Abstract
BACKGROUND
In young adults with acute myeloid leukemia (AML), intensification of the anthracycline dose during induction therapy has improved the rate of complete remission but not of overall survival. We evaluated the use of cytarabine plus either standard-dose or high-dose daunorubicin as induction therapy, followed by intensive consolidation therapy, in inducing complete remission to improve overall survival.
METHODS
In this phase 3 randomized trial, we assigned 657 patients between the ages of 17 and 60 years who had untreated AML to receive three once-daily doses of daunorubicin at either the standard dose (45 mg per square meter of body-surface area) or a high dose (90 mg per square meter), combined with seven daily doses of cytarabine (100 mg per square meter) by continuous intravenous infusion. Patients who had a complete remission were offered either allogeneic hematopoietic stem-cell transplantation or high-dose cytarabine, with or without a single dose of the monoclonal antibody gemtuzumab ozogamicin, followed by autologous stem-cell transplantation. The primary end point was overall survival.
RESULTS
In the intention-to-treat analysis, high-dose daunorubicin, as compared with a standard dose of the drug, resulted in a higher rate of complete remission (70.6% vs. 57.3%, P<0.001) and improved overall survival (median, 23.7 vs. 15.7 months; P = 0.003). The rates of serious adverse events were similar in the two groups. Median follow-up was 25.2 months.
CONCLUSIONS
In young adults with AML, intensifying induction therapy with a high daily dose of daunorubicin improved the rate of complete remission and the duration of overall survival, as compared with the standard dose.
The survival of patients with acute myeloid leukemia (AML) is affected by many variables, including therapy that induces complete remission and appropriate consolidation therapy. Currently, anthracycline plus cytarabine is the usual induction therapy for patients with AML.1 The widely used intravenous combination of daunorubicin (at a dose of 45 mg per square meter of body-surface area), given daily for 3 days, and cytarabine (at a dose of 100 mg per square meter), given daily for 7 days, results in complete remission in 50 to 75% of patients.1,2 Neither the addition of other drugs to daunorubicin and cytarabine3 nor intensification of the dose of cytarabine4–6 has been shown to improve the outcome.
Increasing the anthracycline dose with cytokine support could improve the rate of complete remission and other outcomes.7–10 A high rate of complete remission was reported when daunorubicin was given at a daily dose of 60 mg per square meter for 3 days.11,12 Results of phase 1 and 2 studies7,8 have suggested that daunorubicin doses of 70 to 95 mg per square meter for 3 days are safe and improve the rate of complete remission.
The question of whether intensification of the anthracycline dose for induction therapy might improve survival in patients with AML who are under the age of 60 years remains unresolved. To address this question, the Eastern Cooperative Oncology Group (ECOG) conducted a phase 3 randomized study comparing standard-dose daunorubicin (45 mg per square meter per day) with high-dose daunorubicin (90 mg per square meter per day).
METHODS
PATIENTS
From December 2002 through November 2008, a total of 657 patients between the ages of 17 and 60 years who had untreated AML were enrolled in the study. An antecedent hematologic disorder had been diagnosed in some of the patients up to 6 months before study entry. Eligibility was based on confirmation of AML with the use of central immunophenotyping and morphologic analysis. The ECOG’s Cytogenetic Subcommittee reviewed conventional chromosome studies that were obtained by individual institutions and categorized patients as having a risk profile that was favorable, unfavorable, intermediate, or indeterminate on the basis of a published classification system.13 Fluorescence in situ hybridization (FISH) analysis was performed at the Mayo Clinic’s cytogenetics laboratory on samples from 535 patients, with the use of probes that have been described previously.14 All patients were tested centrally for the most common AML molecular aberrations,15 including the mixed-lineage leukemia gene (MLL), partial tandem duplication (PTD),16 and FMS-like tyrosine kinase 3 (FLT3)–internal tandem duplication (ITD),17 with the use of a semiquantitative polymerase-chain-reaction (PCR) assay.
STUDY DESIGN AND OVERSIGHT
The trial was developed by the ECOG Leukemia Committee. Data were collected and certified by the ECOG Data Coordinating Center and analyzed by the authors. All patients provided written informed consent and had to be candidates for subsequent hematopoietic stem-cell transplantation. The study was approved by the institutional review board at the National Cancer Institute and at each of the study centers.
TREATMENT
Eligible patients were randomly assigned to receive either 45 mg or 90 mg of intravenous daunorubicin per square meter daily for 3 days, together with intravenous cytarabine (100 mg per square meter per day) infused continuously for 7 days. Bone marrow aspiration and biopsy were performed on day 12 to 14 of therapy. If residual leukemic blasts were seen, a second induction course was given with the same dose and duration of cytarabine plus 45 mg of daunorubicin per square meter for 3 days. After bone marrow aplasia was confirmed on the basis of the International Working Group definition of AML,18 patients were given daily sargramostim (at a dose of 250 μg per square meter) until recovery of neutrophils was observed. After recovery from induction therapy and before assignment to consolidation therapy, complete remission was confirmed by bone marrow aspiration and biopsy. Patients in whom complete remission was not achieved after one or two courses of therapy were withdrawn from the study.
