Abstract
Hexavalent chromium (Cr(VI)) in ambient airborne particulate matter (PM) is a known pulmonary carcinogen and may have both soluble and insoluble forms. The sum of the two forms is defined as total Cr(VI). Currently, there were no methods suitable for large-scale monitoring of total Cr(VI) in ambient PM. This study developed a method to measure total Cr(VI) in ambient PM. This method includes PM collection using a Teflon filter, microwave extraction with 3% Na2CO3-2% NaOH at 95°C for 60 minutes, and Cr(VI) analysis by 1,5-diphenylcarbazide colorimetry at 540 nm. The recoveries of total Cr(VI) were 119.5 ± 10.4% and 106.3 ± 16.7% for the Cr(VI)-certified reference materials, SQC 012 and SRM 2700, respectively. Total Cr(VI) in the reference urban PM (NIST 1648a) was 26.0 ± 3.1 mg/kg (%CV = 11.9%) determined by this method. The method detection limit was 0.33 ng/m3. This method and the one previously developed to measure ambient Cr(VI), which is soluble in pH ~9.0 aqueous solution, were applied to measure Cr(VI) in ambient PM10 collected from three urban areas and one suburban area in New Jersey. The total Cr(VI) concentrations were 1.05–1.41 ng/m3 in the winter and 0.99–1.56 ng/m3 in the summer. The soluble Cr(VI) concentrations were 0.03–0.19 ng/m3 in the winter and 0.12–0.37 ng/m3 in the summer. The summer mean ratios of soluble to total Cr(VI) were 14.3–43.7%, significantly higher than 4.2–14.4% in the winter. The winter concentrations of soluble and total Cr(VI) in the suburban area were significantly lower than in the three urban areas. The results suggested that formation of Cr(VI) via atmospheric chemistry may contribute to the higher soluble Cr(VI) concentrations in the summer.
Keywords: Hexavalent chromium, Ambient particulate matter, Microwave extraction, IC-UV, ICPMS
INTRODUCTION
Hexavalent chromium (Cr(VI)) is a human pulmonary carcinogen (Barceloux, 1999; Li et al., 2002; Hazelwood et al., 2004; Pettine and Capri, 2005; Grabarczyk, 2008), which has been listed as a hazardous air pollutant (HAP) by the US EPA (2004). Cr(VI) in ambient air is emitted from anthropogenic sources including metallurgical and refractory industries, chrome plating, cement industry, fuel combustion, corrosion inhibition and pigments manufacture (Barceloux, 1999; Kimbrough et al., 1999; Yoo et al., 2002; Werner et al., 2007). The nature and solubility of Cr(VI) compounds emitted by these sources vary significantly. Soluble Cr(VI) is reported to be the dominant Cr(VI) species in mobile and fuel combustion emissions (Barceloux, 1999). Insoluble Cr(VI) compounds (e.g., PbCrO4, BaCrO4 and ZnCrO4) can be emitted into ambient air from anthropogenic sources that utilize chromate pigments and dyes (Barceloux, 1999; Kimbrough et al., 1999).
The toxicities of Cr(VI) compounds vary by solubility and bioavailability (Barceloux, 1999; Hazelwood et al., 2004). The differential toxicity calls for potentially different environmental regulation strategies. As a result, it is necessary to simultaneously measure Cr(VI) with different solubilities in ambient airborne particulate matter (PM) so that exposure and health risks associated with exposure to ambient Cr(VI) can be better estimated. It is challenging to accurately measure Cr(VI) in ambient PM because Cr(VI) is very reactive, and inter-conversion between Cr species can easily occur during sampling and sample processing. Recently, Meng et al. (2011) developed a method for measuring Cr(VI) in ambient PM. This method collects PM using a NaHCO3-pretreated mixed cellulose ester (MCE) filter and analyzes soluble Cr(VI) in pH ~9 solution using Ion Chromatography-Inductively Coupled Plasma - Mass Spectrometry (IC-ICPMS) (Meng et al., 2011). When using this method, Huang et al. (2013) and Torkmahalleh et al. (2012, 2013a) showed that conversion between Cr(VI) and Cr(III) in ambient PM could be affected by PM matrix, humidity, co-air pollutants such as sulfur dioxide (SO2) and ozone (O3), and reactive oxygen species (ROS) during sampling and analysis processes. Therefore, a Speciated Isotope Dilution Mass Spectrometry (SIDMS) approach was recommended to correct potential inter-conversion of Cr(III) and Cr(VI) in-situ (Huang et al., 2013). A sampling system that can control humidity and temperature of the sample deposit can reduce the potential for interconversion (Torkmahalleh et al., 2013b)
There are a few methods to measure total Cr(VI) in airborne PM, including ASTM Method D 5281-92, NIOSH 7605, and OSHA ID-215 (OSHA, 1998; Boiano et al., 2000). The latter two methods were developed for the measurement of airborne total Cr(VI) in occupational environments (OSHA, 1998; Boiano et al., 2000). The two methods employ a polyvinylchloride (PVC) filter as the collection medium and 1, 5-diphenylcarbazide colorimetry for Cr(VI) detection (OSHA, 1998; Boiano et al., 2000), but differ by extraction conditions. NIOSH 7605 employs hotplate digestion with 2% NaOH-3% Na2CO3 at 95°C (Boiano et al., 2000), while OSHA ID-215 employs hotplate digestion with 10% Na2CO3-2% NaHCO3 (mixed with phosphate buffer and magnesium sulfate) at 95°C (OSHA, 1998). The method detection limits (MDLs) of NIOSH 7605 and OSHA ID-215 are 50 and 3 ng/m3, respectively (OSHA, 1998; Boiano et al., 2000), which may not be sensitive enough for the measurement of trace-level Cr(VI) in ambient PM. In addition, Cr(VI) reduction on PVC filters was observed during sampling process, leading to measurement errors (R.T.I., 1988). The ASTM Method D 5281-92 employs collection of PM with NaHCO3 buffer (pH ~8) filled in three Greenberg-Smith impingers connected in series, hotplate extraction with 2% NaOH-3% Na2CO3, derivatization of Cr(VI) by 1,5-diphenylcarbazitde, and detection by colorimetry at 540 nm (Sheehan et al., 1992). The cumbersome impinger sampling technique limits its application in large-scale ambient air monitoring (Andersson et al., 1979; Beasley et al., 1980; Vairavamurthy et al., 1992). Thereby, a method that uses a filter as the collection medium is needed for the measurement of total Cr(VI) in ambient PM.
Regarding sample extraction, US EPA Method 3060a (USEPA, 1996), i.e., alkaline digestion for Cr(VI), is commonly used to measure total Cr(VI) in soil and sediment (USEPA, 1996). The method uses a hotplate for sample extraction with 2% NaOH-3% Na2CO3 solution at 95°C for 60 minutes. Compared to hotplate digestion, microwave extraction has the advantages of automation, easy control and generation of high pressure (Morales-Munoz et al., 2004), and can enhance the extraction efficiency.
