Highlights
-
•
We related arcuate subtract coherence to longitudinal reading outcomes in children.
-
•
FA along the direct segment was uniquely predictive of reading growth.
-
•
This effect was consistent in both younger and older children.
-
•
Specifically the direct segment's structure may support reading across development.
Keywords: Magnetic resonance imaging, Diffusion tensor imaging, Arcuate fasciculus, Superior longitudinal fasciculus, Longitudinal, Reading development
Abstract
Structural coherence across the arcuate fasciculus has previously been related to reading skill, but the arcuate may be divisible into distinct subtracts which support different functions. Here, we examine longitudinal data from 30 children between the ages of 8 and 14 to determine whether initial coherence in any of the arcuate's subsections is predictive of changes in reading across a longitudinal interval of approximately three years. The arcuate was divided using probabilistic tractography; mean fractional anisotropy across each subtract was extracted for each participant. Time 1 to Time 2 change in reading skill (identification, fluency score average) was significantly and uniquely predicted by only direct fronto-temporal arcuate segment coherence. Participants with lower direct segment FA demonstrated decreases in reading scores, potentially reflecting lessened improvements due to continued inefficient processing. These results were consistent in the older and younger halves of the sample. As such, we demonstrate that it is specifically the direct segment of the arcuate that may support and be predictive of reading skill both initially and longitudinally across development.
The left arcuate fasciculus is one of the most studied white matter tracts in the brain and has been demonstrated to support language skills such as semantic comprehension, phonological sensitivity, and most importantly here, reading (Friederici, 2009). From a functional-anatomical perspective, the arcuate fasciculus serves to connect the temporal lobe and the inferior frontal gyrus, with potential branches stopping in the posterior temporal lobe or inferior parietal lobule. As such, it thus provides a physical connection between regions critically involved in phonological or linguistic processing (see Gazzaniga, 2009), including reading. While connectivity across the arcuate has been related to concurrent reading abilities (see Vandermosten et al., 2012b), whole arcuate coherence has not been consistently predictive of longitudinal reading outcomes in school-aged children (e.g., Hoeft et al., 2011). Recently, it has been proposed that the arcuate may be composed of several separable subtracts that support different functions (Catani et al., 2005), but the longitudinal relationship between initial connectivity in these subsections and future reading ability has not previously been explored. We here aim to determine the relationship between early arcuate connectivity in these three arcuate subsegments, as compared to that across the whole tract, and outcome reading skill in a sample of typically developing children with a range of reading abilities to determine whether these proposed functional relationships are subtract specific and predictive across a multi-year interval.
Fractional anisotropy, or FA, indicates the degree and direction of the diffusivity of water within a voxel. Voxels along major white matter tracts should have a high degree and directionality, as water can diffuse easily in one primary direction. A high FA is taken to reflect high tract coherence, where the individual axonal fibers cohere and travel together; high coherence is thought to indicate increased functional connectivity and processing efficiency, as information can thus travel more effectively along the tract between gray matter regions (Roberts et al., 2013). As such, increased FA generally co-occurs with, or may have a causal relationship with, increased skill.
Left arcuate FA has been consistently demonstrated to be positively correlated with concurrently measured reading and reading-related skills, including word identification (Hoeft et al., 2011, Yeatman et al., 2012), reading fluency (Gold et al., 2007, Nagy et al., 2004), phonological awareness (Saygin et al., 2013, Yeatman et al., 2011), and composite measures of reading ability (Gullick and Booth, 2014). Further, significant differences in arcuate connectivity have been found between reading-skill groups: both adults and children with dyslexia demonstrate decreased fractional anisotropy in the left arcuate (see Vandermosten et al., 2012b for a recent review). As such, reading ability across development may be in part supported by the arcuate.
There is currently some debate within the neuroanatomical literature as to the taxonomy of the arcuate and its potential subtracts. Most prominently, Catani et al. (2005) demonstrated three individual segments: a direct, or long, segment is proposed to make the temporal–parietal–frontal arc, while an anterior section connects frontal and parietal only and a posterior section parietal and temporal only. Other groups have proposed similar subdivisions, such as the two-segment model from Glasser and Rilling (2008), with two parallel frontal to superior versus middle temporal tracts, and the four-segment model from Makris et al. (2005), which was based on non-human primate work and finds segments generally similar to those of Catani et al. (2005). Wakana et al. (2007) has also demonstrated that the superior longitudinal fasciculus, which may be synonymous with the arcuate (Friederici, 2009) or may be a parallel tract (Duffau, 2008), can similarly be reproducibly subdivided into at least an SLF and SLFt (temporal) section. As such, a distinction between frontal–temporal and frontal–parietal sections within the arcuate is consistent with current reports from multiple methodologies, with temporo-parietal segments also demonstrated in some cases.
