Abstract
The marine environment is a valuable resource for drug discovery due to its diversity of life and associated secondary metabolites. However, there is very little published data on the potential application of marine natural products to treat neuropsychiatric disorders. Many natural products derived from chemically defended organisms in the marine environment have pharmacophores related to serotonin or clinically utilized antidepressant drugs. Therefore, in the present study, compounds selected for their structural similarity to serotonin or established antidepressants were evaluated for antidepressant-like activity using the forced swim and tail suspension tests in mice. The antidepressant positive controls, citalopram (selective serotonin reuptake inhibitor) and despiramine (tricyclic antidepressant) both dose-dependently reduced immobility time in the forced swim and tail suspension tests. Two marine natural product compounds tested, aaptamine and 5,6-dibromo-N,N-dimethyltryptamine, also produced significant antidepressant-like activity in the forced swim test. In the tail suspension test, the antidepressant-like effects of 5,6-dibromo-N,N-dimethyltryptamine were confirmed, whereas aaptamine failed to produce significant results. None of the tested compounds induced hyperlocomotion, indicating that nonspecific stimulant effects could not account for the observed antidepressant-like actions of the compounds. These studies highlight the potential to rationally select marine derived compounds for treating depression and other neuropsychiatric disorders.
Keywords: Antidepressant, Tricyclic antidepressant (TCA), Selective serotonin reuptake inhibitor (SSRI), Forced swim test, Tail suspension test (TST), Locomotor activity, Marine natural product
1. Introduction
The marine environment has become an invaluable resource for new drug discoveries due to its diversity of life. Many marine invertebrates utilize bioactive compounds as chemical defense mechanisms because they lack physical protection and ease of mobility (Maplestone et al., 1992; Amsler et al., 2001; Diers et al., 2006). Over the past three decades, bioprospecting of the marine environment has led to numerous drug candidates, with many still in preclinical development and clinical testing for various illnesses, including some cancers (Paul, 1992; Gavagnin and Fontana, 2000; Haefner, 2003; Sipkema et al., 2005). However, there is very little information regarding the potential application of marine natural products to treat neuropsychiatric disorders.
Depression is a particularly debilitating neuropsychiatric disorder that affects about 20% of the total population of the United States (Wong and Lichinio, 2001; Nestler et al., 2002; Society for Neuroscience, 2002; Matthews et al., 2005). Depression leads to functional disabilities that can be more profound than diabetes, chronic lung disease and hypertension, costing the U.S. economy billions of dollars (43 billion as of 2001) annually in direct/indirect costs (Wells et al., 1989; Wong et al., 2001; Matthews et al., 2005).
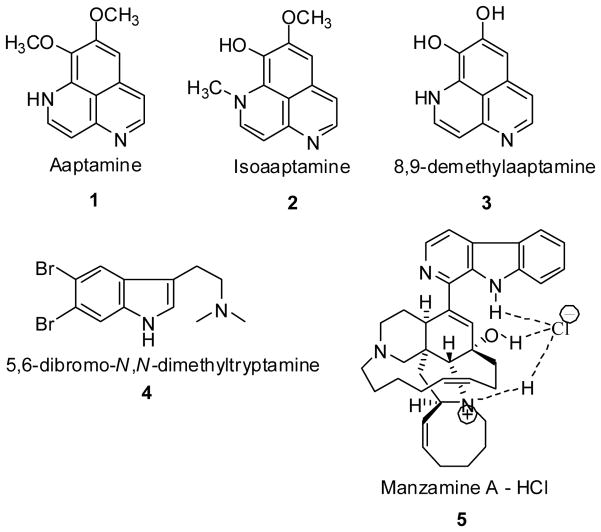
In an effort to further evaluate bioactive marine natural products as prototypes for successful drug leads, we have identified several bioactive compounds from chemically defended organisms in the marine environment with structural similarities to serotonin or known antidepressant drugs (Fig. 1). The selected marine natural products or their semi-synthetic derivatives: aaptamine (1); isoaaptamine (2); 8,9-demethylaaptamine (3); 5,6-dibromo-N,N-dimethyltryptamine (4); and manzamine A (5) were evaluated for antidepressant-like effects in mice using the forced swim test (FST), the most established animal model for assessing the potential clinical antidepressant activity of drugs (Cryan et al., 2002, 2003, 2005). Originally described by Porsolt et al. (1977a) using a rat model, it was later implemented for use with mice (1977b). Inspired by the work of Porsolt, Steru et al. (1985) developed the tail suspension test (TST) as an additional measure of antidepressant-like activity in mice. Decades of research have shown that both the FST and TST procedures are highly predictive of antidepressant-like actions (Porsolt et al., 1977b; Cryan et al., 2002, 2005; Bourin et al., 2005; Crowley et al, 2006). Both tests were employed in the current study since they offer an additional advantage in the field of drug discovery: they are not based on predetermined notions of a drug’s mechanism of action (Porsolt et al., 1977b; Steru et al., 1985). The locomotor activity of those compounds showing antidepressant-like activity in the FST or TST were also tested to demonstrate that reductions in immobility time were not a secondary consequence of nonspecific stimulant actions of the test drugs.
Fig. 1.
Marine compounds (1–5) used for the behavioral studies.
2. Methods
2.1. Subjects
Mice were used for these studies, instead of rats, because their smaller size allowed for more assessments to be made with a given quantity of compound. Male, Swiss Webster mice (Harlan, Indianapolis, IN) weighing 24–40 g at the time of testing were used for the FST. Male, DBA2/J mice (Jackson Laboratory, Bar Harbor, ME) weighing 19–29 g were used for the TST. Both strains were also used for the locomotor behavioral studies. The mice were housed in groups of five with a 12 h light/12 h dark cycle. Food and water were provided ad libitum. All procedures involving animals were performed as approved by the Institutional Animal Care and Use Committee.
