Abstract
This article summarizes anatomical, neurophysiological, pharmacological, and brain imaging studies in humans and animals that have provided insights into the neural circuitry and neurotransmitter mechanisms controlling the lower urinary tract. The functions of the lower urinary tract to store and periodically eliminate urine are regulated by a complex neural control system in the brain, spinal cord, and peripheral autonomic ganglia that coordinates the activity of smooth and striated muscles of the bladder and urethral outlet. The neural control of micturition is organized as a hierarchical system in which spinal storage mechanisms are in turn regulated by circuitry in the rostral brain stem that initiates reflex voiding. Input from the forebrain triggers voluntary voiding by modulating the brain stem circuitry. Many neural circuits controlling the lower urinary tract exhibit switch-like patterns of activity that turn on and off in an all-or-none manner. The major component of the micturition switching circuit is a spinobulbospinal parasympathetic reflex pathway that has essential connections in the periaqueductal gray and pontine micturition center. A computer model of this circuit that mimics the switching functions of the bladder and urethra at the onset of micturition is described. Micturition occurs involuntarily in infants and young children until the age of 3 to 5 years, after which it is regulated voluntarily. Diseases or injuries of the nervous system in adults can cause the re-emergence of involuntary micturition, leading to urinary incontinence. Neuroplasticity underlying these developmental and pathological changes in voiding function is discussed.
Introduction
The storage and periodic elimination of urine depend on the coordinated activity of two functional units in the lower urinary tract (LUT): (1) a reservoir (the urinary bladder) and (2) an outlet consisting of the bladder neck, the urethra, and the urethral sphincter (218). Coordination between these organs is mediated by a complex neural control system located in the brain, spinal cord, and peripheral ganglia (449). Thus, urine storage and release are highly dependent on central nervous system pathways. This distinguishes the LUT from many other visceral structures (e.g., the gastrointestinal tract and cardiovascular system) that maintain a certain level of function even after extrinsic neural input has been eliminated.
The LUT is also unusual in its pattern of activity and organization of neural control mechanisms. For example, the urinary bladder has only two modes of operation: storage and elimination. Thus, many of the neural circuits have switchlike or phasic patterns of activity (142, 156, 173), unlike the tonic patterns characteristic of the autonomic pathways to cardiovascular organs. In addition, micturition is under voluntary control and depends on learned behavior that develops during maturation of the nervous system, whereas many other visceral functions are regulated involuntarily. Micturition also requires the integration of autonomic and somatic efferent mechanisms to coordinate the activity of visceral organs (the bladder and urethra) with that of urethral striated muscles (449).
Due to the complexity of the neural mechanisms regulating the LUT, micturition is sensitive to a wide variety of injuries, diseases, and chemicals that affect the nervous system. Thus, neurologic mechanisms are an important consideration in the diagnosis and treatment of voiding disorders. This article reviews (1) the innervation of the urinary bladder and urethra, (2) the organization of the reflex pathways controlling urine storage and elimination, (3) the neurotransmitters involved in micturition reflex pathways, and (4) neurogenic dysfunctions of the LUT. Abbreviations can be found in Table 1.
Table 1.
Abbreviations
| Full name | Abbreviation |
|---|---|
| Acetylcholine | ACh |
| Adenosine triphosphate | ATP |
| a-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid | AMPA |
| Anterior cingulate cortex | ACC |
| Adenosine | ADS |
| Adenosine monophosphate | AMP |
| Benign prostatic hyperplasia | BPH |
| Bladder outlet obstruction | BOO |
| Brain-derived neurotrophic factor | BDNF |
| Calcitonin gene-related peptide | CGRP |
| Choline acetyltransferase | ChAT |
| Cyclooxygenase-2 | COX-2 |
| Cholecystokinin | CCK |
| Detrusor overactivity | DO |
| Detrusor-sphincter-dyssynergia | DSD |
| Diabetes mellitus | DM |
| Dorsal anterior cingulate cortex | dACC |
| Dorsal root ganglia | DRG |
| Electromyogram | EMG |
| Epinephrine | E |
| Excitatory postsynaptic currents | EPSCs |
| External urethral sphincter | EUS |
| Excitatory postsynaptic potential | EPSP |
| Enkephalin | ENK |
| Prostaglandin E receptor | EP |
| Fast excitatory postsynaptic potential | f-EPSP |
| Functional magnetic resonance imaging | fMRI |
| Gamma aminobutyric acid | GABA |
| Glial cell line-derived neurotrophic factor | GDNF |
| Glutamic acid decarboxylase | GAD |
| Glycine transporters | GlyTs |
| 8-hydroxy-2-(di-n-propylamino)-tetralin | 8-OH-DPAT |
| 5-hydroxtryptamine, Serotonin | 5HT |
| Herpes simplex virus | HSV |
| Inhibitory postsynaptic currents | IPSCs |
| Inhibitory postsynaptic potential | IPSP |
| Isolectin B4 binding sites | IB4 |
| Interstitial cystitis | IC |
| Lateral prefrontal cortex | LPFC |
| Levodopa | L-dopa |
| Locus coeruleus | LC |
| Lumbar splanchnic nerves | LSN |
| Lower urinary tract | LUT |
| Lower urinary tract symptoms | LUTS |
| Mechanosensitive channels | MSC |
| Metabotropic glutamatergic receptors | mGluRs |
| Muscarinic acetylcholine receptors | mACh |
| Myelinated afferents | Aδ |
| Medial prefrontal cortex | mPFC |
| Muscarinic receptor subtype 1 | M1 |
| Muscarinic receptor subtype 2 | M2 |
| Muscarinic receptor subtype 3 | M3 |
| Neurokinin | NK |
| Nerve growth factor | NGF |
| Neurokinin-1 receptor | NK-1 |
| Neurokinin-2 receptor | NK-2 |
| Neuropeptide Y | NPY |
| Neurotrophin-3 | NT-3 |
| Nitric oxide | NO |
| Nitric oxide synthase | NOS |
| Norepinephrine | NE |
| Nucleus raphe magnus | NRM |
| N-methyl-D-aspartic acid | NMDA |
| Overactive bladder | OAB |
| Paraventricular nucleus | PVN |
| Parkinson’s disease | PD |
| Periaqueductal gray | PAG |
| Protein kinase C | PKC |
| Pituitary adenylate cyclase-activating polypeptide | PACAP |
| Pontine micturition center | PMC |
| Pontine urine storage center | PUSC |
| Positron emission tomography | PET |
| Preganglionic neuron | PGN |
| Prostaglandin E2 | PGE2 |
| Prostacyclin | PGI2 |
| Protease-activated receptors | PARs |
| Pseudorabies virus | PRV |
| Purinergic subtype 1 receptor | P1 |
| Purinergic subtype 2X receptor | P2X |
| Purinergic subtype 2Y receptor | P2Y |
| Prefrontal cortex | PFC |
| Resiniferatoxin | RTX |
| Rostral pontine reticular formation | RPRF |
| Somatostatin | SOM |
| Spinal cord injury | SCI |
| Streptozotocin | STZ |
| Substance P | SP |
| Substantia nigra | SN |
| Supplementary motor area | SMA |
| Small intensely fluorescent cells | SIF cells |
| Slow inhibitory postsynaptic potential | s-IPSP |
| Slow excitatory postsynaptic potential | s-EPSP |
| Tetrodotoxin | TTX |
| Transient receptor potential ankyrin receptor 1 | TRPA1 |
| Transient receptor potential melastatin receptor 8 | TRPM8 |
| Transient receptor potential vanilloid receptor 1 | TRPV1 |
| Transient receptor potential vanilloid receptor 4 | TRPV4 |
| Tropomyosin-related kinase A | TrkA |
| Tropomyosin-related kinase B | TrkB |
| Tyrosine hydroxylase | TH |
| Urge urinary incontinence | UUI |
| Vasoactive intestinal polypeptide | VIP |
| Vesicular acetylcholine transporter | VAChT |
| Voltage-gated channels | VGC |
| Ventral tegmental area | VTA |
Peripheral Nervous System
Efferent innervation and neurotransmitters
The LUT receives a bilateral efferent innervation from the thoracic and lumbosacral segments of the spinal cord (Fig. 1A). Efferent axons are carried in three sets of peripheral nerves: sacral parasympathetic (pelvic nerves), thoracolumbar sympathetic (hypogastric nerves and sympathetic chain), and sacral somatic nerves (primarily the pudendal nerves) (175, 449) (Fig. 1A). Preganglionic axons carrying information from the spinal cord to the bladder and urethra synapse with autonomic ganglion cells widely distributed throughout the peripheral nervous system in: (1) the pelvic plexus, (2) prevertebral sympathetic ganglia (inferior mesenteric ganglia, IMG), (3) paravertebral sympathetic chain ganglia, and (4) ganglia on the serosal surface and in the wall (intramural ganglia) of the organs (168, 211, 212, 368, 369, 678). Ganglia on one side are interconnected by numerous fiber tracts and the majority of inputs from the spinal cord occur ipsilaterally. In addition in some species fiber connections between the right and left pelvic plexuses and the IMGs occur (220, 327, 622) and synaptic interactions between the right and left plexuses have been reported (257). The striated muscle of the external urethral sphincter (EUS) is directly innervated by axons originating from motoneurons in the spinal cord.
Figure 1.

Efferent pathways of the lower urinary tract. (A) Innervation of the female lower urinary tract. Sympathetic fibers (shown in blue) originate in the T11-L2 segments in the spinal cord and run through the inferior mesenteric plexus (IMP) and the hypogastric nerve (HGN) or through the paravertebral chain to enter the pelvic nerves at the base of the bladder and the urethra. Parasympathetic preganglionic fibers (shown in green) arise from the S2–S4 spinal segments and travel in sacral roots and pelvic nerves (PEL) to ganglia in the pelvic plexus (PP) and in the bladder wall. This is where the postganglionic nerves that supply parasympathetic innervation to the bladder arise. Somatic motor nerves (shown in yellow) that supply the striated muscles of the external urethral sphincter arise from S2–S4 motor neurons and pass through the pudendal nerves. (B) Efferent pathways and neurotransmitter mechanisms that regulate the lower urinary tract. Parasympathetic postganglionic axons in the pelvic nerve release acetylcholine (ACh), which produces a bladder contraction by stimulating M3 muscarinic receptors in the bladder smooth muscle. Sympathetic postganglionic neurons release noradrenaline (NA), which activates β3 adrenergic receptors to relax bladder smooth muscle and activates α1 adrenergic receptors to contract urethral smooth muscle. Somatic axons in the pudendal nerve also release ACh, which produces a contraction of the external sphincter striated muscle by activating nicotinic cholinergic receptors. Parasympathetic postganglionic nerves also release ATP, which excites bladder smooth muscle, and nitric oxide, which relaxes urethral smooth muscle (not shown). L1, first lumbar root; S1, first sacral root; SHP, superior hypogastric plexus; SN, sciatic nerve; T9, ninth thoracic root (216).
Pelvic and bladder ganglia
The autonomic ganglia contain thousands of postganglionic neurons but are innervated by considerably smaller numbers of preganglionic neurons located in the intermediolateral region of the spinal cord (13, 39, 155,164,278,318,319,443, 444, 455–458). Thus, the preganglionic axons exhibit considerable divergence within the peripheral nervous system to synapse with multiple ganglionic targets. Synaptic transmission in all ganglia is mediated by acetylcholine (Ach) acting on nicotinic receptors; although, as discussed later, other neurotransmitters acting on various types of presynaptic and postsynaptic receptors can modulate cholinergic transmission.
The morphology of the ganglion cells varies markedly in different species. In the rat major pelvic ganglion (606) and mouse hypogastric ganglion (522) the cells are 20 to 30 μm in diameter and have no dendrites or only a few short dendrites. On the other hand, neurons in pelvic and bladder ganglia of the cat are larger (40–60 μm) and have a more complex morphology, exhibiting on the average 6 to 7 dendrites (152).
In the cat bladder ganglia, virtually all neurons are CHAT positive and 50% stain for NOS. VIP-IR is also present in 10% to 15% of cat bladder ganglion cells and is present in neurons that stain heavily for acetylcholinesterase (AChE) (310, 391). Thus, VIP is very likely colocalized with ACh in some bladder ganglion cells. Cat, bladder ganglion cells receive an extensive cholinergic/enkephalinergic input from sacral preganglionic neurons (160, 236, 267, 311). CGRP, substance P and VIP-containing axons and axonal-varicosities are also present in cat pelvic and bladder ganglia, where they represent, in part, afferent pathways (162, 267, 310, 313).
Immunohistochemical studies (318, 319) in the rat pelvic ganglia revealed that neurons are either cholinergic (stained for choline acetyltransferase, ChAT) and vesicular acetylcholine transporter (VAChT) or noradrenergic (stained for tyrosine hydroxylase, TH). Most cholinergic neurons express nitric oxide synthase (NOS) and vasoactive intestinal polypeptide (VIP) and a smaller population also expresses NPY. All pelvic noradrenergic neurons express NPY. Many neurons are surrounded by ENK-IR varicosities (50%–65%) and fewer neurons are surrounded by CCK-or SOM-IR varicosities (30%–35%). The immunohistochemistry of the pelvic ganglia of the male mouse is similar to that of the rat (672).
Intramural ganglion cells in the guinea pig urinary bladder (124, 290, 291) contain either AChE, SOM, NPY, or quinacrine-fluorescence (a marker for purinergic neurons). Studies of the colocalization of peptides in these ganglion cells revealed that 55% to 70% of the total population which is assumed to be primarily cholinergic exhibit both NPY and SOM immunoreactivity. Thus NPY and somatostatin could function as neuromodulators in the cholinergic efferent pathway to the bladder.
Intramural ganglia in the human bladder contain small numbers of neurons (1–36 per ganglia) that exhibit immunoreactivity to VAChT, VIP, NOS, NPY, and galanin (189, 190, 572, 573). Approximately 75% are VAChT positive, 95% NPY positive, and 40% NOS positive. A small percentage of the neurons contain TH-IR. (189). The intramural ganglion cells are surrounded by pericellular baskets of varicose terminals containing CGRP, VIP, enkephalin, NPY, galanin, or substance P.
Pelvic ganglia in some species such as the dog, cat and rabbit also exhibit dense collections of adrenergic varicosities surrounding the principal ganglion cells (188, 204, 255, 256). These varicosities persist after chronic decentralization and therefore must arise from cells within the pelvic plexus, either from adrenergic neurons or SIF cells. Two types of SIF cells have been identified; those with processes (Type I) and those devoid of processes (Type II). It has been suggested that the former can function as interneurons in ganglia and make connections with the principal ganglion cells (491, 626). The latter may have an endocrine function. In the rat, SIF cells can contain 5-hydroxytryptamine, histamine and enkephalins, in addition to norepinephrine (261, 306).
Parasympathetic postganglionic nerves
Parasympathetic neuroeffector excitatory transmission in the bladder is mediated by ACh acting on postjunctional muscarinic receptors (15, 17, 409, 410). Both M2 and M3 muscarinic receptor subtypes are expressed in bladder smooth muscle; however, use of subtype selective muscarinic receptor antagonists and muscarinic receptor knockout mice revealed that the M3 subtype is the principal receptor involved in excitatory transmission (Fig. 1B) (15, 17, 409, 410). Activation of M3 receptors triggers intracellular Ca2+ release; whereas activation of M2 receptors inhibits adenylate cyclase (17). The latter may contribute to bladder contractions by suppressing adrenergic inhibitory mechanisms which are mediated by β adrenergic receptors and stimulation of adenylate cyclase.
In bladders of various animals stimulation of parasympathetic nerves also produces a noncholinergic contraction that is resistant to atropine and other muscarinic receptor blocking agents. Adenosine triphosphate (ATP) is the excitatory transmitter mediating the noncholinergic contractions (15, 17, 87, 515). ATP excites the bladder smooth muscle by acting on P2X receptors which are ligand gated ion channels. Among the seven types of P2X receptors expressed in the bladder, P2X1 is the major subtype in the rat and human bladder smooth muscle (87, 515). Purinergic transmission has an important excitatory role in animal bladders but is not important in the normal human bladder. However, it appears to be involved in bladders from patients with pathological conditions such as detrusor overactivity (DO), chronic urethral outlet obstruction, or interstitial cystitis (87, 494).
Parasympathetic pathways to the urethra induce relaxation during voiding (15, 17, 18, 85, 180, 265). In various species the relaxation is not affected by muscarinic antagonists and therefore is not mediated by ACh. However inhibitors of NOS block the relaxation in vivo during reflex voiding or block the relaxation of urethral smooth muscle strips induced in vitro by electrical stimulation of intramural nerves indicating that NO is the inhibitory transmitter involved in relaxation (15, 85, 180, 451). In some species neurally evoked contractions of the urethra are reduced by muscarinic receptor antagonists or by desensitization of P2X purinergic receptors, indicating that ACh or ATP are involved in excitatory transmission to urethral smooth muscle (738). More detailed information about the actions of neurotransmitters on urinary tract smooth muscle and mechanisms of muscle contraction are available in several review articles (17, 218).
Thoracolumbar sympathetic pathways
Sympathetic preganglionic pathways that arise from the T11 to L2 spinal segments pass to the sympathetic chain ganglia and then to prevertebral ganglia in the superior hypogastric and pelvic plexus (Fig. 1) and also to short adrenergic neurons in the bladder and urethra. Sympathetic postganglionic nerves that release norepinephrine provide an excitatory input to smooth muscle of the urethra and bladder base, an inhibitory input to smooth muscle in the body of the bladder (Fig. 1B), and inhibitory and facilitatory input to vesical parasympathetic ganglia (15, 180, 322). α-adrenergic receptors are concentrated in the bladder base and proximal urethra, whereas β-Adrenergic receptors are most prominent in the bladder body (Fig. 1B) (17,180). These observations are consistent with pharmacological studies showing that sympathetic nerve stimulation or exogenous catecholamines produce β-adrenergic receptor mediated inhibition of the body and α-adrenergic receptor mediated contraction of the base, dome and urethra. Molecular and physiological studies have shown that β3-adrenergic receptors elicit inhibition and α1-adrenergic receptors elicit contractions in the human bladder (17). The α1A-adrenergic receptor subtype is most prominent in the normal bladders but the α1D-subtype is upregulated in bladders from patients with outlet obstruction, raising the possibility that α1-adrenergic receptor excitatory mechanisms in the bladder might contribute to irritative LUT symptoms in patients with benign prostatic hyperplasia (BPH) (17).
Sacral somatic pathways
Somatic efferent pathways to the EUS are carried in the pudendal nerve from anterior horn cells in the third and fourth sacral segments of the human spinal cord and from various caudal lumbo-sacral segments in animals (Fig. 1B). Branches of the pudendal nerve as well as other sacral somatic nerves carry efferent impulses to muscles of the pelvic floor (38, 158, 451, 507, 633).
Afferent innervation
Afferent axons in the pelvic, hypogastric, and pudendal nerves transmit information from the LUT to second-order neurons in the lumbosacral spinal cord (145,292,701). Pelvic nerve afferents that innervate the bladder and urethra originate in caudal lumbosacral dorsal root ganglia (DRG) and are divided into two populations: small myelinated (Aδ) and unmyelinated C-fibers.
Receptor properties of afferents
Aδ mechanoreceptor afferents identified in the pelvic nerve (Fig. 2A) (37, 195, 548, 677) or the sacral dorsal roots (252, 292), of the cat respond to both passive distension as well as active contraction of the bladder indicating that they are in series tension receptors. These afferents which have conduction velocities ranging between 2.5 and 15 m/s (252) are silent when the bladder is empty but during slow filling of the bladder display a graded increase in discharge frequency at intravesical pressures below 25 mmHg (83,292). Multiunit recordings exhibit a successive recruitment of mechanoreceptors with different thresholds during bladder filling. The maximal firing rates range from 15 to 30 Hz. All afferents behave like slowly adapting mechanoreceptors with both a dynamic and static component of their discharge. Pressure thresholds for mechanosensitive afferents in the cat fall on the flat, compliant part of the bladder pressure volume curve at about 25% to 75% of the pressure at which the curve becomes steep. These thresholds are consistent with intravesical pressures at which humans report the first sensation of bladder filling. Electrophysiological studies in cats and rats have revealed that the normal micturition reflex is triggered by myelinated Aδ-fiber afferents (164, 168, 251, 397).
Figure 2.

Classes and distribution of afferent nerves in the LUT. (A) The distribution of the different classes of fibers in the bladder wall and urethra. (B) In the pelvic nerve, four types of mechanosensitive fibers were identified by stretch, stroke, and probe. (C) Proportions of afferent fiber types recorded in the pelvic nerve. (D) Distribution of low- and high-threshold receptive fields of pelvic nerve muscle afferent fibers based on responses to stretch. (E) Distribution of receptive fields of the four classes of pelvic nerve fibers (303).
In contrast to the low threshold mechano-sensitive Aδ-bladder afferents, the C-bladder afferents in cats are generally mechano-insensitive (“silent C-fibers”) (251). Many of these afferents are nociceptive and respond to cold stimuli or chemical/noxious stimuli such as high potassium, low pH, high osmolality, and irritants such as capsaicin and turpentine oil (209, 251, 394, 424). Following exposure to these substances silent afferents become mechanoreceptive and the sensitivity of bladder mechanoreceptors to distension also increases.
In rats, Sengupta and Gebhart (561) reported that both Aδ- and C-fiber afferents are mechanosensitive and respond to bladder distension (Fig. 2B). They also found that 30% of bladder afferents are not responsive to any mechanical stimuli, and these unresponsive bladder afferents include both Aδ-and C-fibers (Fig. 2). Other studies in rats showed that most myelinated Aδ-fiber bladder afferents are mechno-sensitive, while about one-half of unmyelinated C-fiber bladder afferents have no clear mechano-sensitivity (i.e., silent C-fibers), but respond to chemical stimuli (191). Neural activity induced by bladder distention is much lower in mechano-sensitive C-fiber bladder afferent fibers than in myelinated Aδ-fibers, suggesting that C-fiber bladder afferents are less excitable than Aδ-fiber afferents in rats. Another study showed that many C-fiber bladder afferents are volume receptors that do not respond to bladder contractions, a property that distinguishes them from “in series tension receptors” (448).
In the mouse pelvic nerve four classes of bladder afferents (serosal, muscular, muscular/urothelial, and urothelial) have been identified based on responses to receptive field stimulation with different mechanical stimuli including probing, stretch, and stroking the urothelium (Fig. 2B). A low threshold group, representing 65%–80% of the total population, and a high threshold stretch-sensitive population of muscular afferents were identified (132, 679). The muscular afferents can be sensitized by application of a combination of inflammatory mediators (bradykinin, serotonin, prostaglandin, and histamine at pH 6.0) (679).
In the guinea pig bladder four classes of afferents have also been detected (731,732). These include: stretch-sensitive afferents in muscle which behave as in-series tension receptors as well as tension-mucosal mechanoreceptors which can be activated by stretch, mucosal stroking with light von Frey hairs or by hypertonic solutions applied locally to the receptive fields in the mucosa. In addition stretch-insensitive afferents consisting of mucosal mechanoreceptors and chemoreceptors have been identified. Muscle mechanoreceptors are activated by stretch but not by mucosal stroking or by hypertonic solution or capsaicin. Removal of the urothelium does not affect the stretch induced firing. Muscle-mucosal mechanoreceptors are activated by both stretch and mucosal stroking, by hypertonic solution, αβ-methylene-ATP but not by capsaicin. Stroking and stretch induced firing is significantly reduced by removal of the urothelium. The third class of afferents, mucosal high-responding mechanoreceptors, are stretch-insensitive but can be activated by mucosal stroking, hypertonic solution, αβ-methylene-ATP and by capsaicin. Stroking induced activity is reduced by removal of the urothelium. The fourth class of afferents, mucosal low-responding mechanoreceptors, are stretch insensitive but can be weakly activated by mucosal stroking but not by hypertonic solution, αβ-methylene-ATP or capsaicin. Removal of the urothelium reduces stroking induced firing. All four populations of afferents conduct in the C-fiber range and show class-dependent differences in spike amplitude and duration.
Activity of Aδ and C-fiber bladder and urethral afferent axons have been identified in the hypogastric nerves (214,677) lumbar splanchnic nerves (LSNs) or the lumbar white rami (36). The receptive fields of the units are either single or multiple punctuate sites (Fig. 2D and E) on the bladder or urethral surface or associated with blood vessels in the peritoneal attachments to the bladder base. Afferents with receptive fields on or in the bladder wall respond in a graded manner to passive distension or isovolumetric contraction at intravesical pressures from 10 to 70 mmHg with threshold pressures generally below 20 mmHg. Urethral afferents exhibit either no responses to bladder stimulation or low discharge rates at higher intravesical pressures. No functional differences between the Aδ and C-fiber afferent populations in the hypogastric nerve have been reported except that firing rates are lower in the latter group. In contrast to pelvic nerve afferents the hypogastric afferents often are active with the bladder empty (36, 677).
Bladder afferents in the LSNs in the mouse consist of low threshold and high threshold subtypes with receptive fields in the serosal and mucosal layers of the bladder (679). The serosal afferents are the most abundant. Virtually all of these afferents possess small (0.5 mm), punctuate receptive fields that tend to be clustered at the base of the bladder. Some of the afferents exhibit low rates of spontaneous activity. LSN afferents do not exhibit a dynamic response to probing nor adaptation during a maintained force; whereas pelvic afferents in the mouse give dynamic responses at the onset of stimulation and adaptation to a maintained stimulus.
Afferent fibers innervating the urethra are also important for modulating LUT function. In dogs, urethral afferent fibers in the pelvic and pudendal nerves are sensitive to the passage of the fluid through the urethra and pudendal nerve afferents are more sensitive than pelvic nerve afferents (620). Afferents in the pelvic nerves of the rat also are activated by high intraurethral pressures (>60 cm water) (210).
Conduction velocities of cat pudendal nerve afferent fibers responding to electrical stimulation of the urethra are approximately twice as fast (45 m/s vs. 20 m/s) as pelvic nerve afferent fibers responding to the same stimulation (76). In addition, urethral afferents in the pudendal, pelvic, and hypogastric nerves of the cat have different receptor properties. Pudendal nerve afferents responding to urine flow exhibit a slowly adapting firing pattern (643) while small myelinated or unmyelinated urethral afferents in the hypogastric nerves and myelinated urethral afferents in the pelvic nerves responding to urine flow or urethral distention exhibit rapidly adapting responses (36,37). Stimulation of flow-sensing urethral afferents by intraurethral saline infusion enhances volume-induced reflex bladder contractions in rats (294). Electrical stimulation of urethral afferents also evokes reflex bladder contractions in the cat (325, 326, 420).
Nociceptive C-fiber afferents are also present in pelvic and pudendal nerves innervating the urethra (120, 640) and the number of these afferents is higher in the pelvic than in the pudendal nerves (715). Activation of urethral C-fibers by capsaicin application elicits EUS and pelvic floor striated muscle EMG activity and nociceptive behavioral responses that disappear after pudendal nerve transection (120,376,640). Urethral C-fiber activation by capsaicin also suppresses reflex bladder contractions in rats (294,682). Putative C-fiber afferent fibers identified by positive staining for CGRP or substance P are present in the subepithelium, the submucosa, and the muscular layers in all portions of the urethra (267, 673).
Electrophysiological properties of afferent neurons
Functional properties of dissociated bladder and urethral afferent neurons identified by retrograde axonal transport of fluorescent dyes injected into the bladder or urethra have been investigated using patch clamp techniques (Fig. 3) (136, 137, 554, 555, 697, 701, 703, 714–717, 734).
Figure 3.
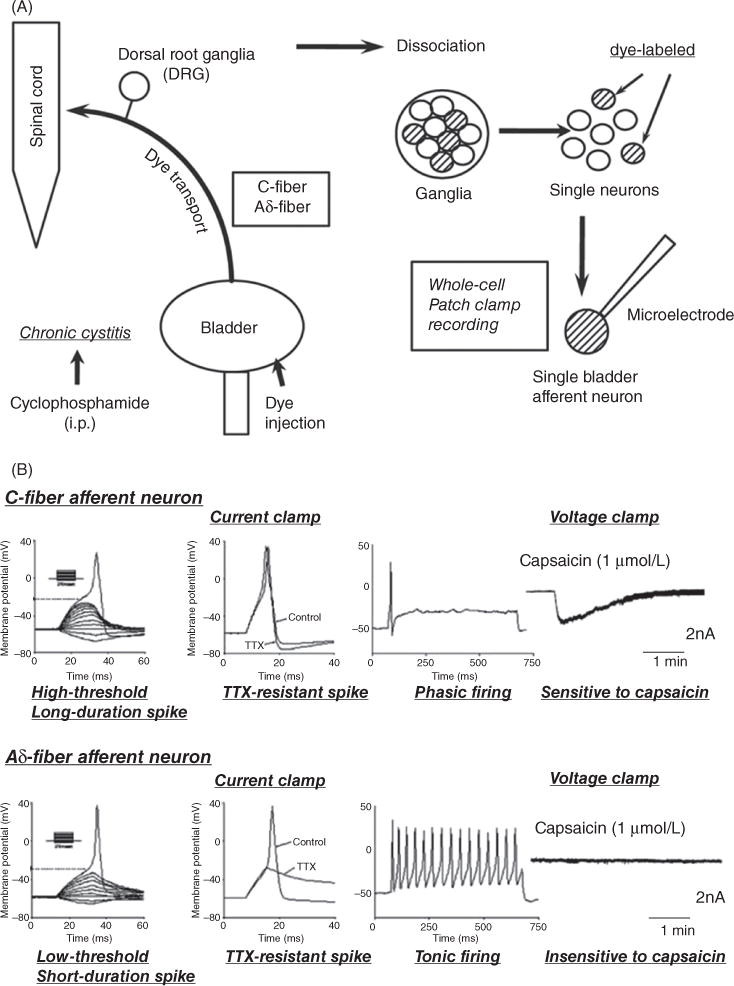
(A) Experimental methods for performing patch-clamp recordings on bladder afferent neurons obtained from rats with chronic cystitis. Chronic cystitis was induced by intraperitoneal injection of cyclophosphamide. Fluorescent dye (fast blue) injected into the bladder wall was transported via Aδ- and C-fiber bladder afferent axons to neurons in the dorsal root ganglia (DRG). L6 and S1 DRG were dissected and dissociated into single neurons by enzymatic methods. Whole cell patch-clamp recordings were then performed on fast blue-labeled bladder afferent neurons that were identified with a fluorescence microscope. (B) Characteristics of a bladder afferent neuron (24-μm diameter, C-fiber afferent neuron, top record) exhibiting tetrodotoxin (TTX)-resistant action potentials and a bladder afferent neuron (33-μm diameter, Aδ-fiber afferent neuron, bottom record) exhibiting TTX-sensitive action potentials. The left panels are voltage responses and action potentials evoked by 30-ms depolarizing current pulses injected through the patch pipette in current-clamp conditions. Asterisks with dashed lines indicate the thresholds for spike activation. The second panels on the left side show the effects of TTX application (1 μmol/L) on action potentials. The third panels from the left show firing patterns during membrane depolarization (700-ms duration). The panels on the right show the responses to extracellular application of capsaicin (1 μmol/L) in voltage-clamp conditions. Note that the C-fiber afferent neuron exhibited TTX-resistant phasic firing (i.e., one to two spikes during prolonged membrane depolarization) and an inward current in response to capsaicin, while A-fiber afferent neuron exhibited TTX-sensitive tonic firing (i.e., repetitive firing during membrane depolarization) and no response to capsaicin (176).
Based on electrical and chemical properties, bladder afferent neurons are divided into two populations (717). The most common population of neurons (greater than 70%) are small in size, sensitive to capsaicin and exhibit high-threshold, longduration action potentials resistant to tetrodotoxin (TTX), a Na+ channel blocker (Fig. 3). The other population of bladder afferent neurons which are larger in size and insensitive to capsaicin exhibit low-threshold, short-duration action potentials which are reversibly blocked by TTX (Fig. 3). Because the majority of bladder afferent neurons with TTX-resistant spikes are sensitive to capsaicin, these neurons are likely to be the origin of C-fiber afferent axons (703).
Chemical properties of afferent neurons
Immunohistochemical studies reveal that bladder afferent neurons contain various neuropeptides such as substance P (SP), calcitonin gene-related peptide (CGRP), pituitary adenylate cyclase-activating polypeptide (PACAP), and VIP (162,321, 394, 659, 660) as well as putative excitatory amino acid transmitters, glutamic and aspartic acid (323), and vesicular glutamate transporters (82). Peptide-containing axons are distributed throughout all layers of the bladder but are particularly dense in the lamina propria just beneath the urothelium. In the spinal cord of rats and cats peptidergic afferents are present in Lissauer’s tract, in lamina I where they are very prominent on the lateral edge of the dorsal horn and in the region of the parasympathetic nucleus (310, 312, 315, 660). This distribution is similar to that of the central projections of bladder afferent neurons labeled by axonal tracers (145, 585). The release of these peptides in the bladder wall is known to trigger inflammatory responses, including plasma extravasation or vasodilation (i.e., neurogenic inflammation).
Bladder afferent neurons and axons, especially C-fiber afferents, also express various receptors including the transient receptor potential vanilloid receptor 1 (TRPV1, the capsaicin receptor) (35, 57, 394), transient receptor potential ankyrin 1 receptor (TRPA1) (208,594), TRPM8, a cold receptor (208), tyrosine kinase receptor A (TrkA) which responds to nerve growth factor (NGF) (511, 512), α and β estrogen receptors (48) and tyrosine kinase receptor B (TrkB) that responds to brain derived neurotrophic factor (BDNF) (511,512), glial cell line-derived neurotrophic factor (GDNF) receptors that respond to GDNF (GRFα1) and artemin (GRFα3) (215), isolectin B4 binding sites (IB4) (715), muscarinic receptors, endothelin receptors, and purinergic receptors (P2X2, P2X3, P2Y) receptors that can be activated by ATP (47,208,379,470,523,594,595,734) (Table 2). Many of these receptors have been detected not only in axons in the bladder but also in the lumbosacral spinal cord in the same locations as the projections of bladder afferent axons. Patchclamp recordings from bladder afferent neurons (703,704) has also demonstrated that a high percentage of these neurons not only from lumbosacral DRG (i.e., pelvic nerve afferents), but also thoracolumber DRG (i.e., hypogastric nerve afferents) respond to ATP, protons, and/or capsaicin (136).
Table 2.
Receptors Localized on Afferent Nerves (303)
| Receptor | Agonist/stimuli | Localization | Physiological role |
|---|---|---|---|
| TRPV1 | Heat and protons, Capsaicin and resiniferatoxin | C-fibers (DRG and terminals) (334) Urothelium and detrusor | Nociception |
| TRPV4 | Osmolarity and pressure, bisandrographolide A | C-fibers (DRG and terminals) (334), urothelium | Osmolarity and pressure sensor |
| TRPA1 | Noxious cold, trans-cinnamaldehyde | C-fibers (DRG and terminals) (334) | Mechano and noxious cold sensor |
| TRPM8 | Cold, menthol | Aδ and C-fibers (DRG and terminals) (334) | Cold sensor |
| P2X2 | ATP | C-fibers (DRG and terminals), urothelium | Enhances afferent firing, nociception (117) |
| P2X3 | ATP | C-fibers (DRG and terminals), urothelium | Enhances afferent firing (665) |
| M3 | Acetylcholine | C-fibers (DRG, and terminals), detrusor | Enhances afferent firing (356) |
| M2 | Acetylcholine | C-fibers (spinal cord) | Inhibitory effect on micturition (405) |
| EP receptors | Prostaglandins | C-fibers (terminals) | Increases bladder afferent firing and sensitization through action on NaV1.9 channel (520) |
| Neurokinin (NK) NK-2, NK-3 receptors |
Tachykinins | C-fibers (DRG and terminals), detrusor | NK1—nociceptive responses (365) NK2—enhances detrusor contractions (378) NK3—inhibition of micturition reflex (302) |
| TrkA | Nerve growth factor (NGF) | Aδ and C-fibers (DRG and terminals) | Responsible for nerve survival, growth and differentiation. May be involved in development of chronic pain following bladder injury (248) |
| 5-HT/serotonin receptors | 5-HT/serotonin | Aδ and C-fibers (DRG and terminals) | Inhibits relaxation of bladder neck/urethra through action on receptors in the spinal cord (637) |
| Guanylate cyclase | Nitric oxide | Afferents, urothelium, interstitial cells | Inhibits afferent firing (714) |
Axonal tracing studies have also revealed that a small percentage of lumbosacral afferent neurons innervate multiple pelvic organs. For example, 3% to 15% of dorsal root ganglion (DRG) neurons are double labeled following injections of different tracers into the colon and bladder (113, 321, 400, 401). The double labeling occurs more frequently in rostral lumbar (L1–L2) than in caudal lumbosacral (L6–S1) DRG, which provide the major innervation to the bladder and colon. It has been speculated that dichotomizing afferents that send axonal branches to different target organs may contribute to viscero-visceral cross-organ sensitization (504). Furthermore the suppression of cross-organ sensitization by capsaicin treatment indicates that a large proportion of the dichotomizing afferents are C-fibers.
Summary
In summary, because capsaicin, the C-fiber afferent neurotoxin, does not block normal micturition reflexes in cats and rats, it is believed that C-fiber afferents are not essential for normal voiding (105, 106, 161, 395). On the other hand, the efficacy of capsaicin in reducing bladder overactivity induced by noxious stimuli indicates that C-fiber afferents do play an important role in LUT dysfunction in pathological conditions (see (107, 175–178, 303) and later sections of this article for more detailed discussions about primary afferents and their role in LUT dysfunction).
Neuron-like properties of the urothelium: Interaction with afferent nerves
While the urothelium has been historically viewed as primarily a “barrier,” there is increasing evidence that urothelial cells display a number of properties similar to sensory neurons (nociceptors/mechanoreceptors), and that both types of cells use diverse signal-transduction mechanisms to detect physiological stimuli (50). Figure 4 depicts possible interactions of urothelial cells with other bladder structures such as bladder nerves, interstitial cells, smooth muscle and blood vessels through autocrine (i.e., autoregulation) or paracrine mechanisms (release from nearby nerves or other cells). Examples of “sensor molecules” (i.e., receptors/ion channels) associated with neurons that have been identified in urothelium include receptors for bradykinin (110) neurotrophins (TrkA and p75) (453), purines (P2X and P2Y) (61, 86, 111, 379, 628), norepinephrine (α and β) (53, 59, 357, 383), acetylcholine (muscarinic and nicotinic) (43, 46, 108, 355, 356) protease activated receptors (PARs) (130,140), amiloride/mechanosensitive Na+ channels (29,576,668), prostaglandin E2 (PGE2) receptors (EP1) (671), and a number of TRP channels (TRPV1, TRPV2, TRPV4, TRPM8, and TRPA1) (51,57,58,200,230,358,440,590,680) (Fig. 4).
Figure 4.

Hypothetical model depicting possible interactions between bladder afferent nerves, urothelial cells, smooth muscle cells, interstitial cells, and blood vessels. Urothelial cells can also be targets for transmitters released from nerves or other cell types. Urothelial cells can be activated by either autocrine (i.e., autoregulation) or paracrine mechanisms (release from nearby nerves or other cells). Bladder stretch releases ATP which acts on P2 receptors on the afferent terminal or the interstitial cell and on P2 receptors on the urothelial cell. Stretch also releases ACh which acts on muscarinic receptors (M3) on the afferent terminal, the interstial cell or the urothelial cell. The latter action can release NO. Epinephrine or norepinephrine also release NO from the urothelial cell by activating β3 adrenergic receptors (50).
When urothelial cells are activated via these receptors/ion channels in response to mechanical or chemical stimuli, they can in turn release chemical mediators such as NO, ATP, acetylcholine, prostaglandins, and substance P (50,53,54,86,87,109,213). These agents are known to have excitatory and inhibitory actions on afferent nerves, which are located close to or in the urothelium (16, 57). Thus, it has been speculated that the urothelium plays a role in bladder sensation by responding to local chemical and mechanical stimuli and then sending chemical signals to the bladder afferent nerves, which convey information to the central nervous system (50, 62, 149, 699) (Fig. 4).
NO can be released by the urothelium, particularly during inflammation (53). The release of NO can be evoked by the calcium ionophore A-23187, norepinephrine, substance P and capsaicin. Release of NO from bladder strips evoked by adrenergic agonists is reduced by 85% after removal of the urothelium. Given that NO has a minimal direct effect on the detrusor muscle but does exert an inhibitory effect on afferent and reflex activity in the bladder (408, 492, 495, 727) and inhibits Ca2+ channels in bladder afferent neurons (714), it is likely that NO is involved in urothelial sensory signaling mechanisms in the bladder and may have a role in modulating inflammatory and nociceptive pathways. Increases in inducible NOS expression in the urothelium and/or NO levels in the bladder have been demonstrated in BPS/IC patients, especially those with Hunner’s lesion (BPS/IC ESSIC Type 3C) (274, 338, 388). In addition NOS expression in afferent neurons is also increased in rats with chronic bladder inflammation (663) raising the possibility that pathological conditions increase the contribution of NO to bladder function.
ATP released from urothelial cells during stretch can activate a population of suburothelial bladder afferents expressing P2X2 and P2X3 receptors, signaling changes in bladder fullness and pain (86,213). Accordingly, P2X3 null mice exhibit urinary bladder hyporeflexia, suggesting that this receptor as well as neural-epithelial interactions is essential for normal bladder function (118). This type of regulation may be similar to epithelial dependent secretion of mediators in airway epithelial cells which are thought to modulate submucosal nerves and bronchial smooth muscle tone and play an important role in inflammation (272, 289). Thus, it is possible that activation of bladder nerves and urothelial cells can modulate bladder function directly or indirectly via the release of chemical factors in the urothelial layer. ATP released from the urothelium facilitates stretch induced bladder afferent firing in cyclophosphamide irritated bladders but does not have a detectable effect in normal bladders, indicating that the role of ATP is unregulated in pathological conditions (726). ATP released from the urothelium or surrounding tissues may also regulate membrane trafficking in urothelial cells. This is supported by recent studies in the urinary bladder where urothelial-derived ATP release purportedly acts as a trigger for exocytosis—in part via autocrine activation of urothelial purinergic (P2X; P2Y) receptors (669). These findings suggest a mechanism whereby urothelial cells sense or respond to ATP and thereby translate extracellular stimuli into changes in afferent and urothelial function.
Prostaglandins are also released from the urothelium and are assigned two possible functions: (1) regulation of detrusor muscle activity and (2) cytoprotection of the urothelium, based on effective treatment of hemorrhagic cystitis by prostaglandins (293). The predominant forms found in biopsies of human urothelium are PGE2 > PGF2α > TXA2. PGI2 (prostacyclin) is also produced. These findings were confirmed and extended in the guinea pig bladder, where the major production of prostaglandins occurs in the urothelium and where production increases greatly with inflammation (530). In mice PGE2 provokes ATP release from cultured urothelial cells, which express EP1 receptors; and bladder overactivity induced by intravesical application of PGE2 is prevented in EP1 receptor-knockout mice, suggesting the involvement of EP1 receptors in the PGE2-mediated urothelial-afferent interaction (670).
The contribution of the muscarinic receptors to bladder function extends beyond detrusor contractility to urothelial-afferent interactions. Muscarinic receptors are expressed in the urothelium at high density (259) and there is a basal release of acetylcholine from the urothelium, which is increased by stretch and aging (694). Activation of the muscarinic receptors in the urothelium releases substances (e.g., ATP) that modulate afferent nerves and smooth muscle activity (50, 148, 259, 355, 356).
Muscarinic agonists also release a substance called urothelium-derived inhibitory factor that decreases the force of detrusor muscle contraction (259,361). The molecular identity of this factor is not known, however, pharmacological studies suggest that it is not NO, a prostaglandin, prostacyclin, adenosine, a catecholamine, GABA or an agent that acts via apamin sensitive, small-conductance K+ channels. It has been shown that the inhibitory response elicited by this factor is attenuated in a fetal model of bladder outlet obstruction (BOO) (631). Further studies are required to identify this substance and its role in bladder function.
Modulation of efferent neurotransmission
Studies in the urinary bladder of several species (rats, rabbits, human, and guinea pig) have revealed that the efficiency of transmission at postganglionic cholinergic and adrenergic neuroeffector junctions (579, 642) as well as at cholinergic synapses in bladder ganglia (152) can vary with the frequency and/or pattern of nerve activity and be modulated by drugs that activate or block receptors for neurotransmitters. This neuroplasticity is dependent in part on homosynaptic and heterosynaptic modulatory mechanisms mediated by the actions of various neurotransmitters (acetylcholine, norepinephrine, neuropeptides, and purines). Postganglionic nerve terminals and ganglonic synapses exhibit frequency-dependent gating mechanisms and are sites of “cross-talk” between sympathetic and parasympathetic nerves (128, 169–171, 549, 581). These properties can alter efferent nerve signals passing from the spinal cord to the bladder.
Prejunctional cholinergic inhibitory mechanisms
Different subtypes of muscarinic receptors on the parasympathetic and sympathetic postganglionic nerve endings have a role in inhibiting or facilitating transmitter release. The first studies of prejunctional modulation in the rat bladder (127, 128, 578) focused on inhibitory receptors that mediate an autoinhibitory control over ACh release. Administration of muscarinic receptor agonists to bladder strips decreases radiolabeled ACh release evoked by low frequency (1–2 Hz) electrical field stimulation. Atropine blocks the inhibition and when administered alone enhances release indicating that endogenously released ACh activates a negative feedback mechanism to suppress its own release. Low concentrations of physostigmine, an anticholinesterase agent, that reduces the metabolism and increases the extracellular concentration of ACh also produces an inhibition that is blocked by atropine. Studies with selective muscarinic receptor antagonists indicate that prejunctional inhibitory mechanisms are mediated by M4 receptors in rabbit, guinea pig, and human bladder and M2 receptors in rat bladder (12,125,126,565,641).
Activation of muscarinic receptors by cholinergic agonists or during low frequencies of electrical field stimulation also suppresses norepinephrine release from noradrenergic terminals in rabbit, cat, and human urinary bladder strips (108,577). Whereas block of muscarinic receptors enhances release indicating that cholinergic nerves can interact functionally with adrenergic nerves.
Prejunctional cholinergic facilitatory mechanisms
Stimulation frequencies higher than those producing prejunctional inhibition facilitates transmitter release in both parasympathetic and sympathetic nerves in the urinary bladder. This effect which has been demonstrated in several species including rat, rabbit, and human is mediated by activation of prejunctional muscarinic receptors (108, 578–580).
ACh release in rat urinary bladder strips is influenced by various stimulation parameters including frequency, pattern, and number of stimuli (108, 232, 580, 582, 641, 642). During intermittent field stimulation (IS), consisting of short trains (10 shocks) separated by 5 s quiescent periods, the duration of stimulation (5–360 shocks) had little effect on the total ACh output (nonfacilitatory stimulation). On the other hand, during continuous stimulation (CS) ACh release per volley markedly increases as the number of shocks are increased from 5 to 70 (facilitatory stimulation). The facilitation of ACh release is blocked by atropine or pirenzepine (an M1 selective antagonist) indicating that it is mediated by M1 muscarinic receptors. The lower range of CS (10 Hz, 100 shocks) which mimics the physiological firing rate of bladder parasympathetic neurons (155) and which produces maximal bladder contractions elicits a marked recruitment of ACh release (580).
Various evidence indicates that the phosphatidylinositolprotein kinase C (PKC) cascade which is known to be a signal transduction pathway linked to M1 muscarinic receptors (627) is involved in the prejunctional facilitation in the rat bladder (582). Activation of PKC by phorbol dibutyrate elicits a concentration dependent increase in ACh release, and the PKC blocker, H-7, suppresses the facilitation of ACh release induced by continuous stimulation. H-7 does not block non-facilitated release of ACh during intermittent stimulation indicating that the PKC system only participates in transmitter release under facilitatory conditions (582). Nifedipine significantly reduces facilitated release but not non-facilitated release indicating a role for L type Ca2+ channels in muscarinic receptor mediated facilitation.
High frequency (10 Hz) continuous stimulation enhances norepinephrine release from noradrenergic terminals (582). This effect is blocked by an M1 muscarinic receptor antagonist (pirenzepine) indicating that heterosynaptic cholinergic facilitation can modulate adrenergic transmission. The facilitation is suppressed by a PKC inhibitor (H7) and reduced by pharmacological suppression of PKC. The function of this facilitatory mechanism is uncertain.
Noncholinergic prejunctional modulatory mechanisms
Noncholinergic prejunctional modulatory mechanisms have also been identified in the urinary tract. Activation of α2 adrenergic receptors by adrenergic agonists or by electrical stimulation of bladder nerves suppresses norepinephrine release in the rat bladder and urethra (418, 419, 454, 577). Block of these receptors with an α2 adrenergic receptor antagonist, enhances release indicating that adrenergic terminals are subject to negative feedback inhibition by endogenous norepinephrine.
Prejunctional α1 facilitatory mechanisms have been identified in cholinergic nerves in the rat urinary bladder. α1 agonists, phenylephrine and methoxamine, enhance neurally mediated bladder contractions and increase release of ACh from cholinergic nerves (581). These effects are blocked by an α1 adrenergic receptor antagonist.
Neuropeptide Y (NPY) which is contained in a large percentage of adrenergic and cholinergic neurons and nerves in the bladder and urethra can suppress neurally evoked contractions as well as ACh and norepinephrine release in the rat urinary tract (648,738). The inhibitory effect of NPY in rat urinary tract is dependent on the frequency of nerve stimulation, being greater (80%–100% inhibition) at low frequencies (2–5 Hz) than at high frequencies (10–50 Hz). This raises the possibility that during urine storage when cholinergic parasympathetic nerve firing is low NPY release from adrenergic nerves can elicit heterosynaptic inhibition of cholinergic nerves and promote urinary continence (648). However, during micturition when the frequency of cholinergic nerve firing is high, NPY should have minimal effects on ACh release and therefore not interfere with efficient voiding.
Hormonal influence on transmitter release in the bladder
ACh release in the urinary bladder is influenced by sex hormone levels in adult female rats (693). Ovariectomy significantly reduces neurally mediated ACh release in bladder strips at frequencies between 5 and 40 Hz, but enhances basal and stretch evoked ACh release which is thought to occur in the urothelium. Estrogen replacement reverses the alterations in ACH release. The sensitivity of transmitter release to estrogen levels may contribute to impaired detrusor contractility that can occur in postmenopausal elderly women.
Modulation of transmission in autonomic ganglia
Transmission in the bladder ganglia is mediated by ACh acting on nicotinic cholinergic receptors. However, considerable differences have been noted between species in regard to: (i) the properties of the preganglionic input to the pelvic ganglia and (ii) facilitatory and inhibitory synaptic mechanisms. Preganglionic pathways to the pelvic ganglia vary in their conduction velocities, patterns of convergence onto ganglion cells and in the characteristics of transmitter release under different physiological conditions (152). In the cat, preganglionic parasympathetic input to the bladder ganglia is carried by myelinated (B-fiber) axons (166, 168, 171). In the rat and mouse preganglionic inputs to the major pelvic ganglion are primarily unmyelinated C-fibers (397,522) and in guinea pig the inputs are a mixture of B- and C-fibers (63).
Frequency dependent homosynaptic facilitation
A major difference between transmission in the parasympathetic ganglia of rat, guinea pig and mouse and transmission in ganglia in cats and rabbits is the magnitude of temporal facilitation and the safety factor for transmission. In the rat, single stimuli to the preganglionic axons in the pelvic nerve elicit large amplitude EPSPs and synaptically mediated postganglionic discharges (397,606). These responses do not change markedly in amplitude during repetitive stimulation with frequencies between 0.25 and 20 Hz. At high frequencies (30–50 Hz) the discharges decrease in amplitude. On the other hand, in the cat, single or low frequency stimuli (<0.25 Hz) elicit small amplitude EPSPs and postganglionic discharges which gradually increase in amplitude during continuous stimulation and are very prominent at frequencies of 1 to 20 Hz (Fig. 5) (74, 142, 151, 153, 154, 171). Maximal facilitation of transmission requires 15 to 25 stimuli in a train and persists for 30 to 60 s after termination of high frequency stimulation.
Figure 5.

