Abstract
Mice sensitized and challenged with OVA were used to investigate the role of innate T cells in the development of allergic airway hyperresponsiveness (AHR). AHR, but not eosinophilic airway inflammation, was induced in T cell-deficient mice by small numbers of cotransferred γδ T cells and invariant NKT cells, whereas either cell type alone was not effective. Only Vγ1+Vδ5+ γδ T cells enhanced AHR. Surprisingly, OVA-specific αβ T cells were not required, revealing a pathway of AHR development mediated entirely by innate T cells. The data suggest that lymphocytic synergism, which is key to the Ag-specific adaptive immune response, is also intrinsic to T cell-dependent innate responses.
Adaptive immunity depends on the synergistic actions of different lymphocyte types. The best-studied example is the development of humoral responses to T-dependent Ag, which requires synergism of Ag-specific T and B cells (1). Likewise, the development of Ag-specific CTL is aided by Agspecific Th cells (2). In addition, the development of the Ag-specific immune responses appears to benefit from the synergistic action of innate T cells (3), but it is not known whether innate T cells also synergize with one another during innate immune responses.
In the pathogenesis of allergic airway diseases, Ag-specific memory T cells and allergen-specific Abs are considered key (4). Studies in humans and rodents indicate important roles for classical CD4+ and CD8+ αβ T cells in allergic inflammation (5, 6), but nonclassical T cells including NKT cells (7, 8) and γδ T cells (9, 10) have been implicated in allergic airway disease as well (11).
NKT cells are innate αβ T cells with a restricted TCR repertoire, which coexpress receptors of the NK lineage (12), and participate in protective and pathological host responses (13, 14), and in allergic airway disease (15). In allergen-sensitized mice, allergennonspecific NKT cells expressing invariant TCRs (iNKT)3 increase airway inflammation and airway hyperresponsiveness (AHR), without a requirement for allergen priming (7, 8). iNKT cells express a semi-invariant TCRα chain (Vα14-Jα18) in association with Vβ8, Vβ7, and Vβ2, and recognize glycolipids presented by the MHC class I-like CD1d molecule (16). They can be detected by staining with tetramerized CD1d/β2-microglobulin (β2m) heterodimeric molecules complexed with the pharmacological ligand α-galactosylceramide (αGalCer) (17). iNKT cells in C57BL/6 mice also express the NK receptor NK1.1, which is acquired during the final stages of their development (18). Like iNKT cells, γδ T cells also play a role in the lung pathology of allergen-sensitized mice (9, 10), particularly in the development of AHR. In OVA-sensitized and challenged mice, γδ T cells expressing Vγ1 enhanced AHR (19), whereas cells expressing Vγ4 strongly suppressed AHR (20, 21). The AHR-regulatory γδ T cells had only minor effects on airway inflammation, however, and they do not appear to recognize OVA (22). Notably, young adult mice (6–12 wk) require γδ T cells for the development of AHR following sensitization and challenge with OVA (19), even though older mice (>6 mo) develop AHR in the absence of γδ T cells (10).
AHR in mice genetically deficient in γδ T cells (B6.TCR-δ−/−) can be restored following adoptive transfer of small numbers of purified Vγ1+ γδ T cells from OVA-sensitized and challenged donors (19). Others have proposed that γδ T cells depend in their functions on interactions with αβ T cells (23). AHR-suppressive γδ T cells do not require αβ T cells (10), but it remained possible that the AHR-enhancing γδ T cells depend on αβ T cells for this function. Our studies suggest that Vγ1+ γδ T cells and iNKT αβ T cells synergize in the development of AHR, and that they depend on each other in this function.
Materials and Methods
Animals
C57BL/6, B6.TCR-β−/−, B6.TCR-δ−/−, and B6.TCR-β−/−δ−/− mice were purchased from The Jackson Laboratory. All mice were maintained on OVA-free diet. The mice were 8–12 wk old at the time of the experiments. All mice were cared for at National Jewish Medical and Research Center, following guidelines for immune-deficient animals. All experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee.
Sensitization and airway challenge
Groups of mice were sensitized by i.p. injection of 20 μg of OVA (OVA grade V; Sigma-Aldrich) emulsified in 2.25 mg of aluminum hydroxide (AlumImuject; Pierce) in a total volume of 100 μl on days 0 and 14 (2ip). Mice were challenged via the airways with OVA (10 mg/ml in saline) for 20 min each on days 28, 29, and 30, by ultrasonic nebulization (particle size 1–5 mm; De Vilbiss) (3N). Lung resistance (RL) and dynamic compliance (Cdyn) were assessed 48 h after the last allergen challenge, and the mice were sacrificed to obtain tissues and cells for additional analysis.
Bronchoalveolar lavage (BAL)
Immediately following measurements of AHR, lungs were lavaged and BAL fluid was recovered. Total leukocyte numbers were measured (Coulter Counter; Coulter Electronics). Differential cell counts were performed by light microscopy of cytocentrifuged preparations (Cytospin2, Cytospin; Thermo Shandon), stained with Leukostat (Fisher Scientific). For each sample, at least 200 cells were counted and differentiated by standard hematological procedures. All BAL cell counts and statistical analysis of differences are provided in Table I.
Table I.
