Abstract
Background
Deficiencies in brain-derived-neurotrophic-factor have been implicated in the pathogenesis of Huntington's disease (HD).
Objective
Glatiramer acetate, an FDA- approved drug used for the treatment of multiple sclerosis, has been shown to increase brain-derived-neurotrophic-factor levels in immune cells; hence, we investigated whether it could have similar effects in striatal cells.
Methods
Wild-type and HD striatal cells were treated with glatiramer acetate for 48 hrs. HD transgenic and wild-type mice were injected with glatiramer acetate (1.5 to1.7 mg/mouse) for five days. These treatments were followed by protein measurements for brain-derived-neurotrophic-factor.
Results
Glatiramer acetate elicited concentration-dependent increases in brain-derived-neurotrophic-factor protein levels in wild-type and HD striatal cells and in striatal tissue from N171-82Q transgenic mice. Glatiramer acetate also improved metabolic activity of HD striatal cells, and significantly reduced the early hyperactivity phenotype exhibited by N171-82Q transgenic mice.
Conclusions
These findings suggest that glatiramer acetate may represent a useful therapeutic approach for HD. The excellent safety and tolerability record of this compound makes it an ideal candidate for drug repurposing efforts.
Keywords: Glatiramer acetate, Copaxone, neurodegenerative, BDNF, striatum, jumping
Introduction
HD is an inherited, progressive neurodegenerative disorder characterized by chorea, movement dysfunction, cognitive impairment, and behavioral disturbances [1]. The prevalence rate in the US is approximately 5 per 100,000 people. Since the identification of the HD gene in 1993, there have been enormous advancements in the diagnosis and understanding of the molecular and pathophysiological features of the disorder [1]. Nonetheless, there is currently no satisfactory treatment or cure for this disease. HD has been the focus of a considerable amount of pre-clinical and clinical research, however, there is only one U.S. FDA- approved agent for the symptomatic management of HD, tetrabenazine [2].
Brain-derived neurotrophic factor (BDNF) plays an important role in survival, growth, differentiation, and death of neurons [3, [4], and is one of the most widely-studied neuroprotective factors. BDNF exerts its functions in the striatum by binding to the tyrosine kinase receptor (TrkB), as well as the p75 neurotrophin receptor [5]. Although BDNF protein in the striatum is thought to be provided primarily by anterograde transport from the cortex [6], recent studies using newer quantitative analyses of protein and mRNA levels in striatal cells revealed similar expression levels of BDNF in striatum compared to cortex [7]. Further, several studies have shown that Bdnf mRNA is widely expressed in striatal tissue [8, [9, [10]. BDNF is also expressed in immune cells and is thought to represent a potent neuroprotective factor in neuroinflammatory disease [11].
Many CNS disorders are associated with low levels of BDNF, in particular, HD in which reduced striatal BDNF is thought to play a crucial role in pathogenesis [12, [13, [14, [15]. Loss of huntingtin-mediated BDNF gene transcription is associated with the striatal degeneration observed in mouse models of HD and in HD patients [13, [16]. Accordingly, BDNF is decreased in brain tissue from human HD patients [13, [17] and in HD transgenic mice [13, [18, [19]. Notably, BDNF administration to the forebrain has shown to be protective in the R6/1 and R6/2 mouse models [12, [20] and BDNF overexpression in vivo was found to rescue HD phenotypes in YAC128 mice [14]. Moreover, BDNF knockout mice display several symptoms reminiscent of HD [21]. These studies have suggested that restoring striatal BDNF levels may have therapeutic effects in this disease. Several strategies to increase BDNF levels in brain have been developed. These include drugs, such as mixed lineage kinase inhibitors [22], engineered cells that overexpress BDNF [23], and gene therapy, which has been tested in clinical trials for Alzheimer’s disease [24, [25, [26, [27]. Finding safe and tolerable drugs that increase BDNF in the brain would be a major breakthrough for HD treatment.