Post-remission consolidation therapy with allogeneic or autologous stem-cell transplantation was based on the risk of relapse. Patients with an unfavorable cytogenetic profile or a white-cell count of more than 100,000 per cubic millimeter at diagnosis were offered allogeneic stem-cell transplantation if they had an HLA-matched sibling. Patients who had an intermediate cytogenetic profile and an HLA-matched sibling were offered the same option. The remaining patients were offered autologous stem-cell transplantation. To proceed with consolidation treatment, patients had to be in complete remission, with no persisting complications of previous chemotherapy, a normal cardiac ejection fraction, an ECOG performance status of 2 or less (on a scale of 0 to 5, with higher scores indicating more severe debility), and adequate hepatic and renal function. Some patients with high-risk disease withdrew from the study to undergo stem-cell transplantation from a matched unrelated donor, but these patients were followed for overall survival.
Patients who underwent autologous stem-cell transplantation received two cycles of high-dose cytarabine therapy (3 g per square meter given intravenously over a 3-hour period every 12 hours every other day for a total of six doses),19 followed by collection of peripheral-blood hematopoietic stem cells. Before transplantation, half of these patients received a single dose of gemtuzumab ozogamicin (at a dose of 6 mg per square meter), according to randomization, performed at the time of enrollment, for post-remission therapy.
STATISTICAL ANALYSIS
We compared demographic factors and disease characteristics using t-tests and chi-square tests. All reported P values are two-sided. Overall survival was measured from the time of randomization for induction therapy. The study was designed according to a cure-rate model. Enrollment and follow-up goals were set to provide the study with a power of at least 85% to detect a 23% decrease in the hazard rate for death in the group receiving the 90-mg dose of daunorubicin with the use of a one sided log-rank test at the 0.025 significance level and assuming 2 years of follow-up. The study was predicated on an estimated enrollment of 830 patients and 563 deaths. The protocol provided for interim efficacy analyses of overall survival with the use of O’Brien–Fleming boundaries at two points, after the occurrence of 282 deaths and after the occurrence of 423 deaths.
A secondary end point was a comparison of rates of complete remission in the two study groups. An enrollment of 747 patients would provide a power of 85% to detect a 10% improvement in the complete-remission rate in the high-dose group with the use of a two-sample binomial test at a one-sided 0.025 significance level. The interim futility analyses for complete remission were conducted when induction-response data were available for 25%, 50%, and 75% of the 747 eligible patients. The study was unblinded at the third interim analysis of complete-remission rates, when significant differences in survival between the two groups became apparent. The study was then terminated by the ECOG data and safety monitoring committee. The survival analysis was performed on the intention-to-treat principle. Overall survival was compared with the use of the log-rank test and a Cox proportional-hazards model. Differences in response rates were evaluated in eligible patients for whom complete data on response status were available. Differences were tested with the use of either Fisher’s exact test or exact methods for ordered categorical data. Exact binomial confidence intervals are provided.
RESULTS
PATIENTS
Of the 657 patients who were enrolled in the study, 30 were ineligible because of an incorrect diagnosis, withdrawal of informed consent, or other reasons. The evaluation of response was incomplete for 45 patients; thus, responses were evaluated in 582 patients. The median age of all 657 patients was 48 years (range, 17 to 60). Median follow-up among survivors was 25.2 months (range, 0.6 to 70.3).
Table 1 provides the demographic and clinical characteristics of the patients at the time of the first randomization. There were no significant differences between the two groups with respect to demographic features, leukemia classification (on the basis of the 2001 criteria of the World Health Organization),20 cytogenetic profiles, or other disease characteristics.
Table 1.
