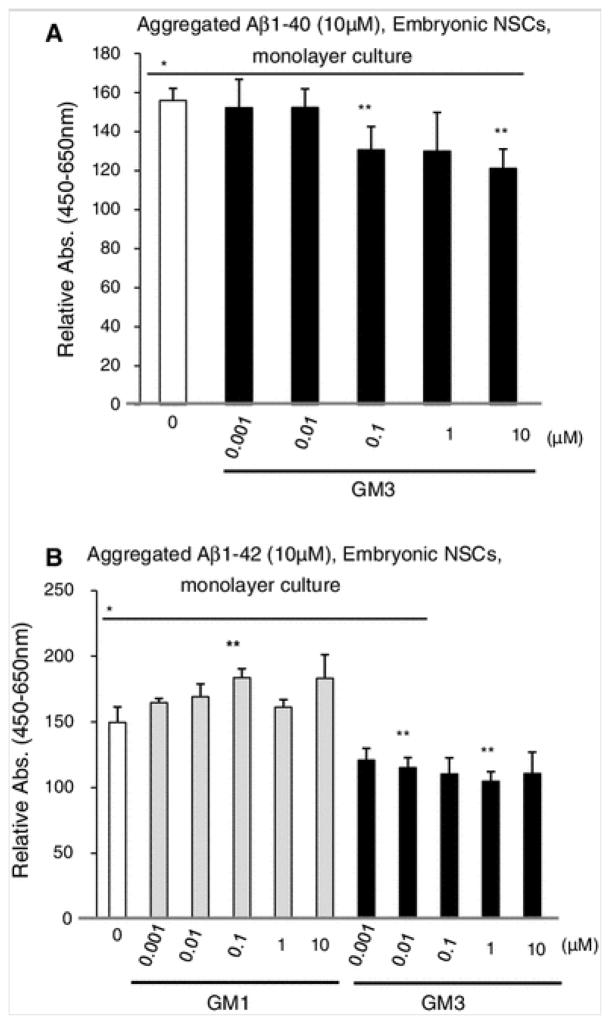
Fig. 4.
Effects of low concentrations of gangliosides and aggregated Aβs (Aβ1–40 or Aβ1–42) on embryonic NSC. The number of NSC on monolayer culture in the presence of gangliosides (0.001, 0.01, 0.1, 1 or 10 μM of GM1 or GM3) and aggregated Aβs (10 μM) was estimated by WST-8 assay. a Aβ1–40, b Aβ1–42. The relative absorbance of the WST-8 assay revealed the percentage of absorbance against vehicle treatment. The bar denotes SEM of 6 independent experiments (n = 6). *p < 0.05: Comparison was made between vehicle versus each treatment. **p < 0.05: Comparison was made between Aβ only (0 μM of ganglioside, white column versus each treatment
Fig. 5.

Apoptosis of NSCs treated with Aβs. Apoptotic cells in embryonic NSCs treated with soluble Aβs (10 μM of Aβ1–40 or Aβ1–42) for 3 days were detected with the TUNEL assay. The bar denotes mean ± SEM of 9 independent experiments (n = 9). Comparison was made between vehicle (0 μM of Aβ) versus each treatment. *p < 0.05
TUNEL Assay
Apoptotic cells were detected with the TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling) assay as previously described [22, 26]. In brief, cells were plated onto chamber slides coated with poly-l-ornithine and fibronectin and cultured for 3 days in Neurobasal A medium containing Aβs in the presence of 20 ng/ml of bFGF and 20 ng/ml of EGF. The cells were then fixed in PBS containing 4 % paraformaldehyde for 1 h at room temperature and permeabilized in 0.1 % sodium citrate containing 0.1 % Triton X-100 for 2 min at 4 °C. The cells were incubated with the fluorescein-conjugated TUNEL reaction mixture (Roche Diagnostics, Mannheim, Germany) for 2 h at 37 °C. Nuclei were stained with Hoechst 33258. The stained cells were photographed under a Nikon Eclipse TE300 fluorescent microscope equipped with a digital CCD camera.
