Abstract
The goal of this study was to simulate in vitro the spontaneous electrical wave activity associated with retinal development and investigate if such biometrically designed signals can enhance differentiation of mouse retinal progenitor cells (mRPC). To this end, we cultured cells on an electroconductive transplantable polymer, polypyrrole (PPy) and measured gene expression and morphology of the cells. Custom-made 8-well cell culture chambers were designed to accommodate PPy deposited onto indium tin oxide-coated (ITO) glass slides, with precise control of the PPy film thickness. mRPCs were isolated from post-natal day 1 (P1) green fluorescent protein positive (GFP+) mice, expanded, seeded onto PPY films, allowed to adhere for 24 hours, and then subjected to electrical stimulation (100 μA pulse trains, 5 s in duration, once per minute) for 4 days. Cultured cells and non-stimulated controls were processed for immunostaining and confocal analysis, and for RNA extraction and quantitative PCR. Stimulated cells expressed significantly higher levels of the early photoreceptor marker cone-rod homebox (CRX, the earliest known marker of photoreceptor identity), and protein kinase-C (PKC), and significantly lower levels of the glial fibrillary acidic protein (GFAP). Consistently, stimulated cells developed pronounced neuronal morphologies with significantly longer dendritic processes and larger cell bodies than non-stimulated controls. Taken together, the experimental evidence shows that the application of an electrical stimulation designed based on retinal development can be implemented to direct and enhance retinal differentiation of mRPCs, suggesting a role for biomimetic electrical stimulation in directing progenitor cells toward neural fates.
I. Introduction
It is has been established that waves of spontaneous cellular electrical activity play essential roles in developmental gene expression and activity-dependent synaptic refinement in retina and other areas of the central nervous system [1-2]. Spontaneous electrical activity during neuronal development is characterized by rhythmic bursts of action potentials lasting for milliseconds, followed by interburst refractory periods lasting from milliseconds to minutes [3] [4].
During the process of retinogenesis, synchronized waves of electrical activity are generated and spread across the immature retina. In mouse, from post-natal day 0 (P0) to P15, these depolarizing waves are triggered by cholinergic starburst of amacrine cells and propagated by ganglion cells [1]. Later in development, from P15 to P30, glutamatergic bipolar cells become the pacemakers that trigger the wave patterning. The depolarizing wave patterns during retinal development in mouse (P0-P4), are characterized by 3-5 second long bursts every 60 s [5-7]. This period of spontaneous bursting activity correlates with the time of peak birth rate for rod photoreceptors [8].
Rhythmic depolarizing stimulation has been shown to influence neuronal differentiation via calcium-dependent mechanisms in a number of cell types [1, 9-12]. We were interested in exploring if such depolarizing effects can be replicated in vitro to study their effects on differentiation of retinal cells under tightly controlled conditions.
Electrical stimulation of stem and progenitor cells on conductive polymers has shown potential for biomedical applications and tissue engineering. [9, 13-18]. In particular, electrically conductive polymers have become an increasingly attractive option for biomedical applications allowing for electrode modification, ease of fabrication and high surface area which facilitates ion exchange between the electrodes and surrounding tissues [17]. Of the electrically conductive polymers, PPy is most widely studied due to its inherent biocompatibility in vitro and in vivo [14-15]. Thin films of PPy have been shown to support cell attachment and growth in vitro, and have been used in vivo to bridge peripheral nerve gaps without any apparent toxic effects [19]. PPy also allows for external control and accurate regulation of electrical stimulation parameters [20]. Furthermore, electrically stimulated cells adherent to PPy show enhanced neurite outgrowth [16-19, 21]. The use of PPy also provides the flexibility of adding dopant ions and growth factors to modify the surface characteristics to support enhanced growth of cells. [9, 13, 21].