2.2. Drugs
Aaptamine (1), isoaaptamine (2), 8,9-demethylaaptamine (3), 5,6-dibromo-N,N-dimethyltryptamine (4), and manzamine A (5) were isolated from sponges collected from Florida and Indonesia as described below, and were tested and compared with two positive controls: citalopram, a selective serotonin reuptake inhibitor (SSRI) (Tocris Bioscience, Ellisville, MO) and desipramine, a tricyclic antidepressant (TCA) drug (Sigma-Aldrich, St. Louis, MO).
Aaptamine (1) and isoaaptamine (2) were extracted from sponge samples collected on reef slopes at a depth of −20 m from Manado and Derawan Island, Indonesia (Diers et al., 2006). The isolation of aaptamine (1) and isoaaptamine (2) along with the semi-synthetic production of 8,9-demethylaaptamine (3) have been described previously (Coutinho et al., 2002; Calcul et al., 2003; Pettit et al., 2004; Diers et al., 2006). The marine natural product 5,6-dibromo-N,N-dimethyltryptamine (4) was obtained from the Florida sponge Verongula rigida. The extraction, purification, and isolation of these types of haloindole compounds were described by Djura et al. (1980). The compound 5,6-dibromo-N,N-dimethyltryptamine (4) was isolated as a light yellow crystal, mp. 171–172°C 1H NMR (MeOD) δ 2.93 (s, 7H), 3.11 (m, 2H) 7.29 (s, 1H), 7.72 (s, 1H), 7.89 (s, 1H) 13C NMR (MeOD) δ 136.53 (s), 127.74 (s), 125.46 (s), 122.23 (s), 116.21(s), 116.00 (s), 113.66 (s), 108.38 (s), 57.56 (s), 42.18 (s, 2C), 20.18 (s) HRMS m/e 344.9673, C12H14N2Br2 requires m/e 343.9525. Manzamine A (5) was extracted from the Indonesian sponge, genus Acanthostrongylophora. The extraction, purification, and physical data of manzamine A have been described in several previous publications (Yousaf et al., 2004; Rao et al., 2006). It should be noted that while all of the aforementioned substances were isolated from sponges, associated bacteria growing within the sponges may be the actual source of the compounds, rather than the sponges per se. Confirmation of the structure and purity of compounds 1–5 were achieved by NMR and MS analysis. The calculated log P (ClogP) values for compounds 1–5, determined with ChemDraw Ultra 10.0 using pH 7.4 and 25° C as parameters, were 2.69, 3.14, 2.59, 3.86, and 8.0, respectively.
Aaptamine (1) and 8,9-demethylaaptamine (3) were dissolved in saline (0.9% NaCl). The compounds 5,6-dibromo-N,N-dimethyltryptamine (4) and isoaaptamine (2) were initially dissolved in 100% EtOH, and administered in a final EtOH concentration of 10%. Manzamine A (5) was initially dissolved in 1 N HCl, and administered in a final HCl concentration of 0.025 N. The intraperitoneal (i.p.) injection dosage for these compounds ranged from 1 to 40 mg/kg, with a 10 ml/kg final drug volume so that each mouse received the same volume of drug according to body weight.
For all compounds used for the first time in mice, the IACUC required monitoring for the following the signs of toxicity: distress vocalization, hunched posture, piloerection, abnormal muscle tone, ocular or nasal discharge, twitching/tremors, lying on side, difficulty breathing, convulsions, or death. The monitoring occurred from the time the compound was administered until the end of the data acquisition period.
2.3. Forced swim test
Swiss Webster mice were selected for the FST based on several studies, including Petit-Demouliere’s review (2005) on mouse strain and antidepressant activity. Bourin et al. (2005) have demonstrated that various classes of antidepressant drugs show significant activity in the FST in this strain of mice. All mice were injected i.p. with one of the following compounds or vehicle control 30 min prior to behavioral testing: citalopram (1, 5 mg/kg, n=20), desipramine (10, 20 mg/kg, n=20), aaptamine (1) (1, 10, 20 mg/kg, n=30), isoaaptamine (2) (1, 5, 10, 15, 20 mg/kg, n=49), 8,9-demethylaaptamine (3) (1, 10, 20 mg/kg, n=29), 5,6-dibromo-N,N-dimethyltryptamine (4) (1, 10, 15, 20 mg/kg, n=40), manzamine A (5) (1, 10, 20, 40 mg/kg, n=34), saline (n=14), 10% EtOH (n=9), 0.025 N HCl (n=14). The treated mice were individually placed into clear plastic cylinders (height 23 cm, internal diameter 10 cm) filled with 8 cm of deionized water at 25° C. Individual mice were recorded with a video camera (height of ~ 30 cm above the cylinder) for a total of 6 min. Quantification of immobility time during the last 4 min of each testing session was conducted by three independent raters. Immobility was operationally defined as when the mouse made no movements other than that required to keep its head/nostrils above the surface of the water (Porsolt et al., 1977a,b; Cryan et al., 2005).