Extracellular recordings on a bladder postganglionic nerve demonstrating facilitation of pelvic ganglionic transmission during repetitive stimulation of preganglionic axons in the pelvic nerve. (A) Action potentials evoked with submaximal (5V, 1 Hz) stimulation recorded with a slow time base. (B) Sample responses from A (a, 1st; b, 5th; c, 10th and d, 20th responses in the series) obtained, respectively, 5, 10, and 20 s later and recorded with a fast time base. (C) Average (20 sweeps) of a facilitated ganglionic response (I.I V, I Hz) showing the nonsynaptic, early response (ER) consisting of axonal volley and the facilitated late synaptic response (LR). (D) Depression by hexamethonium (100 μg, I.A.) of the late (synaptic) response but no effect on the early (nonsynaptic) response. The arrow below C denotes the stimulus artifact. Time calibration in D also applies to C; vertical calibration in A and B is 400 μV and in C and D is 200 μV, negativity upward (171).
Frequency dependent facilitation of transmission has also been noted in bladder ganglia of the rabbit (7, 474). As reported in the cat, low frequencies of preganglionic nerve stimulation (0.1–1 Hz) elicits EPSPs which are subthreshold for evoking action potentials. However, at higher frequencies (10–20 Hz) the EPSPs gradually increase in amplitude and initiate action potentials. The recruitment disappears within 30 s after the termination of repetitive stimulation and is followed by a prolonged facilitation of the EPSP which lasts for more than 10 min. Since the membrane depolarization elicited by exogenous ACh is not altered during recruitment it has been concluded that this phenomenon is dependent upon a presynaptic mechanism and enhancement of ACh release during repetitive preganglionic firing (74,151,474). It has, therefore, been concluded that synapses in cat and rabbit bladder ganglia have a low safety factor for transmission and function as “high pass” filters (142,171) and, therefore, act as a peripheral gating mechanism to suppress the transfer of low-frequency efferent activity from the spinal cord to the bladder during urine storage, but amplify the transfer of high-frequency activity during micturition. Based on the frequency dependence of synaptic transmission, cat and rabbit bladder ganglia can contribute to the maintenance of urinary continence and also promote bladder emptying. On the other hand the mouse, rat, and guinea pig parasympathetic ganglia seem to function as relay rather than integrative centers (63, 522, 606) and frequency-dependent modulation of efferent signals occurs at a more distal location (the postganglionic neuroeffector junction) as described earlier.
Muscarinic modulation of cholinergic transmission
In addition to nicotinic excitatory receptors certain types of pelvic ganglion cells also exhibit muscarinic excitatory receptors that can be stimulated by exogenous cholinergic agonists or by endogenously released ACh. Muscarinic excitatory receptors have been identified in the dog (616), cat (171, 238, 568) and mouse pelvic ganglia (522) but not in the rat (615).
In cat bladder ganglia, muscarinic receptor agonists elicit a postganglionic discharge which is resistant to hexamethonium, a nicotinic receptor antagonist, but is blocked by atropine, a muscarinic receptor antagonist (314). Endogenously released ACh can elicit a similar discharge following the administration of an anticholinesterase agent which blocks ACh metabolism and increases the extracellular concentration of ACh (171). The discharge is markedly enhanced by repetitive stimulation of preganglionic axons in the pelvic nerves and can be facilitated or inhibited by other transmitters (eg., VIP and norepinephrine) (314). These data indicate that under certain conditions endogenous ACh can mediate a muscarinic type of excitatory transmission.
Muscarinic cholinergic inhibitory mechanisms have also been identified in bladder ganglia (314, 549). Intra-arterial injections of muscarinic agonists (ACh, acetyl-B-methylcholine, and bethanechol) depress the postganglionic action potentials elicited by pelvic nerve stimulation. The inhibition is antagonized completely by atropine or in part by alpha adrenergic blocking agents (549). These data indicate that activation of muscarinic receptors can produce a direct inhibition of transmission and also an indirect inhibition through the release of transmitters from adrenergic inhibitory neurons or SIF cells in the pelvic plexus.
Intracellular recording of slow synaptic potentials in cat bladder ganglia (226,227,359,360,567,568) provided further support for muscarinic synaptic transmission. Two types of slow potentials were identified: (i) s-IPSPs and (ii) s-EPSPs. Cholinergic mechanisms have been implicated in both types of potentials, although other transmitters may also initiate slow synaptic responses in bladder ganglia (see next section). s-EPSPs and s-IPSPs are resistant to nicotinic blocking agents but are blocked by atropine; and therefore must be mediated by muscarinic receptors. The muscarinic s-IPSP is mediated by a Ca++-dependent K+ conductance (568); while the s-EPSP is mediated by a decrease in membrane conductance probably due to a decrease in potassium channels as in other autonomic ganglia (5).
Adrenergic modulation of cholinergic transmission
Electrical stimulation of sympathetic nerves (either the hypogastric nerve or the lumbar sympathetic chain) inhibits transmission in bladder ganglia of the cat (169,170,362,549) rabbit (649), guinea pig (3,423), and dog (489) but not in the rat (510, 606) or mouse (522).
Interest in the role of adrenergic transmitters in pelvic ganglia arose from the histochemical studies of Hamberger and Norberg (255, 256) which revealed a dense network of adrenergic varicosities surrounding presumed cholinergic neurons in cat bladder ganglia. The adrenergic varicosities are not altered by chronic decentralization of the ganglia (i.e., transection of the pelvic and hypogastric nerves) and therefore are assumed to arise from local adrenergic neurons or from SIF cells within the pelvic plexus. Histochemical experiments also identified pericellular baskets of adrenergic varicosities surrounding ganglion cells in the rabbit bladder and in the guinea pig hypogastric plexus (649, 685). This type of varicosity is less obvious in the rat major pelvic ganglion (131).
Pharmacological experiments in vivo revealed that transmission in cat bladder ganglia is sensitive to both inhibitory and facilitatory adrenergic modulatory mechanisms mediated by different types of adrenergic receptors. Epinephrine and norepinephrine which activate alpha and beta adrenergic receptors elicit an initial inhibition and a delayed and more prolonged facilitation, whereas isoproterenol, a selective beta adrenergic receptor agonist, elicits only facilitation (154, 169, 170, 172, 549). The inhibitory responses are markedly frequency dependent; being very prominent at low frequencies of stimulation (0.25–2 Hz) and considerably weaker at high frequencies of stimulation (5–10 Hz). Thus adrenergic modulation accentuates the temporal facilitation or “high-pass” characteristics of transmission in cat bladder ganglia (see previous section).
The adrenergic inhibitory responses are antagonized by nonselective alpha adrenergic blocking agents, (dihydroergotamine and phentolamine) and the facilitatory response to isoproterenol is antagonized by beta adrenergic blocking agents (propranolol). The facilitatory effects of epinephrine and norepinephrine are not antagonized by alpha or beta adrenergic blocking agents but are blocked by prazosin a selective α1 adrenergic receptor antagonist. These experiments revealed that two types of α-adrenoceptors are present in cat bladder ganglia: α2 inhibitory and α1 facilitatory (322, 466).
Adrenergic inhibition in bladder ganglia can also be activated by spinal reflex pathways via a sacral-lumbar intersegmental reflex arc consisting of an afferent limb in the pelvic nerve and a lumbar preganglionic cholinergic efferent limb that activates adrenergic inhibitory cells or SIF cells via both nicotinic and muscarinic receptors (170, 172, 549) (Fig. 6). The reflex is triggered by bladder afferent axons during bladder filling and appears to function as a negative feedback mechanism to promote the accommodation of the bladder to increasing volumes of urine. The reflex pathway initiates three responses in the bladder: (i) closure of the bladder neck, (ii) inhibition of the bladder smooth muscle, and (iii) inhibition of transmission in bladder ganglia. During micturition the reflex pathway is turned off by supraspinal mechanisms.
Figure 6.
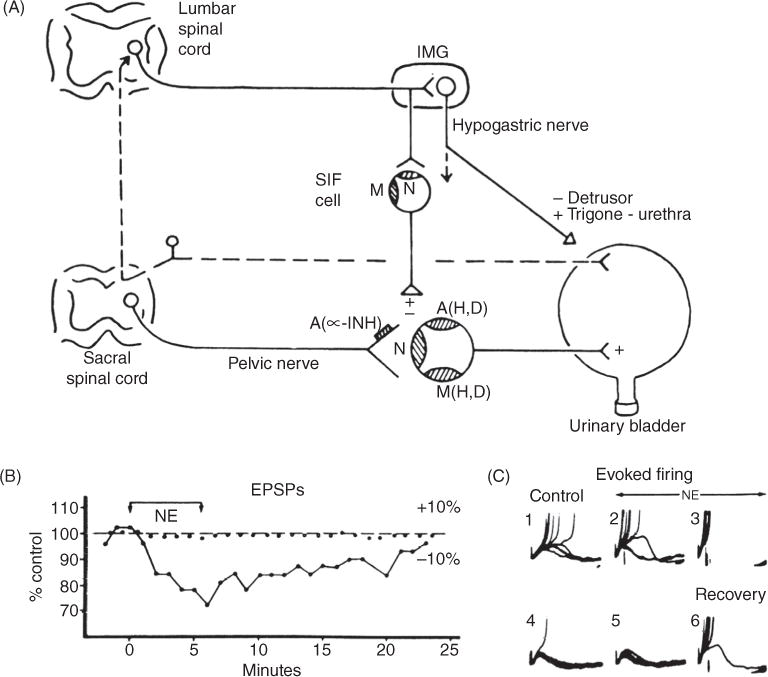
(A) Diagram showing the autonomic innervation of the urinary bladder of the cat and the synaptic mechanisms within bladder ganglia. Nicotinic (N), muscarinic (M), and adrenergic (A) receptors are depicted on a principal ganglion cell and a small intensely fluorescent cell (SIF cell). Receptors mediating hyperpolarization (H), depolarization (D) are also indicated. An α-adrenergic receptor (A, α-INH) mediating presynaptic inhibition is indicated on the preganglionic nerve terminal. Inhibitory and excitatory synaptic mechanisms are designated by − and +, respectively. Postsynaptic adrenergic and muscarinic receptors mediate both hyperpolarizing and depolarizing responses. Sympathetic preganglionic axons make synaptic contact with cells in the inferior mesenteric ganglion (IMG) and also send axons through the IMG to make synaptic contacts with SIF cells in bladder ganglia. SIF cells have both nicotinic and muscarinic excitatory receptors. Sympathetic efferent pathways can be activated by afferent projections from the urinary bladder. (B) Suppression of EPSP amplitude by exogenous norepinephrine (NE, 3 × 10−4). Membrane potential (0) and EPSP amplitude (●) recorded during preganglionic stimulation and following application of NE, (bar). (C) Series of 10 superimposed sweeps showing fast excitatory postsynaptic potentials (f-EPSPs) and action potentials (truncated) elicited before (#1), after start (#2 and #3) of perfusion with NE, (1 × 10−4) and 1 (#4), 8 (#5), and 16 min (#6) following return to the control solution. NE depressed f-EPSP amplitude and spike generation (151).
The mechanisms of adrenergic modulation of pelvic ganglionic transmission have been studied with intracellular recording techniques in vitro in cat (6, 151, 466, 567) and rabbit bladder ganglia (7,10,649). Application of low concentrations of norepinephrine (NE), dopamine or epinephrine (E) depresses orthodromically induced action potentials elicited by stimulation of preganglionic axons but does not affect the depolarization of the ganglion cells induced by the iontophoretic application of ACh. The depression of transmission by low concentrations of NE is accompanied by a decrease in the amplitude of the EPSPs elicited by pelvic nerve stimulation but is not associated with a consistent change in membrane potential (151, 649). These observations indicate that NE has a presynaptic inhibitory action to depress ACh release in bladder ganglia.
The postsynaptic effects of high concentrations of catecholamines on bladder ganglion cells are complex and involve two types membrane potential changes: hyperpolarization and depolarization (6, 7, 9, 151, 466), the former mediated by α2 and the latter by α1 adrenergic receptors.
Purinergic modulation of transmission
Neurons in the cat bladder ganglia stain for all seven P2X receptors and four types of P2Y receptors raising the possibility that ganglionic transmission might be modulated by purinergic agents (528). The most intense staining is obtained with P2X3, P2Y2, P2Y4, P2Y6, and P2Y2 antibodies. Double staining showed that 100%, 50% and 97% of P2X3 neurons coexpressed ChAT and NOS and NF200. This spectrum of receptors differs from that in the rat pelvic ganglion neurons which express high levels of P2X2 and low levels of P2X4 protein and mRNA but no message for P2X1 and P2X3 receptors (735). In the guinea pig pelvic neurons at least three P2X receptors (P2X2, P2X3, and P2X2/3) are present (201,736).
In cat bladder ganglia in situ various purinergic agonists including ATP,α-β methylene ATP, ADP, AMP, adenosine (ADS), and 2-chloroadenosine (2-Cl-ADS) administered intra-arterially depress cholinergic transmission and depress the bladder contractions elicited by stimulation of preganglionic axons in the pelvic nerve (151, 630). All of the nucleotides are equipotent in depressing transmission, except 2-Cl-ADS, an agent more resistant to cellular uptake and metabolism, that is 10-fold more potent than the other agents. This indicates that metabolism has a significant influence on the effectiveness of purinergic agents. Dipyridamole, which slows the cellular uptake of ADS enhances the inhibitory actions of AMP and ADS as well as those of ATP and ADP suggesting that the latter agents can be converted to ADS. Theophylline and caffeine block the inhibitory effects of purinergic agents on ganglionic transmission and on neurally evoked bladder contractions indicating that the inhibition is mediated by P1 receptors. Since purinergic agents inhibit the ganglionic stimulating effects of nicotinic agonists the P1 receptors must be located postsynaptically on the ganglion cells.
A synaptic inhibitory effect of endogenously released purinergic agents was identified using intracellular recordings from cat bladder ganglia in vitro. High-frequency (40 Hz) stimulation of the preganglionic nerve trunk elicits a noncholinergic, slow hyperpolarizing synaptic potential (8,567) which is increased in amplitude and duration by dipyridamole and is reduced in amplitude by adenosine deaminase, an enzyme that metabolizes adenosine. Caffeine, a P1 receptor antagonist, also blocks the synaptic potential. The slow hyperpolarizing synaptic potential is mimicked by the administration of various exogenous purinergic agonists and the relative order of potency of the agonists is consistent with an inhibitory response mediated by a P1 receptor.
Excitatory purinergic responses have also been observed in cat bladder ganglia in vivo after administration of large doses of ATP (50 to 100 times the threshold dose for inhibition). This firing is resistant to nicotinic ganglionic blocking agents and therefore is not mediated via the intraganglionic release of ACh (630). ATP, α,β-methylene-ATP, ATP-γ-S, and UTP also induce an increase in intracellular Ca2+ in a subpopulation of dissociated bladder ganglion cells (528) indicating that P2X3 and P2Y2/4 are functional receptors in these ganglia in the cat. All cells that responded to α,β-methylene-ATP also responded to UTP indicating coexpression of P2X3 and P2Y receptors in the same cells.
Patch clamp recordings in dissociated neurons from the rat and rabbit pelvic ganglia (475), and guinea pig bladder ganglia (737) revealed that ATP evokes a rapid depolarization or inward current via P2X receptors. Although 2-MeSATP and ATP are approximately equipotent in rat neurons α,β-me-ATP evokes only small responses consistent with the immunohistochemical data indicating that high levels of P2X2 receptors but not P2X1 or P2X3 receptors are expressed in these ganglia (737). On the other hand at least three types of P2X receptors are expressed in guinea pig pelvic ganglia (P2X2, P2X3, and P2X2/3) (736). Although ATP can be released from various nerves in ganglia and activation of P2X receptors by exogenously applied ATP can excite ganglion cells, it is still unclear if endogenously released ATP mediates excitatory transmission in bladder ganglia.
Enkephalinergic inhibitory modulation of transmission
In cat bladder ganglia a short train of stimuli (20 Hz for 5–10 s) applied to one preganglionic nerve can elicit a prolonged inhibition lasting for 40 to 60 s of excitatory transmission elicited by stimulation of another preganglionic nerve(159,160). This heterosynaptic inhibition contrasts with the marked facilitation of transmission lasting 700 to 800 ms which occurs with homosynaptic conditioning volleys (74, 171). Because this inhibition is resistant to drugs that block the actions of traditional transmitters, such as ACh, norepinephrine or GABA, but is suppressed by naloxone, an opioid receptor antagonist, the role of opioid peptides in the inhibition was examined.
The most prominent peptidergic system in pelvic ganglia is the enkephalinergic preganglionic pathway arising from neurons in lumbosacral parasympathetic nucleus in the spinal cord (160,236,266,316,320). Exogenous opioid peptides that activate delta opioid receptors mimick heterosynaptic inhibition of transmission (159, 570) whereas agents that activate mu and kappa opioid receptors are ineffective. The inhibitory effects of opioid peptides are frequency dependent, being very prominent at low frequencies of preganglionic nerve stimulation (0.25–0.5 Hz) and negligible at higher frequencies (5–7 Hz).
Intracellular recording from bladder ganglion cells in vitro demonstrated that enkephalinergic inhibition is accompanied by a decrease in the amplitude of EPSPs elicited by preganglionic nerve stimulation, an increase in the number of EPSP failures and a reduced probability of firing without a consistent change in resting membrane potential or resistance indicating that the inhibition is mediated by a presynaptic action (570).
These observations coupled with the immunocytochemical data demonstrating leucine enkephalin in presynaptic terminals (316) and intracellular observations regarding the mechanisms of enkephalinergic inhibition indicate that at high frequencies of preganglionic activity leucine enkephalin is released with ACh and activates δ opioid receptors on preganglionic terminals of the same or adjacent cells to elicit a prolonged reduction of ACh release. Since electron microscopy did not detect enkephalinergic axo-axonic synapses on preganglionic nerve terminals in bladder ganglia, enkephalinergic inhibition must be mediated entirely by “parasynaptic” interactions. In addition it is likely that enkephalins act in an autoinhibitory manner on the terminals from which they are released. Since heterosynaptic inhibition occurs at frequencies of preganglionic stimulation which are within the physiological range of firing of preganglionic neurons (159) it is likely that this mechanism acts synergistically with other inhibitory pathways in bladder ganglia to regulate the transmission of neural activity from the spinal cord to the urinary bladder.
Overview of Lower Urinary Tract Activity During Storage and Voiding
The neural pathways controlling LUT function are organized as simple on-off switching circuits that maintain a reciprocal relationship between the urinary bladder and urethral outlet. Intravesical pressure measurements during bladder filling in both humans and animals reveal low and relatively constant bladder pressures when bladder volume is below the threshold for inducing voiding. The accommodation of the bladder to increasing volumes of urine is primarily a passive phenomenon dependent upon the intrinsic properties of the vesical smooth muscle and quiescence of the parasympathetic efferent pathway. In addition, in some species urine storage is also facilitated by sympathetic reflexes that mediate an inhibition of bladder activity, closure of the bladder neck, and contraction of the proximal urethra. During bladder filling the activity of the sphincter electromyogram (EMG) also increases (Fig. 7), reflecting an increase in efferent firing in the pudendal nerve and an increase in outlet resistance that contributes to the maintenance of urinary continence.
Figure 7.

Reflex voiding responses in an infant, a healthy adult, and a paraplegic patient. Combined cystometrograms and sphincter electromyograms (EMGs, recorded with surface electrodes), allowing a schematic comparison of reflex voiding responses in an infant (A) and in a paraplegic patient (C) with a voluntary voiding response in a healthy adult (B). The abscissa in all recordings represents bladder volume in millilitres; the ordinates represent electrical activity of the EMG recording and detrusor pressure (the component of bladder pressure that is generated by the bladder itself) in cmH2O. On the left side of each trace (at 0 mL), a slow infusion of fluid into the bladder is started (bladder filling). In part B, the start of sphincter relaxation, which precedes the bladder contraction by a few seconds, is indicated (“start”). Note that a voluntary cessation of voiding (“stop”) is associated with an initial increase in sphincter EMG and detrusor pressure (a myogenic response). A resumption of voiding is associated with sphincter relaxation and a decrease in detrusor pressure that continues as the bladder empties and relaxes. In the infant (A), sphincter relaxation is present but less complete. On the other hand, in the paraplegic patient (C) the reciprocal relationship between bladder and sphincter is abolished. During bladder filling, involuntary bladder contractions (detrusor overactivity) occur in association with sphincter activity. Each wave of bladder contraction is accompanied by simultaneous contraction of the sphincter (detrusor-sphincter dyssynergia), hindering urine flow. Loss of the reciprocal relationship between the bladder and the sphincter in paraplegic patients thus interferes with bladder emptying (216).
The storage phase of the urinary bladder can be switched to the voiding phase either involuntarily (Fig. 7A) or voluntarily (Fig. 7B). The former is readily demonstrated in the human infant when the volume of urine exceeds the micturition threshold. At this point, increased afferent firing from tension receptors in the bladder produces firing in the sacral parasympathetic pathways and inhibition of sympathetic and somatic pathways. The expulsion phase consists of an initial relaxation of the urethral sphincter followed by a contraction of the bladder, an increase in bladder pressure, and flow of urine. Relaxation of the urethral outlet is mediated by activation of a parasympathetic reflex pathway to the urethra that triggers the release of NO, an inhibitory transmitter, as well as by removal of adrenergic and somatic excitatory inputs to the urethra.
Model of CNS Lower Urinary Tract Control
The switch of LUT function between storage and voiding is mediated by a long-loop spinobulbospinal voiding reflex which has its rostral terminus in the brainstem (see the lower part of Fig. 8) (216, 244). During urine storage, as the bladder fills, bladder (sacral) afferent signals increase in strength until they exceed a certain threshold in the brainstem, specifically the periaqueductal gray (PAG). In the absence of any controlling influences the reflex then fires: that is, the pontine micturition center (PMC) is activated, the urethral sphincter relaxes, the bladder contracts, and voiding occurs. When the bladder is empty, urine storage resumes. Brain imaging in the rat (614) has confirmed this picture: during storage, the PAG is activated by afferent input from the bladder while the PMC is inactive. When the bladder volume reaches the micturition threshold, the switch from storage to micturition is associated with PMC activation and enhanced PAG activity.
Figure 8.

A simple working model of the lower urinary tract control system, showing the voiding reflex and brainstem (green) and circuits 1, 2, and 3 (red/blue, yellow, and blue, respectively). PAG = periaqueductal gray; PMC = pontine micturition center; th = thalamus; mPFC = medial prefrontal cortex; lPFC = lateral prefrontal cortex; SMA = supplementary motor area; dACC = dorsal anterior cingulate cortex.
If this voiding reflex operated in isolation without restraining influences it would fire whenever the bladder volume (and therefore the bladder afferents) reached the threshold level, precipitating involuntary voiding (incontinence). Normal adults however can postpone or hasten the moment of firing voluntarily and thus ensure that urination occurs only if it is consciously desired, emotionally safe and socially appropriate. Inability to exert such control is abnormal, as exemplified by urgency or urge incontinence [the complaint of involuntary leakage accompanied by or immediately preceded by urgency (4)] or Fowler’s syndrome [inability to empty the bladder with impaired bladder sensation (605)].
A simplified working model that encapsulates our current understanding of the control system is shown in Figure 8. The brainstem contains two principal nuclei concerned with voiding, the PAG and the PMC [also referred to as the “M-region” or “Barrington’s nucleus” (268)]. The PAG projects to many parts of the forebrain, receives ascending afferent activity from the bladder, and sends efferent signals back to the bladder and urethra. It is therefore well placed to perform the signal processing required to set the threshold value for the voiding reflex (45), and thus modulate the onset of voiding. If the threshold is exceeded a signal is sent to the PMC, which in turn provides descending output to the sacral spinal nuclei and initiates voiding. The PMC may also receive a “safe to void” signal from the hypothalamus (268), and—in rats—it may coordinate elimination and voiding behavior (656). The PAG however occupies a pivotal location between brain and bladder: it can both pass information about the bladder to higher parts of the brain, and also receive information back from the cerebral control system so as to suppress or enhance storage or voiding (Fig. 8).
There is some evidence for the involvement of a third brainstem nucleus, lateral and ventral to the PMC and not directly connected to it (67). It is called the L-region, the pontine urine storage center or sometimes the lateral pontine continence center (Fig. 9) (601). Excitation of this region is believed to tighten the urethral sphincter (70).
Figure 9.

Neural connections between the brain and the sacral spinal cord that may regulate the lower urinary tract in the cat. Lower section of the sacral spinal cord shows the location and morphology of a preganglionic neuron in the sacral parasympathetic nucleus (PSN), a sphincter motor neuron in Onuf’s nucleus (ON), and the sites of central termination of afferent projections (shaded area) from the urinary bladder. Upper section of the sacral cord shows the sites of termination (shaded areas) of descending pathways arising from the medial pontine micturition center (PMC), the lateral pontine sphincter or urine storage center, and the paraventricular nuclei of the hypothalamus. Section through the pons shows the projection from the anterior hypothalamic nuclei to the pontine micturition center (PMC).
Anatomy of the Spinal Pathways Controlling the Lower Urinary Tract
The reflex circuitry controlling micturition consists of four basic components: primary afferent neurons, spinal efferent neurons, spinal interneurons, and neurons in the brain that activate or modulate spinal reflex pathways (Figs. 8–11).
Figure 11.

Connections between the lumbosacral spinal cord and brain areas involved in bladder control. The central pathways involved in controlling the urinary bladder can be visualized in rats using transneuronal virus tracing. Injection of pseudorabies virus into the wall of the urinary bladder leads to retrograde transport of the virus (indicated by the dashed arrows) and the sequential infection of postganglionic neurons, preganglionic neurons, spinal interneurons, and then various supraspinal neural circuits that are synaptically linked to the spinal preganglionic neurons and interneurons. The supraspinal sites labeled by the virus transport include the pontine micturition centre (also known as Barrington’s nucleus), the cerebral cortex, the paraventricular nucleus (PVN), the medial preoptic area (MPOA) and periventricular nucleus (PeriVN) of the hypothalamus, the periaqueductal gray (PAG), the locus coeruleus (LC) and subcoeruleus, the red nucleus (Red N.), the raphe nuclei, and the A5 noradrenergic cell group. Synaptic connections are indicated by solid arrows. Synaptic inputs from supraspinal neurons can project to spinal preganglionic neurons or interneurons, as indicated by the bracket (216).
Afferent projections in the spinal cord
Sacral afferent pathways from the cat, monkey and rat bladder passing through the pelvic nerve project into Lissauer’s tract at the apex of the dorsal horn and then send collaterals laterally and medially around the dorsal horn into laminae V–VII and X at the base of the dorsal horn (Figs. 9 and 10A) (445, 455, 458, 585). The lateral pathway terminates in the region of the sacral parasympathetic nucleus. Bladder afferents have not been detected in the center of the dorsal horn (laminae III–IV) or in the ventral horn. Afferents from the pelvic viscera of the cat passing through sympathetic nerves to the rostral lumbar segments have similar sites of termination in laminae I, V–VII, and X (443). The afferents are distributed primarily to the ipsilateral side of the spinal cord with an estimated 10% to 20% projecting to the opposite side (25, 292).
Figure 10.

Primary afferent and spinal interneuronal pathways involved in micturition. (A) Primary afferent pathways to the L6 spinal cord of the rat project to regions of the dorsal commissure (DCM), the superficial dorsal horn (DH), and the sacral parasympathetic nucleus (SPN) that contain parasympathetic preganglionic neurons. The afferent nerves consist of myelinated (Aδ) axons, which respond to bladder distension and contraction, and unmyelinated (C) axons, which respond to noxious stimuli. (B) Spinal interneurons that express c-fos following the activation of bladder afferents by a noxious stimulus (acetic acid) to the bladder are located in similar regions of the L6 spinal segment. (C) Spinal interneurons involved in bladder reflexes (labeled by transneuronal transport of pseudorabies virus injected into the urinary bladder) are localized to the regions of the spinal cord that contain primary afferents and c-fos. Some of these interneurons provide excitatory and inhibitory inputs to the parasympathetic preganglionic neurons located in the SPN. (D) The laminar organization of the cat sacral spinal cord, showing the location of parasympathetic preganglionic neurons in the intermediolateral region of laminae V and VII (shaded area). CC, central canal; IL, intermediolateral nucleus; LT, Lissauer’s tract; VM, ventromedial nucleus (Onuf’s nucleus) (216).
Pudendal nerve afferent pathways as well as those from the EUS of the cat, rat and monkey have central terminations that overlap in part with those of bladder afferents in lateral laminae I, V–VII, and in lamina X (145, 422, 524, 639). These afferents differ markedly from other populations of pudendal nerve afferents innervating the sex organs as well as cutaneous and subcutaneous tissues of the perineum that terminate in the deeper layers of the dorsal horn (laminae II–IV) (317, 639, 653).
Efferent neurons in the spinal cord
Parasympathetic preganglionic neurons (PGNs) are located in the intermediolateral gray matter (laminae V–VII) in the sacral segments of the spinal cord (166, 455, 456, 458); whereas sympathetic preganglionic neurons are located in medial (lamina X) and lateral sites (laminae V–VII) in the rostral lumbar spinal cord. EUS motoneurons are located in lamina IX in Onuf’s nucleus in the cat (Fig. 9) and in the dorsolateral motor nucleus in the rat (422, 633).
Parasympathetic PGN exhibit dendrites projecting to four major areas: (i) the lateral and dorsolateral funiculus, (ii) lamina I on the lateral edge of the dorsal horn, (iii) the dorsal gray commissure, and (iv) the gray matter and lateral funiculus ventral to the autonomic nucleus (Fig. 9) (447). It has been speculated that these dendritic projections reflect the major synaptic inputs to the PGN; that is, lamina I dendrites receiving primary afferent projections, lateral funiculus dendrites receiving bulbospinal projections, and medial dendrites receiving interneuronal projections from the dorsal commissure and inputs from the contralateral side of the spinal cord. EUS motoneurons have a similar dendritic pattern (Fig. 9) (633).
The most striking feature of the sacral PGNs in the cat is an extensive axon collateral system that projects bilaterally to various regions of the dorsal and ventral horns including the area around the central canal, the intermediolateral gray matter, the dorsal commissure and the lateral dorsal horn (446). These axon collaterals are likely to be involved in a bilateral recurrent inhibitory pathway that regulates the parasympathetic outflow to the bladder (143, 167).
Spinal interneurons
Spinal interneurons involved in LUT function have been identified by retrograde transneuronal labeling after injection of pseudorabies virus (PRV) into the urinary bladder, urethra or EUS of the rat. PRV which is taken up and transported from peripheral efferent terminals to efferent neurons in the spinal cord crosses multiple synapses to infect interneuronal circuitry throughout the central nervous system (Figs. 10C and 11). PRV labeled spinal neurons are located in the same general regions of the spinal cord that receive afferent input from the bladder including the dorsal commissure, laminae I and V and lamina VII just dorsal and medial to the PGN (Fig. 10C) (402, 460–463, 604, 664). Spinal interneurons in these locations receiving afferent input from the LUT have also been identified by firing in response to stimulation of bladder afferents (164, 425, 426) or by the expression of the immediate early gene, c-fos after chemical or mechanical stimulation of the bladder and urethra (Fig. 10B) (56, 60). Some of these interneurons make excitatory and inhibitory synaptic connections with PGN (27, 28, 150, 429) and participate in segmental spinal reflexes (150); whereas others send long projections to supraspinal centers, such as the PAG (Figs. 8 and 12), PMC (Barrington’s nucleus), the hypothalamus and thalamus that are involved in the supraspinal control of micturition (60, 68, 71, 184, 202, 270, 425).
Figure 12.

Neural circuits that control continence and micturition. (A) Urine storage reflexes. During the storage of urine, distention of the bladder produces low-level vesical afferent firing. This in turn stimulates the sympathetic outflow in the hypogastric nerve to the bladder outlet (the bladder base and the urethra) and the pudendal outflow to the external urethral sphincter. These responses occur by spinal reflex pathways and represent guarding reflexes, which promote continence. Sympathetic firing also inhibits contraction of the detrusor muscle and modulates neurotransmission in bladder ganglia. A region in the rostral pons (the pontine storage centre) might increase striated urethral sphincter activity. (B) Voiding reflexes. During the elimination of urine, intense bladderafferent firing in the pelvic nerve activates spinobulbospinal reflex pathways (shown in blue) that pass through the pontine micturition centre. This stimulates the parasympathetic outflow to the bladder and to the urethral smooth muscle (shown in green) and inhibits the sympathetic and pudendal outflow to the urethral outlet (shown in red). Ascending afferent input from the spinal cord might pass through relay neurons in the periaqueductal gray (PAG) before reaching the pontine micturition centre. Note that these diagrams do not address the generation of conscious bladder sensations, nor the mechanisms that underlie the switch from storage to voluntary voiding, both of which presumably involve cerebral circuits above the PAG. R represents receptors on afferent nerve terminals (216).
Axonal projections from the brain to the spinal cord
Transneuronal PRV tracing methods have also identified many populations of neurons in the rat brain that are involved in the control of bladder (460, 462, 463, 604), urethra (664), and the EUS (402, 461, 462), including the PMC (Barrington’s nucleus), PAG, medullary raphe nuclei, which contain serotonergic neurons; the locus coeruleus (LC), which contains noradrenergic neurons, and the A5 noradrenergic cell group (Fig. 11). More rostral regions in the hypothalamus (periventricular medial preoptic and paraventricular nuclei), dorsal thalamus, the primary and secondary motor cortices and entorhinal and piriform cortices also exhibit virus-infected cells. In the cat PRV tracing from the urinary bladder or the EUS identified efferent neurons and interneurons in the spinal cord as well as a cluster of neurons extending from the PMC ventrolaterally into the pontine reticular formation (150).
Neurons in Barrington’s nucleus in the rat are also labeled after injections of PRV into the colon (497, 655, 659). Dual PRV tracing in which different tracers were injected into the colon and bladder revealed that three populations of neurons are present in Barrington’s nucleus: (i) bladder labeled, (ii) colon labeled, and (iii) neurons synaptically linked to both organs (526). These experiments suggest that neurons in this nucleus are involved in the control of multiple excretory functions (527, 655, 659).
Other anatomical studies in which anterograde tracers were injected into areas of the cat brain revealed labeled axon terminals in regions of the brain and spinal cord consistent with the virus tracing data (Fig. 9). Tracer injected into the paraventricular nucleus of the hypothalamus labeled terminals in the sacral parasympathetic nucleus as well as the sphincter motor nucleus (44, 270). Injections of tracers into the anterior hypothalamus or PAG (65) labeled terminals in the PMC, whereas tracers in the PMC labeled axonal projections to the sacral parasympathetic nucleus, the lateral edge of the dorsal horn and the dorsal commissure (64,66), areas containing dendrites of preganglionic neurons, sphincter motoneurons, and afferent inputs from the bladder (Fig. 9). Conversely, projections from neurons in the ventrolateral pons in the cat, an area identified as the pontine urine storage center (PUSC) (363), terminate rather selectively in the sphincter motor nucleus (Fig. 9) (269). Thus, the sites of termination of descending projections from the pons are optimally located to regulate reflex mechanisms at the spinal level.
Subcortical urine storage mechanisms
Sympathetic storage reflex
Although the sympathetic input to the LUT is not essential for the performance of micturition, it does contribute to the storage function of the bladder. Surgical interruption or pharmacological blockade of the sympathetic innervation can reduce urethral outflow resistance, reduce bladder capacity, and increase the frequency and amplitude of bladder contractions recorded under constant volume conditions (152).
Sympathetic reflex activity is elicited by a sacrolumbar intersegmental spinal reflex pathway that is triggered by vesical afferent activity in the pelvic nerves (Figs. 6 and 12) (163). The reflex pathway is inhibited when bladder pressure is raised to the threshold for producing micturition. This inhibitory response is abolished by transection of the spinal cord at the lower thoracic level, indicating that it originates at a supraspinal site, possibly the PMC (Fig. 12). Thus, the vesicosympathetic reflex represents a negative feedback mechanism that allows the bladder to accommodate larger volumes during bladder filling but is turned off during voiding to allow the bladder to empty completely.
Urethral sphincter storage reflexes
Motoneurons innervating the striated muscles of the EUS exhibit a tonic discharge that increases during bladder filling (633). This activity is mediated in part by a spinal reflex pathway (the guarding reflex) activated by low-level afferent input from the bladder (Fig. 7). Studies in cats have also suggested that neurons in the ventrolateral region of the pontine reticular formation provide a tonic excitatory input to the EUS motoneurons (269, 270). Electrical stimulation in this region (termed the PUSC) (363, 364) excites the EUS motoneurons and induces contractions of the EUS (269,341, 363).
Contraction of the EUS also induces firing in afferent axons in the pudendal nerve which in turn activate inhibitory interneurons in the spinal cord that suppress reflex bladder activity (158,421) by inhibiting PGN and interneurons on the micturition reflex pathway (144, 155). Thus the bladder-to-EUS-to-bladder reflex pathway represents a second negative feedback mechanism in the spinal cord that promotes urinary continence. Activation of afferents in the pudendal nerve, some of which very likely innervate the EUS, also elicit reflex contractions of the EUS and contribute to continence (633). During micturition the firing of sphincter motoneurons and the negative feedback is inhibited. This inhibition which is mimicked by electrical stimulation of the PMC and activation of bulbospinal pathways (Fig. 12) (346, 348) is less prominent in chronic spinal animals (633); and is, therefore, dependent in part on supraspinal mechanisms.
Rodents exhibit a similar increase in tonic EUS activity during bladder filling and a marked increase just prior to micturition (Fig. 13) (104). This tonic activity is triggered by bladder afferent input and mediated by a spinal segmental reflex pathway (96–98)(Figs. 12 and 14). However, during voiding the EUS exhibits a bursting (phasic) pattern of activity (Fig. 13) that produces pulsatile release of urine. In rats, the bursting EUS activity is mediated by a spinobulbospinal pathway activated by bladder afferents (98). It has been proposed that the descending limb of this pathway passes through a central pattern generator (spinal bursting center) in the third and fourth lumbar segments of the spinal cord (Fig. 14). The EUS bursting is initially eliminated by transection of the thoracic spinal cord (98) but recovers in chronic spinal animals as a result of reorganization of spinal reflex circuitry (Fig. 14) (97, 98, 104, 194). In the rat recordings of single EUS motor units as well as intracellular recordings from EUS motoneurons revealed tonic and burst firing in some units and primarily burst firing in others, raising the possibility of two separate excitatory inputs to EUS motoneurons that allow some to contribute to storage and voiding and others to contribute primarily to voiding (129).
Figure 13.

Bladder (top traces) and EUS EMG activity (bottom traces) recorded during a continuous transvesical infusion CMG in an anesthetized rat. (A) Intravesical pressure and EUS EMG activity were relatively stable during the filling phase. A reflex bladder contraction, indicated by an abrupt, large increase in bladder pressure, was accompanied by large-amplitude EUS EMG activity. (B) Same recording indicated by asterisk in A shown at faster time scale. The bracket in B indicates the recording period in C, and the bracket in C indicates the recording period in D at a faster time scale. Note the decline in intravesical pressure during EUS EMG bursting in B and C, which indicates the period of voiding. (C) Tonic EUS EMG activity precedes the large rise in intravesical pressure and shifts to a bursting pattern at the peak of bladder contraction before the onset of voiding. Small oscillations in intravesical pressure coincide with each burst of EMG activity. (D) Recordings in C shown at very fast time scale showing individual EUS EMG bursts composed of active (AP) and silent periods (SP; brackets) and the small fluctuations in intravesical pressure accompanying each burst. Vertical calibration, intravesical pressure (in cmH2O); horizontal calibration, time (in minutes or seconds); Inf, start of saline infusion (104).
Figure 14.

Reflex pathways to EUS and bladder in rat in spinal intact (A) and after chronic thoracic spinal cord transection (SCT) (B); (C) pelvic afferent to EUS reflexes. (A) Diagram showing putative reflex pathways mediating reflex micturition and tonic and bursting EUS activity in spinal cord-intact (A) and chronic T8–9 SCT rats (B). (A) Spinobulbospinal micturition reflex pathway is shown by the solid line passing through the pontine micturition center (PMC) in the rostral brain stem. The hypothesized pathway mediating EUS bursting is shown by the dotted line also passing through the PMC. In spinal cord-intact rats, when the bladder is distended, afferent input from bladder mechanoreceptors passes via the pelvic nerve to the L6-S1 spinal cord to the spinal EUS-control center to generate tonic EUS activity and the ER. Input from the L6-S1 spinal cord passes to the PMC, which then projects to the lumbosacral micturition center to generate reflex bladder contractions and to L3-4 bursting center to generate EUS bursting. The spinal EUS bursting center provides an excitatory input to the spinal EUS-control center to initiate an excitatory outflow to the EUS. The spinal EUS-control center in the L6-S1 spinal cord consists of interneuronal and motoneuronal circuitry that regulates EUS activity. (B) After SCT, descending input from the PMC to spinal centers is interrupted. This initially eliminates the micturition reflex, the long latency EUS late reflex (LR), and EUS bursting. The short latency EUS early reflex (ER) and tonic EUS activity mediated by a spinal reflex pathway are preserved. However, in chronic SCT rats it is hypothesized that reorganization of synaptic connections in the spinal cord leads to the reemergence of the micturition reflex as well as the LR and EUS bursting. This reorganization depends on the formation of new pathways between pelvic primary afferent nerves and the L3-4 spinal EUS bursting center (dotted line) and spinal micturition center (solid line) or upregulation of pathways that exist in the spinal intact animals. (C) Pelvic afferent to EUS reflexes in spinal intact (top), acute SCI (middle), and chronic SCI rats (bottom tracing). Early reflex (ER) persists after acute SCI but late reflex (LR) is abolished. However, LR recovers in SCT rats if connections between L3-L4 and L6-S1 spinal segments are intact (98).
Brain stem-spinal storage mechanisms
Electrical stimulation of the PUSC located ventrolateral to the PMC not only excites the EUS but also inhibits reflex bladder activity, increases bladder capacity and the inhibits the bladder excitatory effect of PMC stimulation (601). Neurons in the region of the PUSC project to the nucleus raphe magnus (NRM) in the medulla which contains neurons that in turn project to the lumbosacral spinal cord. Electrical (147, 427, 450, 602) or chemical stimulation (103) in the NRM induces serotonergic inhibition of reflex bladder activity. Thus neurons in the PUSC may activate descending inhibitory pathways to the sacral parasympathetic nucleus (601).
Electrical stimulation of the rostral pontine reticular formation (RPRF) ventral to the PMC in an area also known as the nucleus reticularis pontis oralis, inhibits reflex bladder contractions in cats and rats (329, 472, 598, 601). Neurons in this region project to the spinal cord and also to nucleus reticularis gigantocellularis located in the rostrodorsal medulla. The RPRF projects to lumbosacral glycinergic inhibitory neurons that may mediate the inhibitory effects of RPRF stimulation (601).
Voiding Mechanisms
Cerebral control of voiding revealed by human brain imaging
Brain imaging to study the behavior of the LUT is part of the developing field of brain-body medicine (367). It straddles the boundary between the understanding of physiology for its own sake and the hope of improved therapy for disorders of bladder and urethral control. Consequently, considerable attention has been paid to human subjects and to pathophysiological situations, which offer the possibility on the one hand of better treatment of functional disorders, and on the other, by comparison with normal, better understanding of physiology.
Prior to the widespread use of functional brain imaging, animal experiments and clinical observations had revealed numerous brain areas with excitatory or inhibitory effects on voiding (Fig. 15A) (647). Functional brain imaging in humans, starting with single photon emission computed tomography (SPECT) by Fukuyama et al in 1996 (222), has enabled simplification of this picture to a few essential elements (Fig. 8). It has also provided clarity about both normal function and what can go wrong in pathological conditions, particularly conditions implying impaired bladder control such as urgency incontinence or overactive bladder (OAB, a portmanteau expression for urgency incontinence with or without urgency and frequency of micturition (4).
Figure 15.

(A) A representation (prior to the advent of functional brain imaging) of cerebral areas involved in micturition. + = facilitation, − = inhibition; ac = anterior cingulate gyrus, am = amygdala, pl = paracentral lobule, po = preoptic nucleus, rf = pontine reticular formation, sc = subcallosal cingulate gyrus, se = septal area, sfg = superior frontal gyrus. (B) Sagittal section (8 mm off midline) showing medial frontal region (yellow) and presumptive PMC (small yellow region in brainstem) activated during voiding (70, 647).
A potential problem in interpreting imaging data is that neuronal activations (which are derived from local bloodoxygen changes) may represent either excitatory or inhibitory activity: it is usually not known which. Another is that regional deactivation as well as activation may be encountered. Deactivation means that, in response to an event such as bladder filling, activity in the given location falls to below its initial resting value. It is commonly observed in a specific “default mode network” that is active during resting conditions (514). Deactivation is a sign that resting activity is suspended while the brain uses its resources to process an event requiring conscious attention.
In 1997, Holstege’s group published a study of brain activation during filling and voiding in men (70), using positron emission tomography (PET). It established a framework of thinking that has informed studies in this area ever since. Activation was demonstrated in the dorsal pons, the presumptive location of the PMC, in subjects who were able to void in the scanner, and also in the medial prefrontal cortex (Fig. 15B). In those who tried to void but could not do so there was activation in the putative pontine L-region, while medial prefrontal activity was less pronounced (70).
A review of human functional brain imaging experiments conducted using PET or functional magnetic resonance imaging (fMRI) in these and other early studies (241) summarized the midbrain and pontine locations of activation that had been found. Activations were clustered near the PMC (mostly during voiding), more rostrally near the PAG, and also near the putative L-region (Fig. 16A). In PET imaging experiments and also more recent fMRI studies the PAG was activated on bladder filling (34, 240), consistent with the arrangement of bladder control shown in Figure 8. The rat study referred to above has confirmed the pontine location of the storage/voiding switch (614). Table 3 summarizes human brainimaging observations of the principal regions shown in Fig. 8.
Figure 16.

(A) Brainstem and midbrain areas activated during withholding of urine or with full bladder, or during voiding, projected on a lateral view of the brain. Based on PET, fMRI, and SPECT studies in healthy controls, adapted from Ref. 16 (with permission). (B) In urgency-incontinent women, regions activated (a, yellow) and deactivated (b, blue) during the sensation of urgency. SMA = supplementary motor area; SFG = superior frontal gyrus; dACG = dorsal anterior cingulate gyrus; RI = right insula; dlPFC = dorsolateral prefrontal cortex; PFC = prefrontal cortex (ventromedial or medial) [A (217,241), B (609)).
Table 3.
Selected Observations of Principal Regions of the Working Model, Concerned with Bladder Control During the Storage Phase (with Bladder Filling or Withholding, or with Full Bladder and Urgency but Without Bladder Contraction)
| Region | Authors | Coordinates/BA | Subjects | Notes |
|---|---|---|---|---|
| mPFC | Griffiths 2009 (245) | 6, 62, 24 | Women | Deact |
| Tadic 2010 (607) | −8, 52, 22 BA 9 | Women with OAB, urgency | Deact | |
| Tadic 2010 (608) | −6, 38, −4 BA 32 | Women with OAB | Deact | |
| Blok et al. 1997 (70) | 8, 24, 24 BA 24, 32 | Normal men | Deact, PET | |
| dACC/SMA | Griffiths et al. 2007 (242) | −6, 14, 34 | Women with OAB | |
| Griffiths 2009 (245) | 0, 6, 30 | Normal women | ||
| Tadic 2010 (607) | −10, 4, 50 BA 24 | Women with urgency | ||
| Athwal 2001 (34) | −2, 18, 22 | Normal men | PET | |
| Matsuura 2002 (417) | 8, 43, 7 BA32 | Normal men | PET | |
| Insula | Blok et al. 1998 (69) | 38, 10, 12 | Normal women | PET |
| Nour 2000 (481) | −40, 14, 2 | Normal men | PET | |
| Blok 1997 (70) | 32, 24, 12 | Normal men | PET | |
| Matsuura 2002 (417) | −26, −3, 15 | Normal men | PET | |
| Griffiths et al. 2007 (242) | 50, −4, 2 | Normal women | ||
| Griffiths et al. 2007 (242) | 34, 8, 16 | Women with OAB | ||
| Tadic et al. 2008 (610) | 44, 6, 4 | Normal women | ||
| Tadic et al. 2008 (610) | 38, −4, 16 | Women with OAB | ||
| Griffiths et al. 2009 (245) | 34, 8, 16 | Normal women | ||
| Tadic et al. 2010 (607) | 34, 24, 4 BA 13 | Women with urgency | ||
| Seseke et al. 2013 (563) | 36, 8, 8 | Men with prostate ca | ||
| Tadic et al. 2012 (609) | 32, 20, 4 BA 13 | Women with UUI and DO | ||
| lPFC | Tadic et al. 2012 (609) | 38, 30, 6 BA 47 | Women with UUI and DO | |
| Tadic et al. 2008 (610) | 50, 8, 14 | Women with urgency | ||
| Tadic et al. 2008 (610) | 58, 6, 14 | Normal women | ||
| Athwal 2001 (34) | 56, 40, 16 | Normal men | PET | |
| Yin 2008 (684) | 66, 30, −2 BA 45,47 | Normal men | SPECT | |
| Seseke 2013 (563) | 52, 3, 21 | Men with prostate ca | ||
| Blok 1998 (69) | 44, 44, 12 BA 47 | Normal women | PET |
mPFC = medial prefrontal cortex, dACC = dorsal anterior cingulate cortex, SMA = supplementary motor area, lPFC = lateral prefrontal cortex, BA = Brodmann area, OAB = overactive bladder, DO = detrusor overactivity, PET = positron emission tomography, SPECT = single photon emission computed tomography. Based on functional magnetic resonance imaging (fMRI) except where noted.
Recently, fMRI has become the method of choice because it is relatively inexpensive and requires no radioactivity. Ideally, to obtain reliable data from the rather noisy fMRI signal, averaging of numerous repetitions of the behavior to be studied, recorded over several minutes, is desirable. Protocols (“paradigms”) have been developed to do this during storage and during simulated voiding (relaxation of the pelvic floor without actual voiding) (352, 562). An fMRI paradigm to study real voiding has been achieved only recently (342). Most studies however have been made during storage, using a variety of paradigms to examine the brain’s reaction to bladder filling—during repeated, rapid infusion, and withdrawal of an aliquot of liquid via a syringe; or before and after natural or slow bladder filling; or combined with repeated contraction of the pelvic floor muscles. These different solutions to the problems of drift, noise, and physiological accuracy yield different but presumably complementary results. For example, according to PET (70) and SPECT (684) observations in male subjects, the inferior frontal gyrus is activated bilaterally after filling the bladder to capacity, but this region is less evident on fMRI. It has been incorporated under the name “lateral prefrontal cortex” (lPFC) in Figure 8.
Forebrain neural circuits involved in human voiding
Circuit 1: Prefrontal cortex and insula
To understand bladder control and loss of control it is helpful to arrange the relevant brain regions in neural circuits that play different roles (Fig. 8). Of course, these interpretations are speculative and one obvious limitation is that they are based to a large extent on observations made when there is strong desire or urgency to void. A prominent circuit in Figure 8 includes the thalamus, insula, lPFC, and mPFC, as well as the PAG.
The insula has come to be regarded as the homeostatic afferent cortex that registers visceral sensations (121, 352). It receives homeostatic information (“the sense of the physiological condition of the entire body,” including the viscera) (122) via afferent input from small-diameter fibers in lamina 1 of the spinal cord, relayed in the thalamus. A critical element of this information is an appreciation of the degree of bladder filling. Consistent with this concept, insular activation has been observed in most studies of urine storage (see, for example, Fig. 16B), and in healthy controls activation increases with bladder filling and therefore with desire to void (240,352). It is relatively weaker in older subjects (245), who correspondingly have less pronounced bladder sensation (506) and is absent in women with impaired bladder sensation (Fowler’s syndrome) (42, 308). A recently published abstract (609) confirms that the insula is activated in normal elderly women and in those with UUI responsive to behavioral therapy. Lack of insular activation in those with UUI refractory to therapy, however, may indicate that proper use of homeostatic information is important for continence. The insular activations are bilateral (Fig. 16B), although a right-sided preference has been reported (70, 240). The role of the thalamus in processing and relaying bladder signals to the right and left insula (and many other cortical regions) is supported by imaging studies that have shown thalamic excitation in response to bladder filling (309).
The importance of the prefrontal cortex in bladder control was established by clinical studies by Ueki (652). Subsequently Andrew and Nathan (21, 217) highlighted that the location of lesions which were clinically demonstrated to have long-term effects on bladder function was in white matter tracts in the medial prefrontal regions. Medial prefrontal gray-matter lesions led to relatively short-term incontinence (Fig. 17).
Figure 17.