Baseline Demographic and Clinical Characteristics of the Patients.*
| Variable | Standard Dose (45 mg/m2/day) (N = 330) | High Dose (90 mg/m2/day) (N = 327) | P Value |
|---|---|---|---|
| Age
| |||
| Group — no. (%) | 0.27 | ||
|
| |||
| <50 yr | 188 (57.0) | 172 (52.6) | |
|
| |||
| ≥50 yr | 142 (43.0) | 155 (47.4) | |
|
| |||
| Median — yr | 47 | 48 | 0.24 |
|
| |||
| Range — yr | 17–60 | 18–60 | |
|
| |||
| Sex — no. (%) | 0.59 | ||
|
| |||
| Male | 172 (52.1) | 163 (49.8) | |
|
| |||
| Female | 158 (47.9) | 164 (50.2) | |
|
| |||
| Peripheral-blood white-cell count
| |||
| Level — no. (%) | 0.75 | ||
|
| |||
| <10,000/mm3 | 150 (45.5) | 154 (47.1) | |
|
| |||
| ≥10,000/mm3 | 177 (53.6) | 171 (52.3) | |
|
| |||
| Missing data | 3 (0.9) | 2 (0.6) | |
|
| |||
| Median — cells/mm3 × 1000 | 12,000 | 12,000 | 0.85 |
|
| |||
| Range — cells/mm3 × 1000 | 1,000–366,000 | 1,000–192,000 | |
|
| |||
| Hemoglobin
| |||
| Level — no. (%) | 0.61 | ||
|
| |||
| <10 g/dl | 229 (69.4) | 233 (71.3) | |
|
| |||
| ≥10 g/dl | 98 (29.7) | 91 (27.8) | |
|
| |||
| Missing data | 3 (0.9) | 3 (0.9) | |
|
| |||
| Median — g/dl | 9 | 9 | 0.37 |
|
| |||
| Range — g/dl | 5–30 | 5–15 | |
|
| |||
| Peripheral-blood platelet count
| |||
| Level — no. (%) | 0.64 | ||
|
| |||
| <50,000/mm3 | 149 (45.2) | 155 (47.4) | |
|
| |||
| ≥50,000/mm3 | 177 (53.6) | 170 (52.0) | |
|
| |||
| Missing data | 4 (1.2) | 2 (0.6) | |
|
| |||
| Median — platelets/mm3 | 53,000 | 52,000 | 0.33 |
|
| |||
| Range — platelets/mm3 | 9,000–995,000 | 1,000–479,000 | |
|
| |||
| Blasts
| |||
| Peripheral blood | 0.96 | ||
|
| |||
| Median — % | 32 | 31 | |
|
| |||
| Range — % | 0–98 | 0–99 | |
|
| |||
| Bone marrow | 0.58 | ||
|
| |||
| Median — % | 64 | 65 | |
|
| |||
| Range — % | 3–100 | 8–100 | |
|
| |||
| CD33
| |||
| Peripheral blood | 0.29 | ||
|
| |||
| Median — % | 84 | 87 | |
|
| |||
| Range — % | 0–100 | 0–99 | |
|
| |||
| Bone marrow | 0.67 | ||
|
| |||
| Median — % | 87 | 85 | |
|
| |||
| Range — % | 0–100 | 0–100 | |
|
| |||
| Peripheral-blood neutrophils | 0.57 | ||
|
| |||
| Median — cells/mm3 | 9 | 11 | |
|
| |||
| Range — cells/mm3 | 0–784 | 0–951 | |
|
| |||
| Leukemia classification — no. (%) | 0.79 | ||
|
| |||
| AML with minimal differentiation | 14 (4.2) | 15 (4.6) | |
|
| |||
| AML without maturation | 89 (27.0) | 66 (20.2) | |
|
| |||
| AML with maturation | 50 (15.2) | 62 (19.0) | |
|
| |||
| Acute myelomonocytic leukemia | 35 (10.6) | 28 (8.6) | |
|
| |||
| Acute monocytic or monoblastic leukemia | 20 (6.1) | 20 (6.1) | |
|
| |||
| Acute erythroid leukemia | 13 (3.9) | 16 (4.9) | |
|
| |||
| Acute megakaryoblastic leukemia | 1 (0.3) | 2 (0.6) | |
|
| |||
| AML with multilineage dysplasia | 18 (5.5) | 18 (5.5) | |
|
| |||
| CBF-β–positive AML | 24 (7.3) | 33 (10.1) | |
|
| |||
| AML1-ETO–positive AML | 20 (6.1) | 25 (7.6) | |
|
| |||
| AML with MLL rearrangements | 23 (7.0) | 24 (7.3) | |
|
| |||
| AML not otherwise specified | 2 (0.6) | 2 (0.6) | |
|
| |||
| Ineligible leukemia classification† | 9 (2.7) | 7 (2.1) | |
|
| |||
| Not reviewed | 12 (3.6) | 9 (2.8) | |
|
| |||
| Cytogenetic profile — no. (%) | 0.57 | ||
|
| |||
| Favorable | 38 (11.5) | 51 (15.6) | |
|
| |||
| Indeterminate | 89 (27.0) | 85 (26.0) | |
|
| |||
| Intermediate | 142 (43.0) | 127 (38.8) | |
|
| |||
| Unfavorable | 59 (17.9) | 63 (19.3) | |
|
| |||
| Missing data | 2 (0.6) | 1 (0.3) | |
AML denotes acute myeloid leukemia, AML1-ETO expression of the AML1–myeloid translocation gene on chromosome 8 (MTG8), CBF-β core-binding factor β, and MLL mixed-lineage leukemia. Percentages may not total 100 because of rounding.
Patients with certain types of leukemia (e.g., acute promyelocytic or biphenotypic leukemia) were not included in the analysis.