Cell numbers (×1000) and percentages, in the BAL fluid of cell transfer recipients (mean ± SEM)
| Group (treatment) | Total Cell Number | Eosinophil (percentage of total) | Macrophage (percentage of total) | Lymphocyte (percentage of total) | Neutrophil (percentage of total) | Number of Mice | Statistical Significance | |
|---|---|---|---|---|---|---|---|---|
| Fig. 1, a and b | NT | 392 ± 50 | 0 ± 0 (0 ± 0) | 387 ± 60 (100 ± 0) | 6 ± 3 (0 ± 0) | 1 ± 1 (0 ± 0) | 5 | *, p < 0.05 |
| 2ip3n | 212 ± 36** | 106 ± 31 (47 ± 10)* | 100 ± 25** (40 ± 10)** | 4 ± 2 (2 ± 1) | 3 ± 1 (1 ± 0) | 8 | **, p < 0.01 vs NT group | |
| 2ip3n + Vγ1(2ip) | 177 ± 11** | 103 ± 10 (59 ± 4)* | 63 ± 8** (36 ± 5)** | 2 ± 1 (1 ± 0) | 8 ± 3 (4 ± 1) | 8 | #, p < 0.05 | |
| 2ip3n + Vγ1(NT) | 205 ± 108** | 36 ± 9 (29 ± 7)# | 167 ± 105** (70 ± 7)**## | 1 ± 0 (1 ± 0) | 2 ± 1 (1 ± 1) | 7 | ##, p < 0.01 vs Vγ1 (2ip) group | |
| Fig. 1, c and d | 2ip3n | 192 ± 30 | 95 ± 22 (44 ± 10) | 92 ± 9 (53 ± 11) | 3 ± 1 (1 ± 0) | 3 ± 1 (1 ± 0) | 11 | #, p < 0.05 |
| 2ip3n + Vγ1(2ip) | 332 ± 101 | 172 ± 6 (44 ± 7) | 117 ± 29 (47 ± 9) | 18 ± 7 (4 ± 1) | 24 ± 19 (4 ± 2) | 8 | ##, p < 0.01 vs Vγ1 (2ip) group | |
| 2ip3n + Vγ1(NT) | 150 ± 15## | 82 ± 17 (52 ± 8) | 58 ± 10 (42 ± 9)# | 5 ± 1 (3 ± 1) | 5 ± 2 (3 ± 1) | 8 | ||
| Fig. 1, e and f | 2ip3n + no cells | 142 ± 24 | 0 ± 0 | 141 ± 24 | 0 ± 0 | 1 ± 1 | 5 | |
| 2ip3n + αβ | 182 ± 54 | 4 ± 4 | 177 ± 50 | 0 ± 0 | 0 ± 0 | 4 | ||
| 2ip3n + Vγ1 | 240 ± 92 | 1 ± 1 | 237 ± 92 | 0 ± 0 | 2 ± 1 | 6 | ||
| 2ip3n + Vγ1 + αβ | 141 ± 19 | 0 ± 0 | 139 ± 18 | 1 ± 0 | 1 ± 1 | 6 | ||
| Fig. 2, a and b | 2ip3n | 280 ± 51 | 140 ± 56 (48 ± 14) | 131 ± 45 (50 ± 14) | 6 ± 4 (2 ± 1) | 4 ± 2 (1 ± 1) | 4 | *, p < 0.05 |
| NK1.1 depleted, 2ip3n | 276 ± 35 | 37 ± 28 (12 ± 9)* | 240 ± 31* (88 ± 8)** | 0 ± 0 (0 ± 0) | 0 ± 0 (0 ± 0) | 3 | **, p < 0.01 vs 2ip3n group | |
| NK1.1 depleted, 2ip3n + Vγ1 | 215 ± 42 | 27 ± 10 (14 ± 7)* | 187 ± 42* (86 ± 7)** | 0 ± 0 (0 ± 0) | 0 ± 0 (0 ± 0) | 4 | ||
| Fig. 2 e and f | 2ip3n | 212 ± 36 | 106 ± 31 (47 ± 10) | 100 ± 25 (40 ± 10) | 4 ± 2 (2 ± 1) | 3 ± 1 (1 ± 0) | 8 | ***, p < 0.01 vs 2ip3n group |
| Vγ1 from B6 | 205 ± 108 | 36 ± 9 (29 ± 7) | 167 ± 105 (70 ± 7) | 1 ± 0 (1 ± 0) | 2 ± 1 (1 ± 1) | 7 | ||
| Vγ1 from NK1.1 depleted B6 | 164 ± 22 | 20 ± 11 (12 ± 6)*** | 144 ± 25 (88 ± 6)*** | 0 ± 0 (0 ± 0) | 0 ± 0 (0 ± 0) | 6 | ||
| Fig. 3, a and b | No cell transferred | 142 ± 24 | 0 ± 0 | 141 ± 24 | 0 ± 0 | 1 ± 1 | 5 | |
| Vγ1 + NK1.1+αβ | 136 ± 13 | 0 ± 0 | 136 ± 13 | 0 ± 0 | 0 ± 0 | 7 | ||
| NK1.1+αβ | 109 ± 18 | 2 ± 1 | 106 ± 18 | 0 ± 0 | 0 ± 0 | 4 | ||
| Vγ1 + NK1.1–αβ | 154 ± 20 | 0 ± 0 | 154 ± 20 | 0 ± 0 | 0 ± 0 | 3 | ||
| Vγ1 + NK | 148 ± 15 | 0 ± 0 | 148 ± 15 | 0 ± 0 | 0 ± 0 | 5 | ||
| Fig. 3, c and d | No cell transferred | 142 ± 24 | 0 ± 0 | 141 ± 24 | 0 ± 0 | 1 ± 1 | 5 | |
| Vγ1 + Tet+αβ | 155 ± 26 | 0 ± 0 | 155 ± 26 | 0 ± 0 | 0 ± 0 | 5 | ||
| Tet+αβ | 97 ± 15 | 0 ± 0 | 97 ± 15 | 0 ± 0 | 0 ± 0 | 4 | ||
| Vγ1 + Tet–αβ | 142 ± 37 | 0 ± 0 | 142 ± 37 | 0 ± 0 | 0 ± 0 | 5 | ||
| Fig. 3, e and f | No cell transferred | 142 ± 24 | 0 ± 0 | 141 ± 24 | 0 ± 0 | 1 ± 1 | 5 | |
| Vγ1 + NK1.1+Tet+αβ | 96 | 0 | 96 | 0 | 0 | 1a | ||
| Vγ1 + NK1.1+Tet–αβ | 290 ± 120 | 0 ± 0 | 290 ± 120 | 0 ± 0 | 0 ± 0 | 4 | ||
| Vγ1 + NK1.1–Tet–αβ | 115 ± 17 | 2 ± 2 | 113 ± 17 | 0 ± 0 | 0 ± 0 | 4 | ||
| Fig. 4, b and c | No cell transferred | 212 ± 36 | 106 ± 31 (47 ± 10) | 100 ± 25 (40 ± 10) | 4 ± 2 (2 ± 1) | 3 ± 1 (1 ± 0) | 8 | *, p < 0.01 vs no cell transferred group |
| Total Vγ1 | 150 ± 15 | 82 ± 17 (52 ± 8) | 58 ± 10 (42 ± 9) | 5 ± 1 (3 ± 1) | 5 ± 2 (3 ± 1) | 8 | ||
| Vγ1+δ4+ | 200 | 65 | 141 | 2 | 5 | 2a | ||
| Vγ1+δ5+ | 194 ± 36 | 100 ± 21 (39 ± 7) | 159 ± 29 (60 ± 7) | 1 ± 1 (0 ± 0) | 4 ± 2 (1 ± 1) | 4 | ||
| Vγ1+δ6.3+ | 106 ± 23 | 16 ± 5* (14 ± 3) | 87 ± 18 (84 ± 4) | 1 ± 1 (0 ± 0) | 2 ± 1 (1 ± 1) | 4 | ||
| Vγ1+δ5– | 125 ± 14 | 37 ± 19 (26 ± 12) | 88 ± 15 (73 ± 12) | 0 ± 0 (0 ± 0) | 0 ± 0 (0 ± 0) | 5 |
In these cases, not all of the animals that were analyzed for lung function remained available for BAL analysis.