Statistical Evaluation
Data are expressed as mean ± standard error of the mean (SEM) from three to nine independent experiments. Statistical significance was determined using unpaired two-tailed Student’s t test, and p < 0.05 was regarded as significant.
Results
Effects of High Concentrations of Gangliosides on Neurospheres
Neurospheres were generated from dissociated cells from the striata of E14.5 embryos or SVZ of adult mice. To measure the cell numbers, the isolated cells from neurospheres were treated with gangliosides, and living cells were counted 3 days after culture. The relative absorbance of the WST-8 assay revealed the percentage of absorbance against vehicle treatment. Both GM1 and GD3 at higher concentrations (50 and 200 μM) significantly decreased the cell numbers in a dose-dependent manner (Fig. 1b). Smaller neurospheres were observed (data not shown) after treatment with high concentrations of gangliosides. These results indicate that gangliosides GM1 and GD3 can exert an inhibitory effect on cell proliferation on neurospheres in a dose-dependent manner.
Effects of Aβs on Neurospheres
Next, the cell numbers were counted after treatments with Aβs on neurosphere preparations. Treatment with a soluble form of Aβ1–42 (10 μM) significantly increased the number of cells of neurospheres prepared from both embryonic (116 ± 5 %) and adult (109 ± 3 %) brains (data not shown). Soluble Aβ1–40 did not have any effect on the numbers of cells on neurospheres (data not shown).
On the other hand, the aggregated forms of Aβ1–40 and Aβ1–42 significantly increased the numbers of cells from neurospheres in a dose-dependent manner (Fig. 1c). At high concentrations of Aβ1–40 and Aβ1–42 (50 μM), the numbers of cells were elevated to 235 ± 10 % for Aβ1–40 and 170 ± 5 % for Aβ1–42 of the normal growth rate of embryonic neurospheres, respectively. In the presence of aggregated forms of Aβs, the neurospheres appeared larger (data not shown). Increases in the cell number were also found for adult neurospheres treated with the aggregated forms of Aβ1–40 and Aβ1–42 (data not shown). Under these conditions, the aggregated form of Aβ25–35 exhibited inhibitory effects on the number of cells of neurospheres in a dose-dependent manner (Fig. 1c).
Effects of High Concentration of Gangliosides and the Aggregated Forms Of Aβs on Neurospheres
The aggregated forms of Aβ1–40 and Aβ1–42 exhibited proliferative effects of the cells for embryonic and adult neurospheres, as shown in Fig. 1c. Further, we evaluated the additive effects of gangliosides (10, 50, and 100 μM) and aggregated Aβs (10 μM of Aβ1–40 or Aβ1–42) on neurospheres. The addition of GM1 or GD3 gangliosides (50 or 100 μM) inhibited cell growth, with GD3 having greater effects than GM1 in a dose-dependent manner (data not shown).
Effects of Aβs and Low Concentrations of Gangliosides on Embryonic NSCs
Recently, we reported that exogenously added Aβ1–40 (10 μM) was incorporated into embryonic NSCs (monolayer culture), and the combination treatment with Aβ1–40 (10 μM) and GM1 at 40 μM exerted cytotoxic effects on embryonic NSCs [22]. It is unclear, however, whether a lower concentration of gangliosides and Aβs had similar effects on embryonic NSCs. To clarify this issue, we first examined the number of embryonic NSCs in monolayer culture after treatment with lower concentrations (1 nM to 10 μM) of gangliosides. GM1 or GM3 at lower concentrations (1 nM to 1 μM) significantly promoted cell proliferation for embryonic NSCs (Fig. 2), but GD3 did not (data not shown). Next, we evaluated the combinatorial effects of Aβs and low concentrations of gangliosides on cell proliferation of embryonic NSCs. Treatment with soluble Aβ1–40 and GD3 (10 nM to 10 μM) had inhibitory effects for embryonic NSC proliferation (Fig. 3a), but GM1 and GM3 had no effects (data not shown). Soluble Aβ1–42 (10 μM) alone exerted a dramatic stimulatory effect on cell proliferation of embryonic NSC monolayer culture and the number of NSCs increased dramatically (149 ± 10 %) (Fig. 3b). Figure 3b also shows that addition of GM1 and GD3 enhanced proliferative effects of soluble Aβ1–42 on embryonic NSCs, whereas GM3 had no additional effects (data not shown). Similar to neurosphere preparations, the numbers of embryonic NSCs grown as monolayer cultures also increased after treatment with aggregated Aβ1–40 or Aβ1–42 (Figs. 1c, 4). As shown in Fig. 4b, treatment of cells with 100 nM of GM1 significantly increased the cell number on embryonic NSCs treated with aggregated Aβ1–42. GM3, however, exerted inhibitory effects on aggregated Aβ-stimulated cell proliferation (Fig. 4a, b). Similar to neurospheres (Fig. 1c), the number of cells was slightly decreased in monolayer culture of embryonic NSCs treated with both soluble and aggregated forms of Aβ25–35 (data not shown).