INDUCTION THERAPY
Of the 582 eligible patients, 372 (63.9%; 95% confidence interval [CI], 59.9 to 67.8) had a complete remission: 168 of 293 patients (57.3%; 95% CI, 51.5 to 63.1) in the standard-dose group and 204 of 289 (70.6%; 95% CI, 65.0 to 75.8) in the high-dose group (P<0.001 by Fisher’s exact test). Among patients in the two study groups who had a complete remission, 121 of 168 in the standard-dose group (72.0%) and 170 of 204 in the high-dose group (83.3%) had a complete remission after one cycle of induction treatment. Of the 125 patients in the standard-dose group who did not have a response to treatment, 76 (60.8%) had one cycle and 49 (39.2%) had two cycles of induction treatment. Among 85 similar patients in the high-dose group, 56 (65.9%) had one cycle and 29 (34.1%) had two cycles of induction therapy.
ADVERSE EVENTS
There were no significant differences in the rates of grade 3 to 5 hematologic and nonhematologic toxic effects between the two study groups (Table 2). The rate of treatment-related grade 3 to 5 cardiac toxic effects was 7.2% in the standard-dose group and 7.9% in the high-dose group. A reduced left ventricular ejection fraction was reported in none of 318 patients in the standard-dose group and in 4 of 315 patients (1.3%) in the high-dose group (leading to death in 1 of the 4 patients) (P = 0.06). The death rates during induction therapy were 4.5% in the standard-dose group and 5.5% in the high-dose group (P= 0.60). Causes of death were infection (14 patients), pulmonary failure (6), cardiac failure (4), hemorrhage (3), hypotension (2), and ileus (1).
Table 2.
Adverse Events during the Induction Phase.*
| Adverse Event | Standard Dose (45 mg/m2/day) (N = 318) | High Dose (90 mg/m2/day) (N = 315) | ||||
|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | |
| no. of patients with event | ||||||
|
| ||||||
| Low hemoglobin | 212 | 34 | 0 | 200 | 41 | 0 |
|
| ||||||
| Low blood count†
| ||||||
| Leukocytes | 5 | 303 | 0 | 1 | 309 | 0 |
|
| ||||||
| Neutrophils | 11 | 296 | 0 | 7 | 286 | 0 |
|
| ||||||
| Platelets | 51 | 258 | 0 | 56 | 252 | 0 |
|
| ||||||
| Transfusion required
| ||||||
| Platelets | 175 | 17 | 0 | 181 | 20 | 0 |
|
| ||||||
| Packed red cells | 187 | 4 | 0 | 185 | 2 | 0 |
|
| ||||||
| Fatigue | 17 | 1 | 0 | 11 | 8 | 0 |
|
| ||||||
| Fever‡ | 15 | 4 | 0 | 17 | 9 | 0 |
|
| ||||||
| Rash or desquamation | 15 | 1 | 0 | 16 | 0 | 0 |
|
| ||||||
| Anorexia | 12 | 12 | 0 | 16 | 16 | 0 |
|
| ||||||
| Nausea | 19 | 1 | 0 | 17 | 0 | 0 |
|
| ||||||
| Hemorrhage with grade 3 or 4 low platelet count | 25 | 4 | 1 | 31 | 4 | 0 |
|
| ||||||
| Febrile neutropenia | 103 | 8 | 0 | 99 | 14 | 0 |
|
| ||||||
| Infection with grade 3 or 4 neutropenia | 127 | 21 | 1 | 123 | 31 | 8 |
|
| ||||||
| Dyspnea | 12 | 6 | 0 | 3 | 10 | 1 |
|
| ||||||
| Cardiac event | 15 | 5 | 3 | 14 | 10 | 1 |
Listed are all grade 3 to 5 adverse events that occurred in more than 5% of patients during the induction phase of the trial. The cardiac ejection fraction was measured before induction therapy with the use of multiple gated acquisition (MUGA) scanning or echocardiography if MUGA scanning was unavailable. Patients who had a complete remission were required to undergo repeat MUGA scanning before consolidation therapy and stem-cell transplantation.
Blood-count values are based on Common Terminology Criteria for Adverse Events (version 3.0), as follows: leukocytes: grade 3, less than 2000 to 1000 per cubic millimeter; grade 4, less than 1000 per cubic millimeter; grade 5, death; neutrophils: grade 3, less than 1000 to 500 per cubic millimeter; grade 4, less than 500 per cubic millimeter; grade 5, death; platelets: grade 3, less than 50,000 to 25,000 per cubic millimeter; grade 4, less than 25,000 per cubic millimeter; grade 5, death.
Values for fever are as follows: grade 3, more than 40.0°C (104.0°F) for 24 hours or less; grade 4, more than 40.0°C for more than 24 hours.
THERAPY AFTER REMISSION
Of the 657 patients who underwent randomization, 352 (53.6%) entered the consolidation phase: 163 of 330 patients (49.4%) from the standard-dose induction group and 189 of 327 (57.8%) from the high-dose group (P = 0.03). Of the 372 patients who were evaluated for a response and had a complete remission, 69 (18.5%) did not register for the consolidation phase because of a decision by either the patient or a physician to seek stem-cell transplantation from an unrelated donor or because of progressive disease, adverse events, or the patient’s decision not to continue participating in the study.