Glatiramer acetate (GA; Copaxone®) is an FDA- approved drug used as first-line treatment for relapsing-remitting multiple sclerosis. The mechanisms of action of GA are not fully understood, but are thought to involve immunomodulatory effects [28]. Additionally, GA has been shown to release neuroprotective factors from immune cells, suggesting possible direct neuroprotective properties, which could have relevance not only for the treatment of multiple sclerosis, but also other neurological conditions. In particular, studies have shown that GA-reactive T cells can release brain-derived neurotrophic factor (BDNF) [29, [30, [31], and that GA can increase BDNF levels in cultured peripheral blood mononuclear cells [32] and in the brains of an experimental autoimmune encephalomyelitis mouse model treated with GA [33]. In light of these studies, we tested whether GA could increase BDNF levels in striatal cells, in both in vitro and in vivo assays, as a first step in assessing its potential as a therapy for HD.
Methods
Cell culture
Conditionally immortalized wild-type STHdhQ7striatal neuronal progenitor cells expressing endogenous normal huntingtin, with 7 glutamines, and homozygous mutant STHdhQ111 cell lines, expressing endogenous mutant huntingtin with 111-glutamines, were generated from striatal tissue of Hdh knock-in mice [34] and were a kind donation from Dr. Marcy MacDonald. Cells were plated at 3×105 cells per well in six-well plates containing DMEM media supplemented with 10% FBS, grown at 33°C/5% CO2, and treated with GA at concentrations of 3-300 µM or vehicle (40% mannitol) for 24 hrs. At the end of treatment, the culture medium was collected and BDNF levels measured by ELISA.
XTT assay
The metabolic activity of STHdhQ7 and STHdhQ111 cells was determined by using the XTT assay. This assay is based on the conversion of the water-soluble XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) reagent to an orange formazan product, which requires an intact metabolism and respiratory chain. Cells were plated in 96-well tissue culture plates and XTT assays were performed 24 hrs after shifting the cells to 39°C by adding the XTT reagent (Sigma Aldrich), followed by absorbance readings at 490 nm on a multiwell spectrophotometer.
Mice and treatment
Transgenic N171-82Q HD mice were maintained by breeding heterozygous N171-82Q males with C3B6F1 females (Jackson Laboratories). At the age of 4 weeks, mice were genotyped according to the Jackson Laboratories protocols. The CAG repeat length in these mice is 82 ± 1 CAGs (Laragen, Los Angeles, CA). Groups of mice (n=6-7 per genotype and drug condition) were injected s.c. with GA (75 mg/kg/day, equaling 1. 5 to 1.7 mg per mouse) or an equivalent amount of vehicle (40% mannitol) once a day for 5 days, at 10 weeks of age. This dose is similar to that used in previous mouse studies [33, [35]. Mice were sacrificed 5-6 hr after final injection. All procedures were in strict accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Open field exploration
Open field exploration was measured in a square plexiglass chamber (Med Associates INC) 3-4 hrs after the final injection. Several behavioral parameters (ambulatory time, ambulatory distance, average velocity, rearing and jumping) were recorded for each mouse during a 10 minute observation period. The behavioral data were analyzed by two-factor analysis of variance using GraphPad Prism Software (San Diego, CA), with significance accepted at the 95% probability level.
BDNF ELISA
At the end of the drug treatments, brains were removed, and striata and cortex dissected out and immediately frozen on dry ice. Striatal and cortical samples were homogenized in lysis buffer and sonicated. Tissue and cell samples were diluted 1:1 in Block & Sample buffer (1x/Promega). BDNF protein levels were determined by ELISA using BDNF Emax™ ImmunoAssay System (Promega) according to the manufacturers’ protocols. BDNF levels were normalized by total protein in each sample (BCA protein assay reagent; ThermoScientific).