In this study, we have designed electrical stimulation protocols mimicking the electrical activity during early retinal development [1, 22] in the form of pulse trains, 100 μA in amplitude (corresponding to 100 mV amplitude, and shown to influence neurite outgrowth in previous studies [23]), for 5 s, every 60 s (designed to be temporally biomimetic based on the temporal properties of endogenous depolarizing retinal wave patterns observed in vivo [1]). Then, we developed a modular cell culture system allowing cultivation of mouse retinal progenitor cells (mRPCs, derived from GFP+ mice) directly on thin films of PPy, and application of electrical signals over the duration of culture. We collected evidence of increased dendrite length, increased size of cell bodies, and significant changes in protein expression in response to this novel developmental retinal oscillation/PPy stimulation paradigm. We suggest that electrical stimulation of mRPCs on conductive PPy may recapitulate developmental gene expression and axonal refinement in vitro, and result in high percentages of electrophysiologically entrained neural progenitors.
II. Methods
A. Cell Culture Chamber
PPy was deposited onto 25 mm × 75 mm ITO coated glass slides (Delta Technologies) using a standard twoelectrode electropolymerization protocol in 0.2 M pyrrole, 0.2 M sodium dodecylbenzenesulfonate (Sigma-Aldrich). PPy thin-film growth was controlled galvanostatically at 8 mA for 3 min (1.44 C total charge deposition) with the ITO slide as anode and a platinum mesh cathode. In order to create 8 discrete cell culture wells, polydimethylsiloxane (PDMS, Ellsworth Adhesives) was cured around a metal mold. A sheet of polycarbonate (McMaster) 3/16 in thick was machined to fit the cell culture wells. Polycarbonate sheets formed a roof and floor around the PDMS and PPy slides and were tightened with nylon screws to prevent medium leakage. Figure 1 illustrates the fabrication and assembly processes for the cell culture chambers.
Figure 1. Cell culture chamber with electrical stimulation.
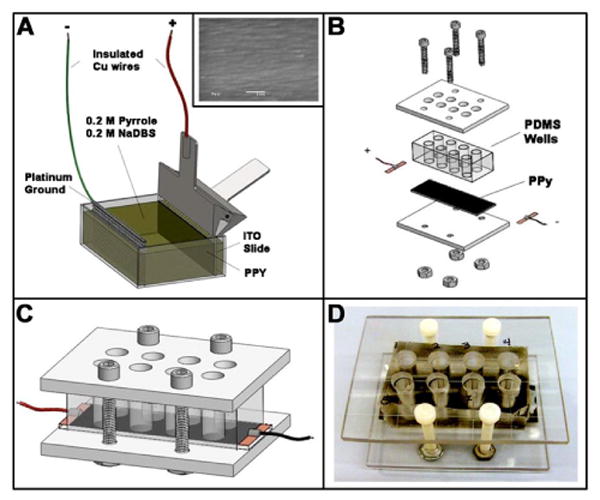
(A) Electropolymerization setup: thin films of electroconductive polymer polypyrrole (PPy) were formed on indium tin oxide (ITO) slides via electropolymerization. Scanning electron micrograph (SEM, 20,000x magnification) image in the inset indicates the smooth PPy surface after electropolymerization; scale: 1 μm. (B) Assembly of cell culture devices: the individual Polydimethylsiloxane (PDMS) wells formed on the PPy slide. Wires soldered to adhesive copper tape were attached to the ends of each side of the slide to enable electrical stimulation. (C-D) Completed cell culture chamber: 3D rendering and photograph, respectively.
B. Mouse Retinal Progenitor Cell (mRPC) Isolation and Culture
All experiments were performed according to the Schepens Eye Research Institute Animal Care and Use Committee and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Isolation of mRPCs was performed as previously described [24]. Briefly, retinas were isolated from postnatal day 1 (P1) enhanced green fluorescent protein positive (GFP+) transgenic mice (C57BL/6 background). Pooled retinas were dissociated by mincing, and digested with 0.1% type 1 collagenase (Sigma-Aldrich, St. Louis, MO) for 20 min.