Coronal (A), sagittal (B), and axial (C) location of lesions causing incontinence (or occasionally retention) in the group of patients studied by Andrew and Nathan (21). The red ellipse shows where white-matter lesions caused lasting urinary tract dysfunction. The cyan ellipse shows the location of gray-matter lesions that caused transient dysfunction [Nathan, personal communication with Dr. Clare Fowler (217)].
The prefrontal cortex lies anterior to the motor strip and supplementary motor areas. Ventromedial regions are involved in decision-making in an emotional and social context (133). They have extensive interconnections with the limbic system—the hypothalamus and amygdala—as well as the insula and ACC. The more lateral parts of the prefrontal cortex are involved in aspects of cognition, especially working memory (42), and several bladder studies have shown lateral PFC activation (Table 3). Connections with the ventromedial PFC give access to the limbic system and other parts of the brain.
PET studies show that, during voiding, the medial prefrontal area is activated (Fig. 15B). During withholding of urine or with full bladder, however, the observations suggest that, especially in urgency incontinence, the medial parts of the prefrontal cortex are deactivated rather than activated in response to bladder filling (Table 3, Fig. 16B) (34, 241,609). Suggestively, the medial prefrontal cortex is part of the default mode network mentioned above. Up to now, it has not been clear whether mPFC deactivation is dysfunctional, a cause of incontinence, or is a sign that the voiding reflex is being suppressed, a mechanism promoting continence. If the latter interpretation proves to be correct, this will suggest a continence mechanism that operates as follows: if involuntary voiding or leakage threatens, excitation of the insula and lateral PFC leads in turn (via an inhibitory connection) to reduced input to the mPFC, reduced input to the PAG (via the return pathway in Fig. 8), and reduction in PAG activity that tends to stabilize the voiding reflex and maintain continence. In normal controls this circuit is not activated. It, therefore, appears to be a mechanism of continence used only by those who need to avoid inappropriate bladder contractions.
Circuit 2: Dorsal anterior cingulate cortex and supplementary motor area
A key feature of interoceptive sensations is their association with an affective, motivational aspect and hence their value in homeostasis. For example, an increasingly unpleasant desire to void as the bladder fills ensures that the bladder is regularly emptied, even though the exact time and place are under voluntary control. The anterior cingulate cortex (ACC) can be considered as the limbic motor cortex (182), responsible for motivation and modulation of bodily arousal states (123). In the cardiac system, dorsal ACC activation is associated with sympathetic control of heart rate (666). By analogy, ACC activation may help to suppress voiding via sympathetic activity that inhibits bladder contraction and contracts urethral smooth muscle, via β- and α-adrenergic receptors, respectively. Moreover, the dorsal ACC (dACC) is usually coactivated with the adjacent supplementary motor cortex (SMA), where activation is associated with contraction of the pelvic floor and striated sphincter muscles (353, 551, 562). Together therefore, if UUI threatens, the dACC and the SMA appear to be able to react by generating both the sensation of urgency and a contraction of the urethral sphincter, thus hastening a toilet visit while reinforcing the ability to postpone voiding until the toilet is reached.
Circuit 2, as sketched in Figure 8, indicates no clear connecting pathways, but in reality the afferent signal from the PAG to the dACC/SMA may be relayed by the thalamus. The efferent signal may return to sympathetic nuclei in the brainstem, or even the L-region, rather than the PAG. As mentioned above, this circuit seems to be a back-up continence mechanism that is employed by patients with urgency incontinence or OAB when they experience urgency. In normal subjects, who have less imperative sensation, the dACC/SMA is activated by bladder filling less strongly or not at all.
Circuit 3: Subcortical mechanisms
In normal individuals for most of the time, the bladder fills slowly, without sensation but presumably with unconscious monitoring by the brain. In such subjects bladder filling, when the bladder volume is small and there is little sensation, leads to activation of a subcortical network that includes the PAG and parts of the inferior or middle temporal (parahippocampal) cortex (Fig. 18) (611). In this situation, the cortical regions described above do not appear to be involved, consistent with absence of bladder filling sensation.
Figure 18.

Top and left panels (red/yellow): subcortical and temporal regions active in normal controls at low bladder volume when there is little bladder sensation, possibly representing circuit 3 in Figure 8; bottom right panel (blue): similar regions where activity changes after improvement of sensation by sacral neuromodulation, in women with Fowler’s syndrome. (Blue image, 308; orange image, our own unpublished work.)
Among women with Fowler’s syndrome (inability to sense or to empty the bladder) restoration of bladder sensation by sacral neuromodulation leads to changes in a rather similar brainstem/parahippocampal network (Fig. 18), (308) suggesting that it may be the route by which the PAG normally monitors bladder behavior and exchanges bladder-related signals with the rest of the brain. Since the parahippocampal cortex is close to the amygdala, the seat of emotion, one would expect this circuit to be particularly concerned with the emotional aspects (“safety”) of voiding, perhaps providing output to the brainstem nuclei via the postulated “safe” signal from the hypothalamus. The subcortical circuitry is included in a schematic way in the working model (Fig. 8).
The cause of incontinence?
The work reviewed so far provides no clear evidence for a cerebral cause of impaired bladder control (OAB or urgency incontinence). It appears likely—and is certainly possible—that the behavior of circuits 1 and 2 described above is a reaction to changes in incontinence rather than the actual cause. The subcortical circuit 3, however, has been little studied and so may be the seat of these common problems.
Another reason for our ignorance regarding causal mechanisms is that the studies performed to date have been based mainly on observations of activity in specific brain regions. They can give only incomplete information about how the cerebral network ultimately controls or fails to control bladder behavior, because the pathways that connect the regions are equally important. Only scattered studies of connectivity have been published: in normal subjects, bladder filling reveals a polysynaptic connection of the dACC and insula with the putamen, while the pattern of connectivity differs between UUI subjects and normal (610); in normal subjects, the connectivity of the right insula is weaker during attempted micturition than at baseline (351). Patterns of functional connectivity differ greatly between empty and full bladder (468).
In fact, reduced integrity of the white-matter connecting pathways in the brain is common and associated with several geriatric syndromes, including urgency incontinence. Sakakibara (535), Kuchel (350) and Tadic (228, 607) have shown that the presence and severity of urgency incontinence in older women, and the corresponding changes in cerebral activity, are correlated with the degree of white-matter damage as assessed by the global volume of inflammatory lesions (white-matter hyperintensities) seen on structural MRI. Moreover there is weaker evidence that focal damage in specific white-matter tracts (e.g., those serving mPFC or dACC/SMA) may contribute causally to incontinence.
To summarize, the picture of LUT control that has been built up is as follows. The brain-bladder control network maintains inhibition of the voiding reflex during urine storage and monitors unusual or unexpected bladder events. At small bladder volumes this occurs largely unconsciously and presumably relies on a subcortical network (circuit 3). Further bladder filling generates a sensation (desire to void) that gradually increases in intensity, reflected in insular activation. Despite the increasing sensation, in normal individuals excitation of the voiding reflex does not occur unless it would be not only mechanically appropriate but also emotionally safe and socially appropriate. In urgency-incontinent patients, however, imperative urgency develops, signaling a threat of leakage. Extra continence mechanisms can then be recruited in an attempt to maintain bladder control. They are based on neural circuits involving respectively activation of the dACC/SMA, deactivation of the mPFC, or (possibly) changes in subcortical function. All three circuits provide input to the PAG or other brainstem nuclei, aimed at suppressing the firing of the voiding reflex.
The preceding discussion suggests that abnormalities in the cortical circuits (1 and 2) are not an underlying cause of incontinence, but rather a reaction to changes in incontinence or the threat of incontinence, caused elsewhere. The underlying cause has not been revealed, but it may involve damage to the cerebral connecting pathways, or dysfunction of the subcortical control circuit—perhaps of the PAG itself, or any dysfunction that compromises the stability of the spinobulbospinal voiding reflex, even including abnormal bladder afferents.
Brain stem circuitry: Spinobulbospinal micturition reflex
Role of PMC
Voiding, which can be initiated voluntarily or reflexly, is mediated by activation of the sacral parasympathetic efferent pathway to the bladder and urethra as well as reciprocal inhibition of the somatic pathway to the urethral sphincter (Fig. 12). In contrast to storage mechanisms that are dependent on spinal reflex pathways, voiding is dependent on neural circuitry in the brain and spinal cord (Figs. 8, 12 and 19) (40, 142, 168, 363, 372, 529).
Figure 19.

Multiunit recordings of reflex activity on a bladder postganglionic nerve in a chloralose anesthetized cat during electrical stimulation (0.8 Hz, 3 v, 0.05 ms duration) of bladder afferent axons in the pelvic nerve. The bladder was distended with saline to a volume below the threshold for inducing micturition. First tracing in the upper right is a recording prior to the onset of stimulation showing that the efferent pathway is inactive. The next tracing shows lack of a response to the first stimulus in a train of stimuli. Further stimulation (lower tracings) induces a gradual increase in the magnitude of a long latency reflex and the eventual emergence of asynchronous firing (last tracing) which indicates the onset of reflex micturition. The diagram on the left shows the spinobulbospinal micturition reflex pathway and the sites of nerve stimulation and recording (173).
Studies in cats using brain-lesioning and electrophysiological techniques revealed that reflex micturition is mediated by a spinobulbospinal pathway consisting of an ascending sensory limb that passes from the sacral spinal cord to circuitry in the rostral brain stem leading to activation of neurons in the PMC that send excitatory signals back to the sacral spinal cord to complete the reflex circuit (Figs. 8 and 12). In animals, reflex micturition is preserved after removal of the forebrain by supracollicular decerebration but is abolished after bilateral destruction of the PMC or transection of the neuraxis at any level caudal to the PMC (363).
Anterograde axonal tracing studies in cats revealed that neurons in the PMC project directly to bladder preganglionic neurons in the sacral spinal cord (66). Labeled fibers from the PMC that are filled with round vesicles and that form a symmetric synaptic cleft, terminate on the soma, and dendrites of the preganglionic neurons. These data suggest that the descending pathway makes monosynaptic connections and has an excitatory function. On the other hand, electrophysiological experiments in cats in which EPSPs were evoked in bladder preganglionic neurons by stimulation of the PMC indicate that the descending pathway from the PMC to bladder preganglionic neurons is polysynaptic and strongly facilitated during the micturition reflex (547). The latter finding is consistent with other electrophysiological studies in cats indicating that the descending PMC-spinal cord limb of the micturition reflex requires afferent feedback from the bladder to induce large amplitude bladder contractions and that it can be modulated at the spinal level possibly at an interneuronal site by segmental afferent inputs (346, 347).
Recordings of electrical activity in bladder efferent nerves (Fig. 19) support the concept that the micturition reflex is mediated by a pathway passing through a switching center in the rostral pons. Stimulation of bladder afferent nerves evokes long latency discharges (120–150 ms) on bladder postganglionic nerves (Fig. 19) that persist after supracollicular decerebration but not after transection of the spinal cord at the thoracic level (142, 168). The evoked reflexes are unmasked by partial filling of the bladder to elicit a basal level of afferent firing. They also exhibit an unusual temporal facilitation in which the first stimulus during a train (0.5–1 Hz frequency) does not evoke a response and the next few stimuli evoke gradually increasing responses (wind-up), eventually producing a self-sustaining micturition reflex (Fig. 19). These observations indicate that even under optimal conditions with tonic afferent input from bladder mechanoceptors, electrical stimulation of bladder afferents only activates the micturition switching circuit after a delay of several seconds.
Bladder afferent nerve stimulation evokes neuronal firing in the PMC at latencies ranging from 30 to 40 ms; and electrical stimulation in the PMC evokes bladder contractions and postganglionic nerve firing at latencies of 60 to 75 ms (142, 480). The sum of the latencies of the putative ascending (afferent-ponto-mesencephalic) and descending limbs (pontine-sacral efferent neuron) of the reflex approximates the latency of the entire reflex pathway (120 ms). The reflex firing elicited in cats and rats is not altered following supracollicular decerebration but is eliminated by acute transection of neuraxis at any level caudal to the PMC (142,164,168).
In the rat, 79% of neurons in the PMC (Barrington’s nucleus) are activated by bladder distension consistent with its role as a PMC (527). Although no neurons are selectively activated by distension of the colon, the majority of bladderresponsive neurons (73%) are also activated by colon distension. These data support the proposals based on PRV tracing experiments that neurons in the PMC coordinate the functions of the colon and the LUT (526, 655, 659). Retrograde axonal tracing experiments in rats in which different color fluorescent beads were injected into the sacral parasympathetic nucleus of the S1–S2 segments (red) and into the thoraco-lumbar sympathetic nucleus at T13-L1 segments (green) revealed two populations spinal projecting neurons in the PMC: preparasympathetic output (PPO) and presympathetic output (PSO) neurons (250). Although it is uncertain which organs are controlled by the PPO and PSO neurons, these findings raise the possibility that separate populations of neurons in the PMC control the parasympathetic and sympathetic outflow to the pelvic viscera.
Properties of neurons in the PMC
Single unit recording in the PMC of the cat (Fig. 20) (77, 150, 337, 543–546, 601, 603, 623) and rat (203, 674) with the bladder distended under isovolumetric conditions revealed several populations of neurons exhibiting firing correlated with reflex bladder contractions including: (i) neurons that are silent in the absence of bladder activity but fire prior to and during reflex bladder contractions (direct neurons, 21%), (ii) neurons that are active during the period between bladder contractions and are inhibited during contractions (inverse neurons, 51%), and (iii) neurons that fire transiently at the beginning of bladder contractions (on-off neurons, 4%) (Fig. 20). Tonic firing that was not correlated with bladder activity was also identified in a large percentage (25%) of PMC neurons (termed independent neurons) (Fig. 20). All of these neurons are localized primarily in the region of the LC complex.
Figure 20.

Relationship between single unit activity in the PMC of a decerebrate, unanesthetized cat, and reflex contractions of the urinary bladder. Top tracings are blood pressure, middle tracings are ratemeter recordings of unit activity in spikes per second and the bottom tracings are bladder pressure in cm H2O. Three types of neuronal activity are illustrated: (A) a direct neuron that only fired during a bladder contraction, (B) an inverse neuron that fired between bladder contractions and was inhibited during contractions, and (C) an independent neuron that exhibited continuous firing unrelated to bladder contractions. Small increases in blood pressure occurred during bladder contractions. The bladder was distended with saline and maintained under isovolumetric conditions. Horizontal calibration represents 1 min. The three neurons were studied at different times in the same animal (173).
Subpopulations of direct and inverse neurons in the cat have also been identified based on slow changes in firing during and between bladder contractions (544). Approximately 50% of direct neurons (type 2) exhibit tonic firing between bladder contractions; whereas the remainder (type 1) are quiescent until 0.5 to 1.2 s prior to a bladder contraction. The majority of inverse neurons (84%) stop firing during a bladder contraction after a delay of 4 to 11 s; whereas a small number exhibit only a reduction in firing. A large percentage of direct neurons project to the lumbosacral spinal cord (543, 546, 603); whereas only a small percentage of inverse neurons send projections to the cord. Thus it has been speculated that inverse neurons function as local inhibitory neurons in the PMC. Both direct and inverse neurons exhibit excitatory synaptic responses to electrical stimulation of afferent axons in the pelvic nerve (150). Direct neurons fire at a mean latency of 62 ms after a stimulus; whereas inverse neurons fire at a shorter latency of 25 to 30 ms followed by an inhibition at a latency of 80 ms and then a late excitation at 250 to 300 ms.
Patch clamp recordings in rat brain slices of the retrogradely labeled PPO and PSO neurons mentioned above revealed marked differences in the electrophysiological properties of the two populations of neurons (250). The membrane potential and action potential threshold are higher and the basal firing rate is lower in PPO than in PSO neurons. In addition A-type K+ currents are significantly larger and blocking these currents increases the excitability more in PPO than in PSO neurons. Synaptic inhibitory mechanisms generated by circuitry within or adjacent to the PMC and which will be discussed later in the neurotransmitter section of this review also contribute to the lower excitability of PPO neurons. Thus, PPO neurons studied under conditions in which afferent inputs from the bladder and other regions of the brain were interrupted exhibit properties consistent with those necessary for urine storage; whereas PSO neurons exhibit a higher level of basal activity that could activate tonic sympathetic input to the bladder and promote urine storage.
Role of the PAG
Early studies in cats (235, 295, 340, 371, 571) revealed that stimulation at sites in the PAG could either excite or inhibit bladder activity. The effects of stimulation were dependent on the state of the bladder. For example when stimulation was applied with the bladder partially full and relatively inactive excitatory effects were commonly elicited; however, when the bladder was full and exhibiting large amplitude reflex contractions stimulation at the same site produced inhibition. Reflex bladder activity was also enhanced by elimination of parts of the PAG by focal lesions or serial transections through the mesencephalon (370, 529, 624). This finding raised the possibility that a mesencephalic bladder inhibitory center tonically controlled micturition. An inhibitory region seems to be located in the dorsolateral margin of the rostral PAG (482) because chemical or electrical stimulation at this site inhibits reflex bladder contractions and the contractions induced by electrical stiumulation of the PMC. Injection of bicuculline, a GABAA receptor antagonist, into the PMC blocks the PAG induced inhibition of PMC stimulation indicating that GABA is the transmitter in the inhibitory pathway (482).
Other sites in the PAG seem to have a facilitatory role in micturition. Electrical stimulation in the ventrolateral region of the PAG evokes bladder contractions (416, 479, 625) and firing on bladder postganglionic nerves (480); while injections of cobalt chloride, a synaptic inhibitory agent (415) or an opioid receptor agonist (413), into this region suppresses reflex micturition. These data raise the possibility that the ventrolateral PAG is an essential component of the micturition reflex.
Electrical recordings in the PAG indicate that it may serve as a relay and coordinating center on the ascending limb of the micturition reflex pathway. In the rat, electrical stimulation of bladder afferents in the pelvic nerve elicits negative field potentials in the dorsal PAG at a mean latency of 13 ms which is considerably shorter than the mean latency of field potentials in the region of the PMC (42 ms) (479). In the cat, a similar difference between latencies of pelvic afferent evoked field potentials in the PAG (11 ms) (202) and PMC in the (30–40 ms) (142) has been noted.
Subsequent studies in the cat and rat provided further support for the idea that bladder afferent information is relayed through the PAG. Axonal tracing studies in the cat, revealed that spinal tract neurons located in lamina I on the lateral edge of the sacral dorsal horn, a region receiving primary afferent input from the bladder (445), send a prominent direct axonal input through the lateral funiculus to the PAG (Fig. 8) (71, 270). Injections of retrograde tracers into the lateral funiculus at the lumbar level labels the same group of sacral spinal tract neurons (164). The PMC, on the other hand, receives a weaker input directly from the spinal cord and this input does not terminate on the PMC output neurons that send information back to the sacral parasympathetic nucleus. Axonal tracing methods also identified projections from the PAG to the PMC (65, 354), raising the possibility that ascending afferent information from the bladder is relayed through synapses in the PAG to the PMC. Thus it has been proposed that the PAG has an essential role in the spinobulbospinal micturition reflex pathway (270, 479).
However experiments in cats by Takasaki et al., (619) have raised questions about the importance of the PAG in reflex micturition. When the mesencephalon was serially transected at various levels to interrupt the connections between the PAG and the PMC reflex bladder contractions persisted after transections at rostral levels that eliminated connections with the dorsal half of the PAG. Reflex micturition also persisted after more caudal transections that eliminated connections with both the dorsal and ventral half of the PAG or eliminated the most rostral part of the PMC. On the other hand, transections caudal to the PMC abolished reflex micturition. The authors concluded that the PAG does not have an essential role in reflex micturition but rather is involved in transmitting bladder filling information to higher brain centers. Subsequently, the techniques used in transection experiments were questioned by other investigators (593) who noted that the PAG lesions in the experiments of Tasaki et al (619) were often incomplete and a part of the caudal ventrolateral PAG was preserved in some experiments.
In the rat the role of the PAG is even less clear because prominent ascending projections from the lumbosacral spinal cord have been detected in the PMC as well as the PAG (68, 184). Thus the organization of the ascending limb of the micturition reflex is uncertain and may vary in different species.
Brain imaging studies (612) in the rat revealed that neuronal activity in the PAG increases during slow bladder filing indicating that afferent activity from the bladder is received and processed in the PAG prior to micturition; however a similar signal was not detected in the PMC during filling (Fig. 21). On the other hand during micturition, signals were detected in the PAG and the PMC. Similar results have been reported during brain imaging in humans (see earlier section). These results suggest that the PAG in the rat serves as a relay station for transmitting afferent information from the bladder to the PMC but that the switch from urine storage to voiding occurs in the PMC.
Figure 21.

Blood oxygen level-dependent (BOLD) images showing brain stem activation associated with switching from the bladder storage phase to the bladder contraction phase. The locations of coronal brain sections (F–G) are indicated in the sagittal brain image at the bottom, which correspond to the Bregma coordinates in the anterior-posterior direction at 2.28, 0.24, 1.80, 3.84, 5.88, 7.80, and 9.84 mm. Region of interest (ROI) analysis was performed on the brain stem at coronal sections F and G to detect the activation. The periaqueductal gray (PAG) and pontine micturition center (PMC) are indicated by the blue arrows. The color scale bars indicate the t value (612).
Properties of neurons in the PAG
Single unit recordings in the PAG and adjacent mesencephalic reticular formation in decerebrate unanesthetized cats during rhythmic reflex bladder contractions under isovolumetric conditions revealed firing patterns similar to those recorded in the PMC including: (i) tonic storage neurons that are partially inhibited during bladder contractions (43%), (ii) phasic storage neurons that are completely inhibited during bladder contractions (15%), similar to inverse neurons in the PMC, and (iii) phasic micturition neurons that are only active during micturition (13%) (387), similar to direct neurons in the PMC. A fourth type of neuron (29%) classified as tonic micturition neurons that are active throughout storage and micturition but increase their firing during bladder contractions may be similar to the transient neurons identified in the PMC. Among the 84 neurons recorded in this study 16 were located in the PAG and the remainder were located just ventral to the PAG. In the PAG storage neurons seemed to be located in the middle part of the PAG (H-C coordinates: P 0–1), whereas micturition neurons were distributed in a broader area. Simultaneous unit recordings in the PAG and PMC or in the PAG and the PUSC did not reveal significant time-correlations in 100 ms windows between unitary activity in the these locations.
The PMC-PAG switch
Pharmacological studies indicate that circuitry in the PMC and PAG allows the spinobulbospinal micturition reflex pathway to function as a switch that is either in a completely “off” mode (storage) or maximally “on” mode (voiding). Injections of excitatory amino acids into the PMC (398) or PAG (625) evokes bladder contractions in cat and rat. On the other hand microinjections of low doses of inhibitory agents such as GABAA receptor agonists (muscimol), or opioid peptides at these sites increases the bladder volume threshold for inducing micturition without altering the magnitude of the micturition reflex measured as the amplitude of voiding contractions (398, 413, 480, 593). Conversely, injections of GABAA receptor (bicuculline) or opioid receptor antagonists (naloxone) reduce the bladder volume threshold indicating that tonic activation of inhibitory receptors in these centers can alter the set point of the micturition switch (398, 480, 593). Because pharmacologic modulation of the PAG circuitry clearly alters the bladder volume threshold it seems reasonable to conclude that PAG input to the PMC switching circuit also regulates the set-point for the micturition switch.
Brain stem modulatory circuits
Properties of neurons in the pontine urine storage center
A region of the pons located ventrolaterally to the PMC has been designated the PUSC because electrical stimulation in this region inhibits micturition and activates the EUS (269, 341, 363, 476). Lesions in this area induce incontinence. Injections of anterograde tracers into the PUSC label axonal projections to the sphincter motor nucleus in the sacral spinal cord (269). In decerebrate, unanesthetized cats when the bladder is distended to induce rhythmic reflex contractions under isovolumetric conditions four major types of neurons can be detected in the PUSC including: (i) tonic storage neurons that are continuously active during storage/voiding cycles with increased firing during storage (38%), (ii) phasic storage neurons that are only active during the storage phase (40%), (iii) tonic micturition neurons that are continuously active throughout the storage/micturition cycle but exhibit increased activity during micturition (9%), and (iv) phasic micturition neurons that are only active during micturition (13%) (536). These neurons have been subclassified into augmenting, constant or decrementing according to the change in their discharge rate during either the storage or micturition phases. Some of the neurons increase their firing rate prior to the onset of the micturition or storage phases. The average interval between the onset of preceding neural activity and the change in bladder activity ranges from 2 to 10 s and is evident in the various types of neurons.
Properties of neurons in the substantia nigra pars compacta and ventral tegmental area
Single unit recordings in the substantia nigra/ventral tegmental area (SN/VTA) in ketamine anesthetized cats during rhythmic reflex bladder contractions under isovolumetric conditions revealed firing patterns similar to those recorded in the PUSC including: (i) tonic (55%) and phasic (22%) storage neurons and (ii) tonic (16%) and phasic (6%) micturition neurons. Augmenting, constant, decrementing, and binary neurons that fired at the beginning and at the end of the storage phase were identified (537). The large percentage of micturition storage neurons is consistent with other observations indicating that the dopaminergic neurons in the SN/VTA have a predominate inhibitory influence on micturition. For example, electrical stimulation in the SN terminates ongoing micturition (711) and destruction of dopaminergic neurons using the toxin MPTP or 6-hydroxydopamine facilitates the micturition reflex (705, 707). Dopaminergic neurons in SN synapse with neostriatal GABAergic neurons that may be involved in micturition inhibitory mechanisms in the forebrain. In addition D1 dopaminergic receptors have been implicated in the control of GABAergic neurons in the PAG (332); where GABAergic inhibition also plays an important role in the control of micturition (593).
Phasic micturition neurons in the SN/VTA might also have a role in the control of micturition because pathological conditions such as middle cerebral artery occlusion or decerebration have been shown to upregulate D2 receptor facilitatory control of micturition in the rat (691).
Cerebellum
Brain imaging studies in humans have shown activation in the cerebellum in response to bladder distension (240, 352, 562, 618). This activation is consistent with studies in cats showing that electrical stimulation of bladder afferent nerves elicits neural activity in the cerebellum (78, 79). In relation to bladder activity both inhibitory and excitatory functions of the cerebellum have been proposed (78, 79, 95, 119, 477). A possible inhibitory role of the cerebellum in the control of micturition is suggested by studies in cats showing that electrical stimulation of the cerebellar fastigial nucleus inhibits reflex bladder activity (78, 79, 403); while ablation of the anterior vermis of the cerebellum results in a hyperactive bladder reflex with increased reflex duration (78, 79). Cerebellectomy in dogs also induces bladder overactivity (477) and cerebellar pathology in humans is accompanied by bladder hyperreflexia (730). These data indicate that the cerebellum has a tonic inhibitory influence over the micturition reflex. A cerebellar-hypothalamic pathway has been mentioned as part of a network for regulating micturition as well as other autonomic functions (183). However this pathway must not be essential for cerebellar inhibitory control because cerebellarinduced bladder inhibition persists after decerebration which eliminates the hypothalamus. Cerebellar projections to the nucleus subcoeruleus, nucleus LC and PAG have also been mentioned as possible pathways for cerebellar modulation of micturition (183). Other evidence suggests that the cerebellum exerts a tonic inhibitory influence on micturition via a pathway passing through the mesencephalic reticular formation. Based on studies in decerebrate cats (477), it was concluded that the cerebellum plays an inhibitory role during the storage phase of the micturition cycle and a facilitatory role during voiding.
Hypothalamus
Transneuronal virus tracing methods have identified virus-infected cells in several regions of the hypothalamus after injection of PRV into the LUT in animals (463, 604, 664). Histochemical studies using tracers also revealed that neurons in the PMC receive input from the caudal hypothalamus and that the paraventricular hypothalamic nucleus projects nonspecifically to all autonomic preganglionic motor neurons in the spinal cord, including the sacral parasympathetic and sphincter motor nuclei (270).
Although both excitatory and inhibitory effects on bladder activity are elicited by stimulation at different sites in the hypothalamus, the overall hypothalamic control seems to be facilitatory according to the studies of Tang and Ruch (529, 624) showing that after supracollicular decerebration, which eliminates the hypothalamic effects, the bladder volume threshold inducing micturition markedly increases (156). A clinical study in three patients with pituitary adenoma compressing the hypothalamus also showed that hypothalamic lesions can cause both DO during urine storage and underactive detrusor during voiding (681).
The hypothalamic influence on bladder function may in turn be modulated by afferent inputs from the bladder because axonal tracing studies in rats have identified a spinohypothalmic pathway arising from neurons in the region of sacral parasympathetic nucleus (60, 88); and 50% of these neurons projecting to the hypothalamus are activated by bladder afferents as evidenced by positive c-fos staining after bladder irritation with dilute acetic acid (60). Brain imaging studies in human subjects also revealed that the caudal hypothalamus responds to changes in bladder volume (34, 242).
Computer model of micturition switching circuit
Computer modeling of the switching circuitry in the PMC and PAG that underlies the spinobulbospinal micturition reflex in a decerebrate animal
Based on neuronal firing patterns recorded in the PMC and PAG during rhythmic bladder contractions as well as antidromic responses to stimulation of the spinal cord and synaptic responses to stimulation of bladder afferent nerves, a neural circuit has been designed in an attempt to model the switching properties of the spinobulbospinal micturition reflex in a decerebrate animal (Fig. 22) (173). The circuit includes the peripheral afferent and efferent pathways between the bladder and the spinal cord plus connections between the spinal cord, PAG, and PMC.
Figure 22.

Computer model of PMC-PAG switching circuits. Diagram illustrating the putative pathways in the periaqueductal gray (PAG) and pontine micturition center (PMC) that contribute to urine storage and voiding. This circuitry shows the neuronal elements and connections used in the computer model. The right side illustrates the ascending afferent limb of the spinobulbospinal micturition reflex that projects to the PAG, and the left side shows the descending limb that connects the PMC direct neuron to the bladder efferent neuron in the sacral spinal cord. During urine storage as the bladder slowly fills low level of afferent activity activates an excitatory neuron (E) in the PAG which relays information (pathway A) to an inverse neuron (I) in the PMC that in turn provides inhibitory input to the type 1 direct neuron (D) to maintain continence. Bladder afferent input is also received by a second neuron in the PAG (E) that is on the excitatory pathway (pathway B) to the PMC type 1 direct neuron (D) and to a transiently active PMC neuron (T) that fires at the beginning of micturition. However, the PAG excitatory relay neuron (E) is not activated during the early stages of bladder filling because it is inhibited by a tonically active independent neuron (I). The PMC type 1 direct neuron is also inhibited by a tonically active independent neuron (I) located in the PMC. Bladder afferent firing gradually increases during bladder filling which increases feedforward inhibition of the direct neuron via the PAG-PMC inverse neuron pathway. However, at a critical level of afferent firing, excitatory input to the PAG excitatory relay neuron surpasses the tonic inhibition of the independent neuron and sends signals to the PMC transient neuron which briefly inhibits the inverse neuron reducing inhibitory input to the direct neuron allowing it to overcome tonic inhibition and fire action potentials which activate by an axon collateral (pathway C) a reciprocal inhibitory neuron (R) that suppresses the inverse neuron (I) and further reduces inhibition of the direct neuron (D). The direct neuron then switches into maximal firing mode and sends excitatory input to the spinal efferent pathway to the bladder inducing a large bladder contraction and more afferent firing which further enhances synaptic transmission in the PAG-PMC micturition reflex pathways. The reflex circuitry returns to storage mode as the bladder empties and afferent firing declines. Excitatory neurons are green and inhibitory neurons are red (173).
The ascending sensory limb of the circuit consists of a mechanosensitive bladder primary afferent neuron that synapses with a second-order spinal tract neuron. The latter projects to excitatory neurons in the PAG that in turn relay information to the PMC. The PMC contains several types of neurons. Direct neurons (indicated by D in Fig. 22) that send information back to the sacral parasympathetic nucleus represent the descending limb of the spinobulbospinal micturition reflex (Fig. 22). These neurons (type 1 direct neurons) are silent during bladder filling but activated prior to and during micturition. In the model the type 1 direct neurons receive tonic inhibitory input from independent neurons and bladder volume dependent inhibition from inverse neurons shown in Figure 20. Inverse neurons (I) that are activated by afferent input from the PAG and fire during bladder filling make inhibitory connections with direct neurons to provide feedforward inhibition of the micturition reflex during bladder filling. The inverse neurons are inhibited during micturition which in turn removes inhibitory input to the direct neurons and facilitates the micturition reflex. Transient neurons (T) which are activated by bladder afferent stimulation via a relay (B) through the PAG and fire at the beginning of a bladder contraction are postulated to inhibit the inverse neurons and play an important role in the initiation of the micturition reflex. Type 2 direct neurons (indicated by R in Fig. 22) which exhibit continuous firing during bladder relaxation but are strongly activated at the onset of micturition are postulated to receive excitatory axon collaterals (pathway C) from type 1 direct neurons and mediate reciprocal inhibition of inverse neurons. This would further enhance the development of the micturition reflex by suppressing inhibitory input to the type 1 direct neurons.
In the PAG, it is postulated that neurons tonically active during bladder filling or between micturition contractions represent relay neurons that transmit excitatory signals (pathway A) to inverse neurons in the PMC that in turn generate feedforward inhibition of the micturition reflex. Conversely excitatory neurons in the PAG that relay bladder afferent information to PMC direct neurons are likely to be “phasic micturition neurons” identified by Sakakibara et al. (536). It is known that a GABAergic inhibitory mechanism in the PAG tonically controls the bladder volume set-point for initiating micturition (593). In the hypothetical circuit in Figure 22, this mechanism is represented by an independent inhibitory neuron synapsing with the PAG relay neuron. Similarly, GABAergic or enkephalinergic independent inhibitory neurons in the PMC are likely to control the set-point for micturition by tonically inhibiting the type 1 direct neurons.
In summary, GABAergic or enkephalinergic inhibitory control of the micturition switching circuit may occur at several sites on the spinobulbospinal pathway including: (i) the ascending limb in the spinal cord, (ii) relay centers in the PAG, and (iii) synapses on type 1 direct neurons in the PMC. However, it is presumed that the switch from storage to voiding occurs at the level of the direct neurons because their firing is closely linked with PGN firing and reflex bladder contractions, indicating that transmission of descending signals through the sacral parasympathetic nucleus occurs with a high safety factor. Therefore, excitatory synapses on the descending limb of the micturition reflex pathway in the spinal cord function as relays rather than switches and transmit signals from the PMC switch to the bladder with high fidelity.
Computer modeling of the spinal urine storage and micturition circuitry
Spinal storage circuitry which is not shown in Figure 22 is also part of the computer model [see (173) for details]. This circuitry includes the spinal vesico-sympathetic and vesicosphincter reflex mechanisms (see Fig. 12) described in a previous section. For simplicity these reflex mechanism have been modeled (Fig. 12) as monosynaptic reflexes although it is probable that they are multisynaptic pathways. As indicated in Figure 12 these two storage reflexes are inhibited by descending input from the type 1 direct neurons in the PMC, thereby promoting urethral outlet relaxation during micturition. The model includes a third storage mechanism in which sphincter afferents activate spinal inhibitory neurons that suppress bladder preganglionic neurons and interneurons on the ascending and descending micturition reflex pathway. The model also includes a recurrent inhibitory circuit in which preganglionic axon collaterals activate interneurons that in turn inhibit excitatory interneurons on the ascending and descending limbs of the micturition reflex pathway (142, 167). A urethral-bladder excitatory mechanism which facilitates micturition in response to flow of urine through the urethra (40, 363) is also included in the model and not shown in Figure 22.
Computer simulation of the storage-voiding cycle using a model of the PMC and PAG switching circuitry
The model of the spinobulbospinal pathway consisting of the supraspinal components shown in Figure 22 and the spinal components which are not shown was used to simulate a reflex storage-voiding cycle and estimate various parameters including bladder pressure, bladder volume, bladder afferent firing, and bladder efferent firing during filling of the bladder at a rate of 30 mL/min (Fig. 23). The model of the urinary tract used in this simulation has two interconnected components: a mechanical component that models the bladder and outlet, and an artificial neural network that models the neural reflex pathways controlling the LUT. This approach is similar to several other attempts to model the LUT (219, 657) but also includes putative supraspinal circuitry based on unit recordings in the PMC and PAG. The mechanical component was taken from the model produced by Bastiaanssen et al. (41). The neural component was based on the electrophysiological properties of individual neuronal groups in the PMC and PAG. The mathematical details for producing the neural component are included in a recent paper (173).
Figure 23.

