Abstract
Study Objectives:
To investigate the correlates of sleep disturbance and to assess escitalopram treatment effects of depression on sleep disturbance in patients with acute coronary syndrome (ACS).
Design:
A cross-sectional study in patients with ACS within 2 w post-ACS, and a 24-w double-blind controlled trial of escitalopram against placebo for patients with ACS who have comorbid depressive disorders.
Setting:
A university hospital in South Korea.
Participants:
There were 1,152 patients with ACS who were consecutively recruited. Of 446 patients with comorbid depressive disorders, 300 were randomized to the trial.
Measurements and Results:
Sleep disturbance was evaluated by the Leeds Sleep Evaluation Questionnaire. Demographic and clinical characteristics were assessed, including cardiovascular risk factors, current cardiac status, and depressive symptoms. Depressive symptoms were most strongly and consistently associated with sleep disturbance. In addition, older age, female sex, hypertension, and more severe ACS status were associated with certain aspects of sleep disturbance. Escitalopram was significantly superior to placebo for improving sleep disturbance over the 24-w treatment period. These effects were substantially explained by improvement in depressive symptoms.
Conclusions:
Depression screening is indicated in patients with acute coronary syndrome with sleep disturbance. Successful treatment of depression has beneficial effects on sleep outcomes in these patients.
Clinical Trials Information:
ClinicalTrial.gov identifier for the 24-w drug trial, NCT00419471.
Citation:
Kim JM, Stewart R, Bae KY, Kang HJ, Kim SW, Shin IS, Hong YJ, Ahn Y, Jeong MH, Yoon JS. Correlates and escitalopram treatment effects on sleep disturbance in patients with acute coronary syndrome: K-DEPACS and EsDEPACS. SLEEP 2015;38(7):1105–1111.
Keywords: acute coronary syndrome, depression, double-blind study, escitalopram, sleep disorders
INTRODUCTION
Sleep disturbance is common in patients with acute coronary syndrome (ACS: acute myocardial infarction or unstable angina). Community studies have reported a higher prevalence of insomnia in patients with ACS compared to those without.1,2 A clinical study reported that moderate or severe insomnia was reported by 37% of patients with ACS during hospitalization.3 Disturbed sleep has been associated with increased morbidity4 and worse quality of life5 in various physical disease contexts. Furthermore, poor sleep has been found to increase the risk for recurrent events in patients with ACS.6 ACS is the leading cause of disease burden worldwide7 and sleep disturbance, which is common and has an adverse effect on prognosis and may considerably aggravate disability in ACS.
Despite the clinical importance of sleep disturbance in ACS, previous research on this issue has been scarce. From studies of correlates of sleep disturbance in ACS, older age, female sex, smoking habits, and concomitant diseases have been implicated.3,8–10 A study investigating the association between cardiac status and sleep disturbance in ACS found that left ventricular ejection fraction (LVEF) was correlated with insomnia in univariate analysis but this association lost significance in multivariate models.3 With respect to psychological factors, depression has consistently been found to be associated with sleep disturbance in ACS even in multivariate analyses,3,9,11 although this may reflect the fact that sleep disturbance is one of the diagnostic criteria for depressive episodes.12 Limitations of previous studies have included small sample sizes, heterogeneous evaluation time points after the cardiac event, and limited measurements.3,8–11
Research into the treatment of sleep disturbance is also an important issue in ACS. However, as far as we aware, there has been no such study in these patients. Treatment of depression is one such intervention, because depression is closely associated with sleep problem and a modifiable factor. Several randomized controlled studies have reported successful depression treatment results with medication or cognitive behavioural therapy in patients with ACS13–16; however, none to date has investigated sleep disturbance as a treatment outcome.
To address these limitations and unanswered questions, we analyzed data from a naturalistic and interventional study in patients with ACS to investigate correlates of sleep disturbance comprehensively in a large sample, and to assess the effect of escitalopram treatment of depression on sleep disturbance using a placebo-controlled design.
METHODS
Study Overview and Participants
The study outline is presented in Figure 1 and its overall design and rationale have been published.17 To investigate the epidemiology of depressive disorders in patients with ACS using a naturalistic prospective design, the Korean DEPression in ACS (K-DEPACS) study was carried out beginning in 2006. Participants were consecutively recruited from patients recently hospitalized with ACS at the Department of Cardiology of Chonnam National University Hospital, Gwangju, South Korea. In 2005, this Department was nominated by the Korean Circulation Society to serve as the central coordinating center for the Korea Acute Myocardial Infarction Registry because of its large number of patients with ACS.18 Patients were treated based on international guidelines for the management of ACS19 by the study cardiologists. For the K-DEPACS study, participants were screened for depressive symptoms using the Beck Depression Inventory (BDI)20 at baseline as inpatients within 2 w post-ACS and thereafter as outpatients every 4 w up to 12 w if not positive for depression screening (BDI ≤ 10). Of these patients, the ones who met the eligibility criteria and agreed to participate comprised the sample for baseline analyses. For a nested trial of depressive disorder treatment, those with depressive symptoms (BDI > 10) on any of these occasions received a clinical evaluation by the study psychiatrists using the Mini-International Neuropsychiatric Interview (MINI),21 a structured diagnostic psychiatric interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV).12 Of the participants with a diagnosis of major or minor depressive disorder according to these criteria, those who agreed to participate were randomized to a 24-w, double-blind, placebo-controlled trial of escitalopram efficacy and safety for treating depressive disorders in ACS: the Escitalopram for DEPression in ACS (EsDEPACS) study (ClinicalTrial.gov registry number: NCT00419471), in which it was found that escitalopram was superior to placebo in both primary depressive and secondary outcomes.22 The first patient was enrolled in May 2007 and the last patient completed the follow-up evaluation in March 2013. Written informed consent was collected for the K-DEPACS and EsDEPACS studies, both of which were approved by the Chonnam National University Hospital Institutional Review Board.