2.4. Tail suspension test
Male DBA2/J mice were selected for the TST based on the research of Liu et al. (2001) and Crowley et al. (2005). The studies demonstrated robust strain differences in the response to various antidepressant drugs in the TST and confirmed the high responsiveness of DBA2/J mice in this test. All mice were injected i.p. with one of the following compounds or vehicle control 30 min prior to behavioral testing: citalopram (5 mg/kg, n=12), desipramine (20 mg/kg, n=14), aaptamine (1) (10, 20, 40 mg/kg, n=23), 5,6-dibromo-N,N-dimethyltryptamine (4) (5, 10, 20, 40 mg/kg, n=34), saline (n=19), 10% EtOH (n=18). Each mouse was hung upside down by the tail and fastened with clear packing tape (2–4 cm from the tip of the tail) to a metal bar attached to a ring-stand. The mice were suspended 35 cm above a protective sponge material (5 cm thick), with the animals at least 15 cm from any object. Individual mice were recorded with a video camera, which was positioned at the same level as the mouse, for a total of 6 min. Quantification of immobility time during the last 4 min of each testing session was conducted by three independent raters. Immobility was operationally defined as when the mouse made no movements (i.e. hung motionless) (Steru et al., 1985).
2.5. Locomotor activity
Locomotor activity was measured using an automated activity monitoring system (San Diego Instruments, San Diego, CA). Swiss Webster were acclimated to laboratory conditions for 30 min and then subsequently placed one mouse per Plexiglas enclosure for an additional 30 min prior to i.p. injection with one of the following treatments: citalopram (5 mg/kg, n=8), desipramine (20 mg/kg, n=8), aaptamine (1) (20 mg/kg, n=8), 5,6-dibromo-N,N-dimethyltryptamine (4) (20 mg/kg, n=8), saline (n=8), 10% EtOH (n=8). The compounds and doses selected were based on statistically significant results in the FST, and corresponding vehicles were included as controls. Locomotor activity was monitored over a 40 min period as the number of interruptions made by the mouse to the 16 × 16 photobeam arrays surrounding each testing enclosure. The data were quantified during the 4 min period that corresponded to the time in which the FST were conducted.
Since DBA2/J mice were used for the TST, locomotor activity was also measured in these mice. To conserve the amount of compound and animals used for these behavioral evaluations, a slightly modified testing procedure was employed in which the locomotor evaluations were conducted immediately prior to the TST. In this modified testing procedure, the DBA2/J mice were acclimated to the laboratory for 30 min and then injected (i.p.) with one the following treatments: citalopram (5 mg/kg, n=4), desipramine (20 mg/kg, n=4), 5,6-dibromo-N,N-dimethyltryptamine (4) (5 mg/kg, n=4), saline (vehicle control, n=4), 10% EtOH (vehicle control, n=4). Each mouse was immediately placed in a Plexiglas enclosure and locomotor activity monitored for the next 30 min. The data during the last 10 min of the testing period was analyzed. Immediately following the locomotor measurements (equivalent to the 30 min pretreatment time), the mice were subject to the TST as described above.
2.6. Statistical analyses
For the FST and TST, the immobility times from the three independent raters were averaged for each mouse. For the TST studies, the two vehicle controls were compared using Student’s t-tests. All other data from the FST and TST studies were evaluated using analysis of variance, followed by post-hoc Dunnett’s tests to determine individual doses that differed significantly from the vehicle control. The effects of the compounds in the locomotor activity studies were evaluated using analysis of variance, followed by Bonferroni’s post-hoc comparisons to identify pairs of treatments that differed significantly from one another. For all of the statistical comparisons, p < 0.05 was considered significant.
3. Results
3.1. Forced swim test
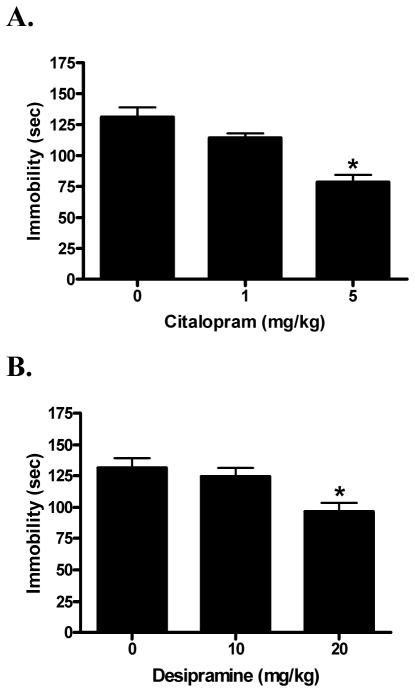
The SSRI antidepressant positive control, citalopram (F[2,31] = 16.49; p < 0.01) and the TCA positive control desipramine (F[2,31] = 5.72; p < 0.01) both dose-dependently reduced immobility time (Fig. 2). Dunnett’s post-hoc comparisons of citalopram confirmed that the 40% reduction in immobility time produced by the 5 mg/kg dose differed significantly from the saline vehicle (q = 5.71; p < 0.01). Post-hoc comparisons of desipramine confirmed that the 27% reduction in immobility time produced by the 20 mg/kg dose differed significantly from the saline vehicle (q = 3.29; p < 0.01).
Fig. 2.
Dose response of the SSRI positive control citalopram (A) and tricyclic antidepressant positive control desipramine (B) in the forced swim test. Saline served as the vehicle control (0 mg/kg). Data were evaluated using analysis of variance, followed by Dunnett’s post-hoc tests, where * (p < 0.05) was considered significantly different from the vehicle control.