These arcuate subtracts are proposed to support distinct functions, based on their gray matter endpoints. The anterior segment is hypothesized to support articulatory processing and the direct section reading (Catani et al., 2005). Vandermosten et al. (2012a) reconstructed these segments in individual control and dyslexic participants through a series of waypoint and exclusion planes with deterministic tractography; mean FA across only the direct segment was positively related to contemporary phonemic awareness skill. In contrast, mean FA in the posterior segment was related to speech-in-noise perception; anterior FA was not related to any of the measured skills. Yeatman et al.’s (2011) probabilistic tracking of the left arcuate effectively included only the direct segment, with anterior and posterior segments referred to as “coherently oriented non-arcuate fibers” tracked from their nodes, and also found that FA in their direct segment was related to phonological awareness skill at that timepoint, though negatively so: participants with higher FAs (and thus lower radial diffusivities) demonstrated lower phonological skills. Interestingly, Thiebaut de Schotten et al. (2014) noted that FA specifically in the posterior segment increased with the late acquisition of literacy in an adult ex-illiterate population, potentially indicating lexical (semantic) and non-lexical (phonemic) processing (see also Myers et al., 2014). This literature establishes initial relationships between particular reading skills and subtract connectivities measured at the same time point.
While these concurrent structure (arcuate)–function (reading skill) relationships have been consistently demonstrated in sizable samples of children across a wide age range, the results from longitudinal investigations of the whole arcuate have been mixed. Hoeft et al. (2011) found that while initial FA in the left superior longitudinal fasciculus (explicitly stated to include the arcuate) was related to initial word identification standard scores for control participants, it was not predictive of longitudinal changes in standard scores over 2.5 years in either dyslexics or controls. Right hemisphere superior longitudinal fasciculus coherence, however, was predictive of reading gains in children with dyslexia, potentially reflecting compensatory use of right hemisphere systems parallel to those typically found on the left side (e.g., Eden et al., 2004). However, this study examined the tract as a whole without segmenting it into subsections: if only particular segments of the arcuate support reading, its longitudinal impact may not be seen when coherence is collapsed across segments.
More recently, Yeatman et al. (2012) found that Time 2 good readers (initial ages 7–12) tend to show increases in arcuate connectivity across the three-year testing period, while poor readers showed decreases in connectivity, indicating that the arcuate's role in reading may be preserved and even strengthened with practice and experience. While an individuals’ rate of arcuate FA change was predictive of reading scores (both initial and average across time points), the predictive power of initial FA on final reading score or change in ability was not tested. These developmental changes were also not related to subsections. Similarly, Myers et al. (2014) demonstrated that change in temporo-parietal coherence from kindergarten to third grade were related to outcome reading scores; post-hoc tractography demonstrated that one of the significant clusters contained both superior corona radiata and direct arcuate fasciculus fibers; anterior streamlines were also included in a subsample of their subjects. The second cluster contained posterior arcuate fibers. This work indicates that changes in the arcuate are critical for successful early reading. However, the impact of initial coherence itself on behavioral outcomes or reading improvement was not examined. As such, early arcuate connectivity may impact later reading ability, but this relationship has not yet been clearly described. To our knowledge, no studies have compared the prospective impact of individual differences in subdivision connectivities on reading outcome, which may be critical for reconciling the previous conflicting whole arcuate results.
Strong connectivity across the arcuate may thus be critical for successful reading, both initially and perhaps longitudinally, but whether this relationship is specific to a subsection or general across the arcuate as a whole remains to be seen. We thus aimed to determine whether Time 1 initial FA across the whole arcuate or in any of the direct, anterior, or posterior segments was predictive of changes in reading from Time 1 to Time 2, in comparison to the predictive ability of Time 1 behavioral measures of performance. This design allows for determination of which sections may be particularly important for successful reading development, and how neural structure may impact educational outcomes beyond what can be predicted by standardized behavioral measures, further specifying the role of these arcuate subsections and informing a causal relationship to behavioral outcomes.
1. Methods
1.1. Participants
Participants were 30 (13 females) children recruited from the Chicago metropolitan area. At Time 1, children's ages were between 8;1 and 13;8 years (mean = 10;7 years); Time 2 ages were between 10;1 and 16;9 years (mean = 13;9 years). Gap period between testing sessions was between two and four years (mean = 33.2 months; see Table 1 for demographic and score information). Children were all right-handed native English speakers with normal hearing and normal or corrected-to-normal vision, and no history of neurological or psychiatric illness or disorder. Informed consent was obtained from participants and their parents, and all procedures were approved by the Institutional Review Board at Northwestern University.