Apoptosis of NSCs Treated with Aβs and Gangliosides
Figure 5 shows the percentage of apoptotic embryonic NSCs after treatment with soluble and aggregated forms of Aβs (1–40 and 1–42). No difference was found in the number of cells between NSCs treated with and without soluble forms of Aβs. This result was consistent with our previous report that expression of active caspase-3 had no difference between intact cells and NSCs treated with soluble form of Aβ1–40 [22]. Taken together, our data suggest that the increase of cell number with soluble Aβ1–42 treatment may cause cell proliferation rather than protection from cell death.
In contrast, the number of NSCs undergoing apoptosis decreased significantly by treatment with the aggregated forms of Aβs (1–40 and 1–42). Those results indicate that the aggregated forms of Aβs, but not the soluble forms, may be involved in the protection of NSCs from undergoing apoptosis. High concentrations of gangliosides (50 μM of GM1 or GD3) and soluble forms of Aβs increased the number of TUNEL+ apoptotic cells in embryonic NSC preparations (data not shown). Those results are also consistent with our previous observations [22] that the combined treatment with high concentrations of gangliosides and Aβs exhibited enhanced apoptotic effects.
Discussion
Although Aβs at elevated concentrations in brain are generally considered to be cytotoxic effect owing to their ability to form fibrils, there has been a growing awareness of the potential beneficial effects on neurogenesis of Aβs at low concentrations. Those observations are, however, controversial as some reports also indicate that they are detrimental to neurogenesis. Some of the discrepancies could be traced to the use of different forms and concentrations of Aβs, either in the presence or absence of gangliosides that bind with those peptides. For this reason, we first undertook this study with the goal of clarifying the effect of various forms of Aβs on NSCs in different conditions using cell proliferation as a criterion. We found that soluble Aβ1–42 and aggregated Aβs (Aβ1–40 and Aβ1–42) stimulated cell proliferation in NSCs. This is consistent with several reports demonstrating the stimulatory effects of Aβs on the proliferation of NSCs [13, 14, 16, 27]. Most remarkably, several in vivo studies also showed that intracerebroventricular infusion of Aβ1–42 in nanomolar concentrations promotes neurogenesis in adult mouse brains [18], and Aβ accumulation increases neurogenesis in the hippocampus and other brain regions in AD transgenic mouse models [28–33]. It is interesting to note that AD patient’s brain revealed increased neuronal precursor markers, such as doublecortin and polysialylated neural cell adhesion molecule (PSA-NCAM) in the hippocampus, presumably in response to the shedding of Aβs [31]. Our current study confirmed that both soluble and aggregated forms of Aβ1–42 have proliferative effects on NSCs.