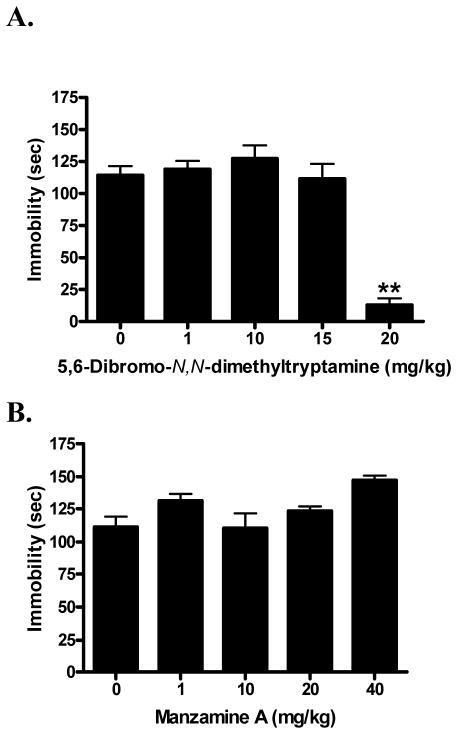
Aaptamine (1) (F[3,40] = 2.74; p < 0.05) dose-dependently reduced immobility time in the FST (Fig. 3A). Post-hoc comparisons of individual doses to the vehicle control showed that aaptamine (1) differed significantly at only the 20 mg/kg dose (q = 2.47; p < 0.05), at which it produced a 36% reduction in immobility time. In contrast, isoaaptamine (2) (F[5,52] = 5.29; p < 0.01) significantly increased immobility time in a dose-dependent manner (Fig. 3B). Post-hoc comparisons of individual doses to vehicle control showed that isoaaptamine (2) at 10 mg/kg (q = 3.12; p < 0.05), 15 mg/kg (q = 3.25; p < 0.01), and 20 mg/kg (q = 3.53; p < 0.01) were significantly different from control, representing changes of 31%, 33%, and 36%, respectively. The semi-synthetic aaptamine derivative 8,9-demethylaaptamine (3) (F[3,39] = 0.31; n.s.) did not show a significant effect in the FST across the studied dose range (Fig. 3C).
Fig. 3.
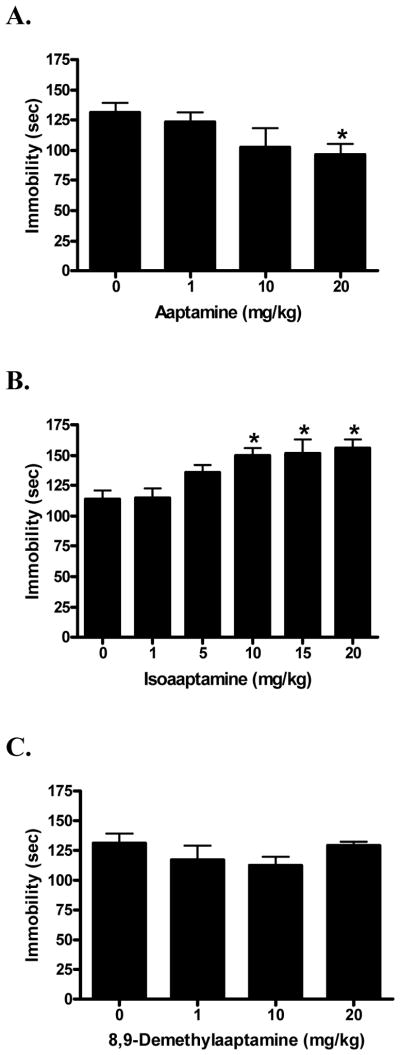
Dose response of aaptamine (A; saline as vehicle control); isoaaptamine (B; 10% EtOH as vehicle control) and 8,9-demethylaaptamine (C; saline as vehicle control) in the forced swim test. Data were evaluated using analysis of variance, followed when applicable by Dunnett’s post-hoc tests, where * (p < 0.05) was considered significantly different from the vehicle control.
The novel compound 5,6-dibromo-N,N-dimethyltryptamine (4) (F[4,44] = 31.56; p < 0.01) exhibited significant antidepressant-like activity (Fig. 4A). Post-hoc comparisons of individual doses to the vehicle control showed that 5,6-dibromo-N,N-dimethyltryptamine (4) was significantly different from control at only the 20 mg/kg dose (q = 8.28; p < 0.01), at which it demonstrated a pronounced, 89% decrease in immobility time.
Fig. 4.
Dose response of 5,6-dibromo-N,N-dimethyltryptamine (A; 10% EtOH as vehicle control) and manzamine A (B; 0.025 N HCl as vehicle control) in the forced swim test. Data were evaluated using analysis of variance, followed when applicable by Dunnett’s post-hoc tests, where ** (p < 0.01) was considered significantly different from the vehicle control.
Manzamine A (5) (F[4,44] = 2.35; n.s.) did not show significant antidepressant-like activity (Fig. 4B). Although manzamine A (5) exhibited a trend to increase immobility time at the highest dose (40 mg/kg) tested, this change was not statistically significant.
All vehicle controls that were used in the FST (saline, 10% EtOH and 0.025 N HCl) were not significantly different from one another (F[7,65] = 1.89; n.s.). However, it should be noted that during methods development and evaluation of drug solubility, 20% EtOH produced a significant reduction in immobility time when compared to the other vehicle controls (q = 3.34; p < 0.01), and was thus not used for behavioral testing.
None of the compounds elicited the following signs of toxicity during the 30 min period leading up to the FST and immediately after it: distress vocalization, hunched posture, piloerection, abnormal muscle tone, ocular or nasal discharge, twitching/tremors, lying on side, difficulty breathing, convulsions, or death. Somnolence was observed in some animals treated with 20 mg/kg of 5,6-dibromo-N,N-dimethyltryptamine.