Table 1.
Demographics and standardized scores.
| N = 30 | Mean (SD) | Range |
|---|---|---|
| T1 age | 10;7 (1;5) | 8;1–13;8 |
| T2 age | 13;4 (1;6) | 10;2–16;8 |
| Testing time gap (ΔAge) | 33.2 months (7.1) | 20–49 months |
| T1 real-word reading standard score | 105.1 (11.8) | 86.0–127.5 |
| T1 word identification | 104.5 (13.8) | 83–129 |
| T1 sight-word efficiency | 105.6 (11.7) | 83–130 |
| T1 pseudoword reading std. score | 106.2 (13.2) | 85–128 |
| T1 word attack | 106.5 (12.2) | 85–126 |
| T1 pseudoword decoding efficiency | 105.9 (15.8) | 79–134 |
| T1 phonological awareness | 106 (10.3) | 76–124 |
| T1 rapid naming | 100.7 (14.6) | 67–127 |
| T1 full-scale IQ | 118.4 (13.8) | 89–144 |
| T2 real-word reading standard score | 103.1 (11.9) | 83.5–124 |
| T2 word identification | 105 (12.4) | 84–131 |
| T2 sight-word efficiency | 101.1 (12.5) | 82–123 |
| ΔReal-word reading standard score | −2 (4.7) | −13.5 to 9 |
| ΔWord identification | 0.5 (6.6) | −13 to 10 |
| ΔSight-word efficiency | −4.5 (7.8) | −21 to 12 |
1.2. Standardized testing
Children participated in standardized testing sessions at both Time 1 and Time 2 to ensure that all participants were of at least average IQ and reading ability. Tests included the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), using two verbal (vocabulary, similarities) and two performance (block design, matrix reasoning) subtests; the Woodcock-Johnson III Tests of Achievement (Woodcock et al., 2001), including the word identification and word attack subtests; the Test of Word Reading Efficiency, including the sight word efficiency and pseudoword efficiency subtests; and the Comprehensive Test of Phonological Processing (Wagner et al., 1999), including the phonological awareness (blending words, elision) and rapid naming (rapid naming letters, digits) subtests. A real-word reading composite score for each timepoint was calculated from the average of that session's word identification and sight-word efficiency subtest standardized scores; this composite measure was used as it includes untimed vocabulary and fluency, which are both critical for successful reading, and further creates a single outcome measure for use in regressions. Similarly, a pseudoword reading score was also calculated from the average of the word attack and pseudoword decoding efficiency scores. All children demonstrated Time 1 full-scale IQ standardized scores between 89 and 144 and real-word reading scores between 85 and 125 (see Table 1; see Table S1 for correlative relationships between these tests and with neural measures).
Change in real-word reading score was calculated as Time 2 minus Time 1 composite score. Many children showed standard score decreases, indicating that the degree of improvement was less than would be expected given the participant's age and Time 1 score, and some showed standard score increases. Notably, all children's real-word reading raw scores increased over the longitudinal interval (between 2 and 18 points), demonstrating that there was improvement in reading over this time, even if not commensurate with starting score and age. None of the participants’ change scores were statistical outliers (more than 2.5 standard deviations from the mean). Importantly, participant's score changes did not simply demonstrate regression to the mean. Under these conditions, initially low scorers would be expected to show greater score gains, while initially high performers might show greater score decreases. Instead, there was no significant relationship between initial score and score change for children in this sample (r = −0.188, p>0.3).
1.3. Experimental procedure
1.3.1. Time 1 procedure
Participants were given a standardized test battery, completed a practice MRI session, and completed the Time 1 MRI sessions, on three separate visits.
MRI images were acquired at the Northwestern University Center for Translational Neuroimaging using a 3.0 T Siemens Trio MRI scanner, with a standard 16-channel headcoil. Participants were positioned in the MRI scanner with their head position secured using foam pads. A diffusion-weighted image (echo-planar spin echo imaging) was acquired for each subject (TR = 9512 ms, TE = 89 ms, matrix size = 128 mm × 128 mm, field of view = 256 mm × 256 mm, slice thickness = 2 mm, b = 1000 s/mm2, 64 non-collinear diffusion-encoding directions, one image b = 0 s/mm2).
1.3.2. Time 2 procedure
Participants were invited back to the lab approximately 3 years after initial participation for administration of a second standardized test battery, from which outcome and change scores were calculated.