The objectives of this study were to 1) develop a sensitive and accurate method to measure total Cr(VI) in ambient PM, and 2) determine soluble and insoluble Cr(VI) concentrations in ambient PM. To achieve the objectives, we developed and evaluated a method to measure total Cr(VI) in ambient PM. The total Cr(VI) concentration includes both soluble and insoluble Cr(VI). The method development involved selection of a proper filter sampling medium, optimization of extraction conditions, and evaluation of the method in the laboratory with the Cr(VI)-certified reference materials and in the field. The method developed for total Cr(VI), and the method developed by Meng et al. (2011) for the measurement of Cr(VI) in ambient PM, which represents Cr(VI) soluble in pH ~9.0 aqueous solution (defined as soluble Cr(VI) and thereafter), was used to determine the concentrations of soluble and total Cr(VI) in ambient PM10 collected from 4 different sites in New Jersey. The insoluble Cr(VI) concentrations were derived from the differences between total Cr(VI) and soluble Cr(VI) concentrations. The ratios of soluble to total Cr(VI) in the winter and summer seasons and the factors affecting the ratios were discussed.
MATERIALS AND METHODS
Materials, Reagents and Instruments
Teflon filters (PTFE membrane with PMP ring, 2.0 μm pores, 47 mm diameter, Pall Life Science, Ann Arbor, MI) were used for the PM collection. The insoluble Cr(VI) compounds used for testing methods included PbCrO4 (ACS grade, Fisher chemical, Fair Lawn, NJ) and BaCrO4 (ACS grade, Coulometrics Inc., Joliet, IL). Other reagents included NaOH (ACS grade, NF/FCC pellets, Fisher Scientific, Fair Lawn, NJ) and Na2CO3 (Anhydrous, HPLC Grade, Powder, Fisher Scientific, Fair Lawn, NJ). Since a Cr(VI) certified reference ambient PM was not available, SQC 012 and SRM 2700 with certified Cr(VI) concentrations were used to evaluate the method accuracy. SQC 012, from the R.T. Corporation (Laramie, WY), was manufactured by homogeneously mixing soluble/Cr(VI) with a common composition soil. The certified concentration of Cr(VI) in SQC 012 is 116.96 ± 17.66 mg/kg. The certified concentration of Cr(VI) in SRM 2700 is 5.51 ± 0.32 mg/kg (Nagourney et al., 2008). SRM 2700 is diluted (1:100) from SRM 2701, which was prepared from the contaminated soil of the chromite ore processing residue (COPR) from the waste sites in Liberty Park, New Jersey (Nagourney et al., 2008). Urban Particulate Matter (NIST 1648a), which is certified for total Cr but Cr(VI), was used for method precision evaluation. The certified total Cr concentration is 402 ± 13 mg/kg.
A Microwave System (MarsX, CEM Corporation, Matthews, NC) with HP 500 plus vessels was used for the microwave extraction. IC-UV was used for the Cr(VI) detection, and the analytical conditions of which were described elsewhere (E.R.G., 2007). Briefly, the IC consisted of a Dionex IonPac® NG1 Guard column, a Dionex RFIC™ IonPac® AS 7 Speciation column and a Dionex GC50 Gradient Pump (Thermo Fisher Scientific, Sunnyvale, CA); the UV-Vis consisted of a Dionex AS50 Autosampler, a Dionex PC10 Pneumatic Controller and a Dionex ICS Series VWD (Thermo Fisher Scientific, Sunnyvale, CA). The eluent solution was a mixture of ammonium sulfate (250 mM) (ACS, VWR) and ammonium hydroxide (100 mM) (Certified A.C.S. Plus, Fisher Scientific, Fair Lawn, NJ). The post-column derivatizing reagent (PCR) of the IC-UV analysis was prepared from 0.5 g of 1,5-deiphenylcarbazide (A.C.S. Reagent, J.T. Baker) diluted with 100 mL of methanol (HPLC grade, Fisher Scientific), 28 mL of 98% sulfuric acid (Baker Analyzed Reagent Grade, J.T. Baker, Philipsboro, NJ) and DI water up to a total of 1 liter in volume.
Development of a Method to Measure total Cr(VI) in Ambient PM
The method development consisted of three steps: selection of a filter as collection medium, optimization of microwave extraction conditions, and lab and field evaluation of the method. A Teflon filter was used as the collection medium given its inert surface, stable physical and chemical properties even under high temperature and pressure. Other filters, such as mixed cellulose ester (MCE) filter and cellulose filter, were previously found to form smaller polysaccharides during extraction with hot alkaline buffers, which can chelate Cr(VI) (Huang, 2012). Cr(VI) in this complex cannot react with 1,5-diphenylcarbazide and thus cannot be analyzed with 1,5-diphenylcarbazide colorimetry method (Huang, 2012).
The development work focused on sample extraction optimization. First, we selected alkaline buffers containing CO32− as the extraction solution for total Cr(VI) in ambient PM. This choice was made because heated alkaline buffer solutions with CO32− or OH− can dissolve insoluble Cr(VI) via the following reactions (Ashley et al., 2003)
| (1) |
| (2) |
Once the insoluble chromate is dissolved, it is unlikely to re-precipitate given it is in a dilute, basic solution containing relatively high concentrations of CO32− and OH−, which will be discussed in detail in the latter section.
Next, a microwave technique was selected to assist in sample extraction. The microwave technique instead of hotplate digestion was used given its advantages of automation, easy control, and generation of high pressure (Morales-Munoz et al., 2004). The extraction conditions tested in the study are described below.
Optimization of Extraction Conditions
The major factors that affect the extraction efficiency were examined: extraction temperature, time, and solution. Experimental conditions are summarized in Table 1. The insoluble Cr(VI) compounds, i.e., PbCrO4 and BaCrO4, were used to assess extraction efficiency under the designated conditions. Two alkaline solutions, 2% NaOH-3% Na2CO3 (pH = 11.75) and 2% NaHCO3-10% Na2CO3 (pH = 10), were tested in the study. Tests 1 to 3 were conducted to determine the optimal extraction temperature (i.e., 95°C and 60°C) and time (i.e., 40 min and 60 min). A comparison between Test 1 and Test 2 shows an effect of extraction temperature on extraction efficiency; whereas a comparison between Test 2 and Test 3 shows the effect of extraction time on extraction efficiency. Extraction with undiluted 3% Na2CO3-2% NaOH solution was reported to damage quartz filters and result in interference for Cr(VI) detection (Swietlik et al., 2011). Thus, Swietlik et al. (2011) recommended the use of diluted alkaline solution for extraction. However, dilution may reduce the extraction efficiency. As a result, Tests 4 and 5 were conducted to test the effects of dilution on extraction efficiency. All sample extracts were diluted with DI-H2O by 104 times prior to IC-UV analysis. The Cr(VI) recovery was calculated as the ratio of the measured Cr(VI) mass (corrected by the dilution factor) in the extract and the original Cr(VI) mass in the sample. The condition yielding the highest Cr(VI) recovery was selected as the optimal extraction condition.
Table 1.