Administration of anti-NK1.1 Abs
Anti-NK1.1 mAb PK136 was purified from hybridoma culture supernatant using a protein G-Sepharose affinity column (Pharmacia). Depletion of NK1.1+ cells was achieved after injection of 200 μg of purified anti-NK1.1 mAb into the tail veins of mice, 3 days before the first OVA challenge and/or cell transfer. Depletion was monitored by immunocytofluorimetry (FACScan).
Cell purification and adoptive transfer of T cells
Donor spleens were homogenized, treated with Gey's solution for RBC removal, and passed through nylon wool columns for T cell enrichment. Nonadherent (NAD) cells were used for further purification. αβ T cells were purified from sensitized TCR-δ−/− mice. NAD cells were stained with FITC-conjugated anti-TCR-β mAb H57.597, with biotinylated anti NK1.1 mAb, followed by PE-streptavidin, or with PE-conjugated anti NK1.1 mAb, or with αGalCer-loaded CD1d/β2m tetramer conjugated to PE, as previously described in detail (17). Stained cells were sorted on a MoFlow cell sorter (DakoCytomation), and collected at a purity of >95%. Vγ1+ γδ T cells were purified from the spleen of C57BL/6 or B6.TCR-β−/− mice. NAD cells were stained with biotinylated anti-Vγ1 mAb (24), and positively selected using streptavidin-conjugated magnetic beads (Streptavidin Microbeads; Miltenyi Biotec), as previously described in detail (22). Repeated selection produced a cell population containing >90% viable Vγ1+ cells, as determined by two-color staining with anti-TCR-δ GL3 and anti-Vγ1 mAbs. The purified cells were washed and resuspended in balanced salt solution, and injected via the tail vein into OVA-sensitized mice (B6.TCR-δ−/− or B6.TCR-β−/−δ−/−), <1 h before the first airway challenge.
Note: Throughout this work, we use the nomenclature for murine Vγ genes introduced by Heilig and Tonegawa (25). We use the term “enhancing” cells to refer to purified Vγ1+ γδ T cells capable of enhancing AHR upon adoptive cell transfer into OVA-sensitized and challenged recipients, and the term “suppressive” cells to refer to purified Vγ4+ γδ T cells derived from OVA-sensitized and challenged mice, which are capable of suppressing AHR.
Determination of airway responsiveness
Airway responsiveness was assessed as a change in lung function after provocation with aerosolized methacholine (MCh) using a method previously described in detail (10). MCh aerosol was administered for 10 s (60 breaths/min, 0.5 ml of tidal volume) in increasing concentrations. Maximum values of RL and minimum values of Cdyn were recorded and expressed as percentage of change from baseline after saline aerosol.
Statistical analysis
Data are presented as means ± SEM. The unpaired t test was used for two-group comparisons, and two-way ANOVA for analysis of differences in three or more groups. Pairwise comparisons were performed using the post-Bonferroni test. Statistically significant levels were set at a p value of <0.05.
Results
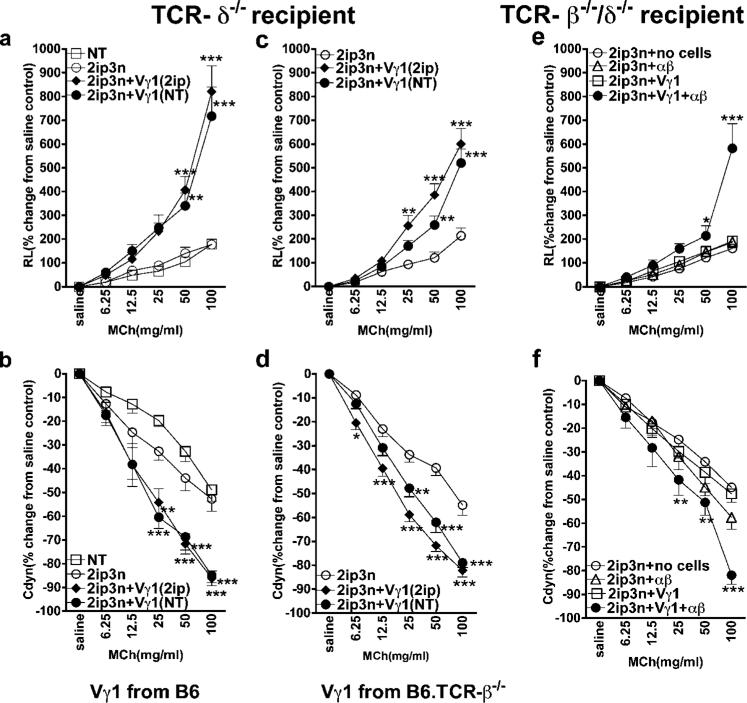
Like NKT cells, γδ T cells are considered part of the innate immune response. To test whether γδ T cells can enhance AHR likewise without allergen priming, we transferred 104 purified Vγ1+ γδ T cells from the spleen of untreated C57BL/6 mice into OVA-sensitized γδ T cell-deficient recipients (B6.TCR-δ−/−), just before OVA challenge. Without the transferred cells, the B6.TCR-δ−/− mice showed only weak responses to inhaled MCh, based upon the changes seen in RL and Cdyn (Fig. 1, a and b). When reconstituted with Vγ1+ cells from either sensitized or nonsensitized donors, they developed AHR (Fig. 1, a and b), indicating that the development of the AHR-enhancing γδ T cells does not require allergen priming or help from allergen-primed αβ T cells. Notably, in this and subsequent experiments, the AHR-enhancing γδ T cells had little or no effect on eosinophilic airway inflammation (Table I).
FIGURE 1.