Computer simulation of the storage-voiding cycle. Simulated bladder volume (top tracing) and pressure (second tracing), bladder afferent firing (third tracing) and bladder efferent firing (bottom tracing) during bladder filling (30 mL/min) and during reflex voiding using the computer model of spinal, PAG and PMC neural pathways and the myocybernetic model of Bastiaanssen et al. 1996 to predict the properties of the bladder, urethra, and the afferent firing arising in these structures. Note that as bladder volume increases, bladder pressure remains low, bladder efferent firing is absent, but bladder afferent firing gradually increases eventually reaching a threshold for inducing a micturition reflex as evidenced by an abrupt increase in efferent firing, which induces an increase in bladder pressure, increased afferent firing and bladder emptying. Bladder efferent firing peaks early during micturition and is maintained until the bladder is empty. The voiding phase is shown on an expanded time scale in the tracings on the right side (173).
Based on the Bastiaanssen et al. model (41) during bladder filling bladder pressure remains low but bladder afferent firing slowly increases as bladder wall tension increases (Fig. 23). Bladder efferent firing which represents activity in the spinobulbospinal micturition reflex pathway remains low during the filling because the PAG-PMC switching circuit is in the off mode. At a critical bladder volume threshold, the PAG-PMC switch is turned on and efferent firing markedly increases inducing a prominent increase in bladder pressure followed by an increase in bladder afferent firing, a relaxation of the urethral outlet (not shown) and then voiding evident as a decrease in bladder volume. The model generates an all-or-none reflex response reflected as maximal efferent discharge throughout voiding even as bladder volume decreases. The model generates efficient voiding resulting in complete bladder emptying. Reducing the strength of the inhibitory input from the PMC tonically active independent neuron to the direct neuron reduces bladder capacity, while increasing the strength of the inhibition increases bladder capacity. The fact that the computer simulation replicates the storage-voiding cycle corroborates the validity of the neural circuitry theorized in this article.
Neurotransmitters in Central Pathways Controlling Micturition
Spinal ascending and descending pathways
Glutamate
Intrathecal or intravenous administration of glutamater-gic NMDA or α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) antagonists in urethane-anesthetized rats depresses reflex bladder contractions and electromyographic activity of the EUS in animals with an intact spinal cord as well as in animals with chronic spinal injury (718, 723). Studies in rats also indicate that activation of bladder PGN by input from the PMC can be blocked by inotropic glutamate receptor antagonists, suggesting that the descending pathways from the PMC utilize glutamate as a neurotransmitter (411, 412). These results indicate that spinal reflex pathways controlling bladder and sphincter function utilize NMDA and AMPA glutamatergic transmitter mechanisms. In spinal cord-injured rats, external sphincter muscle activity is more sensitive than bladder reflexes to glutamatergic antagonists, raising the possibility that the two reflex pathways might have different glutamatergic receptors (722). This was confirmed with in situ hybridization techniques, which revealed that sacral PGNs in the rat express high mRNA levels of GluR-A and GluR-B AMPA receptor subunits and NR1 but not NR2 NMDA receptor subunits (566). Conversely, motoneurons in the urethral sphincter nucleus express all four AMPA receptor subunits (GluR-A, -B, -C, and -D) in conjunction with moderate amounts of NR2A and NR2B as well as high levels of NR1 receptor subunits. It seems likely that this difference in expression accounts for the different sensitivity of bladder and sphincter reflexes to glutamatergic antagonists.
Glutamate also plays a role as an excitatory transmitter in the afferent limb of the micturition reflex. C-fos expression induced in spinal interneurons by activation of bladder afferents is suppressed by the administration of both NMDA and non-NMDA glutamatergic receptor antagonists (55,298,300).
In contrast to excitatory effects of glutamate via ionotropic glutamatergic receptors (NMDA and AMPA/kinate), activation metabotropic glutamatergic receptors (mGluRs) in the spinal cord has inhibitory effects on the descending limb of the micturition reflex because a group I/II mGluR agonist applied to the spinal cord at the lumbosacral level suppresses reflex bladder contractions as well as those induced by PMC stimulation in rats (621). It has also been reported that mGluRs are involved in inhibition of the excitatory pathway to the EUS because a group I/II mGluR antagonist applied into the lumbosacral intrathecal space significantly facilitates the EMG activity of the EUS in rats (719).
During synaptic transmission, glutamate released from presynaptic nerve terminals is cleared from the synaptic cleft into presynaptic nerve terminals and adjacent astrocytes, via glutamate transporters. A recent study demonstrated that intrathecal application of a nonselective inhibitor of glutamate transporters, L-trans-pyrrolidine-2,4-dicarboxylic acid (L-trans-PDC) that increases endogenous glutamate concentration at nerve terminals, delays the onset of micturition by increasing intermicturition intervals and pressure thresholds in rats under urethane anesthesia (273).
Inhibitory amino acids (GABA, glycine, and glycine transporter)
Intrathecal injection of either GABAA or GABAB agonists increases bladder capacity and decreases voiding pressure and efficiency in normal rats (279, 501) and also suppresses DO in rats induced with intravesical application of oxyhemoglobin, an NO scavenger (501) or spinal cord injury (435). In addition, intravenous or intrathecal application of a GABA reuptake inhibitor (tiagabine) that increases endogenous GABA concentrations reportedly inhibits normal micturition in rats (500). In a small clinical study in three subjects, intrathecal administration of a GABAB receptor agonist (baclofen) increased the volume threshold for inducing the micturition reflex (89). Intrathecally administered baclofen also produced phaclofen-sensitive inhibition of distention-evoked micturition in conscious rats that appears to be resistant to capsaicin (substance P depletion) and parachlorophenylalanine (5-hydroxytryptamine depletion) pretreatment (234). Because baclofen also inhibits field stimulation-evoked release of CGRP from primary afferent terminals in dorsal horn slices, one possible mechanism of action of GABAergic inhibition is suppression of transmitter release from primary afferent terminals in the spinal cord.
Previous studies also showed that glycine, another inhibitory amino acid, acting on strychnine-sensitive receptors exerts an inhibitory effect on the micturition reflex pathway (143,437) and is also involved in the inhibition of sphincter motoneurons during micturition (564). Glycine and GABA inhibitory mechanisms have also been identified in the neonatal rat spinal cord in local interneuronal inhibitory pathways projecting directly to the PGN (26). Application of GABAA agonists to sacral parasympathetic PGN inhibits reflex firing, opens chloride channels, and hyperpolarizes the cells and baclofen, a GABAB agonist, suppresses Ca2+ channels in sacral PGN in the rat (26).
In addition, recent studies revealed that the level of glycine in the spinal cord is decreased by approximately 50% in rats with DO induced by chronic spinal cord injury, compared with spinal intact rats (437, 438) and that dietary supplement of glycine can restore bladder function along with an increase in the serum level of glycine in spinal cord injured rats (438). The level of glutamic acid decarboxylase (GAD), the GABA synthetic enzyme, is also reduced in the spinal cord and lumbosacral DRG in spinal cord-injured rats with DO (433) and sphincter-detrusor dyssynergia (DSD) (434), and both impaired functions are suppressed by intrathecal application of GABAA or GABAB receptor agonists (433, 434). These results suggest that downregulation of spinal glycinergic and GABAergic mechanisms may contribute to the emergence of neurogenic DO associated with spinal cord injury.
The extracellular concentration of glycine at synapses is regulated by two types of Na+/Cl− dependent glycine transporters (GlyTs): GlyT1 and GlyT2 (728). GlyT1 is widely distributed in the CNS and predominantly expressed in glial cells near both excitatory and inhibitory neurons, while GlyT2 is specifically distributed in the spinal cord, cerebellum, and brainstem, and localized in the presynaptic terminals of inhibitory glycinergic neurons (729). A recent study reported that intrathecal application of a selective GlyT2 inhibitor, ALX-1393, but not a GlyT1 inhibitor, sarcosine, produced significant increases in intermicturition intervals and pressure thresholds in rats with cyclophosphamide-induced cystitis (696), suggesting that inhibition of GlyT2 is a new approach to enhance the spinal glycinergic inhibitory mechanism controlling the micturition reflex.
Adrenergic
In the spinal cord, descending pathways from noradrenergic brainstem nuclei such as the LC can mediate excitatory and inhibitory influences on the LUT via adrenoceptors. In anesthetized cats, α1-adrenoceptors were implicated in a bulbospinal noradrenergic excitatory pathway from the LC to the sacral parasympathetic outflow to bladder (708–710), although subsequent studies could not confirm these findings in conscious cats (205).
Experiments in conscious or anesthetized rats (179, 286) revealed that intrathecal administration of an α1-adrenergic antagonist (doxazosin) decreases the amplitude of bladder contractions (285, 286). The bladder inhibitory effect of the intrathecal α1-adrenergic antagonist is more prominent in animals with chronic outlet obstruction (286). It was also found that intrathecal administration of doxazosin suppresses DO (unstable bladder contractions) in spontaneously hypertensive rats (503). Although intrathecal injection of doxazosin suppresses the amplitude of reflex bladder contractions in anesthetized rats, it increases the frequency of isovolumetric contractions, indicating the presence of a tonic adrenergic inhibitory mechanism (179). This was supported by the finding that phenylephrine, an α1-adrenergic agonist, applied intrathecally, decreases the frequency of bladder contractions without changing contraction amplitude (179). Overall, it appears that the spinal noradrenergic system has a modulatory role in the control of the micturition reflex and that efferent and afferent limbs of the micturition reflex receive excitatory and inhibitory input, respectively, from this system. Also, it has been reported that intrathecal injection of tamsulosin, an α1A-selective adrenergic antagonist, or naftopidil, a selective α1A/D-adrenergic antagonist, transiently abolish isovolumetric rhythmic bladder contractions in normal rats and (600) and that intrathecal injection of naftopidil prolongs the interval between voiding contractions and decreases the maximum voiding contraction pressure and the number of nonvoiding contractions in spinalized rats (296). Intrathecal application of silodosin, a selective α1A adrenergic antagonist, or naftopidil also increases bladder capacity in a rat model of cerebral infarction induced by middle cerebral occlusion (686). These results suggest that α1A and/or α1D-adrenoceptor subtypes are involved in spinal excitatory mechanism controlling micturition in rats.
Evidence for a role of α2-adrenoceptors in micturition is conflicting because both facilitatory and inhibitory effects of α2-adrenoceptors have been documented (179, 286). Atipamezole, an α2-adrenergic antagonist given intrathecally, can increase micturition pressure in the conscious rat, implying that there is a tonic inhibitory adrenergic control (286). However, yohimbine, an α2-adrenergic antagonist, inhibits micturition in anesthetized rats (336). In paraplegic patients, intrathecal injection of clonidine suppresses DO (181). Conversely, in conscious spinal cats, clonidine, an α2-adrenergic agonist, increases bladder pressures and facilitates voiding (225).
It is also known that LC noradrenergic neurons are activated by visceral stimuli such as bladder and colon distension, and then modulate arousal and attention (493, 525). Previous studies showed that the excitatory response of LC neurons to bladder distention was strongly affected by the state of anesthesia and that the response was accompanied by lightening of the anesthesia, indicative of arousal, detected by EEG recordings in rats (281, 339). Valentino et al. also reported that neurons containing corticotropin-releasing factor in Barrinton’s neucleus (i.e., the PMC) relay input from pelvic visceral afferents to the LC and may serve as a coordinating center of central and peripheral responses to pelvic visceral stimuli (525, 654).
Pharmacologic experiments showed that the bladder-to-sympathetic reflex pathway is also modulated by spinal noradrenergic mechanisms (138, 174, 179). In the chloralose-anesthetized cat, prazosin or doxazosin, α1-adrenergic antagonists, suppress spontaneous firing (516) or the reflex discharge recorded on the hypogastric nerve in response to pelvic nerve afferent stimulation (138). Administration of α2-adrenergic agonists also suppress reflex sympathetic activity (138). These observations suggest that a bulbospinal noradrenergic pathway provides a tonic α1-excitatory control of the bladder-sympathetic reflex in the spinal cord. α2-adrenergic inhibitory mechanisms are not active under control conditions in anesthetized animals but can be upregulated by elevating endogenous norepinephrine levels with an inhibitor (tomoxetine) of norepinephrine reuptake (138). These results suggest that the lumbar sympathetic outflow is controlled by α1-excitatory and α2-inhibitory mechanisms.
The activation of urethral sphincter motoneurons by stimulation of bladder (pelvic nerve) or urethral/perineal (pudendal nerve) afferents is part of a continence-maintaining mechanism. These reflexes recorded as efferent discharges on the pudendal nerve in chloralose-anesthetized cats are suppressed by the α1-adrenoceptor antagonist prazosin (138, 224), but not by the α2 blocker idazoxan (138). Whole-cell patch clamp techniques in rat neonatal spinal cord slices, revealed that norepinephrine depolarizes urethral sphincter motoneurons and evokes action potentials, and that these effects are blocked by prazosin, suggesting that there is a direct facilitatory mechanism increasing urethral sphincter motoneuron excitability by norepinephrine via α1-adrenoceptors (683).
Conversely, clonidine, a α2-adrenoceptor agonist, suppresses the reflex in anesthetized cats (196). The norepinephrine uptake blocker tomoxetine produces a slight inhibition alone and only a slightly greater inhibition after prazosin. However, it greatly facilitates the reflex when given after idazoxan (138). These data indicate the existence of α2-adrenoceptor-mediated inhibition and α1-adrenoceptor-mediated tonic facilitation of sphincter function and that the α2-adrenoceptor-dependent inhibitory mechanism is the dominant adrenergic modulator of the pudendal nerve reflex (634). These α1 and α2-adrenoceptor-mediated facilitatory and inhibitory mechanisms, respectively, also contributeto the urethral continence reflex that prevents stress urinary incontinence because a norepinephrine reuptake inhibitor nisoxetine or a norepinephrine/serotonin reuptake inhibitor duloxetine induces α1-adrenoceptor activation in the lumbosacral spinal cord to enhance reflex contractions of the EUS during sneezing (297, 431) and that α2 adrenergic antagonists, yohimbine or idazoxan, enhance the duloxetine-induced urethral sphincter contraction during sneezing or abdominal compression in rats (223, 333).
Serotonergic
Neurons containing 5-HT in the raphe nucleus of the caudal brain stem send projections to the dorsal horn, as well as to the autonomic and sphincter motor nuclei in the lumbosacral spinal cord. In cats, activation of raphe neurons or 5-HT receptors in the spinal cord inhibits reflex bladder contractions and firing of the sacral efferent pathways to the bladder (103, 147, 165, 287, 427) and also inhibits firing of spinal dorsal horn neurons elicited by stimulation of pelvic nerve afferents (221). Extracellualar recordings of neuronal activity in the raphe nucleus during storage/voiding cycles under isovolumetric conditions revealed that the most common (~50%) neurons were tonic storage type that exhibited increased firing in the interval between reflex bladder contractions in cats (287).
In rats, the administration of m-chlorophenylpiperazine (mCPP), which is an agonist for 5-HT2 receptors, suppresses efferent activity on bladder nerves and reflex bladder contractions (588). These effects are blocked by mesulergine, a 5-HT2 receptor antagonist (247, 588). Intrathecal administration of methysergide, a 5-HT1/2 antagonist, or zatosetron, a 5-HT3 antagonist, decreases the micturition volume threshold in cats (206), implying that descending serotonergic pathways tonically depress the afferent limb of the micturition reflex through 5HT2 and/or 5HT3 receptors.
The role of 5-HT1 receptors in bladder activity is different in cats and rats. Administration of 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT), a 5-HT1A receptor agonist increases bladder capacity in chloralose anesthetized cats, in which the bladder was irritated with acetic acid, but has only moderate effects on bladder activity in the absence of irritation (637). The drug also has a facilitatory effect on activity of the EUS. 8-OH-DPAT also inhibits reflex bladder activity in awake or chloralose-anesthetized, chronic spinal cord-injured cats, but does not alter the somato-bladder excitatory reflex induced in spinal cats by tactile stimulation of the perigenital region (246). The effects of 8-OH-DPAT are blocked by WAY 100635, a 5-HT1A receptor antagonist, which alone has no effect. These results indicate that 8-OH-DPAT acts in the spinal cord to inhibit the micturition reflex triggered by C-fiber bladder afferent axons and has much less effect on the spinobulbospinal reflex elicited by Aδ-afferents.
In contrast, 8-OH-DPAT administered intrathecally facilitates bladder activity in both normal and spinal cord-injured rats but not in rats in which bladder afferents were damaged by treatment with capsaicin at birth (377). Conversely, administration of the 5-HT1A receptor antagonist WAY 100635, which increases the firing rate of raphe neurons by blocking 5-HT1A inhibitory autoreceptors, inhibits reflex bladder contractions in rats (629). The inhibition is antagonized by pretreatment with mesulergine, a 5-HT2 receptor antagonist, indicating that 5-HT2 receptors are involved in descending raphe/spinal inhibitory mechanisms (629). Similar inhibitory effects of another 5-HT1A receptor antagonist, NAD-299, on the micturition reflex have been reported in rats (502).
When the effects of intrathecal administration of WAY 100635 on the ascending and descending limbs of the micturition reflex pathway were examined in anesthetized rats, WAY 100635 depressed bladder contractions evoked by electrical stimulation of the PMC, but did not alter the evoked field potentials in that region during electrical stimulation of afferent axons in the pelvic nerve, indicating that the drug suppresses the pathway from the brainstem to the spinal cord but does not alter the afferent pathway from the bladder to the PMC (147, 299). Thus, micturition in the rat is facilitated by stimulation of 5HT1 inhibitory autoreceptors, whereas in the cat 5HT1 receptor activation appears to act primarily through postsynaptic mechanisms to promote urine storage by enhancing sphincter activity and suppressing bladder activity (149).
The sympathetic autonomic nuclei as well as the sphincter motor nuclei also receive a serotonergic input from the raphe nucleus (153, 206, 634). Serotonergic activity mediated via 5-HT2 and 5-HT3 receptors enhances urine storage by facilitating sphincter reflexes in cats (139, 206). A recent study in rats also reported that activation of 5HT2C receptors enhances the urethral closure reflex induced by pudendal nerve-mediated urethral striated muscle contraction during sneezing at the spinal level whereas 5HT1A receptors inhibit it because intrathecally applied 8-OH-DPAT (a 5HT1A agonist) decreases urethral contractile responses during sneezing and mCPP (a 5HT2 agonist) increases them, and the effects of 8-OH-DPAT and mCPP are antagonized by intrathecal applications of WAY-100635, a selective 5HT1A antagonist, and RS-102221, a selective 5HT2C antagonist, respectively (432).
Duloxetine, a combined norepinephrine/serotonin reuptake inhibitor has been shown, in a bladder-irritated cat model, to increase the neural control of the urethral sphincter and suppress the bladder (634, 636). Thus, duloxetine has been proposed as a treatment for both stress and urge incontinence (90, 634). Duloxetine increases the neural activity to the EUS via 5-HT2 receptors and α1-adrenoceptors and decreases bladder activity via 5-HT1 receptors in the spinal cord (634). Clinical trials have also shown the efficacy of duloxetine for the treatment of stress urinary incontinence, and the drug has been approved in Europe and is already available in several countries (92) although it was withdrawn from the FDA approval process in the US by the manufacturer.
Acetylcholine
Muscarinic acetylcholine (mACh) receptors have an inhibitory effect on the micturition reflex in the spinal cord. In the rat, intrathecal application of an ACh receptor agonist, oxotremorine-M, or a cholinesterase inhibitor, neostigmine, increases bladder capacity and pressure threshold for initiating micturition, and these effects are atropine-sensitive, indicating a mACh receptor-mediated inhibitory action in the spinal cord (282, 405, 407). Since intrathecal application of atropine by itself has no effects on the micturition reflex in normal rats, but decreases intermicturition intervals in rats with cyclophosphamide-induced cystitis, the endogenous mACh mechanism for the inhibitory modulation of micturition, which is not tonically active in the normal condition, might be upregulated after bladder inflammation (407). Nicotinic receptors are also involved in the control of voiding function since intrathecal application of nicotine has a facilitatory effect on the micturition reflex in the rat (406).
Spinal mAChR also modulate the urethral continence reflex that prevents stress urinary incontinence since a cholinesterase inhibitor, neostigimine, administered intrathecally reduces the urethral closure reflex induced by pudendal nerve-mediated urethral striated muscle contraction during sneezing. The neostigmine-induced decrease in sneeze-induced urethral responses was reversed by pretreatment with atropine (nonselective mACh antagonist), methoctramine (M2 receptor antagonist) or 4-DAMP (M3 receptor antagonist), but not with pirenzepine (M1 receptor antagonist), tropicamide (M4 receptor antagonist), or mecamylamine (nicotinic receptor antagonist), suggesting the involvement of M2 and M3 mACh in muscarinic receptor-mediated modulation of urethral function (695).
Opioid peptides
Opioid peptides have an inhibitory action on reflex pathways in the spinal cord. In the cat spinal cord, inhibition of reflex bladder activity is mediated by μ receptors whereas inhibition of sphincter activity is mediated by κ receptors (165, 174, 701). In the rat, both μ and δ receptors mediate bladder inhibition (174, 197–199, 496). The spinal opioid inhibitory system can also be activated by tachykinins via NK3 receptors (301) and by endothelins via endothelin A receptors (487) to inhibit the micturition reflex.
Opioid receptors also seem to be involved in pudendal or tibial nerve neuromodualtion, which has been shown to be effective for the treatment of OAB symptoms, because naloxone, an opioid receptor antagonist, reverses the increasing effect of pudendal or tibial nerve stimulation on bladder capacity during intravesical saline infusion or bladder overactivity induced by intravesical acetic acid infusion, respectively, in cats (101, 399, 613). However, the site of action for opioid receptor activation during neuromodulation may not be limited to the spinal cord as naloxone was administered systemically in these studies.
Pontine micturition center and supraspinal pathways
Glutamate
Glutamic acid has a role in excitatory transmission at supraspinal sites in the micturition reflex pathway. Exogenous L-glutamate or its analogue injected at sites (PMC or parabrachial nucleus) in the brain stem of supracollicular decerebrate or chloralose anesthetized cats where electrical stimulation evoke bladder contractions (348), elicits voiding when the bladder is partially filled or elicits increased frequency and amplitude of rhythmic bladder contractions when the bladder is filled above the micturition threshold volume and maintained under isovolumetric conditions (103,398). On the other hand, injections of glutamic acid at some sites in the PMC elicits inhibition of isovolumetric contractions or initial excitation followed by inhibition (398).
Administration of glutamatergic agonists into the region of the PMC in rats also elicits voiding or increases frequency and amplitude of bladder contractions (416, 521), whereas injection of agonists in the brain of rats and cats at other sites known to have inhibitory functions in micturition elicits inhibitory effects(102, 103, 465, 471, 599). Intracerebroventricular injection of AMPA or NMDA receptor antagonists blocks reflex bladder contractions in anesthetized rats, indicating that glutamatergic transmission in the brain is essential for voiding function (718).
In rat brain slices patch clamp recordings from PPO and PSO neurons projecting, respectively, to the sacral parasympathetic and thoraco-lumbar sympathetic intermediolateral nuclei (250) revealed that spontaneous EPSCs recorded after blocking GABAergic and glycinergic inhibitory receptors with bicuculline and strychnine were blocked by the AMPA glutamatergic receptor antagonist CNQX. This indicates that the neurons receive excitatory inputs from glutamatergic neurons located in the slice. Blocking AMPA and NMDA ionotropic glutamate receptors also decreases the spontaneous firing of PSO neurons but paradoxically increases the firing of PPO neurons indicating that the latter neurons receive a tonic inhibitory input triggered by a glutamatergic mechanism. This is consistent with the observation mentioned above that injections of glutamate at some sites in the cat PMC unexpectedly inhibited reflex bladder activity (398).
A recent study also showed that a nonselective inhibitor of glutamate transporters, L-trans-PDC, administered into the lateral ventricle increases intermicturition intervals and pressure thresholds in anesthetized rats, suggesting that global activation of the glutamatergic system at supraspinal sites has an inhibitory effect on micturition, possibly via activation of glutamate-mediated inhibitory pathways (273).
Acetylcholine
In the rat brain, muscarinic receptor-mediated cholinergic mechanisms may be involved in both inhibitory and facilitatory modulation of the micturition reflex (282, 284, 688), and the muscarinic inhibitory mechanism seems to involve an activation of M1 muscarinic receptors (688) and PKC (467). One site of action can be localized to the midbrainpons region because cholinergic agonists are effective after supracollicular decerebration in rats (569). In the brain stem, microinjection of ACh to the PMC in cats increased or decreased the threshold volume for inducing a reflex contraction of the bladder (598, 701). These effects were blocked by atropine, indicating a role of muscarinic receptors. Nicotinic receptors are also involved in the control of voiding function since nicotinic receptor agonists, epibatidine or nicotine, injected into the lateral ventricle have an inhibitory effect on the micturition reflex in the rat (380, 406). A decreased volume threshold and increased micturition pressure were detected after administration of bethanechol, a muscarinic agonist, into the central circulation of the cross-perfused dog (484).
GABA and Glycine
GABA has been implicated as an inhibitory transmitter at supraspinal sites where it can act on both GABAA and GABAB receptors (152, 174, 179, 304, 701). As mentioned in an earlier section of this paper, injection of GABAA receptor agonists, into the PMC of decerebrate cats or into the PAG of rats suppresses reflex bladder activity and increases the volume threshold for inducing micturition (398). These effects are reversed by bicuculline, a GABAA receptor antagonist; and bicuculline alone stimulates bladder activity and lowers the volume threshold for micturition, indicating that the micturition reflex pathway in the PMC and PAG is tonically inhibited by a GABAergic mechanism. Intracerebroventricular administration of melatonin increases bladder capacity in rats; and this effect is blocked by bicuculline indicating that melatonin activates a GABAergic inhibitory mechanism in the brain (414). Intracerebroventricular injection of baclofen, a GABAB agonist, suppresses distention-evoked micturition in urethane-anesthetized rats but unexpectedly this effect is not blocked by phaclofen, a GABAB receptor antagonist (165, 174).
Patch clamp recordings in rat brain slices showed that blocking GABAA receptors with bicuculline increases the excitability of both PPO and PSO neurons, while blocking glycine receptors with strychnine increases the firing of only PPO neurons (250). Blocking ionotropic glutamatergic receptors which increases firing of PPO neurons in untreated slices does change firing in the presence of strychnine, indicating that glutamatergic excitatory transmission generates the tonic glycinergic inhibitory input to PPO neurons.
Dopamine
In the central nervous system, dopaminergic pathways exert inhibitory and facilitatory effects on the micturition reflex through D1-like (D1 or D5 subtypes) and D2-like (D2, D3, or D4 subtypes) dopaminergic receptors, respectively, (Fig. 24) (11, 258, 335, 558, 689, 704–706, 712). In anesthetized cats, activation of dopaminergic neurons in the substantia nigra inhibits reflex bladder contractions via D1-like receptors (712). In awake rats a D1 dopaminergic antagonist (SCH 23390) facilitates the micturition reflex whereas a D1 agonist (SKF 38393) does not alter reflex bladder contractions, suggesting that D1 receptor-mediated suppression of bladder activity is tonically active in the normal awake state (558). Conversely, activation of central D2-like dopaminergic receptors with quinpirole or bromocriptine facilitates the micturition reflex pathway in rats, cats, and monkeys (335, 689, 704–706). D2-like receptor-mediated facilitation of the micturition reflex may involve actions on spinal cord (Fig. 24) as well as actions on the brain stem because microinjection of quinpirole intrathecally in rats (705) or dopamine into the PMC in cats(165) reduces bladder capacity and facilitates the micturition reflex.
Figure 24.

Hypothetical diagram showing dopaminergic and adenosinergic mechanisms inducing bladder dysfunction in Parkinson’s disease (PD). The micturition reflex is controlled by the spinobulbospinal pathway passing through the PAG in the midbrain and the PMC in the pons. This neural circuit is under the control of higher centers including the striatum and the cortex region. A. Under normal conditions (Intact), tonic firing (+) of dopaminergic neurons in the SN activates dopamine D1 receptors expressed on GABAergic inhibitory neurons in the striatum to induce tonic GABAergic (−) inhibition of the micturition reflex at the level of the PAG. At the same time, D1 receptor stimulation suppresses the activity of adenosinergic neurons, which exert an excitatory effect on micturition via adenosine A2A receptors [Adenosine A2A (+)]. B. In PD, dopaminergic neurons in the SN are lost (lesion), leading to the loss of dopamine D1 receptor activation [D1 (loss of activation)], which results in reduced activation of inhibitory GABAergic neurons in the striatum [GABA (loss of inhibition)]. At the same time, reduced D1 receptor stimulation enhances the adenosinergic mechanism to stimulate adenosine A2A receptors [Adenosine A2A (++)], leading to facilitation of the spinobulbospinal pathway controlling the micturition reflex (activity). Administration of dopamine D1 receptor agonists (D1 agonist) can restore the GABAergic nerve activity and suppress A2A receptor-mediated activation to reduce bladder overactivity in PD. Also, administration of adenosine A2A receptor antagonists (A2A antagonist) can suppress A2A receptor-mediated activation of the micturition reflex to reduce bladder overactivity in PD. Dopamine D2 receptors [D2 (+)] expressed in the spinal cord enhance the micturition reflex. Abbreviations: dopamine D1 receptor (D1); dopamine D2 receptor (D2); gamma-aminobutyric acid (GABA); periaqueductal gray (PAG); pontine micturition center (PMC); substantia nigra pars compacta (SN).
It is also known in cats that neurons in the substantia nigra pars compacta and the ventral tegmental area, which are the major dopamine-containing nuclei in the midbrain, respond to the storage/micturition cycles of isovolumetric cystometry (536) and that dopamine levels in the striatum, where nigrostiatal dopaminergic nerves terminate (Fig. 24), increase during the storage phase of the micturition cycle (681). Thus, central dopaminergic pathways appear to be involved in the control of the bladder function through actions on multiple receptors at different sites in the brain.
Activation of D2-like receptors at a supraspinal site suppresses the activity of the striated sphincter muscle and reduces intraurethral pressure; whereas inhibition of dopamine D1- or D2-like receptors has a minimal effect on urethral function in anesthetized rats, suggesting the dopaminergic control of urethral function is minimally active in the normal condition (488).
Opioid peptides
Intracerebroventricularly administered morphine suppresses isovolumic bladder contractions in rats and cats, and this effect is blocked by naloxone (197–199, 264). Naloxone administered intracerebroventricularly also reverses the effects of systemically administered morphine. Naloxone administered alone intracerebroventricularly or injected directly into the PMC facilitates the micturition reflex, indicating that micturition is tonically inhibited by a supraspinal opioid mechanism (264, 478). Both μ and δ opioid receptors mediate inhibitory effects that are blocked by naloxone (264, 398). In addition, activation of μ and δ1, but not δ2 opioid receptors in the brain increases bladder capacity in both normal rats and rats with cerebral infarction that exhibit frequent voiding; however, κ receptor activation increases bladder capacity only in rats with cerebral infarction (464). In rat brain slices application of DAMGO a specific μ opioid receptor agonist suppresses the firing of PPO and PSO neurons in the PMC (250). DAMGO hyperpolarizes PPO but not PSO neurons.
Developmental Changes in Micturition Reflexes
Reflex changes
The mechanisms involved in storage and periodic elimination of urine undergo marked changes during prenatal and postnatal development (91, 150, 156, 157). In the young fetus prior to maturation of the nervous system, urine is eliminated from the bladder by nonneural mechanisms; however at later stages of development voiding is regulated by primitive reflex pathways organized in the spinal cord. As the central nervous system matures during the postnatal period, reflex voiding is eventually brought under voluntary control which originates in the higher centers of the brain.
In many species (eg., rats and cats) voiding in neonates is dependent on an exteroceptive somato-bladder reflex mechanism triggered when the mother licks the genital or perineal region of the young animal (157, 396, 632, 635, 638). The exteroceptive reflex is organized in the sacral spinal cord and has an afferent limb in the pudendal nerve and an efferent limb in the pelvic nerve. Similar reflexes have been identified in human infants (72). In newborn kittens and rats the exteroceptive perineal-to-bladder reflex is essential for survival because isolation of the newborn from its mother leads to urinary retention (91, 344, 345, 632). This indicates that an adult form of reflex voiding which is induced by bladder distension is not functional in neonatal animals. The latter type of voiding mediated by the spinobulbospinal reflex pathway and by the micturition center in the brain stem, only emerges several weeks after birth (157, 632). During this same period of time the perineal-to-bladder reflex becomes progressively weaker and eventually disappears. Thus postnatal maturation of voiding function is associated with a prominent reorganization of synaptic connections in bladder reflex pathways leading to downregulation of primitive spinal mechanisms and upregulation of mature supraspinal mechanisms. It seems likely that this developmental switching mechanism is dependent upon competition between brain and spinal pathways because spinal cord injury in adult animals and humans which interrupts brain-spinal cord connections causes the reemergence of the neonatal perineal-to-bladder reflex. Recent studies (28,596,597,721) which used tract tracing techniques and electrophysiological methods have provided insights into the synaptic changes underlying developmental neuroplasticity in the parasympathetic pathways to the urinary bladder.
Anatomy
Although voiding in neonatal rats does not depend on neural mechanisms in the brain, a large number of neurons at various sites in the brain are labeled by PRV injected into the urinary bladder of 2 and 10 day old rat pups (604). Indeed, the distribution of PRV-infected neurons is somewhat broader than in adult rats. At the earliest survival times (72 h) the most prominent labeling is in the PMC. Other neuronal populations that are labeled at slightly longer times (78–84 h) include the NRM, A5 and A7 regions, parapyramidal reticular formation, the PAG, LC, the lateral hypothalamus, medial preoptic area and the frontal cortex. These areas are also labeled in adult animals (Fig. 9). Many of these correspond to sites where electrical stimulation facilitates or inhibits bladder activity in adult animals. Thus even in neonatal animals some supraspinal mechanisms may already be functioning, possibly in an inhibitory manner, to suppress the spinobulbospinal micturition reflex pathway allowing micturition to be controlled by primitive spinal reflex mechanisms.
Physiology
In vivo studies
Measurements of bladder activity as well as voiding function in anesthetized or unanesthetized neonatal rats and kittens have revealed strong reflex activation of the parasympathetic outflow to the bladder in response to tactile stimulation of the perineal region (164, 345, 396, 632, 635, 638). The reflex activity is mediated by pathways in the spinal cord because it is unaffected by transection of the cord rostral to the lumbosacral segments. In rats the voiding response to perineal stimulation is depressed by either NMDA or nonNMDA glutamatergic receptor antagonists indicating that glutamic acid, the principal excitatory transmitter in the central nervous system, plays an essential role in the spinal mechanisms involved in the perineal-to-bladder reflex (596, 721).
Although the spinobulbospinal micturition reflex is nonfunctional in young kittens and rat pups, the pathway seems to be “wired-up” and can be activated under certain conditions. For example in 50% of 2-day-old rats and 100% of 6- to 9-day-old rats, removal of the forebrain (decerebration) unmasks reflex bladder activity in response to bladder distension (344). This activity is abolished by transection of the spinal cord rostral to the lumbar level, indicating that it is mediated by a supraspinal pathway. In kittens similar reflexes can be evoked after the administration of certain anesthetics such as chloralose or ketamine. Under general anesthesia the immature spinobulbospinal reflex in kittens exhibits a very long central delay (225–325 ms) (164) in comparison to the delay in adult cats (60–75 ms). This long delay is presumably due to the slow conduction velocity of immature central axons in the newborn animal. The latency of the reflex progressively shortens during the first eight postnatal weeks reaching the adult latency by 2 to 3 months of age. These findings are consistent with the results of PRV tracing and indicate that supraspinal bladder-to-bladder reflexes are organized relatively soon after birth but are tonically suppressed by inhibitory mechanisms in the brain. Suppression of these inhibitory mechanisms by decerebration or anesthetics in young animals or by the natural process of neural maturation in older animals leads to the emergence of the latent reflex pathway and the appearance of mature voiding mechanisms.
In vitro brain stem-spinal cord-bladder preparation
Perineal-to-bladder reflexes have also been studied in vitro using preparations consisting of: (i) the spinal cord or the brain stem-spinal cord, (ii) the bladder and perineal region, and (iii) the relevant peripheral neural pathways (596, 597). Reflexly active preparations have been obtained from rat pups ranging from 4 to 10 days of age. As noted in vivo, tactile stimulation of the perineal region in these preparations induces neurally evoked contractions of the bladder that are blocked by the administration of ganglionic blocking agents or by non-NMDA glutamatergic receptor antagonists as noted in vivo. Transection of the spinal cord above the lumbar segments does not block but commonly enhances the reflex indicating that input from the brain stem tonically inhibits the spinal reflex pathway. Excitatory projections from the brain to the parasympathetic outflow to the bladder are also elicited by microstimulation of a narrow fiber tract running rostrocaudally through the medulla (597). This finding is consistent with in vivo and anatomical experiments showing that the descending limb of the spinobulbospinal micturition reflex is functional in neonatal animals.
In vitro spinal cord slice preparation
The mechanisms underlying the developmental suppression of the perineal-to-bladder reflex and its re-emergence after spinal cord injury were studied using patch clamp recordings in identified PGN in spinal cord slice preparations from 6- to 28-day-old neonatal rats (28). Interneurons located within 100 um of the PGN were stimulated with extracellular micropipettes to elicit postsynaptic currents in the PGN.
Two types of interneurons were identified: (i) interneurons located dorsal and medial to the PGN that elicit excitatory postsynaptic currents (EPSCs) and (ii) interneurons located medial to the PGN that elicit inhibitory postsynaptic currents (IPSCs) (28). Dorsal interneurons receive excitatory synaptic inputs from axonal pathways on the lateral edge of the dorsal horn, an area known to contain primary afferent projections to the parasympathetic nucleus. The EPSCs in PGN evoked by dorsal interneurons consist of fast and slow components that are blocked respectively by nonNMDA (CNQX) and NMDA (APV) glutamatergic receptor antagonists. Repetitive stimulation of dorsal interneurons facilitates the amplitude of EPSCs and elicits action potentials in PGN. Because dorsal interneurons are exclusively excitatory to PGN and receive excitatory inputs from presumptive afferent axons it is likely that these interneurons are part of a disynaptic parasympathetic reflex pathway that underlies the perineal-to-bladder reflex. Thus the effect of postnatal maturation on the efficiency of dorsal interneuron-to-PGN synaptic transmission was evaluated to determine if changes in transmission could account for downregulation of the reflex pathway during postnatal development.
In slice preparations from 1- and 2-week-old rats the dorsal interneuron-evoked EPSCs are large and of constant amplitude. However, in preparations from 3 week old rats, an age when the spinobulbospinal micturition has emerged, the EPSCs are reduced in amplitude by 50%. Quantal analysis of unitary EPSCs indicated that this reduction in amplitude is attributable to a decrease in the presynaptic release of glutamic acid and is not due to a change in the density or sensitivity of postsynaptic glutamatergic receptors (28). Transection of the spinal cord between 1 and 2 weeks of age prevents the reduction in synaptic transmission that occurs at 3 weeks of age.
These observations are most reasonably explained by anatomical changes in the synapses in the sacral parasympathetic nucleus. It seems likely that maturation of the bulbospinal pathways to the PGN during the second to third postnatal week, at the same time when the mature bladder-to-bladder spinobulbospinal reflex is emerging, downregulates the interneuronal-PGN excitatory synapses, possibly by synapse elimination in response to competition for synaptic space between boutons of interneurons and descending axons. Transection of the spinal cord which causes degeneration of the descending axons blocks this synaptic reorganization and maintains the primitive neonatal reflex pathway. A similar degeneration of descending axons in adult animals could stimulate axonal sprouting in interneuronal pathways and cause the re-emergence of the neonatal perineal-to-bladder reflex in chronic spinal animals.
In summary, neuroanatomical and electrophysiological techniques have provided new insights into the organization of the spinal cord circuitry and the neurotransmitter mechanisms involved in primitive voiding reflexes in neonatal animals. In addition, studies of unitary synaptic transmission in spinal cord slice preparations indicate that developmental and spinal cord injury induced plasticity in sacral parasympathetic reflex pathways is due in part to alterations in glutamatergic excitatory transmission between interneurons and PGNs. Thus, synaptic remodeling in the sacral parasympathetic nucleus is likely to be an important factor in the postnatal maturation of voiding reflexes.
Disease-Induced Changes in Micturition
Spinal cord injury and neuropathic bladder
Role of bladder C-fiber afferents
SCI rostral to the lumbosacral level eliminates voluntary and supraspinal control of voiding, leading initially to an areflexic bladder and complete urinary retention followed by a slow development of automatic micturition and bladder overactivity mediated by spinal reflex pathways (28,142,161,164, 175,177,178,697). However, voiding is commonly inefficient due to simultaneous contractions of the bladder and urethral sphincter (DSD). Electrophysiologic studies in animals have shown that the micturition reflex pathways in spinal intact animals and in chronic spinal-injured animals are markedly different (105,141,168). In cats with an intact spinal cord, myelinated (Aδ) afferents activate the micturition reflex, whereas in cats with chronic thoracic spinal cord transection, micturition is induced by unmyelinated (C-fiber) axons (Fig. 25). In normal cats, capsaicin does not block reflex contractions of the bladder or the Aδ-fiber-evoked bladder reflex. However, in cats with chronic spinal injury, capsaicin, a neurotoxin known to disrupt the function of C-fiber afferents, completely blocks C-fiber-evoked bladder reflexes (Fig. 25) (105,161). In chronic spinal cord injured rats capsaicin treatment reduces nonvoiding contractions during bladder filling and partially normalizes external urethra sphincter activity (104).
Figure 25.

Organization of the parasympathetic excitatory reflex pathway to the detrusor muscle. This scheme is based on results from electrophysiological studies in cats. Micturition is initiated by a supraspinal reflex pathway that passes through a center in the brainstem. The pathway is triggered by myelinated afferents (Aδ-fibers), which are connected to the tension receptors in the bladder wall. Injury to the spinal cord above the sacral segments interrupts the connections between the brain and spinal autonomic centers and initially blocks micturition. However, following cord injury a spinal reflex mechanism (shown in green) emerges that is triggered by unmyelinated vesical afferents (C-fibers); the A-fiber afferent inputs are ineffective. The C-fiber reflex pathway is usually weak or undetectable in animals with an intact nervous system. Stimulation of the C-fiber bladder afferents by instillation of ice water into the bladder (cold stimulation) activates voiding responses in patients with spinal cord injury. Capsaicin (20-30 mg, subcutaneously) blocks the C-fiber reflex in cats with spinal lesions but does not block micturition reflexes in spinal intact cats. Intravesical capsaicin also suppresses detrusor hyperreflexia and cold-evoked reflexes in patients with neurogenic bladder dysfunction (216).
Chronic spinal injury in humans also causes the emergence of an unusual bladder reflex that is elicited in response to infusion of cold water into the bladder (229). Studies in animals indicate that cold temperature activates receptors in bladder C-fiber afferents and urothelial cells (209, 590). Contribution of afferent hyperexcitability to the emergence of bladder overactivity in SCI has also been identified by clinical studies using neurotoxins such as botulinum toxin and resiniferatoxin (RTX). For example, suppression of bladder afferent activity with botulinum toxin, which in essence produces chemical denervation, effectively treats DO; mitigates urgency in both neurogenic DO in SCI patients, and, with sustained therapy, reduces the expression of the capsaicin receptor (TRPV1) and the purinergic receptor (P2X3) in C-fiber afferents (23, 24). In patients with SCI-induced DO, the clinical response to intravesical therapy with the C-fiber afferent toxin RTX is accompanied by a marked decrease in the density of nerve fibers positively stained for PGP9.5, and TRPV1. Six of 17 patients showed a satisfactory clinical response to RTX treatment, with marked improvements on cystometry and other parameters whereas SCI patients who did not respond to RTX showed no decrease in nerve fiber staining (80, 81).
The emergence of C-fiber bladder reflexes seems to be mediated by several mechanisms including changes in central synaptic connections and alterations in the properties of the peripheral afferent receptors that lead to sensitization of the “silent” C fibers and the unmasking of responses to mechanical stimuli (146, 175, 177, 178). In rats, it has been shown that bladder afferent neurons undergo both morphologic (neuronal hypertrophy) (343) and physiologic changes including a shift from the high-threshold, tetrodotoxin (TTX)-resistant Na+ channel type to the low-threshold, TTX-sensitive Na+ channel type (697, 702) as well as downregulation of low-threshold A-type K+ channels that is associate with decreased expression of Kv1.4 α-subunit following SCI (617). Measurements of single unit afferent activity in the L6 dorsal root during constant bladder filling in SCI and spinal intact rats, identified a greater number of both Aδ and C type bladder afferent fibers categorized as the accelerated type in SCI rats. These afferents exhibit a sudden increase in firing of more than 10 Hz during small bladder contractions, indicating an increase in bladder afferent excitability after SCI (280).
Role of neurokinins
Destruction of lumbosacral spinal neurons expressing neurokinin-1 (NK-1) receptors using substance P conjugated with saporin does not affect reflex voiding in normal rats, but reduces the bladder irritant effects of intravesical capsaicin (557) and reduces nonvoiding contractions in SCI rats (560). Similarly, intrathecal administration of a selective NK-1 receptor antagonist (L-733060) does not affect the micturition reflex in spinal intact rats but blocks the micturition reflex in SCI rats (733). These data coupled with the increased expression of substance P in the region of the sacral parasympathetic nucleus in SCI rats (733) suggest that activation of NK-1 receptors in the spinal cord plays a role in SCI-induced DO. However, oral administration of selective NK1 (GR 82334) and NK2 receptor antagonists (MEN 11420) which do not penetrate the blood-brain barrier, also suppress DO in SCI rats, suggesting that peripheral NK1 and NK2 receptors, possibly expressed in the bladder, also contribute to the emergence of DO in SCI (1, 2).
Role of VIP and PACAP
The expression of VIP and PACAP in lumbosacral afferent pathways is increased after spinal cord injury (741). The pharmacological effect of VIP on bladder activity is also changed after SCI. Intrathecal administration of VIP, which suppresses reflex bladder activity in cats with an intact spinal cord, enhances or unmasks reflex bladder activity in chronic SCI cats (161, 638). PACAP-27 or PACAP-38 enhances reflex bladder activity when administered intrathecally (283,720) and PACAP-38 has excitatory effects on lumbosacral parasympathetic neurons in the rat (428). Intrathecal administration of PACAP6-38, an agent that blocks one type of PACAP receptor (PAC-1) suppresses bladder overactivity in chronic SCI rats indicating a role for PACAP in neurogenic DO (739).
Role of GABA
As mentioned earlier, reduced GABAergic inhibition could contribute to the development of neurogenic DO because mRNA levels of GAD67, an enzyme involved in GABA synthesis, are decreased in the spinal cord after SCI in rats (433). A possible therapy for neurogenic DO has emerged from experimental studies, in which an HSV vector encoding the GAD gene was injected into the bladder of SCI rats to increase GAD expression in bladder afferent nerves. This treatment reduces DO and DSD in SCI rats (436, 439); an effect similar to that elicited in chronic SCI rats by desensitization of C-fiber bladder afferents with systemic capsaicin administration.
Role of TRP receptors
The number of suburothelial nerve fibers expressing TRPV1 receptors, which are predominantly expressed in C-fiber afferent pathways, is increased in patients with neurogenic DO compared to controls (81). In rats with SCI, duodenal administration of a TRPV1 antagonist (GRC 6211) reduces bladder contraction frequency in SCI rats (540). In addition, intravenous administration of a TRPA1 antagonist (HC-030031) or intrathecal treatment with antisense oligodeoxynucleotide of TRPA1 receptors is effective in suppressing DO in SCI rats (20). These results along with the increased TRPA1 expression in the bladder and L6-S1 DRG in SCI rats (20) suggest that TRP receptors such as TRPV1 and TRPA1 are involved in the emergence of C-fiber bladder afferent hyperexcitability that contributes to neurogenic DO in SCI.
Role of neurotrophic factors
It has been speculated that SCI-induced neuroplasticity is mediated by the actions of neurotrophic factors such as NGF released within the urinary bladder or the spinal cord. Figures 26 and 27, respectively, depict the peripheral and central mechanisms inducing afferent sensitization and bladder overactivity by neurotrophins such as NGF or BDNF. NGF, which can be produced by urothelium, mast cells and detrusor smooth muscle cells in response to stretch or inflammation, binds to TrkA receptors expressed in suburothelial C-fiber afferent nerve terminals, which express TRPV1, mechanosensitive channels, P2X3 purinergic receptors and different types of voltage gated channels. The neurotrophin-Trk complex is retrogradely transported to cell bodies in the lumbosacral DRG to sensitize bladder afferent pathways (Fig. 26) and increase synthesis of excitatory neuromediators, such as substance P, CGRP and BDNF in DRG neurons, which then activate their corresponding receptors (NK1, CGRP receptor and TrkB, respectively) when released from nerve terminals to induce central sensitization of afferent pathways and bladder overactivity (Fig. 27).
Figure 26.

Peripheral mechanisms involved in the neurotrophin-mediated development of bladder overactivity. In urinary bladder, NGF (shown in blue) is produced by several cell types—including urothelium, mast cells, and detrusor smooth muscle cells—upon stretch or inflammation. The urothelium also potentially produces BDNF (shown in red). NGF binding to TrkA receptors on the urothelium might directly activate urothelial sensory ion channels, such as TRPV1 (shown in purple), or increase expression of TRPV1 and mechanosensitive channels (MSC, shown in pink). Increased TRPV1 and MSC activity stimulate the release of urothelial mediators, such as ATP, which sensitize the underlying afferents. In addition, NGF activates TrkA receptors expressed on suburothelial afferent C-fiber terminals, directly sensitizing neuronal TRPV1, MSCs and voltage-gated ion channels (VGCs, shown in orange). The TrkA-NGF complex is internalized (dashed lines) and retrogradely transported to cell bodies in lumbosacral DRG, where de novo transcription of TRPV1, VGCs, MSCs and additional sensory ion channels (including purinergic P2X3 receptor for ATP; shown in green) is initiated. These newly synthesized ion channels are anterogradely transported back to afferent terminals to contribute to peripheral hypersensitivity. Neurotrophin receptors TrkB (shown in red) and p75NTR (shown in black) are also expressed on both urothelium and afferent terminals, although their role has not yet been defined. Abbreviations: ATP, adenosine triphosphate; BDNF, brain-derived nerve factor; BOO, bladder outlet obstruction; BPS, bladder pain syndrome; DRG, dorsal root ganglia; MSC, mechanosensory channel; NGF, nerve growth factor; OAB, overactive bladder syndrome; P2X3, P2X purinoceptor 3; TrkA, tropomyosin-related kinase A; TrkB, tropomyosin-related kinase B; TRPV1, transient receptor potential cation channel vanilloid subfamily member 1; VGC, voltage-gated ion channel (486).
Figure 27.