Figure 1.
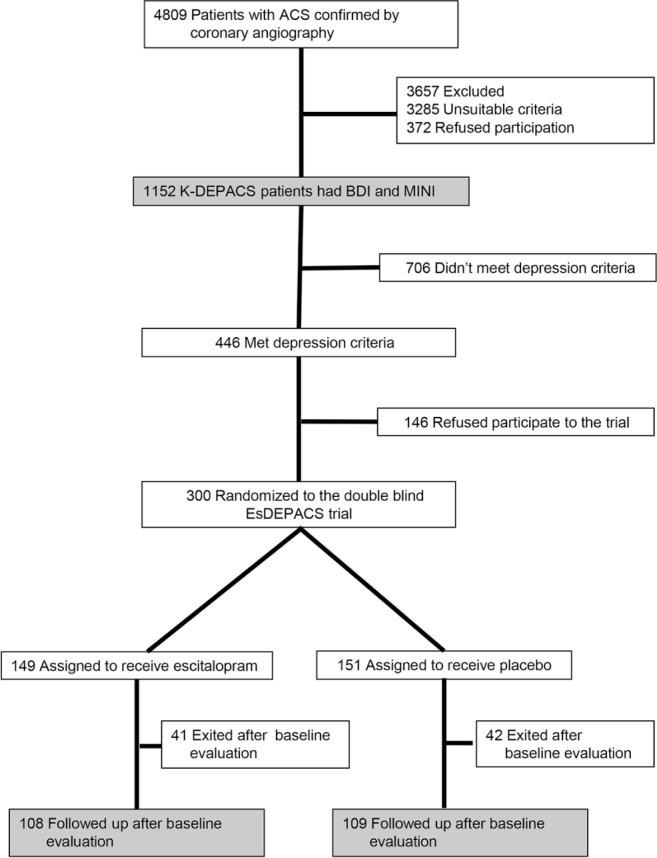
Participant recruitment process. ACS, acute coronary syndrome; BDI, Beck Depression Inventory; MINI, Mini-International Neuropsychiatric Interview; K-DEPACS, Korean DEPression in Acute Coronary Syndrome study; EsDEPACS, Escitalopram for DEPression in Acute Coronary Syndrome study.
Eligibility Criteria
For the K-DEPACS study entry, inclusion criteria were as follows: (1) aged 18∼85 y; (2) confirmed ACS by coronary angiography and laboratory tests; (3) ability to complete study questionnaires; and (4) ability to understand the study objectives and sign informed consent. Exclusion criteria were: (1) occurrence of ACS while hospitalized for another reason; (2) ACS developing less than 3 mo after a coronary artery bypass graft procedure; (3) uncontrolled hypertension (systolic blood pressure (BP) > 180 mmHg or diastolic BP > 100 mmHg); (4) resting heart rate < 40/min; (5) severe physical illnesses threatening life or interfering with the recovery from ACS; (6) persistent clinically significant laboratory abnormalities. For the EsDEPACS study entry, additional inclusion criteria were as follows: (1) BDI > 10; (2) major or minor depressive disorder according to DSM-IV criteria. Additional exclusion criteria were: (1) concomitant use of class I antiarrhythmic medications, reserpine, guanethidine, clonidine, methyldopa, lithium, anticonvulsants, antipsychotics, or antidepressants; (2) history of neuropsychiatric illnesses such as dementia, Parkinson disease, brain tumor, psychosis, bipolar disorder, alcoholism, or other substance dependence; (3) pregnancy; and (4) participating in other drug trials.
Intervention for the EsDEPACS Participants
The efficacy and safety of flexible doses of escitalopram (5 mg, 10 mg, 15 mg or 20 mg) were investigated using a randomized double-blind, placebo-controlled design. The escitalopram and placebo were provided by H. Lundbeck A/S. Patients were randomized on a 1:1 ratio following computer-generated randomization codes provided by a statistician independent of recruiting clinicians. Examinations were scheduled at baseline, and weeks 4, 8, 12, 16, 20, and 24 thereafter, with a 7-day allowable window. The initial escitalopram dose at baseline was 10 mg/day generally, but 5 mg/day for those aged 65 y or older or with hepatic dysfunction. After the second evaluation (week 4), the medication doses could be changed (from 5 mg/day to 20 mg/day) and determined by the investigators' clinical decision considering response and tolerability. Medications were taken once daily by mouth within 30 min after the supper meal. Adherence was checked by pill counts at every visit, and was defined as acceptable if at least 75%. At the end of 24 w of double-blind treatment, the study was completed and study medication was tapered down. Concomitant medication such as any other antidepressant, psychostimulant, antipsychotic, or anticholinergic agent was not permitted. However, transient use was allowed of analgesics, antipyretics, and cold medicines, hypnotics such as zolpidem or triazolam, and benzodiazepines. Subjects evaluated at least once after baseline comprised the sample for the drug trial analysis here.