The isolated mRPCs were passed through a 100 μm mesh filter, centrifuged at 850 rpm for 3 min, re-suspended in Neurobasal culture medium (NB; Invitrogen-Gibco, Rockville, MD) containing 2 mM L-glutamine, 100 mg/ml penicillin-streptomycin, 20 ng/ml epidermal growth factor (EGF; Promega, Madison, Wisconsin) and neural supplement (B27; Invitrogen-Gibco) and plated into culture wells (Multiwell; Becton Dickinson Labware, Franklin Lakes, NJ). P1 isolated cells were expanded with fresh culture medium on alternating days for 3 weeks until mRPCs were visible as proliferating non-adherent spheres. mRPCs were passaged 1:3 every 7 days.
C. Electrical Stimulation of mRPCs on PPy
All components were sterilized in 70% ethanol and/or UV light. Wells were washed with sterile PBS and precoated with laminin 20 μg/ml. Assembly occurred in the sterile biological hood.
Retinal progenitor cells (4 × 105 cells in 800 μL of medium) were seeded into each of the culture wells (surface density 289.9k cells/cm2) and allowed to grow for 24 hours. Starting on day two, cells were stimulated with 100 μA pulse train (5 s pulse, once per minute) for 4 days. 100 μA amplitude corresponded to 100 mV amplitude, which in previous studies has was shown to influence neurite outgrowth [23], and 5 s pulses, delivered once per minute was designed to be temporally biomimetic based on the temporal properties of endogenous depolarizing retinal wave patterns observed in vivo [1]. Stimulation frequency was controlled with a function generator, pulse duration with a ′123 pulse generator and current controlled at 100 μA via a LM334 current source. Control experiments were conducted in identical conditions in the absence of stimulation.
D. Immunofluorescence and image analysis
After culturing mRPCs on PPy films for 7 days, mRPC-PPy composites were harvested from the culture wells, rinsed 3 times with warm PBS (37 °C), fixed in 4% paraformaldehyde for 1 hr, and processed for immunocytochemistry as follows. All samples were rinsed with PBS 3 times for 10 min, blocked, and permeabilized in PBS containing 10% goat serum, 1% BSA, and 0.1% Triton-x for 2 hr. Samples were incubated with the primary antibodies: glial fibrillary acidic protein (GFAP) (Zymed) (1:200), cone-rod homebox (CRX) (Santa Cruz) 1:100, Recoverin (Abcam) 1:200, neural-filament-200 (NF-200) (Sigma) 1:400, Protein kinase C (PKC) (Sigma) 1:200, Ki67 (Sigma) 1:100, in blocking buffer for 12 hr at 4 °C. Samples were then rinsed 3 times for 10 min in PBS and incubated with a rhodamine-conjugated secondary antibody 1:800 (Zymed) and Topro-3 (Molecular Probes) nuclear stain for 2 hr at room temperature. Finally, samples were rinsed 3 times for 10 min in PBS and sealed in mounting medium (Vector Laboratories) for imaging using a Leica TCS SP2 confocal microscope.
Semi-quantitative analysis of protein expression levels was performed using ImageJ Software (NIH) and was used to measure intensity in the red channel. Red always corresponded to the secondary antibody labeling of target proteins, as green was ubiquitously expressed GFP on the actin promoter, and blue the DAPI nuclear stain. Controls of mRPCs cultured on non-stimulated PPy films were used for comparison. To quantify dendritic length, GFP dendrites were imaged at 20x and measured using ImageJ. In addition, we used a custom developed Matlab® script running image analysis functions to measure cell bodies' area. Briefly, images of stimulated and non-stimulated GFP+ cells were processed removing axons and dendrites; measurements of cell bodies were performed on binary images. Significant differences were assessed via one-way ANOVA testing, with p<0.05 considered significant.