Experimental design for the microwave extraction condition optimization.
| Test | Test compounds | Extraction buffer | Microwave extraction condition |
|---|---|---|---|
| 1 | ~20 mg PbCrO4 BaCrO4 |
20 mL | 300 w, 15 min ramping to 95°C and holding for 1 hr, pressure less than 200 psi |
| 2 | 3% Na2CO3-2% NaOH (pH = 11.75) | 300 w, 15 min ramping to 60°C and holding for 1 hr, pressure less than 200 psi | |
| 3 | 2% NaHCO3-10% Na2CO3 (pH = 10) | 300 w, 15 min ramping to 95°C and holding for 40 min, pressure less than 200 psi | |
| 4 | 20 mL 1:2.5 diluted 3% Na2CO3-2% NaOH |
300 w, 15 min ramping to 95°C and holding for 1 hr, pressure less than 200 psi | |
| 5 | 20 mL 1:5 diluted 3% Na2CO3-2% NaOH |
Stability and Inter-Conversion of Cr Species during Extraction
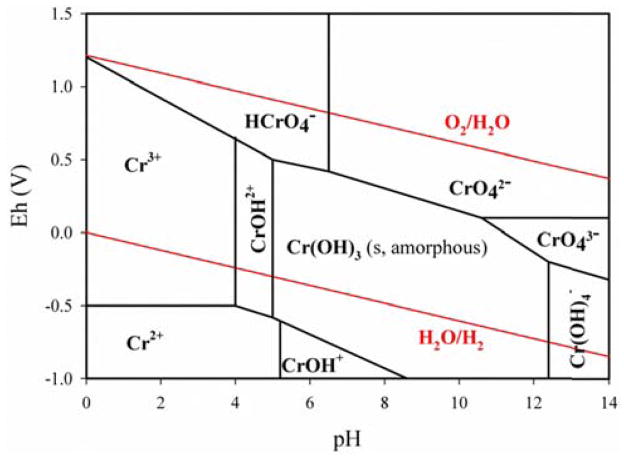
The stability of Cr(VI) and Cr(III) is a major concern for Cr(VI) measurements. The Eh and pH of solution are the two variables under 25°C and 95°C that determine the valence states of Cr species in solution and the concentrations of Cr-containing ions available for any reactions that equilibrate with solids containing Cr (shown in Fig. 1). The temperature will affect chemical reaction rates for reactions such as those outlined in Eqs. (1) and (2). Under the alkaline condition (pH ~12) used in the extraction process, Cr3+ that was in the PM would precipitate as Cr(OH)3 (Fig. 1). Cr(OH)3 has a solubility product of 6.3 × 10−31 M4 (25°C):
| (3) |
Fig. 1.
The Eh-pH diagram of chromium. Note: The data used to draw this figure is from Ball and Nordstrom (1998), Rai et al. (1987), Martell et al. (2004), Tsuchiya a and Umayahara (1963), von Meyenburg et al. (1973).
Soluble Cr(III), i.e., Cr3+, is the only form that can be converted to Cr(VI). The concentration of Cr3+ was 6.3 × 10−25 M under our extraction condition ([OH−] = ~10−2 M), and was negligible. Therefore, soluble Cr(III) in ambient PM, if any, would precipitate and would not convert to Cr(VI) under the our extraction conditions. Other insoluble Cr(III) compounds (e.g., Cr2O3 and Cr-Fe spinel) are only chemically reactive under extreme conditions (e.g., in molten alkali > 300°C) (Lide, 2004). Therefore, the conversion from Cr(III) to Cr(VI) could not be significant under the conditions that were used in our method.
Chromates that were originally in the ambient PM may have solubility products that vary over a wide range. The chromates with high solubility products (e.g., Na2CrO4 and K2CrO4) were defined as soluble Cr(VI) in our study. The valence of Cr(VI) in these substances is stable under alkaline conditions (pH > 10) (Pettine and Capri, 2005), which is also illustrated in the Eh-pH diagram of Cr (Fig. 1). The chromates with low solubility products (e.g., PbCrO4 and BaCrO4) were defined as insoluble Cr(VI) in our study. As shown in Eqs. (1) and (2), Cr(VI) in these compounds can dissolve in the heated alkaline solutions containing CO32− and OH− (US EPA 3060a). The formed precipitates have very low solubility products (e.g., 5.1 × 10−9 M2 (25°C) and 1.2 × 10−15 M3 (25°C) for BaCO3 and Pb(OH)2, respectively). Given the concentrations of CO32− (0.28 M) and OH− (0.5 M) in the alkaline solution, the concentrations of cations (e.g., Ba2+ and Pb2+) in the alkaline solution that equilibrated with the solids were negligible. The reversible reactions of Eqs. (1) and (2) were thus inhibited. As a result, insoluble Cr(VI) that was dissolved during the extraction would not precipitate again when the temperature returned to 25°C after the extraction.
In summary, the inter-conversion of Cr(VI) and Cr(III) will not occur during the extraction, and insoluble Cr(VI) that was dissolved will not precipitate again after the extraction. Given such, the associated measurement errors were expected to be insignificant.
Laboratory Evaluation
The optimized method was first evaluated in the laboratory. Tests were conducted to simulate extraction of total Cr(VI) from the particles collected on the filter. Two Cr(VI) certified reference materials, i.e., SQC 012 and SRM 2700, were used to assess the method accuracy. A reference urban PM, NIST 1648a, was used to assess the method precision.
Prior to the test, ~20 mg SQC 012 or SRM 2700, or ~5 mg NIST 1648a were loaded on a Teflon filter with a vacuum system. The masses selected for these tests were based upon the adequate amount to achieve homogeneity (SQC 012 soil material) and the amount similar to PM collected during 24-hour ambient air sampling. The vacuum system consisted of a TSP sampling head and a Leland Legacy SKC pump (SKC Inc., Eighty Four, PA). The loading procedures were as follows: 1) a pre-weighed Teflon filter was first placed in the TSP sampling head; 2) reference PM was spread on a piece of clean weighing paper; and 3) particles were collected on a Teflon filter by a pump sampling at a flow rate of 15 L/min. The filter samples were extracted using the optimized microwave extraction conditions, and the sample extracts were subsequently analyzed by IC-UV. The amount of total Cr(VI) (mg Cr(VI)/kg particle) in each reference material was calculated from the mass of Cr(VI) in the sample extract divided by the corresponding reference material mass. The recovery of total Cr(VI) was calculated as the ratio of the measured total Cr(VI) concentration in each material and the corresponding certified value. The %RSD of the measurements was used to assess precision.
Measurement of Soluble and Total Cr(VI) in Ambient PM
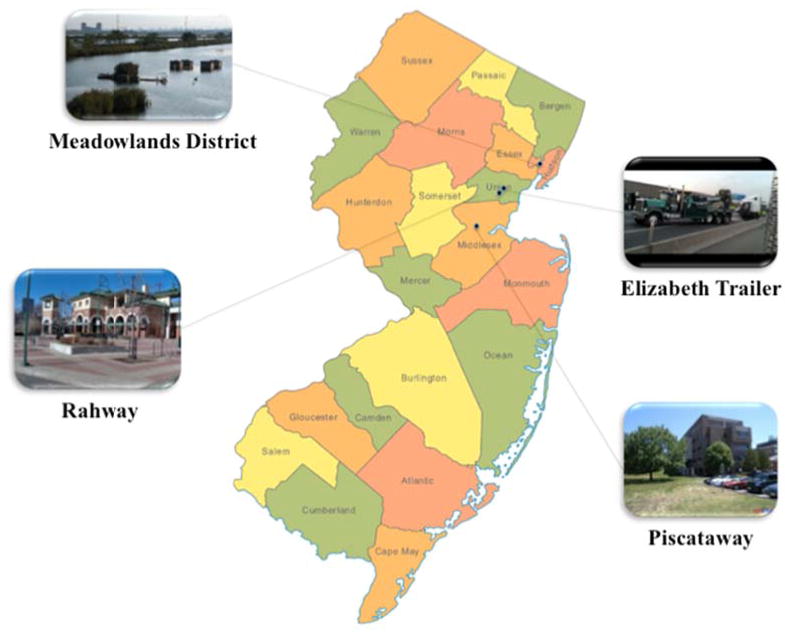
The soluble and total Cr(VI) in ambient PM10 were measured for the samples collected from four sites in New Jersey (see Fig. 2) with different Cr emission sources, i.e., three urban locations (Meadowlands District, Elizabeth Trailer (NJ Turnpike Exist 13) and Rahway) and a suburban area (Piscataway). Specific site information is presented below.