Aβ1–40 and Aβ1–42 are two major forms of Aβ (Fig. 1a), and during normal metabolism of amyloid precursor protein (APP), Aβ1–40 is predominantly produced. The Aβ1–42 species is usually a minor product, but is more cytotoxic and forms aggregates more easily than Aβ1–40 [34]. Aβ1–42 is the major peptide constituent of amyloid plaques, and increased production of the 42-amino acid peptide correlates with earlier onset of AD. Approximately 1 % of freshly dissolved Aβ1–40 forms dimers [35]. However under the same conditions, about 7.4 % of Aβ1–42 is converted to the dimeric form. Thus, it suggests that the soluble Aβ1–42 has a higher tendency to exist as dimers. Aβ1–42 has less configurational entropy and has been found to confer higher rigidity on the C terminus, which may explain the higher amylidogenic ability [36]. Yang and Teplow [37] reported that the two additional hydrophobic residues (isoleucine and alanine) in Aβ1–42 contribute significantly to the contact between the C terminus and the central hydrophobic clusters. Thereby, the β-sheet structure of Aβ1–42 is more stable than Aβ1–40. Those structural distinctions of between Aβ1–40 and Aβ1–42 might affect their biological activities such as their ability to contribute to NSC proliferation. The higher rigidity, less entropy and dimeric stability of Aβ1–42 might increase its interaction with NSC membranes, which in turn, modulate its effect on cellular proliferation.
Significant changes in ganglioside patterns in AD brains have been reported. In AD patients brain, major gangliosides such as GM1, GD1a, GD1b and GT1b are all decreased [38–42] and b-series gangliosides such as GD1b and GT1b are preferentially affected. Additionally, in frontal and parietal cortex simple gangliosides, including GM2, GM3, GM4 and GD3, are elevated. These findings suggest that abnormal ganglioside metabolism coincides with the affected brain region of neurodegeneration in AD patients. However, we and others have not detected significant changes in the major brain ganglioside pattern in the AD mouse brains [43–45] except an increase of minor gangliosides such as GM2 and GM3 in the cerebral cortex [46]. Most interestingly, there is an increase of cholinergic neuronal marker gangliosides such as GT1aα and GQ1bα [43]. Since Aβs have a tendency to bind gangliosides, we also examined the ability of gangliosides and combinations of gangliosides and Aβs on cell proliferation. In this study, we found that lower concentrations of GM1 and GM3 promote cell proliferation on NSCs (Fig. 2). Other investigators also reported that exogenously added gangliosides exhibited stimulatory effects on cellular proliferation of induced pluripotent stem [47] and vascular smooth muscle cells [48]. We have reported NSCs express GD3 as a predominant ganglioside [26,49, 50] and it is co-localized with EGFR to regulate cellular proliferation induced by EGF (unpublished data). Quite contrarily, it has been reported that GD3 induces mitochondrial permeability transition and this event precedes apoptosis [51], and GD3 is required for Fas-induced cell death and Aβ-induced cell death [52, 53]. As mentioned in the Result section, treatment of NSCs with a high concentration of GD3 (50 μM) induced NSCs to undergo apoptosis (>50 %). Thus, the positive or negative effects of gangliosides might depend on culture conditions and ganglioside concentrations. Our results, together with those from other investigators, suggest that gangliosides modulate NSC proliferation or apoptosis depending on the concentrations of gangliosides and the culture conditions of the cells.
With respect to Aβ25–35, it does not occur naturally but has been shown to mimic the adverse effects of Aβ1–42 in several studies [14]. Interestingly, hippocampal neuronal cultures treated with Aβ25–35 showed enhanced metabolism of lipids such as phospholipids (+52 %) and gangliosides (+193 %) [54]. In addition, exposure of rat cultured cortical neurons to Aβ25–35 induced a substantial increase of the intracellular GD3 levels [52]. Those reports suggest that Aβs can modulate ganglioside metabolism in the NSCs and prompted us to propose that a combined use of Aβs and gangliosides in suitable concentrations should promote NSC proliferation. We evaluated the number of embryonic NSCs treated with low concentrations of GM1 and GD3 in the presence of soluble forms of Aβs. In embryonic NSC culture, lower concentrations of GD3, combined with soluble Aβ1–40, had an inhibitory effect on cellular proliferation. On the other hand, lower concentrations of GM1 and GM3 with Aβ1–42 enhanced proliferation of embryonic NSCs. GM1 also promoted cell proliferation with the aggregated form of Aβ1–42. These results indicate that appropriate concentrations of both gangliosides and Aβs are critically involved in stimulating NSC proliferation.