Evaluations for Sleep Disturbance
The Leeds Sleep Evaluation Questionnaire (LSEQ)23 was administered to assess the extent of sleep disturbance at baseline and to evaluate the effect of escitalopram on sleep. The LSEQ captures subjective assessment of four factors using 100 mm line visual analog rating scales: ease of getting to sleep (GTS), quality of sleep (QOS), ease of awakening in the morning from sleep (AFS), and integrity of behavior following wakefulness (BFS). The LSEQ is a sensitive indicator for subjective sleep status change in this. Participants were required to rate how they perceived these four factors at the time of rating, by placing a vertical mark on the line (0–100 mm) to indicate their present self-evaluation. Higher scores indicate worse sleep condition. The LSEQ was evaluated at baseline as inpatients or outpatients and at every follow-up assessment point for the EsDEPACS trial.
Demographic and Clinical Characteristics
Demographic data on age, sex, and educational level were obtained. The following cardiovascular risk factors were ascertained at baseline: diagnosed hypertension and diabetes mellitus, hypercholesterolemia by fasting serum total cholesterol level (> 200 mg/dL), obesity by measured body mass index (BMI) (> 25 kg/m2), and reported current smoking status. Severity of ACS was estimated by the Killip classification,24 left ventricular ejection fraction (LVEF) was estimated using echo-cardiography results, and serum cardiac biomarkers troponin I and creatine kinase-MB (CK-MB) were measured. Depressive symptoms were evaluated by the Montgomery Asberg Depression Rating Scale (MADRS).25 This scale includes 10 items, excluding somatic symptoms of depression, in which higher scores indicate severe pathology. We excluded the scores on item 4, which evaluates reduced sleep, to avoid overlap with the LSEQ. The MADRS was administered at baseline and at every follow-up evaluation point for the escitalopram trial.
Statistical Analysis
To investigate the correlates of sleep disturbance, linear regression analyses were carried out for the associations between patient characteristics and scores on four factors of LSEQ, which scores as continuously distributed dependent variables. Variables significantly associated with each LSEQ score in unadjusted analyses (P < 0.05) were entered simultaneously into multiple regression models respectively to assess independence. To compare the 24-w treatment effect of escitalopram or placebo on sleep outcomes, subjects evaluated at least once after baseline were included in the analysis. Multiple imputation by chained equations was used for missing data due to discontinuation after postbaseline second (week 4) visits by treatment group, demographics (age and sex), and baseline measures on MADRS and Killip scores. Repeated- measures analyses of covariance were used to calculate group by time interactions on the four subscale scores of LSEQ, after adjustment for corresponding baseline scores and for baseline characteristics significantly associated with these (P < 0.05). To investigate effects of changes in depressive symptoms on the group by time interactions, changes in the MADRS score (i.e., score at the last observation minus score at baseline) were included further as an additional covariate in the repeated measures analyses of covariance. Bonferroni corrections were used to maintain an overall type I error rate of 0.05 against the multiple comparisons for four LSEQ outcomes: a two-sided P = 0.0125 (0.05/4) was used to define statistical significance. Statistical analyses were carried out using SPSS 21.0 (IBM) and STATA 12.0 (StataCorp) software.
RESULTS
Recruitment
The recruitment process is described in Figure 1. Having applied eligibility criteria, 1,152 patients with ACS comprised the K-DEPACS baseline sample. The main reason for exclusion for the K-DEPACS study was unsuitable criteria, particularly in severe physical comorbidity and significant laboratory anomalies; for the EsDEPACS trial it was concomitant medications. Of 446 participants with depressive disorders, 300 were randomized to the EsDEPACS trial. Of these, 83 participants (28%) exited from the study after baseline without follow-up, and the remaining 217 (108 on escitalopram and 109 on placebo) formed the sample for the 24-w trial analysis. Of the 217 participants, 142 (65%) enrolled as inpatients, and 75 (35%) as outpatients. The mean time from ACS to the baseline evaluation point was 29.0 days. Baseline characteristics of the remaining two treatment groups are summarized in the first and second columns of Table S1 (supplemental material); there were no significant differences between the two groups in any characteristic (all P > 0.2). The serum CK-MB level was significantly higher in patients exiting after baseline evaluation compared to those followed up (P = 0.043), but there were no significant differences in any other characteristic including scores on LSEQ at baseline between them (P > 0.05), as summarized in Table S1.