Central mechanisms involved in the neurotrophin-mediated development of bladder overactivity. Upon retrograde transport of TrkA-NGF complexes along the afferent fibers from the bladder, DRG neurons increase synthesis of excitatory neuromediators, such as substance P, CGRP, BDNF, and voltage-gated ion channels, which are transported anterogradely to primary afferent terminals in the lumbosacral spinal cord. Following the synaptic release, substance P (shown in green), CGRP (shown in brown), and BDNF (shown in red) activate their corresponding receptors (NK1, CGRP receptor, and TrkB, respectively) to induce central sensitization of nociceptive, and possibly also micturition, pathways. One of the mechanisms of central sensitization involves BDNF-induced activation of the NMDA receptor for excitatory mediator glutamate (shown in gray). Enhanced voltage-gated ion channel activity could contribute towards increased firing of bladder afferents. NGF and TrkA are also detected in the spinal cord, but their origin and function remain unknown. Following sensitization and activation of the central (spinobulbospinal) micturition reflex, excessive efferent stimulation could eventually contribute to DO. Abbreviations: BDNF, brain-derived nerve factor; CGRP, calcitonin gene-related peptide; CGRPR, calcitonin gene-related peptide receptor; DO, detrusor overactivity; DRG, dorsal root ganglia; Glu, Glutamate; NGF, nerve growth factor; NK1, neurokinin-1 receptor; NMDA, N-methyl-D-aspartate; MPG, major pelvic ganglia; SP, substance P; Trk, tropomyosin-related kinase; VGC, voltage-gated ion channel (486).
In clinical studies, NGF production is elevated in the bladder and urine samples of SCI patients with DO. NGF levels can be reduced along with symptom improvement after intradetrusor botulinum toxin treatment (231, 385). Animal studies also demonstrated that the production of neurotrophic factors including NGF increases in the bladder after SCI (661) and chronic administration of NGF into the spinal cord or into the bladder wall in rats induces bladder overactivity and increases excitability of bladder afferent neurons (366,698,740). Increased transport of NGF to DRG cell bodies or central NGF production in the injured spinal cord could modulate the micturition pathway at the spinal level. Intrathecal delivery of an NGF monoclonal antibody diminishes neurogenic DO and DSD in rats with SCI (559, 560). Thus, a combination of peripheral and central NGF actions is likely to be involved in the emergence of neurogenic DO (Figs. 26, 27). Roles of other neurotrophic factors such as BDNF have not yet been defined.
Bladder outlet obstruction
BOO which can occur in patients with BPH often produces detrusor hypertrophy and DO (19, 237). Increased detrusor myogenic activity which is an important contributor to BOO induced DO can be induced by many mechanisms including: denervation supersensitivity of cholinergic (muscarinic) receptors (583), increases in purinergic receptor mediated contractile responses as well as expression of purinergic receptors such as P2X1 (75, 485), changes in the cell-to-cell communication in detrusor muscles due to upregulation of gap-junction proteins such as connexin 43 (112, 253) and increased in the number of interstitial cells (328, 349).
In addition to functional changes in the detrusor, BOO also alters neural networks in the central nervous system. BOO in rats enhances a spinal micturition reflex (587) and clinically enhances the bladder ice water test, which is mediated by a C-fiber afferent-dependent spinal micturition reflex, consistent with considerable upregulation of C-fiber afferent mechanisms in BOO patients with BPH (94,262,263). Among BOO patients with a positive ice-water test, the incidence of DO was significantly greater in those who reported episodes of nocturia greater than or equal to three times the frequency reported by patients with fewer episodes (94,262,263). Within the spinal cord, obstruction stimulates an increased expression of GAP-43, an effect often associated with axonal sprouting after injury (586). These observations suggest an enhancement or de novo development of new spinal circuits after obstruction. Similar to spinal cord injury, obstruction causes hypertrophy of bladder afferent and efferent neurons (584,585). Conversely, relief of obstruction is associated with the reduction of urinary frequency and reversal of these neural changes (589). In animals that fail to revert to a normal voiding pattern after relief of obstruction, this neuroplasticity persists.
Role of neurotrophic factors
Men with OAB symptoms and BOO caused by BPH display increased levels of NGF in bladder tissues (589) and increased levels of urinary NGF (386). Also, NGF elevation in the urine is reduced following successful BPH treatment with an α-adrenoceptor antagonist or a 5α-reductase inhibitor (386). NGF content is increased in obstructed bladders in BOO animals (589), and this increase in NGF content precedes the enlargement of bladder neurons and the developmental of urinary frequency (584,585). Moreover, blockade of NGF action using autoantibodies prevents the neural plasticity and urinary frequency after obstruction (589). In animals with persistent urinary frequency after relief of obstruction, NGF remains elevated in the bladder. These findings suggest a cause-and-effect relationship between NGF-mediated changes in bladder afferents and an enhanced spinal micturition reflex and urinary frequency associated with BOO.
Inflammation/bladder pain syndrome
Bladder pain syndrome/interstitial cystitis (BPS/IC) is a disease with LUT symptoms such as urinary frequency and bladder pain related to bladder filling. Although the etiology of BPS/IC is still not known, there is increasing evidence that the disorder is associated with urothelial dysfunction and afferent hyperexcitability due to neurogenic bladder inflammation (52,699,713). Chronic conditions that involve continuous tissue inflammation or irritation can induce changes in sensory pathways that lead to hyperalgesia (heightened response to painful stimuli) and allodynia (pain in response to normally nonpainful stimuli). Thus, continuous tissue inflammation in visceral organs such as the bladder can lead to sensitization of afferent nerves and increased afferent nerve responses to both noxious and nonnoxious stimuli (251, 561). In a rat model of chronic cystitis induced by cyclophosphamide or hydrochloric acid, it has been shown that capsaicin-sensitive bladder afferent neurons increase their excitability due to decreased density of A-type K+ (KA) currents, associated with the decreased expression of the Kv1.4 α-subunit (260,703). Similar somal hyperexcitability due to reduced KA current expression after chronic tissue inflammation has also been detected in afferent neurons innervating the rat stomach (135) or the guinea pig ileum (592). Thus, the reduction in KA current size could be a key mechanism inducing afferent hyperexcitability and pain in visceral organs including the bladder. Suppression of these K+ currents with drugs such as 4-aminopyridine can acutely increase afferent neuron excitability (553, 556, 703).
A previous study using cats with naturally occurring feline-type IC has also demonstrated that capsaicin-sensitive DRG neurons exhibit an increase in cell size and firing rates to depolarizing current pulses due to a reduction in low-threshold K+ currents elicited by membrane depolarization between–50 to−30 mV (554). If these changes in neuronal cell bodies similarly occur at C-fiber afferent terminals in the bladder wall, such hyperexcitability may represent an important mechanism for inducing pain in the inflamed bladder. Therefore, suppression of C-fiber activity represents a mechanism for treating bladder pain. This is supported by previous findings that C-fiber desensitization induced by intravesical application of high-dose capsaicin or RTX is effective for treating painful symptoms in patients with IC (374, 375) although another prospective, randomized clinical trial using intravesical RTX application failed to show a significant improvement of symptoms in patients with IC (499).
Chemical inflammation of the rat urinary bladder also induces increased expression of various chemical markers in afferent neurons in lumbosacral DRG innervating the bladder (33), such as NOS (663), growth-associated protein (GAP-43) (662), PACAP (659), neuropeptides such as substance P (660), PARs (140), cyclooxygenase-2 (COX-2), and prostaglandins (276). Some of these changes may also contribute to the development of afferent sensitization.
Role of TRP receptors
In patients with BPS/IC, there is a significant increase in suburothelial nerve fibers expressing TRPV1 and the increase is correlated with pain scores (452). There is also evidence that chronic bladder inflammation in animal models can induce changes in functional properties of chemosensitive receptors such as TRPV1 in sensory neurons. Sculptoreanu et al. (554) reported that DRG neurons obtained from cats with feline IC exhibited capsaicin-induced responses that were larger in amplitude and desensitized more slowly compared with those obtained from normal cats, and that altered TRPV1 receptor activity in IC cats was reversed by an application of an inhibitor of PKC, suggesting that BPS/IC could alter TRPV1 activity by enhancing endogenous PKC activity. In rat bladders, increased expression of anandamide that can activateTRPV1 receptors has been proposed as one mechanism that could contribute to the bladder overactivity elicited by CYP-induced cystitis (185). In addition, bladder overactivity elicited in rats by lipopolysaccharide-induced cystitis is inhibited by intraduodenal administration of a TRPV1 antagonist (GRC-6211) (100), and is prevented in TRPV1-knockout mice (99). Therefore, it is assumed that enhanced activity of TRPV1 receptors in bladder afferent pathways contributes to bladder pain in BPS/IC.
Role of neurotrophic factors
In patients with BPS/IC, increased levels of neurotrophic factors, including NGF, neurotrophin-3 (NT-3), and glial-derived neurotrophic factor (GDNF), have been detected in the urine (490). Increased expression of NGF is also present in bladder biopsies from women with BPS/IC (390). In a rat model of chronic cystitis, increased expression of neurotrophic factors (NGF, BDNF, and CTNF) in the bladder as well as phosphorylation of tyrosine kinase receptors (TrkA and TrkB) in bladder-innervating afferent neurons have been detected providing evidence for a contribution of neurotrophin-mediated signaling to chronic bladder inflammation (512, 659). It has also been demonstrated that exogenous NGF can induce bladder nociceptive responses and bladder overactivity in rats when applied acutely into the bladder lumen (114, 192) or chronically to the bladder wall or intrathecal space (366,698). Moreover, it has been shown that an application of NGF sequestering molecules (trkA-IgG or REN1180) can reduce a referred thermal hyperalgesia elicited by bladder inflammation using turpentine oil (288) or bladder overactivity elicited by CYP-induced cystitis (277). Peripheral mechanical hyperalgesia induced by CYP-cystitis in mice is abolished by NGF antiserum or K252a, a nonspecific TrkA antagonist, but these treatments do not affect edema in bladder wall, leukocyte infiltration, or hemorrhage (248, 249).
Intravenous injection of a recombinant TrkB-Ig2 domain, which is designed to neutralize BDNF also reduces the frequency of reflex contractions in rats treated with CYP (509). As TrkB-Ig2 is unlikely to cross the blood–brain barrier, peripheral action of BDNF on the afferent neurons is probably involved in the modulation of micturition pathway plasticity occurring after CYP-induced inflammation. Taken together, target organ-neural interactions mediated by an increase of neurotrophic factors in the bladder and increased transport of neurotrophic factors to the neuronal cell bodies in afferent pathways may contribute to the emergence of bladder pain and other symptoms such as urinary frequency in BPS/IC (Figs. 26, 27).
In clinical studies, the monoclonal NGF neutralizing antibody, tanezumab, has been tested, and encouraging results of the Phase II efficacy study were obtained. Tanezumab improved self-reported pain score and urgency episode frequency six weeks following a single intravenous injection (200 μg/kg) in a cohort of 68 patients with BPS/IC although there was no significant effect on micturition frequency or mean voided volume per micturition (207). Among adverse effects, abnormal peripheral sensation symptoms, such as paresthesia and hyperesthesia were more common in the treatment arm when compared to placebo (207). Thus, proof-of-concept evidence has been provided for effectiveness of systemic targeting of the NGF system in the treatment of BPS/IC; however, clinical studies were put on hold following reports of bone necrosis requiring total joint replacements in clinical trials for ostheoarthritis (www.clinicaltrials.gov). Therefore, the site-specific reduction of NGF would be desirable to reduce the intrinsic toxicity from systemic blockade of NGF. In this regard, a recent study using rats showed that treatment with intravesical liposomal antisense suppresses NGF expression in the urothelium as well as bladder overactivity and chemokine upregulation in a model of acetic acid induced bladder overactivity (307). Thus, local suppression of NGF in the bladder using intravesical liposome-based delivery techniques could be an attractive approach for BPS/IC treatment, which can avoid systemic side effects that may be associated with non-specific blockade of NGF expression.
Role of cross organ sensitization
Recent studies have provided evidence for neural cross-talk and bidirectional cross sensitization between pelvic organs in which chemical irritation of the colon leads to enhancement of reflex bladder activity or irritation of the bladder leads to enhancement of colonic reflexes. Dichotomizing afferents that send axonal branches to different target organs and/or the convergence of sensory innervation from different pelvic organs onto the same second-order neurons in the spinal cord have been put forward as mechanisms inducing this viscerovisceral cross-organ sensitization (113, 400, 504, 513). This mechanism could contribute to the symptoms in some BPS/IC patients.
Aging
LUT symptoms such as increased voiding frequency, urgency, urge incontinence, and poor bladder emptying are common and troublesome problems in older men and women (469, 483, 519, 574). Previous studies have reported various changes in LUT function including a reduction in bladder capacity, increased bladder sensation, and DO (186,187,254, 271,392,393,483). However, there are few studies that address the normal changes in the LUT that occur with aging. Pfisterer et al. (506) have examined age-related changes in bladder function among 85 community-dwelling female volunteers and demonstrated that detrusor contractility, bladder sensation, and intraurethral pressure decline with age and that a reduction in bladder capacity associated with age may be related to DO rather than to aging itself because small bladder capacity associated with DO is similarly seen in younger and older women (505). Thus, aging appears to induce a reduction in bladder and urethral function in humans.
In animal studies, impaired bladder function as evidenced by increased voided volume per micturition associated with a high micturition pressure threshold has also been demonstrated in aged rats in comparison with young rats (93, 115). In addition, aged rats exhibit reduced sensitivity of pelvic nerve afferents in response to increased bladder volume, but not pressure, and a reduction in the maximal bladder pressure generated during pelvic nerve stimulation (275) although the frequency-response curve for electrically evoked contractions of bladder muscle strips is similar in young and old rat bladders (725). A significant linear reduction in the amount of AChE-positive nerves was observed with increasing age in the human bladder (233), suggesting a reduced parasympathetic innervation of the aged bladder. It is also shown that expression of neuropeptides such as CGRP and SP in lumbosacral DRG neurons decreases with age (442), and that there is a marked reduction in the density of PACAP innervation of the subepithelial plexus and of the muscle layer of the bladder base in old rats (441). A recent study using aged mice up to 26 months old also showed that aging is associated with an impaired ability to respond to the challenge of continuous bladder filling while voiding detrusor contraction strength does not degrade with aging (575). Taken together, these results suggest that impaired activity of the aged bladder is likely to be at least in part due to reduced activity of efferent and afferent nerves innervating the bladder.
Changes in the central nervous system in relation to LUT function have also been demonstrated in aged animals. For example, immunohistochemical analyses in aged rats revealed significant age-associated declines in the serotonergic (5-HT) and noradrenergic innervation of various spinal cord regions including the intermediolateral cell nucleus, sacral parasympathetic nucleus, dorsal gray commissure and the ventral horn that contains Onuf’s nucleus, with the exception that 5-HT innervation of the sacral parasympathetic nucleus and noradrenergic innervation in the Onuf’s nucleus were maintained (517). Sympathetic preganglionic neurons in the L1-L2 spinal cord that project to the major pelvic ganglion also exhibit a number of age related degenerative changes such as reductions in the cell number, the length of their dendrites and the synaptic contacts made by glutamate-immunoreactive boutons onto the dendrites, although these changes are not seen in PGNs in the L6–S1 spinal cord (539). Frequent voiding produced by apomorphine-induced dopamine receptor activation is more pronounced in aged rats compared with young rats, suggesting that aged rats are more susceptible to altered central processing to induce bladder overactivity despite the decline in baseline bladder function with aging (93).
In contrast to altered nerve activity, there appears to be no significant difference in detrusor contractile responses to cholinergic stimulation between young and old animals, (116,382,384,389,550) although old rats have a reduced density of muscarinic receptors in the bladder (550). In contrast, there are some reports of age-related changes of the detrusor response to adrenergic stimulation (373). Some studies show that detrusor contractile responses to norepinephrine are increased in old male and female rats (473, 531, 532). Aged female rats also exhibit increases in expression of α1D-adrenoceptors and α1D-receptor-mediated contractions of the bladder (193). However, another study showed no age-dependent changes in α1-adrenoceptor properties such as phenylephrine-induced contractile responses, total receptor density and mRNA expression of α1-adrenoceptor subtypes (α1A, α1B, and α1D) in the rat bladder base and dome (692). The detrusor response to β-adrenergic stimulation is reduced in old male rats (473) along with a reduction in the density of β-adrenergic receptors and a decreased cAMP production (473) in response to β-adrenergic stimulation. The combination of an increase in the α-adrenergic excitatory responses and decreased β-adrenergic inhibitory responses results in a net contracting effect of norepinephrine on the aged bladder in contrast to the relaxing effect of norepinephrine in the young bladder although the contribution of these changes in adrenoceptor properties to age-related alterations in LUT function is still to be determined.
Parkinson’s disease
Parkinson’s disease (PD) is induced by dopamine depletion in the striatum, leading to motor dysfunctions such as bradykinesia or akinesia. PD patients often exhibit irritable bladder symptoms such as urinary urgency, frequency, and incontinence which are induced by DO (30–32, 49, 498). The incidence of LUT dysfunction is estimated to be as high as 50% to 70% in patients with PD (30, 49, 73, 498, 533). Studies also demonstrate that there is a correlation between bladder symptoms and degeneration of the nigrostriatal dopaminergic neurons (538) as well as dopaminergic nerve terminals in the caudate (533, 675, 676). A study using PET scanning shows some similarities and differences in brain activation sites during bladder filling in healthy volunteers and patients with PD who have DO. Activation of the PAG, thalamus, putamen, and insula occurred in both groups, whereas activation of the pons or the anterior cingulate gyrus seen in healthy volunteers is not observed in patients with PD (331).
The underlying mechanisms inducing bladder dysfunction in PD have been investigated in animal studies. Figure 24 shows the potential dopaminergic and adenosinergic mechanisms inducing bladder dysfunction in PD, including a loss of D1-like receptor-mediated inhibition and upregulation of adenosine A2A receptor-mediated facilitation of the micturition reflex that is controlled by the spinobulbospinal pathway. In monkeys, disruption of nigrostriatal dopaminergic pathways induced by the neurotoxin MPTP produces Parkinson-like motor symptoms accompanied by DO (11, 704, 706). Similarly, a rat model of PD induced by a unilateral 6-hydroxydopamine lesion of the nigrostriatal pathway also induces DO (715). In these PD animals, DO was suppressed by stimulation of D1-like receptors with SKF 38393 or pergolide (704, 706, 715), indicating that bladder overactivity in PD is primarily induced by disruption of D1-like dopamine receptor-mediated inhibition of the micturition reflex (Fig. 24).
Although the dopamine replacement using levodopa (L-dopa) is the mainstay of pharmacologic treatments for PD, this drug becomes less effective due to increased incidence of nonmotor symptoms or adverse events in later stages of PD (430, 518), and often worsens bladder overactivity in mild-to-advanced patients due to activation of dopamine D2-receptors (84, 650). However, preclinical and clinical studies of adenosine A2A receptor antagonists have provided promising results for motor dysfunction in PD patients (508, 552) which prompted an examination of the effect of an adenosine A2A receptor antagonist on the micturition reflex in a rat model of PD (330). The study compared the effect of an adenosine A2A receptor antagonist (ZM241385 [ZM]), a dopamine D1 receptor agonist (SKF38393), and a dopamine D2 receptor agonist (quinpirole) on bladder activity in rats with PD induced by 6-hydroxydopamine. Intravenous, intrathecal, and intracerebroventricular administration of ZM reduced voiding frequency in both PD and sham groups. However in the PD rats, the inhibitory effects of intravenous and intracerebroventricular administration were greater than in the sham rats. Sequential administration of intravenous quinpirole and ZM showed that quinpirole consistently reduced ICI, and ZM consistently increased ICI whether administered before or after quinpirole. SKF38393 increased ICI and this effect was further increased with subsequent administration of ZM; however, when administered in the reverse order, a further increase in ICI was not seen. These results indicate that the adenosine A2A receptor-mediated excitatory mechanism is enhanced at a supraspinal site to induce bladder overactivity and that inhibition of A2A receptors is effective in suppressing bladder overactivity in the rat model of PD (Fig. 24). The adenosine A2A receptor-expressing neural pathways are very likely located downstream of D1 receptor-expressing pathways in the control of micturition and inhibition of bladder activity by D1 receptor activation can induce the partial suppression of adenosine A2A receptor-mediated excitatory mechanisms in the rat model of PD (Fig. 24). In addition, since the loss of D1 receptor activation after dopaminergic neuronal degeneration is one of the major causes of bladder overactivity in PD as discussed above, it is possible that the reduction in D1 receptor-mediated inhibition of adenosine A2A receptor activation following degeneration of nigrostriatal dopaminergic nerves contributes at least in part to the enhancement of adenosine A2A receptor-mediated excitatory mechanisms in the brain, resulting in bladder overactivity in the rat model of PD (Fig. 24). Overall, adenosine A2A receptor antagonists could be useful as new pharmacologic treatments for bladder overactivity in PD.
Cerebral infarction
Focal brain lesions cause urinary dysfunction in about 20%–50% of all patients with brain tumor and cerebrovascular accident (CVA). The most common urinary symptoms in cerebrovascular disease are nocturnal urinary frequency and urgency incontinence associated with DO (534). Clinical studies using PET and fMRI imaging techniques have suggested possible mechanisms for urgency incontinence in patients with CVA including impaired striated sphincter control and lack of appreciation of bladder filling and impending bladder contraction (239, 243).
The mechanism underlying DO after CVA remains unclear. However, NMDA glutamatergic mechanisms seem to play a role in the bladder hyperactivity induced by cerebral infarction in rats. Permanent occlusion of the middle cerebral artery produces a dramatic and persistent reduction in bladder capacity in conscious rats (689). This bladder overactivity can be prevented by pretreatment with MK-801, an NMDA receptor antagonist, prior to occlusion of the middle cerebral artery or can be transiently reduced by MK-801 administered after occlusion of the middle cerebral artery (689). The results indicate that bladder overactivity induced by cerebral infarction occurs in two phases, both of which depend on activation of NMDA glutamatergic receptors. An initiation phase that occurs at the time of infarction seems to function like long-term potentiation to induce a persistent facilitation of micturition (the second phase). The initial phase is not affected by pretreatment with an AMPA glutamatergic antagonist (NBQX) but can be blocked by administration of an NMDA antagonist (MK-801) (687), suggesting that plasticity at glutamatergic synapses can trigger this form of neurogenic bladder dysfunction (724). Rats with middle cerebral artery occlusion also exhibit an upregulation of D2-like dopamine receptor-mediated excitatory mechanism that contributes to DO (690) and a disruption of GABAergic inhibitory mechanism in the brain that enhances the micturition reflex (304).
Diabetes mellitus and detrusor underactivity
A large percentage (50%–70%) of patients with diabetes mellitus (DM) exhibit LUT symptoms (LUTS) (324, 381). The most common urodynamic findings, classically referred to as diabetic cystopathy, include impaired sensation of bladder fullness, increased bladder capacity, reduced bladder contractility, and increased residual urine (381, 651, 700). However, DO is also common in patients with DM, especially when they present with LUTS (305, 324).
The pathophysiology of DM-associated LUT complications is multifactorial and can be a result of an alteration in the physiology of the detrusor smooth muscle, the peripheral innervation, reflex mechanisms, or urothelial function (700). A two-step model of diabetic cystopathy progression has been proposed on the basis of experimental animal studies, which suggest that patients initially develop bladder hypertrophy and overactivity, which is presumably a process of adaptation to polyuria-mediated frequent voiding, followed by the decompensated phase inducing classical diabetic cystopathy associated with detrusor underactivity (134). This two-step model of DM progression is also supported by a comparison of biomechanical properties of the bladder from diuretic and streptozotocin (STZ)-induced DM rats, which showed the late-phase (>8 weeks), DM-specific increase in bladder compliance associated with increased bladder capacity while the early changes (<4 weeks) of mechanical properties of the bladder were similar in diuretic and DM rats (667).
Traditionally, diabetic cystopathy was thought to result from polyneuropathy that predominantly affects sensory and autonomic nerve fibers. The neuropathies of DM caused by the metabolic derangement of the Schwann cell result in segmental demyelination and impairment of nerve conduction (404, 459, 658). Some of the proposals for pathogenesis of DM neuropathy include altered metabolism of glucose, ischemia, superoxide-induced free-radical formation and impaired axonal transport (22). DM neuropathy also reportedly affects urethral function in STZ-induced DM rats. DM rats exhibit a reduction in NO-mediated relaxation and an enhancement of α1-adrenoceptor-mediated contraction of the urethral smooth muscle during reflex bladder contractions, both possibly contributing to increased bladder outlet resistance resulting in impaired bladder emptying in DM (645, 646).
Role of neurotropic factors
A two-step model of diabetic cystopathy progression (i.e., initial overactivity followed by underactivity) is also supported by the changes in NGF levels in the bladder and axonal transport of NGF. Increased NGF levels have been reported in the bladders of rats with early experimental DM (591), whereas in more advanced diabetic bladder disease (for example, the decompensated phase of experimental diabetic cystopathy), bladder and DRG levels of NGF drop and animals display increased bladder capacity and decreased peak pressures (542). Loss of NGF production has been implicated in the development of sensory and sympathetic neuronal degeneration associated with diabetic neuropathy (14, 644). Although data regarding bladder function are lacking for DM patients, experimental evidence suggests that NGF improves diabetic bladder dysfunction in the decompensated phase. NGF gene therapy by herpes-virus-mediated NGF gene delivery into the bladder results in long-term elevation of NGF bladder levels, preventing excessive increases in bladder capacity and reducing postvoid residual volume in diabetic rats (541). These data provide interventional evidence for a causal role of NGF in the development of diabetic cystopathy. Thus, NGF levels in the bladder fluctuate during the compensatory and decompensated phases of diabetic bladder dysfunction, possibly actively contributing to functional abnormalities (486).
Conclusion
Storage and periodic release of urine is dependent on a complex neural network located at various levels of the peripheral and central nervous system that coordinates the activity of smooth and striated muscles of the bladder and urethra. The central components of this network have a hierarchical organization in which involuntary spinal storage circuitry is regulated by involuntary brain stem voiding circuitry which is in turn regulated by cortical circuitry responsible for voluntary voiding. Afferent pathways that trigger storage and voiding reflexes as well as the sensations of bladder filling transmit activity from mechanoreceptors in the bladder through second-order neurons in the spinal cord to central processing areas in the mesencephalon (periaqueductal gray, PAG) and forebrain (insula, anterior cingulate) before reaching the frontal cortex. An interaction between the PAG and the PMC generates efferent signals that descend from the PMC to the spinal cord to initiate the appropriate motor responses necessary for voiding (i.e., a bladder contraction and reciprocal urethral sphincter relaxation).
Reflex voiding is activated in an all-or-none, switch-like manner by a spinobulbospinal pathway that is relayed through PAG-PMC circuitry. Electrophysiological, pharmacological and computer modeling experiments indicate that the switching function is mediated by excitatory and inhibitory synapses in the PAG-PMC circuitry. Brain imaging studies revealed that cortical modulation of this circuitry may underlie the voluntary control of voiding. In addition neurons in other subcortical areas (e.g., the nucleus subceruleus, reticularis pontis oralis, cerebellum, hypothalamus, medulllary raphe nucleus) exert direct or indirect modulatory influences on the voiding reflex.
Efferent activity passing from the spinal cord to the bladder is subject to modulation in the periphery at parasympathetic ganglionic synapses and at the neuroeffector junction. In some species (rabbit and cat), the ganglia function as high pass filters blocking transmission of excitatory signals to the bladder smooth muscle at low frequencies of firing but amplifying the transmission of these signals at higher frequencies. Thus ganglionic synapses complement the switching functions of the central pathways. Adrenergic as well as noncholinergic-nonadrenergic inhibitory and facilitatory mechanisms in these ganglia can also influence the transfer of excitatory signals to the bladder. It is not known if these peripheral neuromodulatory mechanisms exist in bladder ganglia in humans.
A number of neurotransmitters have been identified in the peripheral and central neural circuitry. In parasympathetic postganglionic pathways, ACh and ATP are coexcitatory transmitters in the bladder; and NO is the inhibitory transmitter released in the urethra. In sympathetic nerves, norepinephrine has an excitatory role in the bladder base and urethra and an inhibitory role in the bladder dome mediated by α- and β-adrenergic receptors, respectively.
Bladder afferent nerves release transmitters at both peripheral and central terminals. Neuropeptides such as tachykinins, VIP, CGRP, and PACAP are released at both sites and may play a role in peripheral afferent signaling in pathological conditions, but their physiological role in the spinal cord in initiating bladder sensations and/or reflexes is uncertain. On the other hand, there is stronger evidence that glutamic acid is an excitatory transmitter released in spinal cord by bladder afferent nerves.
In the central nervous system, glutamic acid has also been identified as an excitatory transmitter in bladder and sphincter reflex pathways at spinal and supraspinal levels; while GABA, glycine, and enkephalin have been identified as inhibitory transmitters. Monoamines (serotonin, norepinephrine and dopamine) have facilitatory and/or inhibitory modulatory functions that can vary depending on the central pathway and the species.
Owing to the complexity of the neural mechanisms that regulate urine storage and voiding, these processes are sensitive to various neural injuries and diseases. Spinal cord injury has been the focus of considerable attention because it eliminates voluntary and supraspinal control leading to detrusorsphincter-dyssynergia and inefficient voiding, as well as plasticity in central and peripheral neural pathways leading to DO, inefficient urine storage and incontinence. Several types of peripheral and central neuroplasticity have been identified including: (i) emergence of primitive neonatal micturition reflexes and (ii) remodeling of spinal circuitry and sensitization of bladder silent C-fiber afferents leading to the emergence of a spinal micturition reflex. NGF has been implicated in this plasticity because NGF levels increase in the bladder and spinal cord after spinal cord injury; and intrathecal administration of NGF antibodies suppresses neurogenic LUT dysfunctions and afferent sensitization in animal models. Increased levels of neurotrophic factors have also been detected in other types of bladder disorders and, therefore, the role of these agents in bladder pathophysiological mechanisms is a very active research field in neurourology.
References
- 1.Abdel-Gawad M, Dion SB, Elhilali MM. Evidence of a peripheral role of neurokinins in detrusor hyperreflexia: A further study of selective tachykinin antagonists in chronic spinal injured rats. J Urol. 2001;165:1739–1744. [PubMed] [Google Scholar]
- 2.Abdel-Karim AM, Barthlow HG, Bialecki RA, Elhilali MM. Effects of M274773, a neurokinin-2 receptor antagonist, on bladder function in chronically spinalized rats. J Urol. 2005;174:1488–1492. doi: 10.1097/01.ju.0000173004.61302.75. [DOI] [PubMed] [Google Scholar]
- 3.Abe F. Inhibitory presynaptic effect of noradrenaline on the hypogastric ganglion of the guinea pig. J Pharmacol Exp Ther. 1984;231:395–403. [PubMed] [Google Scholar]
- 4.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A, Standardisation Sub-committee of the International Continence S The standardisation of terminology of lower urinary tract function: Report from the Standardisation Subcommittee of the International Continence Society. Neurourol Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 5.Adams PR, Brown DA, Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurons. J Physiol. 1982;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akasu T, Gallagher JP, Nakamura T, Shinnick-Gallagher P, Yoshimura M. Noradrenaline hyperpolarization and depolarization in cat vesical parasympathetic neurones. J Physiol. 1985;361:165–184. doi: 10.1113/jphysiol.1985.sp015639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akasu T, Nishimura T. Synaptic transmission and function of parasympathetic ganglia. Prog Neurobiol. 1995;45:459–522. doi: 10.1016/0301-0082(95)98602-2. [DOI] [PubMed] [Google Scholar]
- 8.Akasu T, Shinnick-Gallagher P, Gallagher JP. Adenosine mediates a slow hyperpolarizing synaptic potential in autonomic neurones. Nature. 1984;311:62–65. doi: 10.1038/311062a0. [DOI] [PubMed] [Google Scholar]
- 9.Akasu T, Shinnick-Gallagher P, Gallagher JP. Evidence for a catecholamine-mediated slow hyperpolarizing synaptic response in parasympathetic ganglia. Brain Res. 1985;365:365–368. doi: 10.1016/0006-8993(86)91651-3. [DOI] [PubMed] [Google Scholar]
- 10.Akasu T, Tsurusaki M, Nishimura T, Tokimasa T. Norepinephrine inhibits calcium action-potential through alpha-2-adrenoceptors in rabbit vesical parasympathetic neurons. Neurosci Res. 1988;6:186–190. doi: 10.1016/0168-0102(88)90022-3. [DOI] [PubMed] [Google Scholar]
- 11.Albanease A, Jenner P, Marsden CD, Stephenson JD. Bladder hyperreflexia induced in marmosets by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurosci Lett. 1988;87:46–50. doi: 10.1016/0304-3940(88)90143-7. [DOI] [PubMed] [Google Scholar]
- 12.Alberts P. Classification of the presynaptic muscarinic receptor subtype that regulates 3H-acetylcholine secretion in the guinea pig urinary bladder in vitro. J Pharmacol Exp Ther. 1995;274:458–468. [PubMed] [Google Scholar]
- 13.Aldskogius H, Elfvin LG. Demonstration of preganglionic fibers in the inferior mesenteric ganglion, pelvic ganglia and related nerves of the guinea pig by anterogradely transported wheat germ agglutininhorseradish peroxidase conjugate. J Auton Nerv Syst. 1987;18:105–109. doi: 10.1016/0165-1838(87)90097-x. [DOI] [PubMed] [Google Scholar]
- 14.Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut RE, Sinicropi DV. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med. 1996;2:703–707. doi: 10.1038/nm0696-703. [DOI] [PubMed] [Google Scholar]
- 15.Andersson K. Pharmacology of lower urinary tract smooth muscle and penile erection tissues. Pharmacol Rev. 1993;45 [PubMed] [Google Scholar]
- 16.Andersson KE. Bladder activation: Afferent mechanisms. Urology. 2002;59:43–50. doi: 10.1016/s0090-4295(01)01637-5. [DOI] [PubMed] [Google Scholar]
- 17.Andersson KE, Arner A. Urinary bladder contraction and relaxation: Physiology and pathophysiology. Physiol Rev. 2004;84:935–986. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 18.Andersson KE, Garcia Pascual A, Persson K, Forman A, Tottrup A. Electrically-induced, nerve-mediated relaxation of rabbit urethra involves nitric oxide. J Urol. 1992;147:253–259. doi: 10.1016/s0022-5347(17)37208-7. [DOI] [PubMed] [Google Scholar]
- 19.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: Basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- 20.Andrade EL, Forner S, Bento AF, Leite DF, Dias MA, Leal PC, Koepp J, Calixto JB. TRPA1 receptor modulation attenuates bladder overactivity induced by spinal cord injury. Am J Physiol Renal Physiol. 2011;300:F1223–F1234. doi: 10.1152/ajprenal.00535.2010. [DOI] [PubMed] [Google Scholar]
- 21.Andrew J, Nathan PW. Lesions on the Anterior Frontal Lobes and Disturbances of Micturition and Defaecation. Brain. 1964;87:233–262. doi: 10.1093/brain/87.2.233. [DOI] [PubMed] [Google Scholar]
- 22.Apfel SC. Neurotrophic factors and diabetic peripheral neuropathy. Eur Neurol. 1999;41(Suppl 1):27–34. doi: 10.1159/000052077. [DOI] [PubMed] [Google Scholar]
- 23.Apostolidis A, Brady CM, Yiangou Y, Davis J, Fowler CJ, Anand P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005;65:400–405. doi: 10.1016/j.urology.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, Dasgupta P, Fowler CJ, Anand P. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005;174:977–982. doi: 10.1097/01.ju.0000169481.42259.54. discussion 982–973. [DOI] [PubMed] [Google Scholar]
- 25.Applebaum AE, Vance WH, Coggeshall RE. Segmental localization of sensory cells that innervate the bladder. J Comp Neurol. 1980;192:203–209. doi: 10.1002/cne.901920202. [DOI] [PubMed] [Google Scholar]
- 26.Araki I. Inhibitory postsynaptic currents and the effects of GABA on visually identified sacral parasympathetic preganglionic neurons in neonatal rats. J Neurophysiol. 1994;72:2903–2910. doi: 10.1152/jn.1994.72.6.2903. [DOI] [PubMed] [Google Scholar]
- 27.Araki I, de Groat WC. Unitary excitatory synaptic currents in preganglionic neurons mediated by two distinct groups of interneurons in neonatal rat sacral parasympathetic nucleus. J Neurophysiol. 1996;76:215–226. doi: 10.1152/jn.1996.76.1.215. [DOI] [PubMed] [Google Scholar]
- 28.Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J Neurosci. 1997;17:8402–8407. doi: 10.1523/JNEUROSCI.17-21-08402.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araki I, Du S, Kamiyama M, Mikami Y, Matsushita K, Komuro M, Furuya Y, Takeda M. Overexpression of epithelial sodium channels in epithelium of human urinary bladder with outlet obstruction. Urology. 2004;64:1255–1260. doi: 10.1016/j.urology.2004.06.064. [DOI] [PubMed] [Google Scholar]
- 30.Araki I, Kitahara M, Oida T, Kuno S. Voiding dysfunction and Parkinson’s disease: Urodynamic abnormalities and urinary symptoms. J Urol. 2000;164:1640–1643. [PubMed] [Google Scholar]
- 31.Araki I, Kuno S. Assessment of voiding dysfunction in Parkinson’s disease by the international prostate symptom score. J Neurol Neurosurg Psychiatry. 2000;68:429–433. doi: 10.1136/jnnp.68.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aranda B, Cramer P. Effects of apomorphine and L-dopa on the parkinsonian bladder. Neurourol Urodyn. 1993;12:203–209. doi: 10.1002/nau.1930120302. [DOI] [PubMed] [Google Scholar]
- 33.Arms L, Vizzard MA. Neuropeptides in lower urinary tract function. Handb Exp Pharmacol. 2011;202:395–423. doi: 10.1007/978-3-642-16499-6_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Athwal BS, Berkley KJ, Hussain I, Brennan A, Craggs M, Sakakibara R, Frackowiak RS, Fowler CJ. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001;124:369–377. doi: 10.1093/brain/124.2.369. [DOI] [PubMed] [Google Scholar]
- 35.Avelino A, Cruz C, Nagy I, Cruz F. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience. 2002;109:787–798. doi: 10.1016/s0306-4522(01)00496-1. [DOI] [PubMed] [Google Scholar]
- 36.Bahns E, Ernsberger U, Janig W, Nelke A. Functional characteristics of lumbar visceral afferent fibres from the urinary bladder and the urethra in the cat. Pflugers Arch. 1986;407:510–518. doi: 10.1007/BF00657509. [DOI] [PubMed] [Google Scholar]
- 37.Bahns E, Halsband U, Janig W. Responses of sacral visceral afferents from the lower urinary tract, colon and anus to mechanical stimulation. Pflugers Arch. 1987;410:296–303. doi: 10.1007/BF00580280. [DOI] [PubMed] [Google Scholar]
- 38.Barber MD, Bremer RE, Thor KB, Dolber PC, Kuehl TJ, Coates KW. Innervation of the female levator ani muscles. Am J Obstet Gynecol. 2002;187:64–71. doi: 10.1067/mob.2002.124844. [DOI] [PubMed] [Google Scholar]
- 39.Baron R, Janig W, McLachlan EM. The afferent and sympathetic components of the lumbar spinal outflow to the colon and pelvic organs in the cat. I. The hypogastric nerve. J Comp Neurol. 1985;238:135–146. doi: 10.1002/cne.902380202. [DOI] [PubMed] [Google Scholar]
- 40.Barrington F. The effect of lesions of the hind- and mid-brani on micturition in the cat. Quart J Exp Physiol Cogn Med. 1925;15:81–102. [Google Scholar]
- 41.Bastiaanssen EH, van Leeuwen JL, Vanderschoot J, Redert PA. A myocybernetic model of the lower urinary tract. J Theor Biol. 1996;178:113–133. doi: 10.1006/jtbi.1996.0011. [DOI] [PubMed] [Google Scholar]
- 42.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 43.Beckel JM, Birder LA. Differential expression and function of nicotinic acetylcholine receptors in the urinary bladder epithelium of the rat. J Physiol. 2012;590:1465–1480. doi: 10.1113/jphysiol.2011.226860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beckel JM, Holstege G. Neuroanatomy of the lower urinary tract. Handb Exp Pharmacol. 2011;202:99–116. doi: 10.1007/978-3-642-16499-6_6. [DOI] [PubMed] [Google Scholar]
- 45.Beckel JM, Holstege G. Neurophysiology of the lower urinary tract. Handb Exp Pharmacol. 2011;202:149–169. doi: 10.1007/978-3-642-16499-6_8. [DOI] [PubMed] [Google Scholar]
- 46.Beckel JM, Kanai A, Lee SJ, de Groat WC, Birder LA. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol. 2006;290:F103–F110. doi: 10.1152/ajprenal.00098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996;206:33–36. doi: 10.1016/0304-3940(96)12418-6. [DOI] [PubMed] [Google Scholar]
- 48.Bennett HL, Gustafsson JA, Keast JR. Estrogen receptor expression in lumbosacral dorsal root ganglion cells innervating the female rat urinary bladder. Auton Neurosci. 2003;105:90–100. doi: 10.1016/S1566-0702(03)00044-4. [DOI] [PubMed] [Google Scholar]
- 49.Berger Y, Blaivas JG, DeLaRocha ER, Salinas JM. Urodynamic findings in Parkinson’s disease. J Urol. 1987;138:836–838. doi: 10.1016/s0022-5347(17)43390-8. [DOI] [PubMed] [Google Scholar]
- 50.Birder L, Andersson KE. Urothelial signaling. Physiol Rev. 2013;93:653–680. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birder L, Kullmann FA, Lee H, Barrick S, de Groat W, Kanai A, Caterina M. Activation of urothelial transient receptor potential vanilloid 4 by 4alpha-phorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J Pharmacol Exp Ther. 2007;323:227–235. doi: 10.1124/jpet.107.125435. [DOI] [PubMed] [Google Scholar]
- 52.Birder LA. Urothelial signaling. Handb Exp Pharmacol. 2011;202:207–231. doi: 10.1007/978-3-642-16499-6_10. [DOI] [PubMed] [Google Scholar]
- 53.Birder LA, Apodaca G, De Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol. 1998;275:F226–F229. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- 54.Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, Buffington CA. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol. 2003;285:F423–F429. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- 55.Birder LA, de Groat WC. The effect of glutamate antagonists on c-fos expression induced in spinal neurons by irritation of the lower urinary tract. Brain Res. 1992;580:115–120. doi: 10.1016/0006-8993(92)90934-2. [DOI] [PubMed] [Google Scholar]
- 56.Birder LA, de Groat WC. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am J Physiol. 1993;265:R326–R333. doi: 10.1152/ajpregu.1993.265.2.R326. [DOI] [PubMed] [Google Scholar]
- 57.Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci U S A. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, de Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 59.Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G, Kanai AJ. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci. 2002;22:8063–8070. doi: 10.1523/JNEUROSCI.22-18-08063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Birder LA, Roppolo JR, Erickson VL, de Groat WC. Increased c-fos expression in spinal lumbosacral projection neurons and preganglionic neurons after irritation of the lower urinary tract in the rat. Brain Res. 1999;834:55–65. doi: 10.1016/s0006-8993(99)01546-2. [DOI] [PubMed] [Google Scholar]
- 61.Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford AP, de Groat WC, Burnstock G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287:F1084–F1091. doi: 10.1152/ajprenal.00118.2004. [DOI] [PubMed] [Google Scholar]
- 62.Birder LA, Wolf-Johnston A, Buffington CA, Roppolo JR, de Groat WC, Kanai AJ. Altered inducible nitric oxide synthase expression and nitric oxide production in the bladder of cats with feline interstitial cystitis. J Urol. 2005;173:625–629. doi: 10.1097/01.ju.0000145900.22849.1d. [DOI] [PubMed] [Google Scholar]
- 63.Blackman JG, Crowcroft PJ, Devine CE, Holman ME, Yonemura K. Transmission from preganglionic fibres in the hypogastric nerve to peripheral ganglia of male guinea-pigs. J Physiol. 1969;201:723–743. doi: 10.1113/jphysiol.1969.sp008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blok BF, de Weerd H, Holstege G. The pontine micturition center projects to sacral cord GABA immunoreactive neurons in the cat. Neurosci Lett. 1997;233:109–112. doi: 10.1016/s0304-3940(97)00644-7. [DOI] [PubMed] [Google Scholar]
- 65.Blok BF, Holstege G. Direct projections from the periaqueductal gray to the pontine micturition center (M-region). An anterograde and retrograde tracing study in the cat. Neurosci Lett. 1994;166:93–96. doi: 10.1016/0304-3940(94)90848-6. [DOI] [PubMed] [Google Scholar]
- 66.Blok BF, Holstege G. Ultrastructural evidence for a direct pathway from the pontine micturition center to the parasympathetic preganglionic motoneurons of the bladder of the cat. Neurosci Lett. 1997;222:195–198. doi: 10.1016/s0304-3940(97)13384-5. [DOI] [PubMed] [Google Scholar]
- 67.Blok BF, Holstege G. Two pontine micturition centers in the cat are not interconnected directly: Implications for the central organization of micturition. J Comp Neurol. 1999;403:209–218. doi: 10.1002/(sici)1096-9861(19990111)403:2<209::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 68.Blok BF, Holstege G. The pontine micturition center in rat receives direct lumbosacral input. An ultrastructural study. Neurosci Lett. 2000;282:29–32. doi: 10.1016/s0304-3940(00)00833-8. [DOI] [PubMed] [Google Scholar]
- 69.Blok BF, Sturms LM, Holstege G. Brain activation during micturition in women. Brain. 1998;121:2033–2042. doi: 10.1093/brain/121.11.2033. [DOI] [PubMed] [Google Scholar]
- 70.Blok BF, Willemsen AT, Holstege G. A PET study on brain control of micturition in humans. Brain. 1997;120:111–121. doi: 10.1093/brain/120.1.111. [DOI] [PubMed] [Google Scholar]
- 71.Blok BFM, Deweerd H, Holstege G. Ultrastructural evidence for a paucity of projections from the lumbosacral cord to the pontine micturition center or M-region in the cat – a new concept for the organization of the micturition reflex with the periaqueductal gray as central relay. J Comp Neurol. 1995;359:300–309. doi: 10.1002/cne.903590208. [DOI] [PubMed] [Google Scholar]
- 72.Boehm JJ, Haynes JL. Bacteriology of “midstream catch” urines. Studies in newborn infants. Am J Dis Child. 1966;111:366–369. doi: 10.1001/archpedi.1966.02090070064007. [DOI] [PubMed] [Google Scholar]
- 73.Bonnet AM, Pichon J, Vidailhet M, Gouider-Khouja N, Robain G, Perrigot M, Agid Y. Urinary disturbances in striatonigral degeneration and Parkinson’s disease: Clinical and urodynamic aspects. Mov Disord. 1997;12:509–513. doi: 10.1002/mds.870120406. [DOI] [PubMed] [Google Scholar]
- 74.Booth AM, de Groat WC. A study of facilitation in vesical parasympathetic ganglia of the cat using intracellular recording techniques. Brain Res. 1979;169:388–392. doi: 10.1016/0006-8993(79)91039-4. [DOI] [PubMed] [Google Scholar]
- 75.Boselli C, Govoni S, Condino AM, D’Agostino G. Bladder instability: A re-appraisal of classical experimental approaches and development of new therapeutic strategies. J Auton Pharmacol. 2001;21:219–229. doi: 10.1046/j.1365-2680.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- 76.Bradley W, Griffin D, Teague C, Timm G. Sensory innervation of the mammalian urethra. Invest Urol. 1973;10:287–289. [PubMed] [Google Scholar]