Vγ1+ γδ T cells from naive donors enhance AHR when αβ T cells are present. AHR was monitored by measuring RL (a, c, and e) and Cdyn (b, d, and f). a and b, Reconstitution of γδ T cell-deficient mice with Vγ1+ cells restores AHR. OVA-sensitized B6.TCR-δ−/− mice received 1 × 104 splenic Vγ1+ γδ T cells from untreated (NT) or sensitized (2ip) C57BL/6 donors, before airway challenge. Untreated recipients (NT) and recipients that were sensitized and challenged, but did not receive cells (2ip3N), are also shown. Results for each group are presented as means ± SEM (n = 8). Significant differences between 2ip3N and 2ip3N + Vγ1 groups are indicated: **, p < 0.01; ***, p < 0.001. c and d, Vγ1+ cells from αβ T cell-deficient mice are still capable of restoring AHR. OVA-sensitized B6.TCR-δ−/− mice received 1 × 104 splenic Vγ1+ γδ T cells from untreated (NT) or sensitized (2ip) B6.TCR-β−/− donors, before airway challenge. Recipients that were sensitized and challenged, but did not receive cells (2ip3N), are also shown. Results for each group are presented as means ± SEM (n = 12). Significant differences between 2ip3N and 2ip3N + Vγ1 groups are indicated as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001. e and f, Vγ1+ cells together with αβ T cells restore AHR in T cell-deficient mice. OVA-sensitized B6.TCR-β−/−δ−/− mice received 1 × 104 splenic Vγ1+ γδ T cells from sensitized B6.TCR-β−/− donors and 2 × 106 αβ T cells from sensitized B6.TCR-δ−/− donors (Vγ1 + αβ), before airway challenge. Recipients that were sensitized and challenged, but did not receive cells (2ip3N), or received only Vγ1+ cells (Vγ1) or only αβ T cells (αβ), are also shown. Results for each group are presented as means ± SEM (n = 4–9). Significant differences between sensitized and challenged mice, which received no cells, or Vγ1+ cells plus αβ T cells, are indicated as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Despite the absence of a priming requirement, it remained possible that αβ T cells influence the normal development of these γδ T cells (26). We therefore repeated the cell-transfer experiment with Vγ1+ γδ T cells derived from B6.TCR-β−/− mice, in which they must develop in the absence of αβ T cells (Fig. 1, c and d). These cells still enhanced AHR, indicating that αβ T cells are not required for the development of the AHR-enhancing γδ T cells. However, because the cell transfer recipients (B6.TCR-δ−/− mice) contain αβ T cells, the possibility remained that αβ T cells are somehow involved in the AHR-enhancing effect of the transferred Vγ1+ cells. To examine this, we used OVA-sensitized mice deficient in both αβ and γδ T cells (B6.TCR-β−/−δ−/−) as recipients (Fig. 1, e and f). In these mice, transferred Vγ1+ cells alone failed to induce AHR, but produced a small AHR response when transferred together with αβ T cells, indicative of a role for αβ T cells in the effector phase of the AHR response. Predictably, αβ T cells transferred alone had no effect.
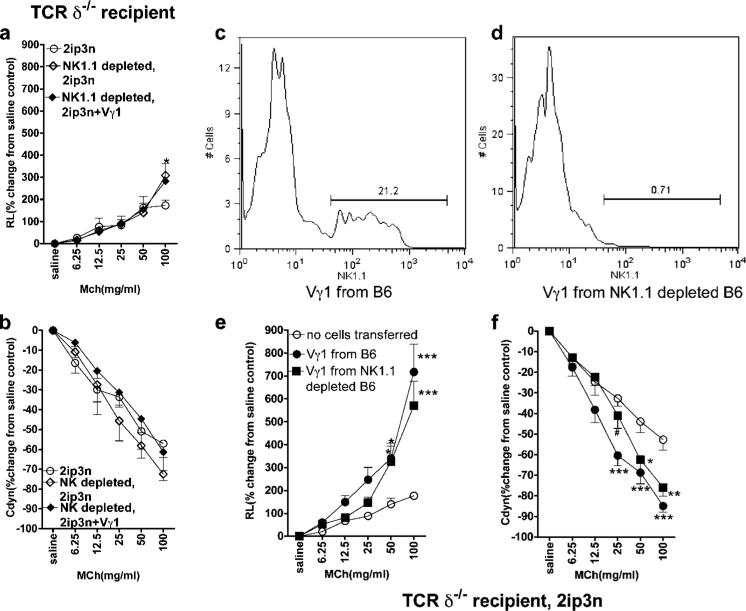
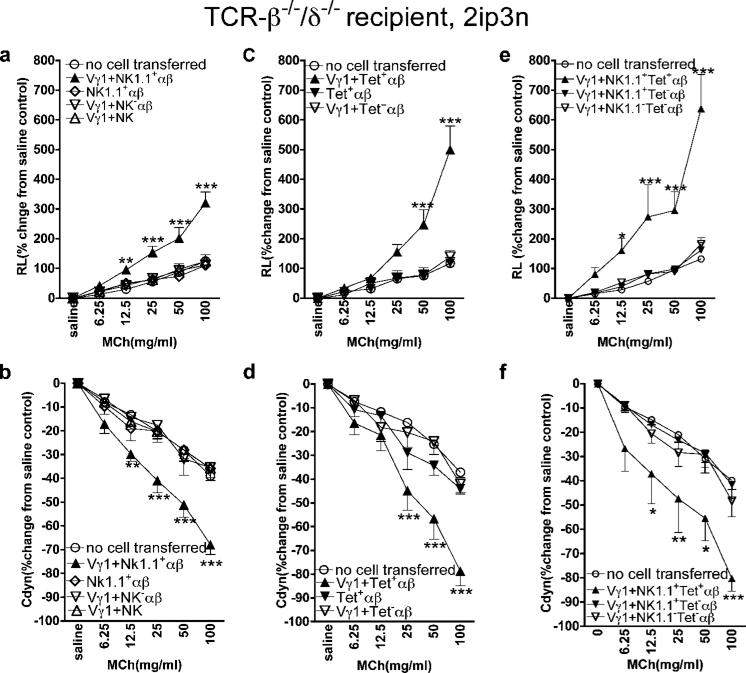
The comparatively weak AHR response in these mice despite a large number of transferred αβ T cells (Fig. 1, e and f) suggested that most αβ T cells were not effective. We reasoned that because NKT cells reportedly mediate AHR (7, 8), they might be the critical αβ T cell component. To test this, we modified the original experiment of transferring Vγ1+ cells into sensitized B6.TCR-δ−/− mice by treating the recipients first with NK1.1-depleting Abs (Fig. 2, a and b) (27). Indeed, these mice no longer developed AHR following transfer of the γδ T cells. Because some γδ T cells themselves express NK1.1 (Fig. 2, c and d) (28, 29), it remained possible that residual Ab in the treated mice had inactivated the transferred γδ T cells. However, there was no significant difference in AHR enhancement when we compared total Vγ1+ cells and NK1.1-depleted Vγ1+ cells (Fig. 2, e and f). This result confirmed that both the transferred Vγ1+ γδ T cells and endogenous NK1.1+ cells are required to elicit AHR in the B6.TCR-δ−/− recipients. Although the requirement for both αβ T cells and NK1.1+ cells in γδ T cell-induced AHR might reflect a need for one type of cellular partner, this result could also indicate that more than one additional cell type is involved, because NK1.1-expressing cells include non-T NK cells, as well as classical and nonclassical NKT cells of the αβ T cell lineage (30). To test the capacity of these different cell types, we cotransferred purified preparations of each type into OVA-sensitized B6.TCR-β−/−δ−/− mice (Fig. 3, a and b). NK1.1+ αβ T cells cotransferred with Vγ1+ γδ T cells elicited a significant AHR response, whereas neither NK1.1+ αβ T cells alone, or either NK1.1− αβ T cells or NK1.1+ TCR-β− NK cells cotransferred with the γδ T cells, enhanced AHR. This indicated that αβ NKT cells (invariant or other) synergize with Vγ1+ γδ T cells to produce AHR. To distinguish between the two, we transferred purified iNKT cells based on staining with αGalCer-loaded CD1d/β2m tetramers together with Vγ1+ γδ T cells (Fig. 3, c and d). This combination elicited a strong AHR response. Notably, iNKT cells alone, or tetramer-negative αβ T cells together with the γδ T cells, had no effect. Finally, to test whether iNKT cells are the only NK1.1+ cell population capable of synergy with the AHR-enhancing γδ T cells, we compared the effect of NK1.1+ αβ T cells that were either tetramer positive or negative (Fig. 3, e and f). Only NK1.1+ αβ T cells that were tetramer positive induced AHR when cotransferred with Vγ1+ γδ T cells, suggesting that the synergism only involves iNKT cells.