Results
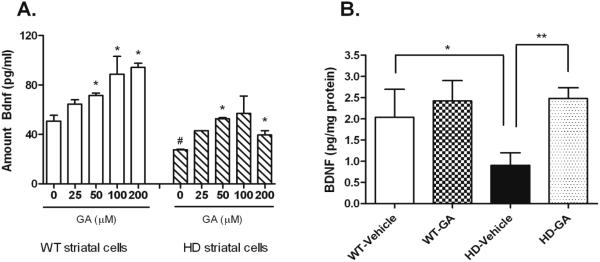
Given the ability of GA to elevate BDNF in immune cells, we tested whether GA could elicit elevation of BDNF levels in striatal cells both in vitro and in vivo. In vitro, GA exposure resulted in concentration-dependent increases in BDNF protein levels in WT STHdhQ7 and mutant STHdhQ111 striatal cells compared to vehicle-treated cells (Figure 1A). Consistent with studies from HD transgenic mice and human HD patients, BDNF levels were lower in the mutant STHdhQ111 striatal cells compared to WT STHdhQ7 cells (Figure 1A).
Figure 1. The effects of GA exposure on striatal cells (A) and striatal tissue (B).
A). Striatal cells were treated with GA for 24 hrs at the indicated concentrations. BDNF levels were measured by ELISA. Data are from two independent experiments performed in duplicate and shown means +/− SEM. Statistically significant differences in BDNF levels were determined by One-way ANOVA, comparing all drug treatment values to the control value. Asterisks denote significant difference between GA-treated and vehicle-treated; *, P<0.05. Hatched mark denotes significant difference between HD vehicle compared to WT vehicle; #, P=0.019. B). Effect of GA treatment (75 mg/kg) on BDNF protein levels in the striatum of wt and N171-82Q transgenic (HD) mice. BDNF levels were measured in striatal samples homogenates from n= 6-7 mice per group. Statistically significant differences in BDNF levels were determined by Student’s t test (unpaired; two-tailed): *, P<0.05; **, P<0.01.
The effects of GA on striatal BDNF levels were next tested in vivo, in the N171-82Q transgenic mouse model of HD. GA treatment resulted in significantly elevated BDNF protein levels in the striatum of N171-82Q transgenic mice (2.48 pg/mg protein vs. 0.90 pg/mg protein; Two-tailed Student’s t test; p=0.003) (Figure 1B). GA-induced increases in BDNF protein were also observed in the cortex, without a concomitant change in cortical BDNF mRNA levels (data not shown).
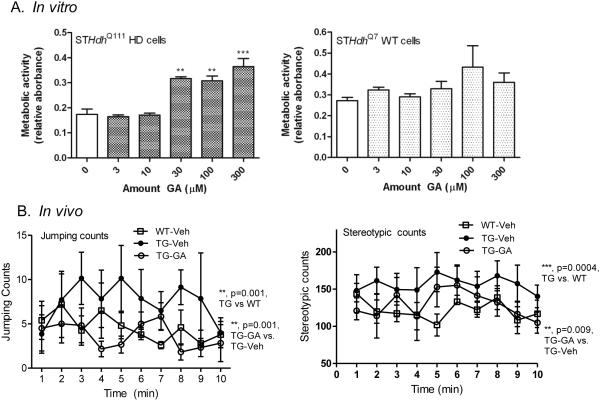
We next tested whether GA could alter HD phenotypes in vitro and in vivo. GA treatment of HD STHdhQ111 striatal cells caused a significant improvement in the metabolic deficit exhibited by these cells, while having no effect on WT STHdhQ7 cells (Figure 2A). In open field activity measurements, there were no statistically significant effects of GA on general locomotion parameters, including ambulatory time, distance or velocity in N171-82Q transgenic mice (data not shown). Although motor deficits between wt and HD mice were minimal at this age (10 weeks), consistent with previous studies in the literature [36, [37, [38], N171-82Q mice did show increased hyperactivity, including erratic jumping activity and stereotypic behavior (Figure 2B), consistent with previous reports [39, [40, [41]. GA treatment results in significant decreases in this activity in N171-82Q mice: jumping counts (F(1,110)=8.81; p=0.001) and stereotypic counts (F(1,68)=7.24; p=0.009) in N171-82Q transgenic mice (Figure 2B).