- 77.Bradley WE, Conway CJ. Bladder representation in the pontinemesencephalic reticular formation. Exp Neurol. 1966;16:237–249. doi: 10.1016/0014-4886(66)90060-4. [DOI] [PubMed] [Google Scholar]
- 78.Bradley WE, Teague CT. Cerebellar influence on the micturition reflex. Exp Neurol. 1969a;23:399–411. doi: 10.1016/0014-4886(69)90087-9. [DOI] [PubMed] [Google Scholar]
- 79.Bradley WE, Teague CT. Cerebellar regulation of the micturition reflex. J Urol. 1969b;101:396–399. doi: 10.1016/s0022-5347(17)62353-x. [DOI] [PubMed] [Google Scholar]
- 80.Brady CM, Apostolidis A, Yiangou Y, Baecker PA, Ford AP, Freeman A, Jacques TS, Fowler CJ, Anand P. P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur Urol. 2004;46:247–253. doi: 10.1016/j.eururo.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 81.Brady CM, Apostolidis AN, Harper M, Yiangou Y, Beckett A, Jacques TS, Freeman A, Scaravilli F, Fowler CJ, Anand P. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int. 2004;93:770–776. doi: 10.1111/j.1464-410X.2003.04722.x. [DOI] [PubMed] [Google Scholar]
- 82.Brumovsky PR, Seal RP, Lundgren KH, Seroogy KB, Watanabe M, Gebhart GF. Expression of vesicular glutamate transporters in sensory and autonomic neurons innervating the mouse bladder. J Urol. 2013;189:2342–2349. doi: 10.1016/j.juro.2012.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruns TM, Gaunt RA, Weber DJ. Multielectrode array recordings of bladder and perineal primary afferent activity from the sacral dorsal root ganglia. J Neural Eng. 2011;8:056010. doi: 10.1088/1741-2560/8/5/056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brusa L, Petta F, Pisani A, Miano R, Stanzione P, Moschella V, Galati S, Finazzi Agro E. Central acute D2 stimulation worsens bladder function in patients with mild Parkinson’s disease. J Urol. 2006;175:202–206. doi: 10.1016/S0022-5347(05)00058-3. discussion 206–207. [DOI] [PubMed] [Google Scholar]
- 85.Burnett AL, Calvin DC, Chamness SL, Liu JX, Nelson RJ, Klein SL, Dawson VL, Dawson TM, Snyder SH. Urinary bladder-urethral sphincter dysfunction in mice with targeted disruption of neuronal nitric oxide synthase models idiopathic voiding disorders in humans. Nat Med. 1997;3:571–574. doi: 10.1038/nm0597-571. [DOI] [PubMed] [Google Scholar]
- 86.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001a;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 87.Burnstock G. Purinergic signaling in the lower urinary tract. In: Abbracchio MP, Williams M, editors. Handbook of Experimental Pharmacology. Berlin: Springer Verlag; 2001b. p. 423. [Google Scholar]
- 88.Burstein R, Wang JL, Elde RP, Giesler GJ., Jr Neurons in the sacral parasympathetic nucleus that project to the hypothalamus do not also project through the pelvic nerve–a double labeling study combining Fluoro-gold and cholera toxin B in the rat. Brain Res. 1990;506:159–165. doi: 10.1016/0006-8993(90)91214-2. [DOI] [PubMed] [Google Scholar]
- 89.Bushman W, Steers WD, Meythaler JM. Voiding dysfunction in patients with spastic paraplegia: Urodynamic evaluation and response to continuous intrathecal baclofen. Neurourol Urodyn. 1993;12:163–170. doi: 10.1002/nau.1930120210. [DOI] [PubMed] [Google Scholar]
- 90.Cannon TW, Yoshimura N, Chancellor MB. Innovations in pharmacotherapy for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:367–372. doi: 10.1007/s00192-003-1093-9. [DOI] [PubMed] [Google Scholar]
- 91.Capek K, Jelinek J. The development of the control of water metabolism. I. The excretion of urine in young rats. Physiol Bohemoslov. 1956;5:91–96. [PubMed] [Google Scholar]
- 92.Castro-Diaz D, Amoros MA. Pharmacotherapy for stress urinary incontinence. Curr Opin Urol. 2005;15:227–230. doi: 10.1097/01.mou.0000172394.60481.47. [DOI] [PubMed] [Google Scholar]
- 93.Chai TC, Andersson KE, Tuttle JB, Steers WD. Altered neural control of micturition in the aged F344 rat. Urol Res. 2000;28:348–354. doi: 10.1007/s002400000135. [DOI] [PubMed] [Google Scholar]
- 94.Chai TC, Gray ML, Steers WD. The incidence of a positive ice water test in bladder outlet obstructed patients: Evidence for bladder neural plasticity. J Urol. 1998;160:34–38. [PubMed] [Google Scholar]
- 95.Chambers WW, Sprague JM. Functional localization in the cerebellum. II. Somatotopic organization in cortex and nuclei. AMA Arch Neurol Psychiatry. 1955;74:653–680. doi: 10.1001/archneurpsyc.1955.02330180071008. [DOI] [PubMed] [Google Scholar]
- 96.Chang H, Peng C, Chen J-J, Cheng C, de Groat W. The time-frequency analysis of pudendo-to-pudendal nerve and pelvic-to-pudendal nerve reflexes in anesthetized intact rats. J Med Biol Eng. 2004;24:17–23. [Google Scholar]
- 97.Chang HY, Cheng CL, Chen JJ, de Groat WC. Roles of glutamatergic and serotonergic mechanisms in reflex control of the external urethral sphincter in urethane-anesthetized female rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R224–R234. doi: 10.1152/ajpregu.00780.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang HY, Cheng CL, Chen JJ, de Groat WC. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am J Physiol Renal Physiol. 2007;292:F1044–F1053. doi: 10.1152/ajprenal.00175.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Charrua A, Cruz CD, Cruz F, Avelino A. Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol. 2007;177:1537–1541. doi: 10.1016/j.juro.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 100.Charrua A, Cruz CD, Narayanan S, Gharat L, Gullapalli S, Cruz F, Avelino A. GRC-6211, a new oral specific TRPV1 antagonist, decreases bladder overactivity and noxious bladder input in cystitis animal models. J Urol. 2009;181:379–386. doi: 10.1016/j.juro.2008.08.121. [DOI] [PubMed] [Google Scholar]
- 101.Chen ML, Shen B, Wang J, Liu H, Roppolo JR, de Groat WC, Tai C. Influence of naloxone on inhibitory pudendal-to-bladder reflex in cats. Exp Neurol. 2010;224:282–291. doi: 10.1016/j.expneurol.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen SY, Chai CY. Coexistence of neurons integrating urinary bladder activity and pelvic nerve activity in the same cardiovascular areas of the pontomedulla in cats. Chin J Physiol. 2002;45:41–50. [PubMed] [Google Scholar]
- 103.Chen SY, Wang SD, Cheng C-L, Kuo JS, de Groat WC, Chai CY. Glutamate activation of neurons in cardiovascular reactive areas of the cat brain stem affects urinary bladder motility. Am J of Physiol. 1993;265:F520–F529. doi: 10.1152/ajprenal.1993.265.4.F520. [DOI] [PubMed] [Google Scholar]
- 104.Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol. 2004;187:445–454. doi: 10.1016/j.expneurol.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 105.Cheng CL, Liu JC, Chang SY, Ma CP, de Groat WC. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am J Physiol. 1999;277:R786–R794. doi: 10.1152/ajpregu.1999.277.3.R786. [DOI] [PubMed] [Google Scholar]
- 106.Cheng CL, Ma CP, de Groat WC. Effects of capsaicin on micturition and associated reflexes in rats. Am J Physiol. 1993;265:R132–R138. doi: 10.1152/ajpregu.1993.265.1.R132. [DOI] [PubMed] [Google Scholar]
- 107.Cheng CL, Ma CP, de Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 1995;678:40–48. doi: 10.1016/0006-8993(95)00212-9. [DOI] [PubMed] [Google Scholar]
- 108.Chess-Williams R. Muscarinic receptors of the urinary bladder: Detrusor, urothelial and prejunctional. Auton Autacoid Pharmacol. 2002;22:133–145. doi: 10.1046/j.1474-8673.2002.00258.x. [DOI] [PubMed] [Google Scholar]
- 109.Chess-Williams R. Potential therapeutic targets for the treatment of detrusor overactivity. Expert Opin Ther Targets. 2004;8:95–106. doi: 10.1517/14728222.8.2.95. [DOI] [PubMed] [Google Scholar]
- 110.Chopra B, Barrick SR, Meyers S, Beckel JM, Zeidel ML, Ford AP, de Groat WC, Birder LA. Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J Physiol. 2005;562:859–871. doi: 10.1113/jphysiol.2004.071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chopra B, Gever J, Barrick SR, Hanna-Mitchell AT, Beckel JM, Ford AP, Birder LA. Expression and function of rat urothelial P2Y receptors. Am J Physiol Renal Physiol. 2008;294:F821–F829. doi: 10.1152/ajprenal.00321.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Christ GJ, Day NS, Day M, Zhao W, Persson K, Pandita RK, Andersson KE. Increased connexin43-mediated intercellular communication in a rat model of bladder overactivity in vivo. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1241–R1248. doi: 10.1152/ajpregu.00030.2002. [DOI] [PubMed] [Google Scholar]
- 113.Christianson JA, Liang R, Ustinova EE, Davis BM, Fraser MO, Pezzone MA. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain. 2007;128:235–243. doi: 10.1016/j.pain.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol. 2001;165:975–979. [PubMed] [Google Scholar]
- 115.Chun AL, Wallace LJ, Gerald MC, Levin RM, Wein AJ. Effect of age on in vivo urinary bladder function in the rat. J Urol. 1988;139:625–627. doi: 10.1016/s0022-5347(17)42546-8. [DOI] [PubMed] [Google Scholar]
- 116.Chun AL, Wallace LJ, Gerald MC, Wein AJ, Levin RM. Effects of age on urinary bladder function in the male rat. J Urol. 1989;141:170–173. doi: 10.1016/s0022-5347(17)40634-3. [DOI] [PubMed] [Google Scholar]
- 117.Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G, Ford AP. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 119.Connor GJ, German WJ. Functional localization within the anterior cerebellar lobe. Trans Am Neurol Ass. 1941;67:181–186. [Google Scholar]
- 120.Conte B, Maggi CA, Giachetti A, Parlani M, Lopez G, Manzini S. Intraurethral capsaicin produces reflex activation of the striated urethral sphincter in urethane-anesthetized male rats. J Urol. 1993;150:1271–1277. doi: 10.1016/s0022-5347(17)35759-2. [DOI] [PubMed] [Google Scholar]
- 121.Craig AD. Interoception: The sense of the physiological condition of the body. Curr Opin Neurobiol. 2003a;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 122.Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003b;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 123.Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 124.Crowe R, Haven AJ, Burnstock G. Intramural neurones of the guineapig urinary bladder: Histochemical localization of putative neurotransmitters in cultures and newborn animals. J Auton Nerv Syst. 1985;15:319–339. doi: 10.1016/0165-1838(86)90018-4. [DOI] [PubMed] [Google Scholar]
- 125.D’Agostino G, Barbieri A, Chiossa E, Tonini M. M4 muscarinic autoreceptor-mediated inhibition of -3H-acetylcholine release in the rat isolated urinary bladder. J Pharmacol Exp Ther. 1997;283:750–756. [PubMed] [Google Scholar]
- 126.D’Agostino G, Bolognesi ML, Lucchelli A, Vicini D, Balestra B, Spelta V, Melchiorre C, Tonini M. Prejunctional muscarinic inhibitory control of acetylcholine release in the human isolated detrusor: Involvement of the M4 receptor subtype. Br J Pharmacol. 2000;129:493–500. doi: 10.1038/sj.bjp.0703080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.D’Agostino G, Chiari MC, Grana E. Prejunctional effects of muscarinic agonists on 3H-acetylcholine release in the rat urinary bladder strip. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:76–81. doi: 10.1007/BF00169210. [DOI] [PubMed] [Google Scholar]
- 128.D’Agostino G, Kilbinger H, Chiari MC, Grana E. Presynaptic inhibitory muscarinic receptors modulating [3H] acetylcholine release in the rat urinary bladder. J Pharmacol Exp Ther. 1986;239:522–528. [PubMed] [Google Scholar]
- 129.D’Amico SC, Collins WF., 3rd External urethral sphincter motor unit recruitment patterns during micturition in the spinally intact and transected adult rat. J Neurophysiol. 2012;108:2554–2567. doi: 10.1152/jn.00927.2011. [DOI] [PubMed] [Google Scholar]
- 130.D’Andrea MR, Saban MR, Nguyen NB, Andrade-Gordon P, Saban R. Expression of protease-activated receptor-1, -2, -3, and -4 in control and experimentally inflamed mouse bladder. Am J Pathol. 2003;162:907–923. doi: 10.1016/S0002-9440(10)63886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dail WG. In: Histochemical and fine structural studies of SIF cells in the major pelvic ganglion of the rat. Eränkö O, editor. Washington, D.C.: Fogarty Int. Cent. Proc. #30 U.S. Govt. Printing Office; 1976. pp. 8–18. [Google Scholar]
- 132.Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2007;583:663–674. doi: 10.1113/jphysiol.2007.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 134.Daneshgari F, Liu G, Birder L, Hanna-Mitchell AT, Chacko S. Diabetic bladder dysfunction: Current translational knowledge. J Urol. 2009;182:S18–S26. doi: 10.1016/j.juro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dang K, Bielefeldt K, Gebhart GF. Gastric ulcers reduce A-type potassium currents in rat gastric sensory ganglion neurons. Am J Physiol Gastrointest Liver Physiol. 2004;286:G573–G579. doi: 10.1152/ajpgi.00258.2003. [DOI] [PubMed] [Google Scholar]
- 136.Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci. 2005;25:3973–3984. doi: 10.1523/JNEUROSCI.5239-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol. 2008;99:49–59. doi: 10.1152/jn.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Danuser H, Thor KB. Inhibition of central sympathetic and somatic outflow to the lower urinary tract of the cat by the alpha 1 adrenergic receptor antagonist prazosin. J Urol. 1995;153:1308–1312. [PubMed] [Google Scholar]
- 139.Danuser H, Thor KB. Spinal 5-HT2 receptor-mediated facilitation of pudendal nerve reflexes in the anaesthetized cat. Br J Pharmacol. 1996;118:150–154. doi: 10.1111/j.1476-5381.1996.tb15378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dattilio A, Vizzard MA. Up-regulation of protease activated receptors in bladder after cyclophosphamide induced cystitis and colocalization with capsaicin receptor (VR1) in bladder nerve fibers. J Urol. 2005;173:635–639. doi: 10.1097/01.ju.0000143191.55468.1d. [DOI] [PubMed] [Google Scholar]
- 141.de Groat C. A neurologic basis for the overactive bladder. Urology. 1997;50(6A Suppl):36–52. doi: 10.1016/s0090-4295(97)00587-6. [DOI] [PubMed] [Google Scholar]
- 142.de Groat WC. Nervous control of the urinary bladder of the cat. Brain Res. 1975;87:201–211. doi: 10.1016/0006-8993(75)90417-5. [DOI] [PubMed] [Google Scholar]
- 143.de Groat WC. Mechanisms underlying recurrent inhibition in the sacral parasympathetic outflow to the urinary bladder. J Physiol. 1976;257:503–513. doi: 10.1113/jphysiol.1976.sp011381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Groat WC. Inhibitory mechanisms in the sacral reflex pathways to the urinary bladder. In: Ryall RW, Kelly JS, editors. Iontophoresis and Transmitter Mechanisms in the Mammalian Central Nervous System. Holland: Elsevier; 1978. pp. 366–368. [Google Scholar]
- 145.de Groat WC. Spinal cord projections and neuropeptides in visceral afferent neurons. Prog Brain Res. 1986;67:165–187. doi: 10.1016/s0079-6123(08)62762-4. [DOI] [PubMed] [Google Scholar]
- 146.de Groat WC. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Paraplegia. 1995;33:493–505. doi: 10.1038/sc.1995.109. [DOI] [PubMed] [Google Scholar]
- 147.de Groat WC. Influence of central serotonergic mechanisms on lower urinary tract function. Urology. 2002;59:30–36. doi: 10.1016/s0090-4295(01)01636-3. [DOI] [PubMed] [Google Scholar]
- 148.de Groat WC. The urothelium in overactive bladder: Passive bystander or active participant? Urology. 2004;64:7–11. doi: 10.1016/j.urology.2004.08.063. [DOI] [PubMed] [Google Scholar]
- 149.de Groat WC. Integrative control of the lower urinary tract: Preclinical perspective. Br J Pharmacol. 2006;147:S25–S40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res. 1998;92:127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 151.de Groat WC, Booth AM. Inhibition and facilitation in parasympathetic ganglia of the urinary bladder. Fed Proc. 1980;39:2990–2996. [PubMed] [Google Scholar]
- 152.de Groat WC, Booth AM. Synaptic transmission in pelvic ganglia. In: Maggi CA, editor. The Autonomic Nervous System. London: Harwood Academic Publishers; 1993. pp. 291–347. [Google Scholar]
- 153.de Groat WC, Booth AM, Krier J. Interaction between sacral parasympathetic and lumbar sympathetic inputs to pelvic ganglia. In: Brooks CM, Koizumi K, Sato A, editors. Integrative functions of the autonomic nervous system. Tokyo: University of Tokyo Press; 1979. pp. 234–247. [Google Scholar]
- 154.de Groat WC, Booth AM, Krier J, Milne RJ, Morgan C, Nadelhaft I. Neural control of the urinary bladder and large intestine. In: Brooks CM, Koizumi K, Sato A, editors. Integrative functions of the autonomic nervous system. Tokyo: Tokyo Univ. Press; 1979. pp. 50–67. [Google Scholar]
- 155.de Groat WC, Booth AM, Milne RJ, Roppolo JR. Parasympathetic preganglionic neurons in the sacral spinal cord. J Auton Nerv Syst. 1982;5:23–43. doi: 10.1016/0165-1838(82)90087-x. [DOI] [PubMed] [Google Scholar]
- 156.de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi C, editor. The Autonomic Nervous System. London: Harwood Academic Publishers; 1993. pp. 227–290. [Google Scholar]
- 157.de Groat WC, Douglas JW, Glass J, Simonds W, Weimer B, Werner P. Changes in somato-vesical reflexes during postnatal development in the kitten. Brain Res. 1975;94:150–154. doi: 10.1016/0006-8993(75)90884-7. [DOI] [PubMed] [Google Scholar]
- 158.de Groat WC, Fraser MO, Yoshiyama M, Smerin S, Tai C, Chancellor MB, Yoshimura N, Roppolo JR. Neural control of the urethra. Scand J Urol Nephrol Suppl. 2001;207:35–43. doi: 10.1080/003655901750174872. discussion 106–125. [DOI] [PubMed] [Google Scholar]
- 159.de Groat WC, Kawatani M. Enkephalinergic inhibition in parasympathetic ganglia of the urinary bladder of the cat. J Physiol. 1989;413:13–29. doi: 10.1113/jphysiol.1989.sp017639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.de Groat WC, Kawatani M, Booth AM. Enkephalinergic modulation of cholinergic transmission in parasympathetic ganglia of the cat urinary bladder. In: Hanin I, editor. Advances in Behavioral Biology, Vol. 30, Dynamics of Cholinergic Function. New York: Plenum Publishing Corp; 1986. pp. 1007–1017. [Google Scholar]
- 161.de Groat WC, Kawatani M, Hisamitsu T, Cheng C-L, Ma C-P, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst. 1990;30:S71–S77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- 162.de Groat WC, Kawatani M, Houston MB, Rutigliano M, Erdman S. Identification of neuropeptides in afferent pathways to the pelvic viscera of the cat. In: Ciriello J, Calaresu F, Renaud L, Polosa C, editors. Organization of the Autonomic Nervous System: Central and Peripheral Mechanism, Neurology and Neurobiology. New York: A.R. Liss, Inc; 1987. pp. 81–90. [Google Scholar]
- 163.de Groat WC, Lalley PM. Reflex firing in the lumbar sympathetic outflow to activation of vesical afferent fibres. J Physiol. 1972;226:289–309. doi: 10.1113/jphysiol.1972.sp009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- 165.de Groat WC, Roppolo JR, Yoshimura N, Sugaya K. Neural control of the urinary bladder and colon. In: Taché Y, Wingate D, Burks T, editors. Proceedings of the Second International Symposium on Brain-Gut Interactions. Boca Raton, FL: CRC Press; 1993. pp. 167–190. [Google Scholar]
- 166.de Groat WC, Ryall RW. The identification and characteristics of sacral parasympathetic preganglionic neurones. J Physiol. 1968;196:563–577. doi: 10.1113/jphysiol.1968.sp008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.de Groat WC, Ryall RW. Recurrent inhibition in sacral parasympathetic pathways to the bladder. J Physiol. 1968;196:579–591. doi: 10.1113/jphysiol.1968.sp008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J Physiol. 1969;200:87–108. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.de Groat WC, Saum WR. Adrenergic inhibition in mammalian parasympathetic ganglia. Nat New Biol. 1971;231:188–189. doi: 10.1038/newbio231188a0. [DOI] [PubMed] [Google Scholar]
- 170.de Groat WC, Saum WR. Sympathetic inhibition of the urinary bladder and of pelvic ganglionic transmission in the cat. J Physiol. 1972;220:297–314. doi: 10.1113/jphysiol.1972.sp009708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.de Groat WC, Saum WR. Synaptic transmission in parasympathetic ganglia in the urinary bladder of the cat. J Physiol. 1976;256:137–158. doi: 10.1113/jphysiol.1976.sp011316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.de Groat WC, Theobald RJ. Reflex activation of sympathetic pathways to vesical smooth muscle and parasympathetic ganglia by electrical stimulation of vesical afferents. J Physiol. 1976;259:223–237. doi: 10.1113/jphysiol.1976.sp011463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.de Groat WC, Wickens C. Organization of the neural switching circuitry underlying reflex micturition. Acta Physiol (Oxf) 2013;207:66–84. doi: 10.1111/apha.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.de Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Ann Rev Pharmacol Toxicol. 2001;41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- 175.de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006;152:59–84. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- 176.de Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol. 2009;194:91–138. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.de Groat WC, Yoshimura N. Changes in afferent activity after spinal cord injury. Neurourol Urodyn. 2010;29:63–76. doi: 10.1002/nau.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp Neurol. 2012;235:123–132. doi: 10.1016/j.expneurol.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.de Groat WC, Yoshiyama M, Ramage AG, Yamamoto T, Somogyi GT. Modulation of voiding and storage reflexes by activation of alpha1-adrenoceptors. [Review] (20 refs] Eur Urol. 1999;36(Suppl 1):68–73. doi: 10.1159/000052324. [DOI] [PubMed] [Google Scholar]
- 180.DeLancey JO, Gosling J, Creed KE. 2nd International Consultation on Incontinence. World Health Organization; 2002. Gross Anatomy and Cell Biology of the Lower Urinary Tract; p. 16. [Google Scholar]
- 181.Denys P, Chartier-Kastler E, Azouvi P, Remy-Neris O, Bussel B. Intrathecal clonidine for refractory detrusor hyperreflexia in spinal cord injured patients: A preliminary report. J Urol. 1998;160:2137–2138. doi: 10.1097/00005392-199812010-00050. [DOI] [PubMed] [Google Scholar]
- 182.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 183.Dietrichs E, Haines DE. Possible pathways for cerebellar modulation of autonomic responses: Micturition. Scand J Urol Nephrol Suppl. 2002;210:16–20. doi: 10.1080/003655902320765917. [DOI] [PubMed] [Google Scholar]
- 184.Ding YQ, Zheng HX, Gong LW, Lu Y, Zhao H, Qin BZ. Direct projections from the lumbosacral spinal cord to Barrington’s nucleus in the rat: A special reference to micturition reflex. J Comp Neurol. 1997;389:149–160. doi: 10.1002/(sici)1096-9861(19971208)389:1<149::aid-cne11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 185.Dinis P, Charrua A, Avelino A, Yaqoob M, Bevan S, Nagy I, Cruz F. Anandamide-evoked activation of vanilloid receptor 1 contributes to the development of bladder hyperreflexia and nociceptive transmission to spinal dorsal horn neurons in cystitis. J Neurosci. 2004;24:11253–11263. doi: 10.1523/JNEUROSCI.2657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Diokno AC, Brock BM, Brown MB, Herzog AR. Prevalence of urinary incontinence and other urological symptoms in the noninstitutionalized elderly. J Urol. 1986;136:1022–1025. [PubMed] [Google Scholar]
- 187.Diokno AC, Brown MB, Brock BM, Herzog AR, Normolle DP. Clinical and cystometric characteristics of continent and incontinent noninstitutionalized elderly. J Urol. 1988;140:567–571. doi: 10.1016/s0022-5347(17)41720-4. [DOI] [PubMed] [Google Scholar]
- 188.Dixon JS, Gosling JA. The distribution of noradrenergic nerves and small, intensely fluorescent (SIF) cells in the cat urinary bladder. A light and electron microscope study. Cell Tissue Res. 1974;150:147–159. doi: 10.1007/BF00222166. [DOI] [PubMed] [Google Scholar]
- 189.Dixon JS, Jen PY, Gosling JA. Tyrosine hydroxylase and vesicular acetylcholine transporter are coexpressed in a high proportion of intramural neurons of the human neonatal and child urinary bladder. Neurosci Lett. 1999;277:157–160. doi: 10.1016/s0304-3940(99)00877-0. [DOI] [PubMed] [Google Scholar]
- 190.Dixon JS, Jen PY, Gosling JA. The distribution of vesicular acetylcholine transporter in the human male genitourinary organs and its colocalization with neuropeptide Y and nitric oxide synthase. Neurourol Urodyn. 2000;19:185–194. doi: 10.1002/(sici)1520-6777(2000)19:2<185::aid-nau9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 191.Dmitrieva N, McMahon SB. Sensitisation of visceral afferents by nerve growth factor in the adult rat. Pain. 1996;66:87–97. doi: 10.1016/0304-3959(96)02993-4. [DOI] [PubMed] [Google Scholar]
- 192.Dmitrieva N, Shelton D, Rice AS, McMahon SB. The role of nerve growth factor in a model of visceral inflammation. Neuroscience. 1997;78:449–459. doi: 10.1016/s0306-4522(96)00575-1. [DOI] [PubMed] [Google Scholar]
- 193.Dmitrieva N, Zhang G, Nagabukuro H. Increased alpha1D adrenergic receptor activity and protein expression in the urinary bladder of aged rats. World J Urol. 2008;26:649–655. doi: 10.1007/s00345-008-0292-x. [DOI] [PubMed] [Google Scholar]
- 194.Dolber PC, Gu B, Zhang X, Fraser MO, Thor KB, Reiter JP. Activation of the external urethral sphincter central pattern generator by a 5-HT(1A) receptor agonist in rats with chronic spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1699–R1706. doi: 10.1152/ajpregu.00142.2006. [DOI] [PubMed] [Google Scholar]
- 195.Downie JW, Armour JA. Mechanoreceptor afferent activity compared with receptor field dimensions and pressure changes in feline urinary bladder. Can J Physiol Pharmacol. 1992;70:1457–1467. doi: 10.1139/y92-206. [DOI] [PubMed] [Google Scholar]
- 196.Downie JW, Bialik GJ. Evidence for a spinal site of action of clonidine on somatic and viscerosomatic reflex activity evoked on the pudendal nerve in cats. J Pharmacol Exp Ther. 1988;246:352–358. [PubMed] [Google Scholar]
- 197.Dray A, Metsch R. Inhibition of urinary bladder contractions by a spinal action of morphine and other opioids. J Pharmacol Exp Ther. 1984a;231:254–260. [PubMed] [Google Scholar]
- 198.Dray A, Metsch R. Opioid receptor subtypes involved in the central inhibition of urinary bladder motility. Eur J Pharmacol. 1984b;104:47–53. doi: 10.1016/0014-2999(84)90367-4. [DOI] [PubMed] [Google Scholar]
- 199.Dray A, Metsch R. Opioids and central inhibition of urinary bladder motility. Eur J Pharmacol. 1984c;98:155–156. doi: 10.1016/0014-2999(84)90127-4. [DOI] [PubMed] [Google Scholar]
- 200.Du S, Araki I, Kobayashi H, Zakoji H, Sawada N, Takeda M. Differential expression profile of cold (TRPA1) and cool (TRPM8) receptors in human urogenital organs. Urology. 2008;72:450–455. doi: 10.1016/j.urology.2007.11.127. [DOI] [PubMed] [Google Scholar]
- 201.Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 202.Duong M, Downie JW, Du HJ. Transmission of afferent information from urinary bladder, urethra and perineum to periaqueductal gray of cat. Brain Res. 1999;819:108–119. doi: 10.1016/s0006-8993(98)01294-3. [DOI] [PubMed] [Google Scholar]
- 203.Elam M, Thoren P, Svensson TH. Locus coeruleus neurons and sympathetic nerves: Activation by visceral afferents. Brain Res. 1986;375:117–125. doi: 10.1016/0006-8993(86)90964-9. [DOI] [PubMed] [Google Scholar]
- 204.Elbadawi A, Schenk EA. Parasympathetic and sympathetic postganglionic synapses in ureterovesical autonomic pathways. Z Zellforsch Mikrosk Anat. 1973;146:147–154. [Google Scholar]
- 205.Espey MJ, Downie JW, Fine A. Effect of 5-HT receptor and adrenoceptor antagonists on micturition in conscious cats. Eur J Pharmacol. 1992;221:167–170. doi: 10.1016/0014-2999(92)90788-6. [DOI] [PubMed] [Google Scholar]
- 206.Espey MJ, Du HJ, Downie JW. Serotonergic modulation of spinal ascending activity and sacral reflex activity evoked by pelvic nerve stimulation in cats. Brain Res. 1998;798:101–108. doi: 10.1016/s0006-8993(98)00401-6. [DOI] [PubMed] [Google Scholar]
- 207.Evans RJ, Moldwin RM, Cossons N, Darekar A, Mills IW, Scholfield D. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol. 2011;185:1716–1721. doi: 10.1016/j.juro.2010.12.088. [DOI] [PubMed] [Google Scholar]
- 208.Everaerts W, Gevaert T, Nilius B, De Ridder D. On the origin of bladder sensing: Tr(i)ps in urology. Neurourol Urodyn. 2008;27:264–273. doi: 10.1002/nau.20511. [DOI] [PubMed] [Google Scholar]
- 209.Fall M, Lindström S, Mazieres L. A bladder-to-bladder cooling reflex in the cat. J Physiol. 1990;427:281–300. doi: 10.1113/jphysiol.1990.sp018172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Feber JL, van Asselt E, van Mastrigt R. Neurophysiological modeling of voiding in rats: Urethral nerve response to urethral pressure and flow. Am J Physiol. 1998;274:R1473–R1481. doi: 10.1152/ajpregu.1998.274.5.R1473. [DOI] [PubMed] [Google Scholar]
- 211.Féher E, Csányi K, Vajda J. Ultrastructure of the nerve cells and fibers in the urinary bladder wall of the cat. Acta Anatomica. 1978;103:109–118. [PubMed] [Google Scholar]
- 212.Fehër E, Csányi K, Vajda J. Intrinsic innervation of the urinary bladder. Acta Anatomica. 1980;106:335–344. [PubMed] [Google Scholar]
- 213.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes–a possible sensory mechanism? J Physiol. 1997;505(Pt 2):503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Floyd K, Hick VE, Morrison JF. Mechanosensitive afferent units in the hypogastric nerve of the cat. J Physiol. 1976;259:457–471. doi: 10.1113/jphysiol.1976.sp011476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Forrest SL, Keast JR. Expression of receptors for glial cell line-derived neurotrophic factor family ligands in sacral spinal cord reveals separate targets of pelvic afferent fibers. J Comp Neurol. 2008;506:989–1002. doi: 10.1002/cne.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fowler CJ, Griffiths DJ. A decade of functional brain imaging applied to bladder control. Neurourol Urodyn. 2010;29:49–55. doi: 10.1002/nau.20740. [DOI] [PubMed] [Google Scholar]
- 218.Fry C, Brading AF, Hussain M. Cell Biology. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Plymouth, UK: Health Publication Ltd; 2005. pp. 313–362. [Google Scholar]
- 219.Fry CH, Sadananda P, Wood DN, Thiruchelvam N, Jabr RI, Clayton R. Modeling the urinary tract-computational, physical, and biological methods. Neurourol Urodyn. 2011;30:692–699. doi: 10.1002/nau.21131. [DOI] [PubMed] [Google Scholar]
- 220.Fukuda H, Fukai K. Fibers in the anastomotic branches connecting the bilateral pelvic rectal plexuses of the dog. Jpn J Physiol. 1985;35:867–870. doi: 10.2170/jjphysiol.35.867. [DOI] [PubMed] [Google Scholar]
- 221.Fukuda H, Koga T. Midbrain stimulation inhibits the micturition, defecation and rhythmic straining reflexes elicited by activation of sacral vesical and rectal afferents in the dog. Exp Brain Res. 1991;83:303–316. doi: 10.1007/BF00231154. [DOI] [PubMed] [Google Scholar]
- 222.Fukuyama H, Matsuzaki S, Ouchi Y, Yamauchi H, Nagahama Y, Kimura J, Shibasaki H. Neural control of micturition in man examined with single photon emission computed tomography using 99mTc-HMPAO. Neuroreport. 1996;7:3009–3012. doi: 10.1097/00001756-199611250-00042. [DOI] [PubMed] [Google Scholar]
- 223.Furuta A, Asano K, Egawa S, de Groat WC, Chancellor MB, Yoshimura N. Role of alpha2-adrenoceptors and glutamate mechanisms in the external urethral sphincter continence reflex in rats. J Urol. 2009;181:1467–1473. doi: 10.1016/j.juro.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gajewski J, Downie JW, Awad SA. Experimental evidence for a central nervous system site of action in the effect of alpha-adrenergic blockers on the external urinary sphincter. J Urol. 1984;132:403–409. doi: 10.1016/s0022-5347(17)49637-6. [DOI] [PubMed] [Google Scholar]
- 225.Galeano C, Jubelin B, Carmel M, Ghazal G. Urodynamic action of clonidine in the chronic spinal cat. Neurourol Urodyn. 1986;5:475–492. [Google Scholar]
- 226.Gallagher JP, Griffith WH, Shinnick-Gallagher P. Cholinergic transmission in cat parasympathetic ganglia. J Physiol. 1982;332:473–486. doi: 10.1113/jphysiol.1982.sp014425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gallagher JP, Shinnick-Gallagher P. Excitatory transmission in parasympathetic ganglia. In: Karczmar AG, Koketsu K, Nishi S, editors. Autonomic and Enteric Ganglia. New York: Plenum Press; 1986. pp. 341–351. [Google Scholar]
- 228.Gary B, Perera S, Murrin A, Aizenstein H, Tadic S. White matter hyperintensity burden and depression are associated with severity and impact of urgency urinary incontinence in functional community-dwelling older women (abstract) Neurourol Urodyn. 2013;32:798–799. [Google Scholar]
- 229.Geirsson G, Lindström S, Fall M. The bladder cooling reflex in man–characteristics and sensitivity to temperature. Brit J Urol. 1993;71:675–680. doi: 10.1111/j.1464-410x.1993.tb16064.x. [DOI] [PubMed] [Google Scholar]
- 230.Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van Leuven F, Voets T, De Ridder D, Nilius B. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest. 2007;117:3453–3462. doi: 10.1172/JCI31766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Giannantoni A, Di Stasi SM, Nardicchi V, Zucchi A, Macchioni L, Bini V, Goracci G, Porena M. Botulinum-A toxin injections into the detrusor muscle decrease nerve growth factor bladder tissue levels in patients with neurogenic detrusor overactivity. J Urol. 2006;175:2341–2344. doi: 10.1016/S0022-5347(06)00258-8. [DOI] [PubMed] [Google Scholar]
- 232.Giglio D, Tobin G. Muscarinic receptor subtypes in the lower urinary tract. Pharmacology. 2009;83:259–269. doi: 10.1159/000209255. [DOI] [PubMed] [Google Scholar]
- 233.Gilpin SA, Gilpin CJ, Dixon JS, Gosling JA, Kirby RS. The effect of age on the autonomic innervation of the urinary bladder. Br J Urol. 1986;58:378–381. doi: 10.1111/j.1464-410x.1986.tb09089.x. [DOI] [PubMed] [Google Scholar]
- 234.Giuliani S, Lecci A, Santicioli P, Del Bianco E, Maggi CA. Effect of the GABAB antagonist, phaclofen, on baclofen-induced inhibition of micturition reflex in urethane-anesthetized rats. Neuroscience. 1992;48:217–223. doi: 10.1016/0306-4522(92)90350-b. [DOI] [PubMed] [Google Scholar]
- 235.Gjone R. Excitatory and inhibitory bladder responses to stimulation of ‘limbic’, diencephalic and mesencephalic structures in the cat. Acta Physiol Scand. 1966;66:91–102. doi: 10.1111/j.1748-1716.1966.tb03171.x. [DOI] [PubMed] [Google Scholar]
- 236.Glazer EJ, Basbaum AI. Leucine enkephalin: Localization in and axoplasmic transport by sacral parasympathetic preganglionic neurons in the cat. Science. 1980;208:1479–1481. doi: 10.1126/science.6155697. [DOI] [PubMed] [Google Scholar]
- 237.Gosling JA, Kung LS, Dixon JS, Horan P, Whitbeck C, Levin RM. Correlation between the structure and function of the rabbit urinary bladder following partial outlet obstruction. J Urol. 2000;163:1349–1356. [PubMed] [Google Scholar]
- 238.Griffith WH, Gallagher JP, Shinnick-Gallagher P. Sucrose-gap recordings of nerve-evoked potentials in mammalian parasympathetic ganglia. Brain Res. 1981;209:446–451. doi: 10.1016/0006-8993(81)90168-2. [DOI] [PubMed] [Google Scholar]
- 239.Griffiths D. Clinical studies of cerebral and urinary tract function in elderly people with urinary incontinence. Behav Brain Res. 1998;92:151–155. doi: 10.1016/s0166-4328(97)00187-3. [DOI] [PubMed] [Google Scholar]
- 240.Griffiths D, Derbyshire S, Stenger A, Resnick N. Brain control of normal and overactive bladder. J Urol. 2005;174:1862–1867. doi: 10.1097/01.ju.0000177450.34451.97. [DOI] [PubMed] [Google Scholar]
- 241.Griffiths D, Tadic SD. Bladder control, urgency, and urge incontinence: Evidence from functional brain imaging. Neurourol Urodyn. 2008;27:466–474. doi: 10.1002/nau.20549. [DOI] [PubMed] [Google Scholar]
- 242.Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage. 2007;37:1–7. doi: 10.1016/j.neuroimage.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Griffiths DJ. Cerebral control of bladder function. Curr Urol Rep. 2004;5:348–352. doi: 10.1007/s11934-004-0081-z. [DOI] [PubMed] [Google Scholar]
- 244.Griffiths DJ, Fowler CJ. The micturition switch and its forebrain influences. Acta Physiol (Oxf) 2013;207:93–109. doi: 10.1111/apha.12019. [DOI] [PubMed] [Google Scholar]
- 245.Griffiths DJ, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the lower urinary tract: How age-related changes might predispose to urge incontinence. Neuroimage. 2009;47:981–986. doi: 10.1016/j.neuroimage.2009.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Gu B, Olejar KJ, Reiter JP, Thor KB, Dolber PC. Inhibition of bladder activity by 5-hydroxytryptamine1 serotonin receptor agonists in cats with chronic spinal cord injury. J Pharmacol Exp Ther. 2004;310:1266–1272. doi: 10.1124/jpet.103.063842. [DOI] [PubMed] [Google Scholar]
- 247.Guarneri L, Angelico P, Ibba M, Poggesi E, Taddei C, Leonardi A, Testa R. Pharmacological in vitro studies of the new 1,4-dihydropyridine calcium antagonist lercanidipine. Arzneimittelforschung. 1996;46:15–24. [PubMed] [Google Scholar]
- 248.Guerios SD, Wang ZY, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett. 2006;392:193–197. doi: 10.1016/j.neulet.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 249.Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R111–R122. doi: 10.1152/ajpregu.00728.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Guo YX, Li DP, Chen SR, Pan HL. Distinct intrinsic and synaptic properties of pre-sympathetic and pre-parasympathetic output neurons in Barrington’s nucleus. J Neurochem. 2013;126:338–348. doi: 10.1111/jnc.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Häbler HJ, Jänig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Häbler HJ, Jänig W, Koltzenburg M. Receptive properties of myelinated primary afferents innervating the inflamed urinary bladder of the cat. J Neurophysiol. 1993;69:395–405. doi: 10.1152/jn.1993.69.2.395. [DOI] [PubMed] [Google Scholar]
- 253.Haferkamp A, Mundhenk J, Bastian PJ, Reitz A, Dorsam J, Pannek J, Schumacher S, Schurch B, Buttner R, Muller SC. Increased expression of connexin 43 in the overactive neurogenic detrusor. Eur Urol. 2004;46:799–805. doi: 10.1016/j.eururo.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 254.Hald T, Horn T. The human urinary bladder in ageing. Br J Urol. 1998;82(Suppl 1):59–64. doi: 10.1046/j.1464-410x.1998.0820s1059.x. [DOI] [PubMed] [Google Scholar]
- 255.Hamberger B, Norberg KA. Adrenergic synaptic terminals and nerve cells in the bladder ganglia of the cat. Int J Neuropharmacol. 1965a;4:41–45. doi: 10.1016/0028-3908(65)90045-6. [DOI] [PubMed] [Google Scholar]
- 256.Hamberger B, Norberg KA. Studies on some systems of adrenergic synaptic terminals in the abdominal ganglia of the cat. Acta Physiol Scand. 1965b;65:235–242. doi: 10.1111/j.1748-1716.1965.tb04266.x. [DOI] [PubMed] [Google Scholar]
- 257.Harji F, Gonzales J, Galindo R, Dail WG. Preganglionic fibers in the rat hypogastric nerve project bilaterally to pelvic ganglia. Anat Rec. 1998;252:229–234. doi: 10.1002/(SICI)1097-0185(199810)252:2<229::AID-AR8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 258.Hashimoto K, Oyama T, Sugiyama T, Park YC, Kurita T. Neuronal excitation in the ventral tegmental area modulates the micturition reflex mediated via the dopamine D1 and D2 receptors in rats. J Pharmacol Sci. 2003;92:143–148. doi: 10.1254/jphs.92.143. [DOI] [PubMed] [Google Scholar]
- 259.Hawthorn MH, Chapple CR, Cock M, Chess-Williams R. Urotheliumderived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol. 2000;129:416–419. doi: 10.1038/sj.bjp.0703068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hayashi Y, Takimoto K, Chancellor MB, Erickson KA, Erickson VL, Kirimoto T, Nakano K, de Groat WC, Yoshimura N. Bladder hyperactivity and increased excitability of bladder afferent neurons associated with reduced expression of Kv1.4 alpha-subunit in rats with cystitis. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1661–R1670. doi: 10.1152/ajpregu.91054.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Helen P, Panula P, Yang H-YT, Rapoport SI. Bombesin/gastrinreleasing peptide (GRP) and met5-enkephalin- arg6-gly7-leu8-like immonoreactivities in small intensely fluorescent (SIF) cells and nerve fibers of the rat sympathetic ganglia. J Histochem Cytochem. 1984;32:1131–1138. doi: 10.1177/32.11.6491253. [DOI] [PubMed] [Google Scholar]
- 262.Hirayama A, Fujimoto K, Matsumoto Y, Hirao Y. Nocturia in men with lower urinary tract symptoms is associated with both nocturnal polyuria and detrusor overactivity with positive response to ice water test. Urology. 2005;65:1064–1069. doi: 10.1016/j.urology.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 263.Hirayama A, Fujimoto K, Matsumoto Y, Ozono S, Hirao Y. Positive response to ice water test associated with high-grade bladder outlet obstruction in patients with benign prostatic hyperplasia. Urology. 2003;62:909–913. doi: 10.1016/s0090-4295(03)00588-0. [DOI] [PubMed] [Google Scholar]
- 264.Hisamitsu T, de Groat WC. The inhibitory effect of opioid peptides and morphine applied intrathecally and intracerebroventricularly on the micturition reflex in the cat. J Physiol Soc Jpn. 1984;46:499–499. doi: 10.1016/0006-8993(84)91146-6. [DOI] [PubMed] [Google Scholar]
- 265.Ho KMT, Ny L, McMurray G, Andersson KE, Brading AF, Noble JG. CO-localization of carbon monoxide and nitric oxide synthesizing enzymes in the human urethral, sphincter. J Urol. 1999;161:1968–1972. [PubMed] [Google Scholar]
- 266.Hökfelt T, Johansson O, Ljungdahl A, Lundberg JM, Schultzberg M. Peptidergic neurons. Nat New Biol. 1980;284:515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- 267.Hökfelt T, Schultzberg M, Elde R, Nilsson G, Terenius L, Said S, Goldstein M. Peptide neurons in peripheral tissues including the urinary tract: Immunohistochemical studies. Acta Pharmacologica et Toxicologica. 1978;43(Suppl. II):79–89. doi: 10.1111/j.1600-0773.1978.tb03224.x. [DOI] [PubMed] [Google Scholar]
- 268.Holstege G. Micturition and the soul. J Comp Neurol. 2005;493:15–20. doi: 10.1002/cne.20785. [DOI] [PubMed] [Google Scholar]
- 269.Holstege G, Griffiths D, de Wall H, Dalm E. Anatomical and physiological observations on supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol. 1986;250:449–461. doi: 10.1002/cne.902500404. [DOI] [PubMed] [Google Scholar]
- 270.Holstege G, Mouton LJ. Central nervous system control of micturition. Int Rev Neurobiol. 2003;56:123–145. doi: 10.1016/s0074-7742(03)56004-4. [DOI] [PubMed] [Google Scholar]
- 271.Homma Y, Imajo C, Takahashi S, Kawabe K, Aso Y. Urinary symptoms and urodynamics in a normal elderly population. Scand J Urol Nephrol Suppl. 1994;157:27–30. [PubMed] [Google Scholar]
- 272.Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol. 2000;150:1349–1360. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Honda M, Yoshimura N, Hikita K, Hinata N, Muraoka K, Saito M, Chancellor MB, Takenaka A. Supraspinal and spinal effects of L-trans-PDC, an inhibitor of glutamate transporter, on the micturition reflex in rats. Neurourol Urodyn. 2013;32:1026–1030. doi: 10.1002/nau.22351. [DOI] [PubMed] [Google Scholar]
- 274.Hosseini A, Ehren I, Wiklund NP. Nitric oxide as an objective marker for evaluation of treatment response in patients with classic interstitial cystitis. J Urol. 2004;172:2261–2265. doi: 10.1097/01.ju.0000144761.69398.be. [DOI] [PubMed] [Google Scholar]
- 275.Hotta H, Morrison JF, Sato A, Uchida S. The effects of aging on the rat bladder and its innervation. Jpn J Physiol. 1995;45:823–836. doi: 10.2170/jjphysiol.45.823. [DOI] [PubMed] [Google Scholar]
- 276.Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;284:R574–R585. doi: 10.1152/ajpregu.00465.2002. [DOI] [PubMed] [Google Scholar]
- 277.Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol. 2005;173:1016–1021. doi: 10.1097/01.ju.0000155170.15023.e5. [DOI] [PubMed] [Google Scholar]
- 278.Hulsebosch CE, Coggeshall RE. An analysis of the axon populations in the nerves to the pelvic viscera in the rat. J Comp Neurol. 1982;211:1–10. doi: 10.1002/cne.902110102. [DOI] [PubMed] [Google Scholar]
- 279.Igawa Y, Mattiasson A, Andersson KE. Effects of GABA-receptor stimulation and blockade on micturition in normal rats and rats with bladder outflow obstruction. J Urol. 1993;150:537–542. doi: 10.1016/s0022-5347(17)35542-8. [DOI] [PubMed] [Google Scholar]
- 280.Iijima K, Igawa Y, Wyndaele JJ, De Wachter S. Mechanosensitive primary bladder afferent activity in rats with and without spinal cord transection. J Urol. 2009;182:2504–2510. doi: 10.1016/j.juro.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 281.Imada N, Koyama Y, Kawauchi A, Watanabe H, Kayama Y. State dependent response of the locus caeruleus neurons to bladder distention. J Urol. 2000;164:1740–1744. [PubMed] [Google Scholar]
- 282.Ishiura Y, Yoshiyama M, Yokoyama O, Namiki M, de Groat WC. Central muscarinic mechanisms regulating voiding in rats. J Pharmacol Exp Ther. 2001;297:933–939. [PubMed] [Google Scholar]
- 283.Ishizuka O, Alm P, Larsson B, Mattiasson A, Andersson KE. Facilitatory effect of pituitary adenylate cyclase activating polypeptide on micturition in normal, conscious rats. Neuroscience. 1995;66:1009–1014. doi: 10.1016/0306-4522(95)00038-k. [DOI] [PubMed] [Google Scholar]
- 284.Ishizuka O, Gu BJ, Yang ZX, Nishizawa O, Andersson KE. Functional role of central muscarinic receptors for micturition in normal conscious rats. J Urol. 2002;168:2258–2262. doi: 10.1016/S0022-5347(05)64367-4. [DOI] [PubMed] [Google Scholar]
- 285.Ishizuka O, Mattiasson A, Steers WD, Andersson KE. Effects of spinal alpha 1-adrenoceptor antagonism on bladder activity induced by apomorphine in conscious rats with and without bladder outlet obstruction. Neurourol Urodyn. 1997;16:191–200. doi: 10.1002/(sici)1520-6777(1997)16:3<191::aid-nau8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 286.Ishizuka O, Persson K, Mattiasson A, Naylor A, Wyllie M, Andersson K-E. Micturition in conscious rats with and without bladder outlet obstruction: Role of spinal a1-adrenoceptors. Br J Pharmacol. 1996;117:962–966. doi: 10.1111/j.1476-5381.1996.tb15288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ito T, Sakakibara R, Nakazawa K, Uchiyama T, Yamamoto T, Liu Z, Shimizu E, Hattori T. Effects of electrical stimulation of the raphe area on the micturition reflex in cats. Neuroscience. 2006;142:1273–1280. doi: 10.1016/j.neuroscience.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 288.Jaggar SI, Scott HC, Rice AS. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth. 1999;83:442–448. doi: 10.1093/bja/83.3.442. [DOI] [PubMed] [Google Scholar]
- 289.Jallat-Daloz IC, Cognard JL, Badet JM, Regnard J. Neural-epithelial cell interplay: In vitro evidence that vagal mediators increase PGE2 production by human nasal epithelial cells. Allergy Asthma Proc. 2001;22:17–23. doi: 10.2500/108854101778249267. [DOI] [PubMed] [Google Scholar]
- 290.James S, Burnstock G. Neuropeptide Y-like immunoreactivity in intramural ganglia of the newborn guinea pig urinary bladder. Regulatory Peptides. 1988;23:237–245. doi: 10.1016/0167-0115(88)90031-6. [DOI] [PubMed] [Google Scholar]
- 291.James S, Burnstock G. Localization of muscarinic receptors on somatostatin-like immunoreactive neurons of the newborn guinea-pig urinary bladder in culture. Neurosci Lett. 1989;106:13–18. doi: 10.1016/0304-3940(89)90194-8. [DOI] [PubMed] [Google Scholar]
- 292.Jänig W, Morrison JFB. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res. 1986;67:87–114. doi: 10.1016/s0079-6123(08)62758-2. [DOI] [PubMed] [Google Scholar]
- 293.Jeremy JY, Tsang V, Mikhailidis P, Rogers H, Morgan RJ, Dandona P. Eicosanoid snthesis by human urinary bladder mucosa: Pathological implications. Brit J Urol. 1987;59:36–39. doi: 10.1111/j.1464-410x.1987.tb04575.x. [DOI] [PubMed] [Google Scholar]
- 294.Jung SY, Fraser MO, Ozawa H, Yokoyama O, Yoshiyama M, De Groat WC, Chancellor MB. Urethral afferent nerve activity affects the micturition reflex; implication for the relationship between stress incontinence and detrusor instability. J Urol. 1999;162:204–212. doi: 10.1097/00005392-199907000-00069. [DOI] [PubMed] [Google Scholar]
- 295.Kabat H, Magoun HW, Ranson SW. Reaction of the bladder to stimulation of points in the forebrain and mid-brain. J Comp Neurol. 1936;63:211–239. [Google Scholar]
- 296.Kadekawa K, Sugaya K, Nishijima S, Ashitomi K, Miyazato M, Ueda T, Yamamoto H. Effect of naftopidil, an alpha1D/A-adrenoceptor antagonist, on the urinary bladder in rats with spinal cord injury. Life Sci. 2013;92:1024–1028. doi: 10.1016/j.lfs.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 297.Kaiho Y, Kamo I, Chancellor MB, Arai Y, de Groat WC, Yoshimura N. Role of noradrenergic pathways in sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol. 2007;292:F639–F646. doi: 10.1152/ajprenal.00226.2006. [DOI] [PubMed] [Google Scholar]
- 298.Kakizaki H, Yoshiyama M, de Groat WC. C-fos expression in spinal neurons after irritation of the lower urinary tract depends on synergistic interactions between NMDA amd AMPA glutamatergic transmission. Am J Physiol. 1996;76:215–226. doi: 10.1152/ajpregu.1996.270.5.R990. [DOI] [PubMed] [Google Scholar]
- 299.Kakizaki H, Yoshiyama M, Koyanagi T, de Groat WC. Effects of WAY100635, a selective 5-HT1A-receptor antagonist on the micturition-reflex pathway in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1407–R1413. doi: 10.1152/ajpregu.2001.280.5.R1407. [DOI] [PubMed] [Google Scholar]
- 300.Kakizaki H, Yoshiyama M, Roppolo JR, Booth AM, de Groat WC. Role of spinal glutamatergic transmission in the ascending limb of the micturition reflex pathway in the rat. J Pharmacol Exp Ther. 1998;285:22–27. [PubMed] [Google Scholar]
- 301.Kamo I, Cannon TW, Conway DA, Torimoto K, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder-to-urethral reflexes in urinary continence mechanisms in rats. Am J Physiol Renal Physiol. 2004;287:F434–F441. doi: 10.1152/ajprenal.00038.2004. [DOI] [PubMed] [Google Scholar]
- 302.Kamo I, Chancellor MB, de Groat WC, Yoshimura N. Differential effects of activation of peripheral and spinal tachykinin neurokinin(3) receptors on the micturition reflex in rats. J Urol. 2005;174:776–781. doi: 10.1097/01.ju.0000164722.08662.c7. [DOI] [PubMed] [Google Scholar]
- 303.Kanai AJ. Afferent mechanism in the urinary tract. Handb Exp Pharmacol. 2011;202:171–205. doi: 10.1007/978-3-642-16499-6_9. [DOI] [PubMed] [Google Scholar]
- 304.Kanie S, Yokoyama O, Komatsu K, Kodama K, Yotsuyanagi S, Niikura S, Nagasaka Y, Miyamoto KI, Namiki M. GABAergic contribution to rat bladder hyperactivity after middle cerebral artery occlusion. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1230–R1238. doi: 10.1152/ajpregu.2000.279.4.R1230. [DOI] [PubMed] [Google Scholar]
- 305.Kaplan SA, Te AE, Blaivas JG. Urodynamic findings in patients with diabetic cystopathy. J Urol. 1995;153:342–344. doi: 10.1097/00005392-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 306.Karhula T, Panula P, Steinbusch H, Häppölä O. Immunohistochemical localization of 5-hydroxytryptamine, histamine and histidine decarboxylase in the rat major pelvic and coeliac-superior mesenteric ganglion. J Auton Nerv Syst. 1990;31:91–100. doi: 10.1016/0165-1838(90)90065-q. [DOI] [PubMed] [Google Scholar]
- 307.Kashyap M, Kawamorita N, Tyagi V, Sugino Y, Chancellor M, Yoshimura N, Tyagi P. Down-regulation of nerve growth factor expression in the bladder by antisense oligonucleotides as new treatment for overactive bladder. J Urol. 2013;190:757–764. doi: 10.1016/j.juro.2013.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kavia R, Dasgupta R, Critchley H, Fowler C, Griffiths D. A functional magnetic resonance imaging study of the effect of sacral neuromodulation on brain responses in women with Fowler’s syndrome. BJU Int. 2010;105:366–372. doi: 10.1111/j.1464-410X.2009.08819.x. [DOI] [PubMed] [Google Scholar]
- 309.Kavia RB, Dasgupta R, Fowler CJ. Functional imaging and the central control of the bladder. J Comp Neurol. 2005;493:27–32. doi: 10.1002/cne.20753. [DOI] [PubMed] [Google Scholar]
- 310.Kawatani M, Erdman SL, de Groat WC. VIP and substance P in primary afferent pathways to the sacral spinal cord of the cat. J Comp Neurol. 1985;241:327–347. doi: 10.1002/cne.902410307. [DOI] [PubMed] [Google Scholar]