Correlates of Sleep Disturbance
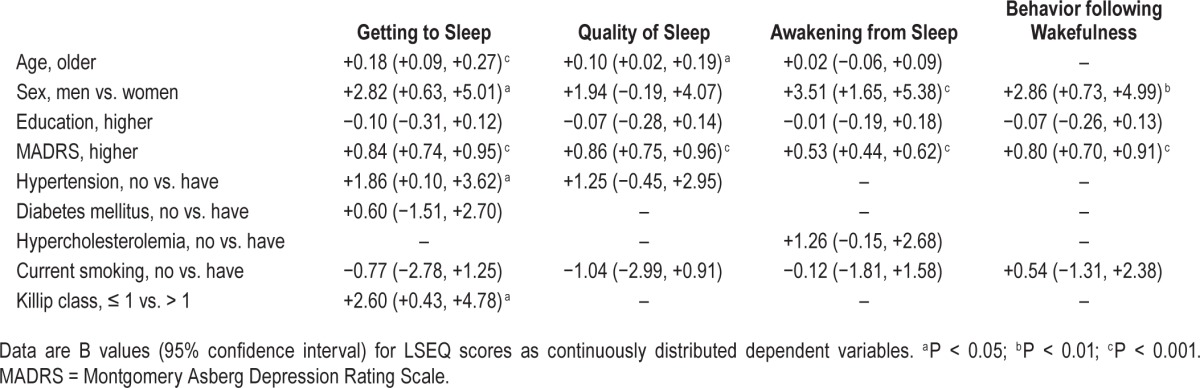
In the 1,152 K-DEPACS participants, the mean (standard deviation, SD) age was 58.6 (11.3) y, most were men (71.4%), the mean (SD) duration of education was 9.8 (4.7) y, the mean (SD) MADRS score was 6.5 (6.9). With respect to cardiovascular risk factors, hypertension was present in 556 participants (48.3%), diabetes in 235 (20.4%), hypercholesterolemia in 572 (49.7%), obesity in 490 (42.5%), and current smoking in 416 (36.1%). With respect to cardiac status, Killip class was > 1 in 206 (17.9%), mean (SD) LVEF was 59.7 (11.4%) and mean (SD) troponin I and CK-MB levels were 8.7 (14.8) and 18.4 (37.6) mg/ dL, respectively. Mean (SD) scores on four factors of LSEQ and unadjusted associations between the baseline characteristics are summarized in Table 1. The GTS factor was significantly associated with older age, female sex, lower education, higher depression scores, hypertension, diabetes, non-smoking status, and more severe ACS; the QOS factor with older age, female sex, lower education, higher depression scores, hypertension, and nonsmoking status; the AFS factor with older age, female sex, lower education, higher depression scores, hypercholesterolemia, and nonsmoking status; and the BFW factor with female sex, lower education, higher depression scores, and non-smoking status. Adjusted associations between simultaneously entered baseline characteristics and LSEQ scores are summarized in Table 2. Higher depression scores were most significantly and consistently associated with all four LSEQ factors, and associations with education, diabetes, hypercholesterolemia, and smoking status were no longer apparent in the adjusted analyses. The GTS factor was independently associated with older age, female sex, higher depression scores, hypertension, and more severe ACS status; the QOS factor with older age and higher depression scores; the AFS factor with female sex and higher depression scores; and the BFW factor with female sex, and higher depression scores. Similar unadjusted analyses were carried out in the EsDEPACS sample (N = 271), the results of which are summarized in Table S2 (supplemental material). The strengths of the associations were weaker in this group in that the GTS factor was associated with lower education and higher depression scores; the QOS factor with higher depression scores; the AFS factor with hypercholesterolemia; and the BFW factor with higher depression scores.
Table 1.
Unadjusted associations between patient characteristics and scores on the four factors of the Leeds Sleep Evaluation Questionnaire (LSEQ) (N = 1,152).
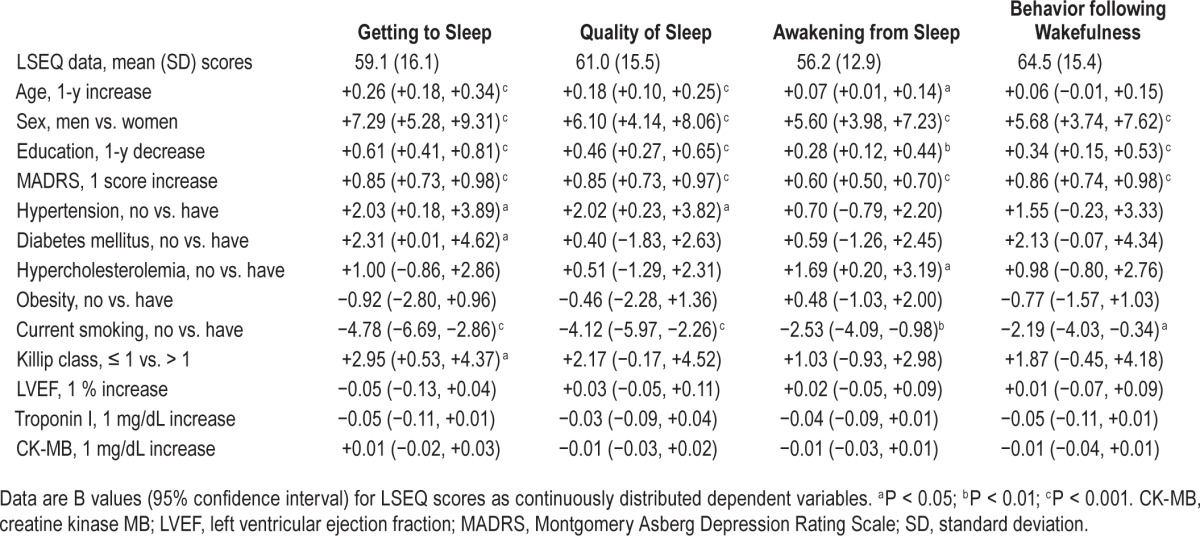
Table 2.
Multivariate associations with scores on four factors of Leeds Sleep Evaluation Questionnaire (LSEQ) (N = 1,152).