1.4. Analysis
1.4.1. DTI analysis
DTI data analysis was performed using FSL software (http://www.fmrib.ox.ac.uk/fsl). All images were first examined for artifact by creating mean, standard deviation, and signal-to-noise maps using the fslmaths command. Between-volume motion was also inspected; all participants demonstrated run motion <5 mm across the scan, indicating minimal movement. Preprocessing steps for all subjects included eddy current correction, brain extraction (fractional intensity threshold 0.25), diffusion tensor fitting, bedpostX (diffusion parameter estimation), and registration to compute transformation matrices between individual and standard spaces. Fractional anisotropy (FA) maps were then calculated for each subject in individual space.
Probabilistic tractography was implemented in individual space to reconstruct left hemisphere arcuate segments using several waypoint and exclusion masks. Several critical waypoints were defined based on those used by Vandermosten et al. (2012a) and Wakana et al. (2007). ROI 1 was located at y = −10; ROI 2 was located at y = −34; ROI 3 was located at z = 10; and ROI 4 was located at z = −2. Direct segment streamlines started from ROI 1 and were required to pass through ROIs 1 and 3. Anterior segment streamlines started from ROI 1 and were required to pass through ROIs 1 and 2, but not ROI 3; posterior segment streamlines started from ROI 4 and were required to pass through ROIs 4 and 3, but not ROI 1. Whole arcuate streamlines started from ROI 1 and were required to pass through ROIs 1 and 2. The whole arcuate tract thus included both the direct and anterior segments, as well as portions of the posterior section. General exclusion planes included x = −25, to confine tractography to the left hemisphere, and y = −60, to truncate streamlines which looped posteriorly beyond the arcuate (see Fig. 1 for examples). One thousand streamlines were sent out from each voxel in this space; streamlines were included in the final tract reconstruction if they met the waypoint and exclusion requirements. Anisotropy was used to constrain tracking, with a curvature threshold of 0.2, step length of 0.5, and a maximum of 2000 steps. For each of the tracts reconstructed, we excluded the least probable paths (the bottom 1%), then derived mean FA values across the remaining path for each participant. These FA values were then used as potential predictors in the regression analyses.
Fig. 1.
Probabilistic tractography waypoints and reconstructions, shown for one participant (on standard brain background). (A) Probabilistic tractography began from a waypoint plane, then passed through or avoided further wayplanes (colored; universal exclusion masks shown in black). (B) Sample direct segment; streamlines were required to pass through ROI 1 (blue, starting plane) and ROI 4 (red) planes. (C) Sample anterior segment; streamlines were required to pass through ROI 1 (blue, starting plane) and ROI 2 (green) but not ROI 4 (yellow) planes. D) Sample posterior segment; streamlines were required to pass through ROIs 3 (red, starting plane) and 4 (yellow) but not ROI 1 (blue) planes (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.).
This procedure was also performed in the right hemisphere, using right homologues of each of these planes and seed regions, to allow for tracking of the right arcuate and its subsections.
1.4.2. Reading outcome regression analyses
The relationships between Time 1 fractional anisotropy across the arcuate and Time 1 to Time 2 changes in reading performance was examined using a series of forward stepwise regressions. These analyses included tractography mean FAs, Time 1 age, Time 1 − Time 2 testing gap period (in months), and several Time 1 reading and related skill standard scores as competing independent predictor variables in an effort to determine whether arcuate FA was significantly predictive of longitudinal change in real-word reading scores in addition to, or beyond, what behavioral measures were able to predict. Real-word reading was chosen as the dependent variable of interest because it includes both identification and fluency skill and thus best reflects reading performance under normal circumstances. These relationships were first explored using direct, anterior, and posterior arcuate mean FAs, then using whole arcuate mean FA and right-hemisphere segment FAs for comparison. These analyses were implemented in SPSS (version 22), with model inclusion criterion set at p < 0.05, and exclusion at p < 0.1. Correlations between participants’ significant tract segment mean FAs and the dependent variable of interest are included to illustrate the direction of these relationships. A further series of regressions validating these results are presented as Supplementary materials (see S3).
2. Results
2.1. Regressions using arcuate segment FAs
Linear regressions were first performed to determine whether initial direct, anterior, or posterior segment mean FAs were predictive of changes in reading scores between testing timepoints. Time 2 − Time 1 change in real-word reading score was used as the dependent variable. Potential predictors included the following: Time 1 real-word reading, pseudoword reading, phonological awareness, and rapid naming scores; Time 1 age, testing time gap; and direct, anterior, and posterior arcuate mean FAs. Stepwise regressions first revealed that only direct arcuate mean FA was significantly predictive of T2–T1 real-word reading change (R = 0.509; Model F(1,28) = 9.772, MSE = 170, p = 0.004) (see Table 2A). Time 1 real word reading, pseudoword reading, phonological awareness, rapid naming, age, testing time gap, and anterior and posterior arcuate FAs were not significantly predictive of change in real-word reading, p's > 0.1, and so were excluded.