FIGURE 2.
Vγ1+ γδ T cells fail to mediate AHR when NK1.1+ cells are absent. AHR was monitored by measuring RL (a and e) and Cdyn (b and f). a and b, Vγ1+ cells fail to restore AHR in γδ T cell-deficient mice pretreated with anti-NK1.1 mAb. OVA-sensitized B6.TCR-δ−/− mice were treated with mAb PK136 (200 μg i.v.), received 1 × 104 splenic Vγ1+ γδ T cells 3 days later, and were then challenged via the airways (NK1.1-depleted, 2ip3N + Vγ1). Recipients that were only sensitized and challenged (2ip3N) and those that were sensitized and challenged and treated with the Ab (NK1.1-depleted, 2ip3N) are also shown. Results for each group are presented as means ± SEM (n = 4). Significant differences between 2ip3N and 2ip3N, NK1.1-depleted groups are indicated as follows: *, p < 0.05. c and d, Depletion of NK1.1+ cells within the Vγ1+ population. C57BL/6 mice were treated with mAb PK136 (200 μg i.v.) and 3 days later, NAD splenocytes were stained for TCR-δ, Vγ1, and NK1.1. Cytofluorimetric analysis shows that ~20% of gated splenic Vγ1+ γδ T cells express NK1.1 (c) and that the treatment with mAb PK136 removes most of these cells (d). e and f, NK1.1− Vγ1+ γδ T cells enhance AHR. OVA-sensitized B6.TCR-δ−/− mice received 1 × 104 splenic Vγ1+ γδ T cells from C57BL/6 donors before airway challenge. The cell donors were either untreated or received mAb PK136 i.v., 3 days before cell transfer (Vγ1 from B6 and Vγ1 from NK1.1-depleted B6). Recipients that were sensitized and challenged, but did not receive cells, are also shown (no cells transferred). Results for each group are presented as means ± SEM (n = 7–8). Significant differences between mice that received no cells or Vγ1 cells are indicated as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001. Significant difference between mice that had received Vγ1+ cells from B6 vs from NK1.1-depleted B6 (f) is as follows: #, p < 0.05.
FIGURE 3.
Vγ1+ γδ T cells synergize with NKT cells in mediating AHR. AHR was monitored by measuring RL (a, c, and e) and Cdyn (b, d, and f). a and b, Vγ1+ cells together with NK1.1+ αβ T cells restore AHR in T cell-deficient mice. OVA-sensitized B6.TCR-β−/−δ−/− mice received 1 × 104 splenic Vγ1+ γδ T cells from sensitized B6.TCR-β−/− donors and 2 × 104 NK1.1+ αβ T cells from sensitized B6.TCR-δ−/− donors (Vγ1 + NK1.1+αβ), before airway challenge. Recipients that were sensitized and challenged, but did not receive cells (no cell transferred), or that received 2 × 104 NK1.1+ αβ T cells (NK1.1+αβ) alone, Vγ1+ cells plus 2 × 106 NK1.1− αβ T cells (Vγ1 + NK1.1−αβ), or Vγ1+ cells plus 9 × 104 NK1.1+ non-T cells (Vγ1 + NK), are also shown. Results for each group are presented as means ± SEM (n = 6–7). Significant differences between mice that received no cells or Vγ1+ cells plus NK1.1+ αβ T cells are indicated as follows: **, p < 0.01; ***, p < 0.001. c and d, Vγ1+ cells together with CD1d tetramer+ αβ T cells restore AHR in T cell-deficient mice. OVA-sensitized B6.TCR-β−/−δ−/− mice received 1 × 104 splenic Vγ1+ γδ T cells from sensitized B6.TCR-β−/− donors and 2 × 104 CD1d tetramer+ αβ T cells from sensitized B6.TCR-δ−/− donors (Vγ1 + Tet+ αβ), before airway challenge. Recipients that were sensitized and challenged, but did not receive cells (no cells), or that received 2 × 104 Tet+ αβ T cells (Tet+ αβ) alone, or Vγ1+ cells plus 2 × 104 Tet- αβ T cells (Vγ1 + Tet− αβ), are also shown. Results for each group are presented as means ± SEM (n = 4–5). Significant differences between mice that received no cells or Vγ1+ plus NK1.1+ αβ T cells are indicated as follows: ***, p < 0.001. e and f, Only NK1.1+ αβ T cells that are also CD1d tetramer+ synergize with Vγ1+ γδ T cells in mediating AHR. OVA-sensitized B6.TCR-β−/−δ−/− mice received 1 × 104 splenic Vγ1+ γδ T cells from sensitized B6.TCR-β−/− mice and 2 × 104 NK1.1+ CD1d tetramer+ αβ T cells from sensitized B6.TCR-δ−/− donors (Vγ1 + NK1.1+Tet+ αβ), before airway challenge. Recipients that were sensitized and challenged, but did not receive cells (no cell transferred), or that received Vγ1+ cells and 2 × 104 NK1.1+Tet− αβ T cells (Vγ1 + NK1.1+Tet− αβ), or Vγ1+ cells plus 2 × 104 NK1.1−Tet− αβ T cells (Vγ1 + NK1.1−Tet− αβ), are also shown. Results for each group are presented as means ± SEM (n = 5–6). Significant differences between mice that received no cells or Vγ1+ cells plus NK1.1+ αβ T cells are indicated as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
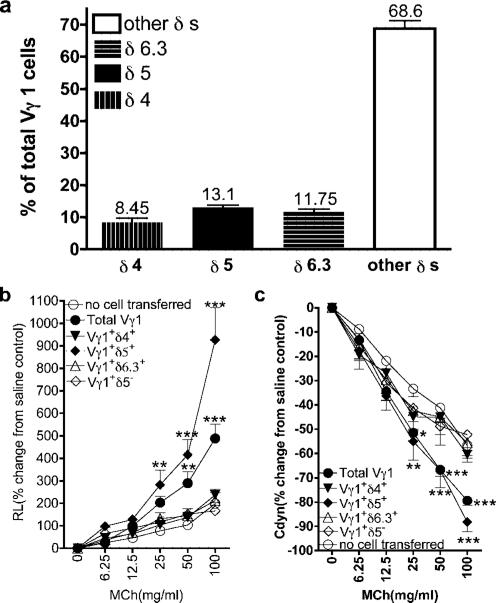
iNKT cells have a limited TCR repertoire (18). However, Vγ1+ cells can express several Vδ genes (Fig. 4a). We purified Vγ1+ cells expressing individual Vδs and examined their ability to induce AHR (Fig. 4, b and c). Only Vγ1+Vδ5+ cells induced AHR, indicating that the TCR repertoire of AHR-enhancing innate γδ T cells is highly limited as well. Thus, as far as T cells are concerned, the combined action of two innate T cell types is sufficient to mediate AHR in the OVA model.