Comparison of Escitalopram and Placebo on LSEQ scores
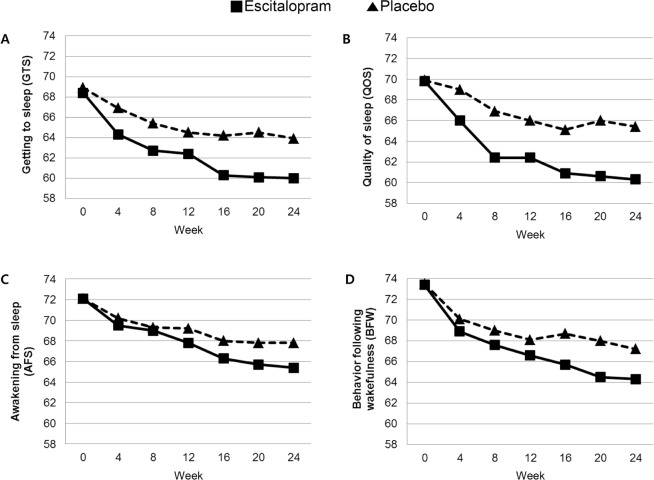
Scores on the four LSEQ factors in the 24-w double-blind trial of escitalopram against placebo are displayed and compared between intervention groups in Figure 2. With respect to treatment characteristics, the mean (SD) doses at the last visit were 7.6 (3.7) mg for the escitalopram group and 8.5 (3.9) mg for the placebo group, and the mean (SD) treatment durations were 138.3 (22.4) and 138.0 (22.9) days, respectively. In addition, there were no significant differences in any aspects related to study drug treatments on doses and duration, concomitant medications, and discontinuation between the escitalopram and placebo groups (Table S3, supplemental material). There were significant group by time interactions on three LSEQ factor scores (GTS, QOS, and BFW) after adjustment for corresponding trial baseline scores and characteristics significantly associated with corresponding LSEQ factor scores at baseline (P < 0.0125, applying a Bonferroni correction for four outcomes). The escitalopram group therefore showed significantly more improvement in three LSEQ factors compared to placebo group in the 24-w treatment period. The mean (SD) changes in MADRS scores were −8.9 (8.8) for the escitalopram group and −4.8 (7.4) for the placebo group, a statistically significant difference (P < 0.001). After further inclusion of MADRS score changes from baseline to week 24 as a covariate in the analyses, the strength of interaction terms for all four LSEQ factor scores were substantially weakened and lost significance when a Bonferroni correction was applied. Hypnotics or benzodiazepines were taken by three and five patients in the escitalopram group and the placebo group, respectively, during the treatment period. When the same analyses were repeated after excluding these participants, the results were not changed substantially (data not shown).
Figure 2.
Adjusted mean scores on the four factors of Leeds Sleep Evaluation Questionnaire (LSEQ) in the 24-w double-blind treatment of escitalopram and placebo (N = 217). Statistical coefficients were driven from repeated -measures analysis of covariance to calculate group by time interactions on the four domain scores of LSEQ. (A) Statistics adjusted for baseline GTS scores, age, sex, education, hypertension, and smoking status: F = 9.447, P = 0.003, and further adjusted for changes in MADRS scores: F = 3.783, P = 0.054. (B) Statistics adjusted for baseline QOS scores, age, sex, education, hypertension, diabetes, smoking status, and Killip class: F = 12.87, P < 0.001, and further adjusted for changes in MADRS scores: F = 4.236, P = 0.039. (C) Statistics adjusted for baseline AFS scores, age, sex, education, hypercholesterolemia, and smoking status: F = 3.156, P = 0.072, and further adjusted for changes in MADRS scores: F = 1.821, P = 0.171. (D) Statistics adjusted for baseline BFW scores, sex, education, and smoking status: F = 6.945, P = 0.009, and further adjusted for changes in MADRS scores: F = 2.139, P = 0.141. MADRS, Montgomery Asberg Depression Rating Scale.
DISCUSSION
Principal findings of this study in patients with recent ACS were that all aspects of sleep disturbance evaluated by LSEQ were strongly associated with depressive symptoms in the acute phase of ACS, and that certain aspects of sleep disturbance were associated with older age, female sex, hypertension, and more severe ACS status. The 24-w escitalopram intervention was significantly more effective for improving sleep disturbance compared with placebo, and these effects were substantially explained by changes in depressive symptoms.
It is noteworthy that most previous studies on correlates of sleep disturbance have focused on long-term outcomes from 4 mo to 7 y following the index ACS episode.8–11 Patients in the current study were ACS survivors who were assessed within 2 w of the index episode during hospitalization. We expected that physical measures such as ACS severity or medical comorbidity would be the primary factors associated with sleep disturbance in these patients, but instead found stronger and more consistent associations with depressive symptoms, even during the acute phase. This finding was thus consistent with those of previous studies evaluating sleep disturbance at the chronic phase of ACS.9,11 These findings suggest that depressive symptoms should be evaluated preferentially in patients with ACS who complain about or report sleep disturbance regardless of the disease phase.