E. Real-Time quantitative RT-PCR (qPCR)
Total RNA was extracted from stimulated and control mRPCs (RNeasy Mini kit Qiagen, CA, USA) followed by column treatment with DNase I (Qiagen, CA, USA). Reverse transcription was performed with Omniscriptase Reverse Transcriptase (Qiagen, CA, USA) and primers (Sigma, MO, USA). Real-Time quantitative PCR was performed with A 7500 qPCR system (Applied Biosystems, Irvine, USA) at 40 cycles with 100 ng of starting cDNA. Power SYBR green was used for amplification and data analyzed by delta CT method, SDS program version 1.4 (Applied Biosystems, Irvine, USA). RNA was quantified with the delta CT method and normalized to ß-Actin as an endogenous control. Each reaction was performed in triplicate.
III Results
A. Immunofluorescence
Figure 2 documents the expression levels for the set of proteins tested on stimulated and control retinal progenitor cells: CRX, PK-C, Ki-67, GFAP, Recoverin, and NF-200, by fluorescent immunostains (distributions of protein expression) and relative levels of fluorescence (semi-quantitative data). Panel A displays representative images of CRX and PK-C immunostainings and GFP images to allow visualization of cells morphologies and distributions (GFP is ubiquitously expressed under an actin promoter). CRX and PK-C expression were rarely seen in non-stimulated cells, while stimulated cells showed robust expression (Figure 2A). GFAP expression decreased 3.8-fold (P<0.005) in stimulated cells, suggesting differentiation away from glial cell fates, whereas CRX expression was increased 26.6-fold and PK-C was increased 83.5-fold (p<0.005), in stimulated cells (Figure 2A). There were no significant differences in Ki-67, Recoverin, and NF-200, although the latter two showed a trend towards increased expression following electrical stimulation (Figure 2B).
Figure 2. Protein Expression Levels in Retinal Progenitor Cells (RPCs) Change with Electrical Stimulation.
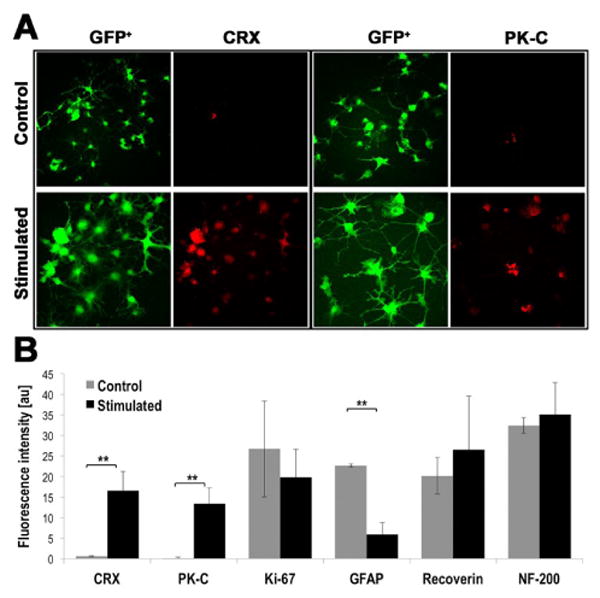
RPCs exposed to stimulation with 5 s, 100 μA pulses once per minute for 24 h exhibit substantial differences in protein expression when compared to non-stimulated controls. (A) Representative images of cone–rod homeobox (CRX) and Protein kinase C (PK-C) immunostainings, 2nd and 4th column; GFP images in 1st and 3rd column ease visualization of all stained cells. (B) Semi-quantitative expression levels measured from the relative fluorescence intensity. Intensity is reported in arbitrary units. **p<0.005, n≥ 3.
B. Real-Time quantitative RT-PCR (qPCR)
qPCR analyses were conducted to quantitate the expression of proteins in stimulated and control RPCs. (Figure 3). The obtained trends for Recoverin, GFAP and PK-C were consistent with the immunofluorescence quantification described above. We also detected a significant increase in Nestin expression (p<0.05) and decrease in NF-200 (p<0.05) in stimulated versus non-stimulated RPCs.
Figure 3. Gene Expression Levels Change with Electrical Stimulation.