Overall, 177 of 352 patients (50.3%) in the consolidation phase underwent stem-cell transplantation. Of these patients, 17 of 163 patients (10.4%) in the standard-dose group and 19 of 189 patients (10.1%) in the high-dose group underwent allogeneic stem-cell transplantation; 62 patients in the standard-dose group (38.0%) and 79 patients in the high-dose group (41.8%) underwent autologous stem-cell transplantation. The reasons that patients did not undergo the intended transplantation procedure included withdrawal from the study or choice of alternative therapy (30.3%), progressive disease (25.5%), an inability to collect stem cells (11.7%), insurance refusal (3.4%), adverse events (11.0%), an unexpected decrease in the blood count (2.8%), other complicating diseases (4.8%), death (2.8%), and other reasons (7.7%). Since the administration of gemtuzumab ozogamicin in the consolidation phase before stem-cell transplantation conferred no improvement in disease-free or overall survival, the subgroup receiving this drug was closed in October 2007.
OVERALL SURVIVAL
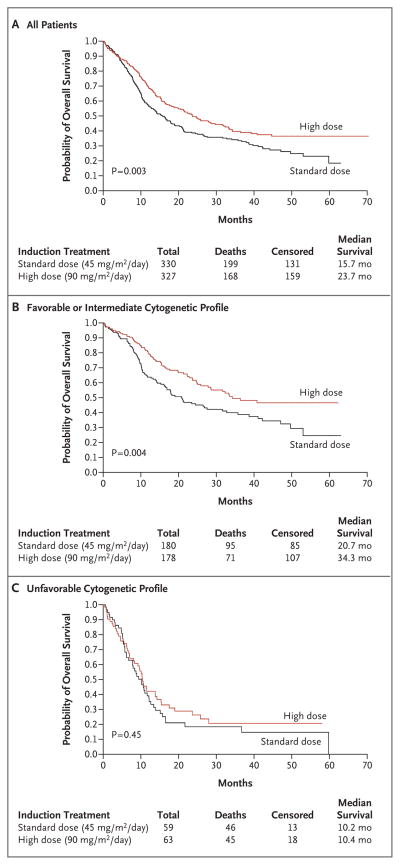
The hazard ratio for death in the high-dose group, as compared with the standard-dose group, was 0.74 (95% CI, 0.60 to 0.90) (Fig. 1). Multivariate proportional-hazards models were used to examine the effect of treatment on overall survival, with adjustment for sex, age, hemoglobin level, leukocyte counts, platelet counts, and cytogenetic profile; older age, an unfavorable cytogenetic profile, and high leukocyte counts are clinically significant risk factors for poor survival. With adjustment for these risk factors as continuous variables, the hazard ratio for death in the high-dose group was 0.74 (95% CI, 0.60 to 0.92; P = 0.005). When these factors were included as categorical variables, the hazard ratio for death in the high-dose group was 0.72 (95% CI, 0.58 to 0.89; P = 0.002). The median overall survival was 15.7 months in the standard-dose group and 23.7 months in the high-dose group (P = 0.003 by the log-rank test) (Fig. 2A).
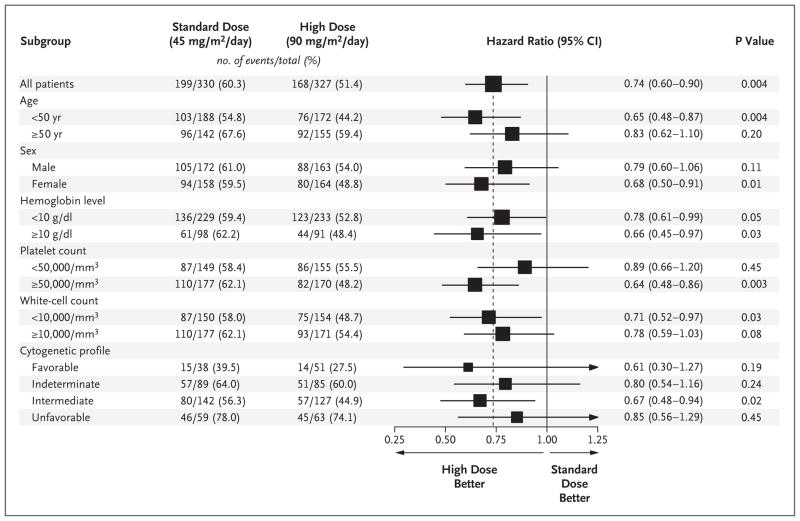
Figure 1. Hazard Ratios for Death, According to Subgroup.
All hazard ratios are for patients who received a 90-mg dose of daunorubicin (high-dose group), as compared with those who received a 45-mg dose (standard-dose group). A univariate Cox proportional-hazards model was used to estimate hazard ratios and the significance of the comparison for overall survival. The horizontal lines represent 95% confidence intervals for the ratios. The box size is proportional to the inverse of the standard error of the hazard-ratio estimates.
Figure 2. Kaplan–Meier Estimates of Overall Survival.