Older age and female sex were associated with certain but not all aspects of sleep disturbance. These findings were consistent with those from previous studies not only in patients with ACS3,8–10 but in other physical disorders1,4 and in general populations.26 Age and sex could be considered as universal risk factors for sleep disturbance in people with and without physical diseases. A previous study in ACS found no significant association between hypertension and insomnia,3 although, insomnia has been found to be associated with hypertension in cross-sectional and prospective analyses of community samples,27,28 and in our study hypertension was independently associated with the GTS factor of the LSEQ. However, sleep disturbance was not ascertained before the index ACS episode and thus the causal relationship underlying the association with hypertension cannot be inferred. We also found an independent association between more severe ACS status, evaluated by the Killip classification, and the GTS factor of LSEQ, which we believe is novel in this field. This association might reflect higher levels of physical discomfort and psychological distress contributing to sleep disturbance.29
Because depression is consistently associated with sleep disturbance, it was thought to be worthwhile to evaluate whether treatment of depression has beneficial effects on sleep in ACS. As stated earlier, no study has reported this outcome despite several randomized controlled trials of depression treatment in ACS.13–16 We found a significantly superior effect of escitalopram over placebo on all aspects of sleep disturbance in a 24-w, double-blind trial irrespective of hypnotics or benzodiazepines use. This was in keeping with a recent meta-analysis of 22 randomized controlled trials in major depressive disorder, which concluded a beneficial effect of escitalopram in the treatment of sleep problems.30 Several mechanisms may underlie this finding. First, the beneficial effects on sleep outcomes are likely to have been mediated at least in part by the depression treatment effect. Depressive symptoms measured by the MADRS were significantly more improved in those receiving escitalopram than in those receiving placebo and treatment effects on sleep were substantially explained by this in adjusted analyses, although the beneficial effect on quality of sleep remained independent. Second, escitalopram may have an indirect effect on improving sleep quality. A trial with healthy, nonde-pressed perimenopausal and postmenopausal women with hot flushes found that escitalopram reduced insomnia symptoms compared with placebo, and improved subjective sleep quality along with improvement in hot flushes over 8 w of follow-up.31 Thus, escitalopram might be associated with better sleep by decreasing somatic discomfort. Third, escitalopram may have direct effects on sleep architecture. An animal study reported that escitalopram had the ability to suppress rapid eye movement sleep (REMS) rebound despite a high REMS pressure caused by REMS deprivation; this suggests a connection between serotonergic and melatonin systems, which are relevant in sleep regulation.32 Our findings could also be interpreted on the other way in that the beneficial effects of escitalopram on sleep resulted in improved depression, since sleep disturbance was closely and reciprocally related to depression.4
Our study had several strengths. The cross-sectional observational element involved the largest sample size to date. Participants were recruited consecutively from all eligible patients with recent ACS and were evaluated within 2 weeks after ACS at the hospital setting, which reduced the risk of error arising from heterogeneous examination times. As well as being the first treatment evaluation of sleep outcomes in ACS, the intervention was a robustly designed randomized placebo-controlled trial. The LSEQ has been well validated for monitoring subjectively perceived changes in sleep during psychopharmacological investigations involving a variety of psychoactive agents23 and is frequently used for measuring sleep outcomes in antidepressant trials.33 All the other measurements of psychiatric and cardiovascular status were also well validated, and a range of covariates were considered in the analyses.
An important limitation of the study was that data on sleep disturbance were obtained by subjective reports only, and future studies using objective sleep measurements such as polysomnography are worth considering. Recruitment was carried out at a single site, which may limit the study's generalizability but add strength in terms of consistency in the evaluation and treatment of patients. In the EsDEPACS trial, 28% of the randomized subjects exited the study shortly after baseline evaluation. Because these patients were not evaluated further, the reasons for attrition remain uncertain. Because serum CK-MB levels of those who exited the study were significantly higher compared to those followed up, it can be assumed that more severe ACS pathology might be associated with the attrition. However, there were no significant differences in any other baseline variables, including sleep measures between them. Because the sleep disturbance was one of the secondary outcomes of the EsDEPACS trial, it would need replication of our findings in a future trial with sleep problems as the primary outcome. Anxiety symptoms were not evaluated in this study, in which the favorable effects of escitalopram on sleep could be explained by benefits to anxiety.34 Finally, sleep apnea is very common in patients with ACS and can contribute significantly to sleep disturbance,35 but unfortunately was not evaluated.
In conclusion, we found that depression was a strong, independent correlate of sleep disturbance even in the acute phase of ACS, and that successful treatment of depression with escitalopram had significant beneficial effects for improving sleep disturbance in those patients. These results underscore the importance of depression screening and treatment in patients with ACS suffering from sleep disturbance even during the acute phase. Also, evaluation of sleep disturbance could be recommended in patients with ACS for decreasing disease burden. Escitalopram can be recommended for this purpose, although its relative efficacy was not evaluated in comparison with other antidepressants. Considering future research, replication of our findings in larger multicenter settings would increase generalizability. Longer term and treatment effects of sleep disturbance on the course and prognosis of ACS are also yet to be determined. A randomized trial comparing antidepressants and hypnotics might be helpful to compare efficacy and tolerability.
DISCLOSURE STATEMENT
This study was supported by grants of the Korea Health 21 R&D, Ministry of Health and Welfare, Republic of Korea (HI12C0003), and was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (NRF-2013R1A2A2A01067367) to Dr. Kim. Dr. Stewart is partly-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. Dr. Kim has received grants from Ministry of Health and Welfare, Republic of Korea, Lundbeck, Lilly, and Otsuka. Dr. Stewart has received research funding from Pfizer, Lundbeck, J&J, and Roche. The other authors have indicated no financial conflicts of interest.
Footnotes
A commentary on this article appears in this issue on page 1001.