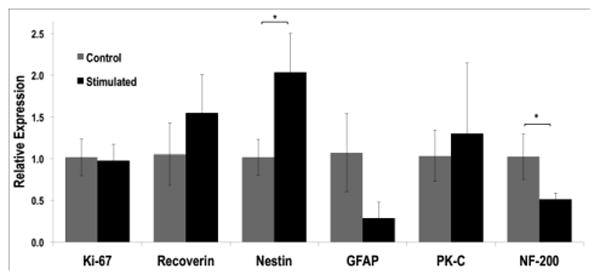
Quantitative PCR was used to evaluate relative changes in mRNA levels between stimulated and non-stimulated mRPCs on PPy. *p<0.05, n≥ 3.
C. Dendritic length and cell body size
Electrically stimulated cells showed an increase in average dendrite length, with RPCs stimulated for 4 days growing dendrites with average lengths of 101.7 ± 3.0 μm, while non-stimulated cells measured 63.7 ± 2.7 μm (p<0.001) (Figure 4A). Representative images of GFP fluorescing cells are shown for control and stimulated cultures, along with the histogram with quantitative data. In parallel, using a custom-developed script running image analysis commands in Matlab®, we evaluated the average size of cell bodies, measured by the projected area. The results are shown in Figure 4B, with GFP images again helping visualization of the differences in cell bodies area, and the histogram on the right reporting the 2.5-fold increase (p<0.005) in stimulated samples versus non-stimulated controls (values in pixels squared are 7.17E+02 ± 235.7 and 2.89E+02 ± 89.2, respectively).
Figure 4. Dendritic Length and Cell Bodies area increase for Electrically Stimulated RPCs.
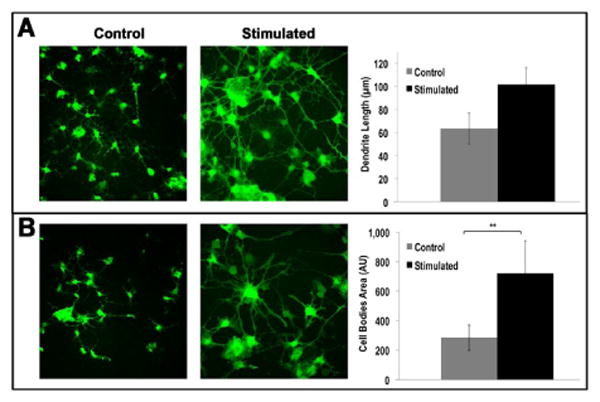
(A) Stimulated GFP+ RPCs (5s duration, 100mV amplitude pulse, once per minute for 24h) have dendrites that were nearly twice as long as those in non-stimulated controls. (B) Stimulated RPCs exhibited a 2.5-fold increase in size of cell bodies (area measured in pixels squared when compared with the non-stimulated controls. Representative images are shown on the left part of panels, histograms on the right quantify the results. *p<0.05.
IV Discussion
To recapitulate electrical wave patterns observed in developing retina we stimulated retinal progenitor cells using a biomimetic pattern of 100 μA pulse train (5 second pulses, once per minute) for 4 days on the electrically conductive polymer PPy. The electrical stimulation induced an increase in CRX and PK-C expression (Figure 2) suggesting that stimulation influences differentiation of RPCs towards early photoreceptor and bipolar cell gene expression patterns [25]. CRX has been shown in vivo to influence the expression of phototransduction genes [26]. Exogenous CRX transfection into RPCs has also been shown to direct differentiation towards photoreceptor fate [27].
Consistent results from both quantification of the fluorescent signals in stained cultures and gene expression analysis via qPCR, highlighted a reduction in GFAP (Figure 2 and Figure 3). During retinal development GFAP expression is decreased in neuronal cell types and upregulated in cells developing toward a Muller glia fate [28]. A decrease in GFAP has also been correlated with increased proliferation and re-enter into the cell cycle [29]. Observed trends of increased expression of Recoverin in stimulated RPCs, also lends support to electrical stimulation influencing retinal neuronal differentiation [30].