Figure 2. Effects of GA treatment on in vitro (STHdh striatal cells) and in vivo (N171-82Q transgenic mice) HD phenotypes.
A). STHdh HD (Q111) and WT (Q7) striatal cells were treated with GA at the indicated concentrations and XTT assays performed after 24 hrs. Significant differences were determined by one-way ANOVA, followed by Dunnett’s post-test. **, P<0.001, ***, P<0.0001. B). Groups of mice were tested in an open field activity chamber over a 10 minute period and jumping activity and stereotypic behavior is shown. Two-way ANOVA revealed significant differences between WT-vehicle and N171-82Q-vehicle mice, as well as GA-treated vs. vehicle-treated N171-82Q transgenic mice in jumping counts (left) (**, P=0.001 and **P=0.001 for transgenic and drug effect, respectively) and stereotypic behavior (right) (**, p=0.0004 and **, P=0.009 for transgenic and drug effect, respectively).
Discussion
Discoveries about the molecular basis of HD have provided opportunities to identify novel therapeutic targets. However, developing a novel drug takes an enormous amount of time, money and effort, largely due to bottlenecks in the therapeutic development process [42]. In contrast, the repurposing of an FDA- approved drug into a new indication would facilitate a rapid path into a critical unmet medical need. Historically, drug repurposing was an unintentional, serendipitous process; in this case, however, the recent studies demonstrating that GA could elevate BDNF levels in immune cells [32] led us to hypothesize that a similar effect of GA in neurons could be beneficial to neurological disorders associated with BDNF deficiency, such as HD. Accordingly, we demonstrated that GA can increase BDNF levels in cultured striatal cells and in striatal tissue after in vivo administration.
Our automated, high-resolution behavioral analyses allows the measure of complex mouse activity, such as jumping and grooming, which have been linked to stress-like behaviors [43], although these specific features are often not reported in HD mice. Previous studies have reported increased stereotypic behavior at early stages of illness in different HD mouse models, including YAC128 and R6/1 mice [39, [40]. An additional study has reported increased erratic jumping activity in the R6/2 HD mouse model, prior to the onset of overt motor deficits [41]; In this study, we report similar phenotypes in the N171-82Q mouse model, prior to the onset of motor deficits and that GA treatment could prevent these behaviors. Increased jumping and stereotypic activity might represent a stress-like phenotype or an uncontrolled, chorea-like movement dysfunction that occurs early in illness. Hence, we suggest that GA might be particularly effective early in the course of illness.
One limitation of testing the N171-82Q mice at an early stage of illness is that we cannot clearly correlate increased BDNF levels to increased motor performance, given that these mice did not show overt motor dysfunction at 10 weeks of age. Previous studies have shown improvement in motor deficits in HD transgenic mice upon BDNF administration [12, [14, [20]. Hence, we expect that longer treatment with GA in this mouse model will improve the motor deficits observed at later stages of illness. Another limitation is that the dose of GA used in this study, and other mouse studies [33, [35], is higher than the human equivalent of 20 mg/day. Hence, the effect of GA on BDNF must be measured at lower doses in order to fully translate these findings into human therapeutics. These studies are the subject of ongoing work by the authors.
The mechanism of how GA elevates BDNF levels is not clear at this time. Given the lack of change in Bdnf mRNA after GA administration, it is possible that the mechanism involves enhancing BDNF protein stability. Nonetheless, in these proof-of-principle studies, we suggest that GA could be a useful therapeutic for HD patients. Over the last 10-15 years, abundant research has characterized the clinical efficacy and safety of GA [44]. GA does not generate neutralizing antibodies and has no known drug interactions. More than 1 million patient-years of experience attest to the safety and tolerability of this compound. The excellent safety and tolerability record of GA makes it an ideal candidate for drug repurposing efforts for HD.