FIGURE 4.
Comparison of Vγ1+ γδ T cells expressing different Vδs for their ability to mediate AHR. a, Vδ expression among Vγ1+ γδ T cells in B6.TCR-β−/− spleen. NAD splenocytes of adult B6.TCR-β−/− mice were stained with Abs against Vγ1, TCR-δ, and several Vδs, and analyzed cytofluorimetrically. Results for each group are presented as means ± SEM (n = 5–11). b and c, Reconstitution of γδ T cell-deficient mice with Vδ+ fractions of Vγ1+ cells. OVA-sensitized B6.TCR-δ−/− mice received 1 × 104 sorted Vγ1+ γδ T cells from untreated B6.TCR-β−/− spleen, expressing the indicated Vδs, before airway challenge (2ip3N + Vγ1). Recipients that were sensitized and challenged, but did not receive cells (no cell transferred), are also shown. AHR was monitored by measuring RL (b) and Cdyn (c). Results for each group are presented as means ± SEM (n = 5–8). Significant differences between mice that received no cells or Vγ1+ cells are indicated as follows: **, p < 0.01; ***, p < 0.001.
Discussion
There is currently some debate concerning the role of different types of lymphocytes in allergic airway disease. With regard to AHR in the OVA models, B cells were found to be required in at least one model (31), as well as in a hapten model of nonatopic asthma (32). However, neither αβ T cells nor γδ T cells are always required for the development of AHR (10, 20), and the role of NKT cells has been controversial (11, 15, 33, 34). In studies investigating the effect of NKT cells on AHR and airway inflammation, contributions of conventional CD4+ αβ T cells have been ruled out (35), but not of γδ T cells. Some of the current controversy therefore might be resolved by taking into account that the NKT cells can depend on γδ T cells to express their AHR-enhancing potential.
The present study confirms that innate lymphocyte populations, including iNKT cells, can play a role in the allergic airway disease induced by OVA. In this role, the innate lymphocytes appear to be independent of the allergen-specific T cells, but they cannot fully replace them. We have previously reported that, although γδ T cells can have a strong effect on AHR, they do not substantially alter airway cytokines or eosinophilic inflammation (19). This difference was maintained in the current study, because γδ T cells whether or not they were cotransferred with iNKT cells did not induce substantial changes in airway eosinophils compared with controls that did not receive transferred cells (Table I). However, treatment with the anti-NK1.1 mAb reduced airway eosinophilic infiltrations.
Most interestingly, our findings show that in the absence of allergen-specific T cells, neither γδ T cells nor iNKT cells alone can mediate AHR. iNKT cells have been previously implicated in allergic airway inflammation and AHR even in mice lacking CD4+ allergen-specific αβ T cells (7, 8), but a dependence on γδ T cells was not noted. In contrast to these studies, and other studies with conventional T cells (36), we have transferred much smaller numbers of cells (104 Vγ1+ γδ T cells and 2 × 104 iNKT cells), which may be crucial in detecting the mutual dependence of these cell types. If quantities of cytokines (e.g., IL-13) are critical in the development of eosinophilic inflammation, the small numbers of transferred innate T cells might also explain why no effect of these cells on eosinophilic airway infiltration was seen (Table I).
We have not addressed the possible involvement of other lymphocytes remaining in the T cell-deficient recipients, including B cells, which might be capable of recognizing OVA. However, if such cells are present, they alone are incapable of mediating AHR. Neither the iNKT cells nor the γδ T cells appear to recognize OVA, although it is difficult to rule out this possibility entirely (37, 38). Whether they recognize other components in the OVA preparation used for sensitization and challenge (e.g., LPS), autologous ligands induced by the treatment of the recipients, or no ligands at all remains to be determined. Candidate ligands might include inducible self-lipids (39), and phospholipids in particular. We found that murine cells expressing Vγ1 exhibit a spontaneous cytokine response in vitro, which might be based on the recognition of self-ligands (40, 41); such cells also responded to certain anionic phospholipids (42). Interestingly, human CD1d-restricted γδ T cells were also stimulated by phospholipids (43), and their response to phosphatidylethanolamine could be correlated with allergic hyperresponsiveness to pollen allergen (44). The limited TCR repertoires of the innate T cells studied in this work might well be shaped entirely by developmental constraints, rather than allergen-driven peripheral selection.