SUPPLEMENTAL MATERIAL
PSYCHIATRIC ASSESSMENT FOR THE K-DEPACS AND ESDEPACS STUDY
Measurements of depressive symptoms included the 17-item Hamilton Depression Rating Scale (HAMD),1 the Montgomery–Asberg Depression Rating Scale (MADRS),2 the Beck Depression Inventory (BDI),3 and the Clinical Global Impression Scale-severity (CGI-s).4 We used four scales because they differed from one another in several ways: the HAMD is the observer-rated scale most widely used in research on depression related to acute coronary syndrome (ACS)5; the MADRS (another observer-rated scale) does not include items for evaluating the somatic symptoms of depression, which may be difficult to differentiate from the physical consequences of ACS; the BDI is a self-report measure; and the CGI-s assesses global symptomatology and can be used quickly in a busy clinical setting. Scales used to assess other psychiatric symptoms included the Social and Occupational Functioning Assessment Scale (SOFAS),6 the World Health Organization Disability Assessment Schedule-12 (WHODAS-12),7 and the World Health Organization Quality of Life Scale-abbreviated form (WHOQOL-BREF).8 We included these measures given recent recommendations that depression outcome research involve multifaceted evaluation (i.e., more than only the HAMD) to address psychological well-being and functioning.9 All assessment scales have been formally translated and standardized in Korean.10–16
Comparison of baseline characteristics between the EsDEPACS participants who were followed up and those who exited after the baseline evaluation.
Unadjusted associations between EsDEPACS patient characteristics and scores on the four factors of the Leeds Sleep Evaluation Questionnaire (LSEQ) (N = 217).
Intervention dosages and cardiovascular medications.
REFERENCES
- 1.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 3.Beck AT, Ware CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 4.Guy W. ECDEU Assessment manual for psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976. Revised. [Google Scholar]
- 5.Davidson KW, Kupfer DJ, Bigger JT, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med. 2006;68:645–50. doi: 10.1097/01.psy.0000233233.48738.22. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Press Inc.; 1994. [Google Scholar]
- 7.Eipping-Jordan J, Ustun TB. Assessment, classification and epidemiology group. The WHODAS II: levelling the playing field for all disorders. WHO Mental Health Bull. 2000;6:5–6. [Google Scholar]
- 8.The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28:551–8. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 9.Fava GA, Ruini C, Belaise C. The concept of recovery in major depression. Psychol Med. 2007;37:307–17. doi: 10.1017/S0033291706008981. [DOI] [PubMed] [Google Scholar]
- 10.Ahn YM, Lee KY, Yi JS, et al. A validation study of the Korean-version of the Montgomery-Asberg Depression Rating Scale. J Korean Neuropsychiatr Assoc. 2005;44:466–76. [Google Scholar]
- 11.Chung YC, Rhee MK, Lee YH, et al. Standardization study of Beck Depression Inventory 1-Korean version (K-BDI): reliability and factor analysis. Korean J Psychopathol. 1995;4:77–95. [Google Scholar]
- 12.Kim JM, Stewart R, Glozier N, et al. Physical health, depression and cognitive function as correlates of disability in an older Korean population. Int J Geriatr Psychiatry. 2005;20:160–7. doi: 10.1002/gps.1266. [DOI] [PubMed] [Google Scholar]
- 13.Lee JY, Cho MJ, Kwon JS. Global assessment of functioning scale and social and occupational functioning scale. Korean J Psychopharmacol. 2006;17:122–7. [Google Scholar]
- 14.Yi JS, Bae SO, Ahn YM, et al. Validity and reliability of the Korean version of the Hamilton Depression Rating Scale (K-HDRS) J Korean Neuropsychiatr Assoc. 2005;44:456–65. [Google Scholar]
- 15.Yoo SW, Kim YS, Noh JS, et al. Validity of Korean version of the Mini-International Neuropsychiatric Interview. Anxiety Mood. 2006;2:50–5. [Google Scholar]
- 16.Kim SY, Kim JM, Yoo JA, et al. Standarization and validation of Big Five Inventory-Korean Version(BFI-K) in elders. Korean J Biol Psychiatry. 2010;17:15–25. [Google Scholar]
REFERENCES
- 1.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 3.Coryell VT, Ziegelstein RC, Hirt K, Quain A, Marine JE, Smith MT. Clinical correlates of insomnia in patients with acute coronary syndrome. Int Heart J. 2013;54:258–65. doi: 10.1536/ihj.54.258. [DOI] [PubMed] [Google Scholar]
- 4.Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS. Insomnia, depression, and physical disorders in late life: a 2-year longitudinal community study in Koreans. Sleep. 2009;32:1221–8. doi: 10.1093/sleep/32.9.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51:229–35. [PubMed] [Google Scholar]
- 6.Leineweber C, Kecklund G, Janszky I, Akerstedt T, Orth-Gomér K. Poor sleep increases the prospective risk for recurrent events in middle-aged women with coronary disease. The Stockholm Female Coronary Risk Study. J Psychosom Res. 2003;54:121–7. doi: 10.1016/s0022-3999(02)00475-0. [DOI] [PubMed] [Google Scholar]
- 7.Murray CJL, Vos T, Lazano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 8.Bengtson A, Karlsson T, Herlitz J. Differences between men and women on the waiting list for coronary revascularization. J Adv Nurs. 2000;31:1361–7. doi: 10.1046/j.1365-2648.2000.01403.x. [DOI] [PubMed] [Google Scholar]