Data from the intention-to-treat analysis are shown for survival of all patients (Panel A), those with a favorable or an intermediate cytogenetic profile (Panel B), and those with an unfavorable cytogenetic profile (Panel C).
Effect of Age
The patient’s age at the time of induction therapy influenced the response rate. Of the 657 patients, 55% were under the age of 50 years. In the standard-dose group, patients under the age of 50 years had a complete-remission rate of 59.4% and a median survival of 19.0 months; in the high-dose group, patients in this age group had a complete-remission rate of 74.3% and a median survival of 34.3 months (hazard ratio for death in the high-dose group, 0.65; P = 0.004). Patients who were 50 years of age or older had no significant benefit from the high dose of daunorubicin; median survival was 12.2 months in the standard-dose group and 16.9 months in the high-dose group (hazard ratio, 0.83; P = 0.20). However, the interaction between treatment assignment and age category was not significant (P = 0.25).
Effect of Cytogenetic Profile
Patients were classified according to the risk associated with their cytogenetic profile, with 13.6% of patients deemed to be at favorable risk, 41.1% at intermediate risk, 26.6% at indeterminate risk, and 18.7% at unfavorable risk. Complete-remission rates for the favorable-risk, intermediate-risk, and unfavorable-risk groups were 81.3%, 58.7%, and 51.4%, respectively (P<0.001 for overall comparisons). Median survival was longest in the favorable-risk group but did not differ significantly between the treatment groups (hazard ratio for death in the high-dose group, 0.61; P = 0.18). At the time of the final analysis, median survival had not been reached among patients in the favorable-risk group who were treated with high-dose daunorubicin. The greatest difference between the two treatment groups was seen in the intermediate-risk subgroup, with a median survival of 17.8 months in the standard-dose group and 32.3 months in the high-dose group (hazard ratio for death in the high-dose group, 0.67; P= 0.02). When patients in the favorable-risk and intermediate-risk subgroups were pooled, the overall median survival was 20.7 months in the standard-therapy group and 34.3 months in the high-dose group (P = 0.004 by the log-rank test) (Fig. 2B). The worst outcome occurred in the subgroup with an unfavorable cytogenetic profile, in which the median survival was less than 11 months for both treatment groups (hazard ratio for the high-dose group, 0.85; P = 0.45) (Fig. 2C). However, the interaction between the treatment assignment and the cytogenetic profile was not significant (P=0.89).
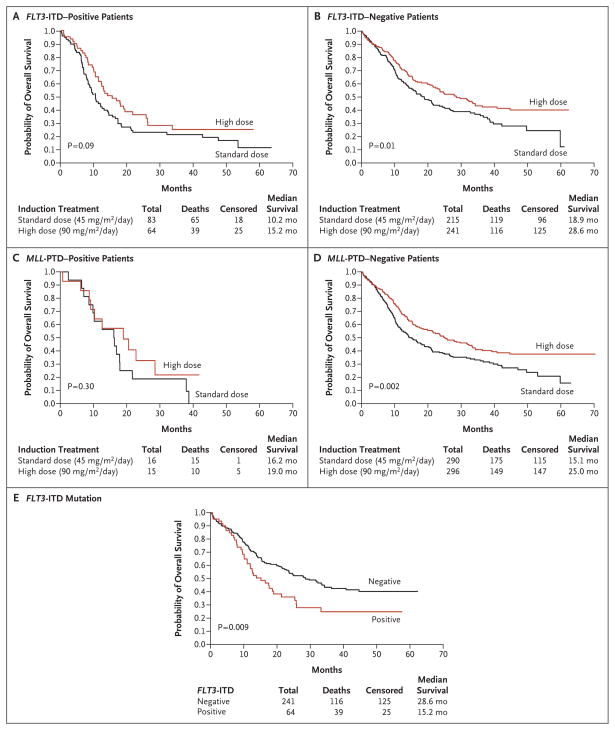
Effect of Mutations
High-dose daunorubicin did not provide a significant survival benefit in patients who had the FLT3-ITD or MLL-PTD mutation (Fig. 3A to 3D). Moreover, in the high-dose group, patients with the FLT3-ITD mutation had a lower median survival (15.2 months) than those without the FLT3-ITD mutation (28.6 months, P = 0.009 by the log-rank test) (Fig. 3E).
Figure 3. Kaplan–Meier Estimates of Overall Survival, According to Mutation Status.
Data are shown for the overall survival of patients with the FLT3-ITD mutation (Panel A), those without the FLT3-ITD mutation (Panel B), those with the MLL-PTD mutation (Panel C), and those without the MLL-PTD mutation (Panel D). Also shown are survival curves for patients who received high-dose daunorubicin (90 mg per square meter of body-surface area per day), according to the presence or absence of the FLT3-ITD genotype (Panel E). ITD denotes internal tandem duplication, and PTD partial tandem duplication.