Observed increases in the area of stimulated RPC soma in comparison to non-stimulated controls (Figure 4) may indicate increases in cellular metabolism and function [31]. It has been suggested that electrical stimulation increases neuronal growth as well as expression of nerve growth factor, glial-derived growth factor and ciliary neurotrophic factor [31]. We also observed increased axon length in stimulated RPCs (Figure 4), which suggests improved neuronal morphology correlated to calcium-mediated electrical activity-dependent mechanisms on PPy substrates [18, 32].
In the present study we focused on in vitro modeling of biomimetic electric wave patterns derived from the developing mammalian retina [1, 7]. Although research evaluating electrical stimulation driven differentiation in a number of cell types [1, 9-12], and although a number of groups have used electrical stimulation targeting ganglion cells in attempts to enhance survival or drive perception toward prosthesis [33-34], to our knowledge this is the first study describing biomimetic electrical stimulation of retinal progenitors to drive differentiation. The work suggests that this in vitro model of RPC stimulation on PPy is capable of guiding multipotent RPCs toward retinal neural genotypic expression and morphology. This work defines changes in cell behavior influenced by rhythmic electrical stimulation potentially driving activity-dependent processes. The experiments reveal changes that may elucidate the influence of electrical activity guiding retinal development and may also be applied to future neural tissue engineering strategies.
Future studies will involve the optimization of the stimulation regimes, implementing more complex spatial and temporal patterns of in vivo-like electrical activities, which we could obtain using programmable electrical stimulators. Further studies will also include analysis of calcium dynamics and biomimetic differentiation media given their established role in influencing neuronal differentiation many cell types. In addition, other biomimetic approaches will be used to investigate the role of electrical stimulation on three-dimensional RPC constructs (neurospheres) better recapitulating the in vivo behavior of cells. Additionally, both Human Embryonic (hESC) and induced Pluripotent Stem Cells (iPSc) differentiated into RPCs may provide expandable cell sources stimulation analysis. These studies will likely be key towards understanding retinal waves during development and toward Tissue Engineering application.
Acknowledgments
Financial support was provided by NIH grants DE013023 and HL060435 (R.L); the Foundation Fighting Blindness and the Department of Defense (M.J.Y.); the National Eye Institute EY07145-06 and NIGMS GM096935-02 (S.R). The National Science Foundation graduate research fellowship (R.S). Elisa Cimetta is a New York Stem Cell Foundation-Druckenmiller Fellow (NYSCF-D-FO2O).
Contributor Information
Rajiv Saigal, Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, USA.
Elisa Cimetta, Columbia University, Department of Biomedical Engineering, 622 west 168th Street, Vanderbilt Clinic 12-234 New York NY, USA.
Nina Tandon, Columbia University, Department of Biomedical Engineering, 622 west 168th Street, Vanderbilt Clinic 12-234 New York NY, USA.
Robert Langer, Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, USA; Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, USA.
Michael Young, Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, USA; Schepens Eye Research Institute, Department of Ophthalmology, Harvard Medical School, 20 Staniford Street, Boston, MA, USA.
Gordana Vunjak-Novakovic, Email: gv2131@columbia.edu, Columbia University, Department of Biomedical Engineering, 622 west 168th Street, Vanderbilt Clinic 12-234 New York NY, USA.
Stephen Redenti, Email: stephen.redenti@lehman.cuny.edu, City University of New York, Lehman College, Molecular Cell and Developmental Biology, 250 Bedford Park Blvd, Davis Hall 217, Bronx, NY, USA.