3.2. Tail suspension test
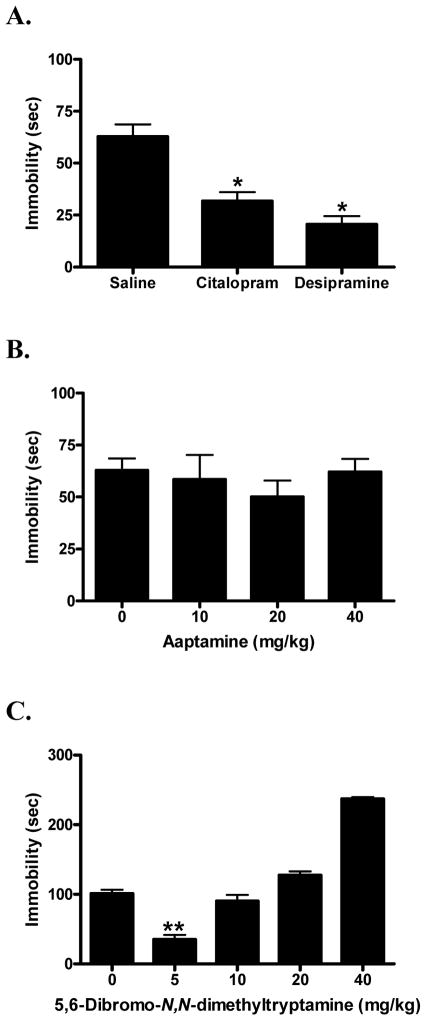
Due to significant differences between saline and 10% ethanol in the TST (t = 4.85; p < 0.0001), compounds were individually compared to their respective vehicle controls. Analysis of variance verified that the antidepressant positive controls, citalopram and desipramine (F[2,42] = 20.40; p < 0.0001), yielded significant effects in the TST (Fig. 5A). Dunnett’s post-hoc comparisons confirmed that the 50% reduction in immobility time produced by 5 mg/kg of the SSRI citalopram was statistically significant (q = 4.27; p < 0.01). Similarly, the 67% reduction in immobility time produced by 20 mg/kg of the TCA desipramine also differed significantly from its vehicle control (q = 6.07; p < 0.01).
Fig. 5.
Effects of the SSRI citalopram (5 mg/kg, i.p.) and the tricyclic antidepressant desipramine (20 mg/kg, i.p.) compared to their saline vehicle control in the tail suspension test (A). Dose response of aaptamine (B; saline as vehicle control) and 5,6-dibromo-N,N-dimethyltryptamine (C; 10% EtOH as vehicle control) in the tail suspension test. Data were evaluated using analysis of variance, followed when applicable by Dunnett’s post-hoc tests, where * (p < 0.05) and ** (p < 0.01) were considered significantly different from the vehicle control.
Aaptamine (1) (F[3,38] = 0.45; n.s.) failed to reduce immobility time at the doses tested (10, 20, 40 mg/kg) in the TST (Fig. 5B). In contrast to the other drug treatments, approximately 20% of the DBA2/J mice injected with aaptamine (1) either climbed their tails or performed other confounding behaviors that required them to be removed from the analysis.
On the other hand, 5,6-dibromo-N,N-dimethyltryptamine (4) (F[4,47] = 67.87; p < 0.0001) exhibited a U-shaped dose response curve (Fig. 5C). Dunnett’s post-hoc comparisons revealed that the 65% reduction in immobility produced by the 5 mg/kg dose was statistically significant (q = 7.00; p < 0.01), reflecting antidepressant-like effects. The 10, 20 and 40 mg/kg doses, however, did not produce antidepressant-like results; the 20 and 40 mg/kg doses significantly increased immobility time (q = 2.92; p < 0.05 and q = 12.13; p < 0.01 respectively), and the 10 mg/kg dose did not differ significantly from the vehicle control (q = 1.28; n.s.).
3.3. Locomotor activity
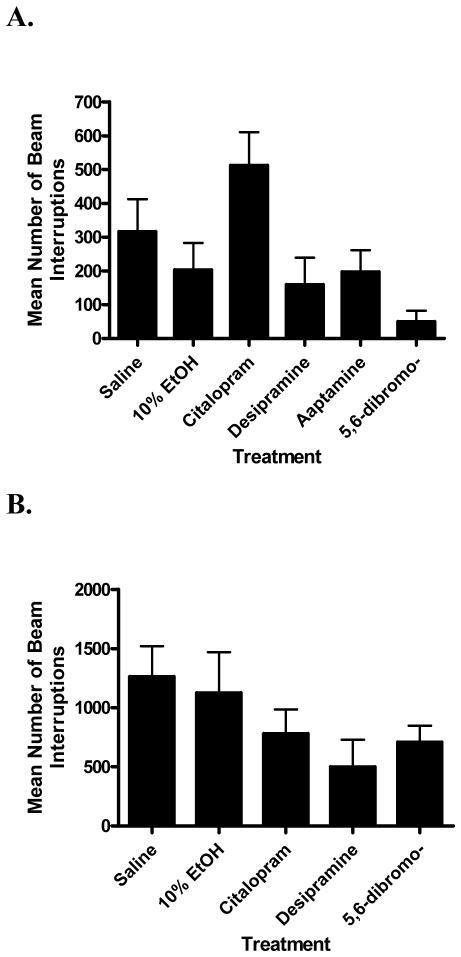
For the Swiss Webster mice, analysis of variance revealed a significant difference between the treatment groups (F[5,42] = 4.07; p < 0.005). However, Bonferroni’s post-hoc tests confirmed that there were no statistical differences between the vehicle controls, or compounds from their respective vehicle controls (Fig. 6A). Compared to their saline vehicle control, there was no significant difference in locomotor activity for citalopram 5 mg/kg (t = 1.77; n.s.), desipramine 20 mg/kg (t = 1.41; n.s.), or aaptamine (1) 20 mg/kg (t = 1.07; n.s.). Likewise, there was no statistically significant difference for 5,6-dibromo-N,N-dimethyltryptamine (4) 20 mg/kg (t = 1.38; n.s.) compared to its 10% EtOH vehicle control, although it produced a noticeable reduction in activity (Fig. 6A).