Our study does not directly address the mechanism underlying the synergy between AHR-enhancing γδ T cells and iNKT cells. However, because of the small numbers of cells transferred, direct cell-cell interactions would appear less likely, although interactions involving cell contacts with an intermediary such as a dendritic cell (DC) might well occur. In other studies, we have colocalized Vγ1+ γδ T cells and DC in lung and spleen (45), and NKT cells are known to interact with DC as well. We have also shown previously that adoptively transferred Vγ1+ cells increase levels of IL-13 and IL-5 in the airways (19). Because iNKT cells can produce IL-13 (7), perhaps the γδ T cells stimulate their cytokine production. Having identified the functional synergy between γδ T cells and iNKT cells, it now seems worthwhile to define the underlying molecular mechanisms.
Cooperation between different lymphocyte types has long been recognized as a hallmark of the adaptive, Ag-specific immune response, exemplified by the classical mechanism of direct T-B cooperation, but also including synergistic interactions between Agspecific T cells and innate lymphocyte types. Whether such interactions are direct or involve cellular intermediates such as the multifunctional DC, all appear to benefit from the different functional potentials of the participating lymphocytes. The example of a synergism between γδ T cells and iNKT cells described in this study probably is based as well upon a complementary functional potential of these innate lymphocytes. The observation that Vγ1+Vδ6+ γδ T cells are not AHR enhancing is consistent with this notion. This subset of the Vγ1+ population contains γδ T cells with NKT-like properties (29) and thus would not be expected to complement the iNKT cells.
Our findings may represent a specific case of the synergism between γδ and αβ T cells, which has been proposed some time ago (23). In addition, because not only iNKT cells (7), but also the AHR-enhancing γδ T cells (this study) could be derived from unprimed donors and express a very limited TCR repertoire, our study suggests that lymphocytic synergism might be a mechanism used not only by adaptive, but also by innate T-dependent immune responses.
Acknowledgments
We thank Drs. Philippa Marrack, Max Cooper, David Talmage, and Katsuyuki Takeda for advice and support, and Joshua Loomis, Shirley Sobus, and William Townend for expert help with cell sorting. Biotinylated mouse CD1d monomers were generously provided by the National Institutes of Health tetramer core facility.
Footnotes
This study was supported by National Institutes of Health Grants HL65410 and AI40611 (to W.K.B.), AI44920 and AI063400 (to R.L.O.), HL36577 and HL61005 (to E.W.G.), and AI057485 (to L.G.), and by Environmental Protection Agency Grant R825702 (to E.W.G.). Support was also provided by a postdoctoral fellowship from the American Cancer Society (to J.L.M.).
Abbreviations used in this paper: iNKT, invariant NKT; αGalCer, α-galactosylceramide; AHR, airway hyperresponsiveness; β2m, β2-microglobulin; BAL, bronchoalveolar lavage; Cdyn, dynamic compliance; DC, dendritic cell; MCh, methacholine; NAD, nonadherent; RL, lung resistance.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Claman HN, Chaperon EA, Triplett RF. Immunocompetence of transferred thymus-marrow cell combinations. J. Immunol. 1966;97:828–832. [PubMed] [Google Scholar]
- 2.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 3.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J. Exp. Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 5.Robinson DS, Hamid O, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 6.Hamelmann E, Oshiba A, Paluh J, Bradley K, Loader J, Potter TA, Larsen GL, Gelfand EW. Requirement for CD8+ T cells in the development of airway hyperresponsiveness in a murine model of airway sensitization. J. Exp. Med. 1996;183:1719–1729. doi: 10.1084/jem.183.4.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 8.Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo LM, Hachem P, Fourneau JM, Sidobre S, Kronenberg M, Taniguchi M, et al. Cutting edge: invariant Vα14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 2003;171:1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 9.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig BB, Pereira P, Pretolani M. Requirement for γδ T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 10.Lahn M, Kanehiro A, Takeda K, Joetham A, Schwarze J, Koehler G, O'Brien R, Gelfand EW, Born W. Negative regulation of airway responsiveness that is dependent on γδ T cells and independent of αβ T cells. Nat. Med. 1999;5:1150–1156. doi: 10.1038/13476. [DOI] [PubMed] [Google Scholar]
- 11.Das Y, Eynott P, Jupp R, Bothwell A, Van Kaer L, Shi Y, Das G. Natural killer T cells and CD8+ T cells are dispensable for T cell-dependent allergic airway inflammation. Nat. Med. 2006;12:1345–1347. doi: 10.1038/nm1206-1345. [DOI] [PubMed] [Google Scholar]
- 12.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 13.Kinjo Y, Kronenberg M. Vα14i NKT cells are innate lymphocytes that participate in the immune response to diverse microbes. J. Clin. Immunol. 2005;25:522–533. doi: 10.1007/s10875-005-8064-5. [DOI] [PubMed] [Google Scholar]
- 14.Yu KO, Porcelli SA. The diverse functions of CD1d-restricted NKT cells and their potential for immunotherapy. Immunol. Lett. 2005;100:42–55. doi: 10.1016/j.imlet.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N. Engl. J. Med. 2006;354:1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 16.Bendelac A, Rivera MN, Park S-H, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuda JL, Gapin L. Developmental program of mouse Vα14i NKT cells. Curr. Opin. Immunol. 2005;17:122–130. doi: 10.1016/j.coi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Hahn Y-S, Taube C, Jin N, Sharp L, Wands JM, Kemal Aydintug M, Lahn M, Huber SA, O'Brien RL, Gelfand EW, Born WK. Different potentials of γδ T cell subsets in regulating airway responsiveness: Vγ1+ cells, but not Vγ4+ cells, promote airway hyperreactivity, TH2 cytokines, and airway inflammation. J. Immunol. 2004;172:2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 20.Lahn M, Kanehiro A, Takeda K, Terry J, Hahn Y-S, Aydintug MK, Konowal A, Ikuta K, O'Brien RL, Gelfand EW, Born WK. MHC class I-dependent Vγ4+ pulmonary T cells regulate αβ T cell-independent airway responsiveness. Proc. Natl. Acad. Sci. USA. 2002;99:8850–8855. doi: 10.1073/pnas.132519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn Y-S, Taube C, Jin N, Takeda K, Park J-W, Wands JM, Aydintug MK, Roark CL, Lahn M, O'Brien RL, et al. Vγ4+ T cells regulate airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J. Immunol. 2003;171:3170–3178. doi: 10.4049/jimmunol.171.6.3170. [DOI] [PubMed] [Google Scholar]