References
- 1.RO W. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- 2.Kamioka H, et al. Spontaneous periodic synchronized bursting during formation of mature patterns of connections in cortical cultures. Neuroscience Letters. 1996;206:109–112. doi: 10.1016/s0304-3940(96)12448-4. [DOI] [PubMed] [Google Scholar]
- 3.Z LI, Poo Mm. Electrical activity and development of neural circuits. nature neuroscience supplement. 2001;4:1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- 4.Moody WJ. Control of spontaneous activity during development. Journal of Neurobiology. 1998;37:97–109. doi: 10.1002/(sici)1097-4695(199810)37:1<97::aid-neu8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.P A, Mooney R, Gallego R, Shatz CJ. Thalamic relay of spontaneous retinal activity prior to vision. Neuron. 1996 Nov;17(5):863–74. doi: 10.1016/s0896-6273(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 6.W DP, Feller Marla B, Stellwagen David, Werblin Frank S, Shatz Carla J. Requirement for Cholinergic Synaptic Transmission in the Propagation of Spontaneous Retinal Waves. Science. 1996;272(5265):1182–1187. doi: 10.1126/science.272.5265.1182. May 24, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Meister M, et al. Synchronous Bursts of Action Potentials in Ganglion Cells of the Developing Mammalian Retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- 8.Bassett EA, Wallace VA. Cell fate determination in the vertebrate retina. Trends in neurosciences. 2012 doi: 10.1016/j.tins.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 9.T K, Yamada M, Okada S, Iwanami A, Nakamura M, Mizuno H, Ozawa M, Ohyama-Goto R, Kitamura N, Kawano M, Tan-Takeuchi K, Ohtsuka C, Miyawaki A, Takashima A, Ogawa M, Toyama Y, Okano H, Kondo T. Electrical stimulation modulates fate determination of differentiating embryonic stem cells. Stem Cells. 2007 Mar;25(3):562–70. doi: 10.1634/stemcells.2006-0011. [DOI] [PubMed] [Google Scholar]
- 10.L NJ, Spitzer Nicholas C, Smith Raymond D, Gomez aTM. Coding of neuronal differentiation by calcium transients. BioEssays. 2000;22(9):811–817. doi: 10.1002/1521-1878(200009)22:9<811::AID-BIES6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 11.Fields RD, et al. Action Potential-Dependent Regulation of Gene Expression: Temporal Specificity in Ca2+, cAMP-Responsive Element Binding Proteins, and Mitogen-Activated Protein Kinase Signaling. The Journal of Neuroscience. 1997 Oct 1;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.E F, Fields RD, Stevens Beth, Itoh Kouichi. Action Potential-Dependent Regulation of Gene Expression: Temporal Specificity in Ca21, cAMP-Responsive Element Binding Proteins, and Mitogen-Activated Protein Kinase Signaling. The Journal of Neuroscience. 1997 Oct 1;17(19):7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong JY, et al. Electrically conducting polymers can noninvasively control the shape and growth of mammalian cells. Proc Natl Acad Sci U S A. 1994 Apr 12;91:3201–4. doi: 10.1073/pnas.91.8.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.G KJ, Liu Xiao, Moulton Simon E, Wallace GG. Electrical stimulation promotes nerve cell differentiation on polypyrrole/poly (2-methoxy-5 aniline sulfonic acid) composites. J Neural Eng. 2009;6:065002. doi: 10.1088/1741-2560/6/6/065002. 2009. [DOI] [PubMed] [Google Scholar]
- 15.S M, Jiang Xiaoping, Marois Yves, Traoré Amidou, Tessier Dominic, Dao LÊH, Guidoin Robert, Zhang ZE. Tissue Reaction to Polypyrrole-Coated Polyester Fabrics: An in Vivo Study in Rats. TISSUE ENGINEERING. 2002;8:635–647. doi: 10.1089/107632702760240553. [DOI] [PubMed] [Google Scholar]
- 16.G X, Wang Xioadong, Yuan Chunwai, Chen Shujian, Zhang Peiyun, Zhang Tianyi, Yao Jian, Chen Fen, Chen Gang. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J Biomed Mater Res A. 2004 Mar 1;68(3):411–22. doi: 10.1002/jbm.a.20065. [DOI] [PubMed] [Google Scholar]