Fig. 2.
Sampling locations in this study.
Meadowlands District
The Meadowlands District is previously part of natural wetlands. It consists of ~34 km2 of undeveloped space and developed area. The developed area includes large landfills, the municipality of Lyndhurst and a portion of Jersey City. The sampler was located on the rooftop of Meadowlands Environmental Research Institute (MERI) building, ~9 m above the ground. The sampling site is sandwiched by NJ Turnpike (I-95) on the east (~3 km) and by NJ-21 on the west (~3.7 km). PSEG Fossil LLC Hudson Generation Station, the largest stationary industrial emission source of ambient Cr in New Jersey in 2008 (NJDEP, 2009), is ~3.7 km southeast. There are more than 100 chromite ore processing residue (COPR) waste sites nearby (Burke et al., 1991). The COPR wastes contain high concentrations of soluble and insoluble Cr(VI) compounds (Burke et al., 1991).
Elizabeth Trailer (NJ Turnpike Exit 13)
Elizabeth Trailer is a New Jersey Department of Environmental Protection (NJDEP) air quality monitoring station. The sampling site is adjacent to NJ Turnpike (I-95) and ~1.6 km east of NJ-1. A PSEG power plant is located ~300 m to the south.
Rahway
Rahway is a typical urban area in the urbanized northeast corridor of New Jersey. Industrial emissions are one of the major sources of ambient Cr in Rahway. The NJDEP has identified three major stationary industrial emission sources of ambient Cr here. Included are API Foils Inc., Covanta Union and Merck & Co Inc. (NJDEP, 2009), which emitted 440 tons, 10 tons, 2.2 tons of Cr compounds into the air in 2008, respectively (NJDEP, 2009). Local mobile emission is another major source of ambient Cr. The sampling site is sandwiched by NJ-1/9 (~400 m) and NJ-27 (~2.3 km) on the west and by NJ Turnpike (I-95) on the east side (~1.9 km).
Piscataway
Piscataway is a suburban area in New Jersey. The sampler was located on the balcony (~5 m above the ground) of Environmental and Occupational Health Science Institute (EOHSI) Building, Rutgers University. There are no identified stationary industrial emission sources of Cr. It is surrounded by local roads and interstates, such as NJ-18, I-287 on the north and NJ-27, NJ-1 and NJ Turnpike on the east.
Method Description
One Partisol Speciation Sampler (Model 2300, Thermo Scientific) was deployed at each site to collect ambient PM10. The sampling flow rate was 16.7 L/min, with variation < 5% during 24-h sampling period (from 12:00 am to 11:59 pm). Two types of filter were used for PM collection, i.e. Teflon filter and NaHCO3-pretreated mixed cellulose ester (MCE) filter. The former was used for measuring total Cr(VI) and the latter was for measuring soluble Cr(VI). The method developed in this study was used for total Cr(VI) measurement. The method that was described in Meng et al. (2011) was used for soluble Cr(VI) measurement. Briefly, a NaHCO3-pretreated MCE filter was used for PM collection, the PM sample was ultrasonically extracted with pH = 4HNO3 solution at 60°C for 40 min, and Cr(VI) in the extract was analyzed by IC-ICPMS (Meng et al. 2011).
Speciated Isotope Dilution Mass Spectrometry (SIDMS) approach was used to monitor inter-conversion of soluble Cr species during sampling and extraction procedures. Prior to field sampling, each MCE filter was spiked with 10 μL each of 1 ng/μL 53Cr(VI) and 1 ng/μL 50Cr(III) to monitor potential inter-conversion of Cr species during sampling and extraction procedures. It is necessary to note that a set of experiments we conducted under weak acid extraction conditions showed that Cr(III) in ambient PM collected in this study was insoluble and inert to conversion (Huang 2012). As a result, conversion of soluble Cr(VI) in ambient PM during sampling is the major concern, which was corrected with SIDMS approach in our study. A Teflon filter is hydrophobic and cannot be spiked with isotope species. Thus, correction of Cr inter-conversion with SIDMS approach could not be applied to the total Cr(VI) measurement. However, significant errors in total Cr(VI) measurement results were not expected. This is because soluble Cr(VI) usually accounted for a small portion (< 30%) of total Cr(VI) in our study, which was demonstrated by the results in the latter section. A ~20% reduction in soluble Cr(VI)was observed in our previous study (Huang et al., 2013). This low fraction of soluble Cr(VI) would maximally lead to ~6% underestimation of the total Cr(VI) concentration. Based on the above tests, interconversion of Cr species during sampling and extraction would not significantly affect the total Cr(VI)measurement results. All samples were shipped to our lab, stored in freezer (−10°C), and analyzed within one week.
The mass of total Cr(VI) in the sample extract was determined by external standards; whereas the mass of soluble Cr(VI) was determined by the SIDMS approach. The Chromium Speciated Analysis Calculation Software (Applied Isotope Technologies Inc.), an Excel add-in, was utilized to correct Cr(VI)-Cr(III) inter-conversion. The sample information was entered into the program, including sample type, sample mass, isotope masses, purity of isotope spikes, isotopic ratios in the isotope spikes and in the sample extract. The program calculates the Cr speciation results after deconvolution. The concentrations of soluble and total Cr(VI) in ambient air were determined as the ratio of Cr(VI) mass in the sample extract and the sampling volume (24 m3). The ratio of soluble and total Cr(VI) was subsequently calculated. The MDL of the total Cr(VI) measurement method was determined as 3 times the standard deviation (SD) of the field blank values.
Descriptive statistical analysis was performed on the concentrations of soluble Cr(VI), the concentrations of total Cr(VI) and the ratios of soluble to total Cr(VI). Unbalanced analysis of variance (ANOVA) with Tukey-Kramer multiple comparison test was performed to examine spatial and seasonal variations of soluble and total Cr(VI) concentrations and the ratio of soluble to total Cr(VI) in ambient PM.