Table 2.
Prediction of Time 2 − Time 1 reading score changes.
| A | Stepwise model | Model R | Model p | R2Δ | R2Δ p |
|---|---|---|---|---|---|
| 1 | T1 direct arcuate FA | 0.509 | 0.004 | 0.259 | 0.004 |
| B | Hierarchical model | Model R | Model p | R2Δ | R2Δ p |
|---|---|---|---|---|---|
| 1 | T1 behavioral measures | 0.504 | >0.4 | 0.254 | |
| 2 |
T1 direct arcuate FA |
0.607 |
>0.2 |
0.114 |
0.045 |
| C | Hierarchical model | Model R | Model p | R2Δ | R2Δ p |
|---|---|---|---|---|---|
| 1 | T1 anterior, posterior arcuate FA | 0.111 | >0.8 | 0.012 | |
| 2 | T1 direct arcuate FA | 0.554 | 0.003 | 0.294 | 0.021 |
In order to determine whether direct section FA was predictive of reading changes beyond what the behavioral measures could determine, we performed a hierarchical regression. In the first step, Time 1 real-word reading, pseudoword reading, phonological awareness, rapid naming, age, and testing time gap were all force-entered as nuisance variable predictors, though the resulting model was not significant (Model F < 1). In the second stepwise step, Time 1 direct, anterior, and posterior arcuate mean FAs were presented as potential predictors. Direct segment arcuate FA was included in the model, significantly improving it (R2 Change = 0.114, Change F(1,21) = 3.779, p = 0.045), and indicating that it accounted for a significant portion of unique variance even after all variance accountable for by behavioral measures had been partialled out, though the final model did not reach significance due to the continued inclusion of non-significant behavioral predictors (Model F(8,21) = 1.530, p > 0.2) (see Table 2B). Neither the anterior nor posterior section mean FAs were predictive (p > 0.8, p > 0.2, respectively).
Finally, mean FA in the direct segment was significantly correlated with both the anterior and posterior segments (see Table S1). This relationship is likely because probabilistic tractography allows voxels to be included in multiple tracts; in particular, the direct segment overlapped with both the anterior and posterior segments (see Table S2). To establish that the direct segment was uniquely predictive of change in reading beyond variability attributable to the other two segments, we performed another hierarchical regression. In the first step, anterior and posterior segment mean FAs were force-entered into the regression, though the resulting model was not significant (Model F < 1, predictor p's > 0.8). In the second stepwise step, direct segment mean FA was presented as a potential predictor. Direct segment arcuate FA was included in the model, significantly improving it (R2 Change = 0.294, Change F(1,26) = 11.023, p = 0.003), and indicating that it accounted for a significant portion of unique variance even after all variance accountable for by the other neural measures had been partialled out (see Table 2C).
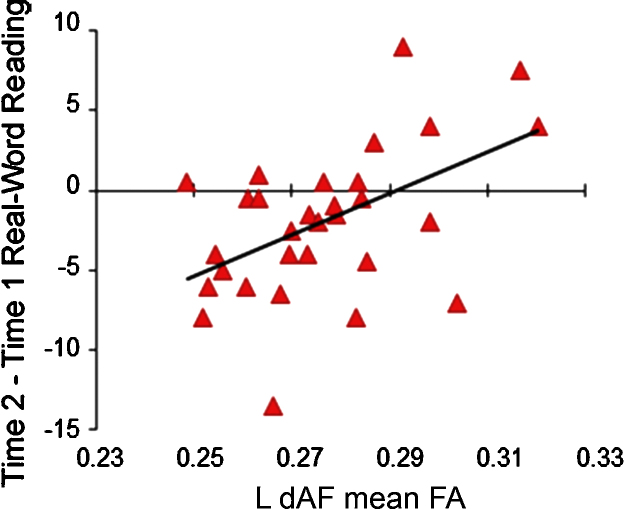
Across the whole sample, then, a correlation between direct arcuate mean FA and T2-T1 change in real-word reading showed that participants with lower initial direct arcuate FA demonstrated greater decreases in real-word reading standard scores between Time 1 and Time 2 testing sessions (see Fig. 2). In summary, initial direct segment arcuate FA was significantly and uniquely predictive of behavioral change in reading performance from Time 1 to Time 2.
Fig. 2.
Relationship between Time 1 direct segment arcuate FA and longitudinal change in reading scores to Time 2. Participants with lower initial FA in the direct segment of the arcuate demonstrated greater decreases in standard scores over the longitudinal interval.