Aβs is produced by cultured cells during normal cellular metabolism [55, 56]. Because Aβ1–40 and Aβ1–42 are present in the brain and cerebrospinal fluid of normal individuals, it suggests that these peptides are likely physiologically active in normal life [57]. Despite extensive efforts for studying Aβs for their cytotoxic effects in AD, the normal biological functions and positive effects of Aβs have remained elusive. Gangliosides are known to interact with Aβs with high affinities and are known to be involved in conformational changes of Aβs. In this study, we evaluated the effect of gangliosides, GM1, GD3 and GM3, and Aβs (Aβ1–40, Aβ1–42, and Aβ25–35) on cell proliferation of the NSCs. Aggregated Aβ1–40 and soluble or aggregated Aβ1–42 were involved in cell proliferation of the NSCs. The lower concentrations of gangliosides and Aβs 1–42 induced cell proliferation of NSCs, suggesting that understanding the roles of gangliosides and Aβs on NSCs may help devise new strategies for treatment of AD.
Acknowledgments
This work was supported in part by a VA Merit Award (1 IO1BX001388 to RKY) and NIH Grants (RO1 NS26994 and RO1 NS11853 to RKY).
Abbreviations
- AD
Alzheimer’s disease
- Aβ
Amyloid β-protein
- SPs
Senile plaques
- NSCs
Neural stem cells
- SVZ
Subventricular zone
- TUNEL
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
References
- 1.Selkoe DJ. Alzheimer’s disease: genotypes, phenotypes, and treatments. Science. 1997;275(5300):630–631. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- 2.Ariga T, McDonald MP, Yu RK. Role of ganglioside metabolism in the pathogenesis of Alzheimer’s disease–a review. J Lipid Res. 2008;49(6):1157–1175. doi: 10.1194/jlr.R800007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu RK, Nakatani Y, Yanagisawa M. The role of glycosphingolipid metabolism in the developing brain. J Lipid Res. 2009;50(Suppl):S440–S445. doi: 10.1194/jlr.R800028-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu RK, Tsai YT, Ariga T. Functional roles of gangliosides in neurodevelopment: an overview of recent advances. Neurochem Res. 2012;37(6):1230–1244. doi: 10.1007/s11064-012-0744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngamukote S, Yanagisawa M, Ariga T, Ando S, Yu RK. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J Neurochem. 2007;103(6):2327–2341. doi: 10.1111/j.1471-4159.2007.04910.x. [DOI] [PubMed] [Google Scholar]
- 6.Yu RK, Macala LJ, Taki T, Weinfield HM, Yu FS. Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J Neurochem. 1988;50(6):1825–1829. doi: 10.1111/j.1471-4159.1988.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 7.Ariga T, Kobayashi K, Hasegawa A, Kiso M, Ishida H, Miyatake T. Characterization of high-affinity binding between gangliosides and amyloid beta-protein. Arch Biochem Biophys. 2001;388(2):225–230. doi: 10.1006/abbi.2001.2304. [DOI] [PubMed] [Google Scholar]
- 8.McLaurin J, Chakrabartty A. Membrane disruption by Alzheimer beta-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J Biol Chem. 1996;271(43):26482–26489. doi: 10.1074/jbc.271.43.26482. [DOI] [PubMed] [Google Scholar]
- 9.Choo-Smith LP, Garzon-Rodriguez W, Glabe CG, Surewicz WK. Acceleration of amyloid fibril formation by specific binding of Abeta-(1–40) peptide to ganglioside-containing membrane vesicles. J Biol Chem. 1997;272(37):22987–22990. doi: 10.1074/jbc.272.37.22987. [DOI] [PubMed] [Google Scholar]
- 10.Matsuzaki K, Horikiri C. Interactions of amyloid beta-peptide (1–40) with ganglioside-containing membranes. Biochemistry. 1999;38(13):4137–4142. doi: 10.1021/bi982345o. [DOI] [PubMed] [Google Scholar]