- 17.P MP, Ghasemi-Mobarakeh Laleh, Morshed Mohammad, et al. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. JOURNAL OF TISSUE ENGINEERING AND REGENERATIVE MEDICINE. 2011;5:e17–e35. doi: 10.1002/term.383. 2011. [DOI] [PubMed] [Google Scholar]
- 18.G N, Guimard Nathalie K, Schmidt Christine E. Conducting polymers in biomedical engineering. Progress in Polymer Science. 2007;32 2007. [Google Scholar]
- 19.S S, Durgam Hymavathi, Deister Curt, Khaing Zin, Chang Emily, Luebben Silvia, Schmidt Christine E. Novel Degradable Co-polymers of Polypyrrole Support Cell Proliferation and Enhance Neurite Out-Growth with Electrical Stimulation. Journal of Biomaterials Science. 2010;21:1265–1282. doi: 10.1163/092050609X12481751806330. 2010. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt CE, et al. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc Natl Acad Sci U S A. 1997 Aug 19;94:8948–53. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.S V, Schmidt CE, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc Natl Acad Sci U S A. 1997 Aug 19;94(17):8948–53. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R A, McCaig CD, Song B, Zhao M. Has electrical growth cone guidance found its potential? Trends Neurosci. 2002 Jul;25(7):354–9. doi: 10.1016/s0166-2236(02)02174-4. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt CE, et al. Stimulation of neurite outgrowth using an electrically conducting polymer. Proceedings of the National Academy of Sciences. 1997 Aug 19;94:8948–8953. doi: 10.1073/pnas.94.17.8948. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redenti S, et al. Retinal tissue engineering using mouse retinal progenitor cells and a novel biodegradable, thin-film poly(e-caprolactone) nanowire scaffold. J Ocul Biol Dis Infor. 2008 Mar;1:19–29. doi: 10.1007/s12177-008-9005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haverkamp S, et al. A comparison of immunocytochemical markers to identify bipolar cell types in human and monkey retina. Visual Neuroscience. 2003;20:589–600. doi: 10.1017/s0952523803206015. [DOI] [PubMed] [Google Scholar]
- 26.Freund CL, et al. Cone-rod dystrophy due to mutations in a novel photoreceptor-specific homeobox gene (CRX) essential for maintenance of the photoreceptor. Cell. 1997 Nov 14;91:543–53. doi: 10.1016/s0092-8674(00)80440-7. [DOI] [PubMed] [Google Scholar]
- 27.Jomary C, Jones SE. Induction of functional photoreceptor phenotype by exogenous Crx expression in mouse retinal stem cells. Invest Ophthalmol Vis Sci. 2008 Jan;49:429–37. doi: 10.1167/iovs.07-0812. [DOI] [PubMed] [Google Scholar]
- 28.Ganat YM, et al. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006 Aug 16;26:8609–21. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyer MA, Cepko CL. Control of Muller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000 Sep;3:873–80. doi: 10.1038/78774. [DOI] [PubMed] [Google Scholar]
- 30.Dizhoor AM, et al. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science. 1991 Feb 22;251:915–8. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- 31.Rana OR, et al. Chronic electrical neuronal stimulation increases cardiac parasympathetic tone by eliciting neurotrophic effects. Circ Res. 2011 May 13;108:1209–19. doi: 10.1161/CIRCRESAHA.110.234518. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg SS, Spitzer NC. Calcium signaling in neuronal development. Cold Spring Harb Perspect Biol. 2011 Oct;3:a004259. doi: 10.1101/cshperspect.a004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morimoto T, et al. Electrical stimulation enhances the survival of axotomized retinal ganglion cells in vivo. NeuroReport. 2002;13:227–230. doi: 10.1097/00001756-200202110-00011. [DOI] [PubMed] [Google Scholar]
- 34.Eickenscheidt M, et al. Electrical stimulation of retinal neurons in epiretinal and subretinal configuration using a multicapacitor array. Journal of Neurophysiology. 2012;107:2742–2755. doi: 10.1152/jn.00909.2011. [DOI] [PubMed] [Google Scholar]