RESULTS AND DISCUSSION
Optimal Extraction Conditions
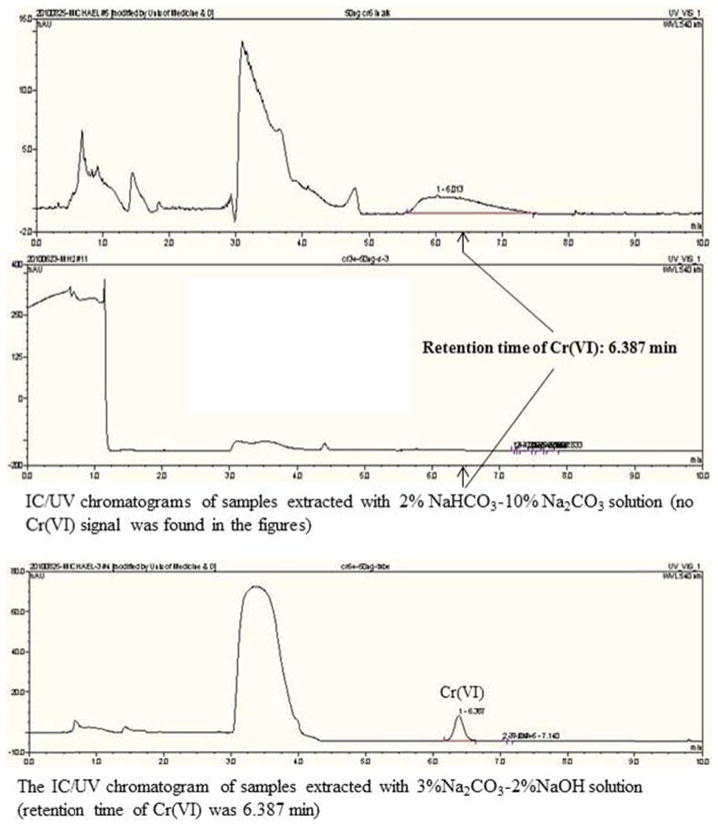
Optimal extraction conditions, i.e., extraction solution, temperature and time, were determined based on the test results. As shown in Fig. 3, the 2% NaHCO3-10% Na2CO3 (pH = 10) solution produced an interference in the IC-UV analysis. Thus, the 3% Na2CO3-2% NaOH (pH = 11.75) solution was selected as the extraction solution. Table 2 presents the Cr(VI) extraction efficiencies obtained from different microwave extraction conditions. The Cr(VI) recoveries were 95% (PbCrO4) and 78% (BaCrO4) when extracted at 95°C for 60 min, noticeably higher than 16.5%–63.6% recoveries when extraction at 60°C for 60 min. The Cr(VI) recoveries when extracted at 95°C for 40 min were lower than at 95°C for 60 min (shown in Table 2). With regards to dilution factor, both 1:2.5 and 1:5 diluted 2% NaHCO3-10% Na2CO3 yielded noticeably lower Cr(VI) recoveries than the undiluted buffer (shown in Table 2), particularly for BaCrO4. These results suggested that 1) a higher temperature favored extraction of Cr(VI) from insoluble chromates and the sufficient Cr(VI) recovery could be obtained at 95°C and higher; 2) extended extraction time favored the extraction of Cr(VI) from insoluble chromates; and 3) dilution indeed reduced the extraction efficiency of alkaline buffer. Based on the above test results, the optimal extraction conditions were determined as the following: microwave extraction with 3% Na2CO3-2% NaOH (pH = 11.75) solution at 95°C for 60 min. In addition, the Cr(VI) recovery for BaCrO4 was lower than that for PbCrO4 in the tests. This result is due to BaCrO4 being more difficult to dissolve than PbCrO4 (James et al., 1995).
Fig. 3.
IC/UV chromatograms of samples extracted with 2% NaHCO3-10% Na2CO3 and 3% Na2CO3-2% NaOH solutions.
Table 2.
Extraction efficiency under designed extraction conditions.
| Test | Extraction condition | Extraction buffer | %recovery of Cr(VI) (Mean ± SD, N = 3)
|
|
|---|---|---|---|---|
| PbCrO4 | BaCrO4 | |||
| 1 | 15 min Ramping to 95°C and holding for 1 hr, pressure less than 220 psi | 3% Na2CO3-2% NaOH | 95.0 ± 5.5% | 78.0 ± 3.1% |
| 2 | 15 min Ramping to 60°C and holding for 1 hr, pressure less than 200 psi | 43.7 ± 8.3% | 16.5 ± 5.4% | |
| 3 | 15 min Ramping to 95°C and holding for 40 min, pressure less than 200 psi | 81.3 ± 1.8% | 42.3 ± 0.5% | |
|
| ||||
| 4 | 15 min Ramping to 95°C and holding for 1 hr, pressure less than 220 psi | 1:2.5 diluted 3% Na2CO3-2% NaOH |
62.8 ± 6.0% | 57.2 ± 8.8% |
| 5 | 1:5 diluted 3% Na2CO3-2% NaOH |
55.1 ± 7.0% | 39.5 ± 6.2% | |
Lab Evaluation Results
The laboratory evaluation results with the three reference materials (i.e., SQC 012, SRM 2700 and NIST 1648a) are presented in Table 3. The Cr(VI) recoveries were close to 100% for both SQC 012 and SRM 2700. As stated previously, Cr(VI) in SQC 012 is in the form of soluble compounds, and Cr(VI) in SRM 2700 is in the form of both soluble and insoluble compounds (Burke et al., 1991; Kimbrough et al., 1999). The high Cr(VI) recoveries (~100%) for both reference materials, obtained from the total Cr(VI) measurement method, suggested that this method could effectively recover total Cr(VI) in particles collected on Teflon filter. The concentration of total Cr(VI) in NIST 1648a determined by this method is presented in Table 3. Good precision was obtained, with %CV (N = 4) of 11.9%. The results demonstrated that the optimized method can effectively recover total Cr(VI) in the particles collected on the filter. The soluble Cr(VI) in NIST 1648a, determined by the method in Meng et al. (2011), was 1.75 ± 0.46 mg/kg (Mean ± SD, N = 6). Thus, about 6.7% of total Cr(VI) in NIST 1648a is in the form of soluble chromates, whereas total Cr(VI) accounts for ~6.5% of total Cr in NIST 1648a.
Table 3.
The concentrations and recoveries of Cr(VI) in three reference materials obtained from the developed method.
| Particle type | Number of Samples | Cr(VI) (mg/kg)
|
Cr(VI) Recovery (%)* | Relative error (%) | ||
|---|---|---|---|---|---|---|
| Mean | SD | RSD (%) | ||||
| NIST1648a | 4 | 26.0 | 3.1 | 11.9% | N/A | N/A |
| SQC012 | 4 | 139.8 | 14.6 | 10.4 | 119.5 | 19.5 |
| SRM2700 | 4 | 5.86 | 0.98 | 16.7 | 106.3 | 6.3 |
recovery was calculated as extracted Cr(VI)/Cr (VI) certified value. The certified Cr(VI) concentration is 116.96 ± 17.66 (acceptable range 64.0–170.0 ng/mg) and 5.51 ± 0.32 ng/mg for SQC012.
Soluble and Total Cr(VI) in Ambient PM in New Jersey
Measurements of soluble and total Cr(VI) in ambient PM10 for the samples collected from the four sites in New Jersey, i.e., Meadowlands District, Elizabeth Trailer (NJ Turnpike Exit 13), Rahway, and Piscataway, are presented below.