- 11.Yanagisawa K, Odaka A, Suzuki N, Ihara Y. GM1 ganglioside-bound amyloid beta-protein (A beta): a possible form of preamyloid in Alzheimer’s disease. Nat Med. 1995;1(10):1062–1066. doi: 10.1038/nm1095-1062. [DOI] [PubMed] [Google Scholar]
- 12.Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Dong C. Abeta40 promotes neuronal cell fate in neural progenitor cells. Cell Death Differ. 2009;16(3):386–394. doi: 10.1038/cdd.2008.94. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Toledano MA, Shelanski ML. Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J Neurosci. 2004;24(23):5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazur-Kolecka B, Golabek A, Nowicki K, Flory M, Frackowiak J. Amyloid-beta impairs development of neuronal progenitor cells by oxidative mechanisms. Neurobiol Aging. 2006;27(9):1181–1192. doi: 10.1016/j.neurobiolaging.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Sotthibundhu A, Li QX, Thangnipon W, Coulson EJ. Abeta(1–42) stimulates adult SVZ neurogenesis through the p75 neurotrophin receptor. Neurobiol Aging. 2009;30(12):1975–1985. doi: 10.1016/j.neurobiolaging.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Caille I, Allinquant B, Dupont E, Bouillot C, Langer A, Muller U, Prochiantz A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131(9):2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- 18.Calafiore M, Battaglia G, Zappala A, Trovato-Salinaro E, Caraci F, Caruso M, Vancheri C, Sortino MA, Nicoletti F, Copani A. Progenitor cells from the adult mouse brain acquire a neuronal phenotype in response to beta-amyloid. Neurobiol Aging. 2006;27(4):606–613. doi: 10.1016/j.neurobiolaging.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Haughey NJ, Liu D, Nath A, Borchard AC, Mattson MP. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer’s disease. Neuromolecular Med. 2002;1(2):125–135. doi: 10.1385/NMM:1:2:125. [DOI] [PubMed] [Google Scholar]
- 20.Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem. 2002;83(6):1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 21.Millet P, Lages CS, Haik S, Nowak E, Allemand I, Granotier C, Boussin FD. Amyloid-beta peptide triggers Fas-independent apoptosis and differentiation of neural progenitor cells. Neurobiol Dis. 2005;19(1–2):57–65. doi: 10.1016/j.nbd.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Yanagisawa M, Ariga T, Yu RK. Cytotoxic effects of G(M1) ganglioside and amyloid beta-peptide on mouse embryonic neural stem cells. ASN Neuro. 2010;2(1):e00029. doi: 10.1042/AN20090063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ariga T, Tao RV, Lee BC, Yamawaki M, Yoshino H, Scarsdale NJ, Kasama T, Kushi Y, Yu RK. Glycolipid composition of human cataractous lenses. Characterization of Lewisx glycolipids. J Biol Chem. 1994;269(4):2667–2675. [PubMed] [Google Scholar]
- 24.Ledeen RW, Yu RK. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- 25.Ren S, Scarsdale JN, Ariga T, Zhang Y, Klein RA, Hartmann R, Kushi Y, Egge H, Yu RK. O-acetylated gangliosides in bovine buttermilk. Characterization of 7-O-acetyl, 9-O-acetyl, and 7,9-di-O-acetyl GD3. J Biol Chem. 1992;267(18):12632–12638. [PubMed] [Google Scholar]
- 26.Nakatani Y, Yanagisawa M, Suzuki Y, Yu RK. Characterization of GD3 ganglioside as a novel biomarker of mouse neural stem cells. Glycobiology. 2010;20(1):78–86. doi: 10.1093/glycob/cwp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heo C, Chang KA, Choi HS, Kim HS, Kim S, Liew H, Kim JA, Yu E, Ma J, Suh YH. Effects of the monomeric, oligomeric, and fibrillar Abeta42 peptides on the proliferation and differentiation of adult neural stem cells from subventricular zone. J Neurochem. 2007;102(2):493–500. doi: 10.1111/j.1471-4159.2007.04499.x. [DOI] [PubMed] [Google Scholar]