Footnotes
Conflicts of interest: Nothing to report.
References
- [1].Huntington's Disease Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. The Huntington's Disease Collaborative Research Group. [DOI] [PubMed] [Google Scholar]
- [2].Guay DR. Tetrabenazine, a monoamine-depleting drug used in the treatment of hyperkinetic movement disorders. Am J Geriatr Pharmacother. 2010;8:331–373. doi: 10.1016/j.amjopharm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- [3].Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- [6].Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- [7].Ma B, Savas JN, Chao MV, Tanese N. Quantitative analysis of BDNF/TrkB protein and mRNA in cortical and striatal neurons using alpha-tubulin as a normalization factor. Cytometry A. 2012;81 doi: 10.1002/cyto.a.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. Embo J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Runne H, Regulier E, Kuhn A, Zala D, Gokce O, Perrin V, Sick B, Aebischer P, Deglon N, Luthi-Carter R. Dysregulation of gene expression in primary neuron models of Huntington's disease shows that polyglutamine-related effects on the striatal transcriptome may not be dependent on brain circuitry. J Neurosci. 2008;28:9723–9731. doi: 10.1523/JNEUROSCI.3044-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tang B, Di Lena P, Schaffer L, Head SR, Baldi P, Thomas EA. Genome-wide identification of Bcl11b gene targets reveals role in brain-derived neurotrophic factor signaling. PLoS ONE. 2011;6:e23691. doi: 10.1371/journal.pone.0023691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hohlfeld R, Kerschensteiner M, Stadelmann C, Lassmann H, Wekerle H. The neuroprotective effect of inflammation: implications for the therapy of multiple sclerosis. Neurol Sci. 2006;27(Suppl 1):S1–7. doi: 10.1007/s10072-006-0537-7. [DOI] [PubMed] [Google Scholar]
- [12].Gharami K, Xie Y, An JJ, Tonegawa S, Xu B. Brain-derived neurotrophic factor over-expression in the forebrain ameliorates Huntington's disease phenotypes in mice. J Neurochem. 2008;105:369–379. doi: 10.1111/j.1471-4159.2007.05137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM, Silani V, Hayden MR, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- [14].Xie Y, Hayden MR, Xu B. BDNF overexpression in the forebrain rescues Huntington's disease phenotypes in YAC128 mice. J Neurosci. 2010;30:14708–14718. doi: 10.1523/JNEUROSCI.1637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zuccato C, Marullo M, Conforti P, MacDonald ME, Tartari M, Cattaneo E. Systematic assessment of BDNF and its receptor levels in human cortices affected by Huntington's disease. Brain Pathol. 2008;18:225–238. doi: 10.1111/j.1750-3639.2007.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L, Cataudella T, Leavitt BR, Hayden MR, Timmusk T, et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35:76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]
- [17].Ferrer I, Goutan E, Marin C, Rey MJ, Ribalta T. Brain-derived neurotrophic factor in Huntington disease. Brain Res. 2000;866:257–261. doi: 10.1016/s0006-8993(00)02237-x. [DOI] [PubMed] [Google Scholar]
- [18].Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci U S A. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang Y, Li M, Drozda M, Chen M, Ren S, Mejia Sanchez RO, Leavitt BR, Cattaneo E, Ferrante RJ, Hayden MR, et al. Depletion of wild-type huntingtin in mouse models of neurologic diseases. J Neurochem. 2003;87:101–106. doi: 10.1046/j.1471-4159.2003.01980.x. [DOI] [PubMed] [Google Scholar]