Method Detection Limit
Eight field blanks were collected during the study. The MDL of the total Cr(VI) measurement method based on the field blanks was 0.33 ng/m3. This value is significantly lower than that of NIOSH 7605 method (50 ng/m3), and OSHA ID-215 method (3 ng/m3), and comparable to that of the impinger-based ASTM D 5281-92 method (0.2–1.0 ng/m3). Given the filter-based sampling in this method, it is adequate for the large-scale air quality monitoring.
Concentrations of Total and Soluble Cr(VI) in Ambient PM
The ambient concentrations of total Cr(VI) for the samples collected from the four sites are shown in Table 4. The mean concentrations of total Cr(VI) in ambient PM10 were in the range of 0.94–1.41 ng/m3 in the winter and 0.86–1.56 ng/m3 in the summer. In the summer, there were no significant differences (p > 0.50, Tukey-Kramer test) in total Cr(VI) concentration among the four sites. In the winter, there were no significant differences (p > 0.50, Tukey-Kramer test) in total Cr(VI) concentration among Meadowlands, NJ Turnpike Exit 13 and Rahway; whereas the winter concentrations of total Cr(VI) in Piscataway (0.86 ± 0.50 ng/m3, N = 12), the suburban area in this study, were significantly lower (p < 0.01, Tukey-Kramer test) than the other 3 sites. In addition, the concentrations of total Cr(VI) in the summer for each site were not significantly different (p > 0.50, Tukey-Kramer test) from those in the winter. The causes for the seasonal and spatial variability of total Cr(VI) concentration are discussed below.
Table 4.
The concentrations of soluble and total Cr(VI) in ambient air, New Jersey and the ratio of soluble to total Cr(VI).
| Location | Soluble Cr(VI)
|
Total Cr(VI)
|
Ratio of soluble to total Cr(VI)
|
|||
|---|---|---|---|---|---|---|
| summer | winter | summer | winter | summer | winter | |
| Meadowlands | 0.30 ± 0.16 | 0.11 ± 0.04 | 1.25 ± 0.58 | 1.32 ± 0.56 | 27.7% ± 18.0% | 10.0% ± 7.4% |
| Elizabeth Trailer | 0.21 ± 0.13 | 0.19 ± 0.09 | 1.56 ± 0.48 | 1.41 ± 0.56 | 14.3% ± 9.0% | 14.4% ± 8.5% |
| Rahway | 0.33 ± 0.36 | 0.14 ± 0.07 | 0.99 ± 0.76 | 1.05 ± 0.36 | 42.7% ± 24.5% | 13.6% ± 5.1% |
| Piscataway | 0.20 ± 0.18 | 0.03 ± 0.01 | 0.86 ± 0.50 | 0.94 ± 0.49 | 24.0% ± 22.3% | 4.2% ± 4.5% |
There are only limited literature data available on total Cr(VI) in ambient PM. Œwietlik et al. (2011) reported the mean concentration of total Cr(VI) as 6.0 ng/m3 in the total suspended ambient PM (TSP) in Radom, a medium-size city in Poland, where local emission sources of chromium were vehicle traffic, metal working plants, and coal-fired municipal heating plants. Talebi (2003) reported 5.4–8.2 ng/m3 of total Cr(VI) in the TSP in Isfahan, a city in Iran next to a large desert, where two large iron and steel factories were located nearby. Due to difference in particle size, it is difficult to make direct comparison between our values and the results from the above two studies.
Total Cr in ambient PM10 was not measured in this study, limited by sampling devices. The data on total Cr in ambient PM10 is scarce for our study area. Only one study analyzed total Cr in ambient PM2.5 collected in New Brunswick (next to Piscataway) and reported a mean value of 1.4 ng/m3 (Gao et al., 2002). Literature data on the ratio of total Cr(VI) to total Cr is also very limited. Talebi (2003) found that the ratio of total Cr(VI) versus total Cr was ~25% in the TSP collected in Isfahan. In Œwietlik et al. (2011), total Cr(VI) accounted for < 11%, 50% and 30–40% of total Cr in TSP from an industrial zone, the city center and transit roads, respectively. The Cr(VI) fraction ranged from non-detected to < 20% of total Cr in PM2.5 collected from Davis, Sacramento and Placerville, California (Werner et al., 2007). Similar to NIST 1648a, Cr(VI) accounted for smaller portion of total Cr in the above studies.
The mean concentrations of soluble Cr(VI) in ambient PM in the four locations, as shown in Table 4, were in the range of 0.03–0.19 ng/m3 in the winter and 0.20–0.33 ng/m3 in the summer. In the winter, the concentration of soluble Cr(VI) in Piscataway (0.03 ± 0.01 ng/m3, Mean ± SD, N = 5), the suburban area in this study, was significantly lower (p < 0.01, Tukey-Kramer test) than the other three sites; whereas there were no significant differences (p > 0.50, Tukey-Kramer test) in the winter concentration among Meadowlands, NJ Turnpike Exit 13 and Rahway. In the summer, there were no significant differences (p > 0.50. Tukey-Kramer test) in soluble Cr(VI) concentrations among the four sites. In addition, the concentrations of soluble Cr(VI) in the summer were significantly higher (p < 0.01, Tukey-Kramer test) than in the winter. The causes for the seasonal and spatial variability of soluble Cr(VI) concentration are discussed below.
The concentrations of soluble Cr(VI) in ambient PM in New Jersey were similar to the reported concentrations of ambient soluble Cr(VI) in the literature. For example, Khlystov and Ma (2006) reported ~0.5 ng/m3 of ambient soluble Cr(VI) (extracted with DI-water) in the summer in Wilmington, DE, U.S. Sheehan et al. (1992) reported 0.08–0.61 ng/m3 of ambient soluble Cr(VI) (extracted with pH ~8.0 NaHCO3 solution) in the area adjacent to a chromium contaminated site in the U.S. In other countries, Li et al. (2002) reported ~0.1 ng/m3 of ambient soluble Cr(VI) (extracted with 0.025 M KOH solution) in the residential areas and 0.2–1.3 ng/m3 in the industrial areas of Sydney Basin in Australia. Krystek and Ritsema (2007) reported 0.5–1.5 ng/m3 of ambient soluble Cr(VI) (extracted with 0.05 mM NaHCO3-Na2CO3 buffer) in the reference living area and 0.5–20.3 ng/m3 in the living area next to an identified foundry emission source. They also found that soluble Cr(VI) accounted for 2%, 0.5–6.5% and 11–21% of total Cr in emission from foundry, emission in living area next to foundry and living area in reference locations, respectively (Krystek and Ritsema, 2007). The values reported in the literature, however, were not corrected for Cr inter-conversion. Given that the pH of the extraction solution varied by study, the inter-conversion of Cr species would likely vary as well.
Soluble and Insoluble Cr(VI) in Ambient PM
The ratios of soluble to total Cr(VI) in ambient PM are presented in Table 4. The ambient total Cr(VI) concentrations were significantly higher (p < 0.01, Wilcoxon signed rank test) than the ambient soluble Cr(VI) concentrations in both summer and winter. The comparison results suggested that both soluble and insoluble Cr(VI) simultaneously presented in ambient PM at the four sites. In the winter, the mean ratios in the four locations were in the range of 4.2%–14.4%. The winter ratios in Piscataway (10.0% ± 7.4%, Mean ± SD, N = 5), the suburban area in this study, were significantly lower (p < 0.05, Tukey-Kramer test) than the other three sites; whereas there were no significant differences (p > 0.50, Tukey-Kramer test) among Meadowlands, NJ Turnpike Exit 13 and Rahway values. In the summer, the mean ratios in the four locations ranged between 14.3% and 42.7% (Table 4), and there were no significant differences (p > 0.50, Tukey-Kramer test) among the four sites.