- 28.Bolos M, Spuch C, Ordonez-Gutierrez L, Wandosell F, Ferrer I, Carro E. Neurogenic effects of beta-amyloid in the choroid plexus epithelial cells in Alzheimer’s disease. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-013-1300-x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esteras N, Bartolome F, Alquezar C, Antequera D, Munoz U, Carro E, Martin-Requero A. Altered cell cycle-related gene expression in brain and lymphocytes from a transgenic mouse model of Alzheimer’s disease [amyloid precursor protein/presenilin 1 (PS1)] Eur J Neurosci. 2012;36(5):2609–2618. doi: 10.1111/j.1460-9568.2012.08178.x. [DOI] [PubMed] [Google Scholar]
- 30.Gan L, Qiao S, Lan X, Chi L, Luo C, Lien L, Yan Liu Q, Liu R. Neurogenic responses to amyloid-beta plaques in the brain of Alzheimer’s disease-like transgenic (pPDGF-APPSw, Ind) mice. Neurobiol Dis. 2008;29(1):71–80. doi: 10.1016/j.nbd.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101(1):343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Y, He J, Zhang Y, Luo H, Zhu S, Yang Y, Zhao T, Wu J, Huang Y, Kong J, Tan Q, Li XM. Increased hippocampal neurogenesis in the progressive stage of Alzheimer’s disease phenotype in an APP/PS1 double transgenic mouse model. Hippocampus. 2009;19(12):1247–1253. doi: 10.1002/hipo.20587. [DOI] [PubMed] [Google Scholar]
- 33.Jin K, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, Greenberg DA. Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APPSw, Ind) mice. Proc Natl Acad Sci U S A. 2004;101(36):13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399(6738 Suppl):A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 35.Roher AE, Chaney MO, Kuo YM, Webster SD, Stine WB, Haverkamp LJ, Woods AS, Cotter RJ, Tuohy JM, Krafft GA, Bonnell BS, Emmerling MR. Morphology and toxicity of Abeta-(1–42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer’s disease. J Biol Chem. 1996;271(34):20631–20635. doi: 10.1074/jbc.271.34.20631. [DOI] [PubMed] [Google Scholar]
- 36.Yan Y, Wang C. Abeta42 is more rigid than Abeta40 at the C terminus: implications for Abeta aggregation and toxicity. J Mol Biol. 2006;364(5):853–862. doi: 10.1016/j.jmb.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 37.Yang M, Teplow DB. Amyloid beta-protein monomer folding: free-energy surfaces reveal alloform-specific differences. J Mol Biol. 2008;384(2):450–464. doi: 10.1016/j.jmb.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crino PB, Ullman MD, Vogt BA, Bird ED, Volicer L. Brain gangliosides in dementia of the Alzheimer type. Arch Neurol. 1989;46(4):398–401. doi: 10.1001/archneur.1989.00520400054019. [DOI] [PubMed] [Google Scholar]
- 39.Kalanj S, Kracun I, Rosner H, Cosovic C. Regional distribution of brain gangliosides in Alzheimer’s disease. Neurol Croat. 1991;40(4):269–281. [PubMed] [Google Scholar]
- 40.Kracun I, Kalanj S, Cosovic C, Talan-Hranilovic J. Brain gangliosides in Alzheimer’s disease. J Hirnforsch. 1990;31(6):789–793. [PubMed] [Google Scholar]
- 41.Kracun I, Kalanj S, Talan-Hranilovic J, Cosovic C. Cortical distribution of gangliosides in Alzheimer’s disease. Neurochem Int. 1992;20(3):433–438. doi: 10.1016/0197-0186(92)90058-y. [DOI] [PubMed] [Google Scholar]
- 42.Svennerholm L, Gottfries CG. Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset form (type II) J Neurochem. 1994;62(3):1039–1047. doi: 10.1046/j.1471-4159.1994.62031039.x. [DOI] [PubMed] [Google Scholar]