DISCUSSION
It has been postulated that one of the most effective ways to achieve higher rates of complete remission and overall survival among patients with AML is to increase the induction dose of anthracyclines.9 Trials of various chemotherapeutic agents have shown an improved rate of complete remission with higher doses of daunorubicin, as compared with the standard 45-mg dose. In aggregate, however, these studies have not definitively shown improved rates of overall survival associated with such increases.5,6,21,22
In our study, we found significant improvements in rates of complete remission and overall survival among patients who received high-dose daunorubicin. The higher dose did not significantly increase the frequency of adverse events or affect the delivery of consolidation therapy.
The rates of complete remission that were attained in this trial were lower than those in several previous studies,2,19,23,24 even in the high-dose group. However, these rates are similar to results of previous ECOG studies, in which the administration of 45-mg to 60-mg doses of daunorubicin resulted in a median complete-remission rate of 62%.25 Our study design allowed the enrollment of patients who had received the diagnosis of an antecedent hematologic disorder within the previous 6 months and patients with multilineage dysplasia, which may have contributed to lower rates of complete remission. Early withdrawal by the patients or their physicians in order to seek alternative therapy could have contributed to the relatively low complete-remission rates.
Patients with favorable or intermediate cytogenetic risk profiles in the high-dose group fared well, with high rates of complete remission and receipt of appropriate consolidation therapy. At the time of the final analysis, median survival had not been reached among patients in the high-dose group who had a favorable cytogenetic profile. The largest survival difference between the two treatment groups was seen in the subgroup of patients who were at intermediate cytogenetic risk. We conclude from these results that in patients with favorable- or intermediate-risk disease, physicians can safely use anthracycline intensification for induction and consolidation (by either cytarabine intensification or stem-cell transplantation) and can anticipate excellent outcomes.
A high dose of daunorubicin provided no benefit in some of the patients in our study, including those who were over the age of 50 years and those with an unfavorable cytogenetic profile. The presence of MDR1 gene overexpression, which is more frequent in older patients with AML26 and causes efflux of daunorubicin from the cell, may have contributed to the poor responses. The high-dose strategy did not significantly improve overall survival among patients with either the FLT3-ITD mutation or the MLL-PTD mutation.
The Food and Drug Administration has approved the 45-mg dose of daunorubicin for patients with untreated AML, and this dose is widely used in the United States. Some cooperative groups have adopted higher doses of daunorubicin for induction therapy (mainly a 60-mg dose) in the absence of supporting data from a phase 3 trial. Whether standard-dose cytarabine with 90 mg of daunorubicin per square meter is better than cytarabine with a 60-mg dose of daunorubicin (or idarubicin or mitoxantrone at equivalent doses) remains to be studied in future trials.
In conclusion, intensifying induction therapy with a high daily dose of anthracycline plus intensive consolidation therapy resulted in a high complete-remission rate and prolonged overall survival in patients with AML. In younger patients, a dose of daunorubicin that exceeds the standard 45-mg dose for induction should be considered the new standard of care.
Acknowledgments
Supported in part by grants from the Public Health Service (CA23318, CA66636, CA21115, CA13650, CA15488, CA17145, and CA14548) and from the National Cancer Institute and the Department of Health and Human Services. Funding for laboratory correlative studies was provided by Wyeth Pharmaceuticals and Immunex through a grant to the ECOG.
Dr. Paietta reports receiving support from Wyeth and Immunex to perform the correlative studies used in this trial.
Footnotes
No other potential conflict of interest relevant to this article was reported.
References
- 1.Rai KR, Holland JF, Glidewell OJ, et al. Treatment of acute myelocytic leukemia: a study by Cancer and Leukemia Group B. Blood. 1981;58:1203–12. [PubMed] [Google Scholar]
- 2.A systematic collaborative overview of randomized trials comparing idarubicin with daunorubicin (or other anthracyclines) as induction therapy for acute myeloid leukaemia. Br J Haematol. 1998;103:100–9. [PubMed] [Google Scholar]
- 3.Preisler H, Davis RB, Kirshner J, et al. Comparison of three remission induction regimens and two postinduction strategies for the treatment of acute nonlymphocytic leukemia: a Cancer and Leukemia Group B study. Blood. 1987;69:1441–9. [PubMed] [Google Scholar]