- 22.Jin N, Taube C, Sharp L, Hahn Y-S, Yin X, Wands JM, Roark CL, O'Brien RL, Gelfand EW, Born WK. Mismatched antigen prepares γδ T cells for suppression of airway hyperresponsiveness. J. Immunol. 2005;174:2671–2679. doi: 10.4049/jimmunol.174.5.2671. [DOI] [PubMed] [Google Scholar]
- 23.Kasahara Y, Chen CH, Cooper MD. Growth requirements for avian γδ T cells include exogenous cytokines, receptor ligation and in vivo priming. Eur. J. Immunol. 1993;23:2230–2236. doi: 10.1002/eji.1830230927. [DOI] [PubMed] [Google Scholar]
- 24.Pereira P, Gerber D, Huang SY, Tonegawa S. Ontogenic development and tissue distribution of Vγ1-expressing γ/δ T lymphocytes in normal mice. J. Exp. Med. 1995;182:1921–1930. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heilig JS, Tonegawa S. Diversity of murine γ genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- 26.Silva-Santos B, Pennington DJ, Hayday AC. Lymphotoxin-mediated regulation of γδ cell differentiation by αβ T cell progenitors. Science. 2005;307:925–928. doi: 10.1126/science.1103978. [DOI] [PubMed] [Google Scholar]
- 27.Sentman CL, Hackett J, Jr., Morre TA, Tutt MM, Bennett M, Kumar V. Pan natural killer cell monoclonal antibodies and their relationship to the NK1.1 antigen. Hybridoma. 1989;8:605–614. doi: 10.1089/hyb.1989.8.605. [DOI] [PubMed] [Google Scholar]
- 28.Vicari AP, Mocci S, Openshaw P, O'Garra A, Zlotnik A. Mouse γδ TCR+NK1.1+ thymocytes specifically produce interleukin-4, are major histocompatibility complex class I independent, and are developmentally related to Ab TCR+NK1.1+ thymocytes. Eur. J. Immunol. 1996;26:1424–1429. doi: 10.1002/eji.1830260704. [DOI] [PubMed] [Google Scholar]
- 29.Azuara V, Levraud JP, Lembezat MP, Pereira P. A novel subset of adult γδ thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell receptor repertoire. Eur. J. Immunol. 1997;27:544–553. doi: 10.1002/eji.1830270228. [DOI] [PubMed] [Google Scholar]
- 30.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat. Rev. Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 31.Hamelmann E, Vella AT, Oshiba A, Kappler JW, Marrack P, Gelfand EW. Allergic airway sensitization induces T cell activation but not airway hyperresponsiveness in B cell-deficient mice. Proc. Natl. Acad. Sci. USA. 1997;94:1350–1355. doi: 10.1073/pnas.94.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawikova I, Palival V, Szczepanik M, Itakura A, Fukui M, Campos RA, Geba GP, Homer RJ, Iliopoulou BP, Pober JS, et al. Airway hyper-reactivity mediated by B-1 cell immunoglobulin M antibody generating complement C5a at 1 day post-immunization in a murine hapten model of nonatopic asthma. Immunology. 2004;113:234–245. doi: 10.1111/j.1365-2567.2004.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vijayanand P, Seumois G, Pickard C, Powell RM, Angco G, Sammut D, Gadola SD, Friedman PS, Djukanovic R. Invariant natural killer T cells and chronic obstructive pulmonary disease. N. Engl. J. Med. 2007;356:1410–1422. doi: 10.1056/NEJMoa064691. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda H, Suda T, Sato E, Nagata T, Koide Y, Chida K, Nakamura H. α-Galactosylceramide, a ligand of natural killer T cells, inhibits allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 2005;33:22–31. doi: 10.1165/rcmb.2004-0010OC. [DOI] [PubMed] [Google Scholar]
- 35.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NKT cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc. Natl. Acad. Sci. USA. 2006;103:2782–2787. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, Dakhama A, Ott VL, Gelfand EW. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat. Med. 2004;10:865–869. doi: 10.1038/nm1081. [DOI] [PubMed] [Google Scholar]
- 37.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γδ T cells. Science. 1994;265:1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 38.Tangri S, Brossay L, Burdin N, Lee DJ, Corr M, Kronenberg M. Presentation of peptide antigens by mouse CD1 requires endosomal localization and protein antigen processing. Proc. Natl. Acad. Sci. USA. 1998;95:14314–14319. doi: 10.1073/pnas.95.24.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Libero G, Moran AP, Gober H-J, Rossy E, Shamshiev A, Chelnokova O, Mazorra Z, Vendetti S, Sacchi A, Prendergast MM, et al. Bacterial infections promote T cell recognition of self-glycolipids. Immunity. 2005;22:763–772. doi: 10.1016/j.immuni.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien RL, Happ MP, Dallas A, Palmer E, Kubo R, Born WK. Stimulation of a major subset of lymphocytes expressing T cell receptor γδ by an antigen derived from Mycobacterium tuberculosis. Cell. 1989;57:667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien RL, Fu Y-X, Cranfill R, Dallas A, Reardon C, Lang J, Carding SR, Kubo R, Born W. Heat shock protein Hsp-60 reactive γδ cells: a large, diversified T lymphocyte subset with highly focused specificity. Proc. Natl. Acad. Sci. USA. 1992;89:4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Born WK, Vollmer M, Reardon C, Matsuura E, Voelker DR, Giclas PC, O'Brien RL. Hybridomas expressing γδ T-cell receptors respond to cardiolipin and β2-glycoprotein 1 (apolipoprotein H). Scand. J. Immunol. 2003;58:374–381. doi: 10.1046/j.1365-3083.2003.01315.x. [DOI] [PubMed] [Google Scholar]
- 43.Agea E, Russano A, Bistoni O, Mannucci R, Nicoletti I, Corazzi L, Postle AD, De Libero G, Porcelli SA, Spinozzi F. Human CD1-restricted T cell recognition of lipids from pollens. J. Exp. Med. 2005;202:295–308. doi: 10.1084/jem.20050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russano AM, Agea E, Corazzi L, Postle AD, De Libero G, Porcelli SA, De Benedictis F, Spinozzi F. Recognition of pollen-derived phosphatidyl-ethanolamine by human CD1d-restricted γδ T cells. J. Allergy Clin. Immunol. 2006;117:1178–1184. doi: 10.1016/j.jaci.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien RL, Roark CL, Jin N, Aydintug MK, French JD, Chain JL, Wands JM, Johnston M, Born WK. γδ T cell receptors: functional correlations. Immunol. Rev. 2007;215:77–88. doi: 10.1111/j.1600-065X.2006.00477.x. [DOI] [PubMed] [Google Scholar]