Fig. 6.
Locomotor activity of compounds producing significant effects in the forced swim test (A) and tail suspension test (B). Panel A: Swiss Webster mice were used and locomotor activity monitored for the 4 min corresponding to the time during which the FST data were quantified. Data were evaluated using analysis of variance, followed by Dunnetts’s post-hoc tests. Compared to their respective vehicle controls, none of the compounds produced statistically significant changes in locomotor activity: citalopram (5 mg/kg; saline as vehicle control), desipramine (20 mg/kg; saline as vehicle control), aaptamine (20 mg/kg; saline as vehicle control), and 5,6-dibromo-N,N-dimethyltryptamine (20 mg/kg; 10% EtOH as vehicle control). Panel B: DBA2/J mice were used and locomotor activity monitored for the 10 min immediately prior to the TST. There were no significant differences between the groups: citalopram (5 mg/kg; saline as vehicle control), desipramine (20 mg/kg; saline as vehicle control), and 5,6-dibromo-N,N-dimethyltryptamine (5 mg/kg; 10% EtOH as vehicle control). Nonspecific stimulant effects could therefore not account for the antidepressant-like effects of the compounds in the FST and TST.
In DBA2/J mice, the pattern of responding in the locomotor studies was similar to that observed in the Swiss Webster mice (Fig. 6B). Analysis of variance revealed that there was no significant difference between the treatment groups (F[4,15] = 1.63; n.s.). Therefore, differences in the mouse strain and slight variations in the time periods during which the locomotor activity data were collected did not significantly affect the pattern of results. The drug/dose combinations that produced antidepressant-like effects in the FST and TST were thus not associated with confounding stimulant effects.
4. Discussion
The invertebrate community of the marine environment has evolved complex bioactive chemistries over billions of years primarily for defensive purposes (Battershill et al., 2005). Some of these compounds display structural similarities to endogenous ligands in mammals, while others represent novel chemical prototypes. Both types of metabolites have become increasingly recognized for their potential to treat a variety of human illnesses that may now include depression.
The current study demonstrated that aaptamine (1) and 5,6-dibromo-N,N-dimethyltryptamine (4) exhibited antidepressant-like activity in the FST, and have the potential to be antidepressant drug leads. Manzamine A (5) did not produce antidepressant-like activity in these studies which is most likely due to its inability to cross the blood brain barrier (ClogP = 8.4). The ability of aaptamine (1) and 5,6-dibromo-N,N-dimethyltryptamine (4) to produce antidepressant-like actions in the FST may involve different mechanisms of action since the former produced a gradual reduction in immobility time with increasing dose, whereas the latter exhibited more of a threshold effect, with a sudden appearance of efficacy with a small increase in dose. It does not appear likely that the antidepressant-like effects of 5,6-dibromo-N,N-dimethyltryptamine (4) in the FST are an artifact of nonspecific toxic actions because: i) the animals did not manifest signs of toxicity that were monitored for during the course of the study, and ii) 5,6-dibromo-N,N-dimethyltryptamine (4) also produced antidepressant-like effects in the TST, further supporting its antidepressant potential.
Since reductions in immobility time are accompanied by increases in escape oriented movements such as swimming or climbing, it was important to confirm that the effects in the FST could not be explained by nonspecific stimulant effects of the test compounds, which would be reflected as an enhancement in locomotor activity. Aaptamine (1) produced no statistically significant changes in locomotor activity at doses that displayed antidepressant-like effects in the FST, suggesting that the compound produced true antidepressant-like actions. 5,6-Dibromo-N,N-dimethyltryptamine (4) in fact produced a non-significant trend toward decreasing locomotor activity, which would not be able to account for its reduction in immobility time in the FST. A similar trend was observed in DBA2/J mice, suggesting that 5,6-dibromo-N,N-dimethyltryptamine also possessed true antidepressant-like properties in the TST.
Although 5,6-dibromo-N,N-dimethyltryptamine (4) elicited antidepressant-like effects in both the FST and TST, the effective doses differed between the two tests and a U-shaped dose response curve was exhibited in the TST. It should therefore be emphasized that differences in the effective doses of compounds in the TST vs. the FST are quite common. Similar trends have been previously reported, and were mostly attributed to interstrain differences. For example, Bai et al. (2001) demonstrated that in C57Bl/6 mice, imipramine produced a U-shaped dose response curve in the FST with no evidence of the biphasic response in the TST. In the same study, NIH Swiss mice showed no evidence of a U-shaped dose response induced by imipramine. Similarly, Li et al. (2001) reported a biphasic response of the AMPA receptor potentiator LY392098 in the FST, but not in the TST. Such interstrain differences may be due to both pharmacokinetic (e.g. strain differences in bioavailability) and pharmacodynamic (e.g. strain differences in neurotransmitters, receptors, or signal transduction) factors. Additionally, it is possible that the U-shaped dose response curve of 5,6-dibromo-N,N-dimethyltryptamine (4) in the TST reflects the sedative actions of the compound at higher doses, with increases in immobility time observed with escalating dose.