- 4.Schiller G, Gajewski J, Nimer S, et al. A randomized study of intermediate versus conventional-dose cytarabine as intensive induction for acute myelogenous leukaemia. Br J Haematol. 1992;81:170–7. doi: 10.1111/j.1365-2141.1992.tb08203.x. [DOI] [PubMed] [Google Scholar]
- 5.Weick JK, Kopecky KJ, Appelbaum FR, et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: a Southwest Oncology Group study. Blood. 1996;88:2841–51. [PubMed] [Google Scholar]
- 6.Bishop JF, Matthews JP, Young GA, Bradstock K, Lowenthal RM. Intensified induction chemotherapy with high dose cytarabine and etoposide for acute myeloid leukemia: a review and updated results of the Australian Leukemia Study Group. Leuk Lymphoma. 1998;28:315–27. doi: 10.3109/10428199809092687. [DOI] [PubMed] [Google Scholar]
- 7.Appelbaum FR, Dahlberg S, Thomas ED, et al. Bone marrow transplantation or chemotherapy after remission induction for adults with acute nonlymphoblastic leukemia: a prospective comparison. Ann Intern Med. 1984;101:581–8. doi: 10.7326/0003-4819-101-5-581. [DOI] [PubMed] [Google Scholar]
- 8.Kolitz JE, George SL, Dodge RK, et al. Dose escalation studies of cytarabine, daunorubicin, and etoposide with and without multidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia younger than 60 years: final induction results of Cancer and Leukemia Group B Study 9621. J Clin Oncol. 2004;22:4290–301. doi: 10.1200/JCO.2004.11.106. [DOI] [PubMed] [Google Scholar]
- 9.Rowe JM, Tallman MS. Intensifying induction therapy in acute myeloid leukemia: has a new standard of care emerged? Blood. 1997;90:2121–6. [PubMed] [Google Scholar]
- 10.Rowe JM, Andersen JW, Mazza JJ, et al. A randomized placebo-controlled phase III study of granulocyte-macrophage colony-stimulating factor in adult patients (> 55 to 70 years of age) with acute myelogenous leukemia: a study of the Eastern Cooperative Oncology Group (E1490) Blood. 1995;86:457–62. [PubMed] [Google Scholar]
- 11.Büchner T, Urbanitz D, Hiddemann W, et al. Intensified induction and consolidation with or without maintenance chemotherapy for acute myeloid leukemia (AML): two multicenter studies of the German AML Cooperative Group. J Clin Oncol. 1985;3:1583–9. doi: 10.1200/JCO.1985.3.12.1583. [DOI] [PubMed] [Google Scholar]
- 12.Schiller G, Gajewski J, Territo M, et al. Long-term outcome of high-dose cytarabine-based consolidation chemotherapy for adults with acute myelogenous leukemia. Blood. 1992;80:2977–82. [PubMed] [Google Scholar]
- 13.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–83. [PubMed] [Google Scholar]
- 14.Vance GH, Kim H, Hicks GA, et al. Utility of interphase FISH to stratify patients into cytogenetic risk categories at diagnosis of AML in an Eastern Cooperative Oncology Group (ECOG) clinical trial (E1900) Leuk Res. 2007;31:605–9. doi: 10.1016/j.leukres.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 15.van Dongen JJ, Macintyre EA, Gabert JA, et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease: report of the BIOMED-1 Concerted Action: investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13:1901–28. doi: 10.1038/sj.leu.2401592. [DOI] [PubMed] [Google Scholar]
- 16.Schnittger S, Kinkelin U, Schoch C, et al. Screening for MLL tandem duplication in 387 unselected patients with AML identify a prognostically unfavorable subset of AML. Leukemia. 2000;14:796–804. doi: 10.1038/sj.leu.2401773. [DOI] [PubMed] [Google Scholar]
- 17.Noguera NI, Breccia M, Divona M, et al. Alterations of the FLT3 gene in acute promyelocytic leukemia: association with diagnostic characteristics and analysis of clinical outcome in patients treated with the Italian AIDA protocol. Leukemia. 2002;16:2185–9. doi: 10.1038/sj.leu.2402723. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe ES, Harris NL, Stein HJWV. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 21.Arlin Z, Case DC, Jr, Moore J, et al. Randomized multicenter trial of cytosine arabinoside with mitoxantrone or daunorubicin in previously untreated adult patients with acute nonlymphocytic leukemia (ANLL) Leukemia. 1990;4:177–83. [PubMed] [Google Scholar]
- 22.Wiernik PH, Banks PL, Case DC, Jr, et al. Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood. 1992;79:313–9. [PubMed] [Google Scholar]
- 23.Cassileth PA, Lee SJ, Litzow MR, et al. Intensified induction chemotherapy in adult acute myeloid leukemia followed by high-dose chemotherapy and autologous peripheral blood stem cell transplantation: an Eastern Cooperative Oncology Group trial (E4995) Leuk Lymphoma. 2005;46:55–61. doi: 10.1080/10428190412331283288. [DOI] [PubMed] [Google Scholar]
- 24.Yates J, Glidewell O, Wiernik P, et al. Cytosine arabinoside with daunorubicin or adriamycin for therapy of acute myelocytic leukemia: a CALGB study. Blood. 1982;60:454–62. [PubMed] [Google Scholar]
- 25.Bennett JM, Young ML, Andersen JW, et al. Long-term survival in acute myeloid leukemia: the Eastern Cooperative Oncology Group experience. Cancer. 1997;80(Suppl 11):2205–9. [PubMed] [Google Scholar]
- 26.Willman CL. Immunophenotyping and cytogenetics in older adults with acute myeloid leukemia: significance of expression of the multidrug resistance gene-1 (MDR1) Leukemia. 1996;10(Suppl 1):S33–S35. [PubMed] [Google Scholar]