- 9.Billing E, Eriksson SV, Hjemdahl P, Rehnqvist N. Psychosocial variables in relation to various risk factors in patients with stable angina pectoris. J Intern Med. 2000;247:240–8. doi: 10.1046/j.1365-2796.2000.00590.x. [DOI] [PubMed] [Google Scholar]
- 10.Edéll-Gustafsson U, Svanborg E, Swahn E. A gender perspective on sleeplessness behavior, effects of sleep loss, and coping resources in patients with stable coronary artery disease. Heart Lung. 2006;35:75–89. doi: 10.1016/j.hrtlng.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Johansson I, Karlson BW, Grankvist G, Brink E. Disturbed sleep, fatigue, anxiety and depression in myocardial infarction patients. Eur J Cardiovasc Nurs. 2010;9:175–80. doi: 10.1016/j.ejcnurse.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Press Inc.; 1994. [Google Scholar]
- 13.Roose SP, Laghrissi-Thode F, Kennedy JS, et al. Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. JAMA. 1998;279:287–91. doi: 10.1001/jama.279.4.287. [DOI] [PubMed] [Google Scholar]
- 14.Strik JJ, Honig A, Lousberg R, et al. Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: findings from a double-blind, placebo-controlled trial. Psychosom Med. 2000;62:783–9. doi: 10.1097/00006842-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Glassman AH, O'Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–9. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 16.Lespérance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297:367–79. doi: 10.1001/jama.297.4.367. [DOI] [PubMed] [Google Scholar]
- 17.Kim JM, Bae KY, Kang HJ, et al. Design and methodology for the Korean observational and escitalopram treatment studies of depression in acute coronary syndrome: K-DEPACS and EsDEPACS. Psychiatry Investig. 2014;11:89–94. doi: 10.4306/pi.2014.11.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong YJ, Jeong MH, Ahn Y, Kang JC. The efficacy and safety of drug-eluting stents in patients with acute myocardial infarction: results from Korea Acute Myocardial Infarction (KAMIR) Int J Cardiol. 2013;163:1–4. doi: 10.1016/j.ijcard.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JL, Adams CD, Antman EM, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e179–347. doi: 10.1016/j.jacc.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 21.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini-international neuropsychiatric interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:S22–33. [PubMed] [Google Scholar]
- 22.Kim JM, Bae KY, Jung BO, et al. Escitalopram treatment for depressive patients with acute coronary syndrome: a 24 week double blind placebo controlled trial. J Clin Psychiatry. 2015;76:62–8. doi: 10.4088/JCP.14m09281. [DOI] [PubMed] [Google Scholar]
- 23.Parrot AC, Hindmarch I. The Leeds Sleep Evaluation Questionnaire in psychopharmacological investigation-a review. Psychopharmacology (Berl) 1980;71:173–9. doi: 10.1007/BF00434408. [DOI] [PubMed] [Google Scholar]
- 24.Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20:457–64. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 26.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 27.Budhiraja R, Roth T, Hudgel DW, Budhiraja P, Drake CL. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34:859–67. doi: 10.5665/SLEEP.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haaramo P, Rahkonen O, Hublin C, Laatikainen T, Lahelma E, Lallukka T. Insomnia symptoms and subsequent cardiovascular medication: a register-linked follow-up study among middle-aged employees. J Sleep Res. 2014;23:281–9. doi: 10.1111/jsr.12116. [DOI] [PubMed] [Google Scholar]
- 29.Vaz Fragoso, Gill TM. Sleep complaints in community-living older persons: a multifactorial geriatric syndrome. J Am Geriatr Soc. 2007;55:1853–66. doi: 10.1111/j.1532-5415.2007.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein DJ, Lopez AG. Effects of escitalopram on sleep problems in patients with major depression or generalized anxiety disorder. Adv Ther. 2011;28:1021–37. doi: 10.1007/s12325-011-0071-8. [DOI] [PubMed] [Google Scholar]
- 31.Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19:848–55. doi: 10.1097/gme.0b013e3182476099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kátai Z, Adori C, Kitka T, et al. Acute escitalopram treatment inhibits REM sleep rebound and activation of MCH-expressing neurons in the lateral hypothalamus after long term selective REM sleep deprivation. Psychopharmacology (Berl) 2013;228:439–49. doi: 10.1007/s00213-013-3046-4. [DOI] [PubMed] [Google Scholar]
- 33.Zajecka J, Schatzberg A, Stahl S, Shah A, Caputo A, Post A. Efficacy and safety of agomelatine in the treatment of major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2010;30:135–44. doi: 10.1097/JCP.0b013e3181d420a7. [DOI] [PubMed] [Google Scholar]
- 34.Stein DJ, Lopez AG. Effects of escitalopram on sleep problems in patients with major depression or generalized anxiety disorder. Adv Ther. 2011;28:1021–37. doi: 10.1007/s12325-011-0071-8. [DOI] [PubMed] [Google Scholar]
- 35.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of baseline characteristics between the EsDEPACS participants who were followed up and those who exited after the baseline evaluation.
Unadjusted associations between EsDEPACS patient characteristics and scores on the four factors of the Leeds Sleep Evaluation Questionnaire (LSEQ) (N = 217).
Intervention dosages and cardiovascular medications.