- 43.Ariga T, Yanagisawa M, Wakade C, Ando S, Buccafusco JJ, McDonald MP, Yu RK. Ganglioside metabolism in a transgenic mouse model of Alzheimer’s disease: expression of Chol-1alpha antigens in the brain. ASN Neuro. 2010;2(4):e00044. doi: 10.1042/AN20100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernardo A, Harrison FE, McCord M, Zhao J, Bruchey A, Davies SS, Jackson Roberts L, 2nd, Mathews PM, Matsuoka Y, Ariga T, Yu RK, Thompson R, McDonald MP. Elimination of GD3 synthase improves memory and reduces amyloid-beta plaque load in transgenic mice. Neurobiol Aging. 2009;30(11):1777–1791. doi: 10.1016/j.neurobiolaging.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 45.Sawamura N, Morishima-Kawashima M, Waki H, Kobayashi K, Kuramochi T, Frosch MP, Ding K, Ito M, Kim TW, Tanzi RE, Oyama F, Tabira T, Ando S, Ihara Y. Mutant presenilin 2 transgenic mice. A large increase in the levels of Abeta 42 is presumably associated with the low density membrane domain that contains decreased levels of glycerophospholipids and sphingomyelin. J Biol Chem. 2000;275(36):27901–27908. doi: 10.1074/jbc.M004308200. [DOI] [PubMed] [Google Scholar]
- 46.Barrier L, Ingrand S, Damjanac M, Rioux Bilan A, Hugon J, Page G. Genotype-related changes of ganglioside composition in brain regions of transgenic mouse models of Alzheimer’s disease. Neurobiol Aging. 2007;28(12):1863–1872. doi: 10.1016/j.neurobiolaging.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Ryu JS, Chang KT, Lee JT, Lim MU, Min HK, Na YJ, Lee SB, Moussavou G, Kim SU, Kim JS, Ko K, Hwang KA, Jeong EJ, Lee JW, Choo YK. Ganglioside GM1 influences the proliferation rate of mouse induced pluripotent stem cells. BMB Rep. 2012;45(12):713–718. doi: 10.5483/BMBRep.2012.45.12.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gouni-Berthold I, Seul C, Ko Y, Hescheler J, Sachinidis A. Gangliosides GM1 and GM2 induce vascular smooth muscle cell proliferation via extracellular signal-regulated kinase 1/2 pathway. Hypertension. 2001;38(5):1030–1037. doi: 10.1161/hy1101.093104. [DOI] [PubMed] [Google Scholar]
- 49.Yanagisawa M, Taga T, Nakamura K, Ariga T, Yu RK. Characterization of glycoconjugate antigens in mouse embryonic neural precursor cells. J Neurochem. 2005;95(5):1311–1320. doi: 10.1111/j.1471-4159.2005.03452.x. [DOI] [PubMed] [Google Scholar]
- 50.Yanagisawa M, Yoshimura S, Yu RK. Expression of GD2 and GD3 gangliosides in human embryonic neural stem cells. ASN Neuro. 2011;3 (2):69–74. doi: 10.1042/AN20110006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scorrano L, Petronilli V, Di Lisa F, Bernardi P. Commitment to apoptosis by GD3 ganglioside depends on opening of the mitochondrial permeability transition pore. J Biol Chem. 1999;274(32):22581–22585. doi: 10.1074/jbc.274.32.22581. [DOI] [PubMed] [Google Scholar]
- 52.Copani A, Melchiorri D, Caricasole A, Martini F, Sale P, Carnevale R, Gradini R, Sortino MA, Lenti L, De Maria R, Nicoletti F. Beta-amyloid-induced synthesis of the ganglioside GD3 is a requisite for cell cycle reactivation and apoptosis in neurons. J Neurosci. 2002;22(10):3963–3968. doi: 10.1523/JNEUROSCI.22-10-03963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Maria R, Lenti L, Malisan F, d’Agostino F, Tomassini B, Zeuner A, Rippo MR, Testi R. Requirement for GD3 ganglioside in CD95- and ceramide-induced apoptosis. Science. 1997;277(5332):1652–1655. doi: 10.1126/science.277.5332.1652. [DOI] [PubMed] [Google Scholar]
- 54.Cazzaniga E, Bulbarelli A, Cassetti A, Lonati E, Re F, Palestini P, Mutoh T, Masserini M. Beta-amyloid (25–35) enhances lipid metabolism and protein ubiquitination in cultured neurons. J Neurosci Res. 2007;85(10):2253–2261. doi: 10.1002/jnr.21354. [DOI] [PubMed] [Google Scholar]
- 55.Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 56.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature. 1992;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 57.Shoji M. Cerebrospinal fluid Abeta40 and Abeta42: natural course and clinical usefulness. Front Biosci. 2002;7:d997–d1006. doi: 10.2741/A826. [DOI] [PubMed] [Google Scholar]