2.2. Comparative tract regressions
For comparison to the existing literature and determination of the specificity of these results, another set of stepwise regressions examined these relationships using the left whole arcuate FA or right hemisphere arcuate segment FAs as independent variables to determine whether the left hemisphere direct segment-driven results were specific. In each case, Time 1 to Time 2 change in real-word reading score was again used as the dependent variable. First, mean FA across the whole left hemisphere arcuate was included as a potential predictor, with the same behavioral and age measures as before. No variables were significantly predictive of change in reading score over this interval, indicating that the relationships seen in the previous analyses are specific to the left direct segment and not consistent or common across the components of the whole left hemisphere tract. Next, we performed a similar tractography and regression procedure in the right hemisphere. The direct segment was not traceable in several participants, and so no FA could be extracted; otherwise, subtract detection was successful across the group. Right hemisphere anterior, direct (where available), and posterior arcuate mean FAs were included as potential predictors, with the same behavioral and age variables. No variables were significantly predictive of change in reading score over this interval. As such, the left direct arcuate relationships found are specific to that segment in the left hemisphere.
2.3. Regressions within younger and older age groups
Given the large age range in the current sample, we performed split-group analyses to ensure that these direct segment-specific results were not driven only by older or younger participants. Younger participants (N = 15) were defined as between 8 and 10;5 years old; older participants (N = 15) were between 10;9 and 13;9 years old. Because of the lessened number of participants in each group, model inclusion criterion was set as p < 0.1, and exclusion at p < 0.15. Regressors included the same behavioral, age, and left hemisphere arcuate segment variables. Consistent with the full cohort analysis, Time 2 − Time 1 reading change scores in both age groups were best predicted by direct arcuate FA, as across the full sample. Direct segment FA was the only significant predictor for younger (R = 0.576, Model F(1,14) = 5.380, p = 0.055) and older participants (R = 0.504, Model F(1,14) = 4.430, p = 0.05) (see Table 3).
Table 3.
Prediction of Time 2 − Time 1 reading score changes in younger and older participants.
| A | Younger children | Model R | Model p | R2Δ | R2Δ p |
|---|---|---|---|---|---|
| 1 | T1 direct arcuate FA | 0.576 | 0.055 | 0.332 | 0.055 |
| B | Older children | Model R | Model p | R2Δ | R2Δ p |
|---|---|---|---|---|---|
| 1 | T1 direct arcuate FA | 0.504 | 0.050 | 0.254 | 0.050 |
3. Discussion
The goal of the current study was to examine the relationship between initial white matter connectivity in separable subsections of the arcuate and longitudinal reading outcomes using standardized reading scores. While previous neuroimaging work has shown specific relationships between concurrent reading skill and arcuate connectivity, and has shown relationships between initial whole-arcuate coherence and future reading, to our knowledge no research has directly compared the predictive abilities of direct, anterior, and posterior segment coherences to determine whether this relationship is driven by any section. Our use of probabilistic tractography with specific waypoint planes allows us to segment the arcuate into three subsections identified in the literature. We demonstrate that initial arcuate FA is predictive of change in reading ability, but importantly that this predictive power is due primarily to the direct segment. Direct section FA is the only significant predictor of change in reading score over the testing interval, and thus predicts reading ability changes beyond what can be accounted for by behavioral measures. The primacy of the direct segment was found in both younger and older children. Initial coherence in the direct segment may thus be an important factor in setting the stage for reading growth.
Initial direct segment coherence was the best, and indeed the only, significant predictor of change in reading score over the longitudinal interval. Improvement, thus, was not simply proportional to initial skill or to the time gap between testing sessions: indeed, these factors were not correlated with each other. This result indicates that a more coherent temporal–frontal tract might support continued processing efficiency and reading growth, while a less coherent connection could impede progress by not allowing for improvements proportional to age. Such slowed or arrested progress could eventually lead these below-average participants to fall out of typical skill bounds, resulting in deficient reading lower than that of their peers, or to remain at the low-normal range of the continuum. Future studies should use even longer intervals with multiple time points to examine growth curves over extended periods of time.