With regard to aaptamine (1), its antidepressant-like effects in the FST involve some specificity since small structural changes that were introduced in isoaaptamine (2) and 8,9-demethylaaptamine (3) resulted in loss of efficacy. The difference in effects between aaptamine (1) vs. isoaaptamine (2) and 8,9-demethylaaptamine (3) suggests that the 3-dimensional shape of the aaptamine molecule was complementary to a protein target mediating antidepressant-like effects in the FST, whereas isoaaptamine (2) and 8,9-demethylaaptamine (3) either did not fit into this binding pocket or docked in a dissimilar manner.
The ability of aaptamine (1) to produce antidepressant-like actions in the FST, but not in the TST, is most likely related to inherent differences in the two animal models. Several hemodynamic, behavioral, physiological, as well as pharmacological studies suggest that the TST is considerably less stressful than the FST (Thierry et al., 1986; Bourin et al., 2005; Cryan et al., 2005; Kulkarni and Dhir, 2007). For example, the added hypothermia induced in the FST when the animal is immersed in water is lacking in the TST and augments the stress level of the model (Thierry et al., 1986). Such differences extend to biochemical and neurochemical mechanisms involved in the two models (Renard et al., 2003). The fact that aaptamine shows antidepressant-like actions in the FST, but not in the TST does not detract from its antidepressant potential. It is well documented that the majority of established antidepressant drugs show significant effects in the FST when Swiss mice are used (Cryan et al., 2002l Bourin et al., 2005). Therefore, the FST in Swiss mice is generally recommended as the primary screen for antidepressant-like effects, and a drug showing positive effects should then be further tested in the TST (Cryan et al., 2002; Bourin et al., 2005). Discrepancies between antidepressant-like efficacy in the TST vs. the FST are most likely related to the mechanism of action of the drug and indeed, several atypical antidepressants such as rolipram and levoprotiline have been reported to reduce immobility time in the FST, but not the TST (Porsolt and Lenegre, 1992). In addition, Mombereau et al. (2004) have recently shown that antagonists or gene knockouts of the GABAB receptor results in antidepressant-like effects in the FST, with no effects in the TST. Hence, further mechanistic studies are needed to fully understand the antidepressant mechanisms of aaptamine (1), as well 5,6-dibromo-N,N-dimethyltryptamine (4).
In addition to having structural similarities to serotonin and known antidepressant drugs, the compounds tested herein also displayed similarities to histamine and morphine, suggesting potential applications for pain management and other neurological and psychiatric disorders. Hu et al. (2002) reported that indole alkaloids similar to 5,6-dibromo-N,N-dimethyltryptamine (4) had high affinity for 5-HT2 receptors. Further studies to identify the protein targets for the tested compounds are therefore needed to fully understand their actions and potential uses.
In summary, this is the first report of antidepressant-like actions of compounds derived from marine natural products. The biodiversity of marine organisms provides a rich source for known and novel prototypes as drug leads, and additional studies in this area are warranted.
Acknowledgments
The authors thank L. Wilson for assistance with the behavioral studies; J.J. Bowling, H. Pennaka, and K.V. Rao for compound purification; C. Dunbar and F. Wiggers for MS and NMR spectral measurements. J.F. Stoker was supported by the Summer Research Institute for Undergraduates at the University of Mississippi. This work was supported in part by grants from the National Institutes of Health: NCRR (P20 RR021929) and NIAID (R01 AI36596).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amsler CD, McClintock JB, Baker BJ. Secondary metabolites as mediators of trophic interactions among antarctic marine organisms. Amer Zoo. 2001;41:17–26. [Google Scholar]
- Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of “behavioral despair. Pharmacol Biochem Behav. 2001;70:187–192. doi: 10.1016/s0091-3057(01)00599-8. [DOI] [PubMed] [Google Scholar]
- Battershill C, Jaspars M, Long P. Marine biodiscovery: new drugs from the ocean depths. Biologist. 2005;52:107–114. [Google Scholar]
- Bourin M, Chenu F, Ripoll N, David DJP. A proposal of decision tree to screen putative antidepressants using forced swim and tail suspension rests. Behav Brain Res. 2005;164:266–269. doi: 10.1016/j.bbr.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Calcul L, Longeon A, Mourabit A, Guyot M, Bourguet-Kondracki M. Novel alkaloids of the aaptamine class from an Indonesian marine sponge of the genus Xestospongia. Tetrahedron. 2003;59:6539–6544. [Google Scholar]
- Coutinho AF, Chanas B, Souza TML, Frugrulhetti ICPP, Epifanio RA. Anti HSV-1 alkaloids from a feeding deterrent marine sponge of the genus Aaptos. Heterocycles. 2002;57:1265–1272. [Google Scholar]
- Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psycopharm. 2005;183:257–264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Brodkin ES, Blendy JA, Berrettini WH, Lucki I. Pharmacogenomic evaluation of the antidepressant citalopram in the mouse tail suspension test. Neuropsychopharmacol. 2006;31:2433–2442. doi: 10.1038/sj.npp.1301065. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry. 2003;54:49–58. doi: 10.1016/s0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacocological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Differential behavior effects of the antidepressants reboxetine, flvoxetine, and moclobemide in a modified forced swim test following chronic treatment. J Psychopharmacol. 2005;182:335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Diers JA, Bowling JJ, Duke SO, Wahyuono S, Kelly M, Hamann MT. Novel zebra mussel antifouling activity of the marine natural product aaptamine and analogs. Mar Biotech. 2006;8:366–372. doi: 10.1007/s10126-005-6055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djura P, Stierle DB, Sullivan B, Faulkner DJ. Some metabolites of the marine sponges Smenospongia aurea and Smenospongia (= Polyfibrospongia) echina. J Org Chem. 1980;80:1435–1441. [Google Scholar]
- Gavagnin M, Fontana A. Diterpenes from marine opisthobranch molluscs. Curr Org Chem. 2000;4(12):1201–1248. [Google Scholar]
- Haefner B. Drugs from the deep: Marine natural products as drug candidates. Drug Disc Tod. 2003;8:536–544. doi: 10.1016/s1359-6446(03)02713-2. [DOI] [PubMed] [Google Scholar]
- Hu JF, Schetz JA, Kelly M, Peng J, Ang KK, Flotow H, Leong CY, Ng SB, Buss AD, Wilkins SP, Hamann MT. New antiinfective and human 5-HT2 receptor binding natural and semisynthetic compounds from the Jamaican sponge Smenospongia aurea. J Nat Prod. 2002;65:476–480. doi: 10.1021/np010471e. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Ashish D. Effect of various classes of antidepressants in behavioral paradigms of despair. Prog Neuro-Psychpharm & Biol Psych. 2007;31(6):1248–1254. doi: 10.1016/j.pnpbp.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator (LY392098) Neuropharmacol. 2001;40:1028–1033. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Xiaoqing Liu, Gershenfeld HK. Genetic differences in the tail-suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biol Psychiatry. 2001;49:575–581. doi: 10.1016/s0006-3223(00)01028-3. [DOI] [PubMed] [Google Scholar]
- Maplestone RA, Stone MJ, Williams DH. The evolutionary role of secondary metabolites – A review. Gene. 1992;115:151–157. doi: 10.1016/0378-1119(92)90553-2. [DOI] [PubMed] [Google Scholar]
- Matthews K, Christmas D, Swan J, Sorrell E. Animal models of depression: navigating through the clinical fog. Neurosci Biobehav Rev. 2005;29:503–513. doi: 10.1016/j.neubiorev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABAB receptors in the modulation of anxiety and antidepressant-like behavior. Neuropsychopharmacol. 2004;29:1050–1062. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- Nestler E, Gould E, Manji H, Bucan M, Duman R, Gershenfeld H, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Paul VJ. In: Ecological roles of marine natural products. Paul VJ, editor. Ithica: Comstock Publishing Associates; 1992. pp. 1–23. [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacol. 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Pettit G, Hoffmann H, Herald D, McNulty J, Murphy A, Higgs K, Hamel E, Lewin N, Pearce L, Blumberg P, Pettit R, Knight J. Antineoplastic agents 491. Synthetic conversion of aaptamine to isoaaptamine, 9-demethylaaptamine, and 4-methylaaptamine. J Org Chem. 2004;69:2251–2256. doi: 10.1021/jo0300486. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977a;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioural despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn. 1977b;229:327–336. [PubMed] [Google Scholar]
- Porsolt R, Lenegre A. Behavioral models of depression. In: Eliott J, Heal D, Mardesen C, editors. Experimental Approaches to Anxiety and Depression. Wiley; London: 1992. pp. 73–85. [Google Scholar]
- Rao KV, Donia MS, Peng J, Garcia-Palomero E, Alonso D, Martinez A, Medina M, Franzblau SG, Tekwani BL, Khan SI, Wahyuono S, Willett KL, Hamann MT. Manzamine B and E and ircinal A related alkaloids from an Indonesian Acanthostrongylophora sponge and their activity against infectious, tropical parasitic, and Alzheimer’s diseases. J Nat Prod. 2006;69:1034–1040. doi: 10.1021/np0601399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard CE, Dailly E, David DJP, Hascoet M, Bourin M. Monoamine metabolism changes following the mouse forced swim test but not the tail suspension test. Fund Clin Pharmacol. 2003;17:449–455. doi: 10.1046/j.1472-8206.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- Sipkema D, Franssen MCR, Osinga R, Tramper J, Wijffels RH. Marine sponges as pharmacy. Mar Biotech. 2005;7:142–162. doi: 10.1007/s10126-004-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey J, editor. Society for Neuroscience. Brain Facts: A Primer on the Brain and Nervous System. 4. Washington, DC: Society for Neuroscience; 2002. p. 32. [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacol. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Thierry B, Steru L, Simon P, Porsolt RD. The tail suspension test: ethical considerations. Psychopharmacol. 1986;90:284–285. doi: 10.1007/BF00181261. [DOI] [PubMed] [Google Scholar]
- Wells KB, Stewart A, Hays RD, Burnam MA, Rogers W, Daniels M, Berry S, Greenfield S, Ware J. The functioning and well-being of depressed patients. JAMA. 1989;262:914–919. [PubMed] [Google Scholar]
- Wong M, Lichinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- Yousaf M, Hammond NL, Peng J, Wahyuono S, McIntosh KA, Charman WN, Mayer AMS, Hamann MT. New manzamine alkaloids from an Indo-Pacific sponge. Pharmacokinetics, oral availability and the significant activity of several manzamines against HIV-I, AIDS opportunistic infections, and inflammatory diseases. J Med Chem. 2004;47:3512–3517. doi: 10.1021/jm030475b. [DOI] [PMC free article] [PubMed] [Google Scholar]