- 311.Kawatani M, Lowe IP, Booth AM, Backes MG, Erdman SL, de Groat WC. The presence of leucine-enkephalin in the sacral preganglionic pathway to the urinary bladder of the cat. Neurosci Lett. 1983;39:143–148. doi: 10.1016/0304-3940(83)90067-8. [DOI] [PubMed] [Google Scholar]
- 312.Kawatani M, Lowe IP, Nadelhaft I, Morgan C, de Groat WC. Vasoactive intestinal polypeptide in visceral afferent pathways to the sacral spinal cord of the cat. Neurosci Lett. 1983;42:311–316. doi: 10.1016/0304-3940(83)90280-x. [DOI] [PubMed] [Google Scholar]
- 313.Kawatani M, Nagel J, de Groat WC. Identification of neuropeptides in pelvic and pudendal nerve afferent pathways to the sacral spinal cord of the cat. J Comp Neurol. 1986;249:117–132. doi: 10.1002/cne.902490109. [DOI] [PubMed] [Google Scholar]
- 314.Kawatani M, Rutigliano M, de Groat WC. Selective facilitatory effect of vasoactive intestinal polypeptide (VIP) on muscarinic firing in vesical ganglia of the cat. Brain Res. 1985;336:223–234. doi: 10.1016/0006-8993(85)90649-3. [DOI] [PubMed] [Google Scholar]
- 315.Kawatani M, Rutigliano M, de Groat WC. Selective facilitatory effects of vasoactive intestinal polypeptide on muscarinic mechanisms in sympathetic and parasympathetic ganglia of the cat. In: Hanin I, editor. Advances in Behavioral Biology, Dynamics of Cholinergic Function. New York: Plenum Press; 1986. pp. 1057–1066. [Google Scholar]
- 316.Kawatani M, Shioda S, Nakai Y, Takeshige C, de Groat WC. Ultrastructural analysis of enkephalinergic terminals in parasympathetic ganglia innervating the urinary bladder of the cat. J Comp Neurol. 1989;288:81–91. doi: 10.1002/cne.902880107. [DOI] [PubMed] [Google Scholar]
- 317.Kawatani M, Tanowitz M, de Groat WC. Morphological and electrophysiological analysis of the peripheral and central afferent pathways from the clitoris of the cat. Brain Res. 1994;646:26–36. doi: 10.1016/0006-8993(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 318.Keast JR. Unusual autonomic ganglia: Connections, chemistry, and plasticity of pelvic ganglia. Int Rev Cytol. 1999;193:1–69. doi: 10.1016/s0074-7696(08)61778-7. [DOI] [PubMed] [Google Scholar]
- 319.Keast JR. Plasticity of pelvic autonomic ganglia and urogenital innervation. Int Rev Cytol. 2006;248:141–208. doi: 10.1016/S0074-7696(06)48003-7. [DOI] [PubMed] [Google Scholar]
- 320.Keast JR, de Groat WC. Immunohistochemical characterization of pelvic neurons which project to the bladder, colon, or penis in rats. J Comp Neurol. 1989;288:387–400. doi: 10.1002/cne.902880303. [DOI] [PubMed] [Google Scholar]
- 321.Keast JR, de Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- 322.Keast JR, Kawatani M, de Groat WC. Sympathetic modulation of cholinergic transmission in cat vesical ganglia is mediated by a1 and a2 adrenoceptors. Am J Physiol. 1990;258:R44–R50. doi: 10.1152/ajpregu.1990.258.1.R44. [DOI] [PubMed] [Google Scholar]
- 323.Keast JR, Stephensen TM. Glutamate and aspartate immunoreactivity in dorsal root ganglion cells supplying visceral and somatic targets and evidence for peripheral axonal transport. J Comp Neurol. 2000;424:577–587. [PubMed] [Google Scholar]
- 324.Kebapci N, Yenilmez A, Efe B, Entok E, Demirustu C. Bladder dysfunction in type 2 diabetic patients. Neurourol Urodyn. 2007;26:814–819. doi: 10.1002/nau.20422. [DOI] [PubMed] [Google Scholar]
- 325.Kennelly MJ, Arena KC, Shaffer N, Bennett ME, Grill WM, Grill JH, Boggs JW. Electrical stimulation of the urethra evokes bladder contractions in a woman with spinal cord injury. J Spinal Cord Med. 2010;33:261–265. doi: 10.1080/10790268.2010.11689704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kennelly MJ, Bennett ME, Grill WM, Grill JH, Boggs JW. Electrical stimulation of the urethra evokes bladder contractions and emptying in spinal cord injury men: Case studies. J Spinal Cord Med. 2011;34:315–321. doi: 10.1179/2045772311Y.0000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Kihara K, de Groat WC. Sympathetic efferent pathways projecting bilaterally to the vas deferens in the rat. Anat Rec. 1997;248:291–299. doi: 10.1002/(SICI)1097-0185(199706)248:2<291::AID-AR16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 328.Kim SO, Oh BS, Chang IY, Song SH, Ahn K, Hwang EC, Oh KJ, Kwon D, Park K. Distribution of interstitial cells of Cajal and expression of nitric oxide synthase after experimental bladder outlet obstruction in a rat model of bladder overactivity. Neurourol Urodyn. 2011;30:1639–1645. doi: 10.1002/nau.21144. [DOI] [PubMed] [Google Scholar]
- 329.Kimura Y, Ukai Y, Kimura K, Sugaya K, Nishizawa O. Inhibitory influence from the nucleus reticularis pontis oralis on the micturition reflex induced by electrical stimulation of the pontine micturition center in cats. Neurosci Lett. 1995;195:214–216. doi: 10.1016/0304-3940(95)11804-6. [DOI] [PubMed] [Google Scholar]
- 330.Kitta T, Chancellor MB, de Groat WC, Kuno S, Nonomura K, Yoshimura N. Suppression of bladder overactivity by adenosine A2A receptor antagonist in a rat model of Parkinson disease. J Urol. 2012;187:1890–1897. doi: 10.1016/j.juro.2011.12.062. [DOI] [PubMed] [Google Scholar]
- 331.Kitta T, Kakizaki H, Furuno T, Moriya K, Tanaka H, Shiga T, Tamaki N, Yabe I, Sasaki H, Nonomura K. Brain activation during detrusor overactivity in patients with Parkinson’s disease: A positron emission tomography study. J Urol. 2006;175:994–998. doi: 10.1016/S0022-5347(05)00324-1. [DOI] [PubMed] [Google Scholar]
- 332.Kitta T, Matsumoto M, Tanaka H, Mitsui T, Yoshioka M, Nonomura K. GABAergic mechanism mediated via D receptors in the rat periaqueductal gray participates in the micturition reflex: An in vivo microdialysis study. Eur J Neurosci. 2008;27:3216–3225. doi: 10.1111/j.1460-9568.2008.06276.x. [DOI] [PubMed] [Google Scholar]
- 333.Kitta T, Miyazato M, Chancellor MB, de Groat WC, Nonomura K, Yoshimura N. Alpha2-adrenoceptor blockade potentiates the effect of duloxetine on sneeze induced urethral continence reflex in rats. J Urol. 2010;184:762–768. doi: 10.1016/j.juro.2010.03.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 335.Kontani H, Inoue T, Sakai T. Dopamine receptor subtypes that induce hyperactive urinary bladder response in anesthetized rats. Jpn J Pharmacol. 1990;54:482–486. doi: 10.1254/jjp.54.482. [DOI] [PubMed] [Google Scholar]
- 336.Kontani H, Sakai T, Maruyama I. Involvement of a 2-adrenoceptors in the sacral micturition reflex in rats. Jpn J Pharmacol. 1992;60:363–368. doi: 10.1254/jjp.60.363. [DOI] [PubMed] [Google Scholar]
- 337.Koshino K. Spontaneous potential activities related to the intravesical pressure in the pontine area of the cat. Jpn J Physiol. 1970;20:272–280. doi: 10.2170/jjphysiol.20.272. [DOI] [PubMed] [Google Scholar]
- 338.Koskela LR, Thiel T, Ehren I, De Verdier PJ, Wiklund NP. Localization and expression of inducible nitric oxide synthase in biopsies from patients with interstitial cystitis. J Urol. 2008;180:737–741. doi: 10.1016/j.juro.2008.03.184. [DOI] [PubMed] [Google Scholar]
- 339.Koyama Y, Imada N, Kayama Y, Kawauchi A, Watanabe H. How does the distention of urinary bladder cause arousal? Psychiatry Clin Neurosci. 1998;52:142–145. doi: 10.1111/j.1440-1819.1998.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 340.Koyama Y, Makuya A, Kuru M. Vesico-motor areas in the cat midbrain. Jpn J Physiol. 1962;12:63–80. doi: 10.2170/jjphysiol.12.63. [DOI] [PubMed] [Google Scholar]
- 341.Koyama Y, Ozaki H, Kuru M. Interference between the pontine detrusor nucleus and the pontine urine-storage nucleus. An electromyographical study of the external urethral sphincter. Jpn J Physiol. 1966;16:291–303. doi: 10.2170/jjphysiol.16.291. [DOI] [PubMed] [Google Scholar]
- 342.Krhut J, Tintera J, Holy P, Zachoval R, Zvara P. A preliminary report on the use of functional magnetic resonance imaging with simultaneous urodynamics to record brain activity during micturition. J Urol. 2012;188:474–479. doi: 10.1016/j.juro.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 343.Kruse MN, Bray LA, de Groat WC. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J Auton Nerv Syst. 1995;54:215–224. doi: 10.1016/0165-1838(95)00011-l. [DOI] [PubMed] [Google Scholar]
- 344.Kruse MN, de Groat WC. Micturition reflexes in decerebrate and spinalized neonatal rats. Am J Physiol. 1990;258:R1508–R1511. doi: 10.1152/ajpregu.1990.258.6.R1508. [DOI] [PubMed] [Google Scholar]
- 345.Kruse MN, de Groat WC. Spinal pathways mediate coordinated bladder/urethral sphincter activity during reflex micturition in decerebrate and spinalized neonatal rats. Neurosci Lett. 1993;152:141–144. doi: 10.1016/0304-3940(93)90503-d. [DOI] [PubMed] [Google Scholar]
- 346.Kruse MN, Mallory BS, Noto H, Roppolo JR, de Groat WC. Properties of the descending limb of the spinobulbospinal micturition reflex pathway in the cat. Brain Res. 1991;556:6–12. doi: 10.1016/0006-8993(91)90541-3. [DOI] [PubMed] [Google Scholar]
- 347.Kruse MN, Mallory BS, Noto H, Roppolo JR, de Groat WC. Modulation of the spinobulbospinal micturition reflex pathway in cats. Am J Physiol. 1992;262:R478–R484. doi: 10.1152/ajpregu.1992.262.3.R478. [DOI] [PubMed] [Google Scholar]
- 348.Kruse MN, Noto H, Roppolo JR, de Groat WC. Pontine control of the urinary bladder and external urethral sphincter in the rat. Brain Res. 1990;532:182–190. doi: 10.1016/0006-8993(90)91758-9. [DOI] [PubMed] [Google Scholar]
- 349.Kubota Y, Hashitani H, Shirasawa N, Kojima Y, Sasaki S, Mabuchi Y, Soji T, Suzuki H, Kohri K. Altered distribution of interstitial cells in the guinea pig bladder following bladder outlet obstruction. Neurourol Urodyn. 2008;27:330–340. doi: 10.1002/nau.20502. [DOI] [PubMed] [Google Scholar]
- 350.Kuchel GA, Moscufo N, Guttmann CR, Zeevi N, Wakefield D, Schmidt J, Dubeau CE, Wolfson L. Localization of brain white matter hyperintensities and urinary incontinence in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2009;64:902–909. doi: 10.1093/gerona/glp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Kuhtz-Buschbeck JP, Gilster R, van der Horst C, Hamann M, Wolff S, Jansen O. Control of bladder sensations: An fMRI study of brain activity and effective connectivity. Neuroimage. 2009;47:18–27. doi: 10.1016/j.neuroimage.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 352.Kuhtz-Buschbeck JP, van der Horst C, Pott C, Wolff S, Nabavi A, Jansen O, Junemann KP. Cortical representation of the urge to void: A functional magnetic resonance imaging study. J Urol. 2005;174:1477–1481. doi: 10.1097/01.ju.0000173007.84102.7c. [DOI] [PubMed] [Google Scholar]
- 353.Kuhtz-Buschbeck JP, van der Horst C, Wolff S, Filippow N, Nabavi A, Jansen O, Braun PM. Activation of the supplementary motor area (SMA) during voluntary pelvic floor muscle contractions–an fMRI study. Neuroimage. 2007;35:449–457. doi: 10.1016/j.neuroimage.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 354.Kuipers R, Mouton LJ, Holstege G. Afferent projections to the pontine micturition center in the cat. J Comp Neurol. 2006;494:36–53. doi: 10.1002/cne.20775. [DOI] [PubMed] [Google Scholar]
- 355.Kullmann FA, Artim D, Beckel J, Barrick S, de Groat WC, Birder LA. Heterogeneity of muscarinic receptor-mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol Renal Physiol. 2008;294:F971–F981. doi: 10.1152/ajprenal.00313.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Kullmann FA, Artim DE, Birder LA, de Groat WC. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci. 2008;28:1977–1987. doi: 10.1523/JNEUROSCI.4694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Kullmann FA, Downs TR, Artim DE, Limberg BJ, Shah M, Contract D, de Groat WC, Rosenbaum JS. Urothelial beta-3 adrenergic receptors in the rat bladder. Neurourol Urodyn. 2011;30:144–150. doi: 10.1002/nau.20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Kullmann FA, Shah MA, Birder LA, de Groat WC. Functional TRP and ASIC-like channels in cultured urothelial cells from the rat. Am J Physiol Renal Physiol. 2009;296:F892–F901. doi: 10.1152/ajprenal.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Kumamoto E, Shinnick-Gallagher P. Soman enhances nicotinic depolarizations, and depresses muscarinic hyperpolarizations in parasympathetic neurons. Brain Res. 1988;458:151–156. doi: 10.1016/0006-8993(88)90508-2. [DOI] [PubMed] [Google Scholar]
- 360.Kumamoto E, Shinnick-Gallagher P. Slow inward and late slow outward currents induced by hyperpolarizing pre-pulses in cat bladder parasympathetic neurones. Pflugers Arch. 1990;416:322–334. doi: 10.1007/BF00392069. [DOI] [PubMed] [Google Scholar]
- 361.Kumar V, Cross RL, Chess-Williams R, Chapple CR. Recent advances in basic science for overactive bladder. Curr Opin Urol. 2005;15:222–226. doi: 10.1097/01.mou.0000172393.52857.92. [DOI] [PubMed] [Google Scholar]
- 362.Kuo DC, de Groat WC. The function of efferent projections from the lumbosacral sympathetic chain to the urinary bladder of the cat. Soc Neurosci Abstr. 1983;9:610–610. [Google Scholar]
- 363.Kuru M. Nervous control of micturition. Physiol Rev. 1965;45:425–494. doi: 10.1152/physrev.1965.45.3.425. [DOI] [PubMed] [Google Scholar]
- 364.Kuru M, Iwanaga T. Ponto-sacral connections in the medial reticulospinal tract subserving storage of urine. J Comp Neurol. 1966;127:241–266. doi: 10.1002/cne.901270208. [DOI] [PubMed] [Google Scholar]
- 365.Laird JM, Olivar T, Roza C, De Felipe C, Hunt SP, Cervero F. Deficits in visceral pain and hyperalgesia of mice with a disruption of the tachykinin NK1 receptor gene. Neuroscience. 2000;98:345–352. doi: 10.1016/s0306-4522(00)00148-2. [DOI] [PubMed] [Google Scholar]
- 366.Lamb K, Gebhart GF, Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain. 2004;5:150–156. doi: 10.1016/j.jpain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 367.Lane RD, Wager TD. The new field of Brain-Body Medicine: What have we learned and where are we headed? Neuroimage. 2009;47:1135–1140. doi: 10.1016/j.neuroimage.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 368.Langley JN, Anderson HK. The innervation of the pelvic and adjoining viscera: Part II. The bladder. Part III. The external generative organs. Part IV. The internal generative organs. Part V. Position of the nerve cells on the course of the efferent nerve fibres. J Physiol. 1895;19:71–139. doi: 10.1113/jphysiol.1895.sp000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Langley JN, Anderson HK. The innervation of the pelvic and adjoining viscera. Part VI. Histological and physiological observations upon the effects of section of the sacral nerves. J Physiol. 1896;20:372–384. doi: 10.1113/jphysiol.1896.sp000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Langworthy OR, Kolb LC. The encephalic control of tones in the musculature of the urinary bladder. Brain. 1933;56:371–382. [Google Scholar]
- 371.Langworthy OR, Kolb LC. Demonstration of Encephalic Control of Micturition by Electrial Stimulation. Vol. 56. Bull Johns Hopkins Hosp; 1935. [Google Scholar]
- 372.Langworthy OR, Kolb LC, Lewis LG. Physiology of Micturition. Baltimore: Williams and Wilkins Company; 1940. [Google Scholar]
- 373.Latifpour J, Kondo S, O’Hollaren B, Morita T, Weiss RM. Autonomic receptors in urinary tract: Sex and age differences. J Pharmacol Exp Ther. 1990;253:661–667. [PubMed] [Google Scholar]
- 374.Lazzeri M, Beneforti P, Benaim G, Maggi CA, Lecci A, Turini D. Intravesical capsaicin for treatment of severe bladder pain: A randomized placebo controlled study. J Urol. 1996;156:947–952. [PubMed] [Google Scholar]
- 375.Lazzeri M, Beneforti P, Spinelli M, Zanollo A, Barbagli G, Turini D. Intravesical resiniferatoxin for the treatment of hypersensitive disorder: A randomized placebo controlled study. J Urol. 2000;164:676–679. doi: 10.1097/00005392-200009010-00014. [DOI] [PubMed] [Google Scholar]
- 376.Lecci A, Giuliani S, Lazzeri M, Benaim G, Turini D, Maggi CA. The behavioral response induced by intravesical instillation of capsaicin rats is mediated by pudendal urethral sensory fibers. Life Sci. 1994;55:429–436. doi: 10.1016/0024-3205(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 377.Lecci A, Giuliani S, Santicioli P, Maggi CA. Involvement of 5-hydroxytryptamine1A receptors in the modulation of micturition reflexes in the anesthetized rat. J Pharmacol Exp Ther. 1992;262:181–189. [PubMed] [Google Scholar]
- 378.Lecci A, Tramontana M, Giuliani S, Criscuoli M, Maggi CA. Effect of tachykinin NK2 receptor blockade on detrusor hyperreflexia induced by bacterial toxin in rats. J Urol. 1998;160:206–209. [PubMed] [Google Scholar]
- 379.Lee HY, Bardini M, Burnstock G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol. 2000;163:2002–2007. [PubMed] [Google Scholar]
- 380.Lee SJ, Nakamura Y, de Groat WC. Effect of (+/−)-epibatidine, a nicotinic agonist, on the central pathways controlling voiding function in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R84–R90. doi: 10.1152/ajpregu.00109.2003. [DOI] [PubMed] [Google Scholar]
- 381.Lee WC, Wu HP, Tai TY, Yu HJ, Chiang PH. Investigation of urodynamic characteristics and bladder sensory function in the early stages of diabetic bladder dysfunction in women with type 2 diabetes. J Urol. 2009;181:198–203. doi: 10.1016/j.juro.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 382.Lieu PK, Sa’adu A, Orugun EO, Malone-Lee JG. The influence of age on isometric and isotonic rat detrusor contractions. J Gerontol A Biol Sci Med Sci. 1997;52:M94–M96. doi: 10.1093/gerona/52a.2.m94. [DOI] [PubMed] [Google Scholar]
- 383.Limberg BJ, Andersson KE, Aura Kullmann F, Burmer G, de Groat WC, Rosenbaum JS. beta-Adrenergic receptor subtype expression in myocyte and non-myocyte cells in human female bladder. Cell Tissue Res. 2010;342:295–306. doi: 10.1007/s00441-010-1053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Lin AT, Yang CH, Chang LS. Impact of aging on rat urinary bladder fatigue. J Urol. 1997;157:1990–1994. [PubMed] [Google Scholar]
- 385.Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur Urol. 2009;56:700–706. doi: 10.1016/j.eururo.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 386.Liu HT, Kuo HC. Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. Urology. 2008;72:104–108. doi: 10.1016/j.urology.2008.01.069. discussion 108. [DOI] [PubMed] [Google Scholar]
- 387.Liu Z, Sakakibara R, Nakazawa K, Uchiyama T, Yamamoto T, Ito T, Hattori T. Micturition-related neuronal firing in the periaqueductal gray area in cats. Neuroscience. 2004;126:1075–1082. doi: 10.1016/j.neuroscience.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 388.Logadottir Y, Hallsberg L, Fall M, Peeker R, Delbro D. Bladder pain syndrome/interstitial cystitis ESSIC type 3C: high expression of inducible nitric oxide synthase in inflammatory cells. Scand J Urol. 2013;47:52–56. doi: 10.3109/00365599.2012.699100. [DOI] [PubMed] [Google Scholar]
- 389.Longhurst PA, Eika B, Leggett RE, Levin RM. Comparison of urinary bladder function in 6 and 24 month male and female rats. J Urol. 1992;148:1615–1620. doi: 10.1016/s0022-5347(17)36981-1. [DOI] [PubMed] [Google Scholar]
- 390.Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997;79:572–577. doi: 10.1046/j.1464-410x.1997.00097.x. [DOI] [PubMed] [Google Scholar]
- 391.Lundberg JM, Hökfelt T, Schultzberg M, Uvnäs-Wallensten K, Köhler L, Said S. Occurence of VIP-like immonoreactivity in cholinergic neurons of the cat: Evidence from combined immunohistochemistry and acetylcholine esterase staining. Neuroscience. 1979;4:1539–1559. doi: 10.1016/0306-4522(79)90018-6. [DOI] [PubMed] [Google Scholar]
- 392.Madersbacher S, Pycha A, Klingler CH, Mian C, Djavan B, Stulnig T, Marberger M. Interrelationships of bladder compliance with age, detrusor instability, and obstruction in elderly men with lower urinary tract symptoms. Neurourol Urodyn. 1999;18:3–15. doi: 10.1002/(sici)1520-6777(1999)18:1<3::aid-nau2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 393.Madersbacher S, Pycha A, Schatzl G, Mian C, Klingler CH, Marberger M. The aging lower urinary tract: A comparative urodynamic study of men and women. Urology. 1998;51:206–212. doi: 10.1016/s0090-4295(97)00616-x. [DOI] [PubMed] [Google Scholar]
- 394.Maggi CA. The dual, sensory and efferent function of the capsaicinsensitive primary sensory nerves in the bladder and urethra. In: Maggi CA, editor. The Autonomic Nervous System. London: Harwood Academic Publishers; 1993. pp. 383–422. [Google Scholar]
- 395.Maggi CA, Conte B. Effect of urethane anesthesia on the micturition reflex in capsaicin -treated rats. J Auton Nerv Syst. 1990;30:247–251. doi: 10.1016/0165-1838(90)90256-i. [DOI] [PubMed] [Google Scholar]
- 396.Maggi CA, Santicioli P, Meli A. Postnatal development of micturition reflex in rats. Am J Physiol. 1986;250:R926–R931. doi: 10.1152/ajpregu.1986.250.5.R926. [DOI] [PubMed] [Google Scholar]
- 397.Mallory B, Steers WD, de Groat WC. Electrophysiological study of micturition reflexes in rats. Am J Physiol. 1989;257:R410–R421. doi: 10.1152/ajpregu.1989.257.2.R410. [DOI] [PubMed] [Google Scholar]
- 398.Mallory BS, Roppolo JR, de Groat WC. Pharmacological modulation of the pontine micturition center. Brain Res. 1991;546:310–320. doi: 10.1016/0006-8993(91)91495-m. [DOI] [PubMed] [Google Scholar]
- 399.Mally AD, Matsuta Y, Zhang F, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Role of opioid and metabotropic glutamate 5 receptors in pudendal inhibition of bladder overactivity in cats. J Urol. 2013;189:1574–1579. doi: 10.1016/j.juro.2012.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Malykhina AP, Qin C, Foreman RD, Akbarali HI. Colonic inflammation increases Na+ currents in bladder sensory neurons. Neuroreport. 2004;15:2601–2605. doi: 10.1097/00001756-200412030-00008. [DOI] [PubMed] [Google Scholar]
- 401.Malykhina AP, Wyndaele JJ, Andersson KE, De Wachter S, Dmochowski RR. Do the urinary bladder and large bowel interact, in sickness or in health? ICI-RS 2011. Neurourol Urodyn. 2012;31:352–358. doi: 10.1002/nau.21228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Marson L. Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: A transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1997;389:584–602. [PubMed] [Google Scholar]
- 403.Martner J. Influences on the defecation and micturition reflexes by the cerebellar fastigial nucleus. Acta Physiol Scand. 1975;94:95–104. doi: 10.1111/j.1748-1716.1975.tb05865.x. [DOI] [PubMed] [Google Scholar]
- 404.Mastri AR. Neuropathology of diabetic neurogenic bladder. Ann Intern Med. 1980;92:316–318. doi: 10.7326/0003-4819-92-2-316. [DOI] [PubMed] [Google Scholar]
- 405.Masuda H, Chancellor MB, Kihara K, Sakai Y, Koga F, Azuma H, de Groat WC, Yoshimura N. Effects of cholinesterase inhibition in supraspinal and spinal neural pathways on the micturition reflex in rats. BJU Int. 2009;104:1163–1169. doi: 10.1111/j.1464-410X.2009.08515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Masuda H, Hayashi Y, Chancellor MB, Kihara K, de Groat WC, de Miguel F, Yoshimura N. Roles of peripheral and central nicotinic receptors in the micturition reflex in rats. J Urol. 2006;176:374–379. doi: 10.1016/S0022-5347(06)00581-7. [DOI] [PubMed] [Google Scholar]
- 407.Masuda H, Ichiyanagi N, Yokoyama M, Sakai Y, Kihara K, Chancellor MB, de Groat WC, Yoshimura N. Muscarinic receptor activation in the lumbosacral spinal cord ameliorates bladder irritation in rat cystitis models. BJU Int. 2009;104:1531–1537. doi: 10.1111/j.1464-410X.2009.08617.x. [DOI] [PubMed] [Google Scholar]
- 408.Masuda H, Kim JH, Kihara K, Chancellor MB, de Groat WC, Yoshimura N. Inhibitory roles of peripheral nitrergic mechanisms in capsaicin-induced detrusor overactivity in the rat. BJU Int. 2007;100:912–918. doi: 10.1111/j.1464-410X.2007.07099.x. [DOI] [PubMed] [Google Scholar]
- 409.Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi S, Manabe T, Taketo MM. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci. 2002;22:10627–10632. doi: 10.1523/JNEUROSCI.22-24-10627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci U S A. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Matsumoto G, Hisamitsu T, de Groat WC. Non-NMDA glutamatergic excitatory transmission in the descending limb of the spinobulbospinal micturition reflex pathway in the rat. Brain Res. 1995;693:246–250. doi: 10.1016/0006-8993(95)00738-c. [DOI] [PubMed] [Google Scholar]
- 412.Matsumoto G, Hisamitsu T, de Groat WC. Role of glutamate and NMDA receptors in the descending limb of the spinobulbospinal micturition reflex pathway of the rat. Neurosci Lett. 1995b;183:58–61. doi: 10.1016/0304-3940(94)11114-x. [DOI] [PubMed] [Google Scholar]
- 413.Matsumoto S, Levendusky MC, Longhurst PA, Levin RM, Millington WR. Activation of mu opioid receptors in the ventrolateral periaqueductal gray inhibits reflex micturition in anesthetized rats. Neurosci Lett. 2004;363:116–119. doi: 10.1016/j.neulet.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 414.Matsuta Y, Yusup A, Tanase K, Ishida H, Akino H, Yokoyama O. Melatonin increases bladder capacity via GABAergic system and decreases urine volume in rats. J Urol. 2010;184:386–391. doi: 10.1016/j.juro.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 415.Matsuura S, Allen GV, Downie JW. Volume-evoked micturition reflex is mediated by the ventrolateral periaqueductal gray in anesthetized rats. Am J Physiol. 1998;275:R2049–R2055. doi: 10.1152/ajpregu.1998.275.6.R2049. [DOI] [PubMed] [Google Scholar]
- 416.Matsuura S, Downie JW, Allen GV. Micturition evoked by glutamate microinjection in the ventrolateral periaqueductal gray is mediated through Barrington’s nucleus in the rat. Neuroscience. 2000;101:1053–1061. doi: 10.1016/s0306-4522(00)00404-8. [DOI] [PubMed] [Google Scholar]
- 417.Matsuura S, Kakizaki H, Mitsui T, Shiga T, Tamaki N, Koyanagi T. Human brain region response to distention or cold stimulation of the bladder: A positron emission tomography study. J Urol. 2002;168:2035–2039. doi: 10.1016/S0022-5347(05)64290-5. [DOI] [PubMed] [Google Scholar]
- 418.Mattiasson A, Andersson K-E, Sjögren C. Adrenoceptors and cholinoceptors controlling noradrenaline release from adrenergic nerves in the urethra of rabbit and man. J Urol. 1984;131:1190–1195. doi: 10.1016/s0022-5347(17)50870-8. [DOI] [PubMed] [Google Scholar]
- 419.Mattiasson A, Andersson KE, Elbadawi A, Morgan E, Sjogren C. Interaction between adrenergic and cholinergic nerve terminals in the urinary bladder of rabbit, cat and man. J Urol. 1987;137:1017–1019. doi: 10.1016/s0022-5347(17)44350-3. [DOI] [PubMed] [Google Scholar]
- 420.McGee MJ, Grill WM. Selective co-stimulation of pudendal afferents enhances bladder activation and improves voiding efficiency. Neurourol Urodyn. 2013 doi: 10.1002/nau.22474. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.McGuire E, Morrissey S, Zhang S, Horwinski E. Control of reflex detrusor activity in normal and spinal injured non-human primates. J Urol. 1983;129:197–199. doi: 10.1016/s0022-5347(17)51982-5. [DOI] [PubMed] [Google Scholar]
- 422.McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- 423.McLachlan EM. Interaction beteen sympathetic and parasympathetic pathays in the pelvic plexus of the guinea pig. Proc Aust Physiol Pharmacol Soc. 1977;8:15P–15P. [Google Scholar]
- 424.McMahon SB, Abel C. A model for the study of visceral pain states: Chronic inflammation of the chronic decerebrate rat urinary bladder by irritant chemicals. Pain. 1987;28:109–127. doi: 10.1016/0304-3959(87)91065-7. [DOI] [PubMed] [Google Scholar]
- 425.McMahon SB, Morrison JF. Spinal neurones with long projections activated from the abdominal viscera of the cat. J Physiol. 1982a;322:1–20. doi: 10.1113/jphysiol.1982.sp014018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.McMahon SB, Morrison JF. Two group of spinal interneurones that respond to stimulation of the abdominal viscera of the cat. J Physiol. 1982b;322:21–34. doi: 10.1113/jphysiol.1982.sp014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.McMahon SB, Spillane K. Brain stem influences on the parasympathetic supply to the urinary bladder of the cat. Brain Res. 1982;234:237–249. doi: 10.1016/0006-8993(82)90865-4. [DOI] [PubMed] [Google Scholar]
- 428.Miura A, Kawatani M, de Groat WC. Effects of pituitary adenylate cyclase activating polypeptide on lumbosacral preganglionic neurons in the neonatal rat spinal cord. Brain Res. 2001;895:223–232. doi: 10.1016/s0006-8993(01)02112-6. [DOI] [PubMed] [Google Scholar]
- 429.Miura A, Kawatani M, De Groat WC. Excitatory synaptic currents in lumbosacral parasympathetic preganglionic neurons evoked by stimulation of the dorsal commissure. J Neurophysiol. 2003;89:382–389. doi: 10.1152/jn.00180.2002. [DOI] [PubMed] [Google Scholar]
- 430.Miyawaki E, Lyons K, Pahwa R, Troster AI, Hubble J, Smith D, Busenbark K, McGuire D, Michalek D, Koller WC. Motor complications of chronic levodopa therapy in Parkinson’s disease. Clin Neuropharmacol. 1997;20:523–530. doi: 10.1097/00002826-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 431.Miyazato M, Kaiho Y, Kamo I, Chancellor MB, Sugaya K, de Groat WC, Yoshimura N. Effect of duloxetine, a norepinephrine and serotonin reuptake inhibitor, on sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol. 2008;295:F264–F271. doi: 10.1152/ajprenal.90241.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Miyazato M, Kaiho Y, Kamo I, Kitta T, Chancellor MB, Sugaya K, Arai Y, de Groat WC, Yoshimura N. Role of spinal serotonergic pathways in sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol. 2009;297:F1024–F1031. doi: 10.1152/ajprenal.00297.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Miyazato M, Sasatomi K, Hiragata S, Sugaya K, Chancellor MB, de Groat WC, Yoshimura N. GABA receptor activation in the lumbosacral spinal cord decreases detrusor overactivity in spinal cord injured rats. J Urol. 2008a;179:1178–1183. doi: 10.1016/j.juro.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Miyazato M, Sasatomi K, Hiragata S, Sugaya K, Chancellor MB, de Groat WC, Yoshimura N. Suppression of detrusor-sphincter dysynergia by GABA-receptor activation in the lumbosacral spinal cord in spinal cord-injured rats. Am J Physiol Regul Integr Comp Physiol. 2008b;295:R336–R342. doi: 10.1152/ajpregu.90315.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Miyazato M, Sasatomi K, Hiragata S, Sugaya K, Chancellor MB, de Groat WC, Yoshimura N. GABA-receptor activation in the lumbosacral spinal cord reduces detrusor overactivity in spinal cord injured rats. J Urol. 2008;179:1178–1183. doi: 10.1016/j.juro.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Miyazato M, Sugaya K, Goins WF, Wolfe D, Goss JR, Chancellor MB, de Groat WC, Glorioso JC, Yoshimura N. Herpes simplex virus vector-mediated gene delivery of glutamic acid decarboxylase reduces detrusor overactivity in spinal cord-injured rats. Gene Ther. 2009;16:660–668. doi: 10.1038/gt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Miyazato M, Sugaya K, Nishijima S, Ashitomi K, Hatano T, Ogawa Y. Inhibitory effect of intrathecal glycine on the micturition reflex in normal and spinal cord injury rats. Exp Neurol. 2003;183:232–240. doi: 10.1016/s0014-4886(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 438.Miyazato M, Sugaya K, Nishijima S, Ashitomi K, Morozumi M, Ogawa Y. Dietary glycine inhibits bladder activity in normal rats and rats with spinal cord injury. J Urol. 2005;173:314–317. doi: 10.1097/01.ju.0000141579.91638.a3. [DOI] [PubMed] [Google Scholar]
- 439.Miyazato M, Sugaya K, Saito S, Chancellor MB, Goins WF, Goss JR, de Groat WC, Glorioso JC, Yoshimura N. Suppression of detrusorsphincter dyssynergia by herpes simplex virus vector mediated gene delivery of glutamic acid decarboxylase in spinal cord injured rats. J Urol. 2010;184:1204–1210. doi: 10.1016/j.juro.2010.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem. 2009;284:21257–21264. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Mohammed H, Hannibal J, Fahrenkrug J, Santer R. Distribution and regional variation of pituitary adenylate cyclase activating polypeptide and other neuropeptides in the rat urinary bladder and ureter: Effects of age. Urol Res. 2002;30:248–255. doi: 10.1007/s00240-002-0261-6. [DOI] [PubMed] [Google Scholar]
- 442.Mohammed HA, Santer RM. Distribution and changes with age of calcitonin gene-related peptide- and substance P-immunoreactive nerves of the rat urinary bladder and lumbosacral sensory neurons. Eur J Morphol. 2002;40:293–301. doi: 10.1076/ejom.40.5.293.28900. [DOI] [PubMed] [Google Scholar]
- 443.Morgan C, de Groat WC, Nadelhaft I. The spinal distribution of sympathetic preganglionic and visceral primary afferent neurons that send axons into the hypogastric nerves of the cat. J Comp Neurol. 1986;243:23–40. doi: 10.1002/cne.902430104. [DOI] [PubMed] [Google Scholar]
- 444.Morgan C, Nadelhaft I, de Groat WC. Location of bladder preganglionic neurons within the sacral parasympathetic nucleus of the cat. Neurosci Lett. 1979;14:189–194. doi: 10.1016/0304-3940(79)96146-9. [DOI] [PubMed] [Google Scholar]
- 445.Morgan C, Nadelhaft I, de Groat WC. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol. 1981;201:415–440. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- 446.Morgan CW, de Groat WC, Felkins LA, Zhang SJ. Axon collaterals indicate broad intraspinal role for sacral preganglionic neurons. Proc Natl Acad Sci U S A. 1991;88:6888–6892. doi: 10.1073/pnas.88.15.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Morgan CW, de Groat WC, Felkins LA, Zhang SJ. Intracellular injection of neurobiotin or horseradish peroxidase reveals separate types of preganglionic neurons in the sacral parasympathetic nucleus of the cat. J Comp Neurol. 1993;331:161–182. doi: 10.1002/cne.903310203. [DOI] [PubMed] [Google Scholar]
- 448.Morrison JF. The physiological mechanisms involved in bladder emptying. Scand J Urol Nephrol Suppl. 1997;184:15–18. [PubMed] [Google Scholar]
- 449.Morrison JF, Birder L, Craggs M. Neural control. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Plymouth, UK: Health Publication Ltd; 2005. pp. 363–422. [Google Scholar]
- 450.Morrison JF, Spillane K. Neuropharmacological studies on decending inhibitory controls over the micturition reflex. J Auton Nerv Syst. 1986;(Suppl):393–397. [Google Scholar]
- 451.Morrison JF, Steers W, Brading AF. 2nd International Consultation on Incontinence. World Health Orgainization; 2002. Neurophysiology and Neuropharmacology; p. 83. [Google Scholar]
- 452.Mukerji G, Yiangou Y, Agarwal SK, Anand P. Transient receptor potential vanilloid receptor subtype 1 in painful bladder syndrome and its correlation with pain. J Urol. 2006;176:797–801. doi: 10.1016/j.juro.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 453.Murray E, Malley SE, Qiao LY, Hu VY, Vizzard MA. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J Urol. 2004;172:2434–2439. doi: 10.1097/01.ju.0000143549.29867.4e. [DOI] [PubMed] [Google Scholar]
- 454.Mutoh S, Ueda S, Fukumoto Y, Machida J, Ikegami K. Effect of adrenergic and cholinergic drugs on the noradrenergic transmission in bladder neck smooth muscle. J Urol. 1987;138:212–215. doi: 10.1016/s0022-5347(17)43047-3. [DOI] [PubMed] [Google Scholar]
- 455.Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: A horseradish peroxidase study. J Comp Neurol. 1984;226:238–245. doi: 10.1002/cne.902260207. [DOI] [PubMed] [Google Scholar]
- 456.Nadelhaft I, de Groat WC, Morgan C. Location and morphology of parasympathetic preganglionic neurons in the sacral spinal cord of the cat revealed by retrograde axonal transport of horseradish peroxidase. J Comp Neurol. 1980;193:265–281. doi: 10.1002/cne.901930118. [DOI] [PubMed] [Google Scholar]
- 457.Nadelhaft I, McKenna KE. Sexual dimorphism in sympathetic preganglionic neurons of the rat hypogastric nerve. J Comp Neurol. 1987;256:308–315. doi: 10.1002/cne.902560210. [DOI] [PubMed] [Google Scholar]
- 458.Nadelhaft I, Roppolo J, Morgan C, de Groat WC. Parasympathetic preganglionic neurons and visceral primary afferents in monkey sacral spinal cord revealed following application of horseradish peroxidase to pelvic nerve. J Comp Neurol. 1983;216:36–52. doi: 10.1002/cne.902160105. [DOI] [PubMed] [Google Scholar]
- 459.Nadelhaft I, Vera PL. Reduced urinary bladder afferent conduction velocities in streptozocin diabetic rats. Neurosci Lett. 1992;135:276–278. doi: 10.1016/0304-3940(92)90455-g. [DOI] [PubMed] [Google Scholar]
- 460.Nadelhaft I, Vera PL. Central nervous system neurons infected by pseudorabies virus injected into the rat urinary bladder following unilateral transection of the pelvic nerve. J Comp Neurol. 1995;359:443–456. doi: 10.1002/cne.903590307. [DOI] [PubMed] [Google Scholar]
- 461.Nadelhaft I, Vera PL. Neurons in the rat brain and spinal cord labeled after pseudorabies virus injected into the external urethral sphincter. J Comp Neurol. 1996;375:502–517. doi: 10.1002/(SICI)1096-9861(19961118)375:3<502::AID-CNE11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 462.Nadelhaft I, Vera PL. Separate urinary bladder and external urethral sphincter neurons in the central nervous system of the rat: Simultaneous labeling with two immunohistochemically distinguishable pseudorabies viruses. Brain Res. 2001;903:33–44. doi: 10.1016/s0006-8993(01)02349-6. [DOI] [PubMed] [Google Scholar]
- 463.Nadelhaft I, Vera PL, Card JP, Miselis RR. Central nervous system neurons labelled following the injection of pseudorabies virus into the rat urinary bladder. Neurosci Lett. 1992;143:271–274. doi: 10.1016/0304-3940(92)90281-b. [DOI] [PubMed] [Google Scholar]
- 464.Nagasaka Y, Yokoyama O, Komatsu K, Ishiura Y, Nakamura Y, Namiki M. Effects of opioid subtypes on detrusor overactivity in rats with cerebral infarction. Int J Urol. 2007;14:226–231. doi: 10.1111/j.1442-2042.2007.01700.x. discussion 232. [DOI] [PubMed] [Google Scholar]
- 465.Naka H, Nishijima S, Kadekawa K, Sugaya K, Saito S. Influence of glutamatergic projections to the rostral pontine reticular formation on micturition in rats. Life Sci. 2009;85:732–736. doi: 10.1016/j.lfs.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 466.Nakamura T, Yoshimura M, Shinnick-Gallagher P, Gallagher JP, Akasu T. Alpha 2 and alpha 1-adrenoceptors mediate opposing actions on parasympathetic neurons. Brain Res. 1984;323:349–353. doi: 10.1016/0006-8993(84)90312-3. [DOI] [PubMed] [Google Scholar]
- 467.Nakamura Y, Ishiura Y, Yokoyama O, Namiki M, De Groat WC. Role of protein kinase C in central muscarinic inhibitory mechanisms regulating voiding in rats. Neuroscience. 2003;116:477–484. doi: 10.1016/s0306-4522(02)00658-9. [DOI] [PubMed] [Google Scholar]
- 468.Nardos R, Gregory WT, Krisky C, Newell A, Nardos B, Schlaggar B, Fair DA. Examining mechanisms of brain control of bladder function with resting state functional connectivity MRI. Neurourol Urodyn. 2013;33:493–501. doi: 10.1002/nau.22458. [DOI] [PubMed] [Google Scholar]
- 469.Naughton MJ, Wyman JF. Quality of life in geriatric patients with lower urinary tract dysfunction. Am J Med Sci. 1997;314:219–227. doi: 10.1097/00000441-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 470.Nishiguchi J, Hayashi Y, Chancellor MB, de Miguel F, de Groat WC, Kumon H, Yoshimura N. Detrusor overactivity induced by intravesical application of adenosine 5′-triphosphate under different delivery conditions in rats. Urology. 2005;66:1332–1337. doi: 10.1016/j.urology.2005.06.099. [DOI] [PubMed] [Google Scholar]
- 471.Nishijima S, Sugaya K, Kadekawa K, Ashitomi K, Yamamoto H. Effect of chemical stimulation of the medial frontal lobe on the micturition reflex in rats. J Urol. 2012;187:1116–1120. doi: 10.1016/j.juro.2011.10.128. [DOI] [PubMed] [Google Scholar]
- 472.Nishijima S, Sugaya K, Miyazato M, Shimabukuro S, Morozumi M, Ogawa Y. Activation of the rostral pontine reticular formation increases the spinal glycine level and inhibits bladder contraction in rats. J Urol. 2005;173:1812–1816. doi: 10.1097/01.ju.0000154646.11570.65. [DOI] [PubMed] [Google Scholar]
- 473.Nishimoto T, Latifpour J, Wheeler MA, Yoshida M, Weiss RM. Age-dependent alterations in beta-adrenergic responsiveness of rat detrusor smooth muscle. J Urol. 1995;153:1701–1705. [PubMed] [Google Scholar]
- 474.Nishimura T, Akasu T. Role of the sodium pump in regulating the excitability of neurons in the vesical parasympathetic ganglia of the rabbit. Kurume Med J. 1988;35:221–224. doi: 10.2739/kurumemedj.35.221. [DOI] [PubMed] [Google Scholar]
- 475.Nishimura T, Tokimasa T. Purinergic cation channels in neurons of rabbit vesical parasympathetic ganglia. Neurosci Lett. 1996;212:215–217. doi: 10.1016/0304-3940(96)12805-6. [DOI] [PubMed] [Google Scholar]
- 476.Nishizawa O, Sugaya K, Noto H, Harada T, Tsuchida S. Pontine urine storage center in the dog. Tohoku J Exp Med. 1987;153:77–78. doi: 10.1620/tjem.153.77. [DOI] [PubMed] [Google Scholar]
- 477.Nishizawa O, Sugaya K, Shimoda N. Pontine and spinal modulation of the micturition reflex. Scand J Urol Nephrol Suppl. 1995;175:15–19. [PubMed] [Google Scholar]
- 478.Noto H, Roppolo JR, de Groat WC, Nishizawa O, Sugaya K, Tsuchida S. Opioid modulation of the micturition reflex at the level of the pontine micturition center. Urol Int. 1991;47(Suppl 1):19–22. doi: 10.1159/000282243. [DOI] [PubMed] [Google Scholar]
- 479.Noto H, Roppolo JR, Steers WD, de Groat WC. Excitatory and inhibitory influences on bladder activity elicited by electrical stimulation in the pontine micturition center in the rat. Brain Res. 1989;492:99–115. doi: 10.1016/0006-8993(89)90893-7. [DOI] [PubMed] [Google Scholar]
- 480.Noto H, Roppolo JR, Steers WD, de Groat WC. Electrophysiological analysis of the ascending and descending components of the micturition reflex pathway in the rat. Brain Res. 1991;549:95–105. doi: 10.1016/0006-8993(91)90604-t. [DOI] [PubMed] [Google Scholar]
- 481.Nour S, Svarer C, Kristensen JK, Paulson OB, Law I. Cerebral activation during micturition in normal men. Brain. 2000;123:781–789. doi: 10.1093/brain/123.4.781. [DOI] [PubMed] [Google Scholar]
- 482.Numata A, Iwata T, Iuchi H, Taniguchi N, Kita M, Wada N, Kato Y, Kakizaki H. Micturition-suppressing region in the periaqueductal gray of the mesencephalon of the cat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1996–R2000. doi: 10.1152/ajpregu.00393.2006. [DOI] [PubMed] [Google Scholar]
- 483.Nuotio M, Jylha M, Luukkaala T, Tammela TL. Urgency, urge incontinence and voiding symptoms in men and women aged 70 years and over. BJU Int. 2002;89:350–355. doi: 10.1046/j.1464-4096.2001.01684.x. [DOI] [PubMed] [Google Scholar]
- 484.O’Donnell PD, Brookover T, Hewett M, al-Juburi AZ. Continence level following radical prostatectomy. Urology. 1990;36:511–512. doi: 10.1016/0090-4295(90)80189-t. [DOI] [PubMed] [Google Scholar]
- 485.O’Reilly BA, Kosaka AH, Chang TK, Ford AP, Popert R, McMahon SB. A quantitative analysis of purinoceptor expression in the bladders of patients with symptomatic outlet obstruction. BJU Int. 2001;87:617–622. doi: 10.1046/j.1464-410x.2001.02179.x. [DOI] [PubMed] [Google Scholar]
- 486.Ochodnicky P, Cruz CD, Yoshimura N, Cruz F. Neurotrophins as regulators of urinary bladder function. Nat Rev Urol. 2012;9:628–637. doi: 10.1038/nrurol.2012.178. [DOI] [PubMed] [Google Scholar]
- 487.Ogawa T, Kamo I, Pflug BR, Nelson JB, Seki S, Igawa Y, Nishizawa O, de Groat WC, Chancellor MB, Yoshimura N. Differential roles of peripheral and spinal endothelin receptors in the micturition reflex in rats. J Urol. 2004;172:1533–1537. doi: 10.1097/01.ju.0000139540.56916.0e. [DOI] [PubMed] [Google Scholar]
- 488.Ogawa T, Seki S, Masuda H, Igawa Y, Nishizawa O, Kuno S, Chancellor MB, de Groat WC, Yoshimura N. Dopaminergic mechanisms controlling urethral function in rats. Neurourol Urodyn. 2006;25:480–489. doi: 10.1002/nau.20260. [DOI] [PubMed] [Google Scholar]
- 489.Ohtsuka M, Mori J, Tsujioka K, Kumada S. Pharmacological study on sympathetic inhibition of the urinary bladder in dogs. Jpn J Pharmacol. 1980;30:187–198. doi: 10.1254/jjp.30.187. [DOI] [PubMed] [Google Scholar]
- 490.Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol. 1999;161:438–441. discussion 441–432. [PubMed] [Google Scholar]
- 491.Owman C, Alm P, Sjöberg NO. Pelvic autonomic ganglia: Structure, transmitters, function and steroid influenc. In: Elfvin L-G, editor. Autonomic Ganglia. New York: John Wiley & Sons Ltd; 1983. pp. 125–143. [Google Scholar]
- 492.Ozawa H, Chancellor MB, Jung SY, Yokoyama T, Fraser MO, Yu Y, de Groat WC, Yoshimura N. Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J Urol. 1999;162:2211–2216. doi: 10.1016/S0022-5347(05)68161-X. [DOI] [PubMed] [Google Scholar]
- 493.Page ME, Valentino RJ. Locus coeruleus activation by physiological challenges. Brain Res Bull. 1994;35:557–560. doi: 10.1016/0361-9230(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 494.Palea S, Artibani W, Ostardo E, Trist DG, Pietra C. Evidence for purinergic neurotransmission in human urinary bladder affected by interstitial cystitis. J Urol. 1993;150:2007–2012. doi: 10.1016/s0022-5347(17)35955-4. [DOI] [PubMed] [Google Scholar]
- 495.Pandita RK, Mizusawa H, Andersson KE. Intravesical oxyhemoglobin initiates bladder overactivity in conscious, normal rats. J Urol. 2000;164:545–550. [PubMed] [Google Scholar]
- 496.Pandita RK, Pehrson R, Christoph T, Friderichs E, Andersson KE. Actions of tramadol on micturition in awake, freely moving rats. Br J Pharmacol. 2003;139:741–748. doi: 10.1038/sj.bjp.0705297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Pavcovich LA, Yang M, Miselis RR, Valentino RJ. Novel role for the pontine micturition center, Barrington’s nucleus: Evidence for coordination of colonic and forebrain activity. Brain Res. 1998;784:355–361. doi: 10.1016/s0006-8993(97)01178-5. [DOI] [PubMed] [Google Scholar]
- 498.Pavlakis AJ, Siroky MB, Goldstein I, Krane RJ. Neurologic findings in Parkinson’s disease. J Urol. 1983;129:80–83. doi: 10.1016/s0022-5347(17)51929-1. [DOI] [PubMed] [Google Scholar]
- 499.Payne CK, Mosbaugh PG, Forrest JB, Evans RJ, Whitmore KE, Antoci JP, Perez-Marrero R, Jacoby K, Diokno AC, O’Reilly KJ, Griebling TL, Vasavada SP, Yu AS, Frumkin LR. Intravesical resiniferatoxin for the treatment of interstitial cystitis: A randomized, double-blind, placebo controlled trial. J Urol. 2005;173:1590–1594. doi: 10.1097/01.ju.0000154631.92150.ef. [DOI] [PubMed] [Google Scholar]
- 500.Pehrson R, Andersson KE. Effects of tiagabine, a gamma-aminobutyric acid re-uptake inhibitor, on normal rat bladder function. J Urol. 2002;167:2241–2246. [PubMed] [Google Scholar]
- 501.Pehrson R, Lehmann A, Andersson KE. Effects of gammaaminobutyrate B receptor modulation on normal micturition and oxyhemoglobin induced detrusor overactivity in female rats. J Urol. 2002;168:2700–2705. doi: 10.1016/S0022-5347(05)64247-4. [DOI] [PubMed] [Google Scholar]
- 502.Pehrson R, Ojteg G, Ishizuka O, Andersson KE. Effects of NAD-299, a new, highly selective 5-HT1A receptor antagonist, on bladder function in rats. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:528–536. doi: 10.1007/s00210-002-0650-y. [DOI] [PubMed] [Google Scholar]
- 503.Persson K, Steers WD, Tuttle JB. Regulation of nerve growth factor secretion in smooth muscle cells cultured from rat bladder body, base and urethra. J Urol. 1997;157:2000–2006. [PubMed] [Google Scholar]
- 504.Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: Implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953–1964. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 505.Pfisterer MH, Griffiths DJ, Rosenberg L, Schaefer W, Resnick NM. The impact of detrusor overactivity on bladder function in younger and older women. J Urol. 2006;175:1777–1783. doi: 10.1016/S0022-5347(05)00985-7. discussion 1783. [DOI] [PubMed] [Google Scholar]
- 506.Pfisterer MH, Griffiths DJ, Schaefer W, Resnick NM. The effect of age on lower urinary tract function: A study in women. J Am Geriatr Soc. 2006;54:405–412. doi: 10.1111/j.1532-5415.2005.00613.x. [DOI] [PubMed] [Google Scholar]
- 507.Pierce LM, Reyes M, Thor KB, Dolber PC, Bremer RE, Kuehl TJ, Coates KW. Innervation of the levator ani muscles in the female squirrel monkey. Am J Obstet Gynecol. 2003;188:1141–1147. doi: 10.1067/mob.2003.329. [DOI] [PubMed] [Google Scholar]
- 508.Pinna A, Wardas J, Simola N, Morelli M. New therapies for the treatment of Parkinson’s disease: Adenosine A2A receptor antagonists. Life Sci. 2005;77:3259–3267. doi: 10.1016/j.lfs.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 509.Pinto R, Frias B, Allen S, Dawbarn D, McMahon SB, Cruz F, Cruz CD. Sequestration of brain derived nerve factor by intravenous delivery of TrkB-Ig2 reduces bladder overactivity and noxious input in animals with chronic cystitis. Neuroscience. 2010;166:907–916. doi: 10.1016/j.neuroscience.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 510.Purinton PT, Fletcher TF, Bradley WE. Innervation of pelvic viscera in the rat. Evoked potentials in nerves to bladder and penis (clitoris) Invest Urol. 1976;14:28–32. [PubMed] [Google Scholar]
- 511.Qiao L, Vizzard MA. Up-regulation of tyrosine kinase (Trka, Trkb) receptor expression and phosphorylation in lumbosacral dorsal root ganglia after chronic spinal cord (T8-T10) injury. J Comp Neurol. 2002;449:217–230. doi: 10.1002/cne.10283. [DOI] [PubMed] [Google Scholar]
- 512.Qiao LY, Vizzard MA. Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J Comp Neurol. 2002;454:200–211. doi: 10.1002/cne.10447. [DOI] [PubMed] [Google Scholar]
- 513.Qin C, Malykhina AP, Akbarali HI, Foreman RD. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology. 2005;129:1967–1978. doi: 10.1053/j.gastro.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 514.Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- 515.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 516.Ramage AG, Wyllie MG. A comparison of the effects of doxazosin and terazosin on the spontaneous sympathetic drive to the bladder and related organs in anaesthetized cats. Eur J Pharmacol. 1995;294:645–650. doi: 10.1016/0014-2999(95)00599-4. [DOI] [PubMed] [Google Scholar]
- 517.Ranson RN, Dodds AL, Smith MJ, Santer RM, Watson AH. Age-associated changes in the monoaminergic innervation of rat lumbosacral spinal cord. Brain Res. 2003;972:149–158. doi: 10.1016/s0006-8993(03)02521-6. [DOI] [PubMed] [Google Scholar]
- 518.Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med. 2000;342:1484–1491. doi: 10.1056/NEJM200005183422004. [DOI] [PubMed] [Google Scholar]
- 519.Resnick NM. Urinary incontinence. Lancet. 1995;346:94–99. doi: 10.1016/s0140-6736(95)92117-6. [DOI] [PubMed] [Google Scholar]
- 520.Ritter AM, Martin WJ, Thorneloe KS. The voltage-gated sodium channel Nav1.9 is required for inflammation-based urinary bladder dysfunction. Neurosci Lett. 2009;452:28–32. doi: 10.1016/j.neulet.2008.12.051. [DOI] [PubMed] [Google Scholar]
- 521.Rocha I, Burnstock G, Spyer KM. Effect on urinary bladder function and arterial blood pressure of the activation of putative purine receptors in brainstem areas. Auton Neurosci. 2001;88:6–15. doi: 10.1016/S1566-0702(00)00284-8. [DOI] [PubMed] [Google Scholar]
- 522.Rogers H, Kennedy C, Henderson G. Characterization of the neurons of the mouse hypogastric ganglion: Morphology and electrophysiology. J Auton Nerv Syst. 1990;29:255–270. doi: 10.1016/0165-1838(90)90152-9. [DOI] [PubMed] [Google Scholar]
- 523.Rong W, Spyer KM, Burnstock G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol. 2002;541:591–600. doi: 10.1113/jphysiol.2001.013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Roppolo JR, Nadelhaft I, de Groat WC. The organization of pudendal motoneurons and primary afferent projections in the spinal cord of the rhesus monkey revealed by horseradish peroxidase. J Comp Neurol. 1985;234:475–488. doi: 10.1002/cne.902340406. [DOI] [PubMed] [Google Scholar]
- 525.Rouzade-Dominguez ML, Curtis AL, Valentino RJ. Role of Barrington’s nucleus in the activation of rat locus coeruleus neurons by colonic distension. Brain Res. 2001;917:206–218. doi: 10.1016/s0006-8993(01)02917-1. [DOI] [PubMed] [Google Scholar]
- 526.Rouzade-Dominguez ML, Miselis R, Valentino RJ. Central representation of bladder and colon revealed by dual transsynaptic tracing in the rat: Substrates for pelvic visceral coordination. Eur J Neurosci. 2003;18:3311–3324. doi: 10.1111/j.1460-9568.2003.03071.x. [DOI] [PubMed] [Google Scholar]
- 527.Rouzade-Dominguez ML, Pernar L, Beck S, Valentino RJ. Convergent responses of Barrington’s nucleus neurons to pelvic visceral stimuli in the rat: A juxtacellular labelling study. Eur J Neurosci. 2003;18:3325–3334. doi: 10.1111/j.1460-9568.2003.03072.x. [DOI] [PubMed] [Google Scholar]