- [20].Giampa C, Montagna E, Dato C, Melone MA, Bernardi G, Fusco FR. Systemic delivery of recombinant brain derived neurotrophic factor (BDNF) in the R6/2 mouse model of Huntington's disease. PLoS ONE. 2013;8:e64037. doi: 10.1371/journal.pone.0064037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Canals JM, Pineda JR, Torres-Peraza JF, Bosch M, Martin-Ibanez R, Munoz MT, Mengod G, Ernfors P, Alberch J. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease. J Neurosci. 2004;24:7727–7739. doi: 10.1523/JNEUROSCI.1197-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Apostol BL, Simmons DA, Zuccato C, Illes K, Pallos J, Casale M, Conforti P, Ramos C, Roarke M, Kathuria S, et al. CEP-1347 reduces mutant huntingtin-associated neurotoxicity and restores BDNF levels in R6/2 mice. Mol Cell Neurosci. 2008;39:8–20. doi: 10.1016/j.mcn.2008.04.007. [DOI] [PubMed] [Google Scholar]
- [23].Dey ND, Bombard MC, Roland BP, Davidson S, Lu M, Rossignol J, Sandstrom MI, Skeel RL, Lescaudron L, Dunbar GL. Genetically engineered mesenchymal stem cells reduce behavioral deficits in the YAC 128 mouse model of Huntington's disease. Behav Brain Res. 2010;214:193–200. doi: 10.1016/j.bbr.2010.05.023. [DOI] [PubMed] [Google Scholar]
- [24].Kells AP, Fong DM, Dragunow M, During MJ, Young D, Connor B. AAV-mediated gene delivery of BDNF or GDNF is neuroprotective in a model of Huntington disease. Mol Ther. 2004;9:682–688. doi: 10.1016/j.ymthe.2004.02.016. [DOI] [PubMed] [Google Scholar]
- [25].Iwasaki Y, Negishi T, Inoue M, Tashiro T, Tabira T, Kimura N. Sendai virus vector-mediated brain-derived neurotrophic factor expression ameliorates memory deficits and synaptic degeneration in a transgenic mouse model of Alzheimer's disease. J Neurosci Res. 2012;90:981–989. doi: 10.1002/jnr.22830. [DOI] [PubMed] [Google Scholar]
- [26].Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- [27].Nilsson P, Iwata N, Muramatsu S, Tjernberg LO, Winblad B, Saido TC. Gene therapy in Alzheimer's disease - potential for disease modification. J Cell Mol Med. 2010;14:741–757. doi: 10.1111/j.1582-4934.2010.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Neuhaus O, Farina C, Wekerle H, Hohlfeld R. Mechanisms of action of glatiramer acetate in multiple sclerosis. Neurology. 2001;56:702–708. doi: 10.1212/wnl.56.6.702. [DOI] [PubMed] [Google Scholar]
- [29].Aharoni R, Kayhan B, Eilam R, Sela M, Arnon R. Glatiramer acetate-specific T cells in the brain express T helper 2/3 cytokines and brain-derived neurotrophic factor in situ. Proc Natl Acad Sci U S A. 2003;100:14157–14162. doi: 10.1073/pnas.2336171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ziemssen T, Kumpfel T, Klinkert WE, Neuhaus O, Hohlfeld R. Glatiramer acetate-specific T-helper 1- and 2-type cell lines produce BDNF: implications for multiple sclerosis therapy. Brain-derived neurotrophic factor. Brain. 2002;125:2381–2391. doi: 10.1093/brain/awf252. [DOI] [PubMed] [Google Scholar]
- [31].Chen M, Valenzuela RM, Dhib-Jalbut S. Glatiramer acetate-reactive T cells produce brain-derived neurotrophic factor. J Neurol Sci. 2003;215:37–44. doi: 10.1016/s0022-510x(03)00177-1. [DOI] [PubMed] [Google Scholar]