The results indicated that soluble Cr(VI) only accounted for small portions of total Cr(VI) in ambient PM when compared to insoluble Cr(VI). Although NJDEP inventory suggested different Cr emission sources present in the four locations, no significant differences in ambient concentrations of soluble Cr(VI) or total Cr(VI) or the ratio of soluble to total Cr(VI) were found among the four sites except in the winter in Piscataway. These observations suggested the same type of sources or the same dominant source(s) for the soluble or insoluble Cr(VI) in the three locations. The limited literature information has suggested some emission sources for ambient Cr(VI). Included are a) diesel exhaust for soluble Cr(VI); b) industries or human facilities related with pigments and paints for insoluble Cr(VI) (Barceloux, 1999); and c) co-precipitation of soluble CrO42− and metal ions such as Pb2+ in atmosphere for insoluble Cr(VI) (Seigneur and Constantinou, 1995). We suspect that ambient soluble Cr(VI) in the four locations may be primarily originated from vehicle emissions and insoluble Cr(VI) may come from precipitation of soluble Cr(VI) from local emission and metal ions in the atmosphere or the PM transported into the region.
The ratios of soluble to total Cr(VI) obtained in the summer were significantly higher (p < 0.01, Tukey-Kramer test) than in the winter. Our study showed that soluble Cr(VI) in ambient PM had higher concentrations and accounted for greater portions of the total Cr(VI) in the summer. This difference may be due to more intense photoreaction activity in the summer, during which higher concentrations of ROS are expected in ambient air. ROS has strong oxidative capacity and thus may lead to higher concentrations of Cr(VI) via oxidizing Cr(III) to Cr(VI) (Nico et al., 2009; Huang et al., 2013, Torkmahallen et al., 2013). As a result, the concentrations of soluble Cr(VI) and the ratios of soluble to total Cr(VI) in the summer were higher than in the winter.
CONCLUSIONS
In this study, we developed a method to measure total Cr(VI) in ambient PM. This method employed a 47-mm Teflon filter as the collection medium, a microwave extraction with 3% Na2CO3-2% NaOH (pH = 11.75) at 95°C for 60 min as the extraction condition, and 1,5-deiphenylcarbazide colorimetry at 540 nm as the Cr(VI) detection condition. The MDL is 0.33 ng/m3. The lab evaluation results suggested that this method can achieve ~~100% recovery of soluble and insoluble Cr(VI) from the Cr(VI)-certified reference particle materials collected on a Teflon filter. The total Cr(VI) measurement method developed in this study and our previous method to measure soluble Cr(VI) in ambient PM (Meng et al., 2011) were used to determine Cr(VI) with different solubility in the Reference Urban Particulate Matter (NIST 1648a) and freshly collected ambient PM. It was found that soluble and insoluble Cr(VI) indeed co-existed in NIST 1648a and ambient PM. Soluble Cr(VI) accounted for 6.7% of total Cr(VI) and total Cr(VI) accounted for 6.5% of total Cr in NIST 1648a; whereas ambient soluble Cr(VI) accounted for 14.3%–42.7% of ambient total Cr(VI) in the summer and 4.2%–14.4% in the winter. Given their potential differences in biological effects, separate quantification and different environmental regulation should be considered for ambient soluble and insoluble Cr(VI) in the future.
Acknowledgments
This study was supported by the USEPA (Agreement #: XA97264906) EPA agreement # with NJDEP XA97264906 and NIEHS Center for Environmental Exposures and Disease (CEED) Grant (P3ES005022). The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the funding agencies.
References
- Andersson G, Andersson K, Nilsson K, Levin JO. Chemosorption of Formaldehyde on Amberlite XAD-2 Coated with 2,4-dinitrophenylhydrazine. Chemosphere. 1979;8:823–827. [Google Scholar]
- Ashley K, Howe AM, Demange M, Nygren O. Sampling and Analysis Considerations for the Determination of Hexavalent Chromium in Workplace Air. J Environ Monit. 2003;5:707–716. doi: 10.1039/b306105c. [DOI] [PubMed] [Google Scholar]
- Ball JW, Nordstrom DK. Critical Evaluation and Selection of Standard State Thermodynamic Properties for Chromium Metal and Its Aqueous Ions, Hydrolysis Species, Oxides, and Hydroxides. J Chem Eng Data. 1998;43:895–918. [Google Scholar]
- Barceloux DG. Chromium. J Toxicol Clin Toxicol. 1999;37:173–194. doi: 10.1081/clt-100102418. [DOI] [PubMed] [Google Scholar]
- Beasley RK, Hoffmann CE, Rueppel ML, Worley JW. Sampling of Formaldehyde in Air with Coated Solid Sorbent and Determination by High Performance Liquid Chromatography. Anal Chem. 1980;52:1110–1114. [Google Scholar]
- Boiano JM, Wallace ME, Sieber WK, Groff JH, Wang J, Ashley K. Comparison of Three Sampling and Analytical methods for the Determination of Airborne Hexavalent Chromium. J Environ Monit. 2000;2:329–333. doi: 10.1039/b002456m. [DOI] [PubMed] [Google Scholar]
- Burke T, Fagliano J, Goldoft M, Hazen RE, Iglewicz R, McKee T. Chromite Ore Processing Residue in Hudson County, New Jersey. Environ Health Perspect. 1991;92:131–137. doi: 10.1289/ehp.9192131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastern Research Group. Collection and Analysis of Hexavalent Chromium in Ambient Air. Eastern Research Group, Inc; Morrisville, California: 2007. [Google Scholar]
- Gao Y, Nelson ED, Field MP, Ding Q, Li H, et al. Characterization of Atmospheric Trace Elements on PM2.5 Particulate Matter over the New York-New Jersey Harbor Estuary. Atmos Environ. 2002;36:1077–1086. [Google Scholar]
- Grabarczyk M. Simultaneous Extraction and Catalytic Adsorptive Stripping Voltammetric Measurement of Cr(VI) in Solid Samples. J Hazard Mater. 2008;158:491–498. doi: 10.1016/j.jhazmat.2008.01.097. [DOI] [PubMed] [Google Scholar]
- Hazelwood KJ, Drake PL, Ashley K, Marcy D. Field Method for the Determination of Insoluble or Total Hexavalent Chromium in Workplace Air. J Occup Environ Hyg. 2004;1:613–619. doi: 10.1080/15459620490493810. [DOI] [PubMed] [Google Scholar]