Analyses within each age group found that fractional anisotropy across the direct segment of the arcuate was a critical predictor of reading skill for both younger and older participants, demonstrating that its importance for reading is continuous across development and not limited to beginning or to mature readers. In contrast, neither the anterior nor posterior segments were significantly related to reading change in either age group or across the full sample. Posterior segment FA trended towards a significant negative correlation with Time 1 (r = −0.334) and Time 2 (r = −0.341) reading, and with Time 1 rapid naming (r = −0.316), though not to change in performance between these sessions. This section has previously been demonstrated to be critical for reading acquisition, though positively so: Thiebaut de Schotten, et al., (2014) proposed that it is involved in phonemic processing, perhaps related to the speech-in-noise perception noted by Vandermosten, et al., (2012a), but also orthographic-semantic translation in the angular gyrus. Myers et al. (2014) also found it to be predictive of rapid naming ability. Since our participants were not naïve, new readers but children with some experience who gained more expertise over the longitudinal interval, increased reliance on the simple phonemic processing or rapid naming supported by this section may indicate a less mature strategy. The anterior section has been posited to support articulation (Catani et al., 2005, Vandermosten et al., 2012a), which may be less critical for reading in this sample of typically developing children where phoneme–grapheme relationships are reasonably mature (Richlan et al., 2009).
The subtract specificity of the relationship to reading outcomes may in part explain why Hoeft et al. (2011) did not find the left hemisphere superior longitudinal fasciculus to predict reading. Their sample included participants with a similar age range (children with dyslexia spanned approximately 8.5–13.5 years of age, typically developing children from 12 to 16), but the whole left superior longitudinal fasciculus and arcuate were tracked together. Because subtracts were not separated, any direct segment specific relationships may have been lost due to the inclusion of nonpredictive anterior or posterior segment variability. Indeed, in our current analyses, the whole arcuate also was not predictive of reading changes. Further, we here used a composite measure of reading ability comprised of identification and fluency, while Hoeft et al. (2011) used only untimed identification. Inclusion of both measures may be important for fully describing reading skill, as both speeded decoding and untimed identification are critical aspects of successful reading, and may be a more ecologically meaningful measure of reading ability than either factor alone.
Mean FAs across the sampled tracts were relatively low, as compared to typical FA values for the arcuate (for example, Gullick and Booth, 2014). This difference may be due to the tracking method used: probabilistic tractography allows for streamlines to track into low-FA gray matter voxels surrounding the white matter, provided that the final path meets the waypoint and exclusionary requirements. As such, some paths may turn into the gray matter for a few voxels before rejoining the white matter stream. We here chose to remove only the 1% least probably streamlines and not “clean” or restrict the created paths any further in order to best describe the full extent of each segment. Additional post-hoc analyses removing the 5% least probable paths demonstrated very similar results, indicating that the current results are not dependent on this liberal threshold. Further, this procedure was implemented uniformly in each arcuate segment, meaning that the direct arcuate-specific results should not be biased by this procedure.
All participants demonstrated gains in raw reading scores, indicating that they learned to read more words, and to read words faster, after the longitudinal interval. However, while some children's standard scores were similar in the pre- and post-testing periods, indicating improvements proportional to their age and thus maintenance of skill level, several showed significant increases or decreases in their standard scores over this period. These changes are unlikely to simply be attributable to a regression to the mean, as both higher- and lower-scoring Time 1 participants showed positive and negative changes in standard scores (i.e., participants’ scores were not simply more similar to the population average at Time 2). As such, children's reading improved over this time, but for some the improvement was more or less than would be expected given their age and initial score. This degree of change in standard scores is relatively large, as the tests reported have high test-retest reliabilities, but it may indicate that the population included represents a broad sample of elementary school children with a variety of reading trajectories and outcomes. This pattern thus demonstrates variability in reading growth even within a typically developing population.
Early FA in the direct segment of the arcuate was demonstrated to be predictive of reading skill changes in both older and younger children, explaining unique variance beyond what behavioral measures could predict, while anterior and posterior arcuate mean FAs were not predictive. This set of results indicates that the relationships previously found between initial reading skill and arcuate coherence (Beaulieu et al., 2005, Gullick and Booth, 2014, Nagy et al., 2004, Yeatman et al., 2012) may be particularly due to contributions from the direct segment. As whole-arcuate effects may be diluted by the inclusion of the anterior and posterior segments, specific examination of the direct subsection is needed. As such, the direct segment of the arcuate may be especially important in reading, potentially via its particular support of the critical crossmodal processing needed for successful fluent mappings between phonemes and their representative letters (see Catani et al., 2005, Gullick and Booth, 2014, Vandermosten et al., 2012a).
4. Conclusions
In summary, this study is the first to demonstrate a predictive relationship specifically between initial direct segment arcuate connectivity and changes in reading ability in both younger and older children. This work thus links behavioral examinations of early crossmodal skill and later reading performance with neuroimaging studies of reading performance and contemporary arcuate connectivity. Further, it clarifies previous work examining the arcuate's longitudinal relationship with reading by establishing that it is particularly the direct segment which is predictive, and so whole-arcuate results may not be significant. By segmenting the arcuate into these subtracts, we can better understand the specific roles of structures supporting the reading network and crossmodal processing, including how the early state of this system may indicate potential for further reading growth.