- [32].Blanco Y, Moral EA, Costa M, Gomez-Choco M, Torres-Peraza JF, Alonso-Magdalena L, Alberch J, Jaraquemada D, Arbizu T, Graus F, et al. Effect of glatiramer acetate (Copaxone) on the immunophenotypic and cytokine profile and BDNF production in multiple sclerosis: a longitudinal study. Neurosci Lett. 2006;406:270–275. doi: 10.1016/j.neulet.2006.07.043. [DOI] [PubMed] [Google Scholar]
- [33].Song F, Bandara M, Deol H, Loeb JA, Benjamins J, Lisak RP. Complexity of trophic factor signaling in experimental autoimmune encephalomyelitis: differential expression of neurotrophic and gliotrophic factors. J Neuroimmunol. 2013;262:11–18. doi: 10.1016/j.jneuroim.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Trettel F, Rigamonti D, Hilditch-Maguire P, Wheeler VC, Sharp AH, Persichetti F, Cattaneo E, MacDonald ME. Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum Mol Genet. 2000;9:2799–2809. doi: 10.1093/hmg/9.19.2799. [DOI] [PubMed] [Google Scholar]
- [35].Aharoni R, Eilam R, Domev H, Labunskay G, Sela M, Arnon R. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc Natl Acad Sci U S A. 2005;102:19045–19050. doi: 10.1073/pnas.0509438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schilling G, Coonfield ML, Ross CA, Borchelt DR. Coenzyme Q10 and remacemide hydrochloride ameliorate motor deficits in a Huntington's disease transgenic mouse model. Neurosci Lett. 2001;315:149–153. doi: 10.1016/s0304-3940(01)02326-6. [DOI] [PubMed] [Google Scholar]
- [37].Schilling G, Jinnah HA, Gonzales V, Coonfield ML, Kim Y, Wood JD, Price DL, Li XJ, Jenkins N, Copeland N, et al. Distinct behavioral and neuropathological abnormalities in transgenic mouse models of HD and DRPLA. Neurobiol Dis. 2001;8:405–418. doi: 10.1006/nbdi.2001.0385. [DOI] [PubMed] [Google Scholar]
- [38].Jia H, Kast RJ, Steffan JS, Thomas EA. Selective histone deacetylase (HDAC) inhibition imparts beneficial effects in Huntington's disease mice: implications for the ubiquitin-proteasomal and autophagy systems. Hum Mol Genet. 2012;21(24):5280–5293. doi: 10.1093/hmg/dds379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Andre VM, Cepeda C, Fisher YE, Huynh M, Bardakjian N, Singh S, Yang XW, Levine MS. Differential electrophysiological changes in striatal output neurons in Huntington's disease. J Neurosci. 2011;31:1170–1182. doi: 10.1523/JNEUROSCI.3539-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bolivar VJ, Manley K, Messer A. Early exploratory behavior abnormalities in R6/1 Huntington's disease transgenic mice. Brain Res. 2004;1005:29–35. doi: 10.1016/j.brainres.2004.01.021. [DOI] [PubMed] [Google Scholar]
- [41].Mochel F, Durant B, Durr A, Schiffmann R. Altered dopamine and serotonin metabolism in motorically asymptomatic R6/2 mice. PLoS ONE. 2011;6:e18336. doi: 10.1371/journal.pone.0018336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- [43].Hines RM, Wu L, Hines DJ, Steenland H, Mansour S, Dahlhaus R, Singaraja RR, Cao X, Sammler E, Hormuzdi SG, et al. Synaptic imbalance, stereotypies, and impaired social interactions in mice with altered neuroligin 2 expression. J Neurosci. 2008;28:6055–6067. doi: 10.1523/JNEUROSCI.0032-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Johnson KP. Glatiramer acetate for treatment of relapsing-remitting multiple sclerosis. Expert Rev Neurother. 2012;12:371–384. doi: 10.1586/ern.12.25. [DOI] [PubMed] [Google Scholar]