- Huang L. Thesis for Doctor of Philosphy. University of Medicine and Dentistry of New Jersey-Rutgers, the State University of New Jersey; Piscataway, New Jersey: 2012. Development and Optimization of Ambient Hexavalent Chromium Measurement Methods. [Google Scholar]
- Huang L, Fan Z, Yu CH, Hopke PK, Lioy PJ, Buckley BT, Lin L, Ma Y. Interconversion of Chromium Species during Air Sampling: Effects of O3, NO2, SO2, Particle Matrices, Temperature, and Humidity. Environ Sci Technol. 2013;47:4408–4415. doi: 10.1021/es3046247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James BR, Petura JC, Vitale RJ, Mussoline GR. Hexavalent Chromium Extraction from Soils - A Comparison of 5 Methods. Environ Sci Technol. 1995;29:2377–2381. doi: 10.1021/es00009a033. [DOI] [PubMed] [Google Scholar]
- Khlystov A, Ma Y. An On-line Instrument for Mobile Measurements of the Spatial Variability of Hexavalent and Trivalent Chromium in Urban Air. Atmos Environ. 2006;40:8088–8093. [Google Scholar]
- Kimbrough DE, Cohen Y, Winer AM, Creelman L, Mabuni C. A Critical Assessment of Chromium in the Environment. Crit Rev Env Sci Technol. 1999;29:1–46. [Google Scholar]
- Krystek P, Ritsema R. Monitoring of Chromium Species and 11 Selected Metals in Emission and Immission of Airborne Environment. Int J Mass Spectrom. 2007;265:23–29. [Google Scholar]
- Li Y, Pradhan NK, Foley R, Low GKC. Selective Determination of Airborne Hexavalent Chromium Using Inductively Coupled Plasma Mass Spectrometry. Talanta. 2002;57:1143–1153. doi: 10.1016/s0039-9140(02)00196-0. [DOI] [PubMed] [Google Scholar]
- Lide DR. CRC Handbook of Chemistry and Physics. 85. CRC Press; 2004. [Google Scholar]
- Martell AE, Smith RM, Motekaitis RJ. NIST Critically Selected Stability Constants of Metal Complexes. NIST; 2004. [Google Scholar]
- Meng Q, Fan Z, Buckley B, Lin L, Huang L, Yu CH, Stiles R, Bonanno L. Development and Evaluation of a Method for Hexavalent Chromium in Ambient Air Using IC-ICP-MS. Atmos Environ. 2011;45:2021–2027. [Google Scholar]
- Morales-Munoz S, Luque-Garcia JL, Castro MDL. A Continuous Approach for the Determination of Cr(VI) in Sediment and Soil Based on the Coupling of Microwave-assisted Water Extraction, Preconcentration, Derivatization and Photometric Detection. Anal Chim Acta. 2004;515:343–348. [Google Scholar]
- Nagourney SJ, Wilson SA, Buckley B, Kingston HMS, Yang SY, Long SE. Development of a Standard Reference Material for Cr(VI) in Contaminated Soil. J Anal At Spectrom. 2008;23:1550–1554. [Google Scholar]
- New Jersey Department of Environmental Protection (NJDEP) 2008 New Jersey Chromium Emission Inventory. NJDEP; Trenton, New Jersey: 2009. [Google Scholar]
- Nico PS, Kumfer BM, Kennedy IM, Anastasio C. Redox Dynamics of Mixed Metal (Mn, Cr, and Fe) Ultrafine Particles. Aerosol Sci Technol. 2009;43:60–70. doi: 10.1080/02786820802482528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occupational Safety and Health Administration (OSHA) OSHA Method Number ID-215: Hexavalent Chromium in Workplace Atmospheres. OSHA; Salt Lake City, UT: 1998. [Google Scholar]
- Pettine M, Capri S. Digestion Treatments and Risks of Cr(III)-Cr(VI) Interconversions during Cr(VI) Determination in Soils and Sediments-A Review. Anal Chim Acta. 2005;540:231–238. [Google Scholar]
- Polytetrafluoroethylene. Available from http://en.wikipedia.org/wiki/Polytetrafluoroethylene#Properties.
- Rai D, Sass BM, Moore DA. Chromium(III) Hydrolysis Constants and Solubility of Chromium(III) Hydroxide. Inorg Chem. 1987;26:345–349. [Google Scholar]
- Research Triangle Institute. The Fate of Hexavalent Chromium in the Atmosphere. Research Triangle Institute; Sacramento, California: 1988. [Google Scholar]
- Seigneur C, Constantinou E. Chemical Kinetic Mechanism for Atmospheric Chromium. Environ Sci Technol. 1995;29:222–231. doi: 10.1021/es00001a029. [DOI] [PubMed] [Google Scholar]
- Sheehan P, Ricks R, Ripple S, Paustenbach D. Field Evaluation of a Sampling and Analytical Method for Environmental Levels of Airborne Hexavalent Chromium. Am Ind Hyg Assoc J. 1992;53:57–68. doi: 10.1080/15298669291359302. [DOI] [PubMed] [Google Scholar]
- Swietlik R, Molik A, Molenda M, Trojanowska M, Siwiec J. Chromium(III/VI) Speciation in Urban Aerosol. Atmos Environ. 2011;45:1364–1368. [Google Scholar]
- Talebi SM. Determination of Total and Hexavalent Chromium Concentrations in the Atmosphere of the City of Isfahan. Environ Res. 2003;92:54–56. doi: 10.1016/s0013-9351(02)00036-1. [DOI] [PubMed] [Google Scholar]
- Torkmahalleh MA, Lin L, Holsen TM, Rasmussen DH, Hopke PK. The Impact of Deliquescence and pH on Cr Speciation in Ambient PM Samples. Aerosol Sci Technol. 2012;46:690–696. [Google Scholar]
- Torkmahalleh MA, Lin L, Holsen TM, Rasmussen DH, Hopke PK. Cr Speciation Changes in the Presence of Ozone and Reactive Oxygen Species at Low Relative Humidity. Atmos Environ. 2013a;71:92–94. [Google Scholar]
- Torkmahalleh MA, Yu CH, Lin L, Fan Z, Swift JL, Bonanno L, Rasmussen DH, Holsen TM, Hopke PK. Improved Atmospheric Sampling of Hexavalent Chromium. J Air Waste Manage Assoc. 2013b;63:1313–1323. doi: 10.1080/10962247.2013.823894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya R, Umayahara A. Formation of Ion-pair Cr3+- SO42− in Aqueous Solution. Bull Chem Soc Jpn. 1963;36:554–559. [Google Scholar]
- United States Environmental Protection Agency (USEPA) Method 3060A:Alkaline Digestion for Hexavalent Chromium. 1996. [Google Scholar]
- Vairavamurthy A, Roberts JM, Newman L. Methods for Determination of Low Molecular Weight Carbonyl Compounds in the Atmosphere: A Review. Atmos Environ. 1992;26:1965–1993. [Google Scholar]
- von Meyenburg U, Siroky O, Schwarzenbach G. Deprotonation of Metal Aquo Ions. 2 Aquochromium(III) Ion-structure of Active Chromium Hydroxides. Helv Chim Acta. 1973;56:1099–1114. [Google Scholar]
- Werner ML, Nico PS, Marcus MA, Anastasio C. Use of Micro-XANES to Speciate Chromium in Airborne Fine Particles in the Sacramento Valley. Environ Sci Technol. 2007;41:4919–4924. doi: 10.1021/es070430q. [DOI] [PubMed] [Google Scholar]
- Yoo JI, Kim KH, Jang HN, Seo YC, Seok KS, Hong JH, Jang M. Emission characteristics of Particulate Matter and Heavy Metals from Samll Incinerators and Boilers. Atmos Environ. 2002;36:5057–5066. [Google Scholar]