Conflict of interest
Neither author on this paper have any interests that may be interpreted as influencing the research. Neither of the funding sources (Northwestern University, NIH) had any role in the conduct or preparation of this work.
Acknowledgements
This research was supported by National Institute of Child Health and Human Development Grant (grant number HD042049 to J.R.B.). The first author was supported by a Ruth L. Kirschstein NRSA Institutional Research T32 Training Grant from the National Institute on Deafness and Other Communication Disorders (grant number T32 DC009399-01A10). We thank MHS for assistance in data analysis.
Footnotes
Available online 13 May 2015
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2015.05.002.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Beaulieu C., Plewes C., Paulson L.A., Roy D., Snook L., Concha L., Phillips L. Imaging brain connectivity in children with diverse reading ability. Neuroimage. 2005;25(4):1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Ffytche D.H. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Duffau H. The anatomo-functional connectivity of language revisited: new insights provided by electrostimulation and tractography. Neuropsychologia. 2008;46(4):927–934. doi: 10.1016/j.neuropsychologia.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Eden G.F., Jones K.M., Cappell K., Gareau L., Wood F.B., Zeffiro T.A., Flowers D.L. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44(3):411–422. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. Pathways to language: fiber tracts in the human brain. Trends in Cognitive Sciences. 2009;13(4):175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Gazzaniga M.S., editor. The Cognitive Neurosciences. 4th ed. Bradford Books; 2009. [Google Scholar]
- Glasser M.F., Rilling J.K. DTI tractography of the human brain's language pathways. Cerebral Cortex. 2008;18(11):2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Gold B.T., Powell D.K., Xuan L., Jiang Y., Hardy P.A. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia. 2007;45(11):2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullick M.M., Booth J. Individual differences in crossmodal brain activity predict arcuate fasciculus connectivity in developing readers. Journal of Cognitive Neuroscience. 2014;26(7):1331–1346. doi: 10.1162/jocn_a_00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F., McCandliss B.D., Black J.M., Gantman A., Zakerani N., Hulme C., Gabrieli J.D.E. Neural systems predicting long-term outcome in dyslexia. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(1):361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Kennedy D.N., McInerney S., Sorensen A.G., Wang R., Caviness V.S., Pandya D.N. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Myers C.A., Vandermosten M., Farris E.A., Hancock R., Gimenez P., Black J.M., Hoeft F. White matter morphometric changes uniquely predict children's reading acquisition. Psychological Science. 2014;25(10):1870–1883. doi: 10.1177/0956797614544511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z., Westerberg H., Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Human Brain Mapping. 2009;30(10):3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R.E., Anderson E.J., Husain M. White matter microstructure and cognitive function. Neuroscientist. 2013;19(1):8–15. doi: 10.1177/1073858411421218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin Z.M., Norton E.S., Osher D.E., Beach S.D., Cyr A.B., Ozernov-Palchik O., Gabrieli J.D.E. Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. Journal of Neuroscience. 2013;33(33):13251–13258. doi: 10.1523/JNEUROSCI.4383-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Cohen L., Amemiya E., Braga L.W., Dehaene S. Learning to read improves the structure of the arcuate fasciculus. Cerebral Cortex. 2014;24(4):989–995. doi: 10.1093/cercor/bhs383. [DOI] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Poelmans H., Sunaert S., Wouters J., Ghesquiere P. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135:935–948. doi: 10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Wouters J., Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neuroscience and Biobehavioral Reviews. 2012;36(6):1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Wagner R.K., Torgesen J.K., Rashotte C.A. PRO-ED; Austin, TX: 1999. Comprehensive Test of Phonological Processing. [Google Scholar]
- Wakana S., Caprihan A., Panzenboeck M.M., Fallon J.H., Perry M., Gollub R.L., Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1999. Wechsler Abbreviated Scale of Intelligence (WASI) [Google Scholar]
- Woodcock R.W., McGrew K.S., Mather N. Riverside; Itasca, IL: 2001. Woodcock-Johnson III Tests of Achievement. [Google Scholar]
- Yeatman J.D., Dougherty R.F., Ben-Shachar M., Wandell B.A. Development of white matter and reading skills. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(44):E3045–E3053. doi: 10.1073/pnas.1206792109. 10/1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Rykhlevskaia E., Sherbondy A.J., Deutsch G.K., Wandell B.A., Ben-Shachar M. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. Journal of Cognitive Neuroscience. 2011;23(11):3304–3317. doi: 10.1162/jocn_a_00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.