- 528.Ruan HZ, Birder LA, Xiang Z, Chopra B, Buffington T, Tai C, Roppolo JR, de Groat WC, Burnstock G. Expression of P2X and P2Y receptors in the intramural parasympathetic ganglia of the cat urinary bladder. Am J Physiol Renal Physiol. 2006;290:F1143–F1152. doi: 10.1152/ajprenal.00333.2005. [DOI] [PubMed] [Google Scholar]
- 529.Ruch TC, Tang PC. Localization of brain stem and diencephalic areas controlling the micturation reflex. J Comp Neurol. 1956;106:213–245. doi: 10.1002/cne.901060108. [DOI] [PubMed] [Google Scholar]
- 530.Saban R, Undem BJ, Keith IM, Saban MR, Tengowski MW, Graziano FM, Bjorling DE. Differential release of prostaglandins and leukotrienes by sensitized guinea pig urinary bladder layers upon antigen challenge. J Urol. 1994;152:544–549. doi: 10.1016/s0022-5347(17)32790-8. [DOI] [PubMed] [Google Scholar]
- 531.Saito M, Gotoh M, Kato K, Kondo A. Influence of aging on the rat urinary bladder function. Urol Int. 1991;47:39–42. doi: 10.1159/000282247. [DOI] [PubMed] [Google Scholar]
- 532.Saito M, Kondo A, Gotoh M, Kato K, Levin RM. Age-related changes in the response of the rat urinary bladder to neurotransmitters. Neurourol Urodyn. 1993;12:191–200. doi: 10.1002/nau.1930120214. [DOI] [PubMed] [Google Scholar]
- 533.Sakaibara R, Uchiyama T, Kuwabara S, Kawaguchi N, Nemoto I, Nakata M, Hattori H. Autonomic dysreflexia due to neurogenic bladder dysfunction; an unusual presentation of spinal cord sarcoidosis. J Neurol Neurosurg Psychiatry. 2001;71:819–820. doi: 10.1136/jnnp.71.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Sakakibara R, Fowler CJ, Hattori T. Voiding and MRI analysis of the brain. [Review] (47 refs] Int Urogynecol J. 1999;10:192–199. doi: 10.1007/s001920050044. [DOI] [PubMed] [Google Scholar]
- 535.Sakakibara R, Hattori T, Uchiyama T, Yamanishi T. Urinary function in elderly people with and without leukoaraiosis: Relation to cognitive and gait function. J Neurol Neurosurg Psychiatry. 1999;67:658–660. doi: 10.1136/jnnp.67.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Sakakibara R, Nakazawa K, Shiba K, Nakajima Y, Uchiyama T, Yoshiyama M, Yamanishi T, Hattori T. Firing patterns of micturitionrelated neurons in the pontine storage centre in cats. Auton Neurosci. 2002;99:24–30. doi: 10.1016/s1566-0702(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 537.Sakakibara R, Nakazawa K, Uchiyama T, Yoshiyama M, Yamanishi T, Hattori T. Micturition-related electrophysiological properties in the substantia nigra pars compacta and the ventral tegmental area in cats. Auton Neurosci. 2002;102:30–38. doi: 10.1016/s1566-0702(02)00180-7. [DOI] [PubMed] [Google Scholar]
- 538.Sakakibara R, Shinotoh H, Uchiyama T, Yoshiyama M, Hattori T, Yamanishi T. SPECT imaging of the dopamine transporter with [(123)I]-beta-CIT reveals marked decline of nigrostriatal dopaminergic function in Parkinson’s disease with urinary dysfunction. J Neurol Sci. 2001;187:55–59. doi: 10.1016/s0022-510x(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 539.Santer RM, Dering MA, Ranson RN, Waboso HN, Watson AH. Differential susceptibility to ageing of rat preganglionic neurones projecting to the major pelvic ganglion and of their afferent inputs. Auton Neurosci. 2002;96:73–81. doi: 10.1016/s1566-0702(01)00366-6. [DOI] [PubMed] [Google Scholar]
- 540.Santos-Silva A, Charrua A, Cruz CD, Gharat L, Avelino A, Cruz F. Rat detrusor overactivity induced by chronic spinalization can be abolished by a transient receptor potential vanilloid 1 (TRPV1) antagonist. Auton Neurosci. 2012;166:35–38. doi: 10.1016/j.autneu.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 541.Sasaki K, Chancellor MB, Goins WF, Phelan MW, Glorioso JC, de Groat WC, Yoshimura N. Gene therapy using replication-defective herpes simplex virus vectors expressing nerve growth factor in a rat model of diabetic cystopathy. Diabetes. 2004;53:2723–2730. doi: 10.2337/diabetes.53.10.2723. [DOI] [PubMed] [Google Scholar]
- 542.Sasaki K, Chancellor MB, Phelan MW, Yokoyama T, Fraser MO, Seki S, Kubo K, Kumon H, Groat WC, Yoshimura N. Diabetic cystopathy correlates with a long-term decrease in nerve growth factor levels in the bladder and lumbosacral dorsal root Ganglia. J Urol. 2002;168:1259–1264. doi: 10.1016/S0022-5347(05)64636-8. [DOI] [PubMed] [Google Scholar]
- 543.Sasaki M. Bladder contractility-related neurons in Barrington’s nucleus: Axonal projections to the spinal cord in the cat. J Comp Neurol. 2002;449:355–363. doi: 10.1002/cne.10290. [DOI] [PubMed] [Google Scholar]
- 544.Sasaki M. Feed-forward and feedback regulation of bladder contractility by Barrington’s nucleus in cats. J Physiol. 2004;557:287–305. doi: 10.1113/jphysiol.2003.059352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Sasaki M. Properties of Barrington’s neurones in cats: Units that fire inversely with micturition contraction. Brain Res. 2005;1033:41–50. doi: 10.1016/j.brainres.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 546.Sasaki M. Role of Barrington’s nucleus in micturition. J Comp Neurol. 2005;493:21–26. doi: 10.1002/cne.20719. [DOI] [PubMed] [Google Scholar]
- 547.Sasaki M, Sato H. Polysynaptic connections between Barrington’s nucleus and sacral preganglionic neurons. Neurosci Res. 2013;75:150–156. doi: 10.1016/j.neures.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 548.Satchell P, Vaughan C. Bladder wall tension and mechanoreceptor discharge. Pflugers Arch. 1994;426:304–309. doi: 10.1007/BF00374786. [DOI] [PubMed] [Google Scholar]
- 549.Saum WR, de Groat WC. Parasympathetic ganglia: Activation of an adrenergic inhibitory mechanism by cholinomimetic agents. Science. 1972;175:659–661. doi: 10.1126/science.175.4022.659. [DOI] [PubMed] [Google Scholar]
- 550.Schneider T, Hein P, Michel-Reher MB, Michel MC. Effects of ageing on muscarinic receptor subtypes and function in rat urinary bladder. Naunyn Schmiedebergs Arch Pharmacol. 2005;372:71–78. doi: 10.1007/s00210-005-1084-0. [DOI] [PubMed] [Google Scholar]
- 551.Schrum A, Wolff S, van der Horst C, Kuhtz-Buschbeck JP. Motor cortical representation of the pelvic floor muscles. J Urol. 2011;186:185–190. doi: 10.1016/j.juro.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 552.Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29:647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 553.Sculptoreanu A, Artim DE, de Groat WC. Neurokinins inhibit low threshold inactivating K +currents in capsaicin responsive DRG neurons. Exp Neurol. 2009;219:562–573. doi: 10.1016/j.expneurol.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Sculptoreanu A, de Groat WC, Buffington CA, Birder LA. Abnormal excitability in capsaicin-responsive DRG neurons from cats with feline interstitial cystitis. Exp Neurol. 2005a;193:437–443. doi: 10.1016/j.expneurol.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 555.Sculptoreanu A, de Groat WC, Buffington CA, Birder LA. Protein kinase C contributes to abnormal capsaicin responses in DRG neurons from cats with feline interstitial cystitis. Neurosci Lett. 2005b;381:42–46. doi: 10.1016/j.neulet.2005.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Sculptoreanu A, Yoshimura N, de Groat WC. KW-7158 [(2S)-(+)-3,3,3-trifluoro-2-hydroxy-2-methyl-N-(5,5,10-trioxo-4,10-dihydrothieno[3,2-c][1]benzothiepin-9-yl)propanamide] enhances A-type K+ currents in neurons of the dorsal root ganglion of the adult rat. J Pharmacol Exp Ther. 2004;310:159–168. doi: 10.1124/jpet.104.065409. [DOI] [PubMed] [Google Scholar]
- 557.Seki S, Erickson KA, Seki M, Nishizawa O, Igawa Y, Ogawa T, de Groat WC, Chancellor MB, Yoshimura N. Elimination of rat spinal neurons expressing neurokinin 1 receptors reduces bladder overactivity and spinal c-fos expression induced by bladder irritation. Am J Physiol Renal Physiol. 2005;288:F466–F473. doi: 10.1152/ajprenal.00274.2004. [DOI] [PubMed] [Google Scholar]
- 558.Seki S, Igawa Y, Kaidoh K, Ishizuka O, Nishizawa O, Andersson KE. Role of dopamine D1 and D2 receptors in the micturition reflex in conscious rats. Neurourol Urodyn. 2001;20:105–113. doi: 10.1002/1520-6777(2001)20:1<105::aid-nau12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 559.Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol. 2002;168:2269–2274. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- 560.Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J Urol. 2004;171:478–482. doi: 10.1097/01.ju.0000088340.26588.74. [DOI] [PubMed] [Google Scholar]
- 561.Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1994;72:2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- 562.Seseke S, Baudewig J, Kallenberg K, Ringert RH, Seseke F, Dechent P. Voluntary pelvic floor muscle control–an fMRI study. Neuroimage. 2006;31:1399–1407. doi: 10.1016/j.neuroimage.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 563.Seseke S, Baudewig J, Ringert RH, Rebmann U, Dechent P. Monitoring brain activation changes in the early postoperative period after radical prostatectomy using fMRI. Neuroimage. 2013;78:1–6. doi: 10.1016/j.neuroimage.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 564.Shefchyk SJ. Sacral spinal interneurones and the control of urinary bladder and urethral striated sphincter muscle function. J Physiol. 2001;533:57–63. doi: 10.1111/j.1469-7793.2001.0057b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Shen A, Mitchelson F. Characterisation of the prejunctional inhibitory muscarinic receptor on cholinergic nerves in the rat urinary bladder. Eur J Pharmacol. 2001;413:179–187. doi: 10.1016/s0014-2999(01)00746-4. [DOI] [PubMed] [Google Scholar]
- 566.Shibata T, Watanabe M, Ichikawa R, Inoue Y, Koyanagi T. Different expressions of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid and N-methyl-D-aspartate receptor subunit mRNAs between visceromotor and somatomotor neurons of the rat lumbosacral spinal cord. J Comp Neurol. 1999;404:172–182. doi: 10.1002/(sici)1096-9861(19990208)404:2<172::aid-cne3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 567.Shinnick-Gallagher P, Gallagher JP, Griffith WH. Inhibition in parasympathetic ganglia. In: Karczmar AG, Koketsu K, Nishi S, editors. Autonomic and Enteric Ganglia. New York: Plenum Press; 1986. pp. 353–367. [Google Scholar]
- 568.Shinnick-Gallagher P, Hirai K, Gallagher JP. Muscarinic receptor activation underlying the slow inhibitory postsynaptic potential (s-I.P.S.P) and the slow excitatory postsynaptic potential (s-E.P.S.P) In: Dun NJ, Perlman RL, editors. Neurobiology of Acetylcholine. New York: Plenum Publishing Co; 1987. pp. 245–254. [Google Scholar]
- 569.Sillen U, Rubenson A, Hjalmas K. Central cholinergic mechanisms in L-DOPA induced hyperactive urinary bladder of the rat. Urol Res. 1982;10:239–243. doi: 10.1007/BF00255929. [DOI] [PubMed] [Google Scholar]
- 570.Simonds WF, Booth AM, Thor KB, Ostrowski NL, Nagel JR, de Groat WC. Parasympathetic ganglia: Naloxone antagonizes inhibition by leucine -enkephalin and GABA. Brain Res. 1983;271:365–370. doi: 10.1016/0006-8993(83)90303-7. [DOI] [PubMed] [Google Scholar]
- 571.Skultety FM. Relation to periaqueductal gray matter to stomach and bladder motility. Neurology. 1959;9:190–198. doi: 10.1212/wnl.9.3.190. [DOI] [PubMed] [Google Scholar]
- 572.Smet PJ, Edyvane KA, Jonavicius J, Marshall VR. Neuropeptides and neurotransmitter-synthesizing enzymes in intrinsic neurons of the human urinary bladder. J Neurocytol. 1996;25:112–124. doi: 10.1007/BF02284790. [DOI] [PubMed] [Google Scholar]
- 573.Smet PJ, Jonavicius J, Marshall VR, de Vente J. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71:337–348. doi: 10.1016/0306-4522(95)00453-x. [DOI] [PubMed] [Google Scholar]
- 574.Smith PP. Aging and the underactive detrusor: A failure of activity or activation? Neurourol Urodyn. 2010;29:408–412. doi: 10.1002/nau.20765. [DOI] [PubMed] [Google Scholar]
- 575.Smith PP, DeAngelis A, Kuchel GA. Detrusor expulsive strength is preserved, but responsiveness to bladder filling and urinary sensitivity is diminished in the aging mouse. Am J Physiol Regul Integr Comp Physiol. 2012;302:R577–R586. doi: 10.1152/ajpregu.00508.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Smith PR, Mackler SA, Weiser PC, Brooker DR, Ahn YJ, Harte BJ, McNulty KA, Kleyman TR. Expression and localization of epithelial sodium channel in mammalian urinary bladder. Am J Physiol. 1998;274:F91–F96. doi: 10.1152/ajprenal.1998.274.1.F91. [DOI] [PubMed] [Google Scholar]
- 577.Somogyi GT, de Groat WC. Modulation of the release of [3H]norepinephrine from the base and body of the rat urinary bladder by endogenous adrenergic and cholinergic mechanisms. J Pharmacol Exp Ther. 1990;255:204–210. [PubMed] [Google Scholar]
- 578.Somogyi GT, de Groat WC. Evidence for inhibitory nicotinic and facilitatory muscarinic receptors in cholinergic nerve terminals of the rat urinary bladder. J Auton Nerv Syst. 1992;37:89–97. doi: 10.1016/0165-1838(92)90237-b. [DOI] [PubMed] [Google Scholar]
- 579.Somogyi GT, de Groat WC. Function, signal transduction mechanisms and plasticity of presynaptic muscarinic receptors in the urinary bladder. Life Sci. 1999;64:411–418. doi: 10.1016/s0024-3205(98)00580-3. [DOI] [PubMed] [Google Scholar]
- 580.Somogyi GT, Tanowitz M, de Groat WC. M1 muscarinic receptormediated facilitation of acetylcholine release in the rat urinary bladder. J Physiol. 1994;480(Pt 1):81–89. doi: 10.1113/jphysiol.1994.sp020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Somogyi GT, Tanowitz M, de Groat WC. Prejunctional facilitatory alpha 1-adrenoceptors in the rat urinary bladder. Br J Pharmacol. 1995;114:1710–1716. doi: 10.1111/j.1476-5381.1995.tb14961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Somogyi GT, Tanowitz M, Zernova G, de Groat WC. M1 muscarinic receptor-induced facilitation of ACh and noradrenaline release in the rat bladder is mediated by protein kinase C. J Physiol. 1996;496:245–254. doi: 10.1113/jphysiol.1996.sp021681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Speakman MJ, Brading AF, Gilpin CJ, Dixon JS, Gilpin SA, Gosling JA. Bladder outflow obstruction–a cause of denervation supersensitivity. J Urol. 1987;138:1461–1466. doi: 10.1016/s0022-5347(17)43675-5. [DOI] [PubMed] [Google Scholar]
- 584.Steers WD, Ciambotti J, Erdman S, de Groat WC. Morphological plasticity in efferent pathways to the urinary bladder of the rat following urethral obstruction. J Neurosci. 1990;10:1943–1951. doi: 10.1523/JNEUROSCI.10-06-01943.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Steers WD, Ciambotti J, Etzel B, Erdman S, de Groat WC. Alterations in afferent pathways from the urinary bladder of the rat in response to partial urethral obstruction. J Comp Neurol. 1991;310:401–410. doi: 10.1002/cne.903100309. [DOI] [PubMed] [Google Scholar]
- 586.Steers WD, Creedon DJ, Tuttle JB. Immunity to nerve growth factor prevents afferent plasticity following urinary bladder hypertrophy. J Urol. 1996;155:379–385. [PubMed] [Google Scholar]
- 587.Steers WD, de Groat WC. Effect of bladder outlet obstruction on micturition reflex pathways in the rat. J Urol. 1988;140:864–871. doi: 10.1016/s0022-5347(17)41846-5. [DOI] [PubMed] [Google Scholar]
- 588.Steers WD, de Groat WC. Effects of m-chlorophenylpiperazine on penile and bladder function in rats. Am J Physiol. 1989;257:R1441–R1449. doi: 10.1152/ajpregu.1989.257.6.R1441. [DOI] [PubMed] [Google Scholar]
- 589.Steers WD, Kolbeck S, Creedon D, Tuttle JB. Nerve growth factor in the urinary bladder of the adult regulates neuronal form and function. J Clin Invest. 1991;88:1709–1715. doi: 10.1172/JCI115488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Stein RJ, Santos S, Nagatomi J, Hayashi Y, Minnery BS, Xavier M, Patel AS, Nelson JB, Futrell WJ, Yoshimura N, Chancellor MB, De Miguel F. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J Urol. 2004;172:1175–1178. doi: 10.1097/01.ju.0000134880.55119.cf. [DOI] [PubMed] [Google Scholar]
- 591.Steinbacher BC, Jr, Nadelhaft I. Increased levels of nerve growth factor in the urinary bladder and hypertrophy of dorsal root ganglion neurons in the diabetic rat. Brain Res. 1998;782:255–260. doi: 10.1016/s0006-8993(97)01287-0. [DOI] [PubMed] [Google Scholar]
- 592.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, Hunt TL, Wein AJ. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–336. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 593.Stone E, Coote JH, Allard J, Lovick TA. GABAergic control of micturition within the periaqueductal grey matter of the male rat. J Physiol. 2011;589:2065–2078. doi: 10.1113/jphysiol.2010.202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, Andersson KE, Hogestatt ED, Zygmunt PM. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol. 2008;53:391–399. doi: 10.1016/j.eururo.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 595.Studeny S, Torabi A, Vizzard MA. P2X2 and P2X3 receptor expression in postnatal and adult rat urinary bladder and lumbosacral spinal cord. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1155–R1168. doi: 10.1152/ajpregu.00234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Sugaya K, de Groat WC. Effects of MK-801 and CNQX, glutamate receptor antagonists, on bladder activity in neonatal rats. Brain Res. 1994;640:1–10. doi: 10.1016/0006-8993(94)91850-3. [DOI] [PubMed] [Google Scholar]
- 597.Sugaya K, de Groat WC. Micturition reflexes in the in vitro neonatal rat brain stem-spinal cord-bladder preparation. Am J Physiol. 1994;266:R658–R667. doi: 10.1152/ajpregu.1994.266.3.R658. [DOI] [PubMed] [Google Scholar]
- 598.Sugaya K, Mori S, Nishizawa O, Noto H, Tsuchida S. Chemical stimulation of the pontine micturition center and the nucleus reticularis pontis oralis. Neurourol Urodyn. 1987;6:143–144. [Google Scholar]
- 599.Sugaya K, Nishijima S, Kadekawa K, Ashitomi K, Ueda T, Yamamoto H. Intravenous or local injections of flavoxate in the rostral pontine reticular formation inhibit urinary frequency induced by activation of medial frontal lobe neurons in rats. J Urol. 2014;192:1278–1285. doi: 10.1016/j.juro.2014.04.092. [DOI] [PubMed] [Google Scholar]
- 600.Sugaya K, Nishijima S, Miyazato M, Ashitomi K, Hatano T, Ogawa Y. Effects of intrathecal injection of tamsulosin and naftopidil, alpha-1A and -1D adrenergic receptor antagonists, on bladder activity in rats. Neurosci Lett. 2002;328:74–76. doi: 10.1016/s0304-3940(02)00459-7. [DOI] [PubMed] [Google Scholar]
- 601.Sugaya K, Nishijima S, Miyazato M, Ogawa Y. Central nervous control of micturition and urine storage. J Smooth Muscle Res. 2005;41:117–132. doi: 10.1540/jsmr.41.117. [DOI] [PubMed] [Google Scholar]
- 602.Sugaya K, Ogawa Y, Hatano T, Koyama Y, Miyazato T, Oda M. Evidence for involvement of the subcoeruleus nucleus and nucleus raphe magnus in urine storage and penile erection in decerebrate rats. J Urol. 1998;159:2172–2176. doi: 10.1016/S0022-5347(01)63300-7. [DOI] [PubMed] [Google Scholar]
- 603.Sugaya K, Ogawa Y, Hatano T, Nishijima S, Matsuyama K, Mori S. Ascending and descending brainstem neuronal activity during cystometry in decerebrate cats. Neurourol Urodyn. 2003;22:343–350. doi: 10.1002/nau.10115. [DOI] [PubMed] [Google Scholar]
- 604.Sugaya K, Roppolo JR, Yoshimura N, Card JP, de Groat WC. The central neural pathways involved in micturition in the neonatal rat as revealed by the injection of pseudorabies virus into the urinary bladder. Neurosci Lett. 1997;223:197–200. doi: 10.1016/s0304-3940(97)13433-4. [DOI] [PubMed] [Google Scholar]
- 605.Swinn MJ, Fowler CJ. Isolated urinary retention in young women, or Fowler’s syndrome. Clin Auton Res. 2001;11:309–311. doi: 10.1007/BF02332976. [DOI] [PubMed] [Google Scholar]
- 606.Tabatabai M, Booth AM, de Groat WC. Morphological and electrophysiological properties of pelvic ganglion cells in the rat. Brain Res. 1986;382:61–70. doi: 10.1016/0006-8993(86)90111-3. [DOI] [PubMed] [Google Scholar]
- 607.Tadic SD, Griffiths D, Murrin A, Schaefer W, Aizenstein HJ, Resnick NM. Brain activity during bladder filling is related to white matter structural changes in older women with urinary incontinence. Neuroimage. 2010;51:1294–1302. doi: 10.1016/j.neuroimage.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Tadic SD, Griffiths D, Schaefer W, Cheng CI, Resnick NM. Brain activity measured by functional magnetic resonance imaging is related to patient reported urgency urinary incontinence severity. J Urol. 2010;183:221–228. doi: 10.1016/j.juro.2009.08.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Tadic SD, Griffiths D, Schaefer W, Murrin A, Clarkson B, Resnick NM. Brain activity underlying impaired continence control in older women with overactive bladder. Neurourol Urodyn. 2012;31:652–658. doi: 10.1002/nau.21240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Tadic SD, Griffiths D, Schaefer W, Resnick NM. Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. Neuroimage. 2008;39:1647–1653. doi: 10.1016/j.neuroimage.2007.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Tadic SD, Tannenbaum C, Resnick NM, Griffiths D. Brain responses to bladder filling in older women without urgency incontinence. Neurourol Urodyn. 2013;32:435–440. doi: 10.1002/nau.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Tai C, Wang J, Jin T, Wang P, Seong-Gi K, Roppolo JR. Brain switch for reflex micturition control detected by fMRI in rats. J Neurophysiol. 2009;102:2719–2730. doi: 10.1152/jn.00700.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Tai C, Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol. 2012;302:F1090–F1097. doi: 10.1152/ajprenal.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Tai C, Wang J, Jin T, Wang P, Kim SG, Roppolo JR, de Groat WC. Brain switch for reflex micturition control detected by FMRI in rats. J Neurophysiol. 2009;102:2719–2730. doi: 10.1152/jn.00700.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Taira N. The autonomic pharmacology of the bladder. Ann Rev Pharmacol. 1972;12:197–208. doi: 10.1146/annurev.pa.12.040172.001213. [DOI] [PubMed] [Google Scholar]
- 616.Taira N, Matsumura S, Hashimoto K. Excitation of the parasympathetic ganglia of the canine urinary bladder through a muscarinic mechanism. J Pharmacol Exp Ther. 1971;176:93–100. [PubMed] [Google Scholar]
- 617.Takahashi R, Yoshizawa T, Yunoki T, Tyagi P, Naito S, de Groat WC, Yoshimura N. Hyperexcitability of bladder afferent neurons associated with Reduction of Kv1.4 alpha-Subunit in Rats with spinal cord injury. J Urol. 2013;190:2296–2304. doi: 10.1016/j.juro.2013.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Takao T, Tsujimura A, Miyagawa Y, Kiuchi H, Ueda T, Hirai T, Komori K, Takada S, Nonomura N, Osaki Y, Enomoto K, Hatazawa J, Okuyama A. Brain responses during the first desire to void: A positron emission tomography study. Int J Urol. 2008;15:724–728. doi: 10.1111/j.1442-2042.2008.02076.x. [DOI] [PubMed] [Google Scholar]
- 619.Takasaki A, Hui M, Sasaki M. Is the periaqueductal gray an essential relay center for the micturition reflex pathway in the cat? Brain Res. 2011;1317:108–115. doi: 10.1016/j.brainres.2009.12.057. [DOI] [PubMed] [Google Scholar]
- 620.Talaat M. Afferent impulses in the nerves supplying the urinary bladder. J Physiol. 1937;89:1–13. doi: 10.1113/jphysiol.1937.sp003458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Tanaka H, Kakizaki H, Shibata T, Ameda K, Koyanagi T. Effects of a selective metabotropic glutamate receptor agonist on the micturition reflex pathway in urethane-anesthetized rats. Neurourol Urodyn. 2003;22:611–616. doi: 10.1002/nau.10138. [DOI] [PubMed] [Google Scholar]
- 622.Tanaka S, Zukeran C, Nakagana S, Nakao T. Innervation of the rat urogenital organs. Acta Anatomica Nippon. 1981;56:413–414. [Google Scholar]
- 623.Tanaka Y, Koyama Y, Kayama Y, Kawauchi A, Ukimura O, Miki T. Firing of micturition center neurons in the rat mesopontine tegmentum during urinary bladder contraction. Brain Res. 2003;965:146–154. doi: 10.1016/s0006-8993(02)04154-9. [DOI] [PubMed] [Google Scholar]
- 624.Tang PC. Levels of brain stem and diencephalon controlling micturition reflex. J Neurophysiol. 1955;18:583–595. doi: 10.1152/jn.1955.18.6.583. [DOI] [PubMed] [Google Scholar]
- 625.Taniguchi N, Miyata M, Yachiku S, Kaneko S, Yamaguchi S, Numata A. A study of micturition inducing sites in the periaqueductal gray of the mesencephalon. J Urol. 2002;168:1626–1631. doi: 10.1016/S0022-5347(05)64532-6. [DOI] [PubMed] [Google Scholar]
- 626.Taxi J, Derer M, Domich A. Morphology and histophysiology of SIF cells in the autonomic ganglia. In: Elfvin L-G, editor. Autonomic Ganglia. New York: John Wiley & Sons Ltd; 1983. pp. 67–95. [Google Scholar]
- 627.Taylor P, Brown JH. Acetylcholine. In: Siegel GJ, Agranoff BW, Albers WR, Mollinoff PB, editors. Basic Neurochemistry. New York: Paven Press; 1993. pp. 231–260. [Google Scholar]
- 628.Tempest HV, Dixon AK, Turner WH, Elneil S, Sellers LA, Ferguson DR. P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int. 2004;93:1344–1348. doi: 10.1111/j.1464-410X.2004.04858.x. [DOI] [PubMed] [Google Scholar]
- 629.Testa R, Guarneri L, Poggesi E, Angelico P, Velasco C, Ibba M, Cilia A, Motta G, Riva C, Leonardi A. Effect of several 5-hydroxytryptamine(1A) receptor ligands on the micturition reflex in rats: Comparison with WAY 100635. J Pharmacol Exp Ther. 1999;290:1258–1269. [PubMed] [Google Scholar]
- 630.Theobald RJ, Jr, de Groat WD. The effects of purine nucleotides on transmission in vesical parasympathetic ganglia of the cat. J Auton Pharmacol. 1989;9:167–181. doi: 10.1111/j.1474-8673.1989.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 631.Thiruchelvam N, Wu C, David A, Woolf AS, Cuckow PM, Fry CH. Neurotransmission and viscoelasticity in the ovine fetal bladder after in utero bladder outflow obstruction. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1296–R1305. doi: 10.1152/ajpregu.00688.2002. [DOI] [PubMed] [Google Scholar]
- 632.Thor KB, Blais DP, de Groat WC. Behavioral analysis of the postnatal development of micturition in kittens. Brain Res Dev Brain Res. 1989;46:137–144. doi: 10.1016/0165-3806(89)90151-x. [DOI] [PubMed] [Google Scholar]
- 633.Thor KB, de Groat WC. Neural control of the female urethral and anal rhabdosphincters and pelvic floor muscles. Am J Physiol Regul Integr Comp Physiol. 2010;299:R416–R438. doi: 10.1152/ajpregu.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Thor KB, Donatucci C. Central nervous system control of the lower urinary tract: New pharmacological approaches to stress urinary incontinence in women. J Urol. 2004;172:27–33. doi: 10.1097/01.ju.0000118381.04432.22. [DOI] [PubMed] [Google Scholar]
- 635.Thor KB, Hisamitsu T, de Groat WC. Unmasking of a neonatal somatovesical reflex in adult cats by the serotonin autoreceptor agonist 5-methoxy-N,N-dimethyltryptamine. Brain Res Dev Brain Res. 1990;54:35–42. doi: 10.1016/0165-3806(90)90062-4. [DOI] [PubMed] [Google Scholar]
- 636.Thor KB, Katofiasc MA. Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor,on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J Pharmacol Exp Ther. 1995;274:1014–1024. [PubMed] [Google Scholar]
- 637.Thor KB, Katofiasc MA, Danuser H, Springer J, Schaus JM. The role of 5-HT(1A) receptors in control of lower urinary tract function in cats. Brain Res. 2002;946:290–297. doi: 10.1016/s0006-8993(02)02897-4. [DOI] [PubMed] [Google Scholar]
- 638.Thor KB, Kawatani M, de Groat WC. Development and Plasticity of Mammalian Spinal Cord. Vol. 3. Padova, Italy: Fidia Press; 1986. Plasticity in the Reflex Pathways to the Lower Urinary Tract of the Cat During Postnatal Development and Following Spinal Cord Injury. (Fidia Research Series). [Google Scholar]
- 639.Thor KB, Morgan C, Nadelhaft I, Houston M, de Groat WC. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989;288:263–279. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- 640.Thor KB, Muhlhauser MA. Vesicoanal, urethroanal, and urethrovesical reflexes initiated by lower urinary tract irritation in the rat. Am J Physiol. 1999;277:R1002–R1012. doi: 10.1152/ajpregu.1999.277.4.R1002. [DOI] [PubMed] [Google Scholar]
- 641.Tobin G, Sjogren C. In vivo and in vitro effects of muscarinic receptor antagonists on contractions and release of [3H]acetylcholine in the rabbit urinary bladder. Eur J Pharmacol. 1995;281:1–8. doi: 10.1016/0014-2999(95)00221-6. [DOI] [PubMed] [Google Scholar]
- 642.Tobin G, Sjogren C. Prejunctional facilitatory and inhibitory modulation of parasympathetic nerve transmission in the rabbit urinary bladder. J Auton Nerv Syst. 1998;68:153–156. doi: 10.1016/s0165-1838(97)00128-8. [DOI] [PubMed] [Google Scholar]
- 643.Todd JK. Afferent impulses in the pudendal nerves of the cat. Q J Exp Physiol Cogn Med Sci. 1964;49:258–267. doi: 10.1113/expphysiol.1964.sp001730. [DOI] [PubMed] [Google Scholar]
- 644.Tomlinson DR, Fernyhough P, Diemel LT. Role of neurotrophins in diabetic neuropathy and treatment with nerve growth factors. Diabetes. 1997;46(Suppl 2):S43–S49. doi: 10.2337/diab.46.2.s43. [DOI] [PubMed] [Google Scholar]
- 645.Torimoto K, Fraser MO, Hirao Y, De Groat WC, Chancellor MB, Yoshimura N. Urethral dysfunction in diabetic rats. J Urol. 2004;171:1959–1964. doi: 10.1097/01.ju.0000121283.92963.05. [DOI] [PubMed] [Google Scholar]
- 646.Torimoto K, Hirao Y, Matsuyoshi H, de Groat WC, Chancellor MB, Yoshimura N. alpha1-Adrenergic mechanism in diabetic urethral dysfunction in rats. J Urol. 2005;173:1027–1032. doi: 10.1097/01.ju.0000146268.45662.36. [DOI] [PubMed] [Google Scholar]
- 647.Torrens M, Feneley RCL. Rehabilitation and management of the neuropathic bladder. In: Illis LS, Sedgwick EM, Glanville HJ, editors. Rehabilitation of the Neurological Patient. Oxford: Blackwell Scientific Publications; 1982. [Google Scholar]
- 648.Tran LV, Somogyi GT, de Groat WC. Inhibitory effect of neuropeptide Y on adrenergic and cholinergic transmission in rat urinary bladder and urethra. Am J Physiol. 1994;266:R1411–R1417. doi: 10.1152/ajpregu.1994.266.4.R1411. [DOI] [PubMed] [Google Scholar]
- 649.Tsurusaki M, Yoshida M, Akasu T, Nagatsu I. Alpha2-Adrenoceptors mediate the inhibition of cholinergic transmission in parasympathetic ganglia of the rabbit urinary bladder. Synapse. 1990;5:233–240. doi: 10.1002/syn.890050309. [DOI] [PubMed] [Google Scholar]
- 650.Uchiyama T, Sakakibara R, Hattori T, Yamanishi T. Short-term effect of a single levodopa dose on micturition disturbance in Parkinson’s disease patients with the wearing-off phenomenon. Mov Disord. 2003;18:573–578. doi: 10.1002/mds.10403. [DOI] [PubMed] [Google Scholar]
- 651.Ueda T, Yoshimura N, Yoshida O. Diabetic cystopathy: Relationship to autonomic neuropathy detected by sympathetic skin response. J Urol. 1997;157:580–584. doi: 10.1016/s0022-5347(01)65209-1. [DOI] [PubMed] [Google Scholar]
- 652.Ueki K. Disturbances of micturition observed in some patients with brain tumour. Neurologica Medica Chirurgica. 1960;2:25–33. [Google Scholar]
- 653.Ueyama T, Mizuno N, Nomura S, Konishi A, Itoh K, Arakawa H. Central distribution of afferent and efferent components of the pudendal nerve in cat. J Comp Neurol. 1984;222:38–46. doi: 10.1002/cne.902220104. [DOI] [PubMed] [Google Scholar]
- 654.Valentino RJ, Chen S, Zhu Y, Aston-Jones G. Evidence for divergent projections to the brain noradrenergic system and the spinal parasympathetic system from Barrington’s nucleus. Brain Res. 1996;732:1–15. doi: 10.1016/0006-8993(96)00482-9. [DOI] [PubMed] [Google Scholar]
- 655.Valentino RJ, Kosboth M, Colflesh M, Miselis RR. Transneuronal labeling from the rat distal colon: Anatomic evidence for regulation of distal colon function by a pontine corticotropin-releasing factor system. J Comp Neurol. 2000;417:399–414. [PubMed] [Google Scholar]
- 656.Valentino RJ, Wood SK, Wein AJ, Zderic SA. The bladder-brain connection: Putative role of corticotropin-releasing factor. Nat Rev Urol. 2011;8:19–28. doi: 10.1038/nrurol.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.van Duin F, Rosier PF, Rijkhoff NJ, van Kerrebroek PE, Debruyne FM, Wijkstra H. A computer model of the neural control of the lower urinary tract. Neurourol Urodyn. 1998;17:175–196. doi: 10.1002/(sici)1520-6777(1998)17:3<175::aid-nau3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 658.Van Poppel H, Stessens R, Van Damme B, Carton H, Baert L. Diabetic cystopathy: Neuropathological examination of urinary bladder biopsies. Eur Urol. 1988;15:128–131. doi: 10.1159/000473412. [DOI] [PubMed] [Google Scholar]
- 659.Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol. 2000;420:335–348. [PubMed] [Google Scholar]
- 660.Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat. 2001;21:125–138. doi: 10.1016/s0891-0618(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 661.Vizzard MA. Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Prog Brain Res. 2006;152:97–115. doi: 10.1016/S0079-6123(05)52007-7. [DOI] [PubMed] [Google Scholar]
- 662.Vizzard MA, Boyle MM. Increased expression of growth-associated protein (GAP-43) in lower urinary tract pathways following cyclophosphamide (CYP)-induced cystitis. Brain Res. 1999;844:174–187. doi: 10.1016/s0006-8993(99)01936-8. [DOI] [PubMed] [Google Scholar]
- 663.Vizzard MA, Erdman SL, de Groat WC. Increase expression of neuronal nitric oxide synthase (NOS) and Fos proteins in neural pathways following acute and chronic irritation of the urinary tract. J Comp Neurol. 1996;370:191–202. doi: 10.1002/(SICI)1096-9861(19960624)370:2<191::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 664.Vizzard MA, Erickson VL, Card JP, Roppolo JR, de Groat WC. Transneuronal labeling of neurons in the adult rat brainstem and spinal cord after injection of pseudorabies virus into the urethra. J Comp Neurol. 1995;355:629–640. doi: 10.1002/cne.903550411. [DOI] [PubMed] [Google Scholar]
- 665.Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: Part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Wang CC, Nagatomi J, Toosi KK, Yoshimura N, Hsieh JH, Chancellor MB, Sacks MS. Diabetes-induced alternations in biomechanical properties of urinary bladder wall in rats. Urology. 2009;73:911–915. doi: 10.1016/j.urology.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Wang EC, Lee JM, Johnson JP, Kleyman TR, Bridges R, Apodaca G. Hydrostatic pressure-regulated ion transport in bladder uroepithelium. Am J Physiol Renal Physiol. 2003;285:F651–F663. doi: 10.1152/ajprenal.00403.2002. [DOI] [PubMed] [Google Scholar]
- 669.Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest. 2005;115:2412–2422. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Wang X, Momota Y, Yanase H, Narumiya S, Maruyama T, Kawatani M. Urothelium EP1 receptor facilitates the micturition reflex in mice. Biomed Res. 2008;29:105–111. doi: 10.2220/biomedres.29.105. [DOI] [PubMed] [Google Scholar]
- 671.Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain. 2008;139:158–167. doi: 10.1016/j.pain.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 672.Wanigasekara Y, Kepper ME, Keast JR. Immunohistochemical characterisation of pelvic autonomic ganglia in male mice. Cell Tissue Res. 2003;311:175–185. doi: 10.1007/s00441-002-0673-1. [DOI] [PubMed] [Google Scholar]
- 673.Warburton AL, Santer RM. Sympathetic and sensory innervation of the urinary tract in young adult and aged rats: A semi-quantitative histochemical and immunohistochemical study. Histochem J. 1994;26:127–133. doi: 10.1007/BF00157961. [DOI] [PubMed] [Google Scholar]
- 674.Willette RN, Morrison S, Sapru HN, Reis DJ. Stimulation of opiate receptors in the dorsal pontine tegmentum inhibits reflex contraction of the urinary bladder. J Pharmacol Exp Ther. 1988;244:403–409. [PubMed] [Google Scholar]
- 675.Winge K, Fowler CJ. Bladder dysfunction in Parkinsonism: Mechanisms, prevalence, symptoms, and management. Mov Disord. 2006;21:737–745. doi: 10.1002/mds.20867. [DOI] [PubMed] [Google Scholar]
- 676.Winge K, Friberg L, Werdelin L, Nielsen KK, Stimpel H. Relationship between nigrostriatal dopaminergic degeneration, urinary symptoms, and bladder control in Parkinson’s disease. Eur J Neurol. 2005;12:842–850. doi: 10.1111/j.1468-1331.2005.01087.x. [DOI] [PubMed] [Google Scholar]
- 677.Winter DL. Receptor characteristics and conduction velocites in bladder afferents. J Psychiatr Res. 1971;8:225–235. doi: 10.1016/0022-3956(71)90021-5. [DOI] [PubMed] [Google Scholar]
- 678.Wozniak W, Skowronska U. Comparative anatomy of pelvic plexus in cat, dog, rabbit, macaque and man. Anat Anz. 1967;120:457–473. [PubMed] [Google Scholar]
- 679.Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2008;99:244–253. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Yamada T, Ugawa S, Ueda T, Ishida Y, Kajita K, Shimada S. Differential localizations of the transient receptor potential channels TRPV4 and TRPV1 in the mouse urinary bladder. J Histochem Cytochem. 2009;57:277–287. doi: 10.1369/jhc.2008.951962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Yamamoto T, Sakakibara R, Hashimoto K, Nakazawa K, Uchiyama T, Liu Z, Ito T, Hattori T. Striatal dopamine level increases in the urinary storage phase in cats: An in vivo microdialysis study. Neuroscience. 2005;135:299–303. doi: 10.1016/j.neuroscience.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 682.Yang Z, Dolber PC, Fraser MO. Differential vulnerabilities of urethral afferents in diabetes and discovery of a novel urethra-to-urethra reflex. Am J Physiol Renal Physiol. 2010;298:F118–F124. doi: 10.1152/ajprenal.00281.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Yashiro K, Thor KB, Burgard EC. Properties of urethral rhabdosphincter motoneurons and their regulation by noradrenaline. J Physiol. 2010;588:4951–4967. doi: 10.1113/jphysiol.2010.197319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Yin Y, Shuke N, Kaneko S, Okizaki A, Sato J, Aburano T, Li Y, Mizunaga M, Yachiku S. Cerebral control of bladder storage in patients with detrusor overactivity. Nucl Med Commun. 2008;29:1081–1085. doi: 10.1097/MNM.0b013e328313bc13. [DOI] [PubMed] [Google Scholar]
- 685.Yokota R, Burnstock G. Decentralisation of neurones in the pelvic ganglion of the guinea-pig: Reinnervation by adrenergic nerves. Cell Tissue Res. 1983;232:399–411. doi: 10.1007/BF00213795. [DOI] [PubMed] [Google Scholar]
- 686.Yokoyama O, Ito H, Aoki Y, Oyama N, Miwa Y, Akino H. Selective alpha1A-blocker improves bladder storage function in rats via suppression of C-fiber afferent activity. World J Urol. 2010;28:609–614. doi: 10.1007/s00345-009-0481-2. [DOI] [PubMed] [Google Scholar]
- 687.Yokoyama O, Mizuno H, Komatsu K, Akino H, Tanase K, Namiki M. Role of glutamate receptors in the development and maintenance of bladder overactivity after cerebral infarction in the rat. J Urol. 2004;171:1709–1714. doi: 10.1097/01.ju.0000104861.73314.fe. [DOI] [PubMed] [Google Scholar]
- 688.Yokoyama O, Ootsuka N, Komatsu K, Kodama K, Yotsuyanagi S, Niikura S, Nagasaka Y, Nakada Y, Kanie S, Namiki M. Forebrain muscarinic control of micturition reflex in rats. Neuropharmacology. 2001;41:629–638. doi: 10.1016/s0028-3908(01)00102-2. [DOI] [PubMed] [Google Scholar]
- 689.Yokoyama O, Yoshiyama M, Namiki M, de Groat WC. Glutamatergic and dopaminergic contributions to rat bladder hyperactivity after cerebral artery occlusion. Am J Physiol. 1999;276:R935–R942. doi: 10.1152/ajpregu.1999.276.4.R935. [DOI] [PubMed] [Google Scholar]
- 690.Yokoyama O, Yoshiyama M, Namiki M, de Groat WC. Interaction between D2 dopaminergic and glutamatergic excitatory influences on lower urinary tract function in normal and cerebral-infarcted rats. Exp Neurol. 2001;169:148–155. doi: 10.1006/exnr.2001.7639. [DOI] [PubMed] [Google Scholar]
- 691.Yokoyama O, Yoshiyama M, Namiki M, de Groat WC. Changes in dopaminergic and glutamatergic excitatory mechanisms of micturition reflex after middle cerebral artery occlusion in conscious rats. Exp Neurol. 2002;173:129–135. doi: 10.1006/exnr.2001.7833. [DOI] [PubMed] [Google Scholar]
- 692.Yono M, Foster HE, Jr, Weiss RM, Latifpour J. Age related changes in the functional, biochemical and molecular properties of alpha1-adrenoceptors in the rat genitourinary tract. J Urol. 2006;176:1214–1219. doi: 10.1016/j.juro.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 693.Yoshida J, Aikawa K, Yoshimura Y, Shishido K, Yanagida T, Yamaguchi O. The effects of ovariectomy and estrogen replacement on acetylcholine release from nerve fibres and passive stretch-induced acetylcholine release in female rat bladder. Neurourol Urodyn. 2007;26:1050–1055. doi: 10.1002/nau.20438. [DOI] [PubMed] [Google Scholar]
- 694.Yoshida M, Inadome A, Murakami S. Pharmacological analysis of neurotransmitters contributing to lower urinary tract function. Nippon Yakurigaku Zasshi. 2003;121:307–316. doi: 10.1254/fpj.121.307. [DOI] [PubMed] [Google Scholar]
- 695.Yoshikawa S, Kitta T, Miyazato M, Sumino Y, Yoshimura N. Inhibitory role of the spinal cholinergic system in the control of urethral continence reflex during sneezing in rats. Neurourol Urodyn. 2014;33:443–448. doi: 10.1002/nau.22431. [DOI] [PubMed] [Google Scholar]
- 696.Yoshikawa S, Oguchi T, Funahashi Y, de Groat WC, Yoshimura N. Glycine transporter type 2 (GlyT2) inhibitor ameliorates bladder overactivity and nociceptive behavior in rats. Eur Urol. 2012;62:704–712. doi: 10.1016/j.eururo.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Yoshimura N. Bladder afferent pathway and spinal cord injury: Possible mechanisms inducing hyperreflexia of the urinary bladder. Prog Neurobiol. 1999;57:583–606. doi: 10.1016/s0301-0082(98)00070-7. [DOI] [PubMed] [Google Scholar]
- 698.Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006;26:10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Yoshimura N, Birder L. Interstitial cystitis and related painful bladder syndromes: Pathophysiology. In: Pasricha PJ, Willis WD, Gebhart GF, editors. Chronic Abdominal and Visceral Pain: Theory and Practice. New York: Informa Healthcare USA; 2007. pp. 495–520. [Google Scholar]
- 700.Yoshimura N, Chancellor MB, Andersson KE, Christ GJ. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 2005;95:733–738. doi: 10.1111/j.1464-410X.2005.05392.x. [DOI] [PubMed] [Google Scholar]
- 701.Yoshimura N, de Groat WC. Neural control of the lower urinary tract. Int J Urol. 1997;4:111–125. doi: 10.1111/j.1442-2042.1997.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 702.Yoshimura N, de Groat WC. Plasticity of Na+ channels in afferent neurones innervating rat urinary bladder following spinal cord injury. J Physiol. 1997;503:269–276. doi: 10.1111/j.1469-7793.1997.269bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Yoshimura N, Erdman SL, Snider MW, de Groat WC. Effects of spinal cord injury on neurofilament immunoreactivity and capsaicin sensitivity in rat dorsal root ganglion neurons innervating the urinary bladder. Neuroscience. 1998;83:633–643. doi: 10.1016/s0306-4522(97)00376-x. [DOI] [PubMed] [Google Scholar]
- 705.Yoshimura N, Kuno S, Chancellor MB, de Groat WC, Seki S. Dopaminergic mechanisms underlying bladder hyperactivity in rats with a unilateral 6-hydroxydopamine (6-OHDA) lesion of the nigrostriatal pathway. Br J Pharmacol. 2003;139:1425–1432. doi: 10.1038/sj.bjp.0705388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Yoshimura N, Mizuta E, Kuno S, Sasa M, Yoshida O. The dopamine D1 receptor agonist SKF 38393 suppresses detrusor hyperreflexia in the monkey with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Neuropharmacology. 1993;32:315–321. doi: 10.1016/0028-3908(93)90151-r. [DOI] [PubMed] [Google Scholar]
- 707.Yoshimura N, Mizuta E, Yoshida O, Kuno S. Therapeutic effects of dopamine D1/D2 receptor agonists on detrusor hyperreflexia in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned parkinsonian cynomolgus monkeys. J Pharmacol Exp Ther. 1998;286:228–233. [PubMed] [Google Scholar]
- 708.Yoshimura N, Sasa M, Ohno Y, Yoshida O, Takaori S. Contraction of urinary bladder by central norepinephrine originating in the locus coeruleus. J Urol. 1988;139:423–427. doi: 10.1016/s0022-5347(17)42448-7. [DOI] [PubMed] [Google Scholar]
- 709.Yoshimura N, Sasa M, Yoshida O, Takaori S. a1-Adrenergic receptormediated excitation from the locus coeruleus of the sacral parasympathetic preganglionic neuron. Life Sci. 1990a;47:789–797. doi: 10.1016/0024-3205(90)90551-2. [DOI] [PubMed] [Google Scholar]
- 710.Yoshimura N, Sasa M, Yoshida O, Takaori S. Mediation of micturition reflex by central norepinephrine from the locus coeruleus in the cat. J Urol. 1990b;143:840–843. doi: 10.1016/s0022-5347(17)40113-3. [DOI] [PubMed] [Google Scholar]
- 711.Yoshimura N, Sasa M, Yoshida O, Takaori S. Dopamine D-1 receptormediated inhibition of micturition reflex by central dopamine from the substantia nigra. Neurourol Urodyn. 1992;11:535–545. [Google Scholar]
- 712.Yoshimura N, Sasa M, Yoshida O, Takaori S. Inhibitory effects of Hachimijiogan on micturition reflex via the locus coeruleus. [Japanese] Nihon Yakurigaku Zasshi. 1992;99:161–166. doi: 10.1254/fpj.99.161. [DOI] [PubMed] [Google Scholar]
- 713.Yoshimura N, Seki S, Chancellor MB, de Groat WC, Ueda T. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology. 2002;59:61–67. doi: 10.1016/s0090-4295(01)01639-9. [DOI] [PubMed] [Google Scholar]
- 714.Yoshimura N, Seki S, de Groat WC. Nitric oxide modulates Ca(2+) channels in dorsal root ganglion neurons innervating rat urinary bladder. J Neurophysiol. 2001;86:304–311. doi: 10.1152/jn.2001.86.1.304. [DOI] [PubMed] [Google Scholar]
- 715.Yoshimura N, Seki S, Erickson KA, Erickson VL, Hancellor MB, de Groat WC. Histological and electrical properties of rat dorsal root ganglion neurons innervating the lower urinary tract. J Neurosci. 2003;23:4355–4361. doi: 10.1523/JNEUROSCI.23-10-04355.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Yoshimura N, Seki S, Novakovic SD, Tzoumaka E, Erickson VL, Erickson KA, Chancellor MB, de Groat WC. The involvement of the tetrodotoxin-resistant sodium channel Na(v)1.8 (PN3/SNS) in a rat model of visceral pain. J Neurosci. 2001;21:8690–8696. doi: 10.1523/JNEUROSCI.21-21-08690.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Yoshimura N, White G, Weight FF, de Groat WC. Different types of Na+ and A-type K+ currents in dorsal root ganglion neurones innervating the rat urinary bladder. J Physiol. 1996;494:1–16. doi: 10.1113/jphysiol.1996.sp021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Yoshiyama M, de Groat WC. Supraspinal and spinal alphaamino-3-hydroxy-5-methylisoxazole-4-propionic acid and N-methyl-D-aspartate glutamatergic control of the micturition reflex in the urethane-anesthetized rat. Neuroscience. 2005;132:1017–1026. doi: 10.1016/j.neuroscience.2005.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Yoshiyama M, de Groat WC. Role of spinal metabotropic glutamate receptors in regulation of lower urinary tract function in the decerebrate unanesthetized rat. Neurosci Lett. 2007;420:18–22. doi: 10.1016/j.neulet.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Yoshiyama M, de Groat WC. The role of vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide in the neural pathways controlling the lower urinary tract. J Mol Neurosci. 2008;36:227–240. doi: 10.1007/s12031-008-9090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Yoshiyama M, Erickson KA, Erdman SL, de Groat WC. Effects of N-methyl-D-aspartate (dizocilpine) and alpha-amino-3-hydroxy-4-isoxazolepropionate (LY215490) receptor antagonists on the voiding reflex induced by perineal stimulation in the neonatal rat. Neuroscience. 1999;90:1415–1420. doi: 10.1016/s0306-4522(98)00545-4. [DOI] [PubMed] [Google Scholar]
- 722.Yoshiyama M, Roppolo JR, de Groat WC. Interations between glutamatergic and monoaminergic systems controlling the micturition reflex in the urethane-anesthetized rat. Brain Res. 1994;639:300–308. doi: 10.1016/0006-8993(94)91743-4. [DOI] [PubMed] [Google Scholar]
- 723.Yoshiyama M, Roppolo JR, de Groat WC. Effects of LY215490, a competitive alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor antagonist, on the micturition reflex in the rat. J Pharmacol Exp Ther. 1997;280:894–904. [PubMed] [Google Scholar]
- 724.Yotsuyanagi S, Yokoyama O, Komatsu K, Kodama K, Niikura S, Namiki M. Expression of neural plasticity related gene in the pontine tegmental area of rats with overactive bladder after cerebral infarction. J Urol. 2001;166:1148–1155. [PubMed] [Google Scholar]
- 725.Yu HJ, Wein AJ, Levin RM. Age-related differential susceptibility to calcium channel blocker and low calcium medium in rat detrusor muscle: Response to field stimulation. Neurourol Urodyn. 1996;15:563–576. doi: 10.1002/(SICI)1520-6777(1996)15:5<563::AID-NAU12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 726.Yu Y, de Groat WC. Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder-pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am J Physiol Renal Physiol. 2008;294:F1146–F1156. doi: 10.1152/ajprenal.00592.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Yu Y, de Groat WC. Nitric oxide modulates bladder afferent nerve activity in the in vitro urinary bladder-pelvic nerve preparation from rats with cyclophosphamide induced cystitis. Brain Res. 2013;1490:83–94. doi: 10.1016/j.brainres.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Zafra F, Aragon C, Olivares L, Danbolt NC, Gimenez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. J Neurosci. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Zafra F, Gomeza J, Olivares L, Aragon C, Gimenez C. Regional distribution and developmental variation of the glycine transporters GLYT1 and GLYT2 in the rat CNS. Eur J Neurosci. 1995;7:1342–1352. doi: 10.1111/j.1460-9568.1995.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 730.Zago T, Pea U, Fumagalli GL, Areta L, Marzorati G, Bianchi F. Cerebellar pathology and micturitional disorders: Anatomotopographic and functional correlations. Arch Ital Urol Androl. 2010;82:177–180. [PubMed] [Google Scholar]
- 731.Zagorodnyuk VP, Costa M, Brookes SJ. Major classes of sensory neurons to the urinary bladder. Auton Neurosci. 2006;126–127:390–397. doi: 10.1016/j.autneu.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 732.Zagorodnyuk VP, Gibbins IL, Costa M, Brookes SJ, Gregory SJ. Properties of the major classes of mechanoreceptors in the guinea pig bladder. J Physiol. 2007;585:147–163. doi: 10.1113/jphysiol.2007.140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Zhang X, Douglas KL, Jin H, Eldaif BM, Nassar R, Fraser MO, Dolber PC. Sprouting of substance P-expressing primary afferent central terminals and spinal micturition reflex NK1 receptor dependence after spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2084–R2096. doi: 10.1152/ajpregu.90653.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Zhong Y, Banning AS, Cockayne DA, Ford AP, Burnstock G, McMahon SB. Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience. 2003;120:667–675. doi: 10.1016/s0306-4522(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 735.Zhong Y, Dunn PM, Burnstock G. Pharmacological comparison of P2X receptors on rat coeliac, mouse coeliac and mouse pelvic ganglion neurons. Neuropharmacology. 2000;39:172–180. doi: 10.1016/s0028-3908(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 736.Zhong Y, Dunn PM, Burnstock G. Multiple P2X receptors on guineapig pelvic ganglion neurons exhibit novel pharmacological properties. Br J Pharmacol. 2001;132:221–233. doi: 10.1038/sj.bjp.0703778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Zhong Y, Dunn PM, Xiang Z, Bo X, Burnstock G. Pharmacological and molecular characterization of P2X receptors in rat pelvic ganglion neurons. Br J Pharmacol. 1998;125:771–781. doi: 10.1038/sj.bjp.0702118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Zoubek J, Somogyi GT, De Groat WC. A comparison of inhibitory effects of neuropeptide Y on rat urinary bladder, urethra, and vas deferens. Am J Physiol. 1993;265:R537–R543. doi: 10.1152/ajpregu.1993.265.3.R537. [DOI] [PubMed] [Google Scholar]
- 739.Zvara P, Braas KM, May V, Vizzard MA. A role for pituitary adenylate cyclase activating polypeptide (PACAP) in detrusor hyperreflexia after spinal cord injury (SCI) Ann N Y Acad Sci. 2006;1070:622–628. doi: 10.1196/annals.1317.092. [DOI] [PubMed] [Google Scholar]
- 740.Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol. 2007;7:9. doi: 10.1186/1472-6793-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Zvarova K, Dunleavy JD, Vizzard MA. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp Neurol. 2005;192:46–59. doi: 10.1016/j.expneurol.2004.10.017. [DOI] [PubMed] [